Abstract
Background
Acute kidney injury (AKI) is associated with increased mortality in patients with acute respiratory distress syndrome (ARDS). However, the epidemiological features and outcomes of AKI among COVID-19 patients with ARDS are unknown.
Methods
We retrospectively recruited consecutive adult COVID-19 patients who were diagnosed with ARDS according to Berlin definition from 13 designated intensive care units in the city of Wuhan, China. Potential risk factors of AKI as well as the relation between AKI and in-hospital mortality were investigated.
Results
A total of 275 COVID-19 patients with ARDS were included in the study, and 49.5% of them developed AKI during their hospital stay. In comparison with patients without AKI, patients who developed AKI were older, tended to have chronic kidney disease, had higher Sepsis-Related Organ Failure Assessment score on day 1, and were more likely to receive invasive ventilation and develop acute organ dysfunction. Multivariate analysis showed that age, history of chronic kidney disease, neutrophil-to-lymphocyte ratio, and albumin level were independently associated with the occurrence of AKI. Importantly, increasing AKI severity was associated with increased in-hospital mortality when adjusted for other potential variables: odds ratio of stage 1 = 5.374 (95% CI: 2.147–13.452; p < 0.001), stage 2 = 6.216 (95% CI: 2.011–19.210; p = 0.002), and stage 3 = 34.033 (95% CI: 9.723–119.129; p < 0.001).
Conclusion
In this multicenter retrospective study, we found that nearly half of COVID-19 patients with ARDS experienced AKI during their hospital stay. The coexistence of AKI significantly increased the mortality of these patients.
Keywords: COVID-19, Acute respiratory distress syndrome, Acute kidney injury, Mortality, Risk factors
Introduction
The rapid spread of COVID-19 has caused a global pandemic since it was firstly reported in Wuhan, China [1]. The total number of coronavirus cases across the world has reached 17,889,134, and over 686,145 deaths occurred as of 3 August 2020 [1]. Although most infected patients showed mild symptoms, around 5% of the patients required intensive cares based on initial studies [2, 3].
Acute respiratory distress syndrome (ARDS) is the principal feature of critically ill patients with COVID-19, which is associated with worse outcome in these patients [4, 5, 6]. However, initial studies reported that critically ill patients with COVID-19 often had multiple organ dysfunction besides ARDS [6, 7, 8]. Previous studies found that 20–40% of intensive care unit (ICU) patients with COVID-19 experienced acute kidney injury (AKI) [6, 9]. However, epidemiological features of AKI as well as its contribution to the risk of death in COVID-19 patients with ARDS are unknown yet. A better understanding of these is essential to facilitate early intervention to improve the outcome of patients with COVID-19, given that the occurrence of AKI may be associated with increased mortality in patients with ARDS [10]. In this multicenter retrospective study including patients from 13 ICUs in the city of Wuhan, we aimed to investigate the epidemiological features of AKI and its related in-hospital mortality in COVID-19 patients with ARDS.
Methods
Participants
This study included consecutive adult patients (aged ≥18 years) from 13 designated ICUs in the city of Wuhan. All patients were confirmed with SARS-CoV-2 infection by quantitative polymerase chain reaction test of throat swab samples or sputum samples according to the WHO guidance. The study periods and number of included patients in each center are listed in online suppl. Table 1; see www.karger.com/doi/10.1159/000512371 for all online suppl. material. These patients were transferred from floors or other hospitals due to worsened conditions that needed intensive care. This study was approved by the Shanghai East Hospital Ethics Committee.
Data Collection
The detailed clinical information of each patient was obtained by physicians using a standard questionnaire after they were admitted to the ICU. Clinical information including demographic data, medical history, comorbidities, symptoms, signs, laboratory findings, chest computed tomographic scans, and treatment of the patients received due to the virus infection were recorded. We also calculated each patient's Sepsis-Related Organ Failure Assessment (SOFA) score (which can range from 0 to 24, with higher scores indicating more severe illness) on day 1 of ICU admission to evaluate the severity of the diseases.
Each measurement of serum creatinine (SCr) and use of renal replacement therapy during their hospital stay (including non-ICU stay) was recorded to determine whether and when AKI occurred in these patients. The medical record of the patients, including the blood gas test, oxygen saturation and fraction of inspired oxygen, and respiratory supports, was independently reviewed by 2 physicians to determine the diagnosis and timing of ARDS.
AKI and ARDS Definition
AKI was determined by the increase of SCr according to the Kidney Disease Improving Global Outcomes (KDIGO) 2012 guidelines [11]. Briefly, AKI was diagnosed if an absolute increase in SCr of ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h or a 50% increase in SCr from baseline within 7 days occurred, by significantly decreased urine output [11]. AKI was further classified to 3 stages based on KDIGO criteria.
The lowest level of in-hospital SCr rather than prehospital creatinine was used as reference creatinine in this study due to several reasons. First, prehospital creatinine was often unavailable or uncollected by medical stuff due to the heavy burden of this disease during outbreak [9]. Second, many patients were already severely ill before they were admitted to the ICU due to lack of medical resources. Therefore, some patients' kidney function was already damaged before admission, which may affect the accurate identification of the occurrence of AKI [11]. Modification of Diet in Renal Disease (MDRD) formula was not used to back-calculate baseline creatinine because this method overestimates the prevalence of AKI [12]. Therefore, we only included patients who had at least 2 creatinine measurements during their hospital stay. Patients who were on regular dialysis due to chronic renal failure were excluded as it would obscure the real change of creatinine. ARDS was defined according to the Berlin definition [13].
Statistical Analysis
Continuous variables were presented as median and interquartile range (IQR), and categorical variables were expressed as percentages. Baseline characteristics between AKI and non-AKI groups were compared with the unpaired Student's t test or Mann-Whitney test for continuous variables and the χ2 or Fisher's exact tests for categorical variables. Univariate logistic regression analyses were performed to examine the association between each of the indicators and in-hospital mortality separately. We also conducted multivariate logistic regression to determine the variables that independently associated with AKI or in-hospital mortality. A criterion of p < 0.05 for entry and a p ≥ 0.10 for removal was imposed in this procedure. Odds ratios (ORs) for continuous variables were described using standardized ORs, which were associated with a 1 standard deviation change in the variable. A two-sided p value of <0.05 was considered to indicate statistical significance. All analyses were performed with SPSS 25.0 software (SPSS Inc., Chicago, IL, USA).
Results
Baseline Characteristics
During the study periods, a total of 275 COVID-19 patients from 13 ICUs who were diagnosed with ARDS according to Berlin criteria were included in the study (online suppl. Table 1). Baseline clinical characteristics of these patients are shown in Table 1. The median age was 69 (IQR: 62–77) years and 58.4% were male. Of those patients, 136 (49.5%) developed AKI based on KDIGO criteria: 46 patients (33.8%) developed stage I AKI, 30 patients (22.1%) developed stage II AKI, and 60 patients (44.1%) developed stage III AKI. A total of 142 (51.6%) patients died during their hospital stay.
Table 1.
Clinical features, laboratory findings on admission, and outcomes of the COVID-19 patients with acute respiratory distress syndrome
| All (n = 275) | No AKI (n = 139) | AKI (n = 136) | p value | |
|---|---|---|---|---|
| Age | 69 (62–77) | 68 (58–74) | 70 (64–78) | 0.007 |
| Sex | ||||
| Male | 161 (58.4) | 80 (57.6) | 81 (59.6) | 0.736 |
| Female | 114 (41.5) | 59 (42.4) | 55 (40.4) | |
| Comorbidities, n (%) | ||||
| Hypertension | 150 (54.5) | 79 (56.8) | 71 (52.2) | 0.441 |
| Diabetes | 62 (22.5) | 32 (23.0) | 30 (22.1) | 0.849 |
| Coronary artery disease | 35 (12.7) | 16 (11.5) | 19 (14.0) | 0.541 |
| Chronic obstructive lung disease | 37 (13.5) | 23 (16.5) | 14 (10.3) | 0.129 |
| Chronic kidney disease | 16 (5.8) | 1 (0.7) | 15 (11.0) | <0.001 |
| Laboratory findings at admission | ||||
| White blood cell count, ×109/L | 9.20 (6.50–12.56) | 8.90 (6.21–11.20) | 9.72 (6.71–14.17) | 0.003 |
| Neutrophil count, ×109/L | 7.68 (5.16–11.36) | 7.32 (4.95–9.79) | 8.64 (5.41–12.88) | 0.001 |
| Lymphocyte count, ×109/L | 0.71 (0.47–0.96) | 0.78 (0.57–0.98) | 0.62 (0.38–0.86) | 0.110 |
| NLR | 11.62 (6.60–21.64) | 9.22 (6.05–14.60) | 15.50 (8.25–26.24) | <0.001 |
| Monocytes, count, ×109/L | 0.45 (0.29–0.64) | 0.47 (0.31–0.65) | 0.43 (0.27–0.64) | 0.979 |
| Platelet count, ×109/L | 201 (130–285) | 246 (156–321) | 165 (102–230) | <0.001 |
| C-reactive protein, mg/L | 57.65 (23.40–141.91) | 41.76 (19.65–111.93) | 90.11 (34.34–168.75) | 0.003 |
| Procalcitonin, ng/mL | 0.30 (0.12–0.96) | 0.23 (0.09–0.44) | 0.44 (0.16–1.57) | 0.589 |
| ALT, U/L | 32.0 (19.8–48.0) | 33.0 (21.5–47.0) | 30.2 (17.1–54.1) | 0.046 |
| AST, U/L | 35.0 (23.2–52.0) | 33.8 (23.2–45.0) | 38.0 (23.2–58.0) | 0.055 |
| Total bilirubin, µmol/L | 13.80 (9.78–19.20) | 13.87 (9.93–17.65) | 13.80 (9.59–20.92) | 0.612 |
| Direct bilirubin, µmol/L | 7.40 (4.28–12.53) | 9.40 (4.40–13.58) | 6.53 (3.89–11.00) | 0.375 |
| Albumin, g/L | 30.95 (27.78–34.02) | 33.00 (29.15–37.00) | 29.85 (26.83–31.90) | <0.001 |
| D-dimer, µg/mL | 1.83 (0.56–7.48) | 0.72 (0.33–4.30) | 3.43 (1.68–17.34) | 0.001 |
| Glucose, mmol/L | 7.90 (5.82–10.48) | 8.10 (5.84–10.21) | 7.65 (5.80–11.17) | 0.220 |
| SOFA score on day 1 | 5 (3–7) | 5 (2–6) | 5 (4–8) | 0.002 |
| Mechanical ventilation, n (%) | ||||
| None | 52 (18.9) | 35 (25.2) | 17 (12.5) | <0.001 |
| Noninvasive | 130 (47.3) | 85 (61.2) | 45 (33.1) | |
| Invasive | 93 (33.8) | 19 (13.7) | 74 (54.4) | |
| Reference SCr, µmol/L | 59.3 (43.0–74.0) | 59.2 (42.6–67.8) | 59.3 (43.9–81.5) | 0.025 |
| Creatinine measurement, times | 6 (3–10) | 3 (2–6) | 7 (4–11) | <0.001 |
| CRRT, n (%) | 37 (13.5) | 0 (0.0) | 37 (27.2) | <0.001 |
| ECMO, n (%) | 7 (2.5) | 1 (0.7) | 6 (4.4) | 0.064 |
| SCrdischarge/SCrReference* | 1.30 (1.20–1.66) | 1.23 (1.16–1.30) | 1.75 (1.65–2.38) | 0.12 |
| Mortality, n (%) | 142 (51.6) | 33 (23.7) | 109 (80.1) | <0.001 |
AKI, acute kidney injury; NLR, neutrophil-to-lymphocyte ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; SOFA, Sepsis-Related Organ Failure Assessment; ARDS, acute respiratory distress syndrome; SCr, serum creatinine; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation.
Calculated in survivors.
Risk Factors Associated with the Development of AKI
Compared with patients without AKI, patients who developed with AKI were older, tended to have chronic kidney disease and worse kidney function at baseline, had higher SOFA score on day 1, and were more likely to receive invasive ventilation (Table 1). Among laboratory parameters on day 1 after ICU admission, AKI patients had higher white cell count, neutrophil count, neutrophil-to-lymphocyte ratio (NLR), C-reactive protein level, and D-dimer. Moreover, patients with AKI had lower platelet count and albumin level and had more organ dysfunction indicated by different laboratory parameters than patients without AKI (Table 1). After adjustment for sex, comorbidity, and SOFA score, age, a history of chronic kidney disease, NLR, and albumin level were independently associated with the occurrence of AKI (Table 2).
Table 2.
Risk factors associated with acute kidney injury
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| odds ratio | 95% CI | p value | odds ratio | 95% CI | p value | |
| Age | 1.027 | 1.007–1.048 | 0.008 | 1.026 | 1.003–1.050 | 0.029 |
| Sex (female) | 0.921 | 0.570–1.488 | 0.736 | 0.996 | 0.565–1.756 | 0.988 |
| Comorbidities | ||||||
| Hypertension | 0.830 | 0.516–1.334 | 0.441 | |||
| Diabetes | 0.946 | 0.537–1.667 | 0.849 | |||
| Coronary artery disease | 1.248 | 0.613–2.543 | 0.541 | |||
| Chronic obstructive lung disease | 0.579 | 0.284–1.179 | 0.132 | |||
| Chronic kidney disease | 17.107 | 2.227–131.423 | 0.006 | 13.019 | 1.624–104.361 | 0.016 |
| NLR | 1.044 | 1.022–1.065 | <0.001 | 1.037 | 1.013–1.061 | 0.002 |
| Albumin | 0.864 | 0.815–0.917 | <0.001 | 0.895 | 0.841–0.953 | 0.001 |
| SOFA score | 1.147 | 1.048–1.254 | 0.003 | 1.058 | 0.955–1.172 | 0.278 |
Variables included in multivariate analysis were age, sex, chronic kidney disease, NLR, albumin, and SOFA score. NLR, neutrophil-to-lymphocyte ratio; SOFA, Sepsis-Related Organ Failure Assessment.
Association between AKI and Invasive Ventilation
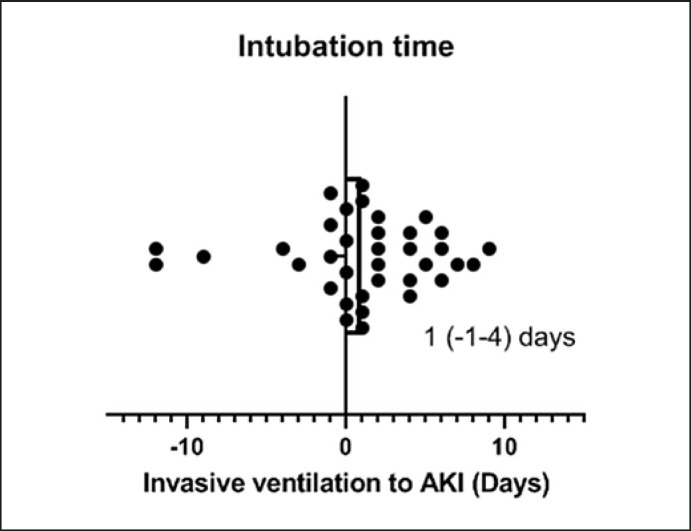
As patients with AKI had more invasive ventilation (54.4 vs. 13.7%) which itself is a risk factor of the development of AKI [10], we analyzed the clinical course of AKI related to intubation time. Among patients with both AKI and invasive ventilation, 75% patients developed AKI after intubation and 25% patients experienced AKI ahead of intubation. Median time from intubation to the occurrence of AKI was 1 (IQR: −1 to 4) day (Fig. 1).
Fig. 1.
Timing of the occurrence of AKI and intubation. AKI, acute kidney injury.
In-Hospital Mortality Related to AKI
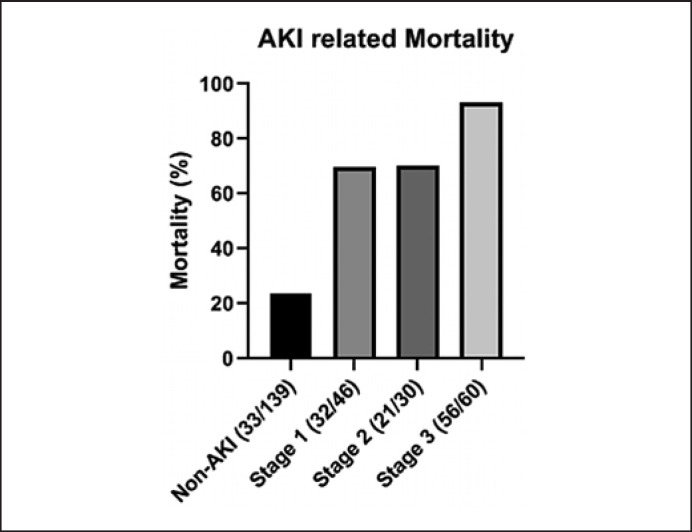
A total of 37 (27.2%) patients with AKI ended up with continuous renal replacement therapy. Those who developed AKI during their hospital stay and survived had more increase in SCr from baseline to discharge than those without AKI development (Table 1), indicating a persistent kidney injury existed. Importantly, the mortality of patients with AKI was significantly higher than those without (80.1 vs. 23.7%, p < 0.001) despite slight difference in the severity of disease indicated by SOFA score between 2 groups (Table 1; Fig. 2). In terms of the KDIGO classification, there were 32 deaths (69.6%) among patients in the stage 1 category, 21 (70.0%) among those in the stage 2 category, and 56 (93.3%) among those in stage 3 category (Fig. 2).
Fig. 2.
Mortality among patients with different stages of AKI. AKI, acute kidney injury.
There was a stepwise increase in mortality with increasing AKI severity (KDIGO stage 1: OR: 7.342, 95% CI: 3.504–15.383; KDIGO stage 2: OR: 7.495, 95% CI: 3.130–17.946; and KDIGO stage 3: OR: 44.970, 95% CI: 15.165–133.354). After adjusting for age, sex, underlying medical conditions, NLR, albumin, and SOFA score, KDIGO stage 1 (OR: 5.374; 95% CI: 2.147–13.452; p < 0.001), KDIGO stage 2 (OR: 6.216; 95% CI: 2.011–19.210; p = 0.002), and KDIGO stage 3 (OR: 34.033; 95% CI: 9.723–119.129; p > 0.001) were still associated with increased in-hospital mortality (Table 3).
Table 3.
Association between acute kidney injury and in-hospital mortality
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| odds ratio | 95% CI | p value | odds ratio | 95% CI | p value | |
| Age | 1.032 | 1.012–1.053 | 0.002 | 1.029 | 0.999–1.061 | 0.056 |
| Sex (female) | 0.623 | 0.384–1.009 | 0.055 | 0.454 | 0.217–0.950 | 0.036 |
| Comorbidities | ||||||
| Hypertension | 0.816 | 0.507–1.313 | 0.403 | |||
| Diabetes | 1.086 | 0.616–1.913 | 0.776 | |||
| Coronary artery disease | 2.256 | 1.058–4.810 | 0.035 | |||
| Chronic obstructive lung disease | 0.677 | 0.337–1.362 | 0.274 | 2.002 | 0.632–6.343 | 0.238 |
| Chronic kidney disease | 4.367 | 1.216–15.687 | 0.024 | 0.340 | 0.070–1.656 | 0.182 |
| NLR | 1.065 | 1.039–1.092 | <0.001 | 1.052 | 1.020–1.086 | 0.001 |
| Albumin | 0.812 | 0.760–0.869 | <0.001 | 0.886 | 0.820–0.958 | 0.002 |
| AKI | ||||||
| Non-AKI | Reference | Reference | ||||
| Stage 1 | 7.342 | 3.504–15.383 | <0.001 | 5.374 | 2.147–13.452 | <0.001 |
| Stage 2 | 7.495 | 3.130–17.946 | <0.001 | 6.216 | 2.011–19.210 | 0.002 |
| Stage 3 | 44.970 | 15.165–133.354 | <0.001 | 34.033 | 9.723–119.129 | <0.001 |
| SOFA score | 1.273 | 1.153–1.406 | <0.001 | 1.361 | 1.164–1.591 | <0.001 |
Variables included in multivariate analysis were age, sex, chronic obstructive lung disease, chronic kidney disease, NLR, albumin, AKI, and SOFA score. NLR, neutrophil-to-lymphocyte ratio; AKI, acute kidney injury; SOFA, Sepsis-Related Organ Failure Assessment.
Discussion
In this multicenter study, we found that AKI is a common comorbidity among COVID-19 patients with ARDS. The risk factors associated with AKI included older age, a history of chronic kidney disease, increased NLR, and decreased albumin level. Importantly, the occurrence of AKI significantly increased the mortality of these patients. To the best of our knowledge, this is the first large-scale study that investigated the epidemiological features of AKI and its related death in COVID-19 patients with acute respiratory distress syndrome.
Respiratory system is the primary target organ of SARS-CoV-2, and the development of ARDS is the principal feature of critically ill patients with COVID-19 [7, 8, 14]. However, respiratory supports such as extracorporeal membrane oxygenation or ventilation treatment resulted in unsatisfied outcomes in these patients [15]. This observation indicates that other organ dysfunctions besides ARDS might be also critical to determine the outcome of these patients. AKI is the most frequent extra-pulmonary organ dysfunction in patients with ARDS, which associated with a higher mortality in these patients [10]. The current study found COVID-19 patients with ARDS who were of older age or had a history of chronic kidney disease were more likely to develop AKI. Moreover, increased NLR and decreased albumin level were also independently associated with the development of AKI. Indeed, recent studies found NLR was the independent risk factor of poor outcomes in COVID-19 patients [16, 17]. Consistently, a study found that hypoalbuminemia is a significant predictor of AKI and the death following AKI development [18]. Thus, our study provided useful information for early recognition of AKI in COVID-19 patients with ARDS in clinical practice.
While the pathogenic features of SARS-CoV-2 have not been fully understood, the virus infection induced cytokine storm was considered playing an important role in the development of organ dysfunction [19]. These uncontrolled inflammatory responses to SARS-CoV-2 infection not only target the respiratory system to cause ARDS but also attack other organs such as kidney to induce multiple organ dysfunction [19]. Moreover, increased intrathoracic pressure induced by mechanical ventilation and gas exchange abnormalities due to respiratory failure can affect renal vascular resistance to reduce kidney perfusion to induce AKI [20, 21]. Another hypothesis is that SARS-CoV-2 can directly attack the kidney. This is evidenced by that the angiotensin-converting enzyme 2, which is the entry receptor for SARS-CoV-2, is expressed not only in the lung but also in the kidney [22]. Therefore, there has been a rising concern that SARS-CoV-2 can directly target the kidney by recognizing its receptor angiotensin-converting enzyme 2 to cause kidney injury. Further studies need to be conducted to investigate the underlying mechanisms of the development of AKI in patients with COVID-19.
Our study found the development of AKI significantly increased the mortality in COVID-19 patients with ARDS in a stepwise mode. Studies showed that the development of AKI could in turn reduce the clearance of cytokines and increase the fluid overload, both of which could deteriorate ARDS [23, 24, 25]. For instance, proinflammatory cytokines IL-6 and IL-8 are increased after the occurrence of AKI, which promote lung injury by facilitating neutrophil accumulation [23, 24, 25, 26]. Therefore, the acute loss of lung and kidney functions could lead to a vicious circle. Our findings indicated that the early recognition and treatment of AKI are essential to improve the outcome of patients with COVID-19.
Several limitations of this study should be mentioned. First, the assessment of AKI and ARDS was not preplanned due to the retrospective nature of the study. Although we conducted intense evaluation of SCr (medium 3 times in non-AKI patients and 7 times in AKI patients) and continuous respiratory monitoring during hospitalization, there is still a possibility that we may underestimate the incidence of AKI and ARDS in these patients. Second, we used the lowest level of in-hospital SCr instead of prehospital SCr as the reference creatinine due to the heavy burden of this disease during outbreak. A very recent study reported that only 15% of patients with COVID-19 had available prehospital creatinine level in the city of New York [9]. This may affect the accuracy of detecting AKI. However, there is no consensus recommendation for choosing reference creatinine [27]. Some studies found that using lowest inpatient SCr as reference creatinine is reasonable and it may even more accurately reflect kidney function, especially in patients with sepsis or prolonged critical illness [28, 29]. Third, urine output was not used as a parameter because it was not very accurately recorded in the heavy burden of COVID-19 during the outbreak in Wuhan, which may also cause an underestimate of the incidence of AKI in these patients.
Conclusion
In this study, we found that nearly half of COVID-19 patients with ARDS developed AKI. The mortality rate was significantly higher in AKI patients than those without. Moreover, the mortality rate in AKI patients increases in a stepwise mode with AKI stages.
Statement of Ethics
This study was approved by the Shanghai East Hospital Ethics Committee (2020-009) and carried out in accordance with the Declaration of Helsinki. Written informed consent was waived by the Ethics Committee due to the retrospective nature of this study and rapid emergence of this infectious disease.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the National Key Research and Development Project of the Ministry of Science and Technology, China (2018YFC1313700), “Gaoyuan” Project of Pudong Health and Family Planning Commission (PWYgy2018-6), and the Research Foundation of Shanghai Science and Technology Commission (No. 18140904100). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions
F.W. conceived and designed the study, analyzed the data, and wrote the paper. L.R., C.Q., J.H., Z.L., M.D., X.Z., W.G., S.G., and W.G. contributed to data acquisition and analysis. C.R., Q.L., Z.L., and C.L. interpreted the data and put expert insights in this study.
Supplementary Material
Supplementary data
Acknowledgments
We acknowledge all the frontline workers in the city of Wuhan for their remarkable efforts to provide care for the critically ill patients with COVID-19.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard (data last updated): 2020 Aug 3. Available from: https://covid19.who.int/?gclid = CjwKCAjw5cL2BRASEiwAENqAPiIP7HmNWk9LVl8TMl39pl3ys2355DnJQ_V4AX6NhmjMWILTV80BARoCx7sQAvD_BwE Accessed 2020 Aug 3.
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020 Feb 24;323((13)):1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.WHO Coronavirus disease 2019 (COVID-19) situation report–195. 2020. Aug 2. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200802-covid-19-sitrep-195.pdf?sfvrsn = 5e5da0c5_2 Accessed 2020 Aug 3.
- 4.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr 1;8((4)):420–2. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020 May 1;8((5)):433–4. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5,700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 May 26;323((20)):2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 May;8((5)):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 11;395((10229)):P1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020 Jul;98((1)):209–18. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panitchote A, Mehkri O, Hastings A, Hanane T, Demirjian S, Torbic H, et al. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019 Dec 1;9((1)):74. doi: 10.1186/s13613-019-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2((1)):1–38. [Google Scholar]
- 12.Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5((7)):1165–73. doi: 10.2215/CJN.08531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Force AD, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012 Jun 20;307((23)):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Jul;180((7)):934–43. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry BM. COVID-19, ECMO, and lymphopenia: a word of caution. Lancet Respir Med. 2020 Apr;8((4)):e24. doi: 10.1016/S2213-2600(20)30119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HH, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020 Jul;81((1)):e6–12. doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagunas‐Rangel FA. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. J Med Virol. 2020 Apr 3; doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: a meta-analysis of observational clinical studies. Intensive Care Med. 2010 Oct 1;36((10)):1657–65. doi: 10.1007/s00134-010-1928-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395((10229)):1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husain-Syed F, Slutsky AS, Ronco C. Lung-kidney cross-talk in the critically ill patient. Am J Respir Crit Care Med. 2016 Aug 15;194((4)):402–14. doi: 10.1164/rccm.201602-0420CP. [DOI] [PubMed] [Google Scholar]
- 21.Hepokoski M, Englert JA, Baron RM, Crotty-Alexander LE, Fuster MM, Beitler JR, et al. Ventilator-induced lung injury increases expression of endothelial inflammatory mediators in the kidney. Am J Physiol Renal Physiol. 2017 Apr 1;312((4)):F654–60. doi: 10.1152/ajprenal.00523.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuiri S, Ohashi Y. ACE and ACE2 in kidney disease. World J Nephrol. 2015 Feb 6;4((1)):74. doi: 10.5527/wjn.v4.i1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ronco C, Reis T, Cozzolino M. Rationale for medium cutoff membranes in COVID-19 patients requiring renal replacement therapy. Nephron. 2020 Apr 9;:1–5. doi: 10.1159/000509807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ronco C, Navalesi P, Vincent JL. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med. 2020 Mar 1;8((3)):240–1. doi: 10.1016/S2213-2600(20)30060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, Ronco C, et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care. 2020 Dec;24((1)):155–3. doi: 10.1186/s13054-020-02872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teixeira JP, Ambruso S, Griffin BR, Faubel S. Pulmonary consequences of acute kidney injury. Semin Nephrol. 2019 Jan 1;39((1)):3–16. doi: 10.1016/j.semnephrol.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Siew ED, Matheny ME. Choice of reference serum creatinine in defining acute kidney injury. Nephron. 2015;131((2)):107–12. doi: 10.1159/000439144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009 Jun 1;20((6)):1217–21. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prowle JR, Kolic I, Purdell-Lewis J, Taylor R, Pearse RM, Kirwan CJ. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014 Jun 6;9((6)):1015–23. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data