Abstract
Background
Voglibose, an α-glucosidase inhibitor, inhibits breakdown of complex carbohydrates into simple sugar units in intestine. Studies showed that voglibose metabolism in the liver might be negligible due to its poor intestinal absorption. Numerous microorganisms live in intestine and have several roles in metabolism and detoxification of various xenobiotics. Due to the limited information, the possible metabolism of voglibose by intestinal microbiota was investigated in vitro and in vivo.
Methods
For the in vitro study, different concentrations of voglibose were incubated with intestinal contents, prepared from both vehicle- and antibiotics-treated mice, to determine the decreased amount of voglibose over time by using liquid chromatography-mass spectrometry. Similarly, in vivo pharmacodynamic effect of voglibose was determined following the administration of voglibose and starch in vehicle- and antibiotic-pretreated non-diabetic and diabetic mice, by measuring the modulatory effects of voglibose on blood glucose levels.
Results
The in vitro results indicated that the remaining voglibose could be significantly decreased when incubated with the intestinal contents from normal mice compared to those from antibiotic-treated mice, which had less enzyme activities. The in vivo results showed that the antibiotic pretreatment resulted in reduced metabolism of voglibose. This significantly lowered blood glucose levels in antibiotic-pretreated mice compared to the control animals.
Conclusion
The present results indicate that voglibose would be metabolized by the intestinal microbiota, and that this metabolism might be pharmacodynamically critical in lowering blood glucose levels in mice.
Keywords: Anti-bacterial agents, Blood glucose, Gastrointestinal microbiome, Metabolism, Voglibose
INTRODUCTION
Gut microbiota has been known as a hidden organ of the human body for a long time [1]. It is estimated that approximately 99% of the intestinal microbial population is of anaerobic type and covers more than 1,000 species of bacteria in human and animals [2,3]. In addition, the composition of intestinal bacteria is affected by age, sex, diet, exercise, and environmental conditions [4]. Over the past few decades, researchers have extensively focused on determining the possible roles of intestinal microbiota in human health and disease, and they found, at least in part, that numerous biological functions including emotion, immunity, and drug metabolism were closely connected with gut microbiota [5]. Among all, drug metabolism by intestinal microbiota might be considered as an important aspect that modulates pharmacodynamic actions [6]. The intestinal microbiota plays a significant role in the metabolism of certain drugs administered orally through a variety of reactions including hydrolysis; thereby, toxicity and efficacy of drugs can be largely modulated [4].
Orally administered drugs are directly absorbed from the gastrointestinal tract into the liver, where extensive metabolism occurs, and the drugs would interact with numerous bacteria present in the gut, leading to further metabolism [7,8]. In addition, the enterohepatic recycling of xenobiotics occurs via certain enzymes such as β-glucuronidase and sulfatase that are produced by intestinal bacteria [9,10]. Through interaction with intestinal microbiota, properties and fate of certain xenobiotics can be largely altered. Most importantly, the formation of new metabolite(s) can lead to changes in pharmacological or toxicological properties of xenobiotics [11,12]. In fact, the impact of gut microbiota on xenobiotic metabolism in several animal models were investigated in previous studies [10,13,14]. Likewise, metabolism of certain drugs by gut microbiota was clinically relevant to their pharmacological effects through the production of pharmacologically active, inactive, or toxic metabolites [6]. Therefore, the study on the possible role of drug metabolism by intestinal microbiota in drug action would be required to reduce possible drug-drug interactions clinically [6].
Voglibose is an α-glucosidase inhibitor developed in 1994 in Japan for lowering post-prandial blood glucose level in people with type 2 diabetes mellitus [15,16]. It was observed that voglibose had minor systemic absorption and negligible metabolism in the liver [15]. In our preliminary experiments with several anti-diabetic drugs, voglibose was significantly metabolized when incubated with intestinal content preparations from mice. Therefore, we studied, for the first time, the metabolism of voglibose by intestinal microbiota in vitro and in vivo. For in vitro study, voglibose was incubated with intestinal contents prepared from vehicle- and antibiotics-treated mice for its metabolism. In addition, for the in vivo study, the pharmacodynamic effects of voglibose on blood glucose levels were determined by orally administering voglibose followed by starch in antibiotics-treated and -untreated diabetic and non-diabetic mice, because the absorption of voglibose in the intestine is reportedly limited [15].
METHODS
Materials
Voglibose (>98.0%) was purchased from Tokyo Chemical Industries (Tokyo, Japan). Erythromycin, oxytetracycline hydrochloride, cefadroxil, 4-nitrophenyl β-D-glucopyranoside, 4-nitrophenyl β-D-glucuronide, 4-nitrophenyl sulfate, soluble starch, ammonium acetate, streptozotocin, and telmisartan, the internal standard (IS), were purchased from Sigma-Aldrich (St. Louis, MO, USA). High-performance liquid chromatography (HPLC)-grade acetonitrile was purchased from J. T. Bakers (Central Valley, PA, USA). An anaerobe gas generating system was purchased from Becton, Dickinson and Company (Sparks, MD, USA). A blood glucose monitoring system was purchased from Bayer Contour TS (Mishawaka, IN, USA). Potassium phosphate monobasic and potassium phosphate dibasic were purchased from Duksan (Seoul, Korea). All other chemicals were of analytical grades and used as received.
Animals
Male Institute of Cancer Research (ICR) mice at 7 weeks of age were purchased from Orient Bio (Seoul, Korea). Animals upon received were randomly placed five in a cage. Animals were acclimatized for at least 1 week under controlled temperature of 22℃±2℃, relative humidity of 50%±10%, and air change of 10 to 20 cycles per hour with a light and dark cycle of 12-hour. The study was performed following procedures of the Institutional Animal Care and Use Committee of Yeungnam University (approved No., 2014-008). Unless specified, all animals were allowed to access food and water ad libitum.
Preparation and extraction of intestinal contents for in vitro study
For in vitro studies, mice were grouped as antibiotics-treated or vehicle-treated groups (n=5). In antibiotics-treated group, mice were orally administered with a mixture of three antibiotics in saline, such as erythromycin (300 mg/kg), oxytetracycline HCl (300 mg/kg), and cefadroxil (100 mg/kg), for 3 successive days. Saline was administered in the vehicle-treated group for 3 days. Twenty-four hours after the final dose of either antibiotics or vehicle, intestinal contents were harvested. Collected intestinal contents were mixed with two volumes of potassium phosphate buffer, pH 7.4, vortexed, and homogenized at 11,000 rpm for 2 minutes. Then, the sample was centrifuged at 500 g for 10 minutes at 4℃, and the supernatant was collected for in vitro tests. Aliquots were prepared and stored at −70℃ until their use.
Determination of intestinal microbial enzyme activity
The enzyme assays were carried out according to a previously published report with some modifications [17]. Briefly, 0.4 mL of either 2.5 mM 4-nitrophenyl β-D-glucopyranoside, 4-nitrophenyl β-D-glucuronide, or 4-nitrophenyl sulfate in potassium phosphate buffer, pH 7.4, was mixed with 0.4 mL of potassium phosphate buffer, pH 7.4, and 0.2 mL of intestinal contents obtained from both antibiotics- and vehicle-treated mice, followed by the incubation at 37℃ for 30 minutes with shaking. The reaction was terminated by adding 0.25 mL of 1 N NaOH. Following the centrifugation at 3,000 ×g for 10 minutes, the absorbance of supernatant was measured at 405 nm by using a UV spectrophotometer (Eppendorf, Hamburg, Germany). Standard calibration curves of 4-nitrophenol were prepared for the calculation of individual enzyme activities.
Analytical conditions
An HPLC connected to a mass spectrometer (API-4000; SCIEX, Framingham, MA, USA) was used. The mobile phase consisted of 10 mM ammonium acetate buffer (mobile phase A) and acetonitrile (mobile phase B). A gradient condition was used for the quantitative analysis of voglibose in which the mobile phase B started at 0.5% was held for 1.0 minute, and increased to 10% during the following 2.0 minutes. Then, mobile phase B was increased to 90% during the following 1.0 minute, held at 90% for 1.5 minutes, and returned to 1% during the following 1.5 minutes. Column equilibration time was 7.0 minutes and the total run time was 14.0 minutes. Analyte peaks were identified with an Agilent Eclipse plus C8 column (2.1×150 mm, 3.5 µm). The column temperature was maintained at 30℃ with a flow rate of 0.25 mL/min and injection volume of 5.0 µL. Multiple reaction monitoring mode was used for the quantitative detection of voglibose using the ratio of areas of analyte to IS peaks. Voglibose and telmisartan (IS) was detected in positive ion modes. Mass transitions for voglibose and telmisartan were m/z 268→92 and m/z 515→276, respectively. The declustering potential, collision energy and collision cell exit potential values were 70, 30, and 18 for voglibose and 156, 65, and 20 for telmisartan, respectively.
Analytical validation
Inter-day and intra-day validations were performed according to the Food and Drug Adminstration guideline [18]. Accuracy and coefficient of variation (CV %) was calculated based on the analysis of spiked quality control (QC) samples of different concentrations. QC samples of 4, 100, and 400 ng/mL voglibose were analyzed. For intra-day validation, five freshly prepared samples of each concentration were analyzed in a single day. For inter-day validation, QC samples were freshly prepared in each day and analyzed for 5 consecutive days. Accuracy and precision of analyzed QC samples were determined.
Stability studies
To investigate the possible metabolism of voglibose by the intestinal microbiota, the remaining level of voglibose was measured following the incubation of voglibose with intestinal contents compared with the initial level of voglibose. Prior to this, the stability of voglibose was tested with a target CV (%) of 15. Voglibose in potassium phosphate buffer, pH 7.4, at 5, 10 and 20 µg/mL was incubated in various conditions for long-term and short-term stability studies. For the short-term stability test, voglibose was incubated in both 25℃ and 37℃ for 24 hours. Similarly, voglibose was tested for long-term stability after storage in a freezer for 30 days at −20℃ and 5 days at 4℃. The stability of voglibose was also tested following three freeze-thaw cycles from −20℃ to room temperature.
Intestinal microbiota-mediated metabolism of voglibose in vitro
For time- and concentration-dependent metabolism of voglibose, 10 µL of either 0.25, 0.5, or 1 mg/mL voglibose was incubated with 490 µL of intestinal contents to prepare the final concentrations of 5, 10, and 20 µg/mL of voglibose. The mixtures were incubated in an anaerobe gas generating system at 37℃ for 3, 6, 12, and 24 hours. Following incubation, 10 µL of each incubation mixture was transferred to another tube containing 490 µL of methanol containing 10 ng/mL of telmisartan (IS). Thus, the final concentrations for liquid chromatography-mass spectrometry (LC-MS/MS) analyses were 100, 200, and 400 ng/mL. The mixture was vortexed and centrifuged at 12,000 ×g for 10 minutes. Then, 200 µL of supernatant was pipetted into vials and analyzed using LC-MS/MS. Calibration curves were prepared accordingly and concentrations of voglibose in incubated samples from antibiotics- and vehicle-treated mice were determined.
Pharmacodynamics of voglibose in non-diabetic mice
To investigate the role of intestinal microbiota in the pharmacodynamics of voglibose in non-diabetic conditions, mice were divided into antibiotics-treated and vehicle-treated groups. In the antibiotic-treated group, a mixture of three antibiotics was administered orally for 3 successive days. Saline was administered to vehicle-treated animals for 3 days. Twenty-four hours after the final treatment, both antibiotics- and vehicle-treated mice were subdivided into two subgroups, i.e., starch+voglibose and starch only, followed by a fasting period of 6 hours. For the main experiment, voglibose at 10 mg/kg was administered orally to the starch+voglibose group and saline to the starch only group. Thirty minutes later, 50 mg/kg of soluble starch was orally administered to all animals and the blood glucose levels were determined at 0, 30, 60, 90, 120, 180, and 240 minutes after starch administration.
Pharmacodynamics of voglibose in diabetic mice
To prepare the diabetic model, mice fasted for 6 hours were intraperitoneally injected with 40 mg/kg streptozotocin for 5 successive days. Body weight and blood glucose levels of individual animals were measured daily before each streptozotocin injection. Once blood glucose level reached >250 mg/dL, mice were considered diabetic and were divided into two groups for antibiotics treatment. Mice in the antibiotic-treated group received the mixture of three antibiotics by oral administration for 3 consecutive days. In the vehicle-treated group, mice received saline for 3 days. Twenty-four hours after the last dose of antibiotics, mice were further divided into two groups, starch+voglibose group and starch only group, followed by a fasting period of 6 hours. For the main experiment, voglibose at 10 mg/kg was administered to the starch+voglibose group and saline was administered to the starch only group to 6-hour fasted animals. After 30 minutes, 50 mg/kg of soluble starch was orally administered to all mice and blood glucose levels were measured at 0, 30, 60, 90, 120, 180, and 240 minutes after starch administration.
Statistics
All data were presented as mean±standard deviation. Student's t-test was used to determine the statistical significance of the data. The results of P<0.05 were represented with alphabets.
RESULTS
Development and optimization of analytical method
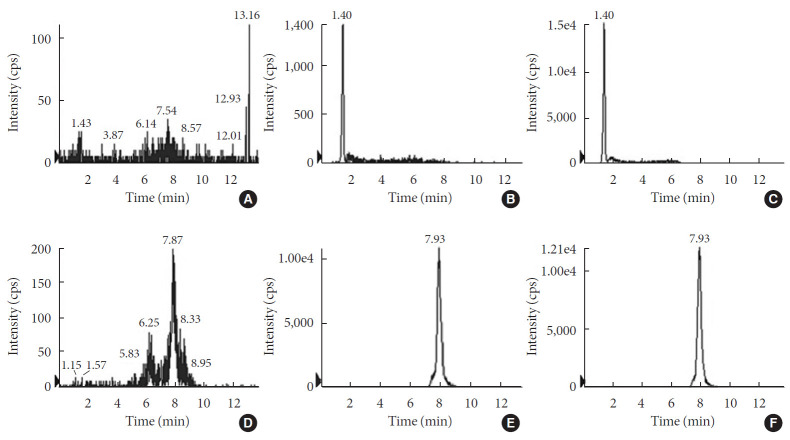
For the accurate quantification of voglibose, an LC-MS/MS method was developed. The separation of voglibose was more effective with C8 column than with C18 or C16 columns (data not shown). In addition, for better resolution, a gradient eluting system was established. As depicted in Fig. 1A, voglibose was ionized in positive ion mode [M+H]+ to form a protonated ion [M+H]+ at m/z 268.0 with a daughter ion at m/z 92.0. Telmisartan, the IS, was detected at m/z 515.2 and further fragmented to the ion at m/z 276.2 (Fig. 1B). There was no interfering peak at the retention time of voglibose at 1.4 minutes and telmisartan at 7.93 minutes (Fig. 2). The inter- and intra-day accuracy and precision of voglibose are summarized in Supplementary Table 1. A good linearity was observed with the calibration curves constructed with the ratio of areas of analyte to IS peaks. Acceptable linearity (R2=0.9938) was observed between the constructed calibration standards with 4 to 400 ng/mL voglibose. The accuracy (%) and CV (%) of inter-day and intra-day were within 15% for the lowest concentration and 10% for other concentrations tested.
Fig. 1. Mass spectra of (A) voglibose with its structure and (B) telmisartan, an internal standard. cps, counts per second; m/z, mass to charge.
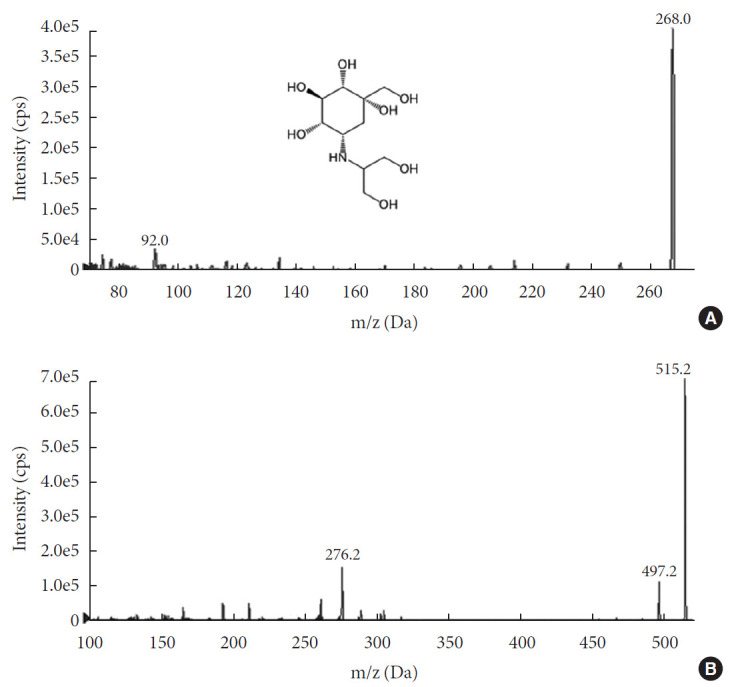
Fig. 2. Representative liquid chromatography-mass spectrometry (LC-MS/MS) chromatograms of (A, B, C) voglibose and (D, E, F) telmisartan. (A, D) blank samples; (B, E) blank samples spiked with analyte at lower limit of quatitation; and (C, F) real samples from in vitro incubation with intestinal contents for 3 hours. cps, counts per second.
A stability test was conducted for three different concentrations of voglibose. Following exposure of voglibose to various ambient conditions, LC-MS/MS analysis was conducted to measure the remaining voglibose from the initial level. As shown in Table 1, voglibose was stable for 24 hours at both room temperature and 37℃. Similarly, voglibose was stable at freezing and refrigerating conditions. However, voglibose was less stable in samples with more than three freeze-thaw cycles. Nevertheless, it was within the limit of 15%. Taken together, the current analytical method was appropriate to study the in vitro metabolism of voglibose by intestinal microbiota.
Table 1.
Stability of voglibose under ambient conditions
| Ambient condition | Voglibose remained, % |
||
|---|---|---|---|
| 0.1 μg/mL | 0.2 μg/mL | 0.4 μg/mL | |
| Short-term at 25°C | 96.3±11 | 97.5±5.7 | 94.4±4.6 |
| Short-term at 37°C | 87.8±4.0 | 95.9±1.2 | 99.2±3.8 |
| Long-term at 4°C | 95.1±8.8 | 96.6±0.8 | 96.6±2.4 |
| Long-term at –20°C | 99.0±8.8 | 98.2±5.6 | 96.9±7.0 |
| Freeze-thaw cycle (–20°C to 25°C) | 89.5±2.1 | 91.4±11.1 | 94.6±1.9 |
Values are presented as mean±standard deviation (n=5). Short-term stability tests were conducted at 25°C and 37°C for 24 hours. Long-term stability tests were conducted after storing voglibose in freezer and refrigerator (4°C) for 30 and 5 days, respectively. Three freeze-thaw cycles were tested for freeze-thaw stability tests from −20°C to room temperature.
In vitro metabolism of voglibose
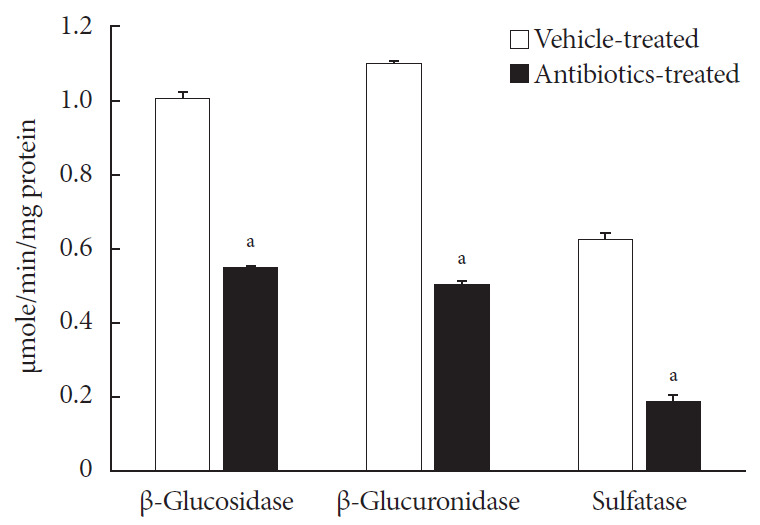
Prior to the characterization of voglibose metabolism by intestinal microbiota, three enzyme activities, i.e., β-D-glucosidase, β-D-glucuronidase and sulfatase, in the intestinal contents isolated from both vehicle- and antibiotics-treated mice were determined. As shown in Fig. 3, the enzyme activities in antibiotics-treated mice were significantly lower than in vehicle-treated mice, indicating that the present antibiotic pretreatment model would be effective to study the role of intestinal microbiota in voglibose metabolism and their effects on the pharmacodynamics of voglibose.
Fig. 3. Enzyme activities of intestinal contents: 0.4 mL of either 2.5 mM 4-nitrophenyl β-D-glucopyranoside, 4-nitrophenyl β-D-glucuronide, or 4-nitrophenyl-sulfate in potassium phosphate buffer, pH 7.4, was mixed with 0.4 mL of potassium phosphate buffer, and 0.2 mL of intestinal contents prepared from both antibiotics- and vehicle-treated mice and incubated at 37℃ for 30 minutes. The enzyme activities were calculated following measuring the sample's absorbance at 405 nm. Each bar represents the mean activity of respective enzymes+standard deviation of triplicate determinations. aThe value significantly different from corresponding vehicle-treated controls at P<0.05.
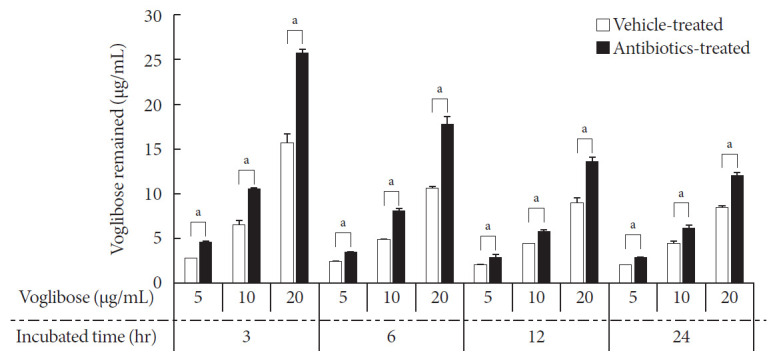
Thereafter, several concentrations of voglibose were incubated with intestinal contents from vehicle- and antibiotics-treated mice at several time points. Then, the remaining voglibose was measured. Following incubation, almost 40% of voglibose in the control was metabolized within 3 hours of incubation compared to the level of voglibose in the antibiotics-treated group. Moreover, the level of voglibose in the antibiotics-treated group was always higher than the level of voglibose in the control up to the period of sample testing (Fig. 4). The results indicated that the intestinal microbiota plays a critical role in the metabolism of voglibose. However, other effects of antibiotics, such as changing the gut epithelial enzyme activity and modulating the blood glucose level in animals, are yet to be studied. In addition, a small but gradual increase in metabolism of voglibose over time in the antibiotics-treated group indicated a reduced number of bacteria in intestinal contents following antibiotic treatment. The results indicated that the pharmacodynamic action of voglibose would be significantly modulated by the condition of intestinal microbiota.
Fig. 4. Time- and concentration-dependent metabolism of voglibose by intestinal microbiota in vitro. Various concentrations of voglibose was incubated with 0.5 g/mL intestinal contents prepared from both antibiotics- and vehicle-treated mice and incubated at 37℃ for various time points. The remained voglibose over time was measured by liquid chromatography-mass spectrometry (LC-MS/MS). Each bar represents the mean concentration of voglibose in the incubated sample+standard deviation of triplicate determinations. aThe value significantly different from corresponding vehicle-treated controls at P<0.05.
To support this hypothesis, the in vivo pharmacodynamic action of voglibose was investigated in conditionally modulated intestinal microbiota by antibiotic treatment.
Pharmacodynamics of voglibose in normal and diabetic mice
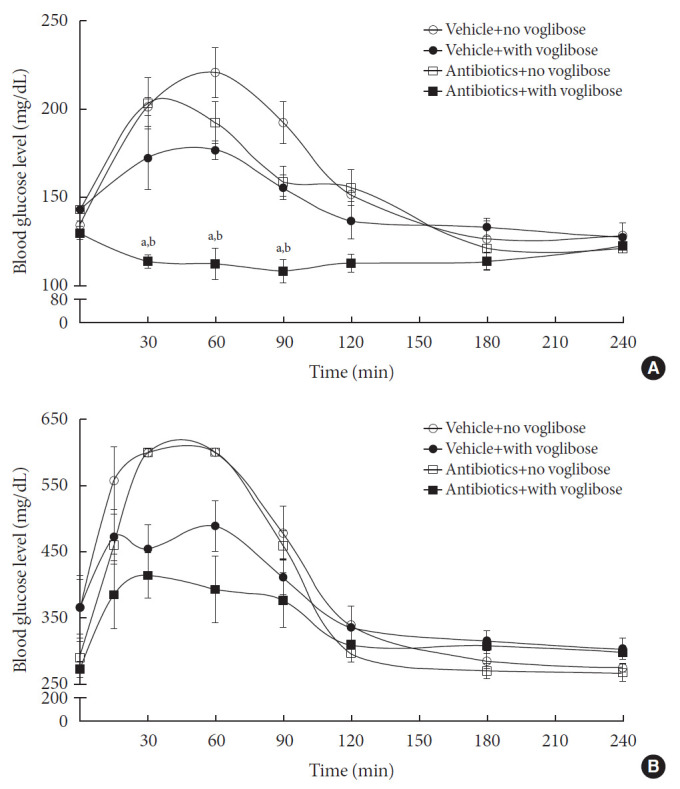
Because antibiotic pretreatment would cause the reduction of the intestinal microbial population, the enzymes available for voglibose metabolism would be reduced and, thereby, the metabolism of voglibose by the intestinal microbiota would be altered. Due to the low intestinal absorption of voglibose, the pharmacodynamic effects of voglibose in starch-supplemented animals were investigated in the present study. To illustrate the effect of the intestinal microbiota on the metabolism of voglibose in vivo, the blood glucose-lowering effect of voglibose was measured in vehicle- and antibiotic-treated non-diabetic and diabetic mice models (Fig. 5). The blood glucose levels rapidly increased following starch administration to 6-hour fasted animals. The changes in blood glucose levels were also clear when the area under the curve (AUC) for 6 hours was calculated from Fig. 5. In addition, the baseline level of blood glucose was much higher in diabetic mice than in non-diabetic mice (AUC=97,457 vs. AUC=38,745). Following voglibose administration in both antibiotics- and vehicle-treated mice of both non-diabetic and diabetic models, the blood glucose levels, even after starch administration, were precisely controlled and similar to the levels at 0 from 2 hours. Particularly, a significant difference in blood glucose level was observed between the voglibose administered antibiotics- and vehicle-treated groups of non-diabetic model (AUC=27,486 vs. AUC=35,262) (Fig. 5A). The blood glucose levels in the antibiotics-treated group were attenuated by voglibose in diabetic mice (AUC=91,837 vs. AUC=81,513), although the results were not statistically significant (Fig. 5B). The results suggested that voglibose could control the blood glucose level by inhibiting α-glucosidase enzyme present in the intestine, particularly in the antibiotics-treated animals, possibly due to the limited availability of microbial enzymes to metabolize voglibose by antibiotics administration. Therefore, more voglibose available showed its effect as a blood glucose-controlling agent via the inhibition of α-glucosidase enzymes.
Fig. 5. Blood glucose level in vehicle-treated and antibiotics-treated non-diabetic normal mice (A) and diabetic mice (B). Six-hr fasted vehicle- and antibiotics-treated non-diabetic and diabetic mice were administered with voglibose followed by an oral administration with starch. And then, the change in the blood glucose level was measured at various time points. Each point represents mean blood glucose level±standard deviation of five animals. aThe value indicates significant difference between antibiotics-treated starch only and antibiotics-treated starch+voglibose groups, bThe value indicates significant difference between vehicle-treated starch+voglibose and antibiotics-treated starch+voglibose groups.
DISCUSSION
The study of drug metabolism is a broad subject that covers liver enzyme- and gut microbiota-mediated metabolism [12]. From studies, it is now generally accepted that metabolism by the intestinal microbiota is important for the formation of new metabolites, which cannot be produced in the liver, and alteration of the pharmacokinetics and pharmacodynamics of many drugs, including amiodarone, amlodipine, aspirin, and lovastatin [8,10,19].
Voglibose has negligible intestinal absorption properties and no liver metabolites are identified yet [15]. In the intestinal lumen, by inhibiting α-glucosidase, complex carbohydrates are not broken down effectively by voglibose. Therefore, glucose absorption is delayed. In our preliminary study, voglibose was metabolized by the intestinal microbiota, although the exact metabolites were not identified yet (Fig. 4). The level of voglibose was dramatically reduced by incubation with intestinal contents prepared from control mice that contain normal microflora than those from antibiotic-treated mice. Therefore, to further explore the action of intestinal microbiota on voglibose action in vivo, pseudo-germ free animals were prepared by antibiotic pretreatment.
For the accurate preparation of pseudo-germ free animals, several in vivo antibiotics-treated models have been used [13,20,21]. Because gut microflora consists of both Gram-positive and Gram-negative bacteria, researchers must use mixtures of broad-spectrum antibiotics to prepare pseudo-germ free mice. In our previous study, an antibiotic treatment model using the mixture of oxytetracycline, erythromycin, and cefadroxil was employed to test the ability of the mixture to reduce the number of bacteria in separated intestinal contents from small intestine, cecum, and large intestine [21]. The results showed that the number of bacteria in intestinal contents collected from all three parts was significantly lowered by antibiotic treatment. Therefore, the same schedule for antibiotic treatment was employed in this study. Following antibiotic administration, the efficacy of the antibiotic treatment was tested by determining various enzyme activities normally produced by the intestinal microbiota in the present study (Fig. 3). Significant decreases in all three enzyme activities tested in the intestinal contents were achieved by antibiotic treatment. These results indicated that the current antibiotics treatment model is effective for studying the possible role of intestinal microbiota in voglibose metabolism.
Thereafter, the in vitro metabolism of voglibose was characterized by incubating various concentrations of voglibose at various time points with the intestinal contents prepared from antibiotics- and vehicle-treated mice. As shown in Fig. 4, the amount of voglibose was significantly reduced within 3 hours of incubation in the control group compared to that in the antibiotic-treated group, and this significant difference remained for up to 24 hours. To show whether the decrease in the level of voglibose in the 24-hour incubated samples was due to the enzymatic action of intestinal microbiota or because of the unstable condition of voglibose at 37℃, a stability test for voglibose was also conducted. The stability results in Table 1 demonstrated that the decreased voglibose was due to the action of intestinal microbiota and not due to the stability related issues of voglibose at 37℃ for 24 hours. A gradual disappearance of voglibose from the intestinal contents prepared from antibiotic-treated mice also indicated that the mice pretreated with oral antibiotics did not completely suppress the enzymes from the intestinal microbiota [21].
Voglibose is neither adequately absorbed nor metabolized by liver enzymes [15]. Therefore, it was impractical to perform the pharmacokinetic study of voglibose in animals. Because voglibose acts as a blood glucose-lowering agent by inhibiting the breakdown of complex carbohydrates in the intestinal lumen, it was reasonable to observe the pharmacological effects of voglibose following antibiotic pretreatment in the starch-supplemented model. Therefore, the alteration of blood glucose levels by voglibose was studied in antibiotic-pretreated non-diabetic and diabetic mice with their respective control groups.
Because voglibose is originally developed as a blood sugar lowering agent, it is normally prescribed to diabetic patients. Therefore, it would be more substantial to perform the voglibose pharmacodynamic study in the diabetic model. For the reason, the role of the intestinal microbiota in the metabolism of voglibose was also tested in diabetic mice in this study. Several methods are available to prepare a diabetic mouse model [22,23,24,25]. Preparation of type I diabetic mice using streptozotocin is one of the simplest and widely accepted methods [23]. From a preliminary study, we found that a single streptozotocin dose higher than 200 mg/kg could destroy pancreatic β-cells rapidly, within 1 or 2 days, but that it was very difficult to control blood glucose levels and the mice died within 10 days of streptozotocin injection (data not shown). Although the result was consistent with a report in which the administration of animals with high doses of streptozotocin yielded the complete death of β-cells and the complete absence of insulin was achieved [26], this was not appropriate for our study. Meanwhile, literatures indicated that multiple low doses of streptozotocin cause delayed onset of hyperglycemia because of the partial damages to the pancreatic islet cells and, thereby, the blood glucose levels are adequately controlled [27,28]. Therefore, we used multiple treatment with streptozotocin at low doses and observed that, with the intraperitoneal injection of mice with 40 mg/kg streptozotocin for consecutive 5 days, blood glucose levels were elevated to >250 mg/dL, which was considered as the diabetic condition in this study [27].
Subsequently, the in vivo pharmacodynamic study of voglibose was performed in antibiotics- and vehicle-treated non-diabetic and diabetic mice. It was hypothesized that, due to the diminished metabolism of voglibose by antibiotic treatment, the blood glucose-controlling activity of voglibose would be significantly elevated and more pronounced in antibiotic-treated animals. Following voglibose and starch administration in the non-diabetic model, the elevation in blood glucose levels was precisely controlled and was similar to the 0-hour values (Fig. 5A). Conversely, the blood glucose levels were highly elevated in starch-supplemented groups in the absence of voglibose. A significant difference was observed between the blood glucose levels in voglibose administered antibiotics- and vehicle-treated non-diabetic mice. These results indicated that the metabolism of voglibose was dependent on the condition of intestinal microbiota, which was largely altered by antibiotic treatment. The same study was also conducted in diabetic mice and the results were similar. Although the patterns of blood glucose levels in mice were similar in both non-diabetic and diabetic mice models, no statistically differences were observed between the groups, probably because of the large blood glucose level variation in diabetic models. The difference in blood glucose levels between antibiotics-treated and -untreated groups showed that the antibiotics pretreatment decreased the intestinal microbial population, and, thereby, resulted in decreased microbial enzyme levels to reduce the metabolism of voglibose. Lesser metabolism of voglibose refers to the availability of more voglibose to show its effect as a blood glucose-controlling agent. Hence, the lowest blood glucose level was observed in antibiotics-pretreated mice administered with voglibose in both diabetic and non-diabetic models.
Following antibiotic treatment, the basal level of blood glucose was slightly lower than in the vehicle-treated group. This was also observed even after starch administration in the antibiotic-treated group at each time point. Therefore, the possible effects of antibiotics employed in the present study on the blood glucose levels could not be completely ruled out. In fact, some reports have suggested that antibiotics treatment could reduce blood glucose levels in exposed individuals with reduced numbers of intestinal microbiota [29,30,31]. In addition, some reports suggested that multiple host factors, as well as the environmental factors, would have direct impacts in host gut microbiome and blood glucose level [32]. Meanwhile, many of the studies employed long-term treatment of animals with antibiotics, which were somewhat different from the present study [30,32]. Therefore, it would be necessary to study the effects of short-term treatment with antibiotics on blood glucose level, which is yet to be determined. Although we could not completely disregard the possibility of decrease in blood glucose level by antibiotics, the role of voglibose metabolism by intestinal microbiota in modulating blood glucose levels might be important at least in part, because of the significantly decreased metabolism of voglibose in vitro in antibiotic-treated intestinal contents (Fig. 4).
Taken together, it was concluded that the metabolism of voglibose was significantly mediated by intestinal microbiota; hence, the pharmacodynamic effects of voglibose were modulated, at least in part, when mice were pretreated with antibiotics. To our knowledge, the role of gut microbiota in voglibose metabolism and the subsequent changes in pharmacodynamic effects was demonstrated for the first time. Because the present results indicated pharmacodynamic interactions of voglibose with antibiotics, a clinical study should be instigated to determine whether the concurrent administration of voglibose and certain antibiotics to patients disturbs the metabolism of voglibose, resulting in the modulation of blood glucose levels in humans similar to the animal models. In addition, it is required to study whether the antibiotics employed in the present study are affecting the blood glucose level or not in the near future, with further studies to identify the possible metabolites produced by the intestinal microbiota.
ACKNOWLEDGMENTS
This research was supported by the grants from National Research Foundation, Korea (NRF-2017RIDIA3B04033313) and Yeungnam University (219-A-380-154).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: M.R.N, T.C.J.
Acquisition, analysis, or interpretation of data: M.R.N., M.J.K., G.H.K., D.H.C., J.H.K.
Drafting the work or revising: M.R.N., J.H.K., T.C.J.
Final approval of the manuscript: M.R.N., M.J.K., G.H.K., D.H.C., J.H.K., T.C.J.
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0147.
Vegetables dietary pattern and risk of gestational diabetes mellitus stratified by parity after propensityscore matching
References
- 1.O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scarpellini E, Ianiro G, Attili F, Bassanelli C, De Santis A, Gasbarrini A. The human gut microbiota and virome: potential therapeutic implications. Dig Liver Dis. 2015;47:1007–1012. doi: 10.1016/j.dld.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hur KY, Lee MS. Gut microbiota and metabolic disorders. Diabetes Metab J. 2015;39:198–203. doi: 10.4093/dmj.2015.39.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang D, Leung RK, Guan W, Au WW. Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018;10:3. doi: 10.1186/s13099-018-0230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dore J, Simren M, Buttle L, Guarner F. Hot topics in gut microbiota. United European Gastroenterol J. 2013;1:311–318. doi: 10.1177/2050640613502477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavhane YN, Yadav AV. Loss of orally administered drugs in GI tract. Saudi Pharm J. 2012;20:331–344. doi: 10.1016/j.jsps.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang MJ, Kim HG, Kim JS, Oh DG, Um YJ, Seo CS, Han JW, Cho HJ, Kim GH, Jeong TC, Jeong HG. The effect of gut microbiota on drug metabolism. Expert Opin Drug Metab Toxicol. 2013;9:1295–1308. doi: 10.1517/17425255.2013.807798. [DOI] [PubMed] [Google Scholar]
- 9.Pellock SJ, Redinbo MR. Glucuronides in the gut: sugar-driven symbioses between microbe and host. J Biol Chem. 2017;292:8569–8576. doi: 10.1074/jbc.R116.767434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noh K, Kang YR, Nepal MR, Shakya R, Kang MJ, Kang W, Lee S, Jeong HG, Jeong TC. Impact of gut microbiota on drug metabolism: an update for safe and effective use of drugs. Arch Pharm Res. 2017;40:1345–1355. doi: 10.1007/s12272-017-0986-y. [DOI] [PubMed] [Google Scholar]
- 11.Jeong HG, Kang MJ, Kim HG, Oh DG, Kim JS, Lee SK, Jeong TC. Role of intestinal microflora in xenobiotic-induced toxicity. Mol Nutr Food Res. 2013;57:84–99. doi: 10.1002/mnfr.201200461. [DOI] [PubMed] [Google Scholar]
- 12.Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 2017;179:204–222. doi: 10.1016/j.trsl.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo DH, Kim IS, Van Le TK, Jung IH, Yoo HH, Kim DH. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 2014;42:1508–1513. doi: 10.1124/dmd.114.058354. [DOI] [PubMed] [Google Scholar]
- 14.Jiao X, Wang Y, Lin Y, Lang Y, Li E, Zhang X, Zhang Q, Feng Y, Meng X, Li B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J Nutr Biochem. 2019;64:88–100. doi: 10.1016/j.jnutbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Dabhi AS, Bhatt NR, Shah MJ. Voglibose: an alpha glucosidase inhibitor. J Clin Diagn Res. 2013;7:3023–3027. doi: 10.7860/JCDR/2013/6373.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh TJ, Yu JM, Min KW, Son HS, Lee MK, Yoon KH, Song YD, Park JY, Jeong IK, Cha BS, Kim YS, Baik SH, Kim IJ, Kim DM, Kim SR, Lee KW, Park JH, Lee IK, Park TS, Choi SH, Park SW. Efficacy and safety of voglibose plus metformin in patients with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Metab J. 2019;43:276–286. doi: 10.4093/dmj.2018.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozes S, Sefcikova Z, Bujnakova D, Racek L. Effect of antibiotic treatment on intestinal microbial and enzymatic development in postnatally overfed obese rats. Obesity (Silver Spring) 2013;21:1635–1642. doi: 10.1002/oby.20221. [DOI] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Guidance for industry: bioanalytical method validation. Rockville: FDA; 2001. [Google Scholar]
- 19.Kim DH. Gut microbiota-mediated drug-antibiotic interactions. Drug Metab Dispos. 2015;43:1581–1589. doi: 10.1124/dmd.115.063867. [DOI] [PubMed] [Google Scholar]
- 20.Hertz FB, Lobner-Olesen A, Frimodt-Moller N. Antibiotic selection of Escherichia coli sequence type 131 in a mouse intestinal colonization model. Antimicrob Agents Chemother. 2014;58:6139–6144. doi: 10.1128/AAC.03021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang MJ, Ko GS, Oh DG, Kim JS, Noh K, Kang W, Yoon WK, Kim HC, Jeong HG, Jeong TC. Role of metabolism by intestinal microbiota in pharmacokinetics of oral baicalin. Arch Pharm Res. 2014;37:371–378. doi: 10.1007/s12272-013-0179-2. [DOI] [PubMed] [Google Scholar]
- 22.Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman HJ. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011;61:356–360. [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes. 2015;8:181–188. doi: 10.2147/DMSO.S82272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877–894. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Belle TL, Taylor P, von Herrath MG. Mouse models for type 1 diabetes. Drug Discov Today Dis Models. 2009;6:41–45. doi: 10.1016/j.ddmod.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70:5. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- 27.Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL, Kudva YC. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45:131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 29.Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, Panda S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9:2872. doi: 10.1038/s41467-018-05336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues RR, Greer RL, Dong X, DSouza KN, Gurung M, Wu JY, Morgun A, Shulzhenko N. Antibiotic-induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol. 2017;8:2306. doi: 10.3389/fmicb.2017.02306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikkelsen KH, Knop FK, Frost M, Hallas J, Pottegard A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J Clin Endocrinol Metab. 2015;100:3633–3640. doi: 10.1210/jc.2015-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujisaka S, Ussar S, Clish C, Devkota S, Dreyfuss JM, Sakaguchi M, Soto M, Konishi M, Softic S, Altindis E, Li N, Gerber G, Bry L, Kahn CR. Antibiotic effects on gut microbiota and metabolism are host dependent. J Clin Invest. 2016;126:4430–4443. doi: 10.1172/JCI86674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vegetables dietary pattern and risk of gestational diabetes mellitus stratified by parity after propensityscore matching