Abstract
The taxonomy of Culex pipiens complex of mosquitoes is still debated, but in North America it is generally regarded to include Culex pipiens pipiens, Culex pipiens molestus, and Culex quinquefasciatus (or Culex pipiens quinquefasciatus). Although these mosquitoes have very similar morphometry, they each have unique life strategies specifically adapted to their ecological niche. Differences include the capability for overwintering diapause, bloodmeal preference, mating behaviors, and reliance on blood meals to produce eggs. Here, we used RNA-seq transcriptome analysis to investigate the differential gene expression and nucleotide polymorphisms that may link to the divergent traits specifically between Cx. pipiens pipiens and Cx. pipiens molestus.
Keywords: Culex pipiens complex, RNA-seq, genetic variants
Culex pipiens complex mosquitoes transmit numerous pathogens responsible for human diseases including West Nile encephalitis, Rift Valley fever, and Lymphatic filariasis (Meegan 1979, Tsai and Mitchell 1989, Atkinson et al. 1995, Hubalek and Halouzka 1999, Lai, Tung et al. 2000, Lanciotti, Kerst et al. 2000, Fonseca, Keyghobadi et al. 2004, Kimura, Darbro et al. 2010, Michalski, Erickson et al. 2010). Culex pipiens pipiens and Cx. pipiens molestus are two biotypes included in the Cx. pipiens complex of mosquitoes (Vinogradova 2000, Shaikevich and Vinogradova 2004, Reusken et al. 2010). Although both mosquitoes are found in urban areas, Cx. pipiens pipiens mosquitoes reside above ground and established worldwide distribution long before the global spread of Cx. pipiens molestus. In contrast, Cx. pipiens molestus mosquitoes were not established until the 20th century and inhabit subterranean, man-made infrastructure such as sewers and tunnels (Byrne and Nichols 1999, Vinogradova 2000, Shaikevich and Vinogradova 2004, Kothera et al. 2010, Reusken et al. 2010). As such, although the biotypes share morphological characteristics, Cx. pipiens pipiens and Cx. pipiens molestus mosquitoes have evolved to unique suites of eco-physiological traits in order to survive two very distinct ecological niches. Key differences between the biotypes include from breeding site preference, bloodmeal preference, mating patterns, egg production, and overwintering diapause (Barr 1957, Spielman 1967, Harbach et al. 1984, Clements 1992, Byrne and Nichols 1999, Sim and Denlinger 2008, 2013, Sim et al. 2015, Kim et al. 2018).
Although the epigenous (above ground) Cx. pipiens pipiens thrive in the open (eurygamy), the hypogenous (below ground) Cx. pipiens molestus have adapted to reproduce in restricted spaces (stenogamy). Consequently, the feeding and oviposition (egg laying) preferences of Cx. pipiens molestus have appropriately shifted from the ornithophilic (bird biting) and anautogeneous (eggs laying requires a bloodmeal) patterns of their pipiens cousins to mammalophilic (mammal biting) and autogenous (egg laying without bloodmeal) behavior that are more appropriate for the type and availability of subterranean mammalian hosts (Mattingly 1967, Harbach et al. 1985, Vinogradova 2000). Finally, diapause is an anticipated, alternative developmental program resulting in dormancy that Cx. pipiens pipiens mosquitoes use to survive harsh winters, but is absent in the below ground Cx. pipiens molestus (Tauber et al. 1986).
Although phenotypic divergence between the two biotypes has been studied in detail, very few studies, with the notable exception of a recent transcriptome study, have investigated the genetic variance of the unique traits from each form (Kang and Sim 2013, Arthofer et al. 2015, Price and Fonseca 2015). Fortunately, the Cx. quinquefasciatus genome is available as a reference for comparative genomics study in Cx. pipiens complex species (Arensburger et al. 2010). In this study, we used RNA-Seq to simultaneously identify sequence polymorphisms and quantify differences in transcript abundance between adult females of the two biotypes 1 wk after adult eclosion. We further validated candidate genes with qRT-PCR and subsequently identified five genes with differences in transcript abundance between the two biotypes. These five genes appear to be involved in diverse biological processes including cuticle formation, juvenile hormone synthesis regulation, olfaction, insecticide resistance, and cellular immunity. From these five genes, 29 single nucleotide polymorphisms (SNPs) were identified in four transcripts (SNPs: Hex-1, Jhest, Cath and Odor; conserved Cut-1). These genes are promising candidates for further functional studies of potential SNPs associated with biotype-specific traits in Cx. pipiens complex species. The deeper understanding of the genetic basis of divergent traits, showed by this study, may provide insights that facilitate development of novel methods for vector-based control of disease.
Materials and Methods
Mosquito Rearing
Culex pipiens pipiens and Cx. pipiens molestus specimens were raised under 75% relative humidity, 25°C, and a 15 h light: 9 h dark (L:D) daily light cycle. Larvae were maintained on Tetramin fish food (Tetra holding Inc., Blacksburg, VA), whereas adults were maintained on honey-soaked sponges. Culex pipiens pipiens colony was established in September 2000 from larvae collected in Columbus, OH, and additional field-collected mosquitoes were added to the laboratory colony in 2009 (Meuti et al 2015). Culex. pipiens molestus colony were provided by Dr. Linda Kothera at the Centers for Disease Control and Prevention Division of Vector Borne Infectious Diseases at Fort Collins, Colorado and originated from the Calumet Water Reclamation Plant in Chicago, Illinois (Mutebi and Savage 2009).
Sequencing, Reference Mapping, and Clustering
Total RNA extraction was performed 7 d after adult eclosion using a standard TRIzol method (Life Technologies, Carlsbad, CA), and RNA concentrations were measured by NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). Each biological replicate was collected from three batches of 15 adult female mosquitoes of Cx. pipiens pipiens and those of Cx. pipiens molestus. Three biological replicates were then pooled for library preparation and sequencing.
After TruSeq mRNA library construction, the samples were sequenced on an Illumina HiSeq platform (Illumina Inc., San Diego, CA). First, the splice junctions were mapped via TopHat (v1.3.3), and then transcripts were assembled and tested for differences in abundance using Cufflinks (v2.0.2) (Langmead et al. 2009, Trapnell et al. 2010). Cuffdiff was then used to examine differences in expression between the two samples. Throughout this process, the genome of Cx. quinquefasciatus (Johannesburg strain version 2.2) served as a reference genome. Differences of transcript abundance between two biotypes were then visualized using a volcano plot based on FPKM (Fragments per Kilobase of exon per Million fragments mapped) and statistical significance threshold (P ≤ 0.05).
The DAVID (Database for Annotation, Visualization and Integrated Discovery) bioinformatics resource v 6.7 was then used to identify and categorize significantly divergent transcripts based on ontology (Huang da et al. 2009 a,b). Genes were further classified by biological process, cellular component, and molecular function via the GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) databases.
Variant Discovery and Annotation
Cleaned and trimmed HiSeq reads were aligned to the genome of Cx. quinquefasciatus (Johannesburg strain version 2.4) with TopHat. Subequently, Freebayes (Garrison and Marth 2012), a haplotype-based variant detector, was utilized in the identification of SNP variation. SNP and indel discovery as well as genotyping between two samples was performed simultaneously using hard filtering parameters appropriate for RNA-seq data. Prior to variant discovery, reads in regions identified as possible indels were realigned in Freebayes as recommended (Garrison and Marth 2012).
To increase the confidence of variant calls, we filtered out low-quality variants with a DP < 10 and QUAL < 40 using VCFannotate and VCFfilter (Danecek et al. 2011). DP combines depth across samples and QUAL is scales PHRED probability of variation (REF/ALT). Thus, combining the two filter sets is expected to produce high-quality results for subsequent analysis.
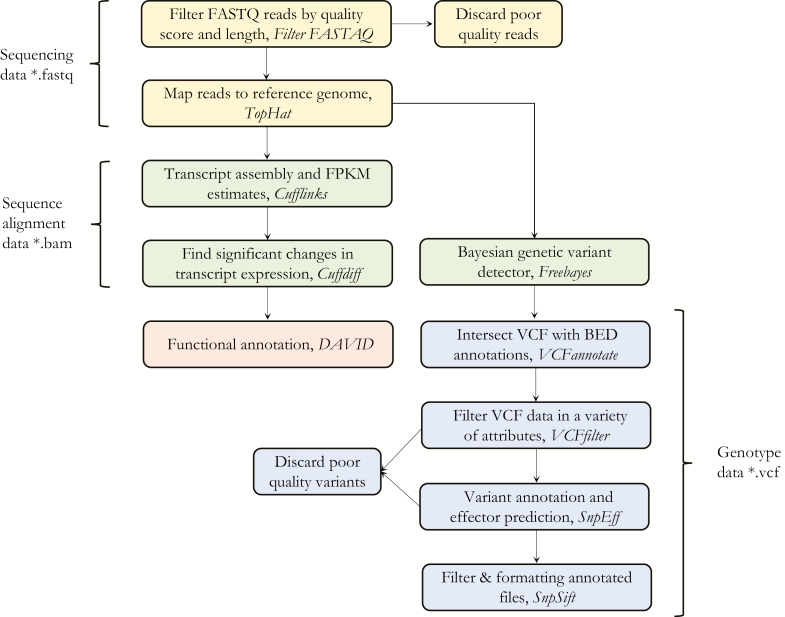
The localization of SNPs/indels in both genic and intergenic regions was based on annotation of gene models provided by the Culex quinquefasciatus genome database. The putative SNPs/indels were tagged and scored as coding/noncoding, silent/missense with strand positions, and ontology via SnpEff (Cingolani et al. 2012) and SnpSift (Kumar et al. 2009). Genes with significant differential expression between the biotypes were given careful consideration while testing for SNPs/indels. The localization of SNPs/indels were categorized based on coding-regions, 5′-upstream or 3′-downstream regions. The bioinformatics tools used study FPKM and the putative SNPs/indels are summarized in the flow charts (Fig. 1).
Fig. 1.
Flow charts for the RNA-sequencing data analysis. Data file types are indicated at the either side (*). Each group of node is also colored by file type. Software platforms are shown in italics.
The Putative SNPs/Indels Among the Five Validated Transcripts by qRT–PCR
Five transcripts were then selected based on fold change, statistical significance, and relevant ontologies as targets for vector control. An Qiagen’s Rotor-Gene Q real-time PCR detection system was then used for qRT-PCR validation with ribosomal protein L19 (RpL19) serving as an internal control. Statistical significance was calculated by Student t test. Primer information can be found in Supp Table 1 (online only).
The putative SNPs/indels were identified among these five genes which show differential expression between two biotypes. The localization of SNPs/indels was tagged by coding/noncoding regions and silent/missense/nonsense mutations.
Results
Data Analysis
After sequencing by HiSeq 2000, Culex pipiens pipiens samples produced 32,916,095 total read pairs with an average read length of 101 base pairs and 6,649,051,190 total bases. Culex pipiens molestus yielded 29,628,110 total read pairs with an average read length of 101 base pairs and 5,984,878,220 total bases read. The number of reads between the two groups was not statistically significant (P = 0.4). After discarding low-quality reads, trimming adaptor sequences, and eliminating poor-quality bases on an Illumina HiSeq platform, 56.76–59.05% of the Cx. pipiens pipiens transcript reads and 37.47–39.25% of the Cx. pipiens molestus uniquely aligned to the reference Cx. quinquefasciatus genome.
Differential Expression
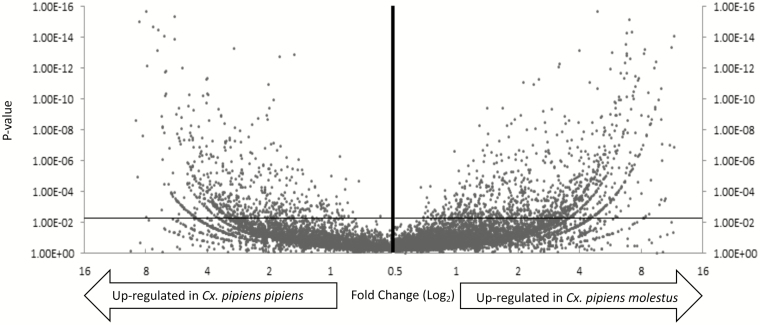
Culex quinquefasciatus, Cx. pipiens pipiens, and Cx. pipiens molestus females shared a high level of homology. When compared with the Cx. quinquefasciatus reference genome Cx. pipiens pipiens only presented 3,789 novel reads out of 32,916,095 total reads and Cx. pipiens molestus expressed 4,784 unique reads out of 29,628,110 total reads. Culex pipiens pipiens yielded 20,041 alternative splices and form molestus yielded 19,367 alternative splices. Finally, the pipiens biotype presented 9,192 potentially novel isoforms while molestus yielded 8,148 novel isoforms. Cufflinks analysis of mapped reads to the reference genome was used to calculate difference in transcript abundance, which was expressed as FPKM (Fig. 2 and Supp File 1 [online only]).
Fig. 2.
Volcano plot of Cx. pipiens molestus specific up- (right arrow) and Cx. pipiens pipiens specific up-regulated (left arrow) genes. The x-axis specifies the fold-changes and the y-axis specifies the negative logarithm to the base 10 of the t-test p-values. The horizontal line indicates the filtering criteria (P-value = 0.05)
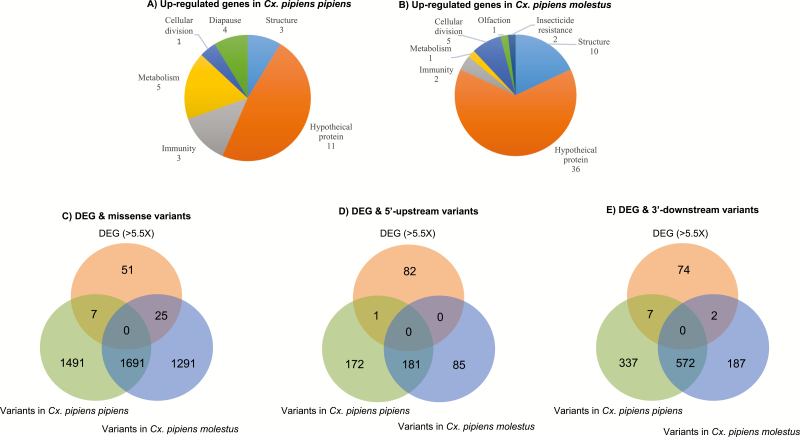
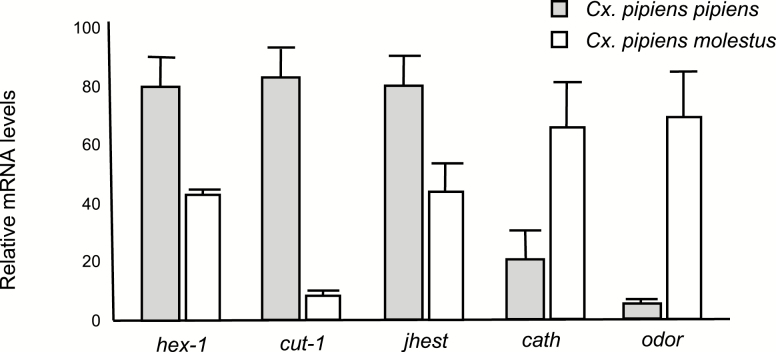
In order to narrow down the realistic pool of candidate genes for future studies, we arbitrarily chose the log2-fold-change threshold of 5.5, at which 83 transcripts had significantly different transcript abundance between adult females of Cx. pipiens pipiens and Cx. pipiens molestus. Interestingly, Cx. pipiens molestus presented a much higher number of upregulated transcripts at this threshold compared with Cx. pipiens pipiens. Ontologies of those genes were classified under different categories, including structure, olfaction, insecticide resistance, cellular division/reproduction, metabolism, immunity, diapause, or hypothetical (Fig. 3). Although the majority of upregulated transcripts were classified as hypothetical proteins, Cx. pipiens pipiens exhibited an increase in genes related to metabolism and immunity, and Cx. pipiens molestus upregulated genes involved with structure, and cell growth/division. Transcripts were assessed based on fold change differences, statistical significance, and relevant ontologies for validation via volcano plot (Fig. 2 and Supp File 2 [online only]) for further analysis. Five transcripts: adult cuticle protein 1 precursor, hexamerin 1.1 precursor, juvenile hormone esterase, cathepsin C, and an odorant receptor were selected for phenotypic relevance, and then validated with qRT–PCR (Fig. 4 and Supp Table 1 [online only]).
Fig. 3.
Distribution of the Gene Ontology (GO) functional categories and transcriptome-wide sequence variants. The differentially expressed genes (DEGs) identified using RNA-Seq of the Cx. pipiens pipiens (A) and Cx. pipiens molestus (B) were classified into GO categories based on biological process, molecular function, and cellular component. Venn diagram depicting overlap between DEGs and missense variants (C), variants in 5′-UTR (D), and variants in 3′-UTR (E) using SnpEff and SnpSift.
Fig. 4.
Expression abundance of female Cx. pipiens pipiens and Cx. pipiens molestus at 7 d after adult eclosion via quantitative real-time PCR. Ribosomal protein large subunit 19 (RpL19) as a loading control. Error bars represent standard error.
Analysis of Genetic Variants From RNA-seq Reads
In total, 73,568 and 73,375 putative SNPs were discovered from Cx. pipiens pipiens and Cx. pipiens molestus, respectively. These SNPs were located in 19,363 genes and 3,171 scaffolds of reference genome Cx. quinquefasciatus. SNPs were then divided into the categories which include 5′-UTR, 3′UTR, CDS, intergenic, splice sites, upstream regions, and downstream regions (Supp Files 3 and 4 [online only]).Culex pipiens pipiens showed 681, 2,319, 42,874, and 513 variants in 5′-UTR, 3′-UTR, coding, and splice sites along with other variant effects, respectively, and Cx. pipiens molestus showed 534, 2,030, 49,811, and 591, respectively (Supp Files 3 and 4 [online only]). As expected, both forms showed the highest number of sequence variants in the silent effect category followed by missense mutation. Culex pipiens pipiens showed 36,113 silent effects and Cx. pipiens molestus had 43,330 (Supp Files 3 and 4 [online only]).
To identify putative SNPs/Indels that can modify the functions or transcription levels, genetic variants were further analyzed among the genes that showed significantly different transcript abundance between the two biotypes. Then, these genetic variants are compared and divided into three categories which include missense, 5′-UTR, and 3′UTR. We found 7 missense variants in Cx. pipiens pipiens and 25 missense variants in Cx. pipiens molestus (Fig. 3 and Supp File 5 [online only]). Among the variants found in 5′-UTR, only 1 SNP was found in Cx. pipiens pipiens. Among the variants found in 3′-UTR, we found 7 SNPs in Cx. pipiens pipiens and 2 SNPs in Cx. pipiens molestus (Fig. 3).
Analysis of Genetic Variants Among the Candidate Genes Validated by qRT–PCR
The five genes that are differentially expressed between adult females of Cx. pipiens pipiens and Cx. pipiens molestus were validated by qRT–PCR. To further identify the putative SNPs/Indels in these genes, we compared the sequences of Cx. pipiens pipiens and Cx. pipiens molestus with the reference genome of Cx. quinquefasciatus. With the exception of cut-1, all transcripts include a high number or SNPs either in the coding and/or noncoding regions.
In Cx. pipiens pipiens, the gene (hex-1, CPIJ001822) encoding hexamerin precursor includes 1 missense and 9 silent mutations in the coding region and 1 silent mutation in the intron, but no variation was detected in Cx. pipiens molestus (Fig. 4 and Supp Fig. 1 [online only]). The missense mutation is a transition (562 A>G) that replaces methionine at position 188 with leucine (Fig. 4 and Supp Fig. 1 [online only]). The gene (jhest, CPIJ0019485) encoding juvenile hormone esterase also includes 1 missense and 8 silent mutations in the coding region and 2 in 5′-UTR, with no variation detected in Cx. pipiens molestus. The missense mutation is a transversion (697 A>T) that replaces glutamine at position 188 with lysine (Fig. 4 and Supp Fig. 1 [online only]).
In Cx. pipiens molestus, the gene (cath, CIPJ000566) encoding cathepsin C includes 1 missense and 2 silent mutations in the coding region, with no variation detected in Cx. pipiens pipiens. The missense mutation is a transversion (373 T>A) that replaces leucine at position 125 with methionine (Fig. 4 and Supp Fig. 1 [online only]). The gene (odor, CPIJ004167) encoding putative odorant receptor includes 1 SNP in splice site and 3 SNPs in 5′-UTR, with no variation detected in Cx. pipiens pipiens (Fig. 4 and Supp Fig. 1 [online only]). This gene showed significantly upregulation in adult females of Cx. pipiens molestus compared with those of Cx. pipiens pipiens.
Discussion
In W. Smithii mosquitoes, natural selection has been suggested to cause a divergence between nonbiting populations and blood-feeding populations that is driven by lower anticipatory costs and more opportunistic behavior (Bradshaw et al. 2018). Differential gene expression (DGE) experiments have shown that this process is also associated with changes in pyruvate metabolism, ribosome proteins, and photo-transduction proteins. These genes are among hundreds of differentially expressed genes found between in biting and nonbiting W. simithii mosquitoes. Our results also found a number of potential biotype-specific genetic variants behind the divergent traits of the Cx. pipiens pipiens (671 genes up) and Cx. pipiens molestus (1018 genes up) that can be utilized in the disruption or overexpression of key traits relevant to disease transmission (Supp File 1 [online only]). Sorting our data by statistical significance and stringent fold change narrowed down our data enough to manually examine transcript ontologies for biological relevance. At the 5.5 log2-fold-change threshold, the increase of metabolic transcripts in the above-ground Cx. pipiens pipiens may be in anticipation of finding a bloodmeal host. The increased expression related to structure, growth, and division in the underground Cx. pipiens molestus may be in preparation for autogenous oogenesis. From the resulting data set, five expression targets were selected for maximum impact upon key eco-physiological traits: adult cuticle protein 1 precursor, hexamerin 1.1 precursor, juvenile hormone esterase, cathepsin C, and an odorant receptor.
Although it is not unusual that cuticle protein would be actively expressed in adult mosquitoes, the marked difference in which transcripts are upregulated between the different biotypes warrants further examination. It is known that in Drosophila melanogaster and Cx. pipiens pipiens the cuticle thickens as a stress tolerance mechanism resulting in harder and less permeable integuments (Baker and Russell 2009, Li and Denlinger 2009). For example, studies in a wide range of insect cuticle proteins in Cx. pipiens pallens were associated with deltamethrin resistance (Fang et al. 2015). As mechanical properties of insect cuticles are highly optimized for biological properties, it seems likely the expression profile differences are related to a desiccation resistance or other protective traits (Andersen et al. 1995). However, for the adult cuticle protein, we did not detect any SNPs between the two biotypes. Other trans-activating factors may play a significant role in gene regulation that may lead to exciting new avenues of vector control.
A hexamerin precursor and a juvenile hormone esterase precursor were selected due to their roles in reproductive programming. Hexamerins are a class of protein around 500 kDa that are synthesized in fat bodies and are associated with adult reproductive development in Anopheles gambiae and Aedes aegypti (Telfer and Kunkel 1991, Zakharkin, Gordadze et al. 1997). The hexamerins known to be involved in the oocyte development by regulating in JH hormone in the fruitfly, Drosophila melanogaster and the termite, Reticulitermes flavipes (Roberts et al. 1991, Zhou et al. 2007). Utilizing RNAi, studies have already demonstrated that the presence or absence of hexamerin varies with sex and life stages and may influence the developmental fate of insects, which makes them excellent candidate targets for vector control (Zhou et al. 2007). Interestingly, we found one missense mutation and one synonymous mutation in the splice site of the gene encoding hexamarin in Cx. pipiens pipiens that were not present in Cx. pipiens molestus (Fig. 4 and Supp Fig. 1 [online only]). The role of each mutation in regulating the gene expression and in altering the protein structure of hexamerin warrants further investigation.
The W. smithii females may be autogenous for their entire adulthood (obligate autogeny), whereas Cx. pipiens molestus mosquitoes will need blood to undergo vitellogenesis after their first or second egg batches (facultative autogeny). Furthermore, Cx. pipiens pipiens always require blood meals to produce progeny (anautogeny). It has been shown that high JH levels are important in halting oocyte maturation in anautogenous females and are a key element in the maintenance of anautogeny (Shapiro et al. 1986, Lassiter et al. 1995, Schomburg and Stephan 1998). It was also found that JH esterase and JH III are inversely correlated in response to blood meals in Ae. aegytpi females (Borovsky et al. 1994). Differences in the SNPs and expression levels were found in the gene (jhest, CPIJ019485) encoding juvenile hormone esterase. Two SNPs occur in 5′-UTR of the jhest gene in Cx. pipiens pipiens that were absent in Cx. pipiens molestus. Differences were observed at the 5′-UTR that either coincide with or are close to transcription factor-binding sites, indicating these SNPs are related to the differential expression level of the jhest gene between the two biotypes. Since the JH hormone esterase modulate the JH synthesis during the female gonotrophic cycles, the different SNPs and expression patterns of the jhest gene may cause a change in JH hormone synthesis between the two forms of Cx. pipiens, which in turn leads to a polymorphism in determining the resting period and maturity of the oocytes. However, it is necessary to study the exact mechanism of action of the jhest gene to determine the maturation process of oocytes between autogenous and anautogenous females.
Although the role of cathepsin C or dipeptidyl peptidase I (DPP-I) is relatively unknown in insects, in general, it is an activator of serine proteases resulting in inflammation (Turk et al. 2001). In Ae. aegypti, serine proteases are known to show a direct link to digestive physiology (Isoe et al. 2009). Specifically, study shows DPP-I’s role in hemoglobin degradation (Hola-Jamriska et al. 2000) potentially supporting the differences in blood feeding patterns in the two biotypes. As another closely related serine protease activator, cathepsin B has been implicated in embryonic degradation of vitellin and is a key to protein catabolism of lysozymes, a cathepsin C was selected for further functional analysis (Cho et al. 1999). Contrary to the cut-1 and jhest genes, only a single missense mutation in cathepsin C (cath) was found in Cx. pipiens molestus (Fig. 4 and Supp Fig. 1 [online only]).
Interfering with the mechanisms underlying mosquito olfaction has been proposed as a vector control strategy (Takken 1991, Hallem et al. 2004, Syed and Leal 2009). In mosquitoes, olfactory receptor neurons (ORNs) receptive to semiochemicals are used to find breeding sites and nutrients, including blood meals (Dethier 1957, Gibson and Torr 1999). DEET (N,N-diethyl-meta-toluamide) has proven to be an effective, topical insect repellent and is known to influence odorant receptors in Cx. quinquefasciatus and An. gambiae (Ditzen et al. 2008, Syed and Leal 2008). Culex quinquefasciatus, in particular, has been shown to possess around 1,300 olfactory sensilla and several Culex odorant binding proteins have already been cloned or mapped to specific sensilla (McIver 1970, Ishida et al. 2002, Leal et al. 2008, Pelletier and Leal 2009). In this study, Cx. pipiens molestus harbors 4 SNPs in noncoding regions of the gene (odor, CPIJ004167) encoding putative odorant receptor absent in Cx. pipiens pipiens. If these mutations do ultimately result in functional changes of olfaction, they may be involved in the adaptation of Cx. pipiens molestus to underground habitats, or differences in pursuing the blood from mammalian hosts instead of birds. Therefore, future experiments are needed to evaluate the role of this gene as a functional genomics tool.
The Culex pipiens complex including Cx. pipiens pipiens and Cx. pipiens molestus, which in spite of displaying a great deal of variation in eco-physiological traits, is nearly morphologically indistinguishable. The genetic basis of these trait divergences between the subspecies is important to understanding the speciation process of the Cx. pipiens complex and epidemiology of vector-borne diseases. Furthermore, our study suggests that an abundance of potentially critical targets is available for genetic approaches to vector control in the Cx. pipiens complex.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health under grant number R15AI139861. Authors’ Contributions: C.S., S.K., and D.K. designed research; S.K., M.C., and D.K. performed research and analyzed data; and C.S, S.K., and D.K. wrote the paper. All authors read and approved the final manuscript.
References Cited
- Andersen S O, Højrup P, and Roepstorff P. . 1995. Insect cuticular proteins. Insect Biochem. Mol. Biol. 25: 153–176. [DOI] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse R M, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, . et al. 2010. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science. 330: 86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthofer W, Banbury B L, Carneiro M, Cicconardi F, Duda T F, Harris R B, Kang D S, Leaché A D, Nolte V, Nourisson C, and Palmieri N. . 2015. Genomic resources notes accepted 1 August 2014–30 September 2014. [DOI] [PubMed] [Google Scholar]
- Atkinson C T, Woods K L, Dusek R J, Sileo L S, and Iko W M. . 1995. Wildlife disease and conservation in Hawaii: pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected iiwi (Vestiaria coccinea). Parasitology. 111: S59–S69. [DOI] [PubMed] [Google Scholar]
- Baker D A, and Russell S. . 2009. Gene expression during Drosophila melanogaster egg development before and after reproductive diapause. BMC Genomics 10: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr A R. 1957. The distribution of Culex p. pipiens and C.P. quinquefasciatus in North America. Am. J. Trop. Med. Hyg. 6: 153–165. [DOI] [PubMed] [Google Scholar]
- Borovsky D, Carlson D A, Ujváry I, and Prestwich G D. . 1994. Biosynthesis of (10R)-juvenile hormone III from farnesoic acid by Aedes aegypti ovary. Arch. Insect. Biochem. Physiol. 27: 11–25. [Google Scholar]
- Bradshaw W E, Burkhart J, Colbourne J K, Borowczak R, Lopez J, Denlinger D L, Reynolds J A, Pfrender M E, and Holzapfel C M. . 2018. Evolutionary transition from blood feeding to obligate nonbiting in a mosquito. Proc. Natl. Acad. Sci. U. S. A. 115: 1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne K, and Nichols R A. . 1999. Culex pipiens in London Underground tunnels: differentiation between surface and subterranean populations. Heredity (Edinb). 82: 7–15. [DOI] [PubMed] [Google Scholar]
- Cho W L, Tsao S M, Hays A R, Walter R, Chen J S, Snigirevskaya E S, and Raikhel A S. . 1999. Mosquito cathepsin B-like protease involved in embryonic degradation of vitellin is produced as a latent extraovarian precursor. J. Biol. Chem. 274: 13311–13321. [DOI] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang L E L, Coon M, Nguyen T, Wang L, Land S J, Lu X, and Ruden D M. . 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 6: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements A N. 1992. The biology of mosquitoes: development, nutrition and reproduction (Vol. 1). 509 Chapman & Hall, London. [Google Scholar]
- Danecek P, Auton A, Abecasis G, Albers C A, Banks E, DePristo M A, Handsaker R E, Lunter G, Marth G T, Sherry S T, . et al. ; 1000 Genomes Project Analysis Group. 2011. The variant call format and VCFtools. Bioinformatics. 27: 2156–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier V G. 1957. The sensory physiology of blood-sucking arthropods. Exp. Parasitol. 6: 68–122. [DOI] [PubMed] [Google Scholar]
- Ditzen M, Pellegrino M, and Vosshall L B. . 2008. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 319: 1838–1842. [DOI] [PubMed] [Google Scholar]
- Fang F, Wang W, Zhang D, Lv Y, Zhou D, Ma L, Shen B, Sun Y, and Zhu C. . 2015. The cuticle proteins: a putative role for deltamethrin resistance in Culex pipiens pallens. Parasitol. Res. 114: 4421–4429. [DOI] [PubMed] [Google Scholar]
- Fonseca D M, Keyghobadi N, Malcolm C A, Mehmet C, Schaffner F, Mogi M, Fleischer R C, and Wilkerson R C. . 2004. Emerging vectors in the Culex pipiens complex. Science. 303: 1535–1538. [DOI] [PubMed] [Google Scholar]
- Garrison E, and Marth G. . 2012. Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:1207.3907.
- Gibson G, and Torr S J. . 1999. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med. Vet. Entomol. 13: 2–23. [DOI] [PubMed] [Google Scholar]
- Hallem E A, Nicole Fox A, Zwiebel L J, and Carlson J R. . 2004. Olfaction: mosquito receptor for human-sweat odorant. Nature. 427: 212–213. [DOI] [PubMed] [Google Scholar]
- Harbach R E, Harrison B A, and Gad A M. . 1984. Culex (Culex) molestus Forskal (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proc. Entomol. Soc. Wash. 86: 521–542. [Google Scholar]
- Harbach R E, Dahl C H R I S T I N E, and White G B. . 1985. Culex (Culex) pipiens Linnaeus (Diptera, Culicidae)-concepts, type designations, and description. Proc. Entomol. Soc. Wash. 87: 24. [Google Scholar]
- Hola-Jamriska L, King L T, Dalton J P, Mann V H, Aaskov J G, and Brindley P J. . 2000. Functional expression of dipeptidyl peptidase I (Cathepsin C) of the oriental blood fluke Schistosoma japonicum in Trichoplusia ni insect cells. Protein Expr. Purif. 19: 384–392. [DOI] [PubMed] [Google Scholar]
- Huang D A W, Sherman B T, and Lempicki R A. . 2009a. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D A W, Sherman B T, and Lempicki R A. . 2009b. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4: 44–57. [DOI] [PubMed] [Google Scholar]
- Hubálek Z, and Halouzka J. . 1999. West Nile fever–a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 5: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Cornel A J, and Leal W S. . 2002. Identification and cloning of a female antenna-specific odorant-binding protein in the mosquito Culex quinquefasciatus. J. Chem. Ecol. 28: 867–871. [DOI] [PubMed] [Google Scholar]
- Isoe J, Rascón A A Jr, Kunz S, and Miesfeld R L. . 2009. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 39: 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, and Sim C. . 2013. Identification of Culex complex species using SNP markers based on high-resolution melting analysis. Mol. Ecol. Resour. 13: 369–376. [DOI] [PubMed] [Google Scholar]
- Kang D S, Cotten M A, Denlinger D L, and Sim C. . 2016. Comparative transcriptomics reveals key gene expression differences between diapausing and non-diapausing adults of Culex pipiens. PLoS One 11: e0154892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Trocke S, and Sim C. . 2018. Comparative studies of stenogamous behaviour in the mosquito Culex pipiens complex. Med. Vet. Entomol. 32: 427–435. [DOI] [PubMed] [Google Scholar]
- Kimura M, Darbro J M, and Harrington L C. . 2010. Avian malaria parasites share congeneric mosquito vectors. J. Parasitol. 96: 144–151. [DOI] [PubMed] [Google Scholar]
- Kothera L, Godsey M, Mutebi J P, and Savage H M. . 2010. A comparison of aboveground and belowground populations of Culex pipiens (Diptera: Culicidae) mosquitoes in Chicago, Illinois, and New York City, New York, using microsatellites. J. Med. Entomol. 47: 805–813. [DOI] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, and Ng P C. . 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Lai C H, Tung K C, Ooi H K, and Wang J S. . 2000. Competence of Aedes albopictus and Culex quinquefasciatus as vector of Dirofilaria immitis after blood meal with different microfilarial density. Vet. Parasitol. 90: 231–237. [DOI] [PubMed] [Google Scholar]
- Lanciotti R S, Kerst A J, Nasci R S, Godsey M S, Mitchell C J, Savage H M, Komar N, Panella N A, Allen B C, Volpe K E, . et al. 2000. Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, and Salzberg S L. . 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassiter M T, Apperson C S, and Roe R M. . 1995. Juvenile hormone metabolism during the fourth stadium and pupal stage of the southern house mosquito, Culex quinquefasciatus Say. J. Insect Physiol. 41: 869–876. [Google Scholar]
- Leal W S, and Ishida Y. . 2008. GP-9s are ubiquitous proteins unlikely involved in olfactory mediation of social organization in the red imported fire ant, Solenopsis invicta. PLoS One 3: e3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, and Denlinger D L. . 2009. Pupal cuticle protein is abundant during early adult diapause in the mosquito Culex pipiens. J. Med. Entomol. 46: 1382–1386. [DOI] [PubMed] [Google Scholar]
- Mattingly P F. 1967. The systematics of the Culex pipiens complex. Bull. World Health Organ. 37: 257–261. [PMC free article] [PubMed] [Google Scholar]
- McIver S B, 1970. Comparative study of antennal sense organs of female culicine mosquitoes. The Canadian Entomologist 102: 1258–1267. [Google Scholar]
- Meegan J M. 1979. The Rift Valley fever epizootic in Egypt 1977-78. 1. Description of the epizzotic and virological studies. Trans. R. Soc. Trop. Med. Hyg. 73: 618–623. [DOI] [PubMed] [Google Scholar]
- Meuti M E, Short C A, and Denlinger D L. . 2015. Mom matters: diapause characteristics of Culex pipiens-Culex quinquefasciatus (Diptera: Culicidae) hybrid mosquitoes. J. Med. Entomol. 52: 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski M L, Erickson S M, Bartholomay L C, and Christensen B M. . 2010. Midgut barrier imparts selective resistance to filarial worm infection in Culex pipiens pipiens. Plos Negl. Trop. Dis. 4: e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutebi J P, and Savage H M. . 2009. Discovery of Culex pipiens pipiens form molestus in Chicago. J. Am. Mosq. Control Assoc. 25: 500–503. [DOI] [PubMed] [Google Scholar]
- Pelletier J, and Leal W S. . 2009. Genome analysis and expression patterns of odorant-binding proteins from the Southern House mosquito Culex pipiens quinquefasciatus. PLoS One 4: e6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D C, and Fonseca D M. . 2015. Genetic divergence between populations of feral and domestic forms of a mosquito disease vector assessed by transcriptomics. PeerJ 3: e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C B, de Vries A, Buijs J, Braks M A, den Hartog W, and Scholte E J. . 2010. First evidence for presence of Culex pipiens biotype molestus in the Netherlands, and of hybrid biotype pipiens and molestus in northern Europe. J. Vector Ecol. 35: 210–212. [DOI] [PubMed] [Google Scholar]
- Roberts D B, Jowett T, Hughes J, Smith D F, and Glover D M. . 1991. The major serum protein of Drosophila larvae, larval serum protein 1, is dispensable. Eur. J. Biochem. 195: 195–201. [DOI] [PubMed] [Google Scholar]
- Schomburg D, and Stephan D. . 1998. Juvenile-hormone esterase. In: Enzyme Handbook 15, pp. 21–26. Springer, Berlin Heidelberg. [Google Scholar]
- Shapiro A B, Wheelock G D, Hagedorn H H, Baker F C, Tsai L W, and Schooley D A. . 1986. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J. Insect Physiol., 32: 867–877. [Google Scholar]
- Sim C, and Denlinger D L. . 2008. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. U. S. A. 105: 6777–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, and Denlinger D L. . 2013. Insulin signaling and the regulation of insect diapause. Front. Physiol. 4: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Kang D S, Kim S, Bai X, and Denlinger D L. . 2015. Identification of FOXO targets that generate diverse features of the diapause phenotype in the mosquito Culex pipiens. Proc. Natl. Acad. Sci. U. S. A. 112: 3811–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A. 1967. Population structure in the Culex pipiens complex of mosquitos. Bull. World Health Organ. 37: 271–276. [PMC free article] [PubMed] [Google Scholar]
- Syed Z, and Leal W S. . 2008. Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl. Acad. Sci. U. S. A. 105: 13598–13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, and Leal W S. . 2009. Acute olfactory response of Culex mosquitoes to a human-and bird-derived attractant. Proc. Natl. Acad. Sci. U.S.A. 106: 18803–18808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W. 1991. The role of olfaction in host-seeking of mosquitoes: a review. Int. J. Trop. Insect Sci. 12: 287–295. [Google Scholar]
- Tauber M J, Tauber C A, and Masaki S. . 1986. Seasonal adaptations of insects. Oxford University Press, New York, NY. [Google Scholar]
- Telfer W H, and Kunkel J G. . 1991. The function and evolution of insect storage hexamers. Annu. Rev. Entomol. 36: 205–228. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Williams B A, Pertea G, Mortazavi A, Kwan G, van Baren M J, Salzberg S L, Wold B J, and Pachter L. . 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai T F, and Mitchell C J. . 1989. St. louis encephalitis. InMonath T P. (ed), The arboviruses: epidemiology and ecology. Vol. 4, Boca Raton, Florida. [Google Scholar]
- Turk D, Janjić V, Stern I, Podobnik M, Lamba D, Dahl S W, Lauritzen C, Pedersen J, Turk V, and Turk B. . 2001. Structure of human dipeptidyl peptidase I (cathepsin C): exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. EMBO J. 20: 6570–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova E B. 2000. Culex pipiens pipiens mosquitoes: taxonomy, distribution, ecology, physiology, genetics, applied importance and control (No. 2). Pensoft Publishers, Bulgaria. [Google Scholar]
- Zakharkin S O, Gordadze A V, Korochkina S E, Mathiopoulos K D, Della Torre A, and Benes H. . 1997. Molecular cloning and expression of a hexamerin cDNA from the malaria mosquito, Anopheles gambiae. Eur. J. Biochem. 246: 719–726. [DOI] [PubMed] [Google Scholar]
- Zhou X, Tarver M R, and Scharf M E. . 2007. Hexamerin-based regulation of juvenile hormone-dependent gene expression underlies phenotypic plasticity in a social insect. Development 134: 601–610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.