Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has rapidly shifted health care needs and delivery internationally, with about 1 in 5 people with COVID-19 requiring hospitalization, including in the intensive care unit (ICU), at pandemic onset.1 ICU survivors in general are at risk for impairments in mental, cognitive, and physical health, collectively known as post-intensive care syndrome;2 , 3 similar challenges have been described post acute hospitalization (post-hospital syndrome).4 Risks may be higher among COVID-19 survivors.2 Given recognized acute pulmonary complications associated with COVID-19, pulmonary sequelae are a prominent concern,5, 6, 7 although COVID-19 has demonstrated an ability to impact multiple organ systems.8 Further, emerging scientific data describe a potential for lingering symptoms post-COVID-19 infection even among those who do not require hospitalization.9 , 10 The etiology and physiologic correlates of potential persistent symptoms require sufficient resource infrastructure for comprehensive supportive care and further insight into the natural history of COVID-19.
We provide a description of early need recognition, resource redistribution, operational experience, and refined multidisciplinary clinic structure to support COVID-19 survivors: the Johns Hopkins Post-Acute COVID-19 Team (JH PACT).
Available System Resources
The first COVID-19 admission to the Johns Hopkins Hospital was reported in March 2020. Swiftly rising inpatient admissions drew heavily on inpatient resources, and Pulmonary and Critical Care Medicine (PCCM) providers were immersed in frontline care. Infection control efforts reduced availability of post-acute and ambulatory rehabilitation centers; provider resources were reallocated to augment in-hospital rehabilitation programs and support safe discharges. Non-COVID-19 research, outside of select clinical trials, was largely halted, and thus, clinical effort was expanded for many faculty members who traditionally serve in dual clinical and research roles.
Anticipated Ambulatory Needs
Potential ambulatory needs of COVID-19 survivors were extrapolated from other viral respiratory diseases, including severe acute respiratory syndrome (SARS) coronavirus, Middle East respiratory syndrome (MERS) coronavirus, and influenza;11, 12, 13, 14, 15 data are notably limited. Patients developing acute respiratory distress syndrome (ARDS) were anticipated to be at risk for long-term respiratory complications,16 and there were emerging reports of potential complications in multiple organ systems.8 Importantly, survivors requiring hospitalization, especially in the ICU, were anticipated to be at risk for markedly impaired strength/physical ability, worsened mood/anxiety/post-traumatic stress disorder symptoms, cognitive impairment, and increased use of health care resources.3 , 4 , 17 , 18 Importantly, aspects of the COVID-19 pandemic, including visitor restrictions, potential limitations on essential rehabilitation services, higher levels and longer duration of sedation during critical illness, and longer lengths of stay had the potential to further complicate recovery.2 Hence, a multidisciplinary approach was needed to address the needs of a rising population of COVID-19 survivors.
A rapidly developed solution was conceived to: 1) support the recovery of patients in the ambulatory setting, 2) prevent the additional burden of predicted readmissions on an already strained inpatient system, 3) understand the natural history of disease, and 4) funnel therapeutic opportunities to patients. A key consideration was the provision of ongoing care to uninsured and underinsured patients and collaboration with language translation services, given the disproportionate burden of COVID-19 in traditionally under-resourced populations.19, 20, 21
Opportunity
The procurement of a physical location for care delivery, which typically requires substantial justification within a formal business plan, can be a barrier to rapid implementation. Coordination for a multidisciplinary model requires harmonization of multiple providers and services in time and space. Rapid adoption of telemedicine on a broad scale, necessitated by infection control measures, overcame these barriers. Specifically, telemedicine allowed for appointments to be scheduled at the mutual convenience of patients and each of the multidisciplinary providers, circumventing the need for schedule alignment at a time of high clinical demand.
Conception and Design
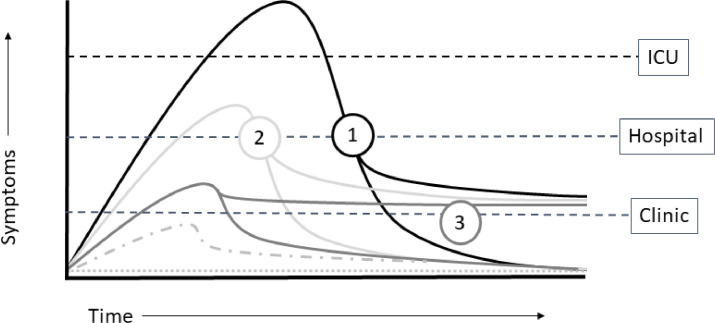
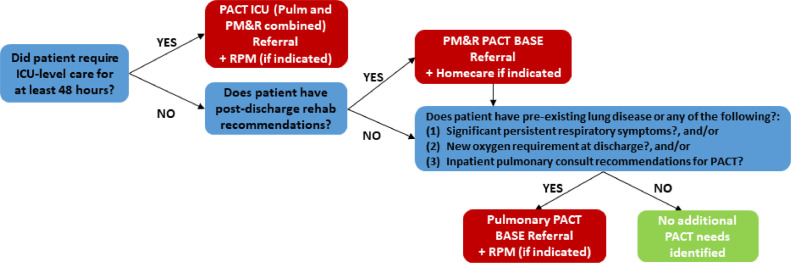
The overall workflow targeted anticipated survivor streams, categorized by initial severity of illness and resultant health care utilization (Figure 1 ). Given predicted patient needs, the PCCM Division partnered with the Department of Physical Medicine and Rehabilitation (PM&R) to provide core assessments and direct services. Patients discharged from the ICU were eligible for JH PACT-ICU, prompting referral to both Pulmonary and PM&R (Figure 2 ). Patients who did not require ICU care for at least 48 hours could be referred to Pulmonary or PM&R (JH PACT-Base), depending on identified needs at the time of discharge. Patients who remained ambulatory but were identified by their primary care or another physician to have residual symptoms at 4-6 weeks post diagnosis were eligible for referral to JH PACT-Base. As an additional service, the Johns Hopkins Homecare Group Remote Patient Monitoring (RPM) team collaborated with the Office of Telemedicine and the PCCM Division to launch the COVID-19 RPM program for post-hospital discharges meeting specific, predefined criteria. Pulse oximeters were deployed at the time of discharge or later delivered to the patient's home. Oxygen saturation, heart rate, and symptoms were transmitted to dedicated clinical staff twice a day for 14 days, with the option of renewal if symptoms or vital sign abnormalities persisted. Clinical staff responded to alerts, contacted patients for abnormalities or missed check-in, and alerted a physician on call, staffed by Johns Hopkins PCCM via a dedicated pager line.
Figure 1.
Schematic of anticipated COVID-19 survivor streams. Simplified depiction of anticipated COVID-19 survivor courses captured for care in the JH PACT clinic. 1) Patients recovering from intensive care unit (ICU) admission for COVID-19. 2) Patient recovering from hospitalization for COVID-19. 3) Patients who remained in an ambulatory care setting but experienced prolonged, nonresolving symptoms post COVID-19 infection.
Figure 2.
Johns Hopkins Post-Acute COVID-19 Team (JH PACT) referral criteria for COVID-19+ hospital discharges. Patients requiring 48 hours or more in the intensive care unit (ICU) were eligible for referral to the JH PACT-ICU, consisting of evaluation by both the Physical Medicine and Rehabilitation (PM&R) and Pulmonary services. Patients requiring hospitalization but no hospital stay were referred to JH PM&R PACT-Base with additional Homecare referral for home physical therapy/occupational therapy services if necessary. Patient then assessed for ongoing pulmonary needs or qualification for remote patient monitoring, and could receive co-referral or independent referral to JH Pulmonary PACT-Base. Patients could also be individually referred to Remote Patient Monitoring without JH PM&R or Pulmonary PACT referral (not pictured). Patients who did not require hospitalization but had ongoing symptoms at 4-6 weeks post diagnosis could qualify for referral to either of the JH PACT-Base teams.
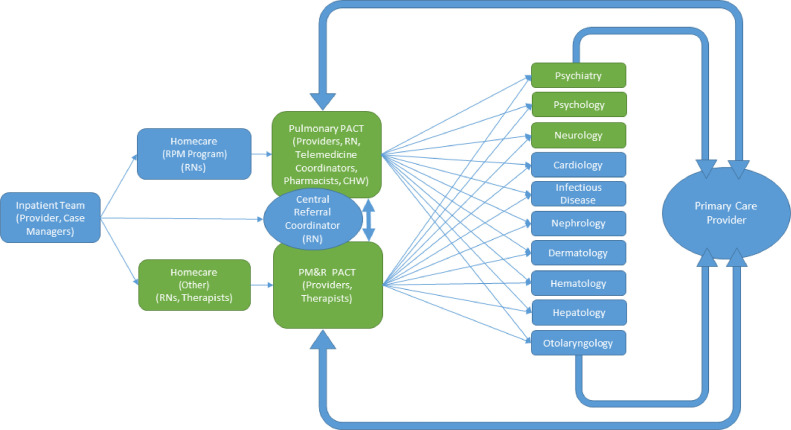
Partnerships with key subspecialties (Figure 3 ) were formed early via grassroots communication with providers willing and interested in receiving referrals from JH PACT. This allowed for streamlined and timely referrals, established key providers for attendance at weekly multidisciplinary meetings, and allowed for development of a shared understanding of the challenges faced by COVID-19 survivors. The JH Pulmonary-PACT team further partnered with Radiology for dedicated, weekly rounds to review chest imaging, revealing typical and atypical findings and enhancing precision of clinical recommendations. Colleagues with a subspecialty focus in interstitial lung disease were invited to attend these rounds, and consulted ad hoc for complicated cases as recommended by the British Thoracic Society.22 , 23 Pharmacists completed medication reconciliation and provided recommendations via phone prior to clinic visits.24 Homecare teams provided nursing and essential therapy services (physical therapy, occupational therapy, speech-language pathology) to homebound patients. Co-management and communication with primary care physicians were prioritized. For patients without primary care, networks were developed with academic and community providers to facilitate establishment of care. Hospital social workers and a community health worker were critical components in light of disproportionate representation among vulnerable populations.
Figure 3.
Key services and staff of the Johns Hopkins Post-Acute COVID-19 Team (JH PACT) clinic. Patient flow and contributing staff members represented above. Green indicates participation in weekly multidisciplinary clinic meetings. Primary care is featured prominently as an essential collaboration and line of communication. Psychology consisted of partners in both neuropsychology and rehabilitation psychology. CHW = community health worker; PMR = Physical Medicine and Rehabilitation; RN = registered nurse.
Concerns were initially raised about access to telemedicine among vulnerable populations and those at risk of marginalization. To address this, research and administrative support staff were trained and re-deployed in telemedicine support to proactively contact patients, assist with software download prior to clinic, and support connection on the clinic day. Nursing staff introduced new patients to the clinic structure and assisted in navigating follow-up testing, which often required repeat COVID-19 test coordination. Clinic workflows and resources (eg, subspecialty referral contacts) were stored in a central, secure drive accessible only by health care team members.
Dissemination
A referral form (Appendix) was disseminated via a collated Department of Medicine protocol for COVID-19+ discharges. Hospital and ambulatory referrals were accepted via a centralized e-mail monitored by a referral coordinator and supported by nursing and physician review for appropriate placement in JH PACT-ICU or PACT-Base. PCCM colleagues were engaged to refer patients at the time of ICU downgrade, and clinic information was disseminated among hospitalist staff and medicine residents. Johns Hopkins Health System partners across the state were engaged in providing care under variably adopted portions of the framework, including RPM, offering an enhanced structure for post-COVID-19 care at participating hospitals.
The first JH PACT patient was seen on April 7, 2020, representing one of the earliest dedicated COVID-19 survivorship clinics in the nation. As of November 11, 2020, 265 unique patients have been seen in 530 visits by the JH PM&R or Pulmonary PACT. New patient JH Pulmonary PACT visits have generated an average of 1 (range 0-3) additional subspecialty referral per patient over the preceding month.
Ongoing Mission
The JH PACT clinic exemplifies the Johns Hopkins tripartite mission: patient care, research, and education. To optimize clinical care and ensure rigor and uniformity of evaluation across the dedicated staff in the clinic, we adopted standardized clinic templates. Assessments were standardized consistent with the Core Outcome Measurement Set for acute respiratory failure25 (improvelto.com); Society of Critical Care Medicine Consensus Statement;26 and COVID-19-specific recommendations by the European Respiratory Society/American Thoracic Society.27 Symptoms are assessed via validated questionnaires preclinic by telemedicine coordinators or via secure messaging and patient self-completion with incorporation into the medical record (Table ).25 , 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 This approach allows for standardization of care, collation of clinical experiences, and description of the natural history of COVID-19 in the JH PACT population. Laboratory, pulmonary function testing, and imaging follow-up are obtained based on individual indications, though most consistent with British Thoracic Society recommendations.22 , 23 Research partnerships have proven synergistic in provision of clinical resources; we are pursuing additional funding to comprehensively characterize outcomes within this population. Further, trainees routinely rotate through the clinic, providing opportunities for clinical education and research. Importantly, trainees voice appreciation for the ability to see the postdischarge trajectory of patients they cared for in the hospital.
Table.
Standardized Functional and Symptomatic Assessments
| Domain | Instrument | Specialty | Visit Type |
|
|---|---|---|---|---|
| Base | ICU | |||
| Health-related quality of life | EQ5D | Pulmonary | x | |
| PROMISƗ | PM&R | x | x | |
| Mental health | ||||
| Depression | PHQ9* | Pulmonary and PM&R | x | x |
| Anxiety | GAD7* | Pulmonary and PM&R | x | x |
| PTSD | IES-6 | Pulmonary | x | |
| Cognition | Telephone cognitive battery | Pulmonary | x | x |
| Pain | EQ5D pain question | Pulmonary | x | x |
| Physical function | AM-PAC surgical short form | PM&R | x | x |
| Respiratory symptoms | BCSS, mMRC | Pulmonary | x | x |
Instruments are assessed at new and follow-up visits.
AM-PAC = Activity Measure for Post-Acute Care;32 BCSS = Breathlessness Cough and Sputum Scale;34 EQ5D = EuroQol 5D;25 GAD-7 = General Anxiety Disorder-730; ICU = intensive care unit; IES-6 = Impact of Event Scale-631; mMRC = modified Medical Research Council;35 MOCA = Montreal Cognitive Assessment25; PHQ-9 = Patient Health Questionnaire-929; PM&R = Department of Physical Medicine and Rehabilitation; PROMIS = Patient-Reported Outcomes Measurement Information System.33
Instruments differ from the core outcome measurement set for clinical research in acute respiratory failure survivors (see improvelto.com) due to licensing and cost associated with the Hospital Anxiety and Depression Scale (HADS).28
Telephone cognitive battery: Cognitive assessments are derived from the Multi-Ethnic Study of Atherosclerosis (MESA);36 battery has been successfully implemented in diverse patient populations and is available in both English and Spanish language translations.37,38
Conclusions
Approaches to support COVID-19 survivors vary across institutions and continents.23 , 39, 40, 41, 42 We have described a successful multidisciplinary approach grounded in a post-intensive care syndrome/post-hospital syndrome framework.3 , 4 The rapid adoption of telemedicine, including ambulatory pulse oximetry monitoring, provided a unique opportunity to overcome traditional barriers and address disparities in care provision. While the American Thoracic Society and European Respiratory Society retain equipoise in recommendations for follow-up in a dedicated multidisciplinary clinic for post-COVID-19 care,43 the present and future benefits to patients, the health system, and knowledge advancements through the JH PACT clinic are tangible. The comprehensive approach described here has proved successful in providing an enduring support network for COVID-19 survivors locally, alongside the provision of data that will inform our understanding of the natural history of COVID-19 in those requiring hospital-level care or with persistent symptoms in the ambulatory setting.
Acknowledgments
Administrative support: Laurie Neisser, MBA; Samuel Boadu, MPH; Andrew Byrd, BFin; Stephen Sisson, MD; Joyce Maygers, DNP, RN
Advisory Board: Christian Merlo, MD, MPH; Dale Needham, MD, PhD; Megan Hosey, PhD; Peiting Lien, DPT, NCS; Jyotsna Supnekar, OTR, CHT, CLT; Amanda Gallagher, MA CCC-SLP; Kelly Daley, PT, MBA; Preeti Raghavan, MD
Clinical Support: Arun Venkatesan, MD, PhD; Esther Oh, MD, PhD; Ashraf Fawzy, MD MPH; Alba Azola, MD; Jennifer Zanni, PT, DScPT, CCS; Laurie Fitz, PT; Norma Wright, RN; Denise Wagner, DT, PT; Jessica Engle, DO; Martin Bishop, PharmD, MS, BCACP; Jenna Blunt, PharmD, BCPS; Caitlin Down-Green, PharmD, BCPS, BCACP; Traci Grucz, PharmD; Erin VanMeter, PharmD, BCACP; Badia Faddoul, MS, RN
Hospital System MD Partners: Carmen Salvaterra, MD; David Holden, MD; Steven Kariya, MD
Footnotes
Funding: Authors receive funding from the National Heart, Lung, and Blood Institute (K23HL138206 [AP]; F32HL143864 [JO]; K12HL143957 [SR]) the National Institute of Environmental Health Sciences (K23ES029105 [EB]), the National Institute of Allergy and Infectious Diseases (P30A1094189 [SR]), and the Joint Artificial Intelligence Center (DoD [AK]).
Conflict of Interest: BH serves on the Board of Directors for local Maryland Medicare Advantage insurance plan, Hopkins Health Advantage (d/b/a AdvantageMD), holds a minor equity interest in a private digital company (TRUE-See Systems, LLC), and within the last year served on an Academic Advisory Committee for a virtual visit research study, receiving a stipend from a Kaiser Permanente affiliate, Mid-Atlantic Permanente Research Institute. The clinic receives financial support via the Maryland State Health Services Cost Review Commission.
Authorship: All listed authors meet International Committee of Medical Journal Editors criteria for co-authorship of the manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2020.12.009.
Appendix
References
- 1.World Health Organization. Media statement: knowing the risks for COVID-19. Available at: https://www.who.int/indonesia/news/detail/08-03-2020-knowing-the-risk-for-covid-19, Accessed September 1, 2020.
- 2.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6(1):60. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 4.Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraser E. Long term respiratory complications of covid-19. BMJ. 2020;370:m3001. doi: 10.1136/bmj.m3001. [DOI] [PubMed] [Google Scholar]
- 6.Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms. Lancet Respir Med. 2020;8(8):750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raghu G, Wilson KC. COVID-19 interstitial pneumonia: monitoring the clinical course in survivors. Lancet Respir Med. 2020;8(9):839–842. doi: 10.1016/S2213-2600(20)30349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yelin D, Margalit I, Yahav D, et al. Long COVID-19-it's not over until [e-pub ahead of print]? Clin Microbiol Infect. 2020 Dec 11 doi: 10.1016/j.cmi.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenforde MW. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network – United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das KM, Lee EY, Singh R, et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27(3):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, Shin H-S, Park HY, et al. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019;16(1):59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam MH-B, Wing Y-K, Yu MW-M, et al. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med. 2009;169(22):2142–2147. doi: 10.1001/archinternmed.2009.384. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020;8:8. doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed H, Patel K, Greenwood DC, et al. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalization of ICU admission; a systematic review and meta-analysis. J Rehabil Med. 2020;52(5):jrm00063. doi: 10.2340/16501977-2694. [DOI] [PubMed] [Google Scholar]
- 16.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 17.Ruhl AP, Huang M, Colantuoni E, et al. Healthcare utilization and costs in ARDS survivors: a 1-year longitudinal national US multicenter study. Intensive Care Med. 2017;43(7):980–991. doi: 10.1007/s00134-017-4827-8. [DOI] [PubMed] [Google Scholar]
- 18.Ruhl AP, Huang M, Colantuoni E, et al. Healthcare resource use and costs in long-term survivors of acute respiratory distress syndrome: a 5-year longitudinal cohort study. Crit Care Med. 2017;45(2):196–204. doi: 10.1097/CCM.0000000000002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macias Gil R, Marcelin JR, Zuniga-Blanco B, et al. COVID-19 pandemic: disparate health impact on the Hispanic/Latinx population in the United States. J Infect Dis. 2020;222(10):1592–1595. doi: 10.1093/infdis/jiaa474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowkwanyun M, Reed AL. Racial health disparities and Covid-19 – caution and context. N Engl J Med. 2020;383(3):201–203. doi: 10.1056/NEJMp2012910. [DOI] [PubMed] [Google Scholar]
- 21.Raifman MA, Raifman JR. Disparities in the population at risk of severe illness from COVID-19 by race/ethnicity and income. Am J Prev Med. 2020;59(1):137–139. doi: 10.1016/j.amepre.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.British Thoracic Society. COVID-19: information for the respiratory community. Available at: https://www.brit-thoracic.org.uk/about-us/covid-19-information-for-the-respiratory-community/. Accessed September 15, 2020.
- 23.George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia|Thorax. Available at: https://thorax.bmj.com/content/early/2020/08/24/thoraxjnl-2020-215314. Accessed September 15, 2020. [DOI] [PubMed]
- 24.Najafzadeh M, Schnipper JL, Shrank WH, Kymes S, Brennan TA, Choudhry NK. Economic value of pharmacist-led medication reconciliation for reducing medication errors after hospital discharge. Am J Manag Care. 2016;22(10):654–661. [PubMed] [Google Scholar]
- 25.Needham DM, Sepulveda KA, Dinglas VD, et al. Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196(9):1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikkelsen ME, Still M, Anderson BJ, et al. Society of Critical Care Medicine's international consensus conference on prediction and identification of long-term impairments after critical illness. Crit Care Med. 2020;48(11):1670–1679. doi: 10.1097/CCM.0000000000004586. [DOI] [PubMed] [Google Scholar]
- 27.Spruit MA, Holland AE, Singh SJ, Tonia T, Wilson KC, Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society- and American Thoracic Society-coordinated international task force. Eur Respir J. 2020;56(6) doi: 10.1183/13993003.02197-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 31.Hosey MM, Leoutsakos J-MS, Li X, et al. Screening for posttraumatic stress disorder in ARDS survivors: validation of the Impact of Event Scale-6 (IES-6) Crit Care. 2019;23(1):276. doi: 10.1186/s13054-019-2553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 suppl):I49–I61. doi: 10.1097/01.mlr.0000103520.43902.6c. [DOI] [PubMed] [Google Scholar]
- 33.HealthMeasures. PROMIS. Available at: https://www.healthmeasures.net/explore-measurement-systems/promis. Accessed November 30, 2020.
- 34.Leidy NK, Rennard SI, Schmier J, et al. The breathlessness, cough, and sputum scale: the development of empirically based guidelines for interpretation. Chest. 2003;124(6):2182–2191. doi: 10.1378/chest.124.6.2182. [DOI] [PubMed] [Google Scholar]
- 35.Bestall J, Paul E, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Multi-Ethnic Study of Atherosclerosis (MESA). MESA overview and protocol. Available at: https://www.mesa-nhlbi.org/aboutMESAOverviewProtocol.aspx. Accessed November 30, 2020.
- 37.Fitzpatrick AL, Rapp SR, Luchsinger J, et al. Sociodemographic correlates of cognition in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Geriatr Psychiatry. 2015;23(7):684–697. doi: 10.1016/j.jagp.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weintraub S, Besser L, Dodge HH, et al. Version 3 of the Alzheimer Disease Centers’ neuropsychological test battery in the Uniform Data Set (UDS) Alzheimer Dis Assoc Disord. 2018;32(1):10–17. doi: 10.1097/WAD.0000000000000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geberhiwot T, Madathil S, Gautam N. After care of survivors of COVID-19—challenges and a call to action. JAMA Health Forum. Available at: https://jamanetwork.com/channels/health-forum/fullarticle/2770074. Accessed January 12, 2021. [DOI] [PubMed]
- 40.O'Brien H, Tracey MJ, Ottewill C, et al. An integrated multidisciplinary model of COVID-19 recovery care [e-pub ahead of print] Ir J Med Sci. 2020:1–8. doi: 10.1007/s11845-020-02354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mount Sinai Health System. Center for post-COVID care. Available at: https://www.mountsinai.org/about/covid19/center-post-covid-care. Accessed September 15, 2020.
- 42.Penn Medicine News. What happens after COVID-19? Penn Medicine's new post-COVID recovery clinic offers support. Available at: https://www.pennmedicine.org/news/news-blog/2020/june/what-happens-after-covid19-penn-medicine. Accessed September 15, 2020.
- 43.Bai C, Chotirmall SH, Rello J, et al. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020) Eur Respir Rev. 2020;29(157) doi: 10.1183/16000617.0287-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]