Abstract
Obesity is among the leading causes of morbidity and mortality worldwide and its prevalence continues to increase globally. Because obesity is a chronic, complex, and heterogeneous disease influenced by genetic, developmental, biological, and environmental factors, it is necessary to approach obesity with an integrated and comprehensive treatment strategy. As it is difficult to achieve and sustain successful long-term weight loss in most patients with obesity through lifestyle modifications (e.g., diet, exercise, and behavioral therapy), pharmacological approaches to the treatment of obesity should be considered as an adjunct therapy. Currently, four drugs (orlistat, naltrexone extended-release [ER]/bupropion ER, phentermine/topiramate controlled-release, and liraglutide) can be used long-term (>12 weeks) to promote weight loss by suppressing appetite or decreasing fat absorption. Pharmacotherapy for obesity should be conducted according to a proper assessment of the clinical evidence and customized to individual patients considering the characteristics of each drug and comorbidities associated with obesity. In this review, we discuss the mechanisms of action, efficacy, and safety of these available long-term anti-obesity drugs and introduce other potential agents under investigation. Furthermore, we discuss the need for research on personalized obesity medicine.
Keywords: Bupropion, Drug therapy, Liraglutide, Naltrexone, Obesity, Orlistat, Phentermine, Topiramate, Weight loss
INTRODUCTION
Obesity, which refers to the state of excessive body fat accumulation owing to an imbalance between energy intake and expenditure, is a major risk factor for non-communicable diseases, such as type 2 diabetes mellitus, hypertension, dyslipidemia, cardiovascular diseases, and some cancers [1,2]. The World Health Organization (WHO) defined obesity as a major global public health problem in 1997 [3].
The prevalence of obesity has steadily increased in response to changes in dietary and physical activity patterns for the past 10 years, most prominently among adults in their 20s and 30s in South Korea. In 2018, the prevalence of obesity, with a body mass index (BMI) of 25 kg/m2 or greater, among adult men and women was 45.4% and 26.5%, respectively. Among adults in their 20s and 30s, 10.8% of men and 4.9% of women were obese, with a BMI of 30 kg/m2 or greater, which reflects a dramatic increase from the past decade [4]. According to the WHO, more than 1.9 billion (39%) adults aged 18 years and over were overweight and 650 million (13%) were obese in 2016; the global prevalence of obesity has nearly tripled between 1975 and 2016 [5].
As a result, the global burden of obesity continues to increase in terms of public health and socioeconomic development. Additionally, obesity is now recognized as a component of the “global syndemic,” which is characterized by obesity, undernutrition, and climate change as the most important health problems faced by humans and the environment in the near future [6].
Nonetheless, therapeutic approaches for patients with obesity are still insufficient. Because obesity is affected by various complex causes, including genetic, physiological, behavioral, sociocultural, and environmental factors, an integrated and comprehensive approach based on diet, exercise, and behavioral therapy, with consideration of pharmacological therapy, is needed to achieve adequate weight loss in patients with obesity.
Lifestyle and behavioral modifications are the cornerstones of obesity management but pharmacological therapy should be promptly considered for those who do not respond to lifestyle modifications or experience difficulty maintaining the initial weight loss caused by lifestyle modifications. Physiologically, as the basal metabolic rate decreases with weight reduction, maintaining weight loss requires an ongoing decrease in energy intake and/or increase in energy expenditure; thus, additional weight loss and maintaining the reduced weight are more difficult with only lifestyle modifications.
The U.S. National Institutes of Health recommends anti-obesity drugs for individuals with BMI ≥30 or ≥27 kg/m2 with comorbidities, such as diabetes, hypertension, dyslipidemia, or sleep apnea [7]. The Asia-Pacific obesity treatment guidelines recommend that anti-obesity drugs should be considered for those with BMI ≥25 or ≥23 kg/m2 who have at least one weight-related comorbidity [8].
The anti-obesity drugs currently approved by the U.S. Food and Drug Administration (FDA) for long-term use (>12 weeks) are orlistat, naltrexone extended-release (ER)/bupropion ER, phentermine/topiramate controlled-release (CR), and liraglutide [9]. The European Medicines Agency has only approved orlistat, naltrexone ER/bupropion ER, and liraglutide for long-term use [10]. Most of these anti-obesity drugs have 3% to 7% efficacy in terms of weight loss. In the case of lorcaserin, the FDA concluded that the benefits do not outweigh the evidence of excess cancer risk for any identifiable patient population [11].
A pharmacological approach should be used according to the efficacy and safety profile of each drug as well as the type of obesity and associated comorbidities. According to FDA regulations, a product should result in at least 5% mean weight loss, which indicates a statistically significant effect compared with the placebo group, and more than 35% of patients should achieve 5% or greater categorical weight loss after 1 year of treatment to be defined as an effective anti-obesity drug [12]. The FDA also requires an anti-obesity drug to improve cardiometabolic parameters, including glycemic control, blood pressure, and lipid levels [12,13].
From this perspective, in this review, we discuss obesity treatment strategies, focusing on pharmacological approaches with anti-obesity drugs approved for long-term use in patients with obesity.
CURRENTLY APPROVED LONG-TERM THERAPIES FOR OBESITY
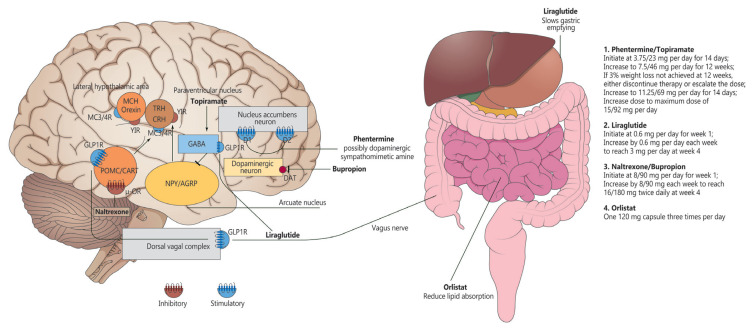
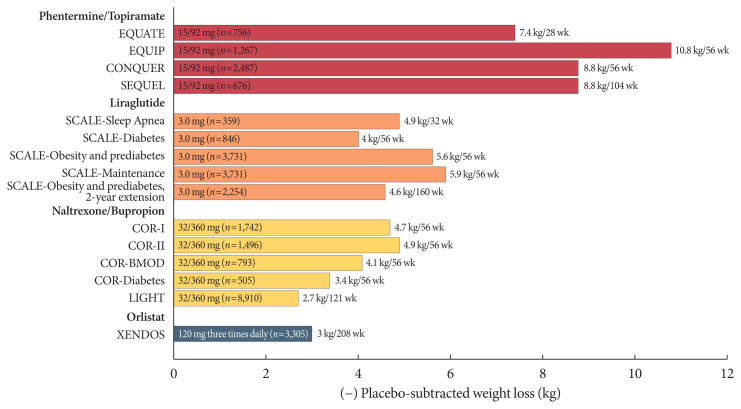
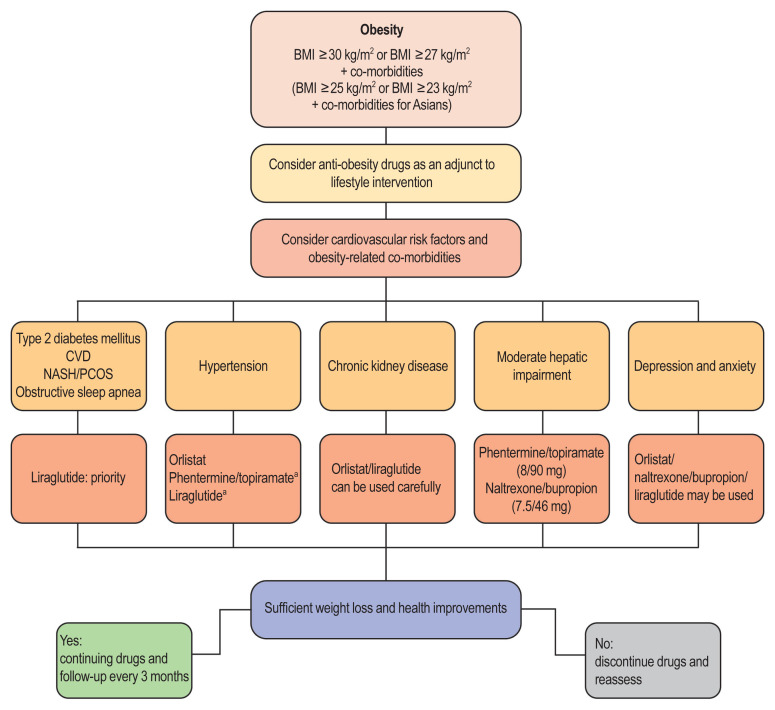
The mechanism of action and dosing schedule of anti-obesity drugs are summarized in Fig. 1. The long-term effects of four approved drugs on weight reduction, cardiometabolic parameters, and safety profiles are summarized in Table 1, Fig. 2. The proposed algorithm for the management of obesity with available long-term anti-obesity drugs is summarized in Fig. 3.
Fig. 1.
Mechanism of action and dosing schedule of anti-obesity drugs. Some images were downloaded from the Smart Servier website. MCH, melanin-concentrating hormone; TRH, thyrotropin-releasing hormone; CRH, corticotropin-releasing hormone; MC3/4R, melanocortin receptor type 3/4 receptor; Y1R, Y1 receptor; GABA, gamma-aminobutyric acid; GLP1R, glucagon-like peptide 1 receptor; D1, dopamine 1 receptor; D2, dopamine 2 receptor; POMC/CART, pro-opiomelanocortin/cocaine amphetamine-related transcript (anorexigenic); μ-OR, μ-opioid receptor; NPY/AGRP, neuropeptide Y/agouti-related peptide (orexigenic); DAT, dopamine active transporter.
Table 1.
Efficacy and safety of currently available long-term anti-obesity drugs for obesity
| Drug | Year of approval | Trial (study duration) | Maximum dose | Mean weight loss from baseline | Proportion of patients losing >5% (10%) of baseline weighta | HbA1c change, %b | Lipid profiles change, %b | SBP/DBP change, mm Hgb | Heart rate change, beats/minc | Common adverse effects | Contraindication | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orlistat | 1999 | XENDOS (208 wk) | 120 mg three times daily | 2.8 kg | 52.8% vs. 37.3% in placebo (26.2% vs. 15.6% in placebo) |
- | TC, −7.5 LDL-C, −9.8 HDL-C, −5.1 TGs, non-significant |
−2.1/−1.0 | No change | Loose, oily stools, fecal incontinence, flatus | Pregnancy, cholestasis, malabsorption syndrome | [17] |
|
| ||||||||||||
| Phentermine/Topiramate ER | 2012 | EQUIP (56 wk) | 15/92 mg | 10.9% | 66.7% vs. 17.3% in placebo (47.2% vs. 7.4% in placebo) |
- | TC, −2.5 LDL-C, −2.9 HDL-C, 3.5 TGs, −14.3 |
−3.8/−1.9 | 1.6 | Dry mouth, paresthesia, insomnia, depression, anxiety | Pregnancy, uncontrolled hypertension, cardiovascular disease, chronic kidney disease, glaucoma, hyperthyroidism, during or within 14 days of treatment with MAOIs | [38] |
| CONQUER (56 wk) | 15/92 mg | 12.4% | 70.0% vs. 21.0% in placebo (48.0% vs. 7.0% in placebo) |
−0.2 | TC, −3.0 LDL-C, −2.8 HDL-C, 5.6 TGs, −15.3 |
−3.2/−1.1 | [37] | |||||
| SEQUEL (104 wk) | 15/92 mg | 10.5% | 79.3% vs. 30.0% in placebo (53.9% vs. 11.5% in placebo) |
−0.2 | TC, non-significant LDL-C, 5.0 HDL-C, 7.7 TGs, −14.5 |
Non-significant | [45] | |||||
|
| ||||||||||||
| Naltrexone SR/Bupropion SR | 2014 | COR-I (56 wk) | 32/360 mg | 6.1% | 48% vs. 16% in placebo (25% vs. 7.0% in placebo) |
- | TC, non-significant LDL-C, non-significant HDL-C, 7.2 TGs, −6.1 |
−0.4/−0.1 | 1.1 (COR-I) | Nausea, dry mouth, constipation, headache, dizziness | Pregnancy, uncontrolled hypertension, seizure, any opioid use, abrupt dis-continuation of alcohol, concomitant administration of MAOIs, severe hepatic impairment, end-stage renal failure | [28] |
| COR-II (56 wk) | 32/360 mg | 6.4% | 50.5% vs. 17.1% in placebo (28.3% vs. 5.7% in placebo) |
- | TC, non-significant LDL-C, 0.03 HDL-C, 0.1 TGs, −9.3 |
1.1/non-significant | [29] | |||||
| COR-BMOD (56 wk) | 32/360 mg | 9.3% | 66.4% vs. 42.5% in placebo (41.5% vs. 20.2% in placebo) |
- | TC, non-significant LDL-C, −2.9 HDL-C, 6.6 TGs, −8.1 |
2.6/1.4 | [30] | |||||
| COR-Diabetes (56 wk) | 32/360 mg | 5.0% | 44.5% vs. 18.9% in placebo (18.5% vs. 5.7% in placebo) |
−0.5 | TC, non-significant LDL-C, non-significant HDL-C, 3,3 TGs, −10.4 |
Non-significant | [31] | |||||
|
| ||||||||||||
| Liraglutide | 2015 | SCALE-Sleep Apnea (32 wk) | 3.0 mg | 5.7% | 46.3% vs. 18.5% in placebo (23.4% vs. 1.7% in placebo) |
−0.2 | Non-significant | −4.1/non-significant | 2.4 (SCALE-Obesity and prediabetes) | Nausea, vomiting, constipation, diarrhea | Pregnancy, history of pancreatitis, personal/family history of medullary thyroid cancer or MEN2 syndrome | [44] |
| SCALE-Diabetes (56 wk) | 3.0 mg | 6.0% | 54.3% vs. 21.4% in placebo (25.2% vs. 6.7% in placebo) |
−1.0 | TC, −4.3 LDL-C, non-significant HDL-C, non-significant TGs, −15.1 |
−2.4/non-significant | [42] | |||||
| SCALE-Obesity and prediabetes (56 wk) | 3.0 mg | 8.0% | 63.2% vs. 27.1% in placebo (33.1% vs. 10.6% in placebo) |
−0.23 | TC, −2.3 LDL-C, −2.4 HDL-C, 1.9 TGs, −9.3 |
−2.8/−0.9 | [41] | |||||
| SCALE-Maintenance (56 wk) | 3.0 mg | 6.2% | 50.5% vs. 21.8% in placebo (26.1% vs. 6.3% in placebo) |
−0.3 | Non-significant | −2.7/non-significant | [43] | |||||
HbA1c, glycosylated hemoglobin; SBP, systolic blood pressure; DBP, diastolic blood pressure; XENDOS, Xenical in the Prevention of Diabetes in Obese Subjects; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; ER, extended-release; EQUIP, controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial; MAOI, monoamine oxidase inhibitor; CONQUER, Controlled-Release Phentermine plus Topiramate Combination in Overweight and Obese Adults; SEQUEL, 2-year Sustained Weight Loss and Metabolic Benefits with Controlled-release Phentermine/Topiramate in Obese and Overweight Adults; SR, sustained-release; COR, Contrave Obesity Research; BMOD, behavior modification; SCALE, Satiety and Clinical Adiposity—Liraglutide Evidence in Nondiabetic and Diabetic Individuals; MEN, multiple endocrine neoplasia.
Treatment vs. placebo,
Placebo-subtracted,
Treatment from baseline.
Fig. 2.
The effect of currently approved long-term therapies for obesity on weight loss. EQUATE, evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults; EQUIP, controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial; CONQUER, Controlled-Release Phentermine plus Topiramate Combination in Overweight and Obese Adults; SEQUEL, 2-year Sustained Weight Loss and Metabolic Benefits with Controlled-release Phentermine/Topiramate in Obese and Overweight Adults; SCALE, Satiety and Clinical Adiposity—Liraglutide Evidence in Nondiabetic and Diabetic Individuals; COR, Contrave Obesity Research; BMOD, behavior modification; LIGHT, long-term intervention with group-wise dietary consulting supported by meal replacements maintaing weight loss in patients with concomitant obesity and knee osteoarthritis; XENDOS, Xenical in the Prevention of Diabetes in Obese Subjects.
Fig. 3.
Choice of anti-obesity drugs based on obesity-associated comorbidities. BMI, body mass index; CVD, cardiovascular disease; NASH, non-alcoholic steatohepatitis; PCOS, polycystic ovary syndrome. aIncrease heart rate.
Orlistat (Xenical)
Mechanism of action
Orlistat induces weight reduction via the inhibition of lipases in the mucous membranes of the stomach, small intestine, and pancreas, thereby preventing the breakdown of triglycerides into fatty acids and their absorption in the intestines (Fig. 1) [14–16]. It is the only available anti-obesity drug that does not involve the mechanisms of appetite.
Efficacy
In the Xenical in the Prevention of Diabetes in Obese Subjects (XENDOS) study, a longitudinal study of patients using orlistat, the mean weight loss from the baseline was significantly greater with orlistat than with the placebo (10.6 kg vs. 6.2 kg) after 1 year and the significantly greater weight loss was maintained after 4 years (5.8 kg vs. 3.0 kg). Among the patients who completed 4 years of treatment, the percentage of patients who achieved at least 5% weight loss was significantly higher in the orlistat group (52.8%) than in the placebo group (37.3%). At the end of the 4-year study, the cumulative incidence of diabetes was 9.0% in the placebo group and 6.2% in the orlistat group, with a risk reduction rate of 37.3% [17].
Additionally, a meta-analysis of 30 studies reported that 21% more participants who use orlistat for 1 year achieve at least 5% or greater weight loss, and 12% more participants achieve a weight loss of 10% or more, than those who use a placebo [18].
Orlistat inhibits the intestinal absorption of 30% of triglycerides; therefore, it exerts a greater weight loss effect than a fat-limited diet. The use of orlistat also results in the improvement of various cardiometabolic parameters, such as decreased insulin resistance, fasting plasma glucose level, low-density lipoprotein cholesterol level, and systolic and diastolic blood pressure [16–18].
Safety
The side effects of orlistat are primarily limited to the intes tines. The side effects experienced by more than 20% of participants who use orlistat for 2 years include fecal incontinence, oily spotting, and fatty stool. In one study, the treatment discontinuation rate was 8.8% in the treatment group and 5.0% in the placebo group [19,20]. Furthermore, when patients with obesity who attempt to lose weight suddenly decrease their food intake, some experience severe constipation owing to reduced dietary fiber intake. Constipation can be treated by orlistat, along with dietary fiber supplementation, via its gastrointestinal side effects.
In patients with chronic malabsorption syndrome or cholestasis, as orlistat can reduce the absorption of fat-soluble vitamins (i.e., vitamins A, D, E, and K), multivitamin supplementation might be needed. Other potential side effects of orlistat include that the oxalic acid content in the urine increases, which can cause renal stones; when administered simultaneously with cyclosporine or thyroid hormone drugs, their effectiveness decreases; and when administered to patients administered warfarin, the decreased absorption of vitamin K causes changes in blood clotting [20,21].
However, because only a small amount of orlistat is absorbed into the body, it is recognized as the safest anti-obesity drug and the only drug that can be used in adolescents [22]; there are insufficient data about the safety of orlistat in elderly patients or those with impaired liver or kidney function.
Naltrexone ER/bupropion ER (Contrave)
Mechanism of action
Bupropion is a norepinephrine and dopamine reuptake inhibitor that is used for depression and smoking cessation treatment. It activates pro-opiomelanocortin (POMC), a neuropeptide that decreases appetite when its concentration increases in the hypothalamus, and supplements dopamine activation, which is lower among patients with obesity. As a result, bupropion inhibits food intake via the reward system and increases energy expenditure for weight reduction [23]. Naltrexone is a mu-opioid receptor antagonist that is used for the treatment of opioid-and alcohol-dependence. Naltrexone inhibits the appetite-enhancing effects of beta-endorphin caused by cannabinoid-1 receptor activation. The combined use of bupropion and naltrexone has a synergistic effect on appetite suppression [24–26]. This may be because POMC, which is self-inhibited by endogenous opioids, can reduce the appetite-suppressing effects of bupropion. However, the addition of naltrexone, which is an opioid antagonist, can maintain POMC activation by bupropion to strengthen its appetite-suppressing effects (Fig. 1) [27].
Efficacy
In the Contrave Obesity Research (COR)-I trial, which was conducted for 56 weeks in individuals with obesity and a BMI of 30 to 45 kg/m2, the naltrexone ER/bupropion ER 32/360 mg group achieved a 6.1% weight reduction and the naltrexone ER/bupropion ER 16/360 mg group achieved a 5.0% weight reduction, which were significant improvements compared with the 1.3% weight reduction in the placebo group. The proportion of participants who achieved ≥5% weight loss was 48% in the naltrexone ER/bupropion ER 32/360 mg group and 39% in the naltrexone ER/bupropion ER 16/360 mg group, which were significantly higher than that (17%) in the placebo group [28]. COR-II, which involved 1,496 individuals with a BMI greater than 30 kg/m2 or a BMI greater than 27 kg/m2 with dyslipidemia (and/or controlled hypertension), also showed that the combination of naltrexone ER/bupropion ER 32/360 mg is associated with a significantly greater weight loss from the baseline body weight than the placebo (−6.4% vs. −1.2%) at week 56 and that the proportion of participants who achieve ≥5% weight loss is significantly higher in the treatment group than in the placebo group (50.5% vs. 17.1%) [29].
The COR-behavior modification (BMOD) study examined the efficacy and safety of naltrexone ER/bupropion ER as an adjunct to intensive BMOD. In this study, all participants were instructed to have a balanced diet of conventional foods and individual goals for energy intake were based on initial weight. The mean weight loss was significantly higher for those who received naltrexone ER/bupropion ER 32/360 mg and BMOD than those who received the placebo and BMOD (11.5% vs. 7.3%) in the completers analysis at the end of the study. At week 56, the proportion of participants who achieved ≥5% weight loss was higher in the naltrexone ER/bupropion ER 32/360 mg and BMOD group than in the placebo and BMOD group (66.4% vs. 42.5%) [30].
In the COR-Diabetes trial, patients using naltrexone ER/bupropion ER lost significantly more weight from the baseline than those using the placebo (5.0% vs. 1.8%) and a greater proportion of patients in the naltrexone ER/bupropion ER group achieved at least 5% weight loss than that in the placebo group (44.5% vs. 18.9%). Naltrexone ER/bupropion ER also resulted in significantly greater glycosylated hemoglobin (HbA1c) reduction (−0.6% vs. −0.1%) than the placebo [31].
In terms of psychosocial status, the naltrexone ER/bupropion ER 32/360 mg and BMOD group showed significant improvements in the physical function and self-esteem subscales compared with the placebo and BMOD group [30].
Moreover, all COR clinical trials showed improvements in cardiometabolic parameters, including glycemic control, insulin resistance, and lipid profiles [28–32].
Safety
The main side effect of naltrexone ER/bupropion ER is nausea, which is so severe in some patients it warrants the discontinuation of the drug. Seizures occur rarely. To prevent these side effects, it is standard to increase the dose gradually. Insomnia is also a common side effect of naltrexone ER/bupropion ER; thus, it is appropriate to take the drug in the morning, at least at the beginning of treatment.
Naltrexone ER/bupropion ER should be used with caution in older patients and is not recommended for those older than 75 years. Its pharmacokinetics in patients with impaired liver and kidney function have not yet been sufficiently studied. If naltrexone ER/bupropion ER is required for patients with impaired liver function, a maximum of one pill per day can be administered and, in patients with impaired kidney function, the maximum dose is two pills per day. The drug is contraindicated in patients with severe hepatic dysfunction or end-stage renal failure [33].
When using naltrexone ER/bupropion ER, the presence of emotional or psychological disorders should be considered. In clinical trials, mild depressive mood and anxiety, which do not require special treatment, were less common in the naltrexone ER/bupropion ER treatment group, which could be attributed to the effects of bupropion. However, the risk of suicidal ideation in individuals aged 18 to 24 years taking bupropion has been reported to the FDA and cases in which bupropion has caused adverse mental and nervous system responses have been reported. The risk of seizures also increases in a dose-dependent manner with bupropion administration, which is increased by prior head trauma, excessive alcohol intake, cocaine or stimulant addiction, diabetes treated with oral hypoglycemic agents or insulin, and the concomitant use of drugs that lower the seizure threshold (antipsychotics, antidepressants, antimalarial drugs, tramadol, theophylline, steroids, quinolones, and sedative antihistamines). Additionally, naltrexone ER/bupropion ER is contraindicated in patients with a history of convulsive seizure or bipolar disorder. For patients with emotional or psychological disorders who take antipsychotics or antidepressants, caution is required owing to the potential for drug interactions and increased risk of seizures [33].
A large-scale study on the cardiovascular safety of naltrexone ER/bupropion ER, the long-term intervention with groupwise dietary consulting supported by meal replacements maintaing weight loss in patients with concomitant obesity and knee osteoarthritis (LIGHT) study, was conducted; however, it was terminated early and did not provide conclusions regarding cardiovascular safety [34].
Phentermine/topiramate CR (Qsymia)
Mechanism of action
Phentermine/topiramate CR is a long-acting combination of phentermine, a short-term appetite suppressant, and topiramate, a nervous system drug. This drug was developed to reduce the incidence of side effects and increase drug efficacy; in combination, these two drugs exert synergistic effects and are used at lower doses than when used independently. Phentermine suppresses appetite by increasing the secretion of epinephrine in the hypothalamus and has been approved for short-term use in obesity treatment. Topiramate is a gamma-aminobutyric acid agonist, glutamate antagonist, and carbonic anhydrase inhibitor that is used to treat epilepsy and prevent migraines, although its mechanism for obesity treatment is uncertain (Fig. 1) [35,36]. However, it exerts weight reduction effects through increased satiety, increased energy expenditure, reduced caloric intake, and taste abnormalities.
Efficacy
The Controlled-Release Phentermine plus Topiramate Combination in Overweight and Obese Adults (CONQUER) study was conducted among 2,487 overweight and obese patients with a BMI of 27 to 45 kg/m2 and two or more cardiometabolic diseases, such as hypertension, dyslipidemia, prediabetes or diabetes, and abdominal obesity. Participants were randomized into three groups. The proportion of participants in the placebo group, phentermine/topiramate CR 7.5/46.0 mg a day group, and phentermine/topiramate CR 15.0/92.0 mg a day group was 2:1:2. At the end of the trial, a greater weight loss effect was observed in the treatment groups than in the placebo group (−1.4 kg vs. −8.1 kg vs. −10.2 kg) and the proportion of patients who achieved ≥5% weight loss was significantly higher in the treatment groups than in the placebo group (21% vs. 62% vs. 70%) at week 56. A similar result was obtained for patients who achieved ≥10% weight loss (7% vs. 37% vs. 48%) [37].
In a randomized controlled trial of phentermine/topiramate CR in adults with severe obesity (BMI greater than 35 kg/m2), patients were randomized into the placebo, low-dose (phentermine/topiramate CR 3.75/23.0 mg), or high-dose (phentermine/topiramate CR 15.0/92.0 mg) group for 56 weeks. The weight loss from the baseline in the placebo group (1.6%) was significantly lower than that in the low-dose (5.1%) and high-dose (10.9%) treatment groups. Additionally, the proportion of patients who achieved ≥5% weight loss was higher in the treatment groups than in the placebo group (17.3% vs. 44.9% vs. 66.7%) [38].
In the secondary endpoint analysis of all clinical trials, the phentermine/topiramate CR group showed significant improvements in cardiometabolic risk factors, including waist circumference, glycemic control, and lipid profile [37,38].
A network meta-analysis that studied differences in the efficacy of anti-obesity drugs showed that phentermine/topiramate CR has the greatest weight loss effect among the currently used anti-obesity drugs [39].
The FDA recommends that if a weight reduction of less than 3% is achieved after 12 weeks of use, the drug should be either discontinued or the dose increased. If the patient does not achieve a 5% weight reduction 12 weeks after a dose increase, it is recommended that this drug should be gradually discontinued.
Safety
As topiramate use during pregnancy increases the risk of cleft palate in babies, for women who might become pregnant, pregnancy testing should be performed before phentermine/topiramate CR initiation and every month during use [40]. The contraindications of phentermine also apply to phentermine/topiramate CR (Table 1) [17,28–31,37,38,41–45].
Many side effects of topiramate are related to the inhibition of carbonic anhydrase activity, including metabolic acidosis, hypokalemia, renal stones, angle-closure glaucoma, myopia, and anhidrosis. Related symptoms should be observed carefully and the drug should be discontinued as soon as symptoms occur. Importantly, topiramate should not be combined with other drugs that inhibit carbonic anhydrase.
The use of phentermine/topiramate CR increases the risk of depression, anxiety, sleep disorder, suicidal ideation, and difficulties in concentration; the drug should be stopped immediately if suicidal ideation or behavior is observed. If a patient handles dangerous machinery, extra caution should be taken. Additionally, phentermine/topiramate CR is associated with taste abnormalities in a dose-dependent manner.
When taking phentermine/topiramate CR, it is recommended that the dose is increased gradually. Additionally, because sudden discontinuation causes seizures in some patients, even in those without a history of epilepsy, it is desirable to discontinue its use gradually by taking a dose every other day for at least 1 week prior to stopping treatment altogether [46].
There are no large-scale studies on the safety and efficacy of phentermine/topiramate CR related to cardiovascular disease, although patients with recent cardio-cerebrovascular disease are recommended not to take this drug. As this drug was approved by the FDA under the condition of further follow-up studies, including an evaluation of long-term safety regarding cardiovascular disease [47], a more accurate assessment of long-term safety will be possible after these results become available. Currently, the Qsymia CardiovascuLAr morbIdity and Mortality study in subjects with documented cardiovascular disease is ongoing.
Liraglutide (Saxenda)
Mechanism of action
Glucagon-like peptide-1 (GLP-1), which is secreted from the intestines in response to carbohydrates and fats digested after a meal, reduces caloric intake by increasing satiety [48]. Liraglutide is 97% identical to human GLP-1 but has a longer action time [49]. Liraglutide acts on the GLP-1 receptor in the hypothalamus and directly stimulates POMC-, cocaine-, and amphetamine-regulated transcript neurons, which suppress appetite and indirectly inhibit neuropeptide-Y/agouti-related protein neurons that stimulate appetite, thereby reducing appetite and promoting weight loss [50,51]. Peripherally, liraglutide delays gastric emptying after a meal and regulates the balance between insulin and glucagon secretion for glycemic control (Fig. 1) [49].
Efficacy
The series of Satiety and Clinical Adiposity-Liraglutide (SCALE) studies were conducted to investigate the efficacy and safety of liraglutide 3.0 mg compared with those of a placebo in combination with a reduced-calorie diet and increased physical activity for weight management in overweight or obese patients with or without comorbidities [41–43,52]. This series consisted of: (1) SCALE-Obesity and Prediabetes trial of 3,731 overweight or obese patients without evidence of type 2 diabetes mellitus; (2) SCALE-Diabetes trial of 846 overweight or obese adults with type 2 diabetes mellitus; (3) SCALE-Maintenance trial of 422 overweight or obese adults who had lost ≥5% of initial body weight during a calorie-restriction period; and (4) the 3-year assessment of the SCALE Obesity and Prediabetes trial of 2,254 overweight or obese patients with prediabetes.
The mean weight loss was significantly higher in the liraglutide group than in the placebo group (SCALE-Obesity and Prediabetes, 8.4 kg vs. 2.8 kg; SCALE-Diabetes, 6.4 kg vs. 2.2 kg; SCALE-Maintenance, additional 6.2% vs. 0.2%, respectively) [41–43]. In terms of categorical weight loss, significantly more patients in the liraglutide group than in the placebo group achieved at least 5% weight loss from the baseline (SCALE-Obesity and Prediabetes, 63.2% vs. 27.1%; SCALE-Diabetes, 54.3% vs. 21.4%; SCALE-Maintenance, 50.5% vs. 21.8%), which suggests that the effect of liraglutide meets the mean and categorical weight loss criteria [41–43].
The 3-year assessment (2-year extension) of the SCALE-Obesity and Prediabetes trial was a double-blind study using 2,254 patients with prediabetes who completed the SCALE-Obesity and Prediabetes study, re-randomized 2:1 to either liraglutide 3.0 mg or the placebo group, with the primary outcome defined as time to diabetes onset within 160 weeks. The time to the onset of diabetes was 2.7 times longer with liraglutide than with the placebo (hazard ratio, 0.21; 95% confidence interval, 0.13 to 0.34; P<0.0001). By week 160, patients in the liraglutide group lost significantly more body weight from the baseline than those in the placebo group (6.1% vs. 1.9%) [52].
In the SCALE-Sleep Apnea trial, 180 non-diabetic patients with obesity who had moderate or severe obstructive sleep apnea (OSA) were randomized to liraglutide 3.0 mg or the placebo group to examine whether liraglutide reduces the severity of OSA using the primary endpoint of the change in the apneahypopnea index (AHI) after 32 weeks. At week 32, the AHI was significantly lower, with weight loss, in the liraglutide group than in the placebo (−12.2±1.8 events h−1 vs. −6.1±2.0 events h−1) [44].
In all SCALE trials, liraglutide resulted in a greater improvement than the placebo in terms of glycemic control, blood pressure, lipid levels, and health-related quality of life in overweight or obese participants [41–44,52].
Liraglutide lowers body weight in humans mainly via the induction of fat mass loss that exceeds lean mass loss [53]. Additionally, liraglutide has been shown to improve hepatic steatosis in patients with non-alcoholic steatohepatitis [54], and after a 26-week intervention, ovarian dysfunction, with 5.2 kg of weight loss, in overweight women with polycystic ovary syndrome [55].
More recently, in the Gauging Responsiveness with A VerifyNow assay-Impact on Thrombosis And Safety (GRAVITAS) trial, liraglutide 1.8 mg as an adjuvant therapy was associated with a significant change in body weight and HbA1c levels from the baseline (−4.23 kg and −1.22% compared with the placebo, respectively) in patients who underwent bariatric surgery and had persistent or recurrent type 2 diabetes mellitus [56].
The FDA recommended that if more than 4% weight reduction is not achieved after 16 weeks of liraglutide administration, it should be discontinued.
Safety
The main side effects of liraglutide are gastrointestinal symptoms, such as nausea, diarrhea, constipation, and vomiting, and it is recommended that the dose is incrementally increased to reduce the incidence of these adverse events. Owing to the delayed gastric emptying caused by liraglutide, the action of other drugs can be affected. Additionally, liraglutide use can cause gallstones and, less commonly, acute pancreatitis [57,58]; it should not be used in patients with a history of pancreatitis. Because there are concerns regarding liraglutide use and medullary thyroid cancer and multiple endocrine neoplasia, it should not be used in patients with a past or family history of such conditions [59–61]. Furthermore, heart rate can be increased when liraglutide is used and, if this symptom persists, the drug might need to be discontinued. The safety of liraglutide has not been demonstrated among those who are older than 75 years; thus, it is not recommended in this population [59].
In the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) study, the cardiovascular safety of liraglutide 1.8 mg was evaluated in patients with diabetes. The time to death owing to cardiovascular disease, myocardial infarction, and cerebral infarction was analyzed in 9,340 patients with diabetes who were randomized to either the liraglutide or placebo group. During an average follow-up period of 3.8 years, the occurrence of the primary composite outcome, including death from cardiovascular disease, non-fatal myocardial infarction, and non-fatal cerebral infarction, was significantly lower in the liraglutide group than in the placebo group (13% vs. 14.9%) [62]. However, there is no direct evidence regarding the safety and efficacy of liraglutide 3.0 mg in cardiovascular disease.
Comparison of available anti-obesity drugs for long-term obesity management
According to the results of a systematic literature review and meta-analysis of 28 randomized clinical studies conducted with 29,018 subjects, all agents are associated with significantly greater weight loss after 1 year than the placebo; phentermine/topiramate CR, liraglutide, naltrexone ER/bupropion ER, and orlistat reduce weight by 8.8, 5.3, 4.9, and 2.6 kg, respectively, compared with the baseline. The proportion of patients with weight loss of at least 5% is 3.4, 2.3, and 1.7 times higher for phentermine/topiramate CR than for orlistat, naltrexone ER/bupropion ER, and liraglutide, respectively. Moreover, the proportion of patients who lost 10% of their weight after 1 year of treatment is also different among phentermine/topiramate CR (54%), liraglutide (34%), naltrexone ER/bupropion ER (30%), and orlistat (20%) groups. In terms of safety, patients are most likely to discontinue liraglutide owing to side effects, followed by naltrexone ER/bupropion ER [39].
Altogether, phentermine/topiramate CR is the most effective of the available drugs, with good tolerability, naltrexone ER/bupropion ER and liraglutide have intermediate efficacy, and orlistat is associated with the fewest side effects [63]. However, there are no head-to-head comparisons of these anti-obesity drugs and the inclusion criteria and background lifestyle and interventions differed among studies; thus, we must interpret these results with caution.
Effects of anti-obesity drugs on eating behavior and neural activity
To personalize pharmacotherapy for obesity in adults, it is critical to target behavioral issues associated with obesity. In a crossover intervention study, functional magnetic resonance imaging (fMRI) data suggested that liraglutide (1.8 mg for 10 days) decreases central nervous system in the insula and putamen compared with insulin in response to viewing pictures of food and high-calorie food during the fasted and postprandial states [64].
In terms of eating behavior, liraglutide (3.0 mg for 5 weeks) also increases feelings of both satiety and fullness and decreases feelings of hunger and prospective food consumption compared with a placebo [65]. In the COR-BMOD trial, there was a significant improvement in the ability to control eating in the naltrexone ER/bupropion ER group compared with the placebo group. Additionally, fMRI data suggest that naltrexone/bupropion treatment may improve the control of eating behavior [66]. Little clinical data are available on the effects of phentermine/topiramate ER on eating behavior.
Considerations for anti-obesity drug use in depression and anxiety
As there is no significant difference in the incidence of depression or anxiety between naltrexone ER/bupropion ER and placebo groups, naltrexone ER/bupropion ER is the recommended drug for patients with obesity and comorbid mood disorders. However, caution is required when using naltrexone ER/bupropion ER in patients taking antidepressants.
Although liraglutide has no effect at a low dose, at a high dose, mood disorders worsen slightly. However, because there is less interaction with antidepressants, liraglutide should be considered first for patients taking antidepressants. As phentermine/topiramate CR can cause mood disorders, it should be avoided in patients with mood disorders.
Sleep disorders have been reported in a significant number of patients taking naltrexone ER/bupropion ER; thus, the deterioration of existing sleep disorders or development of newonset sleep disorders should be monitored when the drug is administered. Additionally, phentermine/topiramate CR should be avoided in patients with sleep disorders [67,68].
Emerging drug therapies in obesity
Setmelanotide
Setmelanotide, a synthetic melanocortin 4 receptor (MC4R) agonist, regulates appetite by selectively binding to and activating MC4Rs in the paraventricular nucleus in the hypothalamus, which is involved in appetite regulation [69]. The MC4Rrelated neural circuit in the hypothalamus is involved in food consumption behavior [70]. The accumulation of fatty acids in adipocytes increases the secretion of leptin, a hormone that induces the feeling of satisfaction. Leptin travels to the hypothalamus through the blood and binds to the leptin receptor (LEPR) in neurons in the hypothalamus. When the LEPR signal pathway is activated by binding with leptin, POMC is converted to alpha-melanocyte-stimulating hormone (α-MSH; also known as alpha-melanotropin). α-MSH is secreted to other neurons to activate the MC4R signaling pathway, which triggers a feeling of satiety that leads to reduced food consumption. If the LEPR and POMC genes, which are involved in the upstream pathways of MC4R-related neural circuits, are deficient, one cannot feel full and continues to eat excessively.
Thus, the unique mechanism of action of setmelanotide can overcome the effects of genetic deficiencies that occur upstream in this pathway in individuals with obesity owing to these rare genetic disorders. Setmelanotide has been shown to reduce body weight and hunger in individuals with obesity owing to POMC or LEPR deficiency in phase 2 trials [71,72] and is currently being investigated in phase 3 trials in subjects with POMC and LERP deficiency (NCT03287960 and NCT02896192). In a recent phase 3 trial, setmelanotide treatment led to a significant reduction in body weight and hunger after 1 year of treatment in individuals with Bardet-Biedl syndrome [73]. The reported adverse reactions related to setmelanotide treatment are injection site reactions, nausea, vomiting, and hyperpigmentation. However, no severe adverse reactions or cardiovascular side effects are related to setmelanotide.
Tesofensine
Tesofensine inhibits the synaptic reuptake of serotonin, noradrenaline, and dopamine. It was originally developed as a treatment for Alzheimer’s and Parkinson’s disease but the treatment effect was not satisfactory. As weight reduction was reported as a side effect, clinical trials on obesity were conducted, and tesofensine was observed to decrease the desire for food, food consumption, and weight [74]. In a small-scale clinical trial with 161 participants, people who received either 0.5 or 1.0 mg of tesofensine for 24 weeks experienced weight reductions of 11.3 and 12.8 kg, respectively. The weight reduction was 2.2 kg in the placebo group, which indicates that tesofensine might have twice the weight reduction effect of previously developed drugs [74]. The weight reduction effect of tesofensine can be attributed to increased overnight energy expenditure and fatty acid oxidization rate [75]. Additionally, the use of tesofensine causes favorable changes in waist circumference, insulin resistance, adiponectin, lipid profiles, and glycemic control. However, the side effects of tesofensine include dry mouth, insomnia, constipation, nausea, and an increased heart rate. As an increase in blood pressure is observed at high doses, it is important to demonstrate the safety of tesofensine in a large-scale clinical trial. Furthermore, phase 3 trials are currently underway [76].
Several other treatment options against obesity are being studied in clinical trials, including cannabinoid type 1 receptor blockers, amylin mimetics, peptide YY, neuropeptide Y inhibitors, fibroblast growth factor 21 analogs, and vaccines [36]. It is possible that the administration of a specific combination of these drugs will have beneficial effects on the complex physiological factors that contribute to the maintenance body weight. Potential anti-obesity drugs in clinical trials are summarized in Table 2.
Table 2.
Emerging anti-obesity drug in clinical trials
| Mechanism of action | Drug | Indication | Registration number | Phase |
|---|---|---|---|---|
| Long-acting amylin analog | NNC0174-0833 | Normal weight, overweight to obesity | NCT02300844 | Phase 1 |
|
| ||||
| FGF21 analog | NNC0194-0499 | Overweight or obesity | NCT03479892 | Phase 1 |
|
| ||||
| Beta 3 adrenergic agonist | Mirabegron | Overweight or obesity | NCT02919176 | Phase 1 |
|
| ||||
| GLP-1R/GCGR/GIPR triple agonists | NNC9204-1706 A | Overweight or obesity | NCT03095807 | Phase 1 |
|
| ||||
| Dual GLP-1R/GCGR agonists | NNC9204-1177 | Overweight or obesity | NCT03308721 | Phase 1 |
| JNJ-64565111 | Severe obesity | NCT03486392 | Phase 2 | |
|
| ||||
| Oxyntomodulin analog | MEDI0382 | Obesity | NCT03625778 | Phase 1 |
|
| ||||
| 5-HT 1 receptor agonist | Cannabidiol | Prader-Willi syndrome | NCT02844933 | Phase 2 |
|
| ||||
| Oxytocin receptor agonist | Oxytocin intranasal | Obesity | NCT03043053 | Phase 2 |
|
| ||||
| GLP-1R agonist | Efpeglenatide (HM11260C) | Obesity | NCT02075281 | Phase 2 |
| Liraglutide | Prader-Willi syndrome | NCT02527200 | Phase 3 | |
| Pubertal adolescent subjects with obesity | NCT02918279 | Phase 3 | ||
| Semaglutide | Overweight or obesity | NCT03552757 | Phase 3 | |
|
| ||||
| SGLT1/2 inhibitor | Licofliglozin (LIK 066) | Obesity | NCT03320941 | Phase 2 |
|
| ||||
| PYY 3-36 analog | Nasal PYY3-36 | Obesity | NCT00537420 | Phase 2 |
|
| ||||
| Type 4 FGF receptor antagonist | ISIS-FGFR4RX | Obesity | NCT02476019 | Phase 2 |
|
| ||||
| PDE-5 inhibitor | Tadalafil | Obesity | NCT02819440 | Phase 2 |
|
| ||||
| Mast cell stabilizer | Amlexanox | Obese type 2 diabetics | NCT01842282 | Phase 2 |
|
| ||||
| Norepinephrine, dopamine, and serotonin transporter inhibitor | Tesofensine | Hypothalamic injury-induced obesity | NCT03845075 | Phase 2 |
|
| ||||
| MCR4R agonist | Setmelanotide | Leptin receptor deficiency obesity | NCT03287960 | Phase 3 |
|
| ||||
| Dopamine reuptake inhibitor | Methylphenidate | Obesity | NCT02754258 | Phase 3 |
FGF, fibroblast growth factor; GLP-1R, glucagon-like peptide-1 receptor; GCGR, glucagon receptor; GIPR, gastric inhibitory peptide receptor; 5-HT, 5-hydroxytryptamine; SGLT1/2, sodium-glucose cotransporter 1/2; PYY, peptide YY; PDE-5, phosphodiesterase type 5; MCR4R, mela-nocortin 4 receptor.
FUTURE PERSPECTIVES: PERSONALIZED MEDICINE IN OBESITY
As obesity is affected by multiple genetic, biological, environmental, and behavioral factors, there are various obesity phenotypes, which affect the response to medications in clinical practice. Although the field of anti-obesity drug therapy has evolved, there are currently no recommendations regarding the type or class of patients for which anti-obesity drugs are more effective in terms of good responders and poor responders; thus, progress remains to be made in personalized medicine in this field.
Additionally, pharmacometabolomic research, including metabolic and genetic profiling, to identify therapeutic gene clusters involved in distinguishing early responders from non-responders to anti-obesity drugs remains inadequate. The identification of response patterns to specific anti-obesity drugs can increase the efficacy of these drugs, which will be an initial step toward personalized medicine for obesity treatment.
Furthermore, pharmacogenetic and mechanistic studies to confirm the effects of drugs on feeding behavior and reward processing would allow the further characterization of good responders to various anti-obesity drugs [77].
CONCLUSIONS
Obesity, a chronic disease associated with significant increases in morbidity and mortality, poses a major public health risk. Currently, four anti-obesity drugs are available for long-term use. The potential weight loss effect of these four anti-obesity drugs can be ranked as follows: phentermine/topiramate CR > liraglutide 3.0 mg > naltrexone ER/bupropion ER > orlistat. Although orlistat has advantages in terms of its ease of administration and tolerability, its potential for weight loss is less than that of other approved anti-obesity drugs. Furthermore, there are more non-responders to orlistat than to other drugs and the cardiovascular safety of orlistat is still unclear.
Phentermine/topiramate CR has shown the strongest weight loss effect of any anti-obesity drug but the risk of neuropsychiatric reactions requires strict pharmacological monitoring. Additionally, the fetal toxicity of topiramate limits its use in women of reproductive age and requires risk assessment.
Naltrexone ER/bupropion ER and liraglutide have an intermediate effect on weight loss. The cardiovascular safety of naltrexone ER/bupropion ER is not yet known, as the only relevant cardiovascular outcome trial was unblinded early. Liraglutide 1.8 mg resulted in significant reductions in cardiovascular outcomes in the LEADER trial, which enrolled patients with type 2 diabetes mellitus and high cardiovascular risk. Although there is no direct evidence regarding the safety and effectiveness of liraglutide 3.0 mg on cardiovascular disease, it is the most preferred drug for patients with obesity and type 2 diabetes mellitus.
Finally, as individual treatment responses to anti-obesity drugs vary, the appropriate classification of patient groups will be the first step toward personalized medicine and the provision of more suitable drug selection and improved treatment algorithms.
ACKNOWLEDGMENTS
None
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.O’Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905–10. doi: 10.2337/db08-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000. WHO Consultation on Obesity. [PubMed] [Google Scholar]
- 4.Korean Society for the Study of Obesity, National Health Insurance Service. 2019 Obesity fact sheet. Seoul: Korean Society for the Study of Obesity; 2019. [Google Scholar]
- 5.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–42. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JR, Brinsden H, Calvillo A, De Schutter O, Devarajan R, Ezzati M, Friel S, Goenka S, Hammond RA, Hastings G, Hawkes C, Herrero M, Hovmand PS, Howden M, Jaacks LM, Kapetanaki AB, Kasman M, Kuhnlein HV, Kumanyika SK, Larijani B, Lobstein T, Long MW, Matsudo VKR, Mills SDH, Morgan G, Morshed A, Nece PM, Pan A, Patterson DW, Sacks G, Shekar M, Simmons GL, Smit W, Tootee A, Vandevijvere S, Waterlander WE, Wolfenden L, Dietz WH. The global syndemic of obesity, undernutrition, and climate change: the Lancet Commission report. Lancet. 2019;393:791–846. doi: 10.1016/S0140-6736(18)32822-8. [DOI] [PubMed] [Google Scholar]
- 7.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, Ryan DH, Still CD Endocrine Society. Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–62. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 9.Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, Daniels SR, Wadden TA, Tsai AG, Hu FB, Jakicic JM, Ryan DH, Wolfe BM, Inge TH. The science of obesity management: an endocrine society scientific statement. Endocr Rev. 2018;39:79–132. doi: 10.1210/er.2017-00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, Toplak H Obesity Management Task Force of the European Association for the Study of Obesity. European guidelines for obesity management in adults. Obes Facts. 2015;8:402–24. doi: 10.1159/000442721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharretts J, Galescu O, Gomatam S, Andraca-Carrera E, Hampp C, Yanoff L. Cancer risk associated with Lorcaserin: the FDA’s Review of the CAMELLIA-TIMI 61 Trial. N Engl J Med. 2020;383:1000–2. doi: 10.1056/NEJMp2003873. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for industry developing products for weight management. Available from: https://www.fda.gov/media/71252/download (cited 2020 Dec 8)
- 13.Srivastava G, Apovian CM. Current pharmacotherapy for obesity. Nat Rev Endocrinol. 2018;14:12–24. doi: 10.1038/nrendo.2017.122. [DOI] [PubMed] [Google Scholar]
- 14.Hvizdos KM, Markham A. Orlistat: a review of its use in the management of obesity. Drugs. 1999;58:743–60. doi: 10.2165/00003495-199958040-00015. [DOI] [PubMed] [Google Scholar]
- 15.Heck AM, Yanovski JA, Calis KA. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20:270–9. doi: 10.1592/phco.20.4.270.34882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drew BS, Dixon AF, Dixon JB. Obesity management: update on orlistat. Vasc Health Risk Manag. 2007;3:817–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155–61. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 18.Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–9. doi: 10.1136/bmj.39385.413113.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. 2000;9:160–7. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 20.Genentech. Xenical (Orlistat) package insert. Available from: https://www.gene.com(cited 2020 Dec 8)
- 21.Ballinger A, Peikin SR. Orlistat: its current status as an anti-obesity drug. Eur J Pharmacol. 2002;440:109–17. doi: 10.1016/s0014-2999(02)01422-x. [DOI] [PubMed] [Google Scholar]
- 22.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. JAMA. 2005;293:2873–83. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 23.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 24.Caixas A, Albert L, Capel I, Rigla M. Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date. Drug Des Devel Ther. 2014;8:1419–27. doi: 10.2147/DDDT.S55587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch M, Varela L, Kim JG, Kim JD, Hernandez-Nuno F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao XB, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutia R, Meece K, Dighe S, Kim AJ, Wardlaw SL. β-Endorphin antagonizes the effects of α-MSH on food intake and body weight. Endocrinology. 2012;153:4246–55. doi: 10.1210/en.2012-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greig SL, Keating GM. Naltrexone ER/bupropion ER: a review in obesity management. Drugs. 2015;75:1269–80. doi: 10.1007/s40265-015-0427-5. [DOI] [PubMed] [Google Scholar]
- 28.Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 29.Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, Kim D, Dunayevich E COR-II Study Group. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21:935–43. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O’Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS, Maier HN, Kim DD, Dunayevich E. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19:110–20. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, Klassen P, Fujioka K COR-Diabetes Study Group. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022–9. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SR, Fujioka K, Gupta AK, Billes SK, Burns C, Kim D, Dunayevich E, Greenway FL. Combination therapy with naltrexone and bupropion for obesity reduces total and visceral adiposity. Diabetes Obes Metab. 2013;15:863–6. doi: 10.1111/dom.12095. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Food Drug Administration. Contrave package insert. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200063s000lbl.pdf(cited 2020 Dec 8)
- 34.Nissen SE, Wolski KE, Prcela L, Wadden T, Buse JB, Bakris G, Perez A, Smith SR. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315:990–1004. doi: 10.1001/jama.2016.1558. [DOI] [PubMed] [Google Scholar]
- 35.Antel J, Hebebrand J. Weight-reducing side effects of the antiepileptic agents topiramate and zonisamide. Handb Exp Pharmacol. 2012:433–66. doi: 10.1007/978-3-642-24716-3_20. [DOI] [PubMed] [Google Scholar]
- 36.Pilitsi E, Farr OM, Polyzos SA, Perakakis N, Nolen-Doerr E, Papathanasiou AE, Mantzoros CS. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism. 2019;92:170–92. doi: 10.1016/j.metabol.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–52. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 38.Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20:330–42. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315:2424–34. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mines D, Tennis P, Curkendall SM, Li DK, Peterson C, Andrews EB, Calingaert B, Chen H, Deshpande G, Esposito DB, Everage N, Holick CN, Meyer NM, Nkhoma ET, Quinn S, Rothman KJ, Chan KA. Topiramate use in pregnancy and the birth prevalence of oral clefts. Pharmacoepidemiol Drug Saf. 2014;23:1017–25. doi: 10.1002/pds.3612. [DOI] [PubMed] [Google Scholar]
- 41.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP SCALE Obesity and Prediabetes NN8022-1839 Study Group. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 42.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, Andreasen AH, Jensen CB, DeFronzo RA NN8022-1922 Study Group. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314:687–99. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 43.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L NN8022-1923 Investigators. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond) 2013;37:1443–51. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 44.Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, Claudius B, Jensen CB, Mignot E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J Obes (Lond) 2016;40:1310–9. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers M, Day WW, Bowden CH. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qsymia. Qsymia (phentermine and topiramate extended-release) package insert. Available from: https://www.qsymia.com(cited 2020 Dec 8)
- 47.Colman E, Golden J, Roberts M, Egan A, Weaver J, Rosebraugh C. The FDA’s assessment of two drugs for chronic weight management. N Engl J Med. 2012;367:1577–9. doi: 10.1056/NEJMp1211277. [DOI] [PubMed] [Google Scholar]
- 48.Torekov SS, Madsbad S, Holst JJ. Obesity: an indication for GLP-1 treatment? Obesity pathophysiology and GLP-1 treatment potential. Obes Rev. 2011;12:593–601. doi: 10.1111/j.1467-789X.2011.00860.x. [DOI] [PubMed] [Google Scholar]
- 49.Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7:507–16. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 51.Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, Schaffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–88. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, Ortiz RV, Wilding JPH, Skjoth TV, Manning LS, Pi-Sunyer X SCALE Obesity Prediabetes NN8022-1839 Study Group. 3 Years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 53.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rossner S, Savolainen MJ, Van Gaal L NN8022-1807 Investigators. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 2012;36:843–54. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hubscher SG, Newsome PN LEAN trial team. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–90. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 55.Nylander M, Frossing S, Clausen HV, Kistorp C, Faber J, Skouby SO. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod Biomed Online. 2017;35:121–7. doi: 10.1016/j.rbmo.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 56.Miras AD, Perez-Pevida B, Aldhwayan M, Kamocka A, Mc-Glone ER, Al-Najim W, Chahal H, Batterham RL, McGowan B, Khan O, Greener V, Ahmed AR, Petrie A, Scholtz S, Bloom SR, Tan TM. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:549–59. doi: 10.1016/S2213-8587(19)30157-3. [DOI] [PubMed] [Google Scholar]
- 57.Chalmer T, Almdal TP, Vilsboll T, Knop FK. Adverse drug reactions associated with the use of liraglutide in patients with type 2 diabetes: focus on pancreatitis and pancreas cancer. Expert Opin Drug Saf. 2015;14:171–80. doi: 10.1517/14740338.2015.975205. [DOI] [PubMed] [Google Scholar]
- 58.Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, Mannucci E. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab. 2017;19:1233–41. doi: 10.1111/dom.12926. [DOI] [PubMed] [Google Scholar]
- 59.U.S. Food Drug Administration. Saxenda (package insert). Liraglutide (rDNA) injection. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf (cited 2020 Dec 8).
- 60.Gallo M. Thyroid safety in patients treated with liraglutide. J Endocrinol Invest. 2013;36:140–5. doi: 10.1007/BF03346749. [DOI] [PubMed] [Google Scholar]
- 61.Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like peptide-1 receptor agonists activate rodent thyroid Ccells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–86. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 62.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bessesen DH, Van Gaal LF. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diabetes Endocrinol. 2018;6:237–48. doi: 10.1016/S2213-8587(17)30236-X. [DOI] [PubMed] [Google Scholar]
- 64.Ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Drent ML, Diamant M, IJzerman RG. Liraglutide reduces CNS activation in response to visual food cues only after short-term treatment in patients with type 2 diabetes. Diabetes Care. 2016;39:214–21. doi: 10.2337/dc15-0772. [DOI] [PubMed] [Google Scholar]
- 65.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:784–93. doi: 10.1038/ijo.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang GJ, Tomasi D, Volkow ND, Wang R, Telang F, Caparelli EC, Dunayevich E. Effect of combined naltrexone and bupropion therapy on the brain’s reactivity to food cues. Int J Obes (Lond) 2014;38:682–8. doi: 10.1038/ijo.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 68.Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012;35:1529–39. doi: 10.5665/sleep.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther. 2014;95:53–66. doi: 10.1038/clpt.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeong JK, Kim JG, Lee BJ. Participation of the central melanocortin system in metabolic regulation and energy homeostasis. Cell Mol Life Sci. 2014;71:3799–809. doi: 10.1007/s00018-014-1650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clement K, Biebermann H, Farooqi IS, Van der Ploeg L, Wolters B, Poitou C, Puder L, Fiedorek F, Gottesdiener K, Kleinau G, Heyder N, Scheerer P, Blume-Peytavi U, Jahnke I, Sharma S, Mokrosinski J, Wiegand S, Muller A, Weib K, Mai K, Spranger J, Gruters A, Blankenstein O, Krude H, Kuhnen P. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med. 2018;24:551–5. doi: 10.1038/s41591-018-0015-9. [DOI] [PubMed] [Google Scholar]
- 72.Kuhnen P, Clement K, Wiegand S, Blankenstein O, Gottesdiener K, Martini LL, Mai K, Blume-Peytavi U, Gruters A, Krude H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375:240–6. doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 73.Haws R, Brady S, Davis E, Fletty K, Yuan G, Gordon G, Stewart M, Yanovski J. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020;22:2133–40. doi: 10.1111/dom.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1906–13. doi: 10.1016/S0140-6736(08)61525-1. [DOI] [PubMed] [Google Scholar]
- 75.Sjodin A, Gasteyger C, Nielsen AL, Raben A, Mikkelsen JD, Jensen JK, Meier D, Astrup A. The effect of the triple monoamine reuptake inhibitor tesofensine on energy metabolism and appetite in overweight and moderately obese men. Int J Obes (Lond) 2010;34:1634–43. doi: 10.1038/ijo.2010.87. [DOI] [PubMed] [Google Scholar]
- 76.Saniona. Tesofensine monotherapy for treatment of obesity. Available from: https://saniona.com(cited 2020 Dec 8)
- 77.Roberts CA, Christiansen P, Halford JCG. Tailoring pharmacotherapy to specific eating behaviours in obesity: can recommendations for personalized therapy be made from the current data? Acta Diabetol. 2017;54:715–25. doi: 10.1007/s00592-017-0994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]