Summary
Background
In 2016, of the estimated 257 million people living with chronic hepatitis B virus (HBV) infection worldwide, only a small proportion was diagnosed and treated. The insufficiency of information on the proportion of people infected with HBV who are eligible for treatment limits the interpretation of global treatment coverage. We aimed to estimate the proportion of people with chronic HBV infection who were eligible for antiviral treatment worldwide, based on the WHO 2015 guidelines.
Methods
In this systematic review and meta-analysis, we searched Medline, EMBASE, and the Cochrane databases from Jan 1, 2007, to Jan 31, 2018, for studies describing HBsAg-positive people in the population or health-care facilities. We extracted information from published studies using a standardised form to estimate the frequency of cirrhosis, abnormal alanine aminotransferase (ALT), HBV DNA exceeding 2000 IU/mL or 20 000 IU/mL, presence of HBeAg, and eligibility for treatment as per WHO and other guidelines as reported in the studies. We pooled proportions through meta-analysis with random effects. The study was registered with PROSPERO, CRD42020132345.
Findings
Of the 13 497 studies, 162 were eligible and included in our analysis. These studies included 145 789 participants. The pooled estimate of the proportion of cirrhosis was 9% (95% CI 8–10), ranging from 6% (4–8) in community settings to 10% (9–11) in clinic settings. Examining the proportion of participants who had characteristics used to determine eligibility in the WHO guidelines, 1750 (10·1%) of 17 394 had HBV DNA exceeding 20 000 IU/mL, and 20 425 (30·8%) of 66 235 had ALT above the upper limit of normal. 32 studies reported eligibility for treatment according to WHO or any other guidelines, with a pooled estimate of eligibility at 19% (95% CI 18–20), ranging from 12% (6–18) for studies in community settings to 25% (19–30) in clinic settings.
Interpretation
Many studies described people with HBV infection, but few reported information in a way that allowed assessment of eligibility for treatment. Although about one in ten of the 257 million people with HBV infection (26 million) might be in urgent need of treatment because of cirrhosis, a larger proportion (12–25%) is eligible for treatment in accordance with different guidelines. Future studies describing people with HBV infection should report on treatment eligibility, according to broadly agreed definitions.
Funding
WHO and US Centers for Disease Control and Prevention.
Introduction
WHO estimates that, in 2015, 257 million people worldwide were living with hepatitis B virus (HBV) infection (defined as being positive for HBsAg).1 68% of these people were living in the WHO-defined African and Western Pacific regions.1 Chronic HBV infection, if left untreated, can progress to cirrhosis and hepatocellular carcinoma.2 WHO also estimates that in 2015, 887 000 people died of chronic HBV infection worldwide.1 Antiviral agents suppress HBV replication, prevent progression to cirrhosis, and reduce the risk of hepatocellular carcinoma and liver-related deaths.3 In 2015, WHO produced its first guidelines on prevention, care, and management of chronic HBV infection, with a focus on low-income and middle-income countries.4 Key recommendations included the use of non-invasive tests for the staging of liver disease; prioritisation of treatment for people at greatest risk of disease progression and mortality, including those with cirrhosis, or, among people without cirrhosis, patients with the combination of high HBV DNA levels (>20 000 IU/mL) and persistently raised alanine aminotransferase (ALT). These guidelines recommended the preferred use of nucleos(t)ide analogues with a high genetic barrier to resistance (ie, tenofovir or entecavir). Other professional guidelines from the Asian-Pacific Association for the Study of the Liver (APASL),5 the American Association for the study of Liver Diseases (AASLD),6 and the European Association for the Study of the Liver (EASL)7 made comparable recommendations for treatment eligibility, although with some differences in thresholds of ALT and HBV DNA for treatment (appendix p 2). The 2012 EASL guidelines7 were revised in 2017,8 expanding the criteria for eligibility. Although many treatment guidelines are available, and the cost of treatment has decreased by 85% between 2004 and 2016, access to and uptake of testing and treatment remains scarce, especially in many low-income and middle-income countries. WHO estimated that in 2016, 27 million (or 10·5%) of people estimated to be HBsAg positive were diagnosed, and, of people diagnosed, 4·5 million (17%) were receiving antiviral treatment.9
Research in context.
Evidence before this study
WHO estimated that, in 2016, among the 257 million people living with chronic hepatitis B virus (HBV) infection worldwide, only 10·5% had been diagnosed and of those, only 17% were receiving treatment. We searched PubMed for systematic reviews published between Jan 1, 2007 and Jan 31, 2018, using “hepatitis B” and “treatment”, but did not identify any estimates, based on empirical data, on the proportion of people with chronic HBV infection eligible for treatment worldwide. The only studies available were studies reporting people with HBV infection, recruited either in the community or in health-care facilities. Although a few of these studies reported the proportion of HBV-infected people who were eligible for treatment, many did not. In the absence of an estimate of the denominator of HBV-infected people eligible for treatment, as per current treatment guidelines, treatment coverage estimates are hard to interpret.
Added value of this study
We systematically reviewed studies describing people with HBV infection to provide a description of their clinical status and assess eligibility. We found that there are many missed opportunities to estimate the proportion of people eligible for treatment. Most studies did not report data that could be used to examine eligibility in a consistent format. We also provided a first estimate of the proportion of people eligible for HBV treatment. About 10% of people with HBV infection are eligible for urgent treatment because of cirrhosis. In addition, overall, between 12% and 25% of people might be eligible for treatment because of either cirrhosis or the combination of raised ALT and viral replication.
Implications of all the available evidence
More opportunities should be seized to estimate the proportion of people who are eligible for HBV treatment. Simple descriptive studies should include estimates of the proportion of participants who are eligible for treatment. Additional efforts are needed to generate disaggregated estimates of the proportion of people eligible for HBV treatment by type of recruitment (community or health-care setting), age, sex, ethnicity, and geographical areas, so as to better understand who is eligible. This information could be used to optimise testing approaches, so that population groups with the highest yield of eligible patients could be tested as a priority. Patients with HBV-related cirrhosis (around 10% people with HBV infection) should be prioritised for treatment initiation. These patients can be treated in the absence of HBV DNA testing.
According to the eligibility criteria of the various treatment guidelines, only a subset of people with chronic infection are in need of treatment, and this subset varies by population, region, and setting. However, no estimates are available for the proportion of people who are infected with HBV who meet these treatment eligibility criteria in different regions.1 Hence, the extent to which the number of people initiated on treatment corresponds to the need is not known. As a consequence, estimates of the treatment gap are difficult to interpret. If available, estimates of the proportion of people positive for HBsAg who were eligible for treatment would allow for better analyses of antiviral treatment gaps and targets, which would support planning of treatment programmes.
To address this knowledge gap, we systematically reviewed the literature to estimate the proportion of HBsAg-positive people who met eligibility criteria for treatment. Our overall goal was to better inform future data collection on eligibility criteria to inform progress in treatment scale-up at national and regional levels. Our aim was to estimate the proportion of people with chronic HBV infection who were eligible for antiviral treatment worldwide, based on the WHO 2015 guidelines.4 We aimed to estimate the proportion of treatment-eligible people according to other professional society guidelines (appendix p 2) and describe the availability of data that would allow assessment of eligibility for hepatitis B antiviral treatment (ie, ALT, HBV DNA, cirrhosis, or liver fibrosis status) in published studies.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we searched MEDLINE, Embase, and the Cochrane database for studies published from Jan 1, 2007, to Jan 31, 2018, that described population of HBsAg-positive people in terms of criteria that can be used to assess eligibility for treatment (ie, ALT, HBV DNA, or cirrhosis; appendix p 3). We also screened bibliographies of relevant articles. We included articles in English, Chinese, French, Spanish, Portuguese, Russian, Korean, and Japanese; although in the final analysis, after inclusion and exclusion criteria were applied, only studies in English, Chinese, French, and Spanish met the inclusion criteria. Two reviewers (MT and ASB) screened titles and abstracts, with inclusions verified by a third reviewer (YH). Disagreements were resolved by discussion and consensus (appendix p 4).
Data analysis
We included studies that reported data on a population of HBsAg-positive people and at least one of the following: liver fibrosis or cirrhosis (F0 to F4, irrespective of test type), data on abnormal ALT levels (more than upper limit of normal of the laboratory, as defined by the study), and data on HBV DNA. We excluded studies focusing entirely or mostly on children (defined as people <18 years); studies with fewer than 20 participants; studies of the complications of HBV infection; case series of liver imaging or biopsy without information on selection of participants; studies focusing on people with HIV, hepatitis C virus, hepatitis D virus co-infection (co-infections affect eligibility and are a minority of all HBV infections10, 11) or focusing on people with other characteristics that would have led to the study population being representative of a subset of the population of people with HBV infection (eg, people who were immunosuppressed);1 studies focusing mainly on participants with a primary condition other than HBV infection; and studies restricted to participants already known to be eligible for therapy and studies that included people who were on treatment. If multiple publications reported the same study population, we included the more recent analysis. For meta-analyses and reviews that included results from several primary studies, we obtained individual studies and extracted data from the primary source if not already included.
We assessed method quality through possible sources of selection and information bias. For selection bias, we reviewed the study design (eg, cohort, cross-sectional, or case control), data collection procedures (eg, prospective or retrospective), and the type of recruitment (eg, from statistical samples in the community, to non-statistical samples in the community, to recruitment in health-care facilities). For information bias, we extracted information on methods used to assess liver fibrosis and viral replication. For methods to assess fibrosis, we considered the range between non-invasive methods (including clinical assessment) to liver biopsy. When more than one method was used to assess liver fibrosis, we extracted data on the best method used, as determined by the assessor. For viral replication, we extracted information about whether HBV DNA or HBeAg was used. We then stratified results for all these determinants of study quality.
We extracted data on summary estimates from the selected articles and verified the information with a second reviewer (appendix p 5). We estimated the proportion of people with individual characteristics by which their treatment eligibility would be determined using WHO criteria (ie, cirrhosis or no cirrhosis, replication based on HBV DNA >20 000 IU/mL, or HBeAg, and presence of abnormal ALT according to the laboratory). We calculated means, medians, and proportions, and corresponding 95% CIs for estimates. We used the total number of studies reporting the information as the denominator to calculate CIs. We considered guidelines of the WHO, APASL, EASL, and AASLD to evaluate eligibility. We extracted information as to whether the study reported a proportion of people eligible for treatment as per any guidelines and reported this proportion.
For two specific outcomes (cirrhosis and eligibility per any guidelines), we did a DerSimonian-Laird random effects meta-analysis with Freeman-Tukey double arcsine transformation. We generated forest plots to estimate pooled estimates, I2 statistics to describe heterogeneity among studies, funnel plots to explore publication bias, and bubble plots to explore variations according to specific characteristics. We stratified the analysis according to year of publication, design of the study from which we extracted cross-sectional data on people with HBV infection (cohort, case-control, and cross-sectional), data collection procedure (prospective, retrospective, and cross-sectional), settings in which study participants had been recruited (outpatient, inpatient, population-based, community, and special groups), and WHO region.
All analyses were done in Epi-Info (version 7.2) and Stata (version 13). The meta-analysis was done using the metaprop, metabias, metafunnel, and metareg programmes in Stata. The protocol of the meta-analysis was registered in PROSPERO (CRD42020132345).
Role of the funding source
The funder who supported WHO for this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The first, second, and corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
The database search returned 13 492 records, and another five records were identified through other sources. 13 403 records were unique, after removal of duplicates, and of these, 465 were selected for full-text screening. Review of the full-text articles led to the exclusion of 303, and the inclusion of 162 articles in the analysis (Figure 1, Figure 2).12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173
Figure 1.
Study selection
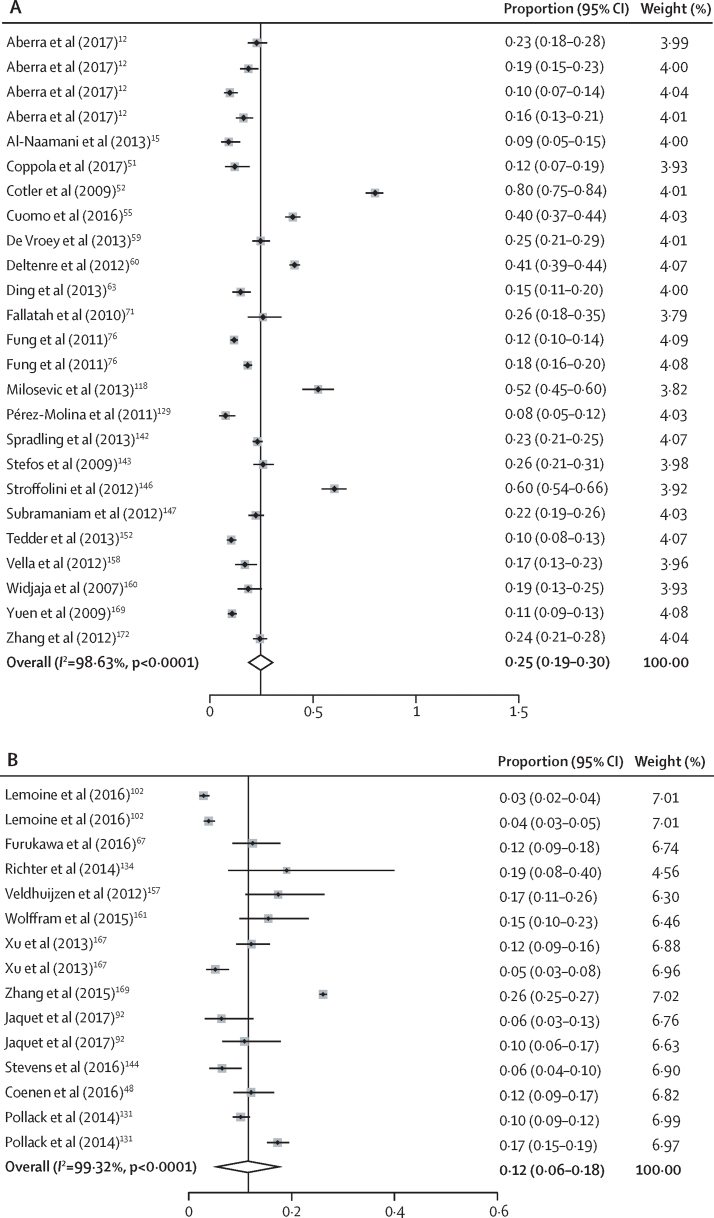
Figure 2.
Pooled estimates of the proportion of HBsAg-positive people eligible for treatment as per any of the known guidelines in studies done in health-care facilities (A) or in the community (B)
Cotler SJ and colleagues52 is an outlier. However, its removal in a sensitivity analysis did not affect the estimate substantially. Studies that appear more than once reported eligibility criteria using more than one set of guidelines.
Of the 162 studies included (table 1), 50 (31%) were published in 2007–11, and 112 (69%) were published in 2012–18. From these 162 studies, all 145 789 participants were included from all WHO regions (median number of HBsAg-positive participants per study 285, IQR 123–818), with the largest median number from the Western Pacific (476 [IQR 226–1394] and smallest from the Americas (185 [144–610]). Participants had a mean age of 41 years (SD 8·8), and cohorts were comprised of a mean of 41·9% (22·5) women.
Table 1.
Characteristics of HBsAg-positive people
| Studies available (N=162) |
Number of HBsAg-positive participants in individual studies (N=162) |
Age in individual studies (n=89) |
Proportion of females in individual studies (n=154) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Range | Total | Mean (SD) | Median (IQR) | Range | Mean (SD) | Range | ||
| Year of publication | ||||||||||
| 2007–11 | 50 (31%) | 728 (1075) | 285 (114–1106) | 25–4405 | 36 408 | 40·6 (7·4) | 40·2 (35·7–46·3) | 26–55 | 34·6 (19·3) | 0–100 |
| 2012–18 | 112 (69%) | 1094 (2473) | 261 (134–841) | 21–13 979 | 109 381 | 41·3 (9·3) | 41·0 (34·5–48·4) | 23–69 | 43·6 (23·0) | 0–100 |
| Recruitment setting | ||||||||||
| Outpatient clinic | 54 (31%) | 720 (1719) | 295 (143–576) | 50–12 016 | 31 568 | 40·3 (6·8) | 40·0 (35·3–45·0) | 29–56 | 35·2 (17·6) | 0–100 |
| Inpatients | 68 (39%) | 757 (1719) | 282 (139–691) | 25–13 210 | 47 709 | 41·0 (9·1) | 40·7 (34·0–48·0) | 26–69 | 43·1 (22·7) | 13–100 |
| Population-based* | 11 (6%) | 1433 (2423) | 400 (168–1542) | 34–8875 | 15 760 | 44·9 (3·2) | 45·0 (41·3–48·4) | 40–49 | 54·1 (18·0) | 34–100 |
| Community† | 21 (12%) | 2146 (3487) | 382 (139–2682) | 21–13 979 | 42 928 | 49·3 (7·5) | 50·2 (45·0–55·2) | 37–64 | 48·5 (14·1) | 34–100 |
| Special groups‡ | 20 (12%) | 455 (924) | 156 (90– 811) | 64–2903 | 9106 | 33·5 (7·8) | 29·2 (28·4–39·8) | 26–52 | 54·0 (34·7) | 0–100 |
| WHO region | ||||||||||
| Americas | 34 (12%) | 899 (2085) | 185 (144–610) | 40–12 016 | 27 872 | 41·4 (5·0) | 40·7 (38·0–45·4) | 31–49 | 42·3 (21·7) | 13–100 |
| African | 46 (17%) | 852 (1840) | 387 (146–756) | 75–12 016 | 34 114 | 37·5 (8·0) | 36·4 (31·0–41·9) | 25–56 | 44·0 (27·5) | 0–100 |
| Eastern Mediterranean | 33 (12%) | 359 (709) | 220 (114–473) | 21–2078 | 11 125 | 37·8 (6·0) | 36·9 (34·0–41·1) | 23–50 | 34·6 (15·7) | 13–100 |
| European | 51 (19%) | 775 (1878) | 291 (135–675) | 21–13 210 | 35 806 | 42·6 (7·7) | 42·0 (38·0–48·0) | 26–57 | 40·6 (20·8) | 0–100 |
| Southeast Asia | 40 (15%) | 610 (704) | 399 (170–691) | 64–3760 | 19 434 | 40·8 (9·0) | 41·0 (31·9–47·0) | 26–56 | 39·0 (21·9) | 0–100 |
| Western Pacific | 72 (26%) | 1425 (2580) | 476 (226–1394) | 21–13 979 | 98 360 | 43·7 (9·6) | 44·0 (38·2–49·1) | 26–68 | 46·0 (22·1) | 0–100 |
| Design§ | ||||||||||
| Cohort | 51 (31%) | 1062 (1857) | 441 (167–1200) | 59–12 016 | 48 875 | 42·4 (9·5) | 41·0 (36·8–45·5) | 27–69 | 44·3 (21·5) | 0–100 |
| Case control | 3 (2%) | 523 (497) | 289 (NA) | 65–1215 | 1569 | 33·8 (NA) | 32·8 (NA) | 33–33 | 27·0 (7·3) | 17–32 |
| Cross-sectional | 107 (67%) | 964 (2295) | 239 (111–602) | 21–13 979 | 95 160 | 40·7 (8·3) | 40·4 (34·8–48·9) | 23–57 | 42·8 (23·2) | 0–100 |
| Data collection¶ | ||||||||||
| Prospective | 88 (55%) | 849 (1397) | 300 (112–1139) | 21–8875 | 66 585 | 41·7 (8·9) | 41·0 (35·5–48·9) | 25–64 | 43·0 (24·1) | 0–100 |
| Retrospective | 44 (27%) | 1244 (2725) | 356 (180–655) | 93–13 210 | 49 772 | 40·5 (7·1) | 40·0 (35·5–45·9) | 26–56 | 42·6 (19·7) | 5–100 |
| Cross-sectional | 29 (18%) | 1012 (2861) | 170 (94–406) | 34–13 979 | 29 346 | 40·4 (10·8) | 40·4 (32·8–48·0) | 23–69 | 38·3 (21·8) | 0–100 |
| Overall | 162 (100%) | 972 (2141) | 285 (123–818) | 21–13 979 | 145 789 | 41·0 (8·8) | 41·0 (34·6–47·0) | 23–69 | 41·9 (22·5) | 0–100 |
Data are n (%), mean (SD), or median (IQR), unless stated otherwise. HBV=hepatitis B virus. NA=not applicable.
Studies of individuals not seeking care sampled using statistically representative methods.
Studies of individuals not seeking care, but recruited without statistically representative methods.
For example, injection drug users and men who have sex with men.
Design of the study from which was extracted information on a population of people with HBV infection. Total exceeds the number of studies because some studies fit more than one category.
One study with missing information on data collection procedure.
11 (6%) studies were based on representative samples of the general population; the remainder were based on either convenience samples in the population (ie, community; n=21 [12%]), outpatient clinics (n=54 [31%]), inpatients (n=68 [39%]) or specific population groups (eg, prisoners or migrants; n=20 [12%]). Only ten studies (6%) included a substantial proportion of pregnant women, representing 2897 (2·0%) of the total population of 145 789 HBsAg-positive people.
Most studies (n=107 [67%]) were cross-sectional in design. 51 (31%) were cohort studies and three (2%) were case-control studies. Data collection was mostly prospective (n=88 [55%]), with the remainder being retrospective (n=44 [27%]) or done in context of a cross-sectional survey (n=29 [18%]).
Methods used to assess liver fibrosis or presence of cirrhosis included clinical assessment (n=43 [26%]), other non-invasive methods (ie, clinical, ultrasound, or transient elastography n=102 [63%]), ultrasound (n=59 [36%]), transient elastography (n=20 [12%]), and biopsy (n=61 [37%]; table 2). 56 (35%) studies used more than one method to assess liver fibrosis.
Table 2.
Liver fibrosis status and assessment methods used in studies describing HBsAg-positive people
|
Proportion of HBsAg-positive participants with selected stages of liver fibrosis |
Proportion of studies using different methods used to assess liver fibrosis |
|||||||
|---|---|---|---|---|---|---|---|---|
| F2 (n=16)* | F3 (n=16)* | F4 (cirrhosis; n=104)* | Clinical assessment (43 studies) | NIM (102 studies) | Ultrasound (59 studies) | Transient elastography (20 studies) | Biopsy (61 studies) | |
| Year of publication | ||||||||
| 2007–11 (n=50) | 26% (24–27) | 20% (18–21) | 11·0% (10·6–11·4) | 29% (16–41) | 67% (54–79) | 43% (30–57) | 4% (1–13) | 41% (27–54) |
| 2012–18 (n=112) | 18% (16–19) | 16% (15–17) | 8·5% (8·2–8·7) | 25% (18–34) | 61% (52–70) | 33% (25–42) | 15% (10–23) | 36% (27–45) |
| Recruitment setting | ||||||||
| Outpatient clinic (n=54) | 31% (28–34) | 33% (30–35) | 9·5% (9·2–9·9) | 46% (32–59) | 62% (55–80) | 45% (33–58) | 13% (6–21) | 51% (38–65) |
| Inpatients (n=68) | 30% (29–32) | 24% (22–25) | 13·9% (13·5–14·4) | 26% (17–38) | 57% (46–69) | 32% (22–44) | 13% (7–23) | 43% (32–54) |
| Population-based (n=11)† | 1% (<1–2) | 3% (2–4) | 22·0% (20·0–24·0) | 9% (2–38) | 50% (19–81) | 18% (5–46) | 9% (2–38) | 9% (2–38) |
| Community (n=21)‡ | .. | 9% (5–13) | 9·6% (8·9–10·2) | 14% (5–35) | 85% (69–100) | 52% (32–72) | 10% (3–29) | 24% (11–45) |
| Special groups (n=20) | 6% (2–9) | 8% (2–14) | 7·7% (6·9–8·6) | 50% (24–76) | 60% (38–81) | 40% (21–61) | 20% (8–42) | 25% (11–47) |
| WHO region | ||||||||
| Americas n=34) | 5% (2–8) | 6% (2–9) | 7·3% (6·9–7·6) | 41% (26–58) | 74% (59–88) | 44% (29–61) | 6% (2–19) | 68% (51–81) |
| African (n=46) | 12% (10–13) | 15% (13–17) | 8·1% (7·8–8·4) | 30% (17–43) | 57% (43–72) | 30% (18–44) | 21% (12–35) | 45% (31–60) |
| Eastern Mediterranean (n=33) | 15% (11–19) | 4% (2–6) | 12·8% (12·1–13·4) | 30% (17–47) | 63% (47–80) | 39% (25–56) | 12% (5–27) | 51% (35–67) |
| European (n=51) | 37% (33–42) | 18% (14–21) | 14·2% (13·7–14·9) | 35% (22–48) | 54% (40–67) | 33% (21–46) | 10% (4–21) | 54% (40–67) |
| Southeast Asia (n=40) | 5% (2–8) | 6% (2–9) | 15·3% (14·7–15·8) | 37% (22–52) | 63% (49–78) | 41% (27–57) | 5% (2–16) | 60% (44–75) |
| Western Pacific (n=72) | 26% (24–28) | 21% (19–23) | 8·6% (8·2–8·8) | 23% (13–33) | 59% (43–71) | 37% (27–48) | 7% (3–15) | 40% (28–51) |
| Overall | 21% (19–23) | 18% (17–19) | 9·5% (9·3–9·7) | 26% (19–33) | 63% (55–70) | 36% (29–44) | 12% (8–17) | 37% (30–45) |
Data are % (95% CI). NIM=non-invasive methods, include clinical criteria, ultrasound, or transient elastography.
Number of studies available that estimated the proportion of people with this specific stage of liver fibrosis. Only a minority of studies reported data on the proportion of persons with F2 and F3 fibrosis.
Studies of individuals not seeking care sampled using statistically representative methods.
Studies of individuals not seeking care but recruited without statistically representative methods.
Overall, 6563 (9·5%) of 69 129 participants had F4 liver fibrosis (cirrhosis). The meta-analysis (appendix p 12) estimated that 9% (95% CI 8–10) of participants had cirrhosis, which was not influenced by the assessment method (appendix p 13). There was a higher proportion of participants with higher prevalence of cirrhosis in larger studies (appendix p 14). In regression with random effect, the proportion of participants with cirrhosis ranged from 6% (95% CI 4–8) in community settings to 10% (9–11) in clinic settings (appendix p 17). We were unable to extract an estimate of the proportion of patients with decompensated cirrhosis.
HBeAg or HBV DNA, or both, were used to assess viral replication (table 3). The proportion of studies with information on HBeAg was higher (n=122 [75%] of 162 studies) than for HBV DNA (n=41 [25%] for 2000 IU/mL threshold and 20 [12%] for the 20 000 IU/mL threshold). Overall, 19 459 (17·7%) of 109 577 participants were HBeAg positive, which was highest in the Western Pacific (19·6% [95% CI 19·1–19·9]) and lowest in the Americas (12·8% [95% CI 12·3–13·1]). 10 101 (28·2%) of 35 826 participants had HBV DNA of more than 2000 IU/mL, which was highest in Europe (31·4% [95% CI 29·6–33·2]) and lowest in the Americas (12·9% [12·4–13·5]). 1750 (10·1%) of 17 394 participants had HBV DNA of more than 20 000 IU/mL, which was highest in southeast Asia (29·2% [24·7–33·7]) and lowest in the Americas (6·4% [6·0–6·9]). The proportions of people with HBeAg and high HBV DNA were also higher in studies done from 2007–11 than 2012–18. Of 162 studies, 80 (49%) reported data on ALT. 20 425 (30·8%) of 66 235 participants had ALT higher than the upper limit of normal, with this number being highest in Europe (54·9% [95% CI 54·0–55·9]) and lowest in the Western Pacific (30·7% [30·4–31·1]). Because information about liver fibrosis, replication, and abnormal ALT were reported in aggregate numbers and without cross tabulation, it was not possible to infer eligibility from these individual criteria or how eligibility was assessed in combination.
Table 3.
HBV replication and abnormal ALT status in HBsAg-positive people
| Proportion of people with HBeAg (n=122) |
HBV DNA |
Abnormal ALT* |
|||
|---|---|---|---|---|---|
| Proportion of people with >2000 IU/mL (n=41) | Proportion of people with >20 000 IU/mL (n=20) | Proportion of people with ALT>ULN (n=80) | Proportion of people with ALT >2 times ULN (n=18) | ||
| Year of publication | |||||
| 2007–11 (n=50) | 20·4% (19·9–21·3) | 43·2% (41·8–44·5) | 30·0% (17·3–42·7) | 35·6% (34·9–36·2) | 26·0% (24·5–27·5) |
| 2012–18 (n=112) | 16·2% (15·9–16·5) | 25·8% (25·3–26·3) | 10·0% (9·5–10·4) | 28·9% (28·5–29·3) | 8·3% (7·9–8·7) |
| Recruitment setting | |||||
| Outpatient clinic (n=54) | 18·7% (18·2–20·2) | 18·7% (18·1–19·4) | 9·3% (8·8–9·8) | 36·9% (36·3–37·5) | 10·1% (9·7–10·6) |
| Inpatients (n=68) | 21·4% (20·9–21·7) | 35·2% (33·8–36·5) | 22·7% (20·9–24·5) | 45·4% (44·6–46·2) | 10·3% (9·4–11·2) |
| Population-based (n=11)† | 19·3% (18·5–20·0) | 6·5% (5·1–7·9) | .. | 28·9% (28·2–29·6) | 6·9% (3·7–10·0) |
| Community (n=21)‡ | 13·5% (13·0–13·9) | 41·1% (40·2–41·9) | 21·0% (18·1–23·9) | 29·2% (28·4–30·1) | 7·6% (5·3–9·9) |
| Special populations (n=20) | 16·8% (15·9–17·8) | 14·5% (13·4–15·7) | 13·7% (12·0–15·4) | 16·0% (15·1–16·9) | 8·3% (4·9–11·7) |
| WHO region | |||||
| Americas (n=34) | 12·8% (12·3–13·1) | 12·9% (12·4–13·5) | 6·4% (6·0–6·9) | 34·7% (34·0–35,5) | 6·4% (6·0–6·9) |
| African (n=46) | 12·2% (11·8–12·6) | 14·9% (14·3–15·4) | 8·2% (7·7–8·6) | 32·7% (32·0–33·3) | 7·1% (6·7–7·5) |
| Eastern Mediterranean (n=33) | 15·1% (14·3–15·8) | 22·1% (19·4–24·8) | 21·3% (18·1–24·4) | 33·6% (32·3–35·0) | 7·6% (5·8–9·4) |
| European (n=51) | 13·7% (13·3–14·0) | 31·4% (29·6–33·2) | 13·3% (11·5–15·1) | 54·9% (54·0–55·9) | 13·3% (12·3–14·4) |
| Southeast Asia (n=40) | 18·2% (17·7–18·6) | 14·5% (12·7–16·2) | 29·2% (24·7–33·7) | 53·6% (52·5–54·7) | 15·6% (14·5–16·8) |
| Western Pacific (n=72) | 19·6% (19·1–19·9) | 24·8% (24·3–25·4) | 6·6% (6·2–7·0) | 30·7% (30·4–31·1) | 10·7% (10·2–11·2) |
| Overall (N=162) | 17·7% (17·5–17·9) | 28·2% (27·7–28·7) | 10·1% (9·6–10·5) | 30·8% (30·5–31·2) | 11·0% (10·6–11·4) |
Data are % (95% CI). HBV=hepatitis B virus. ALT=alanine aminotransferase. ULN=upper limit of normal.
ALT levels were defined as abnormal using the criteria of the individual studies and their laboratories.
Studies of individuals not seeking care sampled using statistically representative methods.
Studies of individuals not seeking care, but recruited without statistically representative methods.
The proportion of participants eligible for treatment was reported using various criteria and only in a small number of studies (table 4). According to EASL 2012 criteria (eight studies), 830 (19·3%) of 4300 participants were eligible for treatment. This number was highest in the Americas (36% [95% CI 33–39]) and in Europe (32% [29–35]) and lowest in the African region (18% [16–19]). According to AASLD criteria (four studies), 246 (8%) of 3030 participants were eligible. This number was highest in the Americas (19% [95% CI 13–24]), with no data from the Eastern Mediterranean or European regions. According to EASL 2017 criteria (two studies, both in Africa), 75 (18%) of 410 participants were eligible. According to WHO criteria (two studies, both in Africa), 40 (10%) of 410 participants were eligible. Overall, of 162 studies, only 32 (20%) assessed eligibility for treatment using WHO or any other guidelines. The pooled estimate of eligibility according to any criteria was 19% (95% CI 18–20), but there was considerable heterogeneity. Stratified analysis led to pooled estimates of treatment eligibility of 12% (6–18) among studies done in community settings and 25% (19–30) in studies from health facilities (hospital or clinic settings). Studies done in health-care facilities that included older participants tended to have a lower proportion of eligibility (p=0·006; appendix p 15). The funnel plots did not suggest any significant publication bias with respect to proportion of eligibility, whether in health-care facilities or the community (appendix p 16).
Table 4.
Eligibility of HBsAg-positive people for treatment according to guidelines
|
AASLD (n=4) |
EASL 2012 (n=8) |
EASL 2017 (n=2) |
WHO (n=2) |
Other*(n=16) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies available | Eligible patients | Studies available | Eligible patients | Studies available | Eligible patients | Studies available | Eligible patients | Studies available | Eligible patients | |
| Year of publication | ||||||||||
| 2007–11 (n=50) | 2 (4%) | 12% (10–14) | 1 (2%) | 18% (16–20) | .. | .. | .. | .. | 4 (8%) | 15% (13–17) |
| 2012–18 (n=112) | 2 (2%) | 3% (2–4) | 7 (6%) | 20% (18–21) | 2 (2%) | 18% (15–22) | 2 (2%) | 10% (7–13) | 12 (11%) | 20% (19–21) |
| Recruitment setting | ||||||||||
| Outpatient (n=54) | 2 (4%) | 12% (11–14) | 4 (7%) | 19% (17–20) | 1 (2%) | 23% (18–27) | 1 (2%) | 10% (6–13) | 7 (13%) | 19% (17–20) |
| Inpatients (n=68) | .. | .. | 2 (3%) | 29% (25–33) | 1 (1%) | 23% (18–27) | 1 (1%) | 10% (6–13) | 9 (13%) | 21% (20–22) |
| Population based (n=11)† | 2 (18%) | 3% (2–4) | 1 (9%) | 4% (3–5) | .. | .. | .. | .. | .. | .. |
| Community (n=21)‡ | 1 (5%) | 6% (3–9) | 1 (5%) | 19% (2–26) | .. | .. | .. | .. | 3 (14%) | 13% (10–16) |
| Special groups (n=20) | .. | .. | 1 (5%) | 12% (6–18) | 1 (5%) | 6% (2–11) | 1 (5%) | 10% (4–16) | 1 (5%) | 12% (8–16) |
| WHO region | ||||||||||
| American (n=34) | 1 (3%) | 19% (13–24) | 2 (6%) | 36% (33–39) | .. | .. | .. | .. | 4 (12%) | 22% (20–24) |
| African (n=46) | 1 (2%) | 3% (2–4) | 5 (11%) | 18% (16–19) | 2 (4%) | 18% (14–22) | 2 (4%) | 10% (7–13) | 4 (9%) | 15% (13–17) |
| Eastern Mediterranean (n=33) | .. | .. | 1 (3%) | 19% (2–26) | .. | .. | .. | .. | 3 (9%) | 16% (14–18) |
| European (n=51) | .. | .. | 4 (8%) | 32% (29–35) | .. | .. | .. | .. | 6 (12%) | 22% (20–24) |
| Southeast Asia (n=40) | 1 (2%) | 6% (3–10) | 2 (5%) | 22% (18–25) | .. | .. | .. | .. | 5 (12%) | 18% (16–19) |
| Western Pacific (n=72) | 2 (3%) | 11% (9–12) | 3 (4%) | 25% (24–27) | .. | .. | .. | .. | 8 (11%) | 17% (16–18) |
| Overall (N=162) | 4 (2%) | 8% (7–9) | 8 (5%) | 19% (19–20) | 2 (1%) | 18% (14–22) | 2 (1%) | 10% (7–13) | 16 (10%) | 19% (18–20) |
Data are n (%) or % (95% CI). AASLD=American Association for the study of Liver Diseases. EASL=European Association for the Study of the Liver.
Studies used guidelines other than AASLD, EASL, or WHO, or used mixed guidelines to document treatment eligibility.
Studies of individuals not seeking care sampled using statistically representative methods.
Studies of individuals not seeking care but recruited without statistically representative methods.
Discussion
Among the 162 studies identified that described people with chronic HBV infection, few provided information that could estimate the proportion of people eligible for treatment. However, among the HBsAg-positive participants, approximately 9% had cirrhosis, 10% had HBV DNA exceeding 20 000 IU/mL, and roughly a third had raised ALT levels on at least one occasion. Estimates of treatment eligibility according to WHO or other guidelines varied between 12% in the community and 25% in clinical settings.
Most studies included some assessment of the stage of liver fibrosis, and according to the data generated through various methods, approximately one in ten people would have a liver fibrosis stage of F4, corresponding to cirrhosis. The methods used in the studies differed, were sometimes based on clinical criteria, and sometimes included tests that can be operator dependent or non-standardised (eg, ultrasound). Some studies relied on ultrasound to diagnose cirrhosis.2 Surface nodularity can be a reliable marker of advanced liver disease in patients with chronic liver disease undergoing liver biopsy, with sensitivity and specificity of more than 90%.174 In a case series without portal hypertension, the positive predictive value of ultrasound to diagnose cirrhosis was 68%.175 However, in the absence of features of portal hypertension, such as varices, ascites, or splenomegaly, the sensitivity can be low.176, 177, 178 WHO guidelines recommend non-invasive tests of acceptable diagnostic accuracy, such as the Fibrosis-4, AST to platelet ratio index, or transient elastography (as well as clinical assessment).4 Our meta-regression analysis did not suggest that this estimate was substantially influenced by the assessment methods. Assessment techniques not considered gold standard might be harder to use to differentiate between earlier stage of liver fibrosis, whereas cirrhosis might lead to changes that might be easier for these various tests to capture. In clinical practice and for individual decision making, the reliability of ultrasound and other less validated tests to diagnose cirrhosis is unclear. However, on a population level, the bubble plot analysis suggests that the assessment methods did not influence the proportion of people with cirrhosis. The combination of these techniques provides some order of magnitude of the proportion of HBV-infected individuals who might require treatment on the basis of cirrhosis, suggesting that roughly one in ten patients with HBV infection would be in that category. There are differences in the frequency of cirrhosis by region, for which we are unable to disentangle selection bias, information bias, and actual differences caused by other factors. The higher frequency of cirrhosis in the European region, for example, might be a result of an information bias. In Europe, there could be a more systematic use of reliable methods of staging and ascertainment of cirrhosis (eg, liver biopsies or transient elastography); however, there could be also more patients with other chronic liver diseases (eg, alcohol or metabolic syndrome).
Although the majority of studies reported on the proportion of people positive for HBeAg, fewer than half used HBV DNA as an assessment of viral replication, and only 20% reported data using the 20 000 IU/mL threshold used by WHO guidelines. Cost might explain, to some extent, why this technique is not more widely available; however, cost is not the only obstacle. Information on ALT was also not available in about half of the studies. Most importantly, although information might be available on liver fibrosis, replication (HBeAg or HBV DNA), and abnormal ALT as separate factors, studies reported data on an aggregated basis and without cross-tabulation. This aggregated data prevented a posteriori calculation of the proportion of people eligible for treatment during this meta-analysis.
Only a minority of studies estimated the proportion of patients eligible for treatment. Given the diversity of guidelines, these studies also reported a diversity of estimates. Our meta-analysis estimate suggested that overall, 19% (95% CI 18–20) of people infected with HBV would need treatment, whereas the pooled estimates of treatment eligibility ranged from 12% (6–18) in the community to 25% (18–20) in clinical settings. However, the representativeness of these studies and the diversity of the criteria used suggest these estimates should be interpreted with caution. The frequency of eligibility was not higher in the highly endemic regions of Africa or the Western Pacific. Differences in the results might be secondary to information bias. Higher frequency of eligibility in the higher income countries of the Americas might be because of diagnosis criteria and more frequent testing services, such as liver biopsies, that result in not only evaluating the degree of liver fibrosis, but also the degree of inflammation (AASLD recommends treatment of people with moderate inflammation or F2 liver fibrosis or greater). In our review, the largest use of biopsies was reported from the Americas. We observed that studies done in health-care facilities that recruited older participants tended to have a lower proportion of eligibility for treatment; this could be because many older participants might already be receiving treatment and a larger proportion of older people in the inactive phase of HBV infection.
This systematic review has several limitations. First, we attempted to retrieve information on eligibility from studies that were not designed to generate it. The information required involved tests that are not often used in low-income and middle-income countries (eg, reliable liver fibrosis assessment techniques or HBV DNA) and data were not always presented in a way that allowed for calculation of eligibility. In addition, because these studies were not done for this objective, information was not presented in a format that reflected the standard algorithm for decision making. As a result, we often missed the proportion that met criteria for treatment initiation, including the presence of cirrhosis, and of those without cirrhosis, the proportion with raised HBV DNA levels combined with ALT levels. Second, the diversity of treatment guidelines made it more difficult to estimate a single proportion of patients eligible for treatment because this varies according to the guidelines. Also, our study cannot be used to compare the proportion of people eligible for treatment according to different guidelines. Such analyses should be done in studies containing individual patient records. Third, we meta-analysed aggregated data from studies that did not disaggregate their study participants by subgroup, such as ethnic group, age, sex, or various other causes of liver diseases. Therefore, the variation in the estimate that we observed according to age, for example, is at the study level and could be explained by selection, information bias, and other factors. As an example of this limitation, we were unable to assess whether other chronic liver diseases (eg, alcohol or metabolic syndrome) would explain the frequency of cirrhosis by regions. Fourth, antiviral treatment for HBV infection is generally lifelong, and so a decision to treat (except for those with identified cirrhosis) is often made on the basis of several visits and repeat investigations over a period of time (eg, persistently raised aminotransferase levels). This is reflected in WHO, EASL,7, 8 and AASLD6 guidelines. Because most studies were cross-sectional in design, only a single timepoint was available to assess eligibility. Finally, we did not estimate the proportion of women eligible for prophylaxis during pregnancy, because criteria differ179 and our studies included very few pregnant women.
Our analysis points to three main conclusions. First, most published studies described populations of people with chronic HBV infection, but did not report data (ie, liver fibrosis staging, HBV DNA level, and ALT) in a consistent format that could be used to examine eligibility for treatment according to different criteria. The adoption of consistent reporting standards in studies of people who are HBsAg positive would facilitate evaluation of the impact of applying different criteria for eligibility. Second, 10% of people with chronic HBV infection across all studies are eligible for treatment based on presence of cirrhosis. Third, using heterogeneous data from studies using different criteria, between a tenth and a quarter of people with HBV infection might be eligible for treatment depending on whether people were recruited in the community or in clinical settings. When applied to the estimated 257 million people with HBV infection worldwide, this would suggest that between 20 million and 64 million people are eligible for treatment. This range could be used as an initial working estimate while awaiting better sources of information.
We propose several actions. First, studies reporting cross-sectional data on people with HBV infection should include estimates of the proportion of participants who are eligible for treatment according to various criteria. Such cross-sectional data would be a useful addition to data from cohorts used to describe natural history and inform guidelines. Descriptions of people with HBV infection should report separately the proportion of cirrhosis (using validated criteria), and the proportion of abnormal ALT and replication (with HBV DNA if possible, or HBeAg if HBV DNA is not available) in patients without cirrhosis. Second, additional efforts are needed to generate disaggregated estimates of the proportion of people eligible for HBV treatment according to the setting where they are identified (ie, population or health-care facilities), age, sex, ethnicity, and geographical areas, as to better understand who is eligible. A better understanding of who is eligible for therapy could also be used to optimise testing approaches, so that population groups with the highest yield of eligible patients could be tested as a priority. Lastly, patients with HBV-related cirrhosis (26 million people, around 10% the 257 million people with HBV infection globally) should be prioritised for treatment initiation to improve survival. Among patients with cirrhosis, according to WHO guidelines, HBV DNA is not needed before treatment initiation, which considerably simplifies management.4 This subset of patients would benefit most from treatment.180 Implementation of such simplified management algorithms by which diagnosis of HBV infection followed by diagnosis of cirrhosis would lead to immediate treatment would be an urgent, efficient, and effective way to start bridging the major HBV treatment gap.
This online publication has been corrected. The corrected version first appeared at thelancet.com/gastrohep on January 11, 2021
Acknowledgments
Acknowledgments
We are grateful to the WHO steering group that piloted this project, including Po-lin Chan, Antons Mozalevskis, Olufunmilayo Lesi, and Nicholas Walsh. We are grateful to the scientific committee who provided expertise on the issue of eligibility to HBV treatment, including Anna Lok, Yusuke Shimakawa, Brian McMahon, Francesco Negro, Saeed Sadiq Hamid, Francisco Averhoff, Seng Gee Lim, and Aaron M Harris. Tomas Allen and Jae Hee Jeong from the WHO library helped with refining the search strategy and terms. Ena Oru compiled the treatment guidelines into a summary table (appendix p 2).
Contributors
MT and QH developed the protocol under the supervision of NF, MB, YH, JVH, and PE. MT, AT, QH, YL and JVH read and abstracted the studies. ASB and FC analysed the data and prepared the tables and figures. YH drafted with paper with NF, MT, AT, YL, and ASB. JVH checked the compliance with the PRISMA reporting guidelines. All authors reviewed, edited, and approved the Article.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.WHO . World Health Organization; Geneva: 2017. Global hepatitis report.https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ [Google Scholar]
- 2.Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B: the REVEAL-HBV study. Clin Liver Dis. 2007;11:797–816. doi: 10.1016/j.cld.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Han Y, Zeng A, Liao H, Liu Y, Chen Y, Ding H. The efficacy and safety comparison between tenofovir and entecavir in treatment of chronic hepatitis B and HBV related cirrhosis: a systematic review and Meta-analysis. Int Immunopharmacol. 2017;42:168–175. doi: 10.1016/j.intimp.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 4.WHO . World Health Organization; Geneva: 2015. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection.https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ [PubMed] [Google Scholar]
- 5.Sarin SK, Kumar M, Lau GK. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Hutin Y, Nasrullah M, Easterbrook P. Access to treatment for hepatitis B virus infection—worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:773–777. doi: 10.15585/mmwr.mm6728a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platt L, French CE, McGowan CR. Prevalence and burden of HBV co-infection among people living with HIV: a global systematic review and meta-analysis. J Viral Hepat. 2020;27:294–315. doi: 10.1111/jvh.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stockdale AJ, Kreuels B, Henrion MYR. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J Hepatol. 2020;73:523–532. doi: 10.1016/j.jhep.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aberra H, Desalegn H, Berhe N. Early experiences from one of the first treatment programs for chronic hepatitis B in sub-Saharan Africa. BMC Infect Dis. 2017;17:438. doi: 10.1186/s12879-017-2549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberra H, Gordien E, Desalegn H. Hepatitis delta virus infection in a large cohort of chronic hepatitis B patients in Ethiopia. Liver Int. 2018;38:1000–1009. doi: 10.1111/liv.13607. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hanafi N, Monem F. Hepatitis B splice-generated protein antibodies in Syrian chronic hepatitis B patients: incidence and significance. Hepat Mon. 2014;14 doi: 10.5812/hepatmon.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Naamani K, Al-Maqbali A, Al-Sinani S. Characteristics of hepatitis B infection in a sample of Omani patients. Sultan Qaboos Univ Med J. 2013;13:380–385. doi: 10.12816/0003259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam S, Ahmad N, Mustafa G, Alam K, Khan M. Characteristics of treatment naive chronic hepatitis B in Bangladesh: younger populations are more affected; HBeAg-negatives are more advanced. Saudi J Gastroenterol. 2008;14:15–19. doi: 10.4103/1319-3767.37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alavian SM, Imanieh MH, Imanieh MH. Predictive factors in the incidence of cirrhosis in chronic hepatitis B virus infections. Hepat Mon. 2016;16 doi: 10.5812/hepatmon.34790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfaresi M, Elkoush A, Alshehhi H, Alzaabi A, Islam A. Hepatitis B virus genotypes and precore and core mutants in UAE patients. Virol J. 2010;7:160. doi: 10.1186/1743-422X-7-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan H, Hayat Z, Ur-Rehman S, Zarif M. Comparative analysis of risk factors and complications of hepatitis B and C infections at Khyber teaching hospital, Peshawar. Hepat Mon. 2007;7:83–86. [Google Scholar]
- 20.Antaki N, Haffar S, Ali Deeb S. High prevalence of HBV genotype D in Syria and the clinical characteristics of hepatitis B e antigen-negative chronic hepatitis B. Epidemiol Infect. 2010;138:40–44. doi: 10.1017/S0950268809990288. [DOI] [PubMed] [Google Scholar]
- 21.Ari A, Çalik Ş, Tosun S, Özsu Yilmaz S. A persistently low HBV DNA level is a predictor of spontaneous HBsAg clearance in patients with chronic hepatitis B. Turk J Med Sci. 2016;46:48–52. doi: 10.3906/sag-1411-156. [DOI] [PubMed] [Google Scholar]
- 22.Assis DR, Tenore SB, Pinho JR, Lewi DS, Ferreira PR. Characteristics of an outpatient chronic hepatitis B virus infection cohort. Einstein. 2015;13:189–195. doi: 10.1590/S1679-45082015AO3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babair YES, Elsafi S, Al-Ghamdi M, Suleiman M, El-Gezery M. Frequency and pattern of chronic hepatitis B infection in the Eastern region of Saudi Arabia: a cross-sectional study. Biosci Biotechnol Res Asia. 2012;9:2. [Google Scholar]
- 24.Bahcecioglu IH, Aygun C, Gozel N, Poyrazoglu OK, Bulut Y, Yalniz M. Prevalence of hepatitis delta virus (HDV) infection in chronic hepatitis B patients in eastern Turkey: still a serious problem to consider. J Viral Hepat. 2011;18:518–524. doi: 10.1111/j.1365-2893.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 25.Baig S. Gender disparity in infections of Hepatitis B virus. J Coll Physicians Surg Pak. 2009;19:598–600. [PubMed] [Google Scholar]
- 26.Baig S, Siddiqui AA, Ahmed W, Qureshi H, Arif A. The association of complex liver disorders with HBV genotypes prevalent in Pakistan. Virol J. 2007;4:128. doi: 10.1186/1743-422X-4-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakhshipour A, Mashhadi M, Mohammadi M, Nezam SK. Seroprevalence and risk factors of hepatitis delta virus in chronic hepatitis B virus infection in Zahedan. Acta Med Iran. 2013;51:260–264. [PubMed] [Google Scholar]
- 28.Balasubramanian S VA, Krishnan A. Spectrum of hepatitis B infection in Southern India: a cross-sectional analysis. Hep B Annual. 2012;9:4–15. [Google Scholar]
- 29.Batoctoy KS, Tseng TC, Kao JH, Quiza FE, Garcia LH, Sr, Lao-Tan J. HBV/A and HBV/C genotype predominance among patients with chronic hepatitis B virus infection in Cebu City, Philippines. Hepatol Int. 2011;5:774–781. doi: 10.1007/s12072-011-9263-1. [DOI] [PubMed] [Google Scholar]
- 30.Becker CE, Mattos AA, Bogo MR, Branco F, Sitnik R, Kretzmann NA. Genotyping of hepatitis B virus in a cohort of patients evaluated in a hospital of Porto Alegre, South of Brazil. Arq Gastroenterol. 2010;47:13–17. doi: 10.1590/s0004-28032010000100003. [DOI] [PubMed] [Google Scholar]
- 31.Bensalem A, Selmani K, Narjes H. Widespread geographical disparities in chronic hepatitis B virus infection in Algeria. Arch Virol. 2017;162:1641–1648. doi: 10.1007/s00705-017-3284-6. [DOI] [PubMed] [Google Scholar]
- 32.Bert F, Rindermann A, Abdelfattah MA, Stahmeyer JT, Rossol S. High prevalence of chronic hepatitis B and C virus infection in a population of a German metropolitan area: a prospective survey including 10 215 patients of an interdisciplinary emergency unit. Eur J Gastroenterol Hepatol. 2016;28:1246–1252. doi: 10.1097/MEG.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya S, O'Donnell K, Dudley T. Ante-natal screening and post-natal follow-up of hepatitis B in the West Midlands of England. QJM. 2008;101:307–312. doi: 10.1093/qjmed/hcn007. [DOI] [PubMed] [Google Scholar]
- 34.Brichler S, Lagathu G, Chekaraou MA. African, Amerindian and European hepatitis B virus strains circulate on the Caribbean Island of Martinique. J Gen Virol. 2013;94:2318–2329. doi: 10.1099/vir.0.055459-0. [DOI] [PubMed] [Google Scholar]
- 35.Brinck-Jensen NS, Erichsen P, Tarp B. Clinical findings in a multi-ethnic adult hepatitis B virus patient population in Denmark with emphasis on genotypic characteristics. Scand J Gastroenterol. 2015;50:1032–1038. doi: 10.3109/00365521.2014.974202. [DOI] [PubMed] [Google Scholar]
- 36.Cadranel JF, Lahmek P, Causse X. Epidemiology of chronic hepatitis B infection in France: risk factors for significant fibrosis--results of a nationwide survey. Aliment Pharmacol Ther. 2007;26:565–576. doi: 10.1111/j.1365-2036.2007.03400.x. [DOI] [PubMed] [Google Scholar]
- 37.Cao ZLGY, Ji Z. Relationship between distribution of HBV genotypes and liver diseases in Lhasa. Shijie Huaren Xiaohua Zazhi. 2009;17:49–52. [Google Scholar]
- 38.Chachá SG, Ferreira SC, Costa TV. Clinical, demographic and epidemiological characteristics of patients with hepatitis B followed at a university hospital in southeastern Brazil: predominance of HBeAg negative cases. Rev Soc Bras Med Trop. 2011;44:13–17. doi: 10.1590/s0037-86822011000100004. [DOI] [PubMed] [Google Scholar]
- 39.Chachá SGF, Gomes-Gouvêa MS, Malta FM. Basal core promoter and precore mutations among hepatitis B virus circulating in Brazil and its association with severe forms of hepatic diseases. Mem Inst Oswaldo Cruz. 2017;112:626–631. doi: 10.1590/0074-02760160540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan HL, Wong GL, Tse CH. Hepatitis B virus genotype C is associated with more severe liver fibrosis than genotype B. Clin Gastroenterol Hepatol. 2009;7:1361–1366. doi: 10.1016/j.cgh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Chang MS, Tuomala R, Rutherford AE. Postpartum care for mothers diagnosed with hepatitis B during pregnancy. Am J Obstet Gynecol. 2015;212:365. doi: 10.1016/j.ajog.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaouch H, Taffon S, Villano U. Naturally occurring surface antigen variants of hepatitis B virus in Tunisian patients. Intervirology. 2016;59:36–47. doi: 10.1159/000445894. [DOI] [PubMed] [Google Scholar]
- 43.Chen P, Xie Q, Chen T. Hepatitis B virus infection in hilly/mountainous regions of southeastern China: a locality-dependent epidemiology. BMC Infect Dis. 2017;17:809. doi: 10.1186/s12879-017-2922-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen QY, Fang ZL, Yang Y. Survey on hepatitis B virus infection and liver function in Guangxi. Guangxi Med J. 2013;11:1432–1434. [Google Scholar]
- 45.Chen P, Yu C, Wu W. Serolological profile among HBsAg-positive infections in southeast China: a community-based study. Hepat Mon. 2013;13 doi: 10.5812/hepatmon.7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen P, Yu C, Ruan B. Prevalence of hepatitis B in insular regions of southeast China: a community-based study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciftci S, Keskin F, Badur S. Clinical features of hepatitis B virus genotypes in Turkish patients. J Pak Med Assoc. 2012;62:759–763. [PubMed] [Google Scholar]
- 48.Coenen S, van Meer S, Vrolijk JM. Clinical impact of five large-scale screening projects for chronic hepatitis B in Chinese migrants in the Netherlands. Liver Int. 2016;36:1425–1432. doi: 10.1111/liv.13125. [DOI] [PubMed] [Google Scholar]
- 49.Conde SR, Rocha LL, Ferreira VM. Absence of correlation between IL-28B gene polymorphisms and the clinical presentation of chronic hepatitis B in an Amazon Brazilian population. Dis Markers. 2014;2014 doi: 10.1155/2014/534534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Contini C, Badia L, Cultrera R, Grilli A, De Togni A. Epidemiological, clinical and laboratory features of chronic hepatitis B infection in a cohort of immigrant and Italian patients from Ferrara, Italy. Ann Hepatol. 2012;11:862–869. [PubMed] [Google Scholar]
- 51.Coppola N, Alessio L, Gualdieri L. Hepatitis B virus infection in undocumented immigrants and refugees in Southern Italy: demographic, virological, and clinical features. Infect Dis Poverty. 2017;6:33. doi: 10.1186/s40249-016-0228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cotler SJ, Dhamija MK, Siqueira F. Hepatitis B seroprevalence and disease characteristics in an urban Chinatown community. Clin Gastroenterol Hepatol. 2009;7:776–780. doi: 10.1016/j.cgh.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Croagh CM, Bell SJ, Locarnini S, Desmond PV. Assessment of chronic hepatitis B: the importance of hepatitis B virus DNA testing. Intern Med J. 2012;42:170–175. doi: 10.1111/j.1445-5994.2011.02435.x. [DOI] [PubMed] [Google Scholar]
- 54.Cuenca-Gómez JA, Salas-Coronas J, Lozano-Serrano AB. Hepatitis B and schistosoma co-infection in a non-endemic area. Eur J Clin Microbiol Infect Dis. 2016;35:1487–1493. doi: 10.1007/s10096-016-2689-6. [DOI] [PubMed] [Google Scholar]
- 55.Cuomo G, Borghi V, Andreone P. Missed treatment in an Italian HBV infected patients cohort: HBV RER. Dig Liver Dis. 2016;48:1346–1350. doi: 10.1016/j.dld.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 56.Da Silva Conde SRS, Pinheiro LM, De Lemos JAR. Prevalence of genotypes and sub-genotypes of the hepatitis B virus in a population of the Brazilian Amazon region (Para State) J Antivir Antiretrovir. 2013;5:108–112. [Google Scholar]
- 57.Datta S, Biswas A, Chandra PK. Molecular epidemiology and clinical significance of hepatitis B virus genotypes, core promoter and precore mutations in eastern India. Intervirology. 2008;51:275–284. doi: 10.1159/000170902. [DOI] [PubMed] [Google Scholar]
- 58.Davies J, Littlejohn M, Locarnini SA. Molecular epidemiology of hepatitis B in the Indigenous people of northern Australia. J Gastroenterol Hepatol. 2013;28:1234–1241. doi: 10.1111/jgh.12177. [DOI] [PubMed] [Google Scholar]
- 59.De Vroey B, Moreno C, Laleman W. Hepatitis B virus and hepatitis C virus infections in Belgium: similarities and differences in epidemics and initial management. Eur J Gastroenterol Hepatol. 2013;25:613–619. doi: 10.1097/MEG.0b013e32835d83a2. [DOI] [PubMed] [Google Scholar]
- 60.Deltenre P, Laleman W, Van Gossum M. HBV infection in Belgium: results of the BASL observatory of 1,456 HBsAg carriers. Acta Gastroenterol Belg. 2012;75:35–41. [PubMed] [Google Scholar]
- 61.Demir M, Nigemeier J, Kütting F. Clinical management of chronic hepatitis B infection: results from a registry at a German tertiary referral center. Infection. 2015;43:153–162. doi: 10.1007/s15010-015-0751-4. [DOI] [PubMed] [Google Scholar]
- 62.Dervisevic S, Ijaz S, Chaudry S, Tedder RS. Non-A hepatitis B virus genotypes in antenatal clinics, United Kingdom. Emerg Infect Dis. 2007;13:1689–1693. doi: 10.3201/eid1311.070578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding Y, Sheng Q, Ma L, Dou X. Chronic HBV infection among pregnant women and their infants in Shenyang, China. Virol J. 2013;10:17. doi: 10.1186/1743-422X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ducancelle A, Abgueguen P, Birguel J. High endemicity and low molecular diversity of hepatitis B virus infections in pregnant women in a rural district of North Cameroon. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dunford L, Carr MJ, Dean J. A multicentre molecular analysis of hepatitis B and blood-borne virus coinfections in Viet Nam. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duong TH, Nguyen PH, Henley K, Peters M. Risk factors for hepatitis B infection in rural Vietnam. Asian Pac J Cancer Prev. 2009;10:97–102. [PMC free article] [PubMed] [Google Scholar]
- 67.Furukawa N, Maeyama K. Clinical course of hepatitis B surface antigen positive subjects following screening: A retrospective observational study from April 2008 to January 2013. Hepatol Res. 2016;46:678–685. doi: 10.1111/hepr.12608. [DOI] [PubMed] [Google Scholar]
- 68.Elefsiniotis IS, Glynou I, Brokalaki H. Serological and virological profile of chronic HBV infected women at reproductive age in Greece. A two-year single center study. Eur J Obstet Gynecol Reprod Biol. 2007;132:200–203. doi: 10.1016/j.ejogrb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 69.Elzouki AN, Bashir SM, Elahmer O, Elzouki I, Alkhattali F. Prevalence and risk factors of hepatitis D virus infection in patients with chronic hepatitis B infection attending the three main tertiary hospitals in Libya. Arab J Gastroenterol. 2017;18:216–219. doi: 10.1016/j.ajg.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Ergunay K, Balaban Y, Cosgun E. Epidemiologic trends in HBV infections at a reference centre in Turkey: an 11-year retrospective analysis. Ann Hepatol. 2012;11:672–678. [PubMed] [Google Scholar]
- 71.Fallatah HI, Akbar HO. Chronic hepatitis B infection in a hepatology clinic at a university hospital in Jeddah. J Infect Dev Ctries. 2010;4:621–628. doi: 10.3855/jidc.751. [DOI] [PubMed] [Google Scholar]
- 72.Fasano M, Saracino A, Carosi G. Hepatitis B and immigrants: a SIMIT multicenter cross-sectional study. Infection. 2013;41:53–59. doi: 10.1007/s15010-012-0384-9. [DOI] [PubMed] [Google Scholar]
- 73.Fouad R, Abdo M, Eldeen HG. Influence of delta virus infection on the virologic status in Egyptian patients with chronic hepatitis B virus genotype D. J Med Virol. 2016;88:837–842. doi: 10.1002/jmv.24412. [DOI] [PubMed] [Google Scholar]
- 74.Fujiko M, Chalid MT, Turyadi Chronic hepatitis B in pregnant women: is hepatitis B surface antigen quantification useful for viral load prediction? Int J Infect Dis. 2015;41:83–89. doi: 10.1016/j.ijid.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 75.Fung J, Lai CL, But D, Wong D, Cheung TK, Yuen MF. Prevalence of fibrosis and cirrhosis in chronic hepatitis B: implications for treatment and management. Am J Gastroenterol. 2008;103:1421–1426. doi: 10.1111/j.1572-0241.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- 76.Fung J, Seto WK, Lai CL, Yuen J, Wong DK, Yuen MF. Profiles of HBV DNA in a large population of Chinese patients with chronic hepatitis B: implications for antiviral therapy. J Hepatol. 2011;54:195–200. doi: 10.1016/j.jhep.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 77.Galizzi F J, Teixeira R, Fonseca JC, Souto FJ. Clinical profile of hepatitis B virus chronic infection in patients of Brazilian liver reference units. Hepatol Int. 2010;4:511–515. doi: 10.1007/s12072-010-9178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghany MG, Perrillo R, Li R. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol. 2015;13:183–192. doi: 10.1016/j.cgh.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giannousis IP, Papatheodoridis GV, Deutsch MJ. The burden and recent epidemiological changes of the main chronic liver diseases in a Greek referral tertiary centre. Eur J Gastroenterol Hepatol. 2010;22:172–179. doi: 10.1097/MEG.0b013e328331115b. [DOI] [PubMed] [Google Scholar]
- 80.Gish RG, Yi DH, Kane S. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol. 2013;28:1521–1525. doi: 10.1111/jgh.12217. [DOI] [PubMed] [Google Scholar]
- 81.Gómez Rodríguez R, Guardiola Arévalo A, Gómez Moreno AZ. Characteristics of patients with chronic hepatitis B virus infection. analysis of a series of 474 patients. Gastroenterol Hepatol. 2013;36:243–253. doi: 10.1016/j.gastrohep.2012.10.006. (in Spanish). [DOI] [PubMed] [Google Scholar]
- 82.Grabarczyk P, Garmiri P, Liszewski G. Molecular and serological characterization of hepatitis B virus genotype A and D infected blood donors in Poland. J Viral Hepat. 2010;17:444–452. doi: 10.1111/j.1365-2893.2009.01192.x. [DOI] [PubMed] [Google Scholar]
- 83.Guo Z, Shi XH, Feng YL. Risk factors of HBV intrauterine transmission among HBsAg-positive pregnant women. J Viral Hepat. 2013;20:317–321. doi: 10.1111/jvh.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta E, Kumar A, Choudhary A, Kumar M, Sarin SK. Serum hepatitis B surface antigen levels correlate with high serum HBV DNA levels in patients with chronic hepatitis B: a cross-sectional study. Indian J Med Microbiol. 2012;30:150–154. doi: 10.4103/0255-0857.96664. [DOI] [PubMed] [Google Scholar]
- 85.Habil FE, Mahdi WK, Abdelwahab SF, Abdel-Hamid M. Hepatitis B virus genotype D predominates HBsAg-positive egyptian blood donors and is mainly associated with a negative HBeAg serostatus. Intervirology. 2013;56:278–283. doi: 10.1159/000353105. [DOI] [PubMed] [Google Scholar]
- 86.Hann HW, Hann RS, Maddrey WC. Hepatitis B virus infection in 6,130 unvaccinated Korean-Americans surveyed between 1988 and 1990. Am J Gastroenterol. 2007;102:767–772. doi: 10.1111/j.1572-0241.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 87.Harkisoen S, Arends JE, van den Hoek JA. Historic and current hepatitis B viral DNA and quantitative HBsAg level are not associated with cirrhosis in non-Asian women with chronic hepatitis B. Int J Infect Dis. 2014;29:133–138. doi: 10.1016/j.ijid.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Hassan MA, Kim WR, Li R. Characteristics of US-born versus foreign-born Americans of African descent with chronic hepatitis B. Am J Epidemiol. 2017;186:356–366. doi: 10.1093/aje/kwx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho E, Deltenre P, Nkuize M, Delwaide J, Colle I, Michielsen P. Coinfection of hepatitis B and hepatitis delta virus in Belgium: a multicenter BASL study. Prospective epidemiology and comparison with HBV mono-infection. J Med Virol. 2013;85:1513–1517. doi: 10.1002/jmv.23653. [DOI] [PubMed] [Google Scholar]
- 90.Ieluzzi D, Covolo L, Donato F, Fattovich G. Progression to cirrhosis, hepatocellular carcinoma and liver-related mortality in chronic hepatitis B patients in Italy. Dig Liver Dis. 2014;46:427–432. doi: 10.1016/j.dld.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 91.Iloeje UH, Yang HI, Jen CL. Risk and predictors of mortality associated with chronic hepatitis B infection. Clin Gastroenterol Hepatol. 2007;5:921–931. doi: 10.1016/j.cgh.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Jaquet A, Nouaman M, Tine J. Hepatitis B treatment eligibility in West Africa: uncertainties and need for prospective cohort studies. Liver Int. 2017;37:1116–1121. doi: 10.1111/liv.13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaquet A, Wandeler G, Tine J. HIV infection, viral hepatitis and liver fibrosis among prison inmates in West Africa. BMC Infect Dis. 2016;16:249. doi: 10.1186/s12879-016-1601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaklikkaya N, Sancaktar M, Guner R. Hepatitis B virus genotypes and subgenotypes in the Eastern Black Sea region of Turkey. Saudi Med J. 2012;33:622–626. [PubMed] [Google Scholar]
- 95.Kant J, Kratzsch J, Maier M, Liebert UG, Berg T, Wiegand J. HBsAg and anti-HCV screening in elderly hospitalized patients of a German tertiary referral centre. Z Gastroenterol. 2016;54:231–237. doi: 10.1055/s-0041-106656. [DOI] [PubMed] [Google Scholar]
- 96.Khelifa F, Thibault V. Characteristics of hepatitis B viral strains in chronic carrier patients from North-East Algeria. Pathol Biol. 2009;57:107–113. doi: 10.1016/j.patbio.2008.07.031. (in French). [DOI] [PubMed] [Google Scholar]
- 97.Kobayashi M, Hosaka T, Suzuki F. Seroclearance rate of hepatitis B surface antigen in 2,112 patients with chronic hepatitis in Japan during long-term follow-up. J Gastroenterol. 2014;49:538–546. doi: 10.1007/s00535-013-0821-2. [DOI] [PubMed] [Google Scholar]
- 98.Kobayashi M, Ikeda K, Arase Y. Change of hepatitis B virus genotypes in acute and chronic infections in Japan. J Med Virol. 2008;80:1880–1884. doi: 10.1002/jmv.21309. [DOI] [PubMed] [Google Scholar]
- 99.Kuo YH, Chang KC, Wang JH. Changing serum levels of quantitative hepatitis B surface antigen and hepatitis B virus DNA in hepatitis B virus surface antigen carriers: a follow-up study of an elderly cohort. Kaohsiung J Med Sci. 2015;31:102–107. doi: 10.1016/j.kjms.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kusakabe A, Tanaka Y, Inoue M. A population-based cohort study for the risk factors of HCC among hepatitis B virus mono-infected subjects in Japan. J Gastroenterol. 2011;46:117–124. doi: 10.1007/s00535-010-0307-4. [DOI] [PubMed] [Google Scholar]
- 101.Lee P-L, Chen J-J, Tung HD. Serum hepatitis B surface antigen level might predict cirrhosis and hepatocellular carcinoma in older patients with chronic hepatitis B. Adv Dig Med. 2015;2:102–107. [Google Scholar]
- 102.Lemoine M, Shimakawa Y, Njie R. Acceptability and feasibility of a screen-and-treat programme for hepatitis B virus infection in The Gambia: the Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) study. Lancet Glob Health. 2016;4:e559–e567. doi: 10.1016/S2214-109X(16)30130-9. [DOI] [PubMed] [Google Scholar]
- 103.Liang F, Sha CX, Fan CS. Qidong Chronic Hepatitis B Cohort: participants enrollment and comparison of baseline characteristics by gender stratification. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38:1569–1573. doi: 10.3760/cma.j.issn.0254-6450.2017.11.026. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 104.Lim TH, Gane E, Moyes C, Borman B, Cunningham C. Serological and clinical outcomes of horizontally transmitted chronic hepatitis B infection in New Zealand Māori: results from a 28-year follow-up study. Gut. 2015;64:966–972. doi: 10.1136/gutjnl-2013-306247. [DOI] [PubMed] [Google Scholar]
- 105.Luca AS, Dorobăţ C, Ursu RG, Luca MC, Vâţă A, Iancu LS. Epidemiological and laboratory features of chronic hepatitis B cases in the interval 2010–2013. Rev Med Chir Soc Med Nat Iasi. 2014;118:479–484. [PubMed] [Google Scholar]
- 106.Lüllau A, Petroff D, Bätz O. Linkage to care of HbsAg-positive and anti-HCV-positive patients after a systematic screening approach in the German primary care setting. Eur J Gastroenterol Hepatol. 2018;30:280–283. doi: 10.1097/MEG.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 107.Luma HN, Eloumou SAFB, Okalla C. Prevalence and characteristics of hepatitis delta virus infection in a tertiary hospital setting in Cameroon. J Clin Exp Hepatol. 2017;7:334–339. doi: 10.1016/j.jceh.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lunel-Fabiani F, Mansour W, Amar AO. Impact of hepatitis B and delta virus co-infection on liver disease in Mauritania: a cross sectional study. J Infect. 2013;67:448–457. doi: 10.1016/j.jinf.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 109.Luo Z, Xie Y, Deng M, Zhou X, Ruan B. Prevalence of hepatitis B in the southeast of China: a population-based study with a large sample size. Eur J Gastroenterol Hepatol. 2011;23:695–700. doi: 10.1097/MEG.0b013e328347322b. [DOI] [PubMed] [Google Scholar]
- 110.Ma LN, Liu XY, Luo X. Serum high-sensitivity C-reactive protein are associated with HBV replication, liver damage and fibrosis in patients with chronic hepatitis B. Hepatogastroenterology. 2015;62:368–372. [PubMed] [Google Scholar]
- 111.Mahmood M, Anwar MA, Khanum A, Zaman N, Raza A. Distribution and clinical significance of hepatitis B virus genotypes in Pakistan. BMC Gastroenterol. 2016;16:104. doi: 10.1186/s12876-016-0513-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manesis EK, Vourli G, Dalekos G. Prevalence and clinical course of hepatitis delta infection in Greece: a 13-year prospective study. J Hepatol. 2013;59:949–956. doi: 10.1016/j.jhep.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 113.Marlen Ivón CF, Zaily DG, Conde-Eduardo Leda Patricia DS, Enrique GG, Enrique AS, Yadina MP. Current condition of chronic hepatitis B virus infection in Cuban adults. Curr Ther Res Clin Exp. 2017;85:15–19. doi: 10.1016/j.curtheres.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masaadeh HA, Hayajneh WA, Alqudah EA. Hepatitis B virus genotypes and lamivudine resistance mutations in Jordan. World J Gastroenterol. 2008;14:7231–7234. doi: 10.3748/wjg.14.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McLernon DJ, Donnan PT, Dillon JF. Prevalence estimates of diagnosed viral hepatitis B, liver condition outcomes and hospitalization costs: a population record-linkage study in Tayside, Scotland. Epidemiol Infect. 2013;141:2122–2130. doi: 10.1017/S095026881200266X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mellen JS, Xia VW, Hashemzadeh M. The clinical presentation of chronic hepatitis B virus infection in Asian Americans: a single center retrospective study. J Clin Gastroenterol. 2010;44:364–370. doi: 10.1097/MCG.0b013e3181b5c7a8. [DOI] [PubMed] [Google Scholar]
- 117.Mihăilă R, Rezi EC, Nedelcu L. The prevalence and the clinical and biological characterstics of the patients with chronic liver diseases in Transylvania—multicentric epidemiological study. Arch Balk Medical Union. 2010;45:111–115. [Google Scholar]
- 118.Milosevic I, Delic D, Lazarevic I. The significance of hepatitis B virus (HBV) genotypes for the disease and treatment outcome among patients with chronic hepatitis B in Serbia. J Clin Virol. 2013;58:54–58. doi: 10.1016/j.jcv.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 119.Minuk GY, Macrury S, Uhanova J. A paucity of liver disease in Canadian Inuit with chronic hepatitis B virus, subgenotype B6 infection. J Viral Hepat. 2013;20:890–896. doi: 10.1111/jvh.12121. [DOI] [PubMed] [Google Scholar]
- 120.Mojiri A, Behzad-Behbahani A, Saberifirozi M. Hepatitis B virus genotypes in southwest Iran: molecular, serological and clinical outcomes. World J Gastroenterol. 2008;14:1510–1513. doi: 10.3748/wjg.14.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nabuco LC, Mello FC, Gomes SA. Hepatitis B virus genotypes in Southeast Brazil and its relationship with histological features. Mem Inst Oswaldo Cruz. 2012;107:785–789. [PubMed] [Google Scholar]
- 122.Nguyen VT, McLaws ML, Dore GJ. Highly endemic hepatitis B infection in rural Vietnam. J Gastroenterol Hepatol. 2007;22:2093–2100. doi: 10.1111/j.1440-1746.2007.05010.x. [DOI] [PubMed] [Google Scholar]
- 123.Niederau C, Amani A, Thiel A. Long-term follow-up of HBsAg-positive patients in Germany. Eur J Gastroenterol Hepatol. 2016;28:48–56. doi: 10.1097/MEG.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 124.Nien HC, Sheu JC, Kao JH, Chou HC, Su CW, Chen CH. Aboriginal Taiwanese hepatitis B carriers have more favorable viral factors than Han Chinese carriers. J Med Virol. 2011;83:1326–1331. doi: 10.1002/jmv.22135. [DOI] [PubMed] [Google Scholar]
- 125.Nita ME, Gaburo N, Jr, Cheinquer H. Patterns of viral load in chronic hepatitis B patients in Brazil and their association with ALT levels and HBeAg status. Ann Hepatol. 2009;8:339–345. [PubMed] [Google Scholar]
- 126.Ntagirabiri R, Munezero B, Nahimana C, Ndabaneze E. Hepatitis B virus genotypes and evolutionary markers in chronic HBsAG patients in Bujumbura. Pan Afr Med J. 2016;23:95. doi: 10.11604/pamj.2016.23.95.8424. (in French). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Odaibo GN, Ola SO, Olaleye OD. Hepatitis B virus DNA in patients with HBsAg in south western Nigeria. J Med Virol. 2013;85:214–218. doi: 10.1002/jmv.23418. [DOI] [PubMed] [Google Scholar]
- 128.Ordieres C, Navascués CA, González-Diéguez ML. Prevalence and epidemiology of hepatitis D among patients with chronic hepatitis B virus infection: a report from Northern Spain. Eur J Gastroenterol Hepatol. 2017;29:277–283. doi: 10.1097/MEG.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 129.Pérez-Molina JA, Herrero-Martínez JM, Norman F. Clinical, epidemiological characteristics and indications for liver biopsy and treatment in immigrants with chronic hepatitis B at a referral hospital in Madrid. J Viral Hepat. 2011;18:294–299. doi: 10.1111/j.1365-2893.2010.01306.x. [DOI] [PubMed] [Google Scholar]
- 130.Poh Z, Goh BB, Chang PE, Tan CK. Rates of cirrhosis and hepatocellular carcinoma in chronic hepatitis B and the role of surveillance: a 10-year follow-up of 673 patients. Eur J Gastroenterol Hepatol. 2015;27:638–643. doi: 10.1097/MEG.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pollack HJ, Kwon SC, Wang SH, Wyatt LC, Trinh-Shevrin C, Coalition A. Chronic hepatitis B and liver cancer risks among Asian immigrants in New York City: results from a large, community-based screening, evaluation, and treatment program. Cancer Epidemiol Biomarkers Prev. 2014;23:2229–2239. doi: 10.1158/1055-9965.EPI-14-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rabbi FJ, Rezwan MK, Shirin T. HBeAg/anti-HBe, alanine aminotransferase and HBV DNA levels in HBsAg positive chronic carriers. Bangladesh Med Res Counc Bull. 2008;34:39–43. doi: 10.3329/bmrcb.v34i2.1173. [DOI] [PubMed] [Google Scholar]
- 133.Raptopoulou M, Papatheodoridis G, Antoniou A. Epidemiology, course and disease burden of chronic hepatitis B virus infection. HEPNET study for chronic hepatitis B: a multicentre Greek study. J Viral Hepat. 2009;16:195–202. doi: 10.1111/j.1365-2893.2008.01057.x. [DOI] [PubMed] [Google Scholar]
- 134.Richter C, Ter Beest G, Gisolf EH. Screening for chronic hepatitis B and C in migrants from Afghanistan, Iran, Iraq, the former Soviet Republics, and Vietnam in the Arnhem region, The Netherlands. Epidemiol Infect. 2014;142:2140–2146. doi: 10.1017/S0950268813003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rufai T, Mutocheluh M, Kwarteng K, Dogbe E. The prevalence of hepatitis B virus E antigen among Ghanaian blood donors. Pan Afr Med J. 2014;17:53. doi: 10.11604/pamj.2014.17.53.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sagnelli EAL, Sagnelli C, Hepatitis B. Virus genotypes, epidemiological characteristics, and clinical presentation of HBV chronic infection in immigrant populations living in southern Italy. Hepat Mon. 2017;17 [Google Scholar]
- 137.Sagnelli E, Stroffolini T, Mele A, Imparato M, Almasio PL. Chronic hepatitis B in Italy: new features of an old disease--approaching the universal prevalence of hepatitis B e antigen-negative cases and the eradication of hepatitis D infection. Clin Infect Dis. 2008;46:110–113. doi: 10.1086/524074. [DOI] [PubMed] [Google Scholar]
- 138.Sagnelli E, Taliani G, Castelli F. Chronic HBV infection in pregnant immigrants: a multicenter study of the Italian Society of Infectious and Tropical Diseases. New Microbiol. 2016;39:114–118. [PubMed] [Google Scholar]
- 139.Sarkar M, Shvachko VA, Ready JB. Characteristics and management of patients with chronic hepatitis B in an integrated care setting. Dig Dis Sci. 2014;59:2100–2108. doi: 10.1007/s10620-014-3142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scotto G, Martinelli D, Di Tullio R, Fazio V. Epidemiological and clinical features of hepatitis B Virus genotypes among immigrants in southern Italy. Hepat Res Treat. 2010;2010 doi: 10.1155/2010/878356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spradling PR, Bulkow L, Teshale EH. Prevalence and causes of elevated serum aminotransferase levels in a population-based cohort of persons with chronic hepatitis B virus infection. J Hepatol. 2014;61:785–791. doi: 10.1016/j.jhep.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 142.Spradling PR, Simons B, Narayanan M. Incidence of diabetes mellitus in a population-based cohort of persons with chronic hepatitis B virus infection. J Viral Hepat. 2013;20:510–513. doi: 10.1111/jvh.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stefos A, Gatselis N, Zachou K, Rigopoulou E, Hadjichristodoulou C, Dalekos GN. Descriptive epidemiology of chronic hepatitis B by using data from a hepatitis registry in Central Greece. Eur J Intern Med. 2009;20:35–43. doi: 10.1016/j.ejim.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 144.Stevens K, Palmo T, Wangchuk T, Solomon S, Dierberg K, Hoffmann CJ. Hepatitis B prevalence and treatment needs among Tibetan refugees residing in India. J Med Virol. 2016;88:1357–1363. doi: 10.1002/jmv.24488. [DOI] [PubMed] [Google Scholar]
- 145.Stroffolini T, Almasio PL, Sagnelli E, Mele A, Gaeta GB. Evolving clinical landscape of chronic hepatitis B: A multicenter Italian study. J Med Virol. 2009;81:1999–2006. doi: 10.1002/jmv.21643. [DOI] [PubMed] [Google Scholar]
- 146.Stroffolini T, Spadaro A, Di Marco V. Current practice of chronic hepatitis B treatment in Southern Italy. Eur J Intern Med. 2012;23:e124–e127. doi: 10.1016/j.ejim.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 147.Subramaniam K, Flexman J, Tarquinio L, Thambiran A, Hopkins S, Cheng W. Hepatitis B status in migrants and refugees: increasing health burden in Western Australia. Intern Med J. 2012;42:880–886. doi: 10.1111/j.1445-5994.2011.02711.x. [DOI] [PubMed] [Google Scholar]
- 148.Sun YT, Zhang YX, Tang H. Clinical characteristics and current management of hepatitis B and C in China. World J Gastroenterol. 2014;20:13582–13590. doi: 10.3748/wjg.v20.i37.13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Suppiah J, Mohd Zain R, Bahari N, Haji Nawi S, Saat Z. G1896A Precore mutation and association with HBeAg status, genotype and clinical status in patients with chronic hepatitis B. Hepat Mon. 2015;15 doi: 10.5812/hepatmon.31490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Świderska M, Pawłowska M, Mazur W. Distribution of HBV genotypes in Poland. Clin Exp Hepatol. 2015;1:1–4. doi: 10.5114/ceh.2015.51372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tang E, Torres S, Liu B, Baden R, Bhuket T, Wong RJ. High prevalence of cirrhosis at initial presentation among safety-net adults with chronic Hepatitis B virus infection. J Clin Exp Hepatol. 2018;8:235–240. doi: 10.1016/j.jceh.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tedder RS, Rodger AJ, Fries L. The diversity and management of chronic hepatitis B virus infections in the United Kingdom: a wake-up call. Clin Infect Dis. 2013;56:951–960. doi: 10.1093/cid/cis1013. [DOI] [PubMed] [Google Scholar]
- 153.Thurnheer MC, Schulz TR, Nguyen T, MacLachlan J, Sasadeusz J. Regional challenges: evaluation of a hepatitis outreach programme using transient elastography (FibroScan) in Victoria. Intern Med J. 2016;46:273–281. doi: 10.1111/imj.12963. [DOI] [PubMed] [Google Scholar]
- 154.Torre F, Basso M, Giannini EG. Clinical and virological survey of patients with hepatitis B surface antigen in an Italian region: clinical considerations and disease burden. J Med Virol. 2009;81:1882–1886. doi: 10.1002/jmv.21523. [DOI] [PubMed] [Google Scholar]
- 155.Traoré F, Gormally E, Villar S. Molecular characteristics of Hepatitis B and chronic liver disease in a cohort of HB carriers from Bamako, Mali. BMC Infect Dis. 2015;15:180. doi: 10.1186/s12879-015-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tufon KA, Meriki HD, Anong DN, Mbunkah HA, Nkuo-Akenji T. Genetic diversity, viraemic and aminotransferases levels in chronic infected hepatitis B patients from Cameroon. BMC Res Notes. 2016;9:117. doi: 10.1186/s13104-016-1916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Veldhuijzen IK, Wolter R, Rijckborst V. Identification and treatment of chronic hepatitis B in Chinese migrants: results of a project offering on-site testing in Rotterdam, The Netherlands. J Hepatol. 2012;57:1171–1176. doi: 10.1016/j.jhep.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 158.Vella RSD, Farrugia A. Hepatitis B infection in Malta: a retrospective cross sectional study Roberta Vella, Danica Spiteri. Malta Med J. 2012;24:13–16. [Google Scholar]
- 159.Wang MLS, Liu Z. The relationship between the HBV DNA quantification and HBV marker, age and gender among chronic patients with hepatitis B virus. Prev Med Trib. 2016;22:7–9. [Google Scholar]
- 160.Widjaja D, Yarlagadda S, Singu BS. Characteristics of patients with chronic hepatitis-B virus infection in an urban hospital. J Natl Med Assoc. 2007;99:384–388. [PMC free article] [PubMed] [Google Scholar]
- 161.Wolffram I, Petroff D, Bätz O. Prevalence of elevated ALT values, HBsAg, and anti-HCV in the primary care setting and evaluation of guideline defined hepatitis risk scenarios. J Hepatol. 2015;62:1256–1264. doi: 10.1016/j.jhep.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 162.Wong GL, Chan HL, Chan HY. Adverse effects of vitamin D deficiency on outcomes of patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2015;13:783–790. doi: 10.1016/j.cgh.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 163.Wu DL, Xu GH, Lu SM. Age versus clinical virological characteristics in chronic hepatitis B virus infection: a case series study in China. Eur J Gastroenterol Hepatol. 2012;24:406–413. doi: 10.1097/MEG.0b013e32834fbf35. [DOI] [PubMed] [Google Scholar]
- 164.Wu W, Zhu Y, Yu C. Clinical features of treatment-naive patients with hepatitis B virus infection: a community-based survey from high- and intermediate-hepatitis B endemicity regions in Southeast China. Medicine. 2017;96 doi: 10.1097/MD.0000000000006660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu Y, Johnson KB, Roccaro G. Poor adherence to AASLD guidelines for chronic hepatitis B Management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109:867–875. doi: 10.1038/ajg.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xie Z-YFY-F, Pu R. Incidence of mother to child transmission of hepatitis B virus and its influencing factors in Pudong New Area of Shanghai. Acad J Second Mil Med Univ. 2014;35:631–636. [Google Scholar]
- 167.Xu JJ, Tien C, Chang M. Demographic and serological characteristics of Asian Americans with hepatitis B infection diagnosed at community screenings. J Viral Hepat. 2013;20:575–581. doi: 10.1111/jvh.12073. [DOI] [PubMed] [Google Scholar]
- 168.Yu MWSW, Shih WL, Lin CL. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol. 2008;26:5576–5582. doi: 10.1200/JCO.2008.16.1075. [DOI] [PubMed] [Google Scholar]
- 169.Yuen MFTY, Tanaka Y, Fong DY. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50:80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 170.Zhang H, Li Q, Sun J. Seroprevalence and risk factors for hepatitis B infection in an adult population in Northeast China. Int J Med Sci. 2011;8:321–331. doi: 10.7150/ijms.8.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang Q, Qi W, Wang X. Epidemiology of hepatitis B and hepatitis C infections and benefits of programs for hepatitis prevention in northeastern China: a cross-sectional study. Clin Infect Dis. 2016;62:305–312. doi: 10.1093/cid/civ859. [DOI] [PubMed] [Google Scholar]
- 172.Zhang S, Ristau JT, Trinh HN, Garcia RT, Nguyen HA, Nguyen MH. Undertreatment of Asian chronic hepatitis B patients on the basis of standard guidelines: a community-based study. Dig Dis Sci. 2012;57:1373–1383. doi: 10.1007/s10620-012-2137-0. [DOI] [PubMed] [Google Scholar]
- 173.Zheng H, Cui FQ, Wang FZ. The epidemiology of hepatitis B virus infection in women of reproductive age in highly endemic areas in China. J Viral Hepat. 2018;25:88–96. doi: 10.1111/jvh.12757. [DOI] [PubMed] [Google Scholar]
- 174.Simonovský V. The diagnosis of cirrhosis by high resolution ultrasound of the liver surface. Br J Radiol. 1999;72:29–34. doi: 10.1259/bjr.72.853.10341686. [DOI] [PubMed] [Google Scholar]
- 175.Kelly EMM, Feldstein VA, Parks M, Hudock R, Etheridge D, Peters MG. An assessment of the clinical accuracy of ultrasound in diagnosing cirrhosis in the absence of portal hypertension. Gastroenterol Hepatol. 2018;14:367–373. [PMC free article] [PubMed] [Google Scholar]
- 176.Ong TZ, Tan HJ. Ultrasonography is not reliable in diagnosing liver cirrhosis in clinical practice. Singapore Med J. 2003;44:293–295. [PubMed] [Google Scholar]
- 177.Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology. 2003;227:89–94. doi: 10.1148/radiol.2272020193. [DOI] [PubMed] [Google Scholar]
- 178.Allan R, Thoirs K, Phillips M. Accuracy of ultrasound to identify chronic liver disease. World J Gastroenterol. 2010;16:3510–3520. doi: 10.3748/wjg.v16.i28.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.WHO . World Health Organization; Geneva: 2020. Prevention of mother-to-child transmission of hepatitis b virus: guidelines on antiviral prophylaxis in pregnancy.https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ [PubMed] [Google Scholar]
- 180.Toy M, Hutton DW, So SK. Cost-effectiveness and cost thresholds of generic and brand drugs in a national chronic hepatitis b treatment program in China. PLoS One. 2015;10 doi: 10.1371/journal.pone.0139876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.