Abstract
Mucosal surfaces constitute the frontiers of the body and are the biggest barriers of our body for the outside world. Immunoglobulin A (IgA) is the most abundant antibody class present at these sites. It passively contributes to mucosal homeostasis via immune exclusion maintaining a tight balance between tolerating commensals and providing protection against pathogens. Once pathogens have succeeded in invading the epithelial barriers, IgA has an active role in host-pathogen defense by activating myeloid cells through divers receptors, including its Fc receptor, FcαRI (CD89). To evade elimination, several pathogens secrete proteins that interfere with either IgA neutralization or FcαRI-mediated immune responses, emphasizing the importance of IgA-FcαRI interactions in preventing infection. Depending on the IgA form, either anti- or pro-inflammatory responses can be induced. Moreover, the presence of excessive IgA immune complexes can result in continuous FcαRI-mediated activation of myeloid cells, potentially leading to severe tissue damage. On the one hand, enhancing pathogen-specific mucosal and systemic IgA by vaccination may increase protective immunity against infectious diseases. On the other hand, interfering with the IgA-FcαRI axis by monovalent targeting or blocking FcαRI may resolve IgA-induced inflammation and tissue damage. This review describes the multifaceted role of FcαRI as immune regulator between anti- and pro-inflammatory responses of IgA, and addresses potential novel therapeutic strategies that target FcαRI in disease.
Keywords: neutrophil, CD89, mucosa, infection, inflammation, autoimmunity
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
The immune system is a central player in protecting the host against infectious diseases. Synergy between both innate and adaptive immunity is essential to induce effective immune responses against invading microbes. Immunoglobulins are major players of the adaptive immune response, and contribute to both immune defense and maintaining homeostasis.1 Based on structure and effector functions, five major immunoglobulin isotypes can be distinguished, ie, IgM, IgD, IgG, IgA, and IgE that differ in the Fc tail. Immunoglobulins can mediate neutralization, thereby preventing invasion of pathogens or toxins. Additionally, immunoglobulins constitute a bridge between pathogens and the innate immune system facilitating complement activation as well as inducing effector cell functions by immune cells.2,3 Binding of the immunoglobulin Fc tail to their cognate Fc receptor, which can be distinguished in receptors for IgG (FcγRs), IgE (FcεRI), IgA (FcαRI), IgM (FcμR), and IgA/IgM (Fcα/μR), induces cellular activation.4
Mucosal surfaces like the respiratory-, urogenital- and gastrointestinal tracts are continuously exposed to environmental factors and therefore considered as the frontiers of the body. Tolerating harmless antigens while providing protection against pathogens is a challenging feature of mucosal immunity. IgA is the predominant immunoglobulin at mucosal surfaces and in external secretions. It contributes to mucosal homeostasis by neutralizing toxins and viruses, preventing colonization and invasion of pathogenic bacteria, clearing unwanted particles, and promoting sampling of luminal antigens.5,6 In serum, IgA is the second most abundant antibody after IgG. Nonetheless, the exact functions of serum IgA are relatively unexplored and ill understood.7
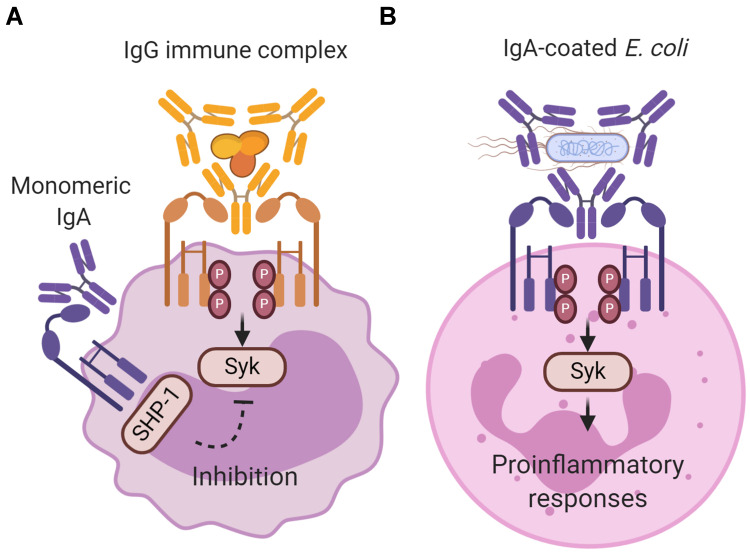
Binding of IgA to its Fc receptor, FcαRI, can initiate either pro- or anti-inflammatory responses. It was demonstrated that interaction of monomeric serum IgA with FcαRI induces inhibitory signals (Figure 1A).8 As such, it is suggested that IgA and FcαRI contribute to homeostatic conditions.9 By contrast, IgA immune complexes (eg, IgA-opsonized bacteria) induce pro-inflammatory responses by cross-linking of FcαRI, which is important in controlling infections (Figure 1B).10,11 The presence of excessive IgA immune complexes or IgA-opsonized bacteria can however lead to uncontrolled and disproportionate FcαRI-mediated immune cell activation, resulting into severe tissue damage as observed during chronic inflammation and autoimmunity.12 Increased serum IgA levels or IgA autoantibodies have been reported in multiple diseases including rheumatoid arthritis, IgA nephropathy, IgA vasculitis, dermatitis herpetiformis, celiac disease, inflammatory bowel disease, Sjögren’s syndrome, ankylosing spondylitis, alcoholic liver cirrhosis, and acquired immunodeficiency syndrome.13–20 The role of FcαRI-mediated inflammation in pathology is still poorly understood. This review summarizes the different functions of FcαRI and its ligand IgA during homeostasis, infection, chronic inflammation, or autoimmunity, and addresses the possibilities of targeting FcαRI for therapeutic strategies.
Figure 1.
Inhibitory and activating signaling via FcαRI after ligand binding. (A) Monomeric IgA (not complexed to an antigen) does not induce FcαRI cross-linking resulting in partial phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) and recruitment of Src homology region 2 domain‐containing phosphatase‐1 (SHP‐1). This results in inhibition of ITAM signaling, and impairs phosphorylation of spleen tyrosine kinase (Syk), LAT and ERK, which is initiated through signaling via other activating Fc receptors (like IgG-mediated Fcγ receptor activation). The exact binding of free dIgA to FcαRI, and concomitant signaling, has not yet been resolved. (B) IgA immune complexes (eg IgA-coated Escherichia coli) induce cross‐linking of FcαRI, resulting in ITAM phosphorylation of the associated FcR γ-chain. Phosphorylated ITAMs subsequently function as a docking site for signaling molecules such as Syk. Syk plays an essential role in initiating signaling pathways, including the Ras/Raf/MEK/MAPK pathway. Activation of signaling pathways results in pro‐inflammatory cellular functions such as phagocytosis, antibody‐dependent cellular cytotoxicity, respiratory burst, degranulation, antigen presentation, and release of NETS, cytokines and inflammatory mediators. Created with BioRender.com.
FcαRI and IgA: The Basics
FcαRI Structure and Expression
Human FcαRI is a member of the Fc receptor immunoglobulin superfamily. Nevertheless, FcαRI has some distinct features compared to other Fc receptors. The FcαRI gene (FCAR) is located on chromosome 19 (19q13.4) within the leukocyte receptor cluster (LRC), whereas other human FcR genes are located on chromosome 1.21,22 LRC also encodes leukocyte Ig-like receptors and natural killer cell immunoglobulin-like receptors. The amino acid (aa) sequence of FcαRI resembles LRC encoded receptors more closely than other Fc receptors.23 FCAR consists of five exons that encode for the leader peptide (S1; 34 base pairs (bp), and S2; 36 bp), two extracellular Ig-like domains (EC1 and EC2; 291 bp and 288 bp) and a combined transmembrane and cytoplasmic region (TM/C; 215 bp).24 FCAR encodes a transmembrane receptor, which consists of two extracellular domains (EC1 and EC2; each 206 aa) that are folded with an angle of approximately 90° to each other.25 The transmembrane region (19 aa) is crucial for association with FcR γ-chain,26 and FcαRI has a short cytoplasmic tail (41 aa). FcαRI is expressed on the surface of myeloid cells, including neutrophils, eosinophils, monocytes, macrophages, Kupffer cells, and human platelets.12,27,28 Additionally, low FcαRI levels were observed on in vitro cultured immature monocyte-derived dendritic cells (DCs) as well as on monocyte-derived CD103+ DCs, which resemble human epithelial interstitial-type DCs.29,30 The molecular weight of FcαRI ranges between 50 and 75 kilodalton (kDa) due to differences in N glycosylation, with the exception of FcαRI on eosinophils, which is between 70 and 100 kDa.31 Orthologues of human FcαRI have been identified in several monkey species, horses, cattle, hamsters, gerbils, and rats, but not in mice due to a gene translocation.32,33
FcαRI is constitutively expressed and independent of its ligand. Expression can be modulated by several mediators, such as lipopolysaccharide (LPS), chemoattractants, cytokines, or adapter protein binding to the intracellular domain of FcαRI.33,34 Upregulation of FcαRI on neutrophils occurs rapidly by either transport from an intracellular pool to the cell surface or via de novo synthesis and is induced by N-formylmethionyl-leucyl-phenylalanine (fMLP), interleukin (IL)-8, tumor necrosis factor-alpha (TNF-α), LPS, and granulocyte-macrophage colony-stimulating factor (GM-CSF).35–38 On monocytes and monocyte-like cell lines FcαRI expression was enhanced by calcitriol, LPS, TNF-α, GM-CSF, and IL-1β.39,40 Downregulation occurs in the presence of transforming growth factor-β (TGF-β), interferon-γ (IFN-γ), or by ligand binding due to FcαRI aggregation and internalization.41,42
IgA Binding to FcαRI
Humans express two closely related IgA subclasses, ie, IgA1 and IgA2, whereas most mammals, except for rabbits and certain primates, express only a single IgA subclass that resembles IgA2.43–45 In serum, IgA1 is the predominant subclass with an IgA1:IgA2 ratio of 9:1, while IgA2 is mainly found in the colon. In other mucosal tissues, IgA1 and IgA2 are more evenly distributed. IgA1 and IgA2 differ in their hinge region and number of glycosylation sites.46 IgA1 contains a hinge region that is 13 amino acids longer compared to IgA2, which results in enhanced antigen recognition capacity but also in increased susceptibility for proteolytic cleavage by bacterial proteases.47 Furthermore, IgA1 contains three to six O-linked glycans in the hinge region, while IgA2 is devoid of O-linked glycosylation.48 O-linked glycans of IgA1 in external secretions can interact with bacterial adhesion molecules, contributing to mucosal homeostasis.49 Altered O-linked glycosylation can cause IgA1 conformational changes resulting in increased immune complex formation, which is a key pathogenic factor in diseases like IgA nephropathy.50
In humans, IgA is expressed in three different forms: ie, monomeric IgA, dimeric IgA (dIgA), and secretory IgA (SIgA). Serum IgA is mostly monomeric and produced by plasma cells in the bone marrow, spleen, and lymph nodes. By contrast, IgA at mucosal sites is predominantly dimeric and produced by local plasma cells in the lamina propria.51,52 Dimeric IgA (dIgA) is composed of two monomers that are linked tail-to-tail with a joining (J-) chain via Cys471-mediated disulfide bonds forming a boomerang-like structure.53 It is transported across the epithelium by binding to the polymeric Ig receptor (pIgR), which is expressed on the basolateral membrane of epithelial cells.53,54 At the luminal side, pIgR is cleaved and a part of this receptor, referred to as secretory component (SC), remains attached to dIgA by binding both Fc-tails and J-chain across the ~50° gap between the two monomers, thereby forming SIgA (Figure 2A).55,56
Figure 2.
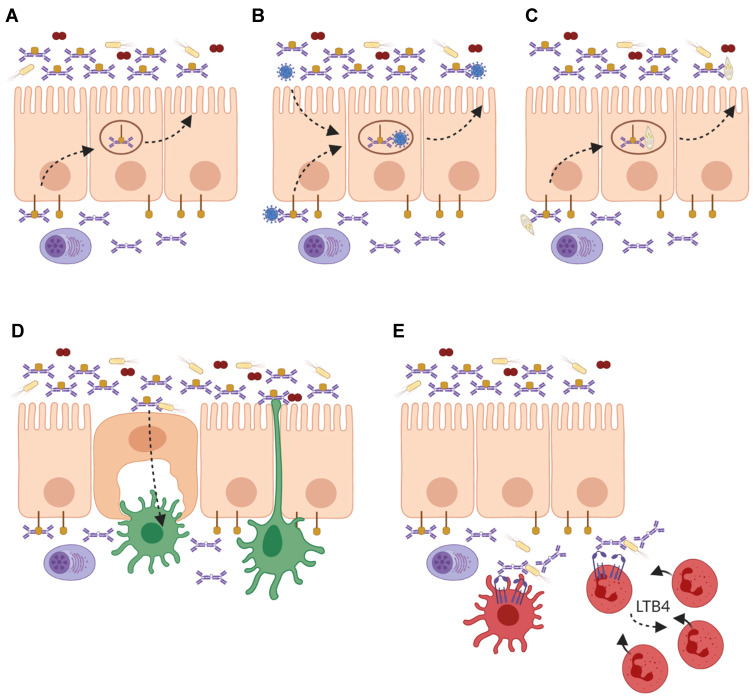
Roles of IgA and FcαRI in homeostasis and infection at mucosal sites. (A) Local plasma cells in the lamina propria produce dimeric IgA (dIgA), which is transported across the epithelium into luminal secretions by binding to the polymeric Ig receptor (pIgR). At the luminal side it is released as secretory IgA (SIgA) where it can neutralize pathogens and toxins. (B) On route of being secreted, dIgA can intercept viruses, which have infected epithelial cells and redirect them into the lumen. (C) Invading pathogens and antigens in the lamina propria are opsonized by dIgA and transported back into the lumen. (D) Microbes that are opsonized with SIgA are shuttled via microfold (M) cells to dendritic cells (DCs) in Peyer’s patches for sampling. Additionally, DCs can extend dendrites through the epithelial layer for sampling of the luminal content. (E) During infection, dIgA-opsonized pathogens are taken up by FcαRI-expressing DCs, and presented to T cells. Additionally, phagocytosis of dIgA-opsonized pathogens by neutrophils results in the release of leukotriene B4 (LTB4), which mediates the chemotaxis of more neutrophils to the site of infection, thereby functioning as a self-contained positive feedback loop of immune cell recruitment to clear invading pathogens. Created with BioRender.com.
All (iso)forms of IgA are ligands for FcαRI, although binding of SIgA to FcαRI is (partially) hampered due to steric hindrance of SC.57 Furthermore, binding of SIgA requires the presence of macrophage-1 antigen (Mac-1, CD11b/CD18).58 Monomeric IgA and dIgA bind with moderate affinity (Ka = 106 M−1), whereas IgA immune complexes bind with higher avidity and induce crosslinking of FcαRI.33 Optimal binding of IgA immune complexes occurs with five to six molecules of IgA per complex.59 In particular residues Pro440-Phe443 and Leu257-Leu258 in the FcαRI EC1 are essential for IgA binding.60 Monomeric IgA binds to FcαRI via its Cα2 and Cα3 domains in a 2:1 stoichiometry (one IgA molecule binds simultaneously two FcαRI molecules).25,61 This is in contrast with other Fc receptors since FcɛRI and FcγRIII bind their ligands in EC2 in a 1:1 stoichiometry.62–65 In theory, dIgA can bind four FcαRI due to its multiplied binding sites, although this may not be possible due to steric hindrance. As such, it still remains unclear how dIgA exactly binds to FcαRI.66 It has been described that pentraxins, including acute phase C reactive protein (CRP) and serum amyloid P (SAP), compete for FcαRI binding since these proteins recognize a similar binding site on FcαRI as IgA.67 Mutations in FcαRI outside the IgA-binding site enhanced pentraxin binding to FcαRI with 2-fold whereas IgA binding was unaffected, suggesting additional binding sites for pentraxins.67
FcαRI harbors six N-glycosylation sites which affect the binding affinity of IgA to FcαRI. Altered glycosylation of FcαRI due to a specific mutation (Asn58 to Glu58) resulted in a nearly 2-fold increased binding of IgA. Furthermore, removal of sialic acids increased IgA binding with nearly 4-fold.68 IgA binding to FcαRI can also be regulated by intracellular signals that are modulated by cytokine stimulation of cells (also referred to as inside-out signaling), independently of FcαRI expression levels.69 Increased binding of IgA to FcαRI in transfected cells, eosinophils, and monocytes was reported in the presence of cytokines like GM-CSF, IL-4, and IL-5 without increasing FcαRI expression levels on the cell surface.70,71
FcαRI Signaling and Cellular Activation
FcαRI does not contain any known signaling motifs. In order to initiate effector functions, FcαRI associates with the FcR γ-chain subunit, which contains an immunoreceptor tyrosine-based activation motif (ITAM) in its intracellular domain.26 In the transmembrane regions, the positively charged arginine on position 209 (R209) in FcαRI associates with the negatively charged aspartic acid 11 (D11) in FcR γ-chain.72 The FcR γ-chain ITAMs contain conserved paired tyrosines and leucines in a consensus sequence (YxxL-x7-12-YxxL). Binding of monomeric serum IgA (not complexed with an antigen) leads to partial phosphorylation of FcR γ‐chain and involves extracellular signal-related kinases (ERK)‐dependent recruitment of tyrosine phosphatase Src homology region 2 domain‐containing phosphatase‐1 (SHP‐1) to sphingolipid–cholesterol‐rich membrane domains.8 Cytoplasmic clusters referred to as inhibisomes hamper spleen tyrosine kinase (Syk), linker for activation of T cells (LAT), and ERK phosphorylation, thereby inhibiting pro-inflammatory responses that are induced by other activating Fc receptors.73 This process is referred to as inhibitory ITAM (ITAMi) signaling (Figure 1A), which may represent an anti-inflammatory mechanism to prevent uncontrolled release of inflammatory responses (see also below).
By contrast, binding of IgA immune complexes to FcαRI induces cross-linking of FcαRI, resulting in pro-inflammatory responses (Figure 1B). After crosslinking, tyrosines in ITAMs are phosphorylated by the Src kinase Fyn, forming docking sites for other tyrosine kinases, including Syk.74–76 Syk plays an essential role in the activation of several proteins, including phosphoinositide 3-kinase (PI3-K), phospholipase Cγ (PLCγ), and Src homology and collagen adaptor protein (Shc). These proteins initiate multiple signaling pathways, including the Ras/Raf/MEK/MAPK pathway and the Rho family GTPase pathway. These pathways are also interconnected and can therefore modulate each other.74–76 Syk additionally induces the release of second messengers such as calcium and diacylglycerol.77 Activation of signaling pathways results in remodeling of the actin cytoskeleton and activation of transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κb), ultimately leading to cellular activation (for detailed description of FcαRI signaling, see Aleyd et al).10
Depending on the cell type, FcαRI activation can result in phagocytosis, degranulation, superoxide generation, release of neutrophil extracellular traps (NETs), antibody-dependent cellular cytotoxicity (ADCC), release of cytokines and chemokines, or antigen presentation.12 It has been shown that phagocytosis of IgA-coated particles by neutrophils induces increased reactive oxygen species (ROS) production and NET release, which can contribute to pathogen elimination.78 Additionally, cross‐linking of FcαRI by serum or dIgA initiates the release of the chemoattractant leukotriene B4 (LTB4) with concomitant neutrophil recruitment.79 SIgA was able to induce respiratory burst in neutrophils, although less efficiently compared to serum IgA. It did not induce efficient uptake of pathogens by either neutrophils or Kupffer cells.27,80 Cross-linking of FcαRI by IgA immune complexes on immature DCs resulted in antigen presentation through the major histocompatibility complex class II pathway, DC maturation, and production of IL-10.81–83 Since IL-10, together with TGF-β, mediates IgA isotype switching in B cells, FcαRI-positive DCs are described to be important for the induction of IgA by B cells in secondary lymphoid organs.84 Additionally, cross-linking of FcαRI on in vitro generated human CD103+ DCs (resembling human epithelial interstitial-type DCs) resulted in the release of pro-inflammatory cytokines like TNF-α, IL-1β, IL-6, and IL-23.29 SIgA was internalized by DCs as well, albeit through carbohydrate-recognizing receptors instead of FcαRI, which did not result in DC maturation.85
FcαRI and IgA: Homeostasis
Immune Exclusion and Immune Regulation
In mucosal areas like the respiratory-, urogenital-, and gastrointestinal tracts, SIgA plays a key role in keeping a tight balance between tolerating commensals and harmless antigens while providing protection against harmful pathogens.86 Due to its multiple binding sites, SIgA opsonizes bacteria with high avidity thereby interfering with bacterial motility and inducing bacterial agglutination. This can block the entrance of bacteria into the mucosal epithelium.87 Additionally, bacterial products like enzymes and toxins are also neutralized by SIgA.88 This process, referred to as immune exclusion, is the main described function of SIgA and prevents local and systemic infection (Figure 2A).86 Not only bacteria but also viruses can be neutralized by mucosal IgA. On route to be secreted, dIgA has the ability to intercept and disarm viruses, which have infected epithelial cells and redirect them back into the lumen (Figure 2B).12 Similarly, pathogens that have invaded the epithelial barrier into the lamina propria are likely opsonized by dIgA and transported back into the lumen (Figure 2C). It has been shown that IgA neutralized several viruses, including sendai, influenza, rota, measles, and human immunodeficiency virus 1 (HIV-1).89–94 Additionally, SIgA plays an important role in shaping and diversifying the gut microbiome.95 Mice lacking IgA showed reduced overall microbial diversity resulting in altered bacterial composition, increased bacterial translocation, and ultimately intestinal inflammation.96–98 Similarly, humans with defective mucosal IgA responses, such as Selective IgA-deficiency (SIgAd), showed modest but significant changes in the gut microbiota composition.99 High-microbiota binding as well as cross-species reactivity of IgA was shown to promote host-microbiota symbiosis, hereby maintaining intestinal mucosal integrity and homeostasis.100–102 Yet, the functional effects of IgA binding to microbiota fitness or physiology remain unclear. IgA production is also microbiota-dependent, as germ-free showed diminished IgA titers compared to specific-pathogen-free mice.103,104 IgA-producing plasma cells were absent in most tissues of germ-free mice and significantly reduced in the small intestine, reflecting the importance of microbiota in initiating humoral responses.101
SIgA immune complexes (eg, IgA-opsonized bacteria) can be transported from the lumen into the lamina propria via the transferrin receptor 1 (TfR1 or CD71) on epithelial cells or via Dectin-1 on microfold cells (M cells) (Figure 2D).105,106 Reverse transcytosis of SIgA is important for the uptake and delivery of antigens from the intestinal lumen to gut-associated lymphoid tissues (GALTs) influencing immune responses.106,107 SIgA immune complexes are taken up by DCs through interaction with Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin (DC-SIGN).85
In the circulation, serum IgA has likely an immunomodulatory role through inhibitory signals via ITAMi signaling (Figure 1A). It was shown that monovalent targeting of FcαRI resulted in the inhibition of oxidative burst activity, chemotaxis and IgG-mediated phagocytosis and cytokine production.108–111 Furthermore, in transgenic mice that express human FcαRI on myeloid cells, IgE-mediated asthma was prevented by the binding of soluble IgA to FcαRI, which inhibited FcεRI-induced degranulation of mast cells.8 ITAMi signaling by FcαRI is therefore suggested to play a role in maintaining homeostasis and protection against enhanced Fcγ receptor- or FcεRI-mediated activation during inflammatory diseases and allergies.9
Early-Life Immunity
SIgA is the predominant antibody in human colostrum and described to protect offspring from infection when the neonatal immune system is still immature.112,113 IgA and IgM are not able to cross the placenta through the neonatal Fc receptor (FcRn) and can therefore only be provided through maternal milk.114 Breastfeeding is important for the development of the neonatal intestinal microbiota and can protect infants from infectious diseases.114–116 Studies in mice have shown that colostrum is the only source of SIgA in the first weeks of life since it takes up to approximately 4 weeks for the neonatal intestine to be populated by IgA-secreting B cells.116,117 In humans, mucosal IgA in the fetal intestine is absent or rarely present until 10 days after birth.118 IgA-producing B cells become pre-dominant 1–2 months after birth and increase in number until 6 to 11 months of age.119 The highest concentration of SIgA is found in colostrum; however, prolonged breastfeeding resulted in increased IgA in maternal milk as well.120,121 Although the opsonic activity of SIgA is poor, early studies demonstrated that SIgA initiated macrophage phagocytosis and neutrophil respiratory burst.122 After antigen interaction, SIgA undergoes conformational changes, which enhanced the binding of SIgA immune complexes to FcαRI.123 In addition to immunoglobulins, maternal milk also facilitates the transfer of leukocytes. IgA-induced cellular effector functions may therefore contribute to early immunity against pathogens in newborns. It has been demonstrated that the majority of leukocytes present in maternal milk have a similar phenotype to blood cells, although leukocyte subsets are present in different frequencies. Myeloid precursors, neutrophils, and immature granulocytes are the main identified cells present in colostrum.124 Colostral neutrophils were able to phagocytose IgA-opsonized bacteria although this did not result in significant bacterial‐killing and release of superoxide anion. This is likely due to FcαRI expression without γ subunit association.125 The exact contribution of FcαRI-mediated cellular responses initiated by SIgA or leukocytes in maternal milk remains incompletely understood.
FcαRI and IgA: Infection
The production of IgA, with a synthesis rate of 66mg/kg, exceeds that of all other antibodies combined supporting its importance in host-pathogen defense.12 Pathogen-specific IgA is found at mucosal surfaces and in circulation during several infectious diseases, while the exact function of IgA remains unclear.6,11 Passive immunity (via neutralization) as well as immune activating properties of IgA have been described for infections in the respiratory- and reproductive tracts such as Mycobacterium tuberculosis (Mtb), HIV-1, and more recently severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections.126–128
Passive Immunity
Respiratory infections often occur via inhalation of air-borne droplets containing pathogens or via direct contact with respiratory secretions.129,130 The nasopharyngeal mucosa is furthermore a natural reservoir for several pathogens like Streptococcus pneumoniae (S. pneumoniae) and Neisseria meningitides (N. meningitides).131 In individuals who lack protective antibody titers, infections can occur causing various pathologies like pneumonia, meningitis, bacteremia, and sepsis.132,133 In mice, SIgA against Mtb or S. pneumoniae has been associated with protection against tuberculosis (TB) or pneumococcal disease respectively.134,135 The neutralizing capacities of IgA are not only described for bacteria since IgA against HIV-1 or rotavirus was able to neutralize either virus. Monomeric IgA1 or dIgA2 against envelope proteins (Env) of HIV-1 showed neutralizing activity in vitro.136 Moreover, mucosally applied Env-specific dIgA1 and dIgA2 protected rhesus macaques against an intrarectal challenge with simian-human immunodeficiency virus (SHIV).137,138 IgA against rotavirus (RV-IgA) neutralized both virus in solution and virus that had bound to epithelial cells in vitro.139 Furthermore, it was shown that RV-IgA was produced in all rotavirus animal models (horse, cow, sheep, gnotobiotic piglet, rat, rabbit, and mouse) which correlated with clearance of infection and protective immunity.140–142 Generation of specific IgA in coronavirus disease 2019 (COVID-19) was described as well.143–146 It was suggested that SIgA antibodies against SARS-CoV-2 were able to neutralize the spike protein or nucleocapsid protein thereby providing protective mucosal immunity.147 It is unknown whether serum IgA anti-SARS-CoV-2 antibodies contribute to protection against COVID-19. On the one hand, it was demonstrated that severe COVID-19 illness was significantly associated with increased total serum IgA but not with total serum IgG levels.148 Furthermore, IgA levels correlated with disease score in critically ill patients.149 On the other hand, the presence of IgA and IgG antibodies against SARS-CoV-2 spike protein subunit 1 (S1) showed an inverse correlation with viral load. Additionally, enhanced titers of specific anti-S1 IgA in serum correlated with significant increased survival in COVID-19 patients 28 days post intensive care unit admission.150 Passive antibody therapy using convalescent plasma from recovered COVID-19 patients resulted in a reduction in viral load and better disease outcomes, most notably in less severely ill COVID-19 patients.151–153 Nonetheless, since IgA-specific SARS-CoV-2 neutralizing antibodies were not determined in convalescent plasma that was transferred to COVID-19 patients, the exact contribution of IgA neutralizing antibodies in COVID-19 remains unknown.154–156 Longitudinal investigation of potentially protective functions of mucosal and systemic IgA is needed to determine their role in COVID-19.
Active Immunity
Although few FcαRI-positive cells are observed in mucosal areas in homeostatic conditions, FcαRI-positive neutrophils are the first cells that are recruited after infection.79,157 Once pathogens have successfully invaded the epithelial barrier, they can become opsonized by dIgA in the lamina propria. It was shown that dIgA induced efficient phagocytosis by neutrophils.80 As such, a pro-inflammatory role for dIgA in eliminating infiltrating pathogens was proposed.33 Enhanced phagocytosis of Escherichia coli, Streptococcus pneumonia, Staphylococcus aureus, Porphyromonas gingivalis, Candida albicans, Bordetella pertussis, and Neisseria meningitides by neutrophils after targeting FcαRI has been demonstrated in vitro.27,79,80,158–161 Specific IgA antibodies against meningococcal capsular polysaccharides were shown to induce moderate phagocytosis by neutrophils but initiated respiratory burst more potently than IgG.80 Cross-linking of FcαRI on neutrophils furthermore results in the release of LTB4, which is a potent neutrophil chemoattractant, and additionally mediates the migration of monocytes and monocyte-derived DCs.79,162,163 LTB4 release after neutrophil activation through FcαRI has therefore been proposed to function as a self-contained positive feedback loop of immune cell recruitment to clear invading pathogens and maintain homeostasis (Figure 2E).33 Alveolar macrophages, which express an alternatively spliced variant of FcαRI, also contribute to host-pathogen defense by clearance of inhaled antigens that were opsonized by IgA.164 A recent study showed that antibodies from patients with latent TB induced enhanced macrophage killing of intracellular Mtb.165 Since IgA levels against TB antigens distinguished patients with pulmonary TB, patients with latent TB, and non-infected individuals, a role for FcαRI-mediated effector functions in Mtb control is predicted, although this is formally not yet established.166 Interestingly, a recent study showed that FcαRI also acts as an innate receptor, by binding bacteria directly independent of its ligands IgA or CRP. Binding of bacteria to FcαRI induced phagocytosis by CD11c+ DCs and monocytes/macrophages. Moreover, FcαRI transgenic mice were protected against two different sepsis-induced models, identifying FcαRI as a first-line innate receptor for bacterial clearance.167
The contribution of antibody-dependent cellular phagocytosis (ADCP) in protective immune responses against viruses remains debatable. Nonetheless, there is substantial evidence that supports the involvement of ADCP in protection against several types of viruses and reduction of disease.168 It has been described that human NK cells and neutrophils perform ADCC and ADCP of HIV-1 gp120-pulsed target CEM-NKr cells or gp120-coated beads, respectively, in the presence of polyclonal antibodies from different HIV-positive subject groups.169 Moreover, HIV-1 gp41 envelope-specific IgA induced FcαRI-mediated ADCC of HIV-1 Clade A- and B-infected target cells by monocytes.170 HIV-1 gp41 envelope-specific IgA additionally triggered ADCP of HIV-1 infected CD4+ T cells by monocytes and neutrophils more efficiently than anti-gp41 IgGs.171 FcαRI-mediated neutrophil phagocytosis initiated by vaccine-induced IgA was associated with reduced risk of infection against simian immunodeficiency virus (SIV) after immunization via the nasal, but not the intramuscular route in non-human primates.172 These studies may provide new insights into FcαRI-mediated immune responses against HIV-1 infected cells and address the potential functions of IgA during viral infections. By contrast, the RV144 HIV-1 vaccine trial showed that IgA interfered with IgG-mediated ADCC by NK cells of HIV-1 infected cells.173 It was proposed that Env-specific IgA competed with IgG for NK cell-mediated ADCC of HIV-1 infected cells, while enhancing ADCP by FcαRI-expressing cells like neutrophils, which represent the dominant phagocyte population in tissues from the lower female reproductive tract.
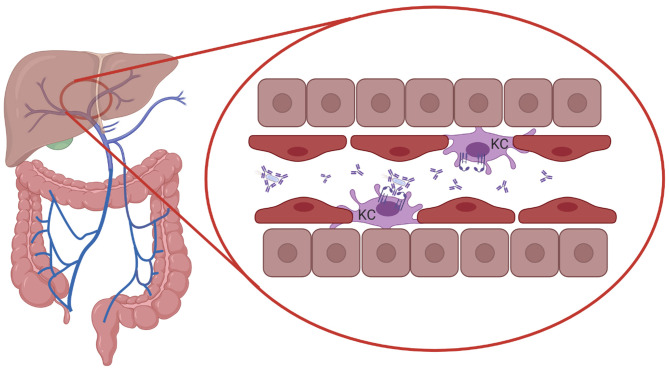
The severity of infections increases when invading pathogens from the respiratory- or gastrointestinal tract enter the bloodstream. In mice it was shown that serum IgA directed against commensal bacteria protected mice against lethal sepsis when the intestinal barrier was damaged, suggesting that serum IgA provided protection against systemic infection.103 Mice lacking pIgR and SIgA have epithelial barrier disruption, enhanced numbers of IgA-secreting plasma cells, and increased levels of serum IgG and IgA.174 It was furthermore demonstrated that in human FcαRI-transgenic mice IgA-opsonized bacteria in the circulation were phagocytosed by Kupffer cells, which express FcαRI.27 FcαRI cross-linking by serum IgA immune complexes and cross-talk with pathogen recognition receptors (PRRs) on Kupffer cells initiated the release of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6.175,176 As such, clearance of serum IgA-coated bacteria by Kupffer cells may act as a systemic line of defense, through elimination of invasive bacteria that have escaped mucosal immune responses (Figure 3).
Figure 3.
Systemic protection by serum IgA and FcαRI-expressing Kupffer cells. Serum IgA-opsonized bacteria in the circulation are transported to the liver through the portal vein and phagocytosed by Kupffer cells (KC), which express FcαRI, providing protection against systemic infection. Cross-talk between FcαRI and pathogen recognition receptors (PRRs) may break the tolerance of Kupffer cells to bacterial structures. Created with BioRender.com.
Bacterial Evasion Mechanisms
Pathogens have evolved and developed strategies to either disarm IgA or evade FcαRI-mediated activation of immune cells, emphasizing the importance of IgA-FcαRI interactions in the elimination of pathogens and prevention of infection.177 Pathogens that colonize the oral and upper respiratory mucosa like S. pneumoniae and N. meningitides produce specific proteases cleaving both mucosal and serum IgA1 at the hinge region, which abrogates its protective effects and interferes with host antibacterial immunity.178,179 Binding of pneumococcal surface protein (SpsA) to SIgA is suggested to recruit SIgA to the bacterial surface to promote its degradation by IgA proteases, hinder SIgA from clearing bacteria, or block the interaction between SIgA and FcαRI thereby preventing immune responses.53 Additionally, IgA1 proteases were shown to degrade lysosomal-associated membrane protein 1 (LAMP-1) promoting intracellular bacterial survival in epithelial cells in vitro.180 Furthermore, it was described that serine proteases do not only cleave IgA1, which prevents FcαRI-mediated immune responses but also reduce the binding avidity of IgA1 thereby reducing the neutralizing ability of IgA1.181 Closely related strains of N. meningitides, as well as S. pneumoniae that lack IgA1 proteases are considered nonvirulent.182 Furthermore, Staphylococcus aureus (S. aureus) and group A and B streptococci developed evasion mechanisms to circumvent FcαRI-mediated elimination by secreting decoy proteins that inhibit binding of IgA to FcαRI. Decoy proteins Sir22, Arp4, and an unrelated β protein from group B streptococci, as well as staphylococcal superantigen-like protein seven that is produced by S. aureus, bind to specific Fc residues in the Cα2 and Cα3 domains of IgA.183,184 The ability of bacteria to escape FcαRI-mediated immunity enhances survival in mucosal environments and may contribute to systemic infections.
FcαRI and IgA: Chronic Inflammation and Autoimmunity
Because FcαRI potently activates immune cells, aberrant IgA responses may contribute to pathogenesis in inflammatory and/or autoimmune diseases. The presence of IgA autoantibodies, aberrant IgA glycosylation, or excessive IgA immune complexes can contribute to chronic inflammation and tissue damage. The detrimental role of IgA and FcαRI-mediated immune responses has been proposed in several inflammatory diseases like IgA nephropathy, rheumatoid arthritis, IgA vasculitis, dermatitis herpetiformis, linear IgA bullous disease, and inflammatory bowel disease.
IgA Nephropathy
IgA nephropathy (IgAN) is the most common IgA-mediated autoimmune disease. It is characterized by primary glomerulonephritis, resulting in chronic renal failure in 30% of the patients.185,186 Infections or microbiome dysbiosis prior to IgAN have been suggested to initiate abnormal IgA1 glycosylation as glycosyltransferases are regulated by bacterial products.187 Elevated synthesis of galactose-deficient IgA1 (gd-IgA1) triggers the production of anti-glycan antibodies. IgA immune complexes can deposit in the glomeruli inducing mesangial proliferation and matrix expansion, which eventually initiates renal injury.188 The release of pro-inflammatory cytokines such as TNF-α, IL-6, and TGF-β by mesangial cells after IgA immune complex binding was suggested to induce inflammation and glomerulosclerosis.187,189 In mice, gd-IgA1 immune complexes were not cleared from the circulation and deposited in the kidney. Shedding of FcαRI in transgenic mice however resulted in the deposition of soluble FcαRI-IgA immune complexes in the mesangium, which induced glomerular and interstitial macrophage infiltration, hematuria, mesangial matrix expansion, and mild proteinuria.190–192 Soluble FcαRI-IgA complexes additionally mediated kidney inflammation by interacting with TfR1 on mesangial cells, resulting in the release of pro-inflammatory mediators.190 Human FcαRI transgenic mice developed IgAN after injection of serum IgA of IgAN patients. Furthermore, human IgA1 knock-in mice that had been crossed with FcαRI transgenic mice already developed IgAN in 6 weeks.193 These data clearly support the contribution of IgA and FcαRI-mediated inflammation in IgAN.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a systemic and chronic autoimmune disorder characterized by inflammation in the joints, which frequently leads to joint destruction and disability.194 Although the etiology of RA is unknown, the presence of autoantibodies is a distinctive feature of RA. In particular, IgM rheumatoid factor (RF) and IgG anti-citrullinated protein antibodies (ACPA) are commonly used for diagnosing and classifying RA.195 Interestingly, elevated levels of IgA RF, but not of IgM- or IgG RF, correlated with worse disease prognosis and extra-articular manifestations in RA.196 Additionally, high levels of IgA RF were associated with a poor response rate to TNF‐α blockers as therapeutic agents in advanced RA.197 In IgM RF negative patients it was shown that IgA ACPA was associated with disease severity based on disease activity score.15 RF or ACPA can be elevated in the serum years prior to the onset of clinical disease, referred to as preclinical RA.198,199 An increased number of IgA+ plasmablasts was identified in preclinical RA without a history of or current inflammatory arthritis.200 In both clinical and preclinical RA, IgA ACPA was highly specific for RA.201,202 IgA ACPA and RF immune complexes furthermore increased the release of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 by myeloid cells via cross-linking of FcαRI, whereas blocking of FcαRI on macrophages resulted in reduced levels of TNF-α.203 Additionally, increased NET release by neutrophils in the presence of plasma of RA patients containing IgA RF was demonstrated, which was dependent on FcαRI.78 Altogether, this supports that IgA contributes to disease, as activation of FcαRI-expressing neutrophils and macrophages in the joint via IgA auto-immune complexes likely enhances inflammation and pathology of RA.
IgA Vasculitis
IgA vasculitis, formerly known as Henoch-Schönlein purpura, is the most common form of vasculitis involving the small vessels of the joints, kidneys, gastrointestinal tract, and the skin.204 Although the etiology of IgA vasculitis remains unknown, the disease is characterized by IgA1 immune deposits and neutrophil infiltrates damaging the small vessels. Abnormal glycosylation of the hinge region of IgA1 is suggested to cause aggregation into macromolecular immune complexes.204 FcαRI-mediated cross-linking of neutrophils induces inflammatory processes like ROS production, NET formation, and LTB4 release. As such, it is hypothesized that FcαRI-mediated activation of neutrophils results in vessel damage and leakage of red blood cells into the skin causing typical cutaneous hemorrhages.14 Antigen recognition sites of accumulated IgA1 in IgA vasculitis have not yet been identified. However, it was proposed that these antibodies can recognize epitopes on endothelial cells as serum IgA from IgA vasculitis patients was shown to bind to human but not bovine glomerular endothelial cells in vitro.205 Notably, sera of IgA vasculitis patients had increased levels of soluble FcαRI-IgA complexes, which was associated with decreased FcαRI expression on monocytes.206 A positive feedback loop of inflammation in IgA vasculitis was proposed, since binding of IgA1 antibodies to endothelial cells induced the release of IL-8, thereby attracting more neutrophils that can be activated through FcαRI.16
Skin Blistering Diseases
Dermatitis herpetiformis (DH) is an autoimmune disease that often occurs in combination with gluten-sensitive enteropathy (celiac disease). Anti-transglutaminase IgA antibodies (mainly anti-epidermal transglutaminase 3; TGase3) play a key role in disease pathogenesis of DH as it was shown that serum anti-epidermal transglutaminase IgA positively correlated with disease activity.207 IgA anti-tissue transglutaminase antibodies form immune complexes and deposit in the dermis of patients with DH.12 In the skin, transglutaminase/IgA immune complexes induce fibrinolysis, which directly contributes to blister formation in DH, and results in chemotaxis of neutrophils and monocytes.208,209 Accumulating FcαRI-positive neutrophils in the skin of DH had increased ability to bind IgA. As such, they presumably bind transglutaminase/IgA immune complexes and induce cellular activation contributing to tissue damage.210,211 Released proteolytic enzymes by activated neutrophils can induce sub-epidermal split by cleaving adhesion molecules such as collagen VII.212 Circulating epidermal transglutaminase/IgA immune complexes have been detected in patients with DH which, together with disease manifestations, decrease over time after following a gluten-free diet.213 Some DH patients also showed IgA immune complex deposition in the kidney, which ultimately can lead to IgA nephropathy (IgAN).213
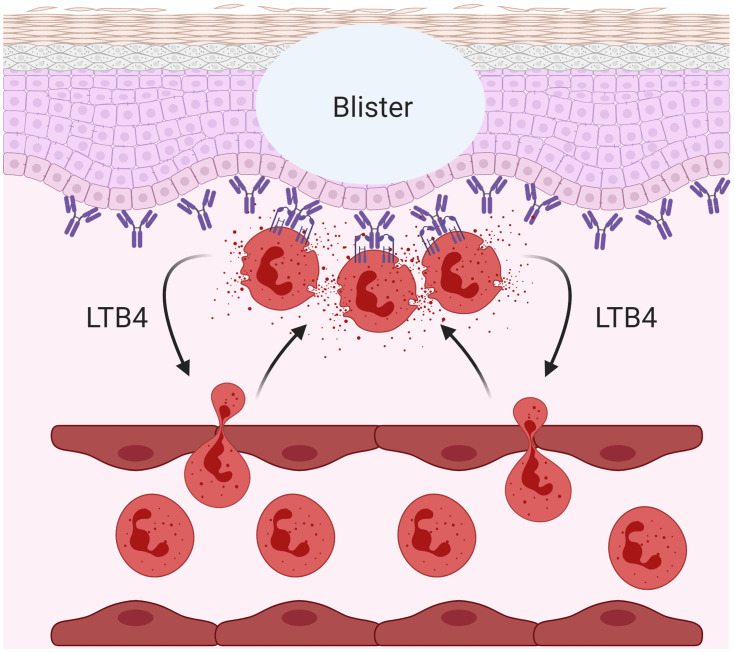
Similar to DH, linear IgA bullous disease (LABD) is a skin blistering disease characterized by IgA autoantibodies against collagen XVII and FcαRI-mediated neutrophil activation.214 It was shown in an ex vivo skin model that activated neutrophils in the presence of serum of LABD patients (containing anti-collagen XVII IgA) caused the separation of dermis from epidermis (Figure 4). This separation, reflecting blister formation in LABD patients, was prevented by blocking IgA-FcαRI interaction on neutrophils.215 Additionally, eosinophil influx has also been observed in the skin of LABD, which may contribute to LABD pathology through FcαRI-mediated respiratory burst activity.214,215
Figure 4.
IgA-FcαRI induced pathology in skin blistering diseases. Cross-linking of FcαRI on neutrophils with IgA immune complexes results in the release of leukotriene B4 (LTB4) inducing enhanced neutrophil influx, causing tissue damage in a variety of inflammatory- and autoimmune diseases. An example is the blister formation in linear IgA bullous disease (LABD). Created with BioRender.com.
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is characterized by chronic inflammation of the gastrointestinal tract and disruption of the epithelium. IBD can be subdivided into two major forms, ulcerative colitis (UC) and Crohn’s disease (CD).216 The etiology of IBD is ill-understood, but likely involves derailed immune responses against commensal bacteria. Fecal bacteria of patients with IBD, that were highly opsonized with IgA,induced colitis in germ-free mice.217 Furthermore, fecal bacteria in IBD patients showed increased opsonization with IgA compared to healthy individuals, suggesting that leakage of serum IgA or dIgA through the disrupted epithelial layer contributes to enhanced opsonization.217,218 In line with this, it was shown that IBD patients have increased levels of specific IgA against microbiota in their serum.219 The percentage of IgA-opsonized bacteria in CD strongly correlated with multiple clinical indexes of disease activity and may therefore be useful for monitoring CD.220 Due to the disruption of the intestinal epithelial barrier in these patients, bacteria are able to invade the sub-epithelial lamina propria and are likely opsonized with dIgA. This may subsequently induce neutrophil activation via FcαRI cross-linking, since uptake of IgA complexes by neutrophils was observed in the mucosa of patients with UC.79 FcαRI-mediated neutrophil activation induces migration through the release of LTB4. It was therefore hypothesized that in UC patients a continuous neutrophil recruitment loop is initiated. Newly recruited neutrophils will be activated by IgA‐opsonized bacteria and initiate a feedback loop of neutrophil chemotaxis, which eventually results in tissue damage.79 Moreover, cross-talk of FcαRI and toll-like receptor 4 (TLR4) on neutrophils resulted in enhanced release of pro-inflammatory TNF-α, which plays a central role in IBD pathogenesis.221,222 Excessive influx of neutrophils causing major tissue damage was demonstrated in UC patients.222 As such, a role for IgA and FcαRI is proposed in especially UC, but their exact roles still need to be established.
FcαRI and IgA: Therapeutic Opportunities
IgA Vaccination Strategies
Inducing pathogen-specific mucosal and systemic IgA through passive or active immunization may be an attractive strategy to combat bacterial and viral infections.223 Licensed oral and nasal vaccines have demonstrated to induce mucosal SIgA responses as well as system responses contributing to protection.223,224 Furthermore, mucosal application of the TB protein subunit vaccine H56/CAF01 followed by intramuscular priming induced high levels of antigen-specific lung mucosal and systemic IgA.225 Mtb-specific IgA may contribute to prevention of TB, since mucosally administered human IgA antibodies against Mtb in mice provided passive protection.226 Additionally, vaccine-induced pulmonary SIgA in mice was associated with passive protection against TB.134,227 Recombinant monoclonal antibodies (mAb), generated from single isolated B cells of untreated adult patients with acute pulmonary TB and from MTB‐exposed healthcare workers, revealed that IgA against Mtb inhibited mycobacterial infection of human epithelial-like and macrophage-like cells, whereas IgG promoted infection.126 Thus, it is suggested that IgA may be particularly important for protection against TB infection.228 This is supported by experiments with human FcαRI transgenic mice, which had a lower Mtb infection rate compared to control WT mice after inoculation of human IgA mAbs, implicating an important role for FcαRI in clearing Mtb infection.226 Nasal immunization with pneumococcal surface protein A (PspA) and cholera toxin as adjuvant elicited PspA-specific mucosal IgA and systemic IgG antibody responses, which provided protection against colonization of S. pneumoniae and pneumococcal infection in mice.229 PspA-specific SIgA effectively neutralized colonized S. pneumoniae, diminished nasal carriage and prevented subsequent infection with S. pneumoniae. IgA-deficient mice failed to prevent nasal colonization by S. pneumoniae, in spite of having functional anti-PspA IgG antibodies in the nasal cavity, stating the necessity of specific IgA to prevent infection.230
Vaccine-induced IgA also provides effective protection against viral infections. Both the inactivated and the live-attenuated oral poliovirus vaccines, which played a tremendous role in the elimination of the poliovirus, induced potent-specific IgA responses.231 Oral vaccines consisting of live attenuated rotavirus (RV) mediate significantly reduced disease. Serum rotavirus-specific IgA (RV-IgA) correlated with vaccine efficacy and protective immunity.232 IgA knockout, as well as J-chain knockout mice, showed delayed viral clearance and absence of protective immunity, supporting the critical role of IgA in RV immunity.233,234 Interestingly, in both humans and animals, RV-IgA can persist for a long time, suggesting the presence of long-lived IgA+ plasma cells.235 Similar to RV, it has been shown that vaccination with live attenuated poliovirus induced long-lived IgA memory immune responses in elderly.236 Intravenous administration of polymeric IgA against influenza showed protection against infection in mice. Inhibition of influenza virus shedding by IgA was 10 times more effective than IgG.237 Additionally, IgA antibodies against influenza A virus hemagglutinin, purified from respiratory tract washings of hemagglutinin immunized mice, were able to protect naïve mice from influenza infection.238 Similar protective effects of IgA were shown in mice that received passive oral administration of IgA against reovirus-specific adhesion molecule sigma1, which prevented entry of reovirus into the Peyer’s patches. Since IgG mAbs against sigma1 did not prevent Peyer’s patch infection by reovirus, this effect was IgA specific.239 Thus, inducing systemic and mucosal IgA responses through vaccination may play an important role in either passive or active protection against pathogens (Figure 5). Notably, the adjuvant alum, which is currently used in many injectable vaccines does not effectively induce IgA class-switching.240 Newly developed mucosal adjuvants like TLR agonists and toxin derivatives (ADP-ribosyltransferase enterotoxins and adenylate cyclase toxins) are suggested to improve IgA responses in vaccination strategies.241 Thus, inducing enhanced IgA responses through vaccination represents a promising therapeutic strategy for future vaccine development.
Figure 5.
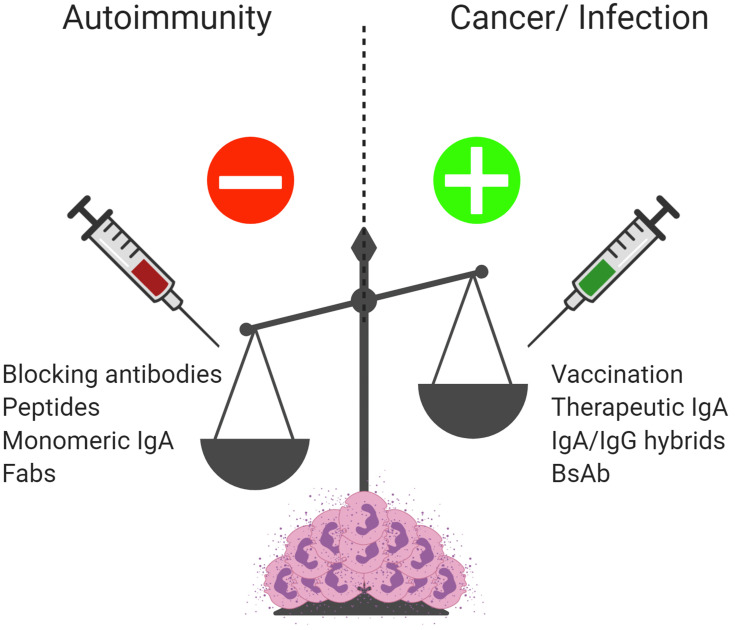
IgA and FcαRI as therapeutic targets. Enhanced IgA complexes or autoantibodies result in excessive activation of immune cells contributing to chronic inflammation and tissue damage in autoimmune diseases. Blocking IgA-FcαRI interactions by either monoclonal antibodies or peptides may reduce inflammation and tissue damage in these diseases. Treatment with monomeric IgA or anti-FcαRI Fabs may dampen immune responses by inducing inhibitory ITAM signaling and inhibiting IgG-induced phagocytosis and IgE-mediated allergic diseases. To combat bacterial and viral infections, inducing pathogen-specific IgA via passive or active vaccination may result in enhanced protective immunity. Enhancing pro-inflammatory responses by FcαRI-expressing immune cells via IgA monoclonal antibody therapy, bi-specific antibodies, or cross-isotype hybrid antibodies may result in efficient killing of tumor cells and represent a promising therapeutic opportunity for cancer patients. Created with BioRender.com.
Promoting FcαRI-Mediated Anti-Tumor Immunity
IgA or FcαRI bispecific antibodies have been proposed as novel drugs to treat cancer by enhancing activation of FcαRI-expressing immune cells.242–245 Tumor cell killing by neutrophils was superior after targeting FcαRI, compared with targeting to IgG Fc receptors, which was demonstrated for several tumor antigens such as EGFR, HER2, EpCAM, HLA-II, CD20, CD30, and carcinoembryonic antigen.242,245,246 Treatment of FcαRI transgenic mice with anti-tumor IgA mAbs against CD20, HER2, or EGFR resulted in enhanced migration of FcαRI-expressing immune cells and significantly increased anti-tumor cytotoxicity, which was mostly mediated by FcαRI-expressing macrophages.243,247,248 However, in contrast to IgG, IgA mAbs cannot activate natural killer cells, which do not express FcαRI. Moreover, IgA is a poor complement activator and has a shorter half-life compared to IgG. IgA therapeutic antibodies that are produced in non-human systems contain different glycosylation profiles, which may also enhance immunogenicity and are likely to be cleared from the human circulation.12 Alternatively, the efficacy of FcαRI bispecific antibodies targeting both tumor antigens and FcαRI has been investigated. FcαRI bispecific antibodies efficiently induced neutrophil migration in vitro, which was not observed after targeting Fcγ receptors.249 Additionally, tumor cell killing was more effective.250,251 Engineering of a cross-isotype antibody that contains merged Fc domain residues from IgG1 and IgA, combining the effector functions of both isotypes, mediated increased complement-dependent cytotoxicity and Her2+ tumor cell killing by both neutrophils and macrophages.252 Furthermore, it was shown that anti-epidermal growth factor receptor-2 IgG1/IgA2 cross-isotype antibodies induced increased recruitment of neutrophils, resulting in enhanced ADCC of human breast cancer cells by neutrophils.253 Pharmacokinetics of IgG and IgA cross-isotype antibodies were similar to the parental IgG antibodies and may therefore augment IgG-based antibody therapies.253 Thus, antibody-based strategies that target FcαRI represent a promising therapeutic opportunity for cancer patients (Figure 5).
Inhibiting FcαRI-Mediated Immunity
Naturally occurring serum IgA was shown to dampen immune responses by inducing ITAMi signaling via FcαRI (Figure 1A).8 Enhancing ITAMi signaling by monovalent targeting of FcαRI has been proposed as a promising strategy to inhibit IgG-induced phagocytosis and IgE-mediated allergic diseases (Figure 5).254 Monovalent targeting of FcαRI with the anti-FcαRI mAb A77 inhibited degranulation of RBL-2H3 transfected cells.8 In in vivo models for allergic asthma and arthritis, FcαRI transgenic mice had reduced airway inflammation or resolution of arthritis after monovalent targeting of FcαRI.254,255 Additionally, targeting of FcαRI by mAb A77 suppressed inflammation in transgenic mice with IgG immune complex-induced glomerulonephritis and obstructive nephropathy.256 Renal inflammation induced by CpG dinucleotides (TLR9 agonist) in FcαRI transgenic mice was downregulated by monomeric targeting of FcαRI as well.257 Thus, monomeric targeting of FcαRI was shown to induce anti-inflammatory properties, which could be used as treatment of inflammatory diseases with involvement of myeloid cells (Figure 5). Alternatively, blocking FcαRI with mAb MIP8a inhibited cytokine production, leukocyte recruitment, and inflammation in a lupus nephritis model.258 FcαRI blocking also reduced NET release by neutrophils that had been stimulated with IgA immune complexes obtained from serum and synovial fluid of RA patients.259 Similarly, in an ex vivo LABD skin model, FcαRI blocking with MIP8a prevented IgA-induced neutrophil-mediated blister formation.215 In a recent study, peptides targeting the interaction sites of IgA and FcαRI were suggested as novel therapeutic strategy for IgA-mediated skin autoimmune diseases, as these peptides were able to penetrate into human skin ex vivo and reduced IgA-induced neutrophil migration.260 As such, blocking FcαRI-IgA interactions represents a promising therapeutic strategy for IgA-associated inflammatory diseases and autoimmunity (Figure 5).
Concluding Remarks and Future Perspectives
IgA and FcαRI-mediated cell activation is important for maintaining homeostasis and preventing infections. SIgA contributes to passive immunity at mucosal surfaces by neutralizing pathogens, whereas active immunity is provided by dIgA and serum IgA through induction of FcαRI-mediated activation of myeloid cells. Future research regarding vaccination and infectious diseases should therefore also include serological and functional studies of IgA and FcαRI-mediated immune responses. It is becoming clear that the presence of overabundant IgA complexes or autoantibodies can result in excessive activation and recruitment of immune cells contributing to tissue damage and the development of chronic inflammation in multiple diseases. A better understanding of the IgA-FcαRI axis in health and disease can provide multiple options for therapeutic interventions. On the one hand, immune responses against pathogens or cancer cells may be enhanced by vaccination or passive transfer of therapeutic IgA. On the other hand, initiating inhibitory ITAM signaling by monomeric targeting of FcαRI or blocking IgA-FcαRI interactions with mAbs or peptides can dampen inflammation and disease.
Highlights
SIgA contributes to mucosal homeostasis and passive immunity through immune exclusion and by diversifying the gut microbiome
Monomeric IgA induces inhibitory signaling, whereas active immunity is provided by complexed dIgA and serum IgA through cross-linking of FcαRI on myeloid cells
Pathogen-specific IgA is associated with protection against infections
Altered IgA glycosylation or excessive IgA immune complexes contribute to chronic inflammation and autoimmunity through FcαRI-mediated immune activation
Understanding the IgA-FcαRI axis during infection and autoimmunity will provide new therapeutic opportunities.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46(4):562–576. doi: 10.1016/j.immuni.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18(1):46–61. doi: 10.1038/nri.2017.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimitrov JD, Lacroix-Desmazes S. Noncanonical functions of antibodies. Trends Immunol. 2020;41(5):379–393. doi: 10.1016/j.it.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 4.Bruhns P, Jonsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268(1):25–51. doi: 10.1111/imr.12350 [DOI] [PubMed] [Google Scholar]
- 5.Macpherson AJ, Yilmaz B, Limenitakis JP, Ganal-Vonarburg SC. IgA function in relation to the intestinal microbiota. Annu Rev Immunol. 2018;36(1):359–381. doi: 10.1146/annurev-immunol-042617-053238 [DOI] [PubMed] [Google Scholar]
- 6.Pabst O, Slack E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020;13(1):12–21. doi: 10.1038/s41385-019-0227-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leong KW, Ding JL. The unexplored roles of human serum IgA. DNA Cell Biol. 2014;33(12):823–829. doi: 10.1089/dna.2014.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasquier B, Launay P, Kanamaru Y, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22(1):31–42. doi: 10.1016/j.immuni.2004.11.017 [DOI] [PubMed] [Google Scholar]
- 9.Mkaddem SB, Christou I, Rossato E, Berthelot L, Lehuen A, Monteiro RC. IgA, IgA receptors, and their anti-inflammatory properties. Curr Top Microbiol Immunol. 2014;382:221–235. doi: 10.1007/978-3-319-07911-0_10 [DOI] [PubMed] [Google Scholar]
- 10.Aleyd E, Heineke MH, van Egmond M. The era of the immunoglobulin A Fc receptor FcalphaRI; its function and potential as target in disease. Immunol Rev. 2015;268(1):123–138. doi: 10.1111/imr.12337 [DOI] [PubMed] [Google Scholar]
- 11.Davis SK, Selva KJ, Kent SJ, Chung AW. Serum IgA Fc effector functions in infectious disease and cancer. Immunol Cell Biol. 2020;98(4):276–286. doi: 10.1111/imcb.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breedveld A, van Egmond M. IgA and FcalphaRI: pathological roles and therapeutic opportunities. Front Immunol. 2019;10:553. doi: 10.3389/fimmu.2019.00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallustio F, Curci C, Di Leo V, Gallone A, Pesce F, Gesualdo L. A new vision of IgA nephropathy: the missing link. Int J Mol Sci. 2019;21(1):189. doi: 10.3390/ijms21010189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heineke MH, Ballering AV, Jamin A, Ben Mkaddem S, Monteiro RC, Van Egmond M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schonlein purpura). Autoimmun Rev. 2017;16(12):1246–1253. doi: 10.1016/j.autrev.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 15.Karimifar M, Moussavi H, Babaei M, Akbari M. The association of immunoglobulin A, immunoglobulin G and anti-cyclic citrullinated peptide antibodies with disease activity in seronegative rheumatoid arthritis patients. J Res Med Sci. 2014;19(9):823–826. [PMC free article] [PubMed] [Google Scholar]
- 16.Yang YH, Huang YH, Lin YL, et al. Circulating IgA from acute stage of childhood Henoch-Schonlein purpura can enhance endothelial interleukin (IL)-8 production through MEK/ERK signalling pathway. Clin Exp Immunol. 2006;144(2):247–253. doi: 10.1111/j.1365-2249.2006.03076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Niro R, Mesin L, Zheng NY, et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med. 2012;18(3):441–445. doi: 10.1038/nm.2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercieca C, van der Horst-bruinsma IE, Borg AA. Pulmonary, renal and neurological comorbidities in patients with ankylosing spondylitis; implications for clinical practice. Curr Rheumatol Rep. 2014;16(8):434. doi: 10.1007/s11926-014-0434-7 [DOI] [PubMed] [Google Scholar]
- 19.Torruellas C, French SW, Medici V. Diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20(33):11684–11699. doi: 10.3748/wjg.v20.i33.11684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez E, Shattock RJ, Kent SJ, Chung AW. The multifaceted nature of immunoglobulin A and its complex role in HIV. AIDS Res Hum Retroviruses. 2018;34(9):727–738. doi: 10.1089/aid.2018.0099 [DOI] [PubMed] [Google Scholar]
- 21.Kremer EJ, Kalatzis V, Baker E, Callen DF, Sutherland GR, Maliszewski CR. The gene for the human IgA Fc receptor maps to 19q13.4. Hum Genet. 1992;89(1):107–108. [DOI] [PubMed] [Google Scholar]
- 22.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24(1):19–28. doi: 10.1016/j.immuni.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 23.Monteiro RC, Van De Winkel JG. IGAFC receptors. Annu Rev Immunol. 2003;21(1):177–204. doi: 10.1146/annurev.immunol.21.120601.141011 [DOI] [PubMed] [Google Scholar]
- 24.de Wit TP, Morton HC, Capel PJ, van de Winkel JG. Structure of the gene for the human myeloid IgA Fc receptor (CD89). J Immunol. 1995;155(3):1203–1209. [PubMed] [Google Scholar]
- 25.Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature. 2003;423(6940):614–620. doi: 10.1038/nature01685 [DOI] [PubMed] [Google Scholar]
- 26.Pfefferkorn LC, Yeaman GR. Association of IgA-Fc receptors (Fc alpha R) with Fc epsilon RI gamma 2 subunits in U937 cells. Aggregation induces the tyrosine phosphorylation of gamma 2. J Immunol. 1994;153(7):3228–3236. [PubMed] [Google Scholar]
- 27.van Egmond M, van Garderen E, van Spriel AB, et al. FcαRI-positive liver kupffer cells: reappraisal of the function of immunoglobulin A in immunity. Nat Med. 2000;6(6):680–685. doi: 10.1038/76261 [DOI] [PubMed] [Google Scholar]
- 28.Qian K, Xie F, Gibson AW, Edberg JC, Kimberly RP, Wu J. Functional expression of IgA receptor FcalphaRI on human platelets. J Leukoc Biol. 2008;84(6):1492–1500. doi: 10.1189/jlb.0508327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen IS, Krabbendam L, Bernink JH, et al. FcalphaRI co-stimulation converts human intestinal CD103(+) dendritic cells into pro-inflammatory cells through glycolytic reprogramming. Nat Commun. 2018;9(1):863. doi: 10.1038/s41467-018-03318-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heystek HC, Moulon C, Woltman AM, Garonne P, van Kooten C. Human immature dendritic cells efficiently bind and take up secretory IgA without the induction of maturation. J Immunol. 2002;168(1):102–107. [DOI] [PubMed] [Google Scholar]
- 31.Monteiro RC, Cooper MD, Kubagawa H. Molecular heterogeneity of Fc alpha receptors detected by receptor-specific monoclonal antibodies. J Immunol. 1992;148(6):1764–1770. [PubMed] [Google Scholar]
- 32.Reljic R. In search of the elusive mouse macrophage Fc-alpha receptor. Immunol Lett. 2006;107(1):80–81. doi: 10.1016/j.imlet.2006.04.014 [DOI] [PubMed] [Google Scholar]
- 33.Bakema JE, van Egmond M. The human immunoglobulin A Fc receptor FcalphaRI: a multifaceted regulator of mucosal immunity. Mucosal Immunol. 2011;4(6):612–624. doi: 10.1038/mi.2011.36 [DOI] [PubMed] [Google Scholar]
- 34.Bakema JE, Hiemstra IH, Bakker J, et al. c-Jun activating binding protein 1 binds to the IgA receptor and modulates protein levels of FcalphaRI and FcRgamma-chain. Eur J Immunol. 2010;40(7):2035–2040. doi: 10.1002/eji.200939985 [DOI] [PubMed] [Google Scholar]
- 35.Hostoffer RW, Krukovets I, Berger M. Increased Fc alpha R expression and IgA-mediated function on neutrophils induced by chemoattractants. J Immunol. 1993;150(10):4532–4540. [PubMed] [Google Scholar]
- 36.Nikolova EB, Russell MW. Dual function of human IgA antibodies: inhibition of phagocytosis in circulating neutrophils and enhancement of responses in IL-8-stimulated cells. J Leukoc Biol. 1995;57(6):875–882. doi: 10.1002/jlb.57.6.875 [DOI] [PubMed] [Google Scholar]
- 37.Yin N, Peng M, Xing Y, Zhang W. Intracellular pools of FcalphaR (CD89) in human neutrophils are localized in tertiary granules and secretory vesicles, and two FcalphaR isoforms are found in tertiary granules. J Leukoc Biol. 2007;82(3):551–558. doi: 10.1189/jlb.0207112 [DOI] [PubMed] [Google Scholar]
- 38.Wehrli M, Schneider C, Cortinas-Elizondo F, et al. IgA triggers cell death of neutrophils when primed by inflammatory mediators. J Immunol. 2020. [DOI] [PubMed] [Google Scholar]
- 39.Maliszewski CR, Shen L, Fanger MW. The expression of receptors for IgA on human monocytes and calcitriol-treated HL-60 cells. J Immunol. 1985;135(6):3878–3881. [PubMed] [Google Scholar]
- 40.Shen L, Collins JE, Schoenborn MA, Maliszewski CR. Lipopolysaccharide and cytokine augmentation of human monocyte IgA receptor expression and function. J Immunol. 1994;152(8):4080–4086. [PubMed] [Google Scholar]
- 41.Reterink TJ, Levarht EW, Klar-Mohamad N, Van Es LA, Daha MR. Transforming growth factor-beta 1 (TGF-beta 1) down-regulates IgA Fc-receptor (CD89) expression on human monocytes. Clin Exp Immunol. 1996;103(1):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossetete B, Launay P, Lehuen A, Jungers P, Bach JF, Monteiro RC. Down-regulation of Fc alpha receptors on blood cells of IgA nephropathy patients: evidence for a negative regulatory role of serum IgA. Kidney Int. 1998;53(5):1321–1335. doi: 10.1046/j.1523-1755.1998.00885.x [DOI] [PubMed] [Google Scholar]
- 43.Kawamura S, Saitou N, Ueda S. Concerted evolution of the primate immunoglobulin alpha-gene through gene conversion. J Biol Chem. 1992;267(11):7359–7367. [PubMed] [Google Scholar]
- 44.Pinheiro A, de Sousa-pereira P, Strive T, et al. Identification of a new European rabbit IgA with a serine-rich hinge region. PLoS One. 2018;13(8):e0201567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Sousa-pereira P, Woof JM. IgA: structure, function, and developability. Antibodies (Basel). 2019;8(4). doi: 10.3390/antib8040057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steffen U, Koeleman CA, Sokolova MV, et al. IgA subclasses have different effector functions associated with distinct glycosylation profiles. Nat Commun. 2020;11(1):120. doi: 10.1038/s41467-019-13992-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen EV. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104(5):321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x [DOI] [PubMed] [Google Scholar]
- 48.Tarelli E, Smith AC, Hendry BM, Challacombe SJ, Pouria S. Human serum IgA1 is substituted with up to six O-glycans as shown by matrix assisted laser desorption ionisation time-of-flight mass spectrometry. Carbohydr Res. 2004;339(13):2329–2335. doi: 10.1016/j.carres.2004.07.011 [DOI] [PubMed] [Google Scholar]
- 49.Royle L, Roos A, Harvey DJ, Wormald MR, van Gijlswijk-janssen D. Secretory IgA N- and O-glycans provide a link between the innate and adaptive immune systems. J Biol Chem. 2003;278(22):20140–20153. doi: 10.1074/jbc.M301436200 [DOI] [PubMed] [Google Scholar]
- 50.Novak J, Barratt J, Julian BA, Renfrow MB. Aberrant glycosylation of the IgA1 molecule in IgA nephropathy. Semin Nephrol. 2018;38(5):461–476. doi: 10.1016/j.semnephrol.2018.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerr MA. The structure and function of human IgA. Biochem J. 1990;271(2):285–296. doi: 10.1042/bj2710285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunker JJ, Bendelac A. IgA responses to microbiota. Immunity. 2018;49(2):211–224. doi: 10.1016/j.immuni.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Wang G, Li Y, et al. Structural insights into secretory immunoglobulin A and its interaction with a pneumococcal adhesin. Cell Res. 2020;30(7):602–609. doi: 10.1038/s41422-020-0336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunol. 2011;4(6):598–602. doi: 10.1038/mi.2011.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandtzaeg P, Prydz H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature. 1984;311(5981):71–73. doi: 10.1038/311071a0 [DOI] [PubMed] [Google Scholar]
- 56.Kumar N, Arthur CP, Ciferri C, Matsumoto ML. Structure of the secretory immunoglobulin A core. Science. 2020;367(6481):1008–1014. doi: 10.1126/science.aaz5807 [DOI] [PubMed] [Google Scholar]
- 57.Herr AB. Secret(ory) revealed: the long-awaited structures of secretory IgA. Cell Res. 2020;30(7):558–559. doi: 10.1038/s41422-020-0351-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Spriel AB, Leusen JH, Vile H. Mac-1 (CD11b/CD18) as accessory molecule for Fc alpha R (CD89) binding of IgA. J Immunol. 2002;169(7):3831–3836. doi: 10.4049/jimmunol.169.7.3831 [DOI] [PubMed] [Google Scholar]
- 59.Reterink TJ, van Zandbergen G, van Egmond M, et al. Size-dependent effect of IgA on the IgA Fc receptor (CD89). Eur J Immunol. 1997;27(9):2219–2224. doi: 10.1002/eji.1830270915 [DOI] [PubMed] [Google Scholar]
- 60.Pleass RJ, Dehal PK, Lewis MJ, Woof JM. Limited role of charge matching in the interaction of human immunoglobulin A with the immunoglobulin A Fc receptor (FcalphaRI) CD89. Immunology. 2003;109(3):331–335. doi: 10.1046/j.1365-2567.2003.01677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Posgai MT, Tonddast-Navaei S, Jayasinghe M, Ibrahim GM, Stan G, Herr AB. FcalphaRI binding at the IgA1 CH2-CH3 interface induces long-range conformational changes that are transmitted to the hinge region. Proc Natl Acad Sci U S A. 2018;115(38):E8882–E91. doi: 10.1073/pnas.1807478115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Boesen CC, Radaev S, et al. Crystal structure of the extracellular domain of a human Fc gamma RIII. Immunity. 2000;13(3):387–395. doi: 10.1016/S1074-7613(00)00038-8 [DOI] [PubMed] [Google Scholar]
- 63.Keown MB, Henry AJ, Ghirlando R, Sutton BJ, Gould HJ. Thermodynamics of the interaction of human immunoglobulin E with its high-affinity receptor Fc epsilon RI. Biochemistry. 1998;37(25):8863–8869. doi: 10.1021/bi972354h [DOI] [PubMed] [Google Scholar]
- 64.Keown MB, Ghirlando R, Mackay GA, Sutton BJ, Gould HJ. Basis of the 1:1 stoichiometry of the high affinity receptor Fc epsilon RI-IgE complex. Eur Biophys J. 1997;25(5–6):471–476. doi: 10.1007/s002490050062 [DOI] [PubMed] [Google Scholar]
- 65.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406(6793):267–273. doi: 10.1038/35018508 [DOI] [PubMed] [Google Scholar]
- 66.Bonner A, Furtado PB, Almogren A, Kerr MA, Perkins SJ. Implications of the near-planar solution structure of human myeloma dimeric IgA1 for mucosal immunity and IgA nephropathy. J Immunol. 2008;180(2):1008–1018. doi: 10.4049/jimmunol.180.2.1008 [DOI] [PubMed] [Google Scholar]
- 67.Lu J, Marjon KD, Mold C, Marnell L, Du Clos TW, Sun P. Pentraxins and IgA share a binding hot-spot on FcalphaRI. Protein Sci. 2014;23(4):378–386. doi: 10.1002/pro.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xue J, Zhao Q, Zhu L, Zhang W. Deglycosylation of FcalphaR at N58 increases its binding to IgA. Glycobiology. 2010;20(7):905–915. doi: 10.1093/glycob/cwq048 [DOI] [PubMed] [Google Scholar]
- 69.Bakema JE, Bakker A, de Haij S, Honing H, Bracke M, Koenderman L. Inside-out regulation of Fc alpha RI (CD89) depends on PP2A. J Immunol. 2008;181(6):4080–4088. doi: 10.1038/s41467-017-00294-0 [DOI] [PubMed] [Google Scholar]
- 70.Weisbart RH, Kacena A, Schuh A, Golde DW. GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature. 1988;332(6165):647–648. doi: 10.1038/332647a0 [DOI] [PubMed] [Google Scholar]
- 71.Bracke M, Dubois GR, Bolt K, et al. Differential effects of the T helper cell type 2-derived cytokines IL-4 and IL-5 on ligand binding to IgG and IgA receptors expressed by human eosinophils. J Immunol. 1997;159(3):1459–1465. [PubMed] [Google Scholar]
- 72.Morton HC, van den Herik-oudijk IE, Vossebeld P, et al. Functional association between the human myeloid immunoglobulin A Fc receptor (CD89) and FcR gamma chain. Molecular basis for CD89/FcR gamma chain association. J Biol Chem. 1995;270(50):29781–29787. doi: 10.1074/jbc.270.50.29781 [DOI] [PubMed] [Google Scholar]
- 73.Pfirsch-Maisonnas S, Aloulou M, Xu T, et al. Inhibitory ITAM signaling traps activating receptors with the phosphatase SHP-1 to form polarized “inhibisome” clusters. Sci Signal. 2011;4(169):ra24. doi: 10.1126/scisignal.2001309 [DOI] [PubMed] [Google Scholar]
- 74.Lang ML, Chen YW, Shen L, et al. IgA Fc receptor (FcalphaR) cross-linking recruits tyrosine kinases, phosphoinositide kinases and serine/threonine kinases to glycolipid rafts. Biochem J. 2002;364(Pt 2):517–525. doi: 10.1042/bj20011696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gulle H, Samstag A, Eibl MM, Wolf HM. Physical and functional association of Fc alpha R with protein tyrosine kinase Lyn. Blood. 1998;91(2):383–391. doi: 10.1182/blood.V91.2.383 [DOI] [PubMed] [Google Scholar]
- 76.Mkaddem SB, Murua A, Flament H, et al. Lyn and Fyn function as molecular switches that control immunoreceptors to direct homeostasis or inflammation. Nat Commun. 2017;8(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Getahun A, Cambier JC. Of ITIMs, ITAMs, and ITAMis: revisiting immunoglobulin Fc receptor signaling. Immunol Rev. 2015;268(1):66–73. doi: 10.1111/imr.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aleyd E, van Hout MW, Ganzevles SH, et al. IgA enhances NETosis and release of neutrophil extracellular traps by polymorphonuclear cells via Fcalpha receptor I. J Immunol. 2014;192(5):2374–2383. doi: 10.4049/jimmunol.1300261 [DOI] [PubMed] [Google Scholar]
- 79.van der Steen L, Tuk CW, Bakema JE, et al. Immunoglobulin A: Fc(alpha)RI interactions induce neutrophil migration through release of leukotriene B4. Gastroenterology. 2009;137(6):2018-29e1–3. doi: 10.1053/j.gastro.2009.06.047 [DOI] [PubMed] [Google Scholar]
- 80.Vidarsson G, van Der Pol WL, van Den Elsen JM, et al. Activity of human IgG and IgA subclasses in immune defense against neisseria meningitidis serogroup B. J Immunol. 2001;166(10):6250–6256. doi: 10.4049/jimmunol.166.10.6250 [DOI] [PubMed] [Google Scholar]
- 81.Geissmann F, Launay P, Pasquier B, et al. A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J Immunol. 2001;166(1):346–352. doi: 10.4049/jimmunol.166.1.346 [DOI] [PubMed] [Google Scholar]
- 82.Chen YW, Lang ML, Wade WF. Protein kinase C-alpha and -delta are required for FcalphaR (CD89) trafficking to MHC class II compartments and FcalphaR-mediated antigen presentation. Traffic. 2004;5(8):577–594. doi: 10.1111/j.1600-0854.2004.00202.x [DOI] [PubMed] [Google Scholar]
- 83.Pasquier B, Lepelletier Y, Baude C, Hermine O, Monteiro RC. Differential expression and function of IgA receptors (CD89 and CD71) during maturation of dendritic cells. J Leukoc Biol. 2004;76(6):1134–1141. doi: 10.1189/jlb.0204101 [DOI] [PubMed] [Google Scholar]
- 84.Fayette J, Dubois B, Vandenabeele S, et al. Human dendritic cells skew isotype switching of CD40-activated naive B cells towards IgA1 and IgA2. J Exp Med. 1997;185(11):1909–1918. doi: 10.1084/jem.185.11.1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baumann J, Park CG, Mantis NJ. Recognition of secretory IgA by DC-SIGN: implications for immune surveillance in the intestine. Immunol Lett. 2010;131(1):59–66. doi: 10.1016/j.imlet.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. doi: 10.3389/fimmu.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rogier EW, Frantz AL, Bruno ME, Kaetzel CS. Secretory IgA is concentrated in the outer layer of colonic mucus along with gut bacteria. Pathogens. 2014;3(2):390–403. doi: 10.3390/pathogens3020390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4(6):603–611. doi: 10.1038/mi.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan H, Lamm ME, Bjorling E, Huang YT. Multiple functions of immunoglobulin A in mucosal defense against viruses: an in vitro measles virus model. J Virol. 2002;76(21):10972–10979. doi: 10.1128/JVI.76.21.10972-10979.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci U S A. 1992;89(15):6901–6905. doi: 10.1073/pnas.89.15.6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mantis NJ, Palaia J, Hessell AJ, et al. Inhibition of HIV-1 infectivity and epithelial cell transfer by human monoclonal IgG and IgA antibodies carrying the b12 V region. J Immunol. 2007;179(5):3144–3152. doi: 10.4049/jimmunol.179.5.3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wright A, Lamm ME, Huang YT. Excretion of human immunodeficiency virus type 1 through polarized epithelium by immunoglobulin A. J Virol. 2008;82(23):11526–11535. doi: 10.1128/JVI.01111-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kulkarni V, Ruprecht RM. Mucosal IgA responses: damaged in established HIV infection-yet, effective weapon against HIV transmission. Front Immunol. 2017;8:1581. doi: 10.3389/fimmu.2017.01581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blutt SE, Conner ME. The gastrointestinal frontier: IgA and viruses. Front Immunol. 2013;4:402. doi: 10.3389/fimmu.2013.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sutherland DB, Suzuki K, Fagarasan S. Fostering of advanced mutualism with gut microbiota by immunoglobulin A. Immunol Rev. 2016;270(1):20–31. doi: 10.1111/imr.12384 [DOI] [PubMed] [Google Scholar]
- 96.Kawamoto S, Maruya M, Kato LM, et al. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41(1):152–165. doi: 10.1016/j.immuni.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 97.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural salmonella typhimurium infection. J Exp Med. 2006;203(1):21–26. doi: 10.1084/jem.20052093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reikvam DH, Derrien M, Islam R, et al. Epithelial-microbial crosstalk in polymeric Ig receptor deficient mice. Eur J Immunol. 2012;42(11):2959–2970. doi: 10.1002/eji.201242543 [DOI] [PubMed] [Google Scholar]
- 99.Catanzaro JR, Strauss JD, Bielecka A, et al. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci Rep. 2019;9(1):13574. doi: 10.1038/s41598-019-49923-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kabbert J, Benckert J, Rollenske T, et al. High microbiota reactivity of adult human intestinal IgA requires somatic mutations. J Exp Med. 2020;217(11):11. doi: 10.1084/jem.20200275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bunker JJ, Erickson SA, Flynn TM, et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017;358(6361):6361. doi: 10.1126/science.aan6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakajima A, Vogelzang A, Maruya M, et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J Exp Med. 2018;215(8):2019–2034. doi: 10.1084/jem.20180427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilmore JR, Gaudette BT, Gomez Atria D, et al. Commensal microbes induce serum IgA responses that protect against polymicrobial sepsis. Cell Host Microbe. 2018;23(3):302–11 e3. doi: 10.1016/j.chom.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kozakova H, Schwarzer M, Tuckova L, et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cell Mol Immunol. 2016;13(2):251–262. doi: 10.1038/cmi.2015.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205(1):143–154. doi: 10.1084/jem.20071204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rochereau N, Drocourt D, Perouzel E, et al. Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol. 2013;11(9):e1001658. doi: 10.1371/journal.pbio.1001658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alpan O, Rudomen G, Matzinger P. The role of dendritic cells, B cells, and M cells in gut-oriented immune responses. J Immunol. 2001;166(8):4843–4852. doi: 10.4049/jimmunol.166.8.4843 [DOI] [PubMed] [Google Scholar]
- 108.Van Epps DE, Williams RC Jr. Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med. 1976;144(5):1227–1242. doi: 10.1084/jem.144.5.1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilton JM. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978;34(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- 110.Wolf HM, Fischer MB, Puhringer H, Samstag A, Vogel E, Eibl MM. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood. 1994;83(5):1278–1288. doi: 10.1182/blood.V83.5.1278.1278 [DOI] [PubMed] [Google Scholar]
- 111.Olas K, Butterweck H, Teschner W, Schwarz HP, Reipert B. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol. 2005;140(3):478–490. doi: 10.1111/j.1365-2249.2005.02779.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rogier EW, Frantz AL, Bruno ME, et al. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A. 2014;111(8):3074–3079. doi: 10.1073/pnas.1315792111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3(4):442–474. doi: 10.3390/nu3040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gopalakrishna KP, Hand TW. Influence of maternal milk on the neonatal intestinal microbiome. Nutrients. 2020;12(3):823. doi: 10.3390/nu12030823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. Bellagio child survival study G. How many child deaths can we prevent this year? Lancet. 2003;362(9377):65–71. doi: 10.1016/S0140-6736(03)13811-1 [DOI] [PubMed] [Google Scholar]
- 116.Gopalakrishna KP, Macadangdang BR, Rogers MB, et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat Med. 2019;25(7):1110–1115. doi: 10.1038/s41591-019-0480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rognum TO, Thrane S, Stoltenberg L, Vege A, Brandtzaeg P. Development of intestinal mucosal immunity in fetal life and the first postnatal months. Pediatr Res. 1992;32(2):145–149. doi: 10.1203/00006450-199208000-00003 [DOI] [PubMed] [Google Scholar]
- 118.Brandtzaeg P, Nilssen DE, Rognum TO, Thrane PS. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol Clin North Am. 1991;20(3):397–439. [PubMed] [Google Scholar]
- 119.Blanco E, Perez-Andres M, Arriba-Mendez S, et al. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J Allergy Clin Immunol. 2018;141(6):2208–19 e16. doi: 10.1016/j.jaci.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 120.Miller EM, McConnell DS. Milk immunity and reproductive status among Ariaal women of northern Kenya. Ann Hum Biol. 2015;42(1):76–83. doi: 10.3109/03014460.2014.941398 [DOI] [PubMed] [Google Scholar]
- 121.Fujita M, Wander K, Paredes Ruvalcaba N, Brindle E. Human milk sIgA antibody in relation to maternal nutrition and infant vulnerability in northern Kenya. Evol Med Public Health. 2019;2019(1):201–211. doi: 10.1093/emph/eoz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gorter A, Hiemstra PS, Leijh PC, et al. IgA- and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology. 1987;61(3):303–309. [PMC free article] [PubMed] [Google Scholar]
- 123.Duc M, Johansen F-E, Corthesy B. Antigen binding to secretory immunoglobulin A results in decreased sensitivity to intestinal proteases and increased binding to cellular Fc receptors. J Biol Chem. 2010;285(2):953–960. doi: 10.1074/jbc.M109.059220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trend S, de Jong E, Lloyd ML, et al. Leukocyte populations in human preterm and term breast milk identified by multicolour flow cytometry. PLoS One. 2015;10(8):e0135580. doi: 10.1371/journal.pone.0135580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Honorio-Franca AC, Launay P, Carneiro-Sampaio MM, Monteiro RC. Colostral neutrophils express Fc alpha receptors (CD89) lacking gamma chain association and mediate noninflammatory properties of secretory IgA. J Leukoc Biol. 2001;69(2):289–296. [PubMed] [Google Scholar]
- 126.Zimmermann N, Thormann V, Hu B, et al. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol Med. 2016;8(11):1325–1339. doi: 10.15252/emmm.201606330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Magri G, Cerutti A. IgA summons IgG to take a hit at HIV-1. Cell Host Microbe. 2020;27(6):854–856. doi: 10.1016/j.chom.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chao YX, Rotzschke O, Tan EK. The role of IgA in COVID-19. Brain Behav Immun. 2020;87:182–183. doi: 10.1016/j.bbi.2020.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Riley RL, Mills CC, Nyka W, et al. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. 1959. Am J Epidemiol. 1995;142(1):3–14. doi: 10.1093/oxfordjournals.aje.a117542 [DOI] [PubMed] [Google Scholar]
- 130.Bijlsma MW, Bekker V, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. Epidemiology of invasive meningococcal disease in the Netherlands, 1960–2012: an analysis of national surveillance data. Lancet Infect Dis. 2014;14(9):805–812. doi: 10.1016/S1473-3099(14)70806-0 [DOI] [PubMed] [Google Scholar]
- 131.Zhang Q, Finn A. Mucosal immunology of vaccines against pathogenic nasopharyngeal bacteria. J Clin Pathol. 2004;57(10):1015–1021. doi: 10.1136/jcp.2004.016253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tuomanen EI, Austrian R, Masure HR. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332(19):1280–1284. doi: 10.1056/NEJM199505113321907 [DOI] [PubMed] [Google Scholar]
- 133.Musher DM, Groover JE, Reichler MR, et al. Emergence of antibody to capsular polysaccharides of Streptococcus pneumoniae during outbreaks of pneumonia: association with nasopharyngeal colonization. Clin Infect Dis. 1997;24(3):441–446. doi: 10.1093/clinids/24.3.441 [DOI] [PubMed] [Google Scholar]
- 134.Wu M, Zhao H, Li M, Yue Y, Xiong S, Xu W. Intranasal vaccination with mannosylated chitosan formulated DNA vaccine enables robust IgA and cellular response induction in the lungs of mice and improves protection against pulmonary mycobacterial challenge. Front Cell Infect Microbiol. 2017;7:445. doi: 10.3389/fcimb.2017.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferreira DM, Darrieux M, Silva DA, et al. Characterization of protective mucosal and systemic immune responses elicited by pneumococcal surface protein PspA and PspC nasal vaccines against a respiratory pneumococcal challenge in mice. Clin Vaccine Immunol. 2009;16(5):636–645. doi: 10.1128/CVI.00395-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jia M, Liberatore RA, Guo Y, et al. VSV-displayed HIV-1 envelope identifies broadly neutralizing antibodies class-switched to IgG and IgA. Cell Host Microbe. 2020;27(6):963–75 e5. doi: 10.1016/j.chom.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Watkins JD, Sholukh AM, Mukhtar MM, et al. Anti-HIV IgA isotypes: differential virion capture and inhibition of transcytosis are linked to prevention of mucosal R5 SHIV transmission. AIDS. 2013;27(9):F13–20. doi: 10.1097/QAD.0b013e328360eac6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sholukh AM, Watkins JD, Vyas HK, et al. Defense-in-depth by mucosally administered anti-HIV dimeric IgA2 and systemic IgG1 mAbs: complete protection of rhesus monkeys from mucosal SHIV challenge. Vaccine. 2015;33(17):2086–2095. doi: 10.1016/j.vaccine.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Johansen K, Svensson L. Neutralization of rotavirus and recognition of immunologically important epitopes on VP4 and VP7 by human IgA. Arch Virol. 1997;142(7):1491–1498. doi: 10.1007/s007050050175 [DOI] [PubMed] [Google Scholar]
- 140.To TL, Ward LA, Yuan L, Saif LJ. Serum and intestinal isotype antibody responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Gen Virol. 1998;79(Pt 11):2661–2672. [DOI] [PubMed] [Google Scholar]
- 141.Crawford SE, Patel DG, Cheng E, et al. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J Virol. 2006;80(10):4820–4832. doi: 10.1128/JVI.80.10.4820-4832.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Feng N, Burns JW, Bracy L, Greenberg HB. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol. 1994;68(12):7766–7773. doi: 10.1128/JVI.68.12.7766-7773.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5(52):52. doi: 10.1126/sciimmunol.abe0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kowitdamrong E, Puthanakit T, Jantarabenjakul W, et al. Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019. PLoS One. 2020;15(10):e0240502. doi: 10.1371/journal.pone.0240502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sterlin D, Mathian A, Miyara M, et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. medRxiv. 2020;2020.06.10.20126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xue M, Zhang T, Hu H, et al. Predictive effects of IgA and IgG combination to assess pulmonary exudation progression in COVID-19 patients. J Med Virol. 2020. doi: 10.1002/jmv.26437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ejemel M, Li Q, Hou S, et al. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat Commun. 2020;11(1):4198. doi: 10.1038/s41467-020-18058-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hasan Ali O, Bomze D, Risch L, et al. Severe COVID-19 is associated with elevated serum IgA and antiphospholipid IgA-antibodies. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu HQ, Sun BQ, Fang ZF, et al. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur Respir J. 2020;56(2):2001526. doi: 10.1183/13993003.01526-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fourati S, Hue S, Pawlotsky JM, Mekontso-Dessap A, de Prost N. SARS-CoV-2 viral loads and serum IgA/IgG immune responses in critically ill COVID-19 patients. Intensive Care Med. 2020;46(9):1781–1783. doi: 10.1007/s00134-020-06157-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545–1548. doi: 10.1172/JCI138003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu STH, Lin HM, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9 [DOI] [PubMed] [Google Scholar]
- 154.Ye M, Fu D, Ren Y, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92(10):1890–1901. doi: 10.1002/jmv.25882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680–1690. doi: 10.1016/j.ajpath.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Perotti C, Baldanti F, Bruno R, et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020. doi: 10.3324/haematol.2020.261784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602–611. doi: 10.1038/ni.2921 [DOI] [PubMed] [Google Scholar]
- 158.Hellwig SM, van Spriel AB, Schellekens JF, Mooi FR, van de Winkel JG. Immunoglobulin A-mediated protection against bordetella pertussis infection. Infect Immun. 2001;69(8):4846–4850. doi: 10.1128/IAI.69.8.4846-4850.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van Spriel AB, van den Herik-oudijk IE, van Sorge NM, Vile HA, van Strijp JA, van de Winkel J. Effective phagocytosis and killing of Candida albicans via targeting FcγRI (CD64) or FcαRI (CD89) on neutrophils. J Infect Dis. 1999;179(3):661–669. doi: 10.1086/314643 [DOI] [PubMed] [Google Scholar]
- 160.van der Pol W, Vidarsson G, Vile HA, van de Winkel JG, Rodriguez ME. Pneumococcal capsular polysaccharide-specific IgA triggers efficient neutrophil effector functions via FcalphaRI (CD89). J Infect Dis. 2000;182(4):1139–1145. doi: 10.1086/315825 [DOI] [PubMed] [Google Scholar]
- 161.Kobayashi T, Yamamoto K, Sugita N, et al. Effective in vitro clearance ofPorphyromonas gingivalis by Fcα receptor I (CD89) on gingival crevicular neutrophils. Infect Immun. 2001;69(5):2935–2942. doi: 10.1128/IAI.69.5.2935-2942.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shin EH, Lee HY, Bae YS. Leukotriene B4 stimulates human monocyte-derived dendritic cell chemotaxis. Biochem Biophys Res Commun. 2006;348(2):606–611. doi: 10.1016/j.bbrc.2006.07.084 [DOI] [PubMed] [Google Scholar]
- 163.Subramanian BC, Majumdar R, Parent CA. The role of the LTB4-BLT1 axis in chemotactic gradient sensing and directed leukocyte migration. Semin Immunol. 2017;33:16–29. doi: 10.1016/j.smim.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patry C, Sibille Y, Lehuen A, Monteiro RC. Identification of Fc alpha receptor (CD89) isoforms generated by alternative splicing that are differentially expressed between blood monocytes and alveolar macrophages. J Immunol. 1996;156(11):4442–4448. [PubMed] [Google Scholar]
- 165.Lu LL, Chung AW, Rosebrock TR, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433–43 e14. doi: 10.1016/j.cell.2016.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Legesse M, Ameni G, Medhin G, et al. IgA response to ESAT-6/CFP-10 and Rv2031 antigens varies in patients with culture-confirmed pulmonary tuberculosis, healthy Mycobacterium tuberculosis-infected and non-infected individuals in a tuberculosis endemic setting, Ethiopia. Scand J Immunol. 2013;78(3):266–274. doi: 10.1111/sji.12080 [DOI] [PubMed] [Google Scholar]
- 167.de Tymowski C, Heming N, Correia MDT, et al. CD89 is a potent innate receptor for bacteria and mediates host protection from sepsis. Cell Rep. 2019;27(3):762–75 e5. doi: 10.1016/j.celrep.2019.03.062 [DOI] [PubMed] [Google Scholar]
- 168.Tay MZ, Wiehe K, Pollara J. Antibody-dependent cellular phagocytosis in antiviral immune responses. Front Immunol. 2019;10:332. doi: 10.3389/fimmu.2019.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ackerman ME, Mikhailova A, Brown EP, et al. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 2016;12(1):e1005315. doi: 10.1371/journal.ppat.1005315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Duchemin M, Khamassi M, Xu L, Tudor D, Bomsel M. IgA targeting human immunodeficiency virus-1 envelope gp41 triggers antibody-dependent cellular cytotoxicity cross-clade and cooperates with gp41-specific IgG to increase cell lysis. Front Immunol. 2018;9:244. doi: 10.3389/fimmu.2018.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Duchemin M, Tudor D, Cottignies-Calamarte A, Bomsel M. Antibody-dependent cellular phagocytosis of HIV-1-infected cells is efficiently triggered by IgA targeting HIV-1 envelope subunit gp41. Front Immunol. 2020;11:1141. doi: 10.3389/fimmu.2020.01141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ackerman ME, Das J, Pittala S, et al. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat Med. 2018;24(10):1590–1598. doi: 10.1038/s41591-018-0161-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492 [DOI] [PubMed] [Google Scholar]
- 174.Uren TK, Johansen FE, Wijburg OL, Koentgen F, Brandtzaeg P, Strugnell RA. Role of the polymeric Ig receptor in mucosal B cell homeostasis. J Immunol. 2003;170(5):2531–2539. doi: 10.4049/jimmunol.170.5.2531 [DOI] [PubMed] [Google Scholar]
- 175.Hansen IS, Hoepel W, Zaat SAJ, Baeten DLP, den Dunnen J. Serum IgA immune complexes promote proinflammatory cytokine production by human macrophages, monocytes, and kupffer cells through FcalphaRI-TLR cross-talk. J Immunol. 2017;199(12):4124–4131. doi: 10.4049/jimmunol.1700883 [DOI] [PubMed] [Google Scholar]
- 176.Hansen IS, Baeten DLP, den Dunnen J. The inflammatory function of human IgA. Cell Mol Life Sci. 2019;76(6):1041–1055. doi: 10.1007/s00018-018-2976-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Woof JM. Immunoglobulins and their receptors, and subversion of their protective roles by bacterial pathogens. Biochem Soc Trans. 2016;44(6):1651–1658. doi: 10.1042/BST20160246 [DOI] [PubMed] [Google Scholar]
- 178.Fasching CE, Grossman T, Corthesy B, Plaut AG, Weiser JN, Janoff EN. Impact of the molecular form of immunoglobulin A on functional activity in defense against Streptococcus pneumoniae. Infect Immun. 2007;75(4):1801–1810. doi: 10.1128/IAI.01758-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Janoff EN, Rubins JB, Fasching C, et al. Pneumococcal IgA1 protease subverts specific protection by human IgA1. Mucosal Immunol. 2014;7(2):249–256. doi: 10.1038/mi.2013.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lin L, Ayala P, Larson J, et al. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol. 1997;24(5):1083–1094. doi: 10.1046/j.1365-2958.1997.4191776.x [DOI] [PubMed] [Google Scholar]
- 181.Vidarsson G, Overbeeke N, Stemerding AM, et al. Working mechanism of immunoglobulin A1 (IgA1) protease: cleavage of IgA1 antibody to neisseria meningitidis PorA requires de novo synthesis of IgA1 protease. Infect Immun. 2005;73(10):6721–6726. doi: 10.1128/IAI.73.10.6721-6726.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Polissi A, Pontiggia A, Feger G, et al. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66(12):5620–5629. doi: 10.1128/IAI.66.12.5620-5629.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pleass RJ, Areschoug T, Lindahl G, Woof JM. Streptococcal IgA-binding proteins bind in the Calpha 2-Calpha 3 interdomain region and inhibit binding of IgA to human CD89. J Biol Chem. 2001;276(11):8197–8204. doi: 10.1074/jbc.M009396200 [DOI] [PubMed] [Google Scholar]
- 184.Ramsland PA, Willoughby N, Trist HM, et al. Structural basis for evasion of IgA immunity by staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1. Proc Natl Acad Sci U S A. 2007;104(38):15051–15056. doi: 10.1073/pnas.0706028104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.D’Amico G. Natural history of idiopathic IgA nephropathy and factors predictive of disease outcome. Semin Nephrol. 2020;12(3):823–896. doi: 10.1016/j.semnephrol.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 186.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant. 2011;26(2):414–430. doi: 10.1093/ndt/gfq665 [DOI] [PubMed] [Google Scholar]
- 187.Novak J, Julian BA, Tomana M, Mestecky J. IgA glycosylation and IgA immune complexes in the pathogenesis of IgA nephropathy. Semin Nephrol. 2019;10(1):78–87. doi: 10.1016/j.semnephrol.2007.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen A, Yang SS, Lin TJ, Ka SM. IgA nephropathy: clearance kinetics of IgA-containing immune complexes. Semin Immunopathol. 2018;40(6):539–543. doi: 10.1007/s00281-018-0708-7 [DOI] [PubMed] [Google Scholar]
- 189.Gutierrez E, Carvaca-Fontan F, Luzardo L, Morales E, Alonso M, Praga M. A personalized update on IgA nephropathy: a new vision and new future challenges. Nephron. 2020;144(11):555–571. doi: 10.1159/000509997 [DOI] [PubMed] [Google Scholar]
- 190.Berthelot L, Papista C, Maciel TT, et al. Transglutaminase is essential for IgA nephropathy development acting through IgA receptors. J Exp Med. 2012;209(4):793–806. doi: 10.1084/jem.20112005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Launay P, Grossetete B, Arcos-Fajardo M, et al. Fcalpha receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger’s disease). Evidence for pathogenic soluble receptor-Iga complexes in patients and CD89 transgenic mice. J Exp Med. 2000;191(11):1999–2009. doi: 10.1084/jem.191.11.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)A1 and IgA2 isotypes. J Exp Med. 2020;27(6):963–975.e5. doi: 10.1084/jem.191.12.2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xu L, Li B, Huang M, et al. Critical role of kupffer cell CD89 expression in experimental IgA nephropathy. PLoS One. 2016;11(7):e0159426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 195.Moeez S, John P, Bhatti A. Anti-citrullinated protein antibodies: role in pathogenesis of RA and potential as a diagnostic tool. Rheumatol Int. 2013;33(7):1669–1673. doi: 10.1007/s00296-012-2635-6 [DOI] [PubMed] [Google Scholar]
- 196.Jonsson T, Arinbjarnarson S, Thorsteinsson J, et al. Raised IgA rheumatoid factor (RF) but not IgM RF or IgG RF is associated with extra-articular manifestations in rheumatoid arthritis. Scand J Rheumatol. 1995;24(6):372–375. doi: 10.3109/03009749509095183 [DOI] [PubMed] [Google Scholar]
- 197.Bobbio-Pallavicini F, Caporali R, Alpini C, et al. High IgA rheumatoid factor levels are associated with poor clinical response to tumour necrosis factor alpha inhibitors in rheumatoid arthritis. Ann Rheum Dis. 2007;66(3):302–307. doi: 10.1136/ard.2006.060608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50(2):380–386. doi: 10.1002/art.20018 [DOI] [PubMed] [Google Scholar]
- 199.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–2749. doi: 10.1002/art.11223 [DOI] [PubMed] [Google Scholar]
- 200.Kinslow JD, Blum LK, Deane KD, et al. Elevated IgA plasmablast levels in subjects at risk of developing rheumatoid arthritis. Arthritis Rheumatol. 2016;68(10):2372–2383. doi: 10.1002/art.39771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Svard A, Kastbom A, Soderlin MK, Reckner-Olsson A, Skogh T. A comparison between IgG- and IgA-class antibodies to cyclic citrullinated peptides and to modified citrullinated vimentin in early rheumatoid arthritis and very early arthritis. J Rheumatol. 2011;38(7):1265–1272. doi: 10.3899/jrheum.101086 [DOI] [PubMed] [Google Scholar]
- 202.Kokkonen H, Mullazehi M, Berglin E, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther. 2011;13(1):R13. doi: 10.1186/ar3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Anquetil F, Clavel C, Offer G, Serre G, Sebbag M. IgM and IgA rheumatoid factors purified from rheumatoid arthritis sera boost the Fc receptor- and complement-dependent effector functions of the disease-specific anti-citrullinated protein autoantibodies. J Immunol. 2015;194(8):3664–3674. doi: 10.4049/jimmunol.1402334 [DOI] [PubMed] [Google Scholar]
- 204.Du L, Wang P, Liu C, Li S, Yue S, Yang Y. Multisystemic manifestations of IgA vasculitis. Clin Rheumatol. 2020. doi: 10.1007/s10067-020-05166-5 [DOI] [PubMed] [Google Scholar]
- 205.Yang YH, Wang SJ, Chuang YH, Lin YT, Chiang BL. The level of IgA antibodies to human umbilical vein endothelial cells can be enhanced by TNF-alpha treatment in children with Henoch-Schonlein purpura. Clin Exp Immunol. 2002;130(2):352–357. doi: 10.1046/j.1365-2249.2002.01964.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Berthelot L, Jamin A, Viglietti D, et al. Value of biomarkers for predicting immunoglobulin A vasculitis nephritis outcome in an adult prospective cohort. Nephrol Dial Transplant. 2018;33(9):1579–1590. doi: 10.1093/ndt/gfx300 [DOI] [PubMed] [Google Scholar]
- 207.Sardy M, Karpati S, Merkl B, Paulsson M, Smyth N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J Exp Med. 2002;195(6):747–757. doi: 10.1084/jem.20011299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Taylor TB, Schmidt LA, Meyer LJ, Zone JJ. Transglutaminase 3 present in the IgA aggregates in dermatitis herpetiformis skin is enzymatically active and binds soluble fibrinogen. J Invest Dermatol. 2015;135(2):623–625. doi: 10.1038/jid.2014.368 [DOI] [PubMed] [Google Scholar]
- 209.Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. [DOI] [PubMed] [Google Scholar]
- 210.Smith AD, Streilein RD, Hall RP 3rd. Neutrophil CD11b, L-selectin and Fc IgA receptors in patients with dermatitis herpetiformis. Br J Dermatol. 2002;147(6):1109–1117. doi: 10.1046/j.1365-2133.2002.05004.x [DOI] [PubMed] [Google Scholar]
- 211.Gornowicz-Porowska J, Seraszek-Jaros A, Bowszyc-Dmochowska M, Kaczmarek E, Dmochowski M. Immunoexpression of IgA receptors (CD89, CD71) in dermatitis herpetiformis. Folia Histochem Cytobiol. 2017;55(4):212–220. doi: 10.5603/FHC.a2017.0024 [DOI] [PubMed] [Google Scholar]
- 212.Russo V, Klein T, Lim DJ, et al. Granzyme B is elevated in autoimmune blistering diseases and cleaves key anchoring proteins of the dermal-epidermal junction. Sci Rep. 2018;8(1):9690. doi: 10.1038/s41598-018-28070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Coppo R, Amore A, Roccatello D. Dietary antigens and primary immunoglobulin A nephropathy. J Am Soc Nephrol. 1992;2(10 Suppl):S173–80. [DOI] [PubMed] [Google Scholar]
- 214.Sitaru C, Zillikens D. Mechanisms of blister induction by autoantibodies. Exp Dermatol. 2005;14(12):861–875. doi: 10.1111/j.1600-0625.2005.00367.x [DOI] [PubMed] [Google Scholar]
- 215.van der Steen LP, Bakema JE, Sesarman A, et al. Blocking Fcalpha receptor I on granulocytes prevents tissue damage induced by IgA autoantibodies. J Immunol. 2012;189(4):1594–1601. doi: 10.4049/jimmunol.1101763 [DOI] [PubMed] [Google Scholar]
- 216.Park JH, Peyrin-Biroulet L, Eisenhut M, Shin JI. IBD immunopathogenesis: a comprehensive review of inflammatory molecules. Autoimmun Rev. 2017;16(4):416–426. doi: 10.1016/j.autrev.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 217.Palm NW, de Zoete MR, Cullen TW, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–1010. doi: 10.1016/j.cell.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.van der Waaij LA, Kroese FG, Visser A, et al. Immunoglobulin coating of faecal bacteria in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16(7):669–674. doi: 10.1097/01.meg.0000108346.41221.19 [DOI] [PubMed] [Google Scholar]
- 219.Mitsuyama K, Niwa M, Takedatsu H, et al. Antibody markers in the diagnosis of inflammatory bowel disease. World J Gastroenterol. 2016;22(3):1304–1310. doi: 10.3748/wjg.v22.i3.1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rengarajan S, Vivio EE, Parkes M, et al. Dynamic immunoglobulin responses to gut bacteria during inflammatory bowel disease. Gut Microbes. 2020;11(3):405–420. doi: 10.1080/19490976.2019.1626683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bakema JE, Tuk CW, van Vliet SJ, et al. Antibody-opsonized bacteria evoke an inflammatory dendritic cell phenotype and polyfunctional Th cells by cross-talk between TLRs and FcRs. J Immunol. 2015;194(4):1856–1866. doi: 10.4049/jimmunol.1303126 [DOI] [PubMed] [Google Scholar]
- 222.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208 [DOI] [PubMed] [Google Scholar]
- 223.Miquel-Clopes A, Bentley EG, Stewart JP, Carding SR. Mucosal vaccines and technology. Clin Exp Immunol. 2019;196(2):205–214. doi: 10.1111/cei.13285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev. 2017;114:116–131. doi: 10.1016/j.addr.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Thakur A, Rodriguez-Rodriguez C, Saatchi K, et al. Dual-isotope SPECT/CT imaging of the tuberculosis subunit vaccine H56/CAF01: induction of strong systemic and mucosal IgA and T-cell responses in mice upon subcutaneous prime and intrapulmonary boost immunization. Front Immunol. 2018;9:2825. doi: 10.3389/fimmu.2018.02825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Balu S, Reljic R, Lewis MJ, et al. A novel human IgA monoclonal antibody protects against tuberculosis. J Immunol. 2011;186(5):3113–3119. doi: 10.4049/jimmunol.1003189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Williams A, Reljic R, Naylor I, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111(3):328–333. doi: 10.1111/j.1365-2567.2004.01809.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Casadevall A. Antibodies to mycobacterium tuberculosis. N Engl J Med. 2017;376(3):283–285. doi: 10.1056/NEJMcibr1613268 [DOI] [PubMed] [Google Scholar]
- 229.Briles DE, Ades E, Paton JC, et al. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000;68(2):796–800. doi: 10.1128/IAI.68.2.796-800.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fukuyama Y, King JD, Kataoka K, Kobayashi R, Gilbert RS, Oishi K. Secretory-IgA antibodies play an important role in the immunity to Streptococcus pneumoniae. J Immunol. 2010;185(3):1755–1762. doi: 10.4049/jimmunol.1000831 [DOI] [PubMed] [Google Scholar]
- 231.Parker EP, Molodecky NA, Pons-Salort M, O’Reilly KM, Grassly NC. Impact of inactivated poliovirus vaccine on mucosal immunity: implications for the polio eradication endgame. Expert Rev Vaccines. 2015;14(8):1113–1123. doi: 10.1586/14760584.2015.1052800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis. 2013;208(2):284–294. doi: 10.1093/infdis/jit166 [DOI] [PubMed] [Google Scholar]
- 233.Blutt SE, Miller AD, Salmon SL, Metzger DW, Conner ME. IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol. 2012;5(6):712–719. doi: 10.1038/mi.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.McNeal MM, Stone SC, Basu M, et al. Protection against rotavirus shedding after intranasal immunization of mice with a chimeric VP6 protein does not require intestinal IgA. Virology. 2006;346(2):338–347. doi: 10.1016/j.virol.2005.11.016 [DOI] [PubMed] [Google Scholar]
- 235.McNeal MM, Ward RL. Long-term production of rotavirus antibody and protection against reinfection following a single infection of neonatal mice with murine rotavirus. Virology. 1995;211(2):474–480. doi: 10.1006/viro.1995.1429 [DOI] [PubMed] [Google Scholar]
- 236.Buisman AM, Abbink F, Schepp RM, Sonsma JA, Herremans T, Kimman TG. Preexisting poliovirus-specific IgA in the circulation correlates with protection against virus excretion in the elderly. J Infect Dis. 2008;197(5):698–706. doi: 10.1086/527487 [DOI] [PubMed] [Google Scholar]
- 237.Renegar KB, Small PA Jr., Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173(3):1978–1986. doi: 10.4049/jimmunol.173.3.1978 [DOI] [PubMed] [Google Scholar]
- 238.Tamura S, Funato H, Hirabayashi Y, et al. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21(6):1337–1344. doi: 10.1002/eji.1830210602 [DOI] [PubMed] [Google Scholar]
- 239.Hutchings AB, Helander A, Silvey KJ, et al. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer’s patches. J Virol. 2004;78(2):947–957. doi: 10.1128/JVI.78.2.947-957.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Boyaka PN. Inducing mucosal IgA: a challenge for vaccine adjuvants and delivery systems. J Immunol. 2017;199(1):9–16. doi: 10.4049/jimmunol.1601775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sterlin D, Gorochov G. When therapeutic IgA antibodies might come of age. Pharmacology. 2020;1–11. doi: 10.1159/000510251 [DOI] [PubMed] [Google Scholar]
- 242.Heemskerk N, van Egmond M. Monoclonal antibody-mediated killing of tumour cells by neutrophils. Eur J Clin Invest. 2018;48(Suppl 2):e12962. doi: 10.1111/eci.12962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lohse S, Loew S, Kretschmer A, Jansen JHM, Meyer S, Ten Broeke T. Effector mechanisms of IgA antibodies against CD20 include recruitment of myeloid cells for antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. Br J Haematol. 2018;181(3):413–417. doi: 10.1111/bjh.14624 [DOI] [PubMed] [Google Scholar]
- 244.Dechant M, Valerius T. IgA antibodies for cancer therapy. Crit Rev Oncol Hematol. 2001;39(1–2):69–77. doi: 10.1016/S1040-8428(01)00105-6 [DOI] [PubMed] [Google Scholar]
- 245.Valerius T, Stockmeyer B, van Spriel AB, et al. FcalphaRI (CD89) as a novel trigger molecule for bispecific antibody therapy. Blood. 1997;90(11):4485–4492. doi: 10.1182/blood.V90.11.4485 [DOI] [PubMed] [Google Scholar]
- 246.Bakema JE, van Egmond M. Immunoglobulin A: a next generation of therapeutic antibodies? MAbs. 2011;3(4):352–361. doi: 10.4161/mabs.3.4.16092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Boross P, Lohse S, Nederend M, et al. IgA EGFR antibodies mediate tumour killing in vivo. EMBO Mol Med. 2013;5(8):1213–1226. doi: 10.1002/emmm.201201929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Meyer S, Nederend M, Jansen JH, et al. Improved in vivo anti-tumor effects of IgA-Her2 antibodies through half-life extension and serum exposure enhancement by FcRn targeting. MAbs. 2016;8(1):87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Otten MA, Bakema JE, Tuk CW, et al. Enhanced FcalphaRI-mediated neutrophil migration towards tumour colonies in the presence of endothelial cells. Eur J Immunol. 2012;42(7):1815–1821. [DOI] [PubMed] [Google Scholar]
- 250.Otten MA, Rudolph E, Dechant M, et al. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol. 2005;174(9):5472–5480. doi: 10.4049/jimmunol.174.9.5472 [DOI] [PubMed] [Google Scholar]
- 251.Bakema JE, Ganzevles SH, Fluitsma DM, et al. Targeting FcalphaRI on polymorphonuclear cells induces tumor cell killing through autophagy. J Immunol. 2011;187(2):726–732. doi: 10.4049/jimmunol.1002581 [DOI] [PubMed] [Google Scholar]
- 252.Kelton W, Mehta N, Charab W, et al. IgGA: a “cross-isotype” engineered human Fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem Biol. 2014;21(12):1603–1609. doi: 10.1016/j.chembiol.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 253.Borrok MJ, Luheshi NM, Beyaz N, et al. Enhancement of antibody-dependent cell-mediated cytotoxicity by endowing IgG with FcalphaRI (CD89) binding. MAbs. 2015;7(4):743–751. doi: 10.1080/19420862.2015.1047570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ben Mkaddem S, Rossato E, Heming N, Monteiro RC. Anti-inflammatory role of the IgA Fc receptor (CD89): from autoimmunity to therapeutic perspectives. Autoimmun Rev. 2013;12(6):666–669. doi: 10.1016/j.autrev.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 255.Rossato E, Ben Mkaddem S, Kanamaru Y, et al. Reversal of arthritis by human monomeric IgA through the receptor-mediated SH2 domain-containing phosphatase 1 inhibitory pathway. Arthritis Rheumatol. 2015;67(7):1766–1777. doi: 10.1002/art.39142 [DOI] [PubMed] [Google Scholar]
- 256.Kanamaru Y, Pfirsch S, Aloulou M, et al. Inhibitory ITAM signaling by Fc alpha RI-FcR gamma chain controls multiple activating responses and prevents renal inflammation. J Immunol. 2008;180(4):2669–2678. doi: 10.4049/jimmunol.180.4.2669 [DOI] [PubMed] [Google Scholar]
- 257.Watanabe T, Kanamaru Y, Liu C, et al. Negative regulation of inflammatory responses by immunoglobulin A receptor (FcalphaRI) inhibits the development of toll-like receptor-9 signalling-accelerated glomerulonephritis. Clin Exp Immunol. 2011;166(2):235–250. doi: 10.1111/j.1365-2249.2011.04452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu C, Kanamaru Y, Watanabe T, et al. Targeted IgA Fc receptor I (FcalphaRI) therapy in the early intervention and treatment of pristane-induced lupus nephritis in mice. Clin Exp Immunol. 2015;181(3):407–416. doi: 10.1111/cei.12647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Aleyd E, Al M, Tuk CW, van der Laken CJ, van Egmond M. IgA complexes in plasma and synovial fluid of patients with rheumatoid arthritis induce neutrophil extracellular traps via FcalphaRI. J Immunol. 2016;197(12):4552–4559. doi: 10.4049/jimmunol.1502353 [DOI] [PubMed] [Google Scholar]
- 260.Heineke MH, van der Steen LPE, Korthouwer RM, et al. Peptide mimetics of immunoglobulin A (IgA) and FcalphaRI block IgA-induced human neutrophil activation and migration. Eur J Immunol. 2017;47(10):1835–1845. doi: 10.1002/eji.201646782 [DOI] [PMC free article] [PubMed] [Google Scholar]