Abstract
Pea (Pisum sativum L.) is a widely grown pulse crop that is a source of protein, starch and micronutrients in both human diets and livestock feeds. There is currently a strong global focus on making agriculture and food production systems more sustainable, and pea has one of the smallest carbon footprints of all crops. Multiple genetic loci have been identified that influence pea seed protein content, but protein composition is also important nutritionally. Studies have previously identified gene families encoding individual seed protein classes, now documented in a reference pea genome assembly. Much is also known about loci affecting starch metabolism in pea, with research especially focusing on improving concentrations of resistant starch, which has a positive effect on maintaining blood glucose homeostasis. Diversity in natural germplasm for micronutrient concentrations and mineral hyperaccumulation mutants have been discovered, with quantitative trait loci on multiple linkage groups identified for seed micronutrient concentrations. Antinutrients, which affect nutrient bioavailability, must also be considered; mutants in which the concentrations of important antinutrients including phytate and trypsin inhibitors are reduced have already been discovered. Current knowledge on the genetics of nutritional traits in pea will greatly assist with crop improvement for specific end uses, and further identification of genes involved will help advance our knowledge of the control of the synthesis of seed compounds.
Keywords: Genetics, Nutrition, Quality traits, Pea
Highlights
-
•
There is huge potential to use genetic diversity to modify pea seed composition.
-
•
Such modifications can help meet demands for novel plant-based products.
-
•
Concentrations of protein, resistant starch and micronutrients can be improved.
-
•
Amounts of so-called antinutrients can be reduced.
-
•
Genomic resources are accelerating the rate at which changes can be made.
1. Introduction
Pea (Pisum sativum L.) is an economically important cool season legume crop, grown widely across Europe, North America, China and Australia, having been domesticated in the Fertile Crescent around 10,000 years ago (Zohary and Hopf, 2000). It is the fourth most cultivated legume crop worldwide (Mertens et al., 2012). Estimates suggest 280,000 tonnes of peas were produced in the UK in 2017, including both dried pea and vining (immature) pea seeds for the canning and fresh frozen vegetable markets (PGRO, 2018). Global production of dry pea is dominated by Canada, Russia and China, followed by India, Ukraine and the United States (FAOSTAT, 2020). Dried peas of marrowfat varieties are commonly used for “mushy peas” in the UK, and a proportion of UK grown peas is exported to the Far East to be used in snack products (Robinson et al., 2019).
Peas are a nutritional source of protein, beneficial starch and micronutrients, and have significant environmental benefits when compared to other crops cultivated within a cereal-dominated agriculture. There is currently a strong spotlight on the environmental footprint of agriculture, with the mass farming of livestock being a major contributor to global greenhouse gas emissions (Steinfeld et al., 2006). Agriculture must be made more sustainable if global climate change is to be mitigated, and the production of pea has one of the lowest carbon footprints of any crop (Poore and Nemecek, 2018). Despite already having significant nutritional benefits as a foodstuff, the total nutritional potential of pea has not yet been realised, with studies on wild species and genetic mutants suggesting that great diversity exists or can be generated with regard to nutritionally important traits (Demirbaş, 2018; Warkentin et al., 2012). This review discusses current knowledge of the genetic bases of seed quality traits, encompassing seed protein, starch, micronutrients and antinutritional compounds. The importance of protein and starch quality, in addition to their actual concentrations, is emphasised, highlighting nutritional and human health benefits. Additionally, knowledge gaps for the genetics of seed quality traits in pea are discussed in order to identify target areas for future research.
2. Protein
Protein is one of the major food groups essential for health and is vital for maintaining muscle mass and strength in later life. It is estimated that 0.5–1.0% of muscle mass is lost annually in humans from the age of 50, and therefore protein intake is especially important for the proportion of the population above this age threshold (Lonnie et al., 2018). However, it has been found that increasing protein intake at all stages of life may reduce muscle decline and its associated health conditions (Stevenson et al., 2018). Protein makes up around 15–30% of dry pea seeds, more typically 20–28% in cultivars (Burstin et al., 2007; PGRO, 2020), with this fraction mainly comprised of globulin and albumin storage proteins (Tao et al., 2017; Tömösközi et al., 2001; Tzitzikas et al., 2006). Pea seed protein composition is complex genetically, with multigene families encoding different proteins, which are then subject to considerable post-translational processing (Bourgeois et al., 2011). These authors showed that pea seed protein composition is predominantly under genetic control, with 60% of the protein ‘spots’ from a two-dimensional electrophoresis analysis varying in abundance between genotypes (Bourgeois et al., 2011). However, environmental factors have also been shown to have an effect on the pea seed proteome; heat and drought stress can interrupt seed maturation and may result in lower accumulation of those seed proteins that typically accumulate later in development (Bourgeois et al., 2009).
An earlier study used a linkage map and genetic crosses to detect quantitative trait loci (QTLs) for total seed protein concentration in pea (Tar'an et al., 2004). In a study involving a cross between parents differing in seed protein concentration by just 9 g kg−1 (0.9%), a QTL was detected on linkage group VI that explained 45% of the variation in seed protein concentration (i.e. up to 0.4% difference in protein concentration between the two parental lines), suggested to be linked to the synthesis of the Albumin a protein in pea. Additionally, a single-nucleotide polymorphism (SNP) conveying a significant effect on seed protein concentration has previously been discovered within the O2like gene in pea. O2like in pea shows similarity to Opaque2 (O2) in maize, a bZIP transcriptional regulatory factor that affects starch and protein content (Jha et al., 2015). An earlier study examined the effect of developmental genes on pea seed protein concentration and yield, and identified 14 QTLs across five environments (Burstin et al., 2007). These loci corresponded to eight genomic regions, explaining 9–46% of genetic variation. QTLs found at 160 cM and 170 cM on linkage group V showed opposite effects, suggesting that there may be two separate QTLs at this location, despite their overlapping confidence intervals (Burstin et al., 2007). Another study (Irzykowska and Wolko, 2004) also reported significant QTLs for total protein content on linkage group V, accounting for 18.3–25.5% of phenotypic variance, using a population of recombinant inbred lines (RILs) made from a cross between large-seeded and small-seeded parents. Additionally, a QTL study using two hybrid populations created by crossing a small-seeded high-protein line with two distinct large-seeded high-yielding cultivars also revealed QTLs for total seed protein content on linkage group V (Krajewski et al., 2012), including one locus in close proximity to the marker tl, also noted previously (Irzykowska and Wolko, 2004).
The predominant storage proteins in pea are the globulins legumin and vicilin, thought to be synthesised by at least 40 genes (Casey et al., 2001). Loci related to the synthesis of legumin (Lycett et al., 1984), vicilin (Lycett et al., 1983), and convicilin (Cvc) (Newbigin et al., 1990), have been identified, and may be considered as suitable targets for mutagenesis for altering seed protein composition (Domoney et al., 1986; Ellis et al., 1986). However, pea seed protein content is a quantitatively variable trait, with globulins encoded by multigene families (Bourgeois et al., 2009), and so mutations affecting single genes have little influence on total protein concentration (Chinoy et al., 2011; Rayner et al., 2018), unless the mutation is at a locus which controls a large proportion of phenotypic variation. Up to four gene classes have been described for legumin biosynthesis in pea, defined by distinct genetic loci, and there are thought to be around 10–15 genes in total that synthesise this protein (Casey et al., 2001). In general, genes within a locus are highly homologous, whereas those at different loci have lower homology (Domoney et al., 1986; Ellis et al., 1986). Loci involved in starch synthesis can also have an effect on the production of legumin, with mutant alleles at r and rb encoding starch-branching enzyme I (SBEI) and the large subunit of ADP-glucose pyrophosphorylase (AgpL), respectively, resulting in considerably reduced legumin synthesis. Double mutants (rrrbrb) at these loci show drastically reduced seed concentrations of legumin proteins (Casey et al., 2001); the mechanism behind this effect is thought to be the preferential destabilisation of legumin mRNAs due to elevated cellular sucrose concentrations and osmotic pressure in cotyledon cells (Casey et al., 2001).
Vicilin proteins have lower concentrations of sulphur-containing amino acids than legumins (Shewry et al., 1995). These amino acids have been identified as limiting in pea and increasing their concentrations has been identified as a primary target for the nutritional improvement of pea and other pulse crops (Robinson et al., 2019). Vicilin has also been shown to form amyloids in pea cotyledon cells (Antonets et al., 2020); amyloids are protein aggregates with unique physicochemical properties. Amyloids are resistant to the action of proteases and vicilin amyloids show resistance to gastrointestinal digestion (Antonets et al., 2020). The same term has been used to describe protein depositions that can cause health problems in humans and other organisms (Kyle, 2001). Proteomic studies (Bourgeois et al., 2009) suggested that there are at least 24 genes encoding vicilin in the pea genome, following construction of a pea seed proteome reference map. Another study demonstrated how the biosynthesis of vicilin and other proteins could be impacted by knocking out multiple genes at single loci through fast-neutron mutagenesis (Domoney et al., 2013). A mutant allele at the Vc-2 locus has been shown to impact the production of major vicilin polypeptides and affect seed nitrogen concentrations (Chinoy et al., 2011). A vicilin-encoding gene, and genes encoding legumin and convicilin, were also identified in an analysis of protein quantity loci (Bourgeois et al., 2011). Another study (Le Signor et al., 2017) used the model legume Medicago truncatula to identify molecular determinants of seed protein composition in legumes, and found the ABI5 transcription factor to be an important player in determining seed globulin abundance. The same study then characterised loss of function abi5 mutants in pea, where mutants showed decreased vicilin concentrations and upregulation of genes encoding other major seed proteins (Le Signor et al., 2017). Mertens and co-workers used gel permeation chromatography to show that, although ratios are predominantly under genetic control, agronomic factors have an influence on legumin/vicilin ratios, and that this ratio positively correlates with total protein content (Mertens et al., 2012).
Genome sequencing has provided seed protein annotations for a reference genome based on the pea cultivar (cv.) Caméor (Kreplak et al., 2019). The assembly was searched for genes listed on UNIPROT as coding for seed storage proteins, and sequences for proteins including legumin (12 genes), vicilin (nine genes) and convicilin (two genes) were identified (Kreplak et al., 2019). The number of genes found for legumin synthesis broadly agrees with previous work (Casey et al., 2001), but for vicilin the number is considerably lower than deduced from previous studies (Casey and Domoney, 1999; Ellis et al., 1986). Various motifs upstream of seed storage protein genes are thought to regulate their expression based on developmental and environmental clues and, in this reference genome, so-called RY motifs (CATGCATG) were found upstream of all but three of the seed storage protein genes identified (Kreplak et al., 2019). RY motifs have been shown previously to be involved in seed-specific transcriptional activation in soybean and faba bean (Reidt et al., 2000; Yoshino et al., 2006).
The lectin protein in pea seeds has been associated with poor digestibility (Le Gall et al., 2007), and is encoded by LecA, a gene that is also involved in the response of pea roots to Rhizobium symbiotic bacteria (Díaz et al., 1989). LecA was knocked-out using mutagenesis in a previous study (Domoney et al., 2013), resulting in a loss of lectin synthesis, but the function of this gene in symbiotic root interactions raised concerns that the mutant could show deleterious effects on the pea-Rhizobium symbiosis. However, a subsequent study showed that the nodulation efficiency of the LecA mutant was equivalent to that of wild-type pea, suggesting that a lack of lectin does not have an overall negative effect on the plant-bacterial symbiosis (Rayner et al., 2018). Pea albumin 2 (PA2) is also considered to be an undesirable protein, due to its potentially allergenic effects (Vioque et al., 1998) and resistance to digestion (Le Gall et al., 2007). The reference genome for cv. Caméor identified nine gene sequences coding for PA2, and eight for a second major albumin, PA1 (Kreplak et al., 2019). Previously, a screen of pea germplasm identified a naturally occurring null mutant for PA2, a pea line with wild characteristics that has a deletion spanning across most of the structural genes of the protein (Vigeolas et al., 2008). The mutant line was crossed with the commercial cultivar Birte and a recombinant inbred population developed; analysis of this population showed that lines lacking PA2 had a higher seed nitrogen content, possibly due to an over-compensatory increase in the synthesis of other seed proteins (Vigeolas et al., 2008). Backcrossing into the cv. Birte removed the effects of differences in plant height and seed size and showed that lines lacking PA2 have altered polyamine synthesis, suggesting that PA2 also has a role in plant development and stress responses (Vigeolas et al., 2008). This effect was later substantiated by a study which showed that the polyamine, spermine, could bind to the homolog of PA2 in grass pea (Lathyrus sativus) (Gaur et al., 2010). Alternative binding of spermine or heme was linked to dimerization and dissociation of this hemopexin-type protein, where the different oligomeric states were suggested to play a role in sensing oxidative stress (Gaur et al., 2010). The N-terminus of the PA2 peptide has been shown to have 77.7% peptide sequence identity with PsEND1, the product of an anther-specific gene that displays early expression in pea flowers (Gómez et al., 2004). PsEND1 is present in the naturally occurring mutant lacking PA2, mapping to a genetic locus that is distinct from that of the PA2 genes (Vigeolas et al., 2008).
Lipoxygenases (LOX) are abundant seed proteins, which are also iron-containing enzymes that catalyse the synthesis of hydroperoxides from fatty acids; two major LOX polypeptides exist in pea seeds (LOX-2 and LOX-3), and one of these catalyses the production of compounds that are considered as off-flavours by consumers (Forster et al., 1999). LOX-2 and LOX-3 in pea are each encoded by two or three genes at the lox locus on linkage group IV (North et al., 1989). A Pisum fulvum line from the John Innes Centre pea collection has been identified as a null mutant for LOX-2. Investigation of the lox locus in this line showed that the reduction in LOX-2 mRNA was not the result of a deletion of LOX genes, but was likely due to one or multiple insertions, deletions or substitutions that are present in the LOX-2 promoter of the null mutant (Forster et al., 1999).
Pea albumins have been shown to contain disproportionately high concentrations of sulphur-containing amino acids compared with globulin protein fractions (Croy et al., 1984); these amino acids are essential for human health, and concerns are frequently raised over the lower concentrations of these compounds in pulses. Therefore, pea albumins may convey a nutritional benefit with respect to amino acid composition, despite concerns over possible allergenic effects and resistance to digestion of these proteins. Pea albumin 1 subunit b (PA1b) from pea and other legumes has been shown to have insecticidal effects on a range of pests and so may confer biological protection to the seed (Louis et al., 2004). Six isoforms of PA1b exist in pea, five of which have shown similar insecticidal activity (Eyraud et al., 2013). PA1b subunits are derived by post-translational modification of the product of PA1 genes (Eyraud et al., 2013) and there are approximately eight PA1 genes (Kreplak et al., 2019).
3. Starch
Starch contributes around 45–50% of the dry weight of pea seeds (Bhattacharyya et al., 1990) and is comprised of multiple fractions, commonly classified by their structure as well as by digestibility in the gut (Lockyer and Nugent, 2017). Resistant starch, considered in some classifications as a component of dietary fibre (Stephen et al., 2017), has significant benefits for human health, as it cannot be broken down by amylolytic enzymes in the small intestine and is fermented in the large intestine by gut microflora (Petropoulou et al., 2016). This process produces short chain fatty acids, including acetate and butyrate, which help maintain blood glucose homeostasis in humans through interactions with insulin-producing β-cells in the pancreas (Petropoulou et al, 2016, 2020).
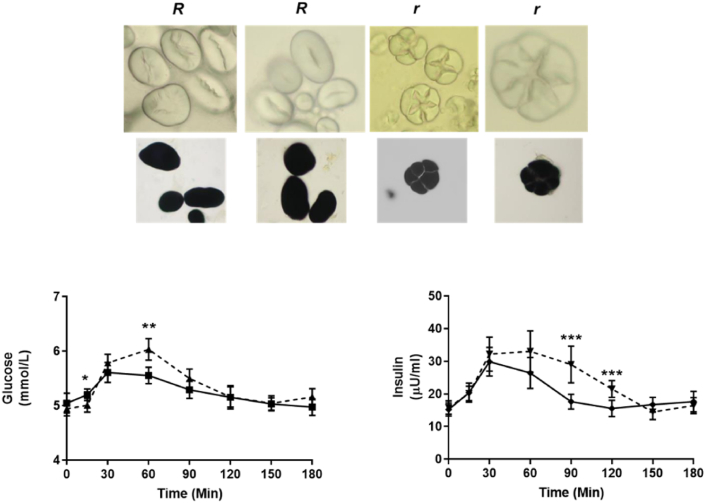
The pea varieties used in studies of inheritance by Gregor Mendel (1886) are very likely to include genotypes which produce seeds with different concentrations of resistant starch. Mutations which give rise to wrinkled seeds generally result in seeds which contain less total starch than is present in round seeds, but mutations in some starch biosynthetic genes result in higher relative concentrations of dietary resistant starch (Kozłowska, 2001). The higher level of resistant starch in one class of wrinkled-seeded pea is due to a naturally occurring mutation in the SBEI gene at the r locus, which gives rise to both the increase in resistant starch and the wrinkled-seeded phenotype (Bhattacharyya et al., 1990; Rayner et al., 2017); the seed phenotype has been associated with alterations to starch granule morphology (Fig. 1) and sucrose metabolism (Wang and Hedley, 1991). The increase in resistant starch has been shown to lead to lower blood glucose levels and insulin responses, when compared with the response to a near-isogenic control line in human intervention studies (Fig. 1; Petropoulou et al., 2020). The naturally occurring r mutation is a consequence of an insertion (at least 0.8 kb) into the coding region of SBEI, that predicts the production of an abnormal transcript and a disruption to the carboxy-terminal region of the encoded protein. The end-result is a reduction in amylopectin synthesis, leading to starch which is amylose-rich, with fewer branched amylopectin molecules, conveying increased resistance to digestion (Bhattacharyya et al., 1990; Rayner et al., 2017). Therefore, although total starch is reduced in seeds of sbeI mutants, the proportion of resistant starch is much higher, a consequence of an increase in amylose from 33 to 49% up to 72% of pea starch (Ratnayake et al., 2002). Similar work has been conducted in durum wheat (Triticum turgidum L. var. durum), where the knock-out of a homologous enzyme, SBEIIa, increases the amylose content of grain (Sestili et al., 2010). Genetic markers for the sbeI allele in pea have been developed (Rayner et al, 2017, 2018) and although no other naturally occurring mutations have been discovered at the r locus (Rayner et al., 2017), studies using chemical mutagenesis have generated a set of induced mutations (Wang et al., 1990).
Fig. 1.
Changes in starch morphology in r mutant pea seeds compared with round-seeded (R) pea seeds, unstained or stained (top panel). Comparison of glucose and insulin response curves in humans following consumption of wrinkled (solid lines), compared with control near-isogenic round (dotted lines), pea seeds (lower panel). The glucose and insulin response data are re-plotted from Petropoulou et al. (2020).
Mutations at other loci affecting enzymes in the starch biosynthesis pathway have been identified that also change pea seed starch composition and result in the wrinkled-seeded phenotype. One such mutation is a nine-base pair deletion in AgpL at the rb locus (Rayner et al., 2017); this mutation results in an approximately 50% reduction in the concentration of pea seed starch (Hylton and Smith, 1992). Studies using plants with mutations at either or both the r and rb loci have shown them to differ in starch composition and content; rrRbRb mutant seeds had the greatest concentrations of amylose, higher than the double mutant (rrrbrb), as mutations at the rb locus reduce amylose levels and total starch (Wang and Hedley, 1991). Four other loci have been identified which affect starch metabolism: rug3, rug4, rug5, and lam. rug3 encodes plastidial phosphoglucomutase, and mutations at this locus can give rise to almost starchless pea plants (Harrison et al., 1998). rug4 affects the supply of substrate for starch biosynthesis, as this affects a sucrose synthase enzyme (Bogracheva et al., 1999). rug5 encodes starch synthase II, and mutations result in alterations to starch granule morphology and the structure of amylopectin (Craig et al., 1998). The lam mutation also acts on a starch synthase enzyme (starch synthase I), and produces starch with low amylose and very high concentrations of amylopectin (Tahir et al., 2011). Genes relating to starch biosynthesis are clearly well studied in pea, and this provides good potential for the manipulation of pea starch content and composition for various applications. A further study showed that although total starch content is reduced in peas high in protein, amylose content is significantly higher, increasing the resistance to digestion of the starch fraction (Shen et al., 2016).
Certain starch fractions that are enzyme resistant, such as ungelatinized starch and starch enclosed in food structures, can behave like dietary fibre (Asp et al., 1987). Other types of dietary fibre also exist in pea, and have physiological effects in the gastrointestinal tract (De Almeida Costa et al., 2006). These include long-chain soluble and insoluble polysaccharides, and galacto-oligosaccharides including the raffinose family of oligosaccharides; insoluble fractions are associated with laxation, whereas soluble fibre is more closely linked to lowering cholesterol levels and alleviating blood glucose fluctuations (Brummer et al., 2015). Raffinose and related oligosaccharides are resistant to digestion in upper regions of the gastrointestinal tract and are instead fermented in the colon by microflora (Tosh and Yada, 2010). Genes encoding raffinose and stachyose synthases have been mapped to linkage groups III and V, respectively, and quantitative variation in these compounds related to genetic map locations (Ellis et al., 2018). Dietary fibre makes up 14–26% of pea seeds by dry weight, slightly less than the concentrations found in beans (23–32%) (Brummer et al., 2015).
Two recent studies have attempted to identify and map QTLs for dietary fibre (Gali et al, 2018, 2019), using either acid or neutral detergent as digestion solutions to classify the fibre fractions. One of these studies (Gali et al., 2018) used three RIL populations derived from crosses between pea cultivars and breeding lines from Canada and Europe: PR-02 (Orb x CDC Striker), PR-07 (Carrera x CDC Striker) and PR-15 (1–2347-144 x CDC Meadow). In trials of the PR-02 RIL population, QTLs for acid detergent fibre (ADF) were identified on four linkage groups, including two QTLs on linkage group VII that separately explained 28.0% and 26.2% of phenotypic variance. For the PR-07 population, significant QTLs for ADF were found on linkage group IV and linkage group VIIa (Gali et al., 2018). For neutral detergent fibre (NDF), QTLs on linkage group Va were identified in all trials using the PR-02 population, and QTLs on linkage groups Ia, IV and VIIa were identified in multiple trials in the PR-07 RIL population, explaining up to 44% of phenotypic variance (Gali et al., 2018). A further study (Gali et al., 2019) conducted genome-wide association mapping of seed quality traits including dietary fibre on a collection of 135 pea accessions from 23 different breeding programmes from across the world. Five SNPs were identified as being associated with concentrations of ADF (on chromosomes 5, 6 and 7), and eight with concentrations of NDF (chromosomes 2, 3, 5, 6 and 7). Two of these markers, on chromosomes 6 and 7, were associated with both fractions of fibre, and three other markers identified were found to be linked to total starch concentration (Gali et al., 2019). In comparing the results of different authors, the historical and numerical relationships between linkage group and chromosome number in pea needs to be considered (Kreplak et al., 2019).
4. Micronutrients
Pea seeds are rich in a range of micronutrients including iron, zinc, and selenium. Iron is an essential micronutrient for seed embryo development, and a large proportion of the iron in pea seeds is stored as ferritin, which acts as a store for the seedling following germination (Ravet et al., 2009). Plant ferritin is located in plastids, and so ferritin-iron in seeds is surrounded by multiple membranes, which can hinder the release of iron during digestion (Moore et al., 2018); however, ferritin-iron in its purified form has been shown to exhibit good bioavailability in both pea and soybean (Lönnerdal et al., 2006; Perfecto et al., 2018).
brz (bronze) and dgl (degenerate leaves) pea mutants, displaying greatly increased iron uptake, have been identified in mutant screens. However, although brz plants showed increased iron uptake, there was no increase in seed iron concentration. Iron overaccumulates in other parts of the plant in this mutant and causes phytotoxicity, as well as reduced root nodulation (Kneen et al., 1990). In contrast, dgl mutants show a significant increase in seed iron concentration compared to wild type when grown in conditions with excess iron (Marentes and Grusak, 1998). Embryonic iron concentrations in the dgl mutants are 163 mg kg−1, compared to 65 mg kg−1 in wild-type seeds. dgl shows no deleterious effects on embryos (Marentes and Grusak, 1998) although, as with the brz mutants, the mutation leads to reduced root nodulation and the accumulation of ferritin in the leaves. The accumulation of ferritin in the leaves is thought to be the result of a plant defence mechanism against iron-mediated oxidative stress (Becker et al., 1998). Increased root iron (III) reductase activity and proton efflux cause the iron accumulation in dgl, and high concentrations of nicotianamine, a chelator for iron (II), are associated with both mutations (Becker et al., 1998). The specific genes relating to the dgl and brz mutants have not yet been identified (García et al., 2013), although a gene controlling a Fe(III)-chelate-reductase (FRO1) has been observed to be expressed constitutively in brz and dgl, as opposed to specifically under iron-deficient conditions in a line with correctly regulated iron accumulation (Waters et al., 2002).
Mineral accumulation mutants have otherwise not yet been described for pea or other legume species, despite work elucidating the role of ascorbate in iron loading in seeds (Grillet et al., 2013). It has been suggested that the remobilisation of minerals stored in vegetative tissue occurs during seed mineral accumulation, but that continued uptake and translocation of mineral micronutrients still play a larger role during seed filling (Sankaran and Grusak, 2014).
Mean seed iron concentrations were reported to range from 45 to 54 mg kg−1 in commercial cultivars grown in North Dakota (Amarakoon et al, 2012, 2015), values that are similar to those of Canadian grown peas (48–58 mg kg−1) (Ray et al., 2014). A study on marker-trait association using SNPs in 94 diverse accessions found that genotype contributed 60.3% of the total variation in seed iron concentration (Diapari et al., 2015). Another study presented data showing a very large range of diversity in seed iron concentration in 152 Turkish land races and five commercial cultivars, with concentrations ranging from 38.6 mg kg−1, to 320.9 mg kg−1 (Demirbaş, 2018). The mean iron concentration in all lines studied was 67.9 mg kg−1, similar to the average concentrations recorded for commercial varieties (Amarakoon et al., 2012; Ray et al., 2014). The accession with the highest seed iron concentration (320.9 mg kg−1) was a land race from Tekirdağ in western Turkey with almost double the concentration of seed iron measured in the dgl iron hyperaccumulation mutant (Marentes and Grusak, 1998). The Turkish land race accession appeared to be an outlier with respect to seed iron concentration (Demirbaş, 2018), and it is likely to be another micronutrient hyperaccumulation mutant. Seven other accessions in the study were found to have seeds containing over 100 mg kg−1 iron (Demirbaş, 2018), demonstrating that Turkish germplasm may be a useful resource for biofortification of commercial pea cultivars. As seed nutritional quality studies on pea land races are relatively rare, further exploration of accessions from around the world would likely provide even further useful diversity in seed iron concentrations.
Various studies have examined the genetic basis of seed iron content in current germplasm stocks, to identify genetic markers and QTLs to aid breeding programmes. Ma et al. (2017) identified five QTLs for seed iron concentration (μg g−1 dry weight), and a further five QTLs for iron content (μg seed−1) using a population of RILs developed from a cross of cultivars Aragorn and Kiflica. The QTLs explaining the most phenotypic variance for seed iron concentration and content were on linkage groups VII (19.4%) and II (12.3%), respectively (Ma et al., 2017). Three of the QTLs for iron concentration were found to be in close proximity to markers previously linked to iron concentration in a study examining micronutrient concentrations across a panel of 94 diverse accessions (Diapari et al., 2015). Two of these QTLs were on linkage group V and the other, explaining the most phenotypic variance, was on linkage group VII (Ma et al., 2017). QTLs for seed iron concentration have also been investigated in three RIL populations developed from crosses using Canadian and European varieties grown in multiple environments (Gali et al., 2018). QTLs for seed iron concentration were detected on multiple linkage groups, including a QTL on linkage group IIIb explaining 20–26% of variation. No significant QTLs were found on linkage group VII, in contrast to other studies (Diapari et al., 2015, Ma et al., 2017). This may be due to the different parental lines used, or the fact that multiple environments were used for field trials (Gali et al., 2018), which may mask genetic effects.
For other essential micronutrients such as zinc and selenium, studies on their concentrations in pea seeds are rare. One study (Diapari et al., 2015) identified two SNPs associated with seed zinc concentration on linkage group III, explaining 11.5% and 9.2% of the genetic variation respectively. Another study also found that a QTL on linkage group III explained the highest amount of phenotypic variance for seed zinc concentration (Ma et al., 2017); however, the SNPs identified in the previous study (Diapari et al., 2015) are not in close proximity to the QTL on linkage group III. A recent study to map QTLs for multiple traits of breeding importance in pea identified four QTLs relating to seed zinc concentration, including a significant locus on linkage group III explaining 42.7% of phenotypic variance (Gali et al., 2018). One of the QTLs identified was mapped to an interval of 91.8–109.1 on linkage group IIIb, a region encompassing the location of a seed zinc QTL previously found (Ma et al., 2017). Overall, these studies suggested that regions of the Pisum genome on linkage group III have real potential for increasing seed zinc concentration and identifying the underlying genes would be of great interest for biofortification programmes. Seed selenium concentration has been studied using three RIL populations; multiple QTLs were identified in two of the populations studied, PR-02 (Orb x CDC Striker) and PR-7 (Carrera x CDC Striker) (Gali et al., 2018). Strong environmental influence on seed selenium content was also observed in this study, but QTLs were identified on two linkage groups of one population, PR-02, including a locus on linkage group VII explaining 15% of phenotypic variance. Studies of the second population, PR-7, revealed QTLs on linkage group IV and linkage group Vb, with the locus on the latter group explaining 17.1% of variance (Gali et al., 2018). Due to the chemical similarities between selenium and sulphur, it is likely that the QTLs identified in this work are loci that encode sulphate transporters (White, 2016).
5. Antinutrients in pea
Antinutrients are compounds that reduce the bioavailability of other nutrients in foods (Hurrell and Egli, 2010), and have a big effect on the nutritional value of many pulse crops including pea. Phytic acid is an abundant antinutrient in pea, and decreasing the concentrations of this compound is seen as a potential means of improving pea nutrient bioavailability. In mature pea seeds, iron bioavailability measured through Caco-2 cell assays is reduced, due to high concentrations of phytate (Moore et al., 2018).
Chemical mutagenesis was used to produce low-phytate (lpa) pea lines from the cultivar CDC Bronco, in which the phytic acid-phosphorus content was reduced by 60–90% to 1.0–1.6 mg g−1 (Warkentin et al., 2012). The low-phytate trait was stable over nine generations and in several different growing environments (Warkentin et al., 2012). The lpa mutants have similar agronomic characteristics to their progenitor, except for a slightly slower time to maturity and a 6% decrease in seed weight that is thought to cause a yield reduction of 8.1–17.6% (Shunmugam et al., 2014; Warkentin et al., 2012). Iron bioavailability was improved by 50–100% in lpa lines compared to controls (Liu et al., 2015). The tight relationship between phytate concentration and iron bioavailability was further supported by a later study which showed that lower phytate levels in immature pea seeds correlated with better iron bioavailability when compared with that of mature seeds (Moore et al., 2018). Removal of phytate would not only enhance the bioavailability of micronutrients, but also improve the digestibility of protein (Carnovale et al., 1988). A population of RILs generated from a cross between a low-phytate mutant and a wild-type cultivar was used to create a linkage map, confirming the single-gene inheritance of phytate concentration, and mapping the lpa locus to linkage group V (Shunmugam et al., 2015). Further proof for the presence of a significant locus for seed phytate on linkage group V was provided by a study using an expanded set of genetic markers (Gali et al., 2018).
Commercial pea varieties that appear to be naturally low in phytate have also been identified (Amarakoon et al., 2012). In six ‘low-phytate’ commercial lines studied in the USA, phytic acid phosphorus ranged from 1.4 to 2.0 mg g−1, concentrations that are just slightly higher on average than those of the two low-phytate mutants developed by chemical mutagenesis (1.0–1.6 mg g−1) (Liu et al., 2015). Phytate concentrations and its degradation have been investigated in European pea lines (Fredrikson et al., 2001). Phytic acid phosphorus concentrations varied from 1.2 to 3.2 mg g−1 in 27 pea lines used in this study, showing that there is considerable variation in the phytate levels of European pea cultivars (Fredrikson et al., 2001). It should be noted that, in the different studies, phytate levels are cited as the concentration of phytate or as the concentration of phytic acid phosphorus, and different units are used (e.g. mg g−1 and μmol g−1) (Bangar et al., 2017; Fredrikson et al., 2001; Wang et al., 2008; Warkentin et al., 2012), which leads to difficulty when comparing data between studies.
Seed protease inhibitors constitute another family of antinutrients and these can reduce the nutritional quality of pea seeds with implications for various applications in the food and feed industries (Clemente et al., 2015). Trypsin and chymotrypsin inhibitors (TI) interfere with the breakdown of proteins by digestive enzymes in the gut, often leading to a requirement for significant processing of seeds for their inactivation (Clemente et al., 2015). Analysis of 17 Canadian pea lines found mean levels of trypsin inhibitory activity to vary from 2.2 to 7.7 trypsin inhibitor units (TIU) mg−1 of dry matter, with the cultivar ‘Danto’ showing the lowest mean activity (Wang et al., 1998). A subsequent study on a set of four Spanish pea cultivars found trypsin inhibitor activity to range from 6.0 to 15.0 TIU mg−1 (Guillamón et al., 2008), similar to results from a much older study encompassing eight pea cultivars ranging from 3.0 to 11.0 TIU mg−1 (Valdebouze et al., 1980). These studies agree with previous work that showed significant variation in trypsin inhibitor activity among different Pisum genotypes; a study of 63 genotypes showed there to be a tenfold range in the extent of trypsin inhibition (Domoney and Welham, 1992). Variation in genes at the Tri locus in pea were shown to co-segregate with quantitative variation in inhibitor activity (Domoney et al., 1994) and these genes were characterised to determine the genetic basis of the variation in trypsin inhibitor activity (Page et al., 2002). Two cultivars with relatively high trypsin inhibitor activity were found to contain additional copies of the TI genes, in comparison to a cultivar displaying low activity. Additionally, there was significant variation in the promoters of these genes, leading to the suggestion that polymorphisms in coding and promoter sequences was linked to quantitative variation in trypsin inhibitor activity (Page et al., 2002).
The most abundant trypsin inhibitors in pea seeds are Bowman-Birk inhibitors, a specific class of protease inhibitors discovered more than 70 years ago (Birk, 1985). High concentrations of these inhibitors can cause increased secretion of trypsin and chymotrypsin from the pancreas, increasing demand for the amino acids methionine and cysteine (Hill, 2003), and further exacerbating the issue of sulphur-containing amino acid deficiency (Guillamón et al., 2008). Two genes in pea, TI1 and TI2, have been identified as those expressed predominantly in seeds, whereas two additional genes (TI6 and TI9) make a smaller contribution to seed trypsin inhibitory activity, and are also expressed in non-seed organs (Domoney et al., 2002). A study which investigated TILLING mutants and an extensive pea germplasm collection showed the impact of mutations which could reduce trypsin inhibitor activity in pea seeds (Clemente et al., 2015). Among the TILLING mutants, three mutations predicted to affect inhibitor activity were identified; of these, C77Y, a missense mutation of TI1, predicted to affect the extent of intra-molecular disulphide bond formation, reduced trypsin inhibitor activity by 60% (Clemente et al., 2015). The same study used a genetic marker screen on 2822 accessions from the JIC pea germplasm collection and identified a naturally-occurring TI1/TI2 double null mutant, which showed a much greater reduction in seed trypsin inhibitor activity than had been achieved through mutagenesis or transgenesis (Welham and Domoney, 2000). The natural mutant is a Pisum elatius line (John Innes Centre accession JI 262) originating from Turkey, and chemical analysis of its seeds showed an extreme reduction in trypsin inhibitor activity (0.15–0.20 TIU mg−1 meal in comparison to 2.60–2.70 TIU mg−1 in a wild-type control). JI 262 has been crossed successfully with commercial cultivars (Fig. 2), to produce double-null progeny lines for comparative studies (Clemente et al., 2015).
Fig. 2.
Introgression of the trypsin inhibitor null allele from Pisum elatius (JI 262) into a cultivar of Pisum sativum (JI 3253), showing the selection for seed size and seed coat phenotypes in lines carrying the null allele through successive backcross (Bc) generations (F2, F3).
Despite their frequently cited status as antinutrients, Bowman-Birk inhibitors have been shown to have potential health-promoting properties in humans; inhibitors from pea (Clemente et al., 2005), soybean (Clemente et al., 2010) and cowpea (Souza et al., 2014) have been shown to have cytotoxic effects in vitro using a number of cancer cell models. Trypsin/chymotrypsin inhibitors also have beneficial concentrations of sulphur-containing amino acids, containing 20% cysteine residues (Domoney et al., 1993). It has been suggested that, in pea lines containing high concentrations of these protease inhibitors, up to 3% of total seed sulphur-containing amino acids might be contributed by trypsin inhibitors, despite the fact that these proteins are in relatively low abundance in comparison to major globulin and albumin storage proteins (Domoney et al., 1993).
Protease inhibitors including Bowman-Birk inhibitors have also been implicated in plant defence against insect herbivory (Domoney et al., 2002). An inhibitor isoform purified from pea seeds (PsTI-2) has been shown to exhibit toxicity to the pea aphid, Acyrthosiphon pisum, with the chymotrypsin-inhibiting domain of the inhibitor showing greater toxicity than the trypsin-inhibitory one (Rahbé et al., 2003).
Additional compounds in pea may also be classified as antinutrients. For example, saponins can interact with proteins, influencing the taste of foods. Both vicilin and legumin have been shown to bind flavour compounds, although the extent of this binding is governed by temperature and pH conditions (Heng et al., 2004). Saponins are non-volatile triterpene glycosides that occur in pea and other seeds, and can impart a bitter taste during consumption, especially in isolated protein-rich fractions (Heng et al., 2006). Pea lines have been shown to vary significantly in seed saponin concentrations, from 0.7 to 1.5 g kg−1 and 0.0–0.4 g kg−1 for DDMP saponin (containing the moiety 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one) and saponin B, respectively (Heng et al., 2006). Despite the negative effects on consumption, both DDMP and group B saponins have been shown to have health benefits for humans, notably the prevention of high cholesterol in the blood and liver-protecting functions (Yano et al., 2017). A naturally occurring mutant in soybean, sg-5, deficient in group A saponins, has led to the identification of a locus involved in the biosynthesis of different classes of saponin. This mutant has a loss-of-function mutation in a gene encoding the cytochrome P450 enzyme CYP72A69; induced mutations at the same locus also prevented the biosynthesis of group A saponins (Yano et al., 2017). Concentrations of DDMP saponins are higher in sg-5 mutants due to the loss of enzyme activity converting aglycone moieties found in DDMP saponins to moieties found in saponins characterised as group A. A saponin isolate from Medicago truncatula seeds has also been shown to display specific insecticidal activity against the rice weevil (Sitophilus oryzae), an economically important pest of stored grain (Da Silva et al., 2012).
6. Conclusions and future prospects
Pea seeds have high nutritional value with respect to protein, starch, dietary fibre and micronutrients, and many studies have aimed to determine the genetic bases for variation in these nutritional traits. A simplified summary of current knowledge of genetic control of nutritional traits is available in Table 1. For protein and starch, composition as well as total concentrations must be considered, as different fractions of these compounds can convey certain health benefits or functional properties. Protein composition is largely under genetic control, and is complex genetically, with multiple genes involved in the synthesis of individual proteins. QTLs for total seed protein concentration have been found on several linkage groups, and the release of a chromosome-level reference genome assembly for pea has enriched the genetic information for loci encoding individual pea seed proteins. However, the prevalence of repetitive sequences in the pea genome causes difficulties when conducting genomic analysis and assembling genomes such as pea (Kreplak et al., 2019) and greater clarity will be achieved through development of a pan-genome for pea. There is a good deal of knowledge available for genetic loci affecting pea seed starch content and composition, most notably a mutation in the SBEI gene causing wrinkled seeds with higher concentrations of resistant starch and potential health benefits, as well as other mutations affecting the starch biosynthesis pathway. Significant QTLs in multiple RIL populations have been identified for fibre on linkage group VII. SNPs linked to the concentration of dietary fibre were also identified on chromosomes 6 and 7 (LG II and VII, respectively), using a diverse panel of pea accessions.
Table 1.
A simplified summary of the genetic basis for variation in seed quality traits in pea.
Note that the numbering of linkage groups has changed during the research period covered (Ellis and Poyser, 2002). Where possible, currently accepted linkage group numbers are cited, with reference to chromosome number (Kreplak et al., 2019) for some studies. Linkage groups cited for several starch-related loci are unpublished data.
Hyperaccumulation mutants for iron have been discovered in pea (dgl, brz), but the usefulness of these lines is diminished by the iron-toxicity symptoms the plants display. Nevertheless, these hyperaccumulation mutants are important resources and will help increase understanding of micronutrient uptake and translocation within the plant. Great diversity in seed iron concentrations has additionally been found in landraces and significant QTLs have been located on multiple linkage groups. QTLs for seed zinc concentration have also been identified. When examining nutrient concentrations, it is also important to consider antinutrients, which can reduce the bioavailability of many nutritional compounds in the gut. For a number of relevant antinutrients in pea, mutants have already been discovered, either through chemical mutagenesis (e.g. phytate) or in natural germplasm (e.g. trypsin inhibitors).
Pleiotropic effects must be considered when applying knowledge of pea genetics to improve current cultivars, as changes in certain seed properties may have large impacts on agronomic traits. For example, if attempting to increase pea seed protein content or the concentration of resistant starch, then ideally yields must not be eventually impacted, in order to maintain attractiveness to growers. Much recent research has identified QTLs and SNPs that correlate with certain nutritional traits; further investigation into these markers using genome sequences will support fundamental research into the causal genes conveying these effects and support breeding programmes aimed at a variety of end uses.
Contribution
G.H.J. Robinson drafted the article; both authors contributed to editing and refining the text.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding is acknowledged from UKRI (BBSRC) for the John Innes Centre Institute Strategic Programme grant (BBS/E/J/000PR799) and from Defra for the Pulse Crop Genetic Improvement Network (CH0111). We are grateful to Carol Moreau, John Innes Centre (JIC), and Dr Katerina Petropoulou, Imperial College, London, for images and data, respectively, presented in Fig. 1 and to Tracey Rayner, JIC, for providing the image for Fig. 2. We thank Dr Noel Ellis, JIC, for advice on Table 1.
References
- Amarakoon D., Thavarajah D., Gupta D., McPhee K., DeSutter T., Thavarajah P. Genetic and environmental variation of seed iron and food matrix factors of North-Dakota-grown field peas (Pisum sativum L.) J. Food Compos. Anal. 2015;37:67–74. doi: 10.1016/j.jfca.2014.09.001. [DOI] [Google Scholar]
- Amarakoon D., Thavarajah D., McPhee K., Thavarajah P. Iron-, zinc-, and magnesium-rich field peas (Pisum sativum L.) with naturally low phytic acid: a potential food-based solution to global micronutrient malnutrition. J. Food Compos. Anal. 2012;27:8–13. doi: 10.1016/j.jfca.2012.05.007. [DOI] [Google Scholar]
- Antonets K.S., Belousov M.V., Sulatskaya A.I., Belousova M.E., Kosolapova A.O., Sulatsky M.I., Andreeva E.A., Zykin P.A., Malovichko Y.V., Shtark O.Y., Lykholay A.N., Volkov K.V., Kuznetsova I.M., Turoverov K.K., Kochetkova E.Y., Bobylev A.G., Usachev K.S., Demidov O.N., Tikhonovich I.A., Nizhnikov A.A. Accumulation of storage proteins in plant seeds is mediated by amyloid formation. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp N.G., Björck I., Holm J., Nyman M., Siljeström M. Enzyme resistant starch fractions and dietary fibre. Scand. J. Gastroenterol. Suppl. 1987;129:29–32. doi: 10.3109/00365528709095847. [DOI] [PubMed] [Google Scholar]
- Bangar P., Glahn R.P., Liu Y., Arganosa G.C., Whiting S., Warkentin T.D. Iron bioavailability in field pea seeds: correlations with iron, phytate, and carotenoids. Crop Sci. 2017;57:891–902. doi: 10.2135/cropsci2016.08.0682. [DOI] [Google Scholar]
- Becker R., Manteuffel R., Neumann D., Scholz G. Excessive iron accumulation in the pea mutants dgl and brz: subcellular localization of iron and ferritin. Planta. 1998;207:217–223. doi: 10.1007/s004250050475. [DOI] [Google Scholar]
- Bhattacharyya M.K., Smith A.M., Ellis T.H.N., Hedley C., Martin C., Lane C. The wrinkled-seed character of pea described by Mendel is caused by a transposon-like insertion in a gene encoding starch-branching enzyme. Cell. 1990;60:115–122. doi: 10.1002/9781118191323.ch4. [DOI] [PubMed] [Google Scholar]
- Birk Y. The Bowman‐Birk inhibitor. Trypsin‐ and chymotrypsin‐inhibitor from soybeans. Int. J. Pept. Protein Res. 1985;25:113–131. doi: 10.1111/j.1399-3011.1985.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Bogracheva T.Y., Cairns P., Noel T.R., Hulleman S., Wang T.L., Morris V.J., Ring S.G., Hedley C.L. Effect of mutant genes at the r, rb, rug3, rug4, rug5 and lam loci on the granular structure and physico-chemical properties of pea seed starch. Carbohydr. Polym. 1999;39:303–314. doi: 10.1016/S0144-8617(99)00020-X. [DOI] [Google Scholar]
- Bourgeois M., Jacquin F., Cassecuelle F., Savois V., Belghazi M., Aubert G., Quillien L., Huart M., Marget P., Burstin J. A PQL (protein quantity loci) analysis of mature pea seed proteins identifies loci determining seed protein composition. Proteomics. 2011;11:1581–1594. doi: 10.1002/pmic.201000687. [DOI] [PubMed] [Google Scholar]
- Bourgeois M., Jacquin F., Savois V., Sommerer N., Labas V., Henry C., Burstin J. Dissecting the proteome of pea mature seeds reveals the phenotypic plasticity of seed protein composition. Proteomics. 2009;9:254–271. doi: 10.1002/pmic.200700903. [DOI] [PubMed] [Google Scholar]
- Brummer Y., Kaviani M., Tosh S.M. Structural and functional characteristics of dietary fibre in beans, lentils, peas and chickpeas. Food Res. Int. 2015;67:117–125. doi: 10.1016/j.foodres.2014.11.009. [DOI] [Google Scholar]
- Burstin J., Marget P., Huart M., Moessner A., Mangin B., Duchene C., Desprez B., Munier-Jolain N., Duc G. Developmental genes have pleiotropic effects on plant morphology and source capacity, eventually impacting on seed protein content and productivity in pea. Plant Physiol. 2007;144:768–781. doi: 10.1104/pp.107.096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnovale E., Lugaro E., Lombardi-Boccia G. Phytic acid in faba bean and pea: effect on protein availability. Cereal Chem. 1988;65:114–117. [Google Scholar]
- Casey R., Christou P., Domoney C., Hedley C., Hitchin E., Parker M., Stoger E., Wang T., Zasiura C. Expression of legumin and vicilin genes in pea mutants and the production of legumin in transgenic plants. Nahrung-Food. 2001;45:385–387. doi: 10.1002/1521-3803(20011001)45:6%3C385::AID-FOOD385%3E3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Casey R., Domoney C. Pea globulins. In: Shewry P.R., Casey R., editors. Seed Proteins. 1999. pp. 171–208. [Google Scholar]
- Chinoy C., Welham T., Turner L., Moreau C., Domoney C. The genetic control of seed quality traits: effects of allelic variation at the Tri and Vc-2 genetic loci in Pisum sativum L. Euphytica. 2011;180:107–122. doi: 10.1007/s10681-011-0363-8. [DOI] [Google Scholar]
- Clemente A., Arques M.C., Dalmais M., Le Signor C., Chinoy C., Olias R., Rayner T., Isaac P.G., Lawson D.M., Bendahmane A., Domoney C. Eliminating anti-nutritional plant food proteins: the case of seed protease inhibitors in pea. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente A., Gee J.M., Johnson I.T., MacKenzie D.A., Domoney C. Pea (Pisum sativum L.) protease inhibitors from the Bowman - birk class influence the growth of human colorectal adenocarcinoma HT29 cells in vitro. J. Agric. Food Chem. 2005;53:8979–8986. doi: 10.1021/jf051528w. [DOI] [PubMed] [Google Scholar]
- Clemente A., Moreno F.J., Marín-Manzano M.C., Jiménez E., Domoney C. The cytotoxic effect of Bowman-Birk isoinhibitors, IBB1 and IBBD2, from soybean (Glycine max) on HT29 human colorectal cancer cells is related to their intrinsic ability to inhibit serine proteases. Mol. Nutr. Food Res. 2010;54:396–405. doi: 10.1002/mnfr.200900122. [DOI] [PubMed] [Google Scholar]
- Craig J., Lloyd J.R., Tomlinson K., Barber L., Edwards A., Wang T.L., Martin C., Hedley C.L., Smith A.M. Mutations in the gene encoding starch synthase II profoundly alter amylopectin structure in pea embryos. Plant Cell. 1998;10:413–426. doi: 10.1016/S1369-5266(98)80075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy R.R.D., Hoque M.S., Gatehouse J.A., Boulter D. The major albumin proteins from pea (Pisum sativum L) Biochem. J. 1984;218:795–803. doi: 10.1042/bj2180795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva P., Eyraud V., Carre-Pierrat M., Sivignon C., Rahioui I., Royer C., Gressent F. High toxicity and specificity of the saponin 3-GlcA-28-AraRhaxyl- medicagenate, from Medicago truncatula seeds, for Sitophilus oryzae. BMC Chem. Biol. 2012;12:3. doi: 10.1186/1472-6769-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida Costa G.E., Da Silva Queiroz-Monici K., Pissini Machado Reis S.M., De Oliveira A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006;94:327–330. doi: 10.1016/j.foodchem.2004.11.020. [DOI] [Google Scholar]
- Demirbaş A. Micro and macronutrients diversity in Turkish pea (Pisum sativum) germplasm. Int. J. Agric. Biol. 2018;20:701–710. doi: 10.17957/IJAB/15.0545. [DOI] [Google Scholar]
- Diapari M., Sindhu A., Warkentin T.D., Bett K., Tar’an B. Population structure and marker-trait association studies of iron, zinc and selenium concentrations in seed of field pea (Pisum sativum L.) Mol. Breed. 2015;35:30. doi: 10.1007/s11032-015-0252-2. [DOI] [Google Scholar]
- Díaz C.L., Melchers L.S., Hooykaas P.J.J., Lugtenberg B.J.J., Kijne J.W. Root lectin as a determinant of host-plant specificity in the Rhizobium-legume symbiosis. Nature. 1989 doi: 10.1038/338579a0. [DOI] [Google Scholar]
- Domoney C., Ellis T.H.N., Davies D.R. Organization and mapping of legumin genes in Pisum. Mol. Gen. Genet. 1986;202:280–285. doi: 10.1007/BF00331651. [DOI] [Google Scholar]
- Domoney C., Knox M., Moreau C., Ambrose M., Palmer S., Smith P., Christodoulou V., Isaac P.G., Hegarty M., Blackmore T., Swain M., Ellis N. Exploiting a fast neutron mutant genetic resource in Pisum sativum (pea) for functional genomics. Funct. Plant Biol. 2013;40 doi: 10.1071/FP13147. [DOI] [PubMed] [Google Scholar]
- Domoney C., Welham T. Trypsin inhibitors in Pisum: variation in amount and pattern of accumulation in developing seed. Seed Sci. Res. 1992;2:147–154. [Google Scholar]
- Domoney C., Welham T., Ellis N., Hellens R. Inheritance of qualitative and quantitative trypsin inhibitor variants in Pisum. Theor. Appl. Genet. 1994;89:387–391. doi: 10.1007/BF00225370. [DOI] [PubMed] [Google Scholar]
- Domoney C., Welham T., Ellis N., Mozzanega P., Turner L. Three classes of proteinase inhibitor gene have distinct but overlapping patterns of expression in Pisum sativum plants. Plant Mol. Biol. 2002;48:319–329. doi: 10.1023/A:1013379430582. [DOI] [PubMed] [Google Scholar]
- Domoney C., Welham T., Sidebottom C. Purification and characterization of Pisum seed trypsin inhibitors. J. Exp. Bot. 1993;44:701–709. doi: 10.1093/jxb/44.4.701. [DOI] [Google Scholar]
- Ellis T.H.N., Domoney C., Castleton J., Cleary W., Davies D.R. Vicilin genes of pisum. Mol. Gen. Genet. 1986;205:164. doi: 10.1007/BF02428047. [DOI] [Google Scholar]
- Ellis T.H.N., Poyser S.J. An integrated and comparative view of pea genetic and cytogenetic maps: research review. New Phytol. 2002;153:17–25. doi: 10.1046/j.0028-646X.2001.00302.x. [DOI] [Google Scholar]
- Ellis N., Hattori C., Cheema J., Donarski J., Charlton A., Dickinson M., Venditti G., Kaló P., Szabó Z., Kiss G.B., Domoney C. NMR metabolomics defining genetic variation in pea seed metabolites. Front. Plant Sci. 2018;9:1022. doi: 10.3389/fpls.2018.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyraud V., Karaki L., Rahioui I., Sivignon C., Da Silva P., Rahbé Y., Royer C., Gressent F. Expression and biological activity of the cystine knot bioinsecticide PA1b (Pea Albumin 1 subunit b) PLoS One. 2013;8:1–9. doi: 10.1371/journal.pone.0081619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations FAOSTAT statistical database. 2020. http://www.fao.org/faostat/en/#data/QC (date accessed 25/10/20)
- Forster C., North H., Afzal N., Domoney C., Hornostaj A., Robinson D.S., Casey R. Molecular analysis of a null mutant for pea (Pisum sativum L.) seed lipoxygenase-2. Plant Mol. Biol. 1999;39:1209–1220. doi: 10.1023/A:1006173313548. [DOI] [PubMed] [Google Scholar]
- Fredrikson M., Alminger M.L., Carlsson N.G., Sandberg A.S. Phytate content and phytate degradation by endogenous phytase in pea (Pisum sativum) J. Sci. Food Agric. 2001;81:1139–1144. doi: 10.1002/jsfa.918. [DOI] [Google Scholar]
- Gali K.K., Liu Y., Sindhu A., Diapari M., Shunmugam A.S.K., Arganosa G., Daba K., Caron C., Lachagari R.V.B., Tar’an B., Warkentin T.D. Construction of high-density linkage maps for mapping quantitative trait loci for multiple traits in field pea (Pisum sativum L.) BMC Plant Biol. 2018;18:1–25. doi: 10.1186/s12870-018-1368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gali K.K., Sackville A., Tafesse E.G., Lachagari V.B.R., McPhee K., Hybl M., Mikić A., Smýkal P., McGee R., Burstin J., Domoney C., Ellis T.H.N., Tar’an B., Warkentin T.D. Genome-wide association mapping for agronomic and seed quality traits of field pea (Pisum sativum L.) Front. Plant Sci. 2019;10:1–19. doi: 10.3389/fpls.2019.01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García M.J., Romera F.J., Stacey M.G., Stacey G., Villar E., Alcántara E., Pérez-Vicente R. Shoot to root communication is necessary to control the expression of iron-acquisition genes in Strategy I plants. Planta. 2013;237:65–75. doi: 10.1007/s00425-012-1757-0. [DOI] [PubMed] [Google Scholar]
- Gaur V., Qureshi I.A., Singh A., Chanana V., Salunke D.M. Crystal structure and functional insights of hemopexin fold protein from grass pea. Plant Physiol. 2010;152:1842–1850. doi: 10.1104/pp.109.150680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez M.D., Beltrán J.P., Cañas L.A. The pea END1 promoter drives anther-specific gene expression in different plant species. Planta. 2004;219:967–981. doi: 10.1007/s00425-004-1300-z. [DOI] [PubMed] [Google Scholar]
- Gottschalk W. Improvement of the selection value of gene dgl through recombination. Pisum Newslett. 1987;19:9–11. [Google Scholar]
- Grillet L., Ouerdane L., Flis P., Hoang M.T.T., Isaure M.-P., Lobinski R., Curie C., Mari S. Ascorbate efflux as a new strategy for iron reduction and transport in plants. J. Biol. Chem. 2013;289:2515–2525. doi: 10.1074/jbc.m113.514828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusak M.A., Welch R.M., Kochian L.V. Physiological characterization of a single-gene mutant of Pisum sativum exhibiting excess iron accumulation. I. Root iron reduction and iron uptake. Plant Physiol. 1990;93:976–981. doi: 10.1104/pp.93.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamón E., Pedrosa M.M., Burbano C., Cuadrado C., Sánchez M. de C., Muzquiz M. The trypsin inhibitors present in seed of different grain legume species and cultivar. Food Chem. 2008;107:68–74. doi: 10.1016/j.foodchem.2007.07.029. [DOI] [Google Scholar]
- Harrison C.J., Hedley C.L., Wang T.L. Evidence that the rug3 locus of pea (Pisum sativum L.) encodes plastidial phosphoglucomutase confirms that the imported substrate for starch synthesis in pea amyloplasts is glucose-6-phosphate. Plant J. 1998;13:753–762. doi: 10.1046/j.1365-313X.1998.00077.x. [DOI] [Google Scholar]
- Heng L., Van Koningsveld G.A., Gruppen H., Van Boekel M.A.J.S., Vincken J.P., Roozen J.P., Voragen A.G.J. Protein-flavour interactions in relation to development of novel protein foods. Trends Food Sci. Technol. 2004;15:217–224. doi: 10.1016/j.tifs.2003.09.018. [DOI] [Google Scholar]
- Heng L., Vincken J.P., Van Koningsveld G., Legger A., Gruppen H., Van Boekel T., Roozen J., Voragen F. Bitterness of saponins and their content in dry peas. J. Sci. Food Agric. 2006;86:1225–1231. doi: 10.1002/jsfa.2473. [DOI] [Google Scholar]
- Hill G.D. Encyclopedia of Food Sciences and Nutrition. 2003. Plant antinutritional factors | characteristics; pp. 4578–4587. [DOI] [Google Scholar]
- Hurrell R., Egli I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010;91:1461S–1467S. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- Hylton C., Smith A.M. The rb mutation of peas causes structural and regulatory changes in ADP glucose pyrophosphorylase from developing embryos. Plant Physiol. 1992;99:1626–1634. doi: 10.1104/pp.99.4.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irzykowska L., Wolko B. Interval mapping of QTLs controlling yield-related traits and seed protein content in Pisum sativum. J. Appl. Genet. 2004;45:297–306. [PubMed] [Google Scholar]
- Jha A.B., Tar’an B., Diapari M., Warkentin T.D. SNP variation within genes associated with amylose, total starch and crude protein concentration in field pea. Euphytica. 2015;206:459–471. doi: 10.1007/s10681-015-1510-4. [DOI] [Google Scholar]
- Kneen B.E., Larue T.A., Welch R.M., Weeden N.F. Pleiotropic effects of brz: a mutation in Pisum sativum (L) cv. “Sparkle” conditioning decreased nodulation and increased iron uptake and leaf necrosis. Plant Physiol. 1990;93:717–722. doi: 10.1104/pp.93.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozłowska H. Nutrition. In: Hedley C.L., editor. Carbohydrates in Grain Legume Seeds: Improving Nutritional Quality and Agronomic Characteristics. CABI Publishing; Wallingford: 2001. pp. 61–89. [Google Scholar]
- Krajewski P., Bocianowski J., Gawłowska M., Kaczmarek Z., Pniewski T., Święcicki W., Wolko B. QTL for yield components and protein content: a multienvironment study of two pea (Pisum sativum L.) populations. Euphytica. 2012;183:323–336. doi: 10.1007/s10681-011-0472-4. [DOI] [Google Scholar]
- Kreplak J., Madoui M., Cápal P., Novák P., Labadie K., Aubert G., Bayer P.E., Gali K.K., Syme R.A., Main D., Klein A., Bérard A., Vrbová I., Fournier C., Agata L., Belser C., Berrabah W., Toegelová H., Milec Z., Vrána J., Lee H., Kougbeadjo A., Térézol M., Huneau C., Turo C.J., Mohellibi N., Neumann P., Falque M., Gallardo K., Mcgee R., Tar B., Bendahmane A., Aury J., Batley J., Paslier M. Le, Ellis N., Warkentin T.D., Coyne C.J., Salse J., Edwards D., Lichtenzveig J., Macas J., Doležel J., Wincker P., Burstin J. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019;51:1411–1422. doi: 10.1038/s41588-019-0480-1. [DOI] [PubMed] [Google Scholar]
- Kyle R.A. Amyloidosis: a convoluted story. Br. J. Nutr. 2001;114:529–538. doi: 10.1046/j.1365-2141.2001.02999.x. [DOI] [PubMed] [Google Scholar]
- Le Gall M., Quillien L., Sève B., Guéguen J., Lallès J.P. Weaned piglets display low gastrointestinal digestion of pea (Pisum sativum L.) lectin and pea albumin 2. J. Anim. Sci. 2007;85:2972–2981. doi: 10.2527/jas.2006-795. [DOI] [PubMed] [Google Scholar]
- Le Signor C., Aimé D., Bordat A., Belghazi M., Labas V., Gouzy J., Young N.D., Prosperi J.-M., Leprince O., Thompson R.D., Buitink J., Burstin J., Gallardo K. Genome-wide association studies with proteomics data reveal genes important for synthesis, transport and packaging of globulins in legume seeds. New Phytol. 2017;214:1597–1613. doi: 10.1111/nph.14500. [DOI] [PubMed] [Google Scholar]
- Liu X., Glahn R.P., Arganosa G.C., Warkentin T.D. Iron bioavailability in low phytate pea. Crop Sci. 2015;55:320. doi: 10.2135/cropsci2014.06.0412. [DOI] [Google Scholar]
- Lockyer S., Nugent A.P. Health effects of resistant starch. Nutr. Bull. 2017;42:10–41. doi: 10.1111/nbu.12244. [DOI] [Google Scholar]
- Lönnerdal B., Bryant A., Liu X., Theil E.C. Iron absorption from soybean ferritin in nonanemic women. Am. J. Clin. Nutr. 2006;83:103–107. doi: 10.1093/ajcn/83.1.103. [DOI] [PubMed] [Google Scholar]
- Lonnie M., Hooker E., Brunstrom J.M., Corfe B.M., Green M.A., Watson A.W., Williams E.A., Stevenson E.J., Penson S., Johnstone A.M. Protein for life: review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients. 2018;10:360. doi: 10.3390/nu10030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis S., Delobel B., Gressent F., Rahioui I., Quillien L., Vallier A., Rahbé Y. Molecular and biological screening for insect-toxic seed albumins from four legume species. Plant Sci. 2004;167:705–714. doi: 10.1016/j.plantsci.2004.04.018. [DOI] [Google Scholar]
- Lycett G.W., Croy R.R.D., Shirsat A.H., Boulter D. The complete nucleotide sequence of a legumin gene from pea (Pisum sativum L.) Nucleic Acids Res. 1984;12:4493–4506. doi: 10.1093/nar/12.11.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett G.W., Delauney A.J., Gatehouse J.A., Gilroy J., Croy R.R.D., Boulter D. The vicilin gene family of pea (Pisum sativum L.): a complete cDNA coding sequence for preprovicilin. Nucleic Acids Res. 1983;11:2367–2380. doi: 10.1093/nar/11.8.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Coyne C.J., Grusak M.A., Mazourek M., Cheng P., Main D., McGee R.J. Genome-wide SNP identification, linkage map construction and QTL mapping for seed mineral concentrations and contents in pea (Pisum sativum L.) BMC Plant Biol. 2017;17:1–17. doi: 10.1186/s12870-016-0956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marentes E., Grusak M.A. Iron transport and storage within the seed coat and embryo of developing seeds of pea (Pisum sativum L.) Physiol. Biochem. 1998;8:367–375. doi: 10.1017/S0960258500004293. [DOI] [Google Scholar]
- Mendel G. Versuche über Pflanzenhybriden. Verhandlungen des naturforschenden Vereines Brünn, Bd. IV für das Jahr 1865 Abhandlung. 1886. 3-47. [DOI]
- Mertens C., Dehon L., Bourgeois A., Verhaeghe-Cartrysse C., Blecker C. Agronomical factors influencing the legumin/vicilin ratio in pea (Pisum sativum L.) seeds. J. Sci. Food Agric. 2012;92:1591–1596. doi: 10.1002/jsfa.4738. [DOI] [PubMed] [Google Scholar]
- Moore K.L., Rodríguez-Ramiro I., Jones E.R., Jones E.J., Rodríguez-Celma J., Halsey K., Domoney C., Shewry P.R., Fairweather-Tait S., Balk J. The stage of seed development influences iron bioavailability in pea (Pisum sativum L.) Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-25130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbigin E.J., DeLumen B.O., Chandler P.M., Gould A., Blagrove R.J., March J.F., Kortt A.A., Higgins T.J.V. Pea convicilin: structure and primary sequence of the protein and expression of a gene in the seeds of transgenic tobacco. Planta. 1990;180:461–470. doi: 10.1007/BF02411442. [DOI] [PubMed] [Google Scholar]
- North H., Casey R., Domoney C. Inheritance and mapping of seed lipoxygenase polypeptides in Pisum. Theor. Appl. Genet. 1989;77:805–808. doi: 10.1007/BF00268330. [DOI] [PubMed] [Google Scholar]
- Page D., Aubert G., Duc G., Welham T., Domoney C. Combinatorial variation in coding and promoter sequences of genes at the Tri locus in Pisum sativum accounts for variation in trypsin inhibitor activity in seeds. Mol. Genet. Genomics. 2002;267:359–369. doi: 10.1007/s00438-002-0667-4. [DOI] [PubMed] [Google Scholar]
- Perfecto A., Rodriguez-Ramiro I., Rodriguez-Celma J., Sharp P., Balk J., Fairweather-Tait S. Pea ferritin stability under gastric pH conditions determines the mechanism of iron uptake in Caco-2 cells. J. Nutr. 2018;148:1229–1235. doi: 10.1093/jn/nxy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petropoulou K., Chambers E.S., Morrison D.J., Preston T., Godsland I.F., Wilde P., Narbad A., Parker R., Salt L., Morris V.J., Domoney C., Persaud S.J., Holmes E., Penson S., Watson J., Stocks M., Buurman M., Luterbacher M., Frost G. Identifying crop variants with high resistant starch content to maintain healthy glucose homeostasis. Nutr. Bull. 2016;41:372–377. doi: 10.1111/nbu.12240. [DOI] [Google Scholar]
- Petropoulou K., Salt L.J., Edwards C.H., Warren F.J., Garcia-Perez I., Chambers E.S., Alshaalan R., Khatib M., Perez-Moral N., Cross K.L., Kellingray L., Stanley R., Koev T., Khimyak Y.Z., Narbad A., Penney N., Serrano-Contreras J.I., Charalambides M.N., Blanco J.M., Seoane R.C., McDonald J.A.K., Marchesi J.R., Holmes E., Godsland I.F., Morrison D.J., Preston T., Domoney C., Wilde P.J., Frost G.S. A natural mutation in Pisum sativum L. (pea) alters starch assembly and improves glucose homeostasis in humans. Nat. Food. 2020 doi: 10.1038/s43016-020-00159-8. [DOI] [PubMed] [Google Scholar]
- PGRO Blueprint for UK Pulses in a post-Brexit world. 2018. https://www.pgro.org/blueprint-for-uk-pulses/ Executive summary. 1-20.
- PGRO Recommended lists | PGRO. 2020. https://www.pgro.org/recommended-lists-2017/
- Poore J., Nemecek T. Reducing food's environmental impacts through producers and consumers. Science (80- 2018;360:987–992. doi: 10.1126/science.aaq0216. [DOI] [PubMed] [Google Scholar]
- Rahbé Y., Ferrasson E., Rabesona H., Quillien L. Toxicity to the pea aphid Acyrthosiphon pisum of anti-chymotrypsin isoforms and fragments of Bowman-Birk protease inhibitors from pea seeds. Insect Biochem. Mol. Biol. 2003;33:299–306. doi: 10.1016/S0965-1748(02)00244-8. [DOI] [PubMed] [Google Scholar]
- Ratnayake W.S., Hoover R., Warkentin T. Pea starch: composition, structure and properties - a review. Starch Staerke. 2002;54:217–234. doi: 10.1002/1521-379X(200206)54:6%3C217::AID-STAR217%3E3.0.CO;2-R. [DOI] [Google Scholar]
- Ravet K., Touraine B., Boucherez J., Briat J.F., Gaymard F., Cellier F. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J. 2009;57:400–412. doi: 10.1111/j.1365-313X.2008.03698.x. [DOI] [PubMed] [Google Scholar]
- Ray H., Bett K., Tar’an B., Vandenberg A., Thavarajah D., Warkentin T. Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Canada. Crop Sci. 2014;54:1698–1708. doi: 10.2135/cropsci2013.08.0568. [DOI] [Google Scholar]
- Rayner T., Moreau C., Ambrose M., Isaac P.G., Ellis N., Domoney C. Genetic variation controlling wrinkled seed phenotypes in Pisum: how lucky was Mendel? Int. J. Mol. Sci. 2017;18:1205. doi: 10.3390/ijms18061205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner T., Moreau C., Isaac P.G., Domoney C. Genetic diversity and strategies for seed quality enhancement in Pisum (pea) In: Carlton R., Smith B., Topp K., Watson C., editors. Aspects of Applied Biology 138: Advances in Legume Science and Practice. The Association of Applied Biologists; Wellesbourne: 2018. pp. 141–148. [Google Scholar]
- Reidt W., Wohlfarth T., Ellerström M., Czihal A., Tewes A., Ezcurra I., Rask L., Bäumlein H. Gene regulation during late embryogenesis: the RY motif of maturation-specific gene promoters is a direct target of the FUS3 gene product. Plant J. 2000;21:401–408. doi: 10.1046/j.1365-313X.2000.00686.x. [DOI] [PubMed] [Google Scholar]
- Robinson G.H.J., Balk J., Domoney C. Improving pulse crops as a source of protein, starch and micronutrients. Nutr. Bull. 2019;44:202–215. doi: 10.1111/nbu.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran R.P., Grusak M.A. Whole shoot mineral partitioning and accumulation in pea (Pisum sativum) Front. Plant Sci. 2014;5:1–8. doi: 10.3389/fpls.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestili F., Janni M., Doherty A., Botticella E., D'Ovidio R., Masci S., Jones H.D., Lafiandra D. Increasing the amylose content of durum wheat through silencing of the SBEIIa genes. BMC Plant Biol. 2010;10:144. doi: 10.1186/1471-2229-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Hou H., Ding C., Bing D.-J., Lu Z.-X. Protein content correlates with starch morphology, composition and physicochemical properties in field peas. Can. J. Plant Sci. 2016;96:404–412. doi: 10.1139/cjps-2015-0231. [DOI] [Google Scholar]
- Shewry P.R., Napier J.A., Tatham A.S. Seed storage proteins: structures and biosynthesis. Plant Cell. 1995;7:945–956. doi: 10.2307/3870049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shunmugam A.S., Bock C., Arganosa G., Georges F., Gray G., Warkentin T. Accumulation of phosphorus-containing compounds in developing seeds of low-phytate pea (Pisum sativum L.) mutants. Plants. 2014;4:1–26. doi: 10.3390/plants4010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shunmugam A.S., Liu X., Stonehouse R., Tar’An B., Bett K.E., Sharpe A.G., Warkentin T.D. Mapping seed phytic acid concentration and iron bioavailability in a pea recombinant inbred line population. Crop Sci. 2015;55:828–836. doi: 10.2135/cropsci2014.08.0544. [DOI] [Google Scholar]
- Souza L.D.C., Camargo R., Demasi M., Santana J.M., De Sá C.M., De Freitas S.M. Effects of an anticarcinogenic Bowman-Birk protease inhibitor on purified 20S proteasome and MCF-7 breast cancer cells. PLoS One. 2014;9:1–10. doi: 10.1371/journal.pone.0086600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld H., Gerber P., Wassenaar T., Castel V., Rosales M., de Haan C. Food and Agriculture Organization of the United Nations; Rome: 2006. Livestock's long shadow: environmental issues and options.http://www.fao.org/3/a0701e/a0701e00.htm [Google Scholar]
- Stephen A.M., Champ M.M.-J., Cloran S.J., Fleith M., van Lieshout L., Mejborn H., Burley V.J. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr. Res. Rev. 2017;30:149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- Stevenson E.J., Watson A.W., Brunstrom J.M., Johnstone A.M., Green M.A., Corfe B.M., Williams E.A. Protein for life: towards a focussed dietary framework for healthy ageing. Nutr. Bull. 2018;43:97–102. doi: 10.1111/nbu.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir R., Ellis P.R., Bogracheva T.Y., Meares-Taylor C., Butterworth P.J. Study of the structure and properties of native and hydrothermally processed wild-type, lam and r variant pea starches that affect amylolysis of these starches. Biomacromolecules. 2011;12:123–133. doi: 10.1021/bm101070r. [DOI] [PubMed] [Google Scholar]
- Tao A., Afshar R.K., Huang J., Mohammed Y.A., Espe M., Chen C. Variation in yield, starch, and protein of dry pea grown across Montana. Agron. J. 2017;109:1491–1501. doi: 10.2134/agronj2016.07.0401. [DOI] [Google Scholar]
- Tar’an B., Warkentin T., Somers D.J., Miranda D., Vandenberg A., Blade S., Bing D. Identification of quantitative trait loci for grain yield, seed protein concentration and maturity in field pea (Pisum sativum L.) Euphytica. 2004;136:297–306. doi: 10.1023/B:EUPH.0000032721.03075.a0. [DOI] [Google Scholar]
- Tömösközi S., Lásztity R., Haraszi R., Baticz O. Isolation and study of the functional properties of pea proteins. Nahrung-Food. 2001;45:399–401. doi: 10.1002/1521-3803(20011001)45:6<399::AID-FOOD399>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Tosh S.M., Yada S. Dietary fibres in pulse seeds and fractions: characterization, functional attributes, and applications. Food Res. Int. 2010;43:450–460. doi: 10.1016/j.foodres.2009.09.005. [DOI] [Google Scholar]
- Turner L., Hellens R.P., Lee D., Ellis T.H.N. Genetic aspects of the organization of legumin genes in pea. Plant Mol. Biol. 1993;22:101–112. doi: 10.1007/BF00038999. [DOI] [PubMed] [Google Scholar]
- Turner S.R., Barratt D.H.P., Casey R. vol. 14. 1990. The effect of different alleles at the r locus on the synthesis of seed storage proteins; pp. 793–803. (Pisum sativum. Plant Mol. Biol). [DOI] [PubMed] [Google Scholar]
- Tzitzikas E.N., Vincken J.P., De Groot J., Gruppen H., Visser R.G.F. Genetic variation in pea seed globulin composition. J. Agric. Food Chem. 2006;54:425–433. doi: 10.1021/jf0519008. [DOI] [PubMed] [Google Scholar]
- Valdebouze P., Bergeron E., Gaborit T., Delort-Laval J. Content and distribution of trypsin inhibitors and hemagglutinins in some legume seeds. Can. J. Plant Sci. 1980;60:695–701. doi: 10.4141/cjps80-097. [DOI] [Google Scholar]
- Vigeolas H., Chinoy C., Zuther E., Blessington B., Geigenberger P., Domoney C. Combined metabolomic and genetic approaches reveal a link between the polyamine pathway and albumin 2 in developing pea seeds. Plant Physiol. 2008;146:74–82. doi: 10.1104/pp.107.111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vioque J., Clemente A., Sánchez-Vioque R., Pedroche J., Bautista J., Millán F. Comparative study of chickpea and pea PA2 albumins. J. Agric. Food Chem. 1998;46:3609–3613. doi: 10.1021/jf980351l. [DOI] [Google Scholar]
- Wang N., Hatcher D.W., Gawalko E.J. Effect of variety and processing on nutrients and certain anti-nutrients in field peas (Pisum sativum) Food Chem. 2008;111:132–138. doi: 10.1016/j.foodchem.2008.03.047. [DOI] [Google Scholar]
- Wang T.L., Hadavizideh A., Harwood A., Welham T.J., Harwood W.A., Faulks R., Hedley C.L. An analysis of seed development in Pisum sativum. XIII. The chemical induction of storage product mutants. Plant Breed. 1990;105:311–320. doi: 10.1111/j.1439-0523.1990.tb01290.x. [DOI] [Google Scholar]
- Wang T.L., Hedley C.L. Seed development in peas: knowing your three ‘r's’ (or four, or five) Seed Sci. Res. 1991;1:3–14. doi: 10.1017/S096025850000057X. [DOI] [Google Scholar]
- Wang X., Warkentin T.D., Briggs C.J., Oomah B.D., Campbell C.G., Woods S. Trypsin inhibitor activity in field pea (Pisum sativum L.) and grass pea (Lathyrus sativus L.) J. Agric. Food Chem. 1998;46:2620–2623. doi: 10.1021/jf971007b. [DOI] [Google Scholar]
- Warkentin T.D., Delgerjav O., Arganosa G., Rehman A.U., Bett K.E., Anbessa Y., Rossnagel B., Raboy V. Development and characterization of low-phytate pea. Crop Sci. 2012;52:74–78. doi: 10.2135/cropsci2011.05.0285. [DOI] [Google Scholar]
- Waters B.M., Blevins D.G., Eide D.J. Characterization of FRO1, a pea ferric-chelate reductase involved in root iron acquisition. Plant Physiol. 2002;129:85–94. doi: 10.1104/pp.010829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welham T., Domoney C. Temporal and spatial activity of a promoter from a pea enzyme inhibitor gene and its exploitation for seed quality improvement. Plant Sci. 2000;159:289–299. doi: 10.1016/S0168-9452(00)00358-7. [DOI] [PubMed] [Google Scholar]
- White P.J. Selenium accumulation by plants. Ann. Bot. 2016;117:217–235. doi: 10.1093/aob/mcv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R., Takagi K., Takada Y., Mukaiyama K., Tsukamoto C., Sayama T., Kaga A., Anai T., Sawai S., Ohyama K., Saito K., Ishimoto M. Metabolic switching of astringent and beneficial triterpenoid saponins in soybean is achieved by a loss-of-function mutation in cytochrome P450 72A69. Plant J. 2017;89:527–539. doi: 10.1111/tpj.13403. [DOI] [PubMed] [Google Scholar]
- Yoshino M., Nagamatsu A., Tsutsumi K.I., Kanazawa A. The regulatory function of the upstream sequence of the β-conglycinin α subunit gene in seed-specific transcription is associated with the presence of the RY sequence. Genes Genet. Syst. 2006;81:135–141. doi: 10.1266/ggs.81.135. [DOI] [PubMed] [Google Scholar]
- Zohary D., Hopf M. third ed. Oxford University Press; Oxford: 2000. Domestication of Plants in the Old World. [Google Scholar]