Abstract
Introduction
During the COVID-19 pandemic, Lombardy (Northern Italy) Regional Health Council created hubs for cancer care, meant to be SARS-CoV-2-free pathways for cancer patients. The workflow of breast cancer (BC) radiotherapy (RT) in one of the hubs is presented here.
Methods
Candidates to adjuvant RT during the pandemic peak of March-April 2020 were compared to those treated in the same period of 2019, and patient volume, deferral rate, and type of RT were analyzed. Statistics were calculated with χ<sup>2</sup> or Fisher exact tests for categorical variables, and the Wilcoxon rank test for continuous variables.
Results
In March-April 2020 the BC patient volume increased by 28% compared to the same period in 2019 (scheduled patients: 175 vs. 137) and amid travel restrictions it was kept high (treated patients: 136 vs. 133), mainly due to an influx from across Lombardy. RT schemes basically did not change, being already centered on hypofractionation. The increase of median time (67 vs. 74.5 days in 2019 and 2020, respectively) to the commencement of RT for low-risk patients was clinically negligible yet statistically significant (p = 0.03), and in line with the pertinent recommendations. No significant difference was found in the time interval between treatments and RT for high-risk patients. Concomitant chemoradiotherapy was avoided throughout the pandemic peak. Twenty-one women (13.6%) delayed either computed tomography simulation or RT commencement mainly because of COVID-19-related concerns and mobility restrictions.
Conclusion
The workload for BC was high during the pandemic peak. Hubs allowed the continuation of oncologic treatments, while mitigating the strain on frontline COVID-19 hospitals.
Keywords: COVID-19, Pandemic, Breast cancer, Oncologic care, Hub
Introduction
As the outbreak of the global pandemic of COVID-19 (coronavirus disease 2019) has been ravaging countries, radiation oncologists have had to face the challenges of treating outpatients on a daily basis. Distress stemmed from the need for continuing radiotherapy (RT) while ensuring the safety and protection of patients and healthcare workers. A number of guidelines [1, 2, 3, 4, 5] have been published to help physicians act in compliance with the World Health Organization (WHO) recommendations [6]. Oncologic patients are by definition frail and concerns about worse infection-related outcomes compared to immunocompetent subjects seemed legitimate [7]. Therefore, the pressure of treating cancer patients in the safest environment possible was intense. Lombardy, Italy, has been severely hit by the COVID-19 pandemic, with 96,330 testing positive and more than 16,818 deaths as of August 3, 2020, which accounted for about half Italy's total [8]. The vast majority of hospitals in Lombardy have been entirely dedicated to COVID-19 patients. The European Institute of Oncology IRCCS (IEO) was given the status of a hub for cancer care and has been devoted to maintaining the continuity of oncologic treatments. Measures to contain the virus spread activated in the RT Division are described in this report, with an in-depth look at the workflow of breast cancer (BC) RT, which accounted for 30% of the workload (about 1,000 patients per year).
Materials and Methods
Patients scheduled to receive RT for adjuvant BC treatment in the 2-month interval comprising March and April 2020 (the 2 months with the highest incidence of COVID-19 contagion in Italy since the outbreak) were compared to those treated in the same period of 2019. All patients gave written informed consent for the treatment and anonymous use of their data for research purposes. Data were extracted from the dedicated RT databank (RTP R039-000-tomotherapy-breast), which is part of the research project on BC notified to the IEO Ethics Committee. Statistical significance was evaluated with χ2 or Fisher exact tests for categorical variables, and with the Wilcoxon rank test for continuous variables. All the analyses were approved by the institutional review board.
Results
Resources Reorganization
A number of protective measures [9, 10, 11] were taken in response to the pandemic, as described in online supplementary Table 1 (Table 1S; for all online suppl. material, see www.karger.com/doi/10.1159/000513227). Figures and numbers refer to the 2-month study period of March-April 2020.
Twenty-nine nasopharyngeal swabs were performed among patients (4 with BC), and 5 subjects tested positive (none with BC). Three patients were hospitalized for severe respiratory syndrome and 2 died (none with BC). As the RT Division worked at full capacity during the pandemic peak, the permanent ward staff was not significantly reduced. In the 2-month study period, 3 radiation technologists and 2 radiation oncology residents tested positive after contracting mild symptoms, and 4 were quarantined after coming in close contact with potential/known COVID-19 carriers.
RT Schedules for BC
The RT Division of the IEO has been committed to delivering hypofractionated schemes since the early 2000s. Therefore, the RT schedules did not basically change throughout the pandemic. RT treatments for BC performed at the IEO during the pandemic peak with respect to National and International recommendations are described in online supplementary Table 2S.
Comparison of Treatments and Schedules for BC between 2020 and 2019
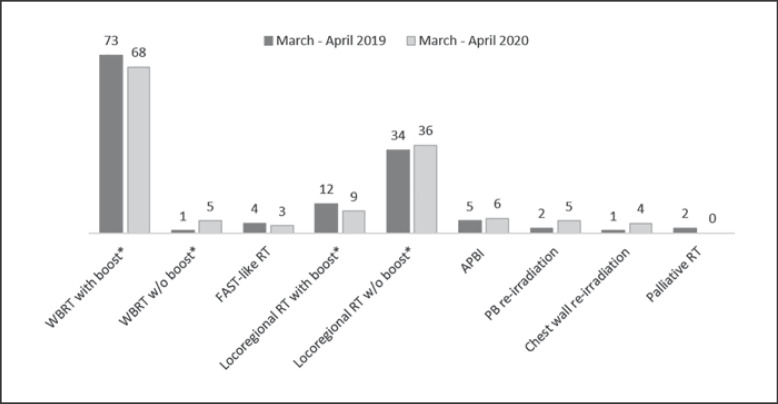
In 2020, over the 2-month study period, 175 BC patients were scheduled to have RT at IEO, with an increase by 28% compared with the same period in 2019. Of them, 19 eventually decided to be irradiated near home (referrals elsewhere: 11%), 1 declined RT because of the difficult conditions caused by the pandemic (renounce: 0.6%), while 1 had their treatment strategy changed for regional progression. One hundred and fifty-four patients remained on the RT waiting list, registering a 16% increase compared to March-April 2019. This greater influx was due to women coming from Milan and from across Lombardy. A comparison of clinical characteristics between the two time frames are summarized in Table 1. The types of schedules and BC treatments remained basically the same between the two study periods (Fig. 1).
Table 1.
Summary and comparison of patient and tumor characteristics of BC treatments between the two study periods
| March-April 2019 | % | March-April 2020 | % | Δ% | p value | |
|---|---|---|---|---|---|---|
| Patients scheduled | 137 | 175 | +28 | |||
| Patients treated | 133a | 136 | +2.2 | |||
| Median patient age, years Region of residence | 56 (48–63) | 56 (49–64) | ||||
| Lombardy | 66 | 49.6 | 84 | 61.8 | +12.1 | 0.04 |
| Other Italian regions | 67 | 50.4 | 52 | 38.2 | −12.1 | |
| Tumor stage | ||||||
| pTis | 8 | 6.0 | 8 | 5.9 | −0.1 | 0.99 |
| pT1 | 83 | 61.9 | 85 | 62.5 | +0.6 | |
| pT2 | 19 | 14.2 | 21 | 15.4 | +1.3 | |
| pT3/pT4 | 16 | 11.9 | 14 | 10.3 | −1.6 | |
| pTx | 4 | 3.0 | 5 | 3.7 | +0.7 | |
| ypT0/ypTis/ypTx | 4 | 3.0 | 3 | 2.2 | −0.8 | |
| Nodal stage | ||||||
| pN0/ypN0 | 69 | 51.5 | 81 | 59.6 | +8.1 | 0.33 |
| pN+ | 50 | 37.3 | 45 | 33.1 | −4.2 | |
| pNx | 15 | 11.2 | 10 | 7.4 | −3.8 | |
| Receptor status | ||||||
| ER | ||||||
| ER >1% | 110 | 82.1 | 108 | 79.4 | −2.7 | 0.42 |
| ER ≤1% | 21 | 15.7 | 27 | 19.9 | +4.2 | |
| NE | 3 | 2.2 | 1 | 0.7 | −1.5 | |
| Ki-67 | ||||||
| Ki-67 <20% | 65 | 48.5 | 78 | 57.4 | +8.8 | 0.17 |
| Ki-67 ≥20% | 65 | 48.5 | 57 | 41.9 | −6.6 | |
| NE | 4 | 3.0 | 1 | 0.7 | −2.2 | |
| HER2 | ||||||
| Absent | 114 | 85.1 | 118 | 86.8 | +1.7 | 0.86 |
| Present | 17 | 12.7 | 16 | 11.8 | −0.9 | |
| NE | 3 | 2.2 | 2 | 1.5 | −0.8 | |
| Grade | ||||||
| 1–2 | 83 | 61.9 | 86 | 63.2 | +1.3 | 0.16 |
| 3 | 33 | 24.6 | 23 | 16.9 | −7.7 | |
| NE | 18 | 13.4 | 27 | 19.9 | +6.4 |
Data are presented as n and the percentage, or the median interval (IQR). p values were obtained with the χ2 or Fisher exact tests. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; NE, not evaluable.
One bilateral BC.
Fig. 1.
Comparison of the types of BC treatment delivered between the two study periods. * Simultaneous integrated boost.
Timing of RT
Although statistically significant, the increase of the median time gap between surgery and the start of RT for low-risk patients (67 days in 2019 vs. 74.5 days in 2020, p = 0.03) was limited to within 12 weeks (Table 2). For 16 patients treated in 2020 the surgery-RT interval exceeded 90 days compared to 4 patients treated in 2019 (p = 0.002). It should be noted that some high-risk patients received RT concomitantly with chemotherapy in 2019, while in 2020 all patients completed systemic therapy before starting RT. No difference was found in the time interval between treatments (either surgery, p = 0.10 or the end of chemotherapy, p = 0.26) and the commencement of RT in the two time frames for high-risk patients.
Table 2.
Comparison of the median time interval of different steps of RT programs between March-April 2019 and March-April 2020
| March-April 2019, days |
March-April 2020, days |
p value | |
|---|---|---|---|
| CHT to RT | 28 (19–32.5) | 29 (22–38) | 0.16 |
| Surgery to RT for low-risk patients | 67 (56–80) | 74.5 (64–96) | 0.03 |
| Surgery to RT for high-risk patients | 72 (58–88) | 85 (68–92) | 0.10 |
| CHT to RT for high-risk patients | 28 (19–33) | 27.5 (22–38) | 0.26 |
| 1st RT visit to RT for patients without CHT | 39 (33–48.5) | 47.5 (39–61) | 0.001 |
| 1st RT visit to RT for patients with CHT | 147 (90.5–199) | 147.5 (109.5–201) | 0.61 |
Data are presented as the median (IQR). p values were obtained with the Wilcoxon rank test. Bold values are significant. High-risk patients included those: aged <40 years, triple negative, HER2 positive, with inflammatory cancer, nodal positive, and with residual disease after neoadjuvant chemotherapy. Low-risk patients were those: stage I/II, aged under 65 years, with luminal tumors grade 1–2, and absent LVI. CHT, chemotherapy; RT, radiotherapy.
RT Discontinuity and Reasons
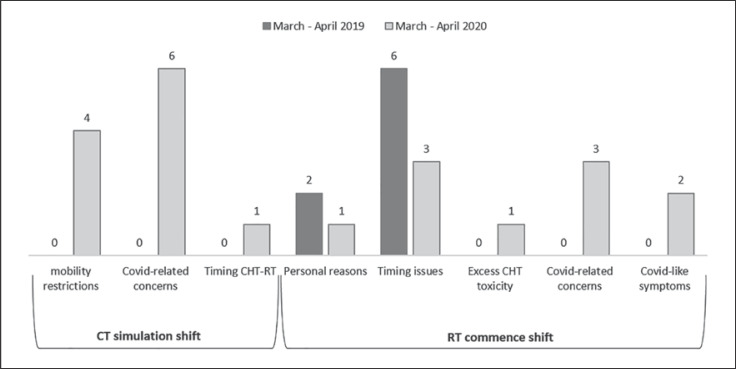
In March-April 2020, 21 BC patients (13.6%, of whom 18 were treated in the following months) postponed either the computed tomography (CT) simulation or the commencement of RT compared to 8 women (6%) in 2019 (Fig. 2). The CT simulation shift was seen only in 2020 and was attributed to travel restrictions and COVID-19-related concerns in 10 out of 11 patients. Of them, 7 women experienced a delay of more than 30 days, 3 of more than 15, and 1 of less than 15 days compared to the scheduled time. The RT commencement shift affected 8 women in 2019, mainly due to the timing of chemotherapy or expander inflation procedure, and was limited to within 15 days in 7 out of 8 cases. In 2020, 5 out of 10 women postponed their RT commencement for COVID-19-related issues (1 due to the travel ban, 2 for fear of COVID-19 infection, and 2 because of quarantine following suspicious symptoms), while for the remaining 5 women the delay was due to timing issues between therapies, personal reasons, and expander inflation. The delay was of more than 30 days in 3 cases, more than 15 days for 6, and less than 15 days for 1 patient.
Fig. 2.
Comparison of the proportion of discontinuity of the RT program and reasons for treatment deferral between the two study periods.
Discussion
Management of patients with cancer during the pandemic peak was a major concern for health professionals, especially in the area of Milan, the capital of Lombardy, which was the worst affected Italian region [12]. A survey addressed to the RT departments in Lombardy [13] showed that more than 75% of hospitals became COVID-19 centers and about half of them reduced their clinical activity by 10–50%, which was in line with the national average [14]. The need to create a SARS-CoV-2 free pathway was pressing, based on the belief that that Oncology and Radiotherapy Divisions should have ideally been COVID-19-free sanctuaries to provide a safe environment for patients frail by definition [15].
As a contingency plan, the regional authorities integrated all public and private healthcare activity into a network in order to better coordinate the healthcare workforce and resource allocation. Three comprehensive cancer centers in Milan (IEO, Istituto Nazionale dei Tumori, and Humanitas Cancer Center) were identified as specialized hubs for oncology and became the referral of general hospitals for cancer patients. Recent investigations on cancer patients with a presumptive or confirmed diagnosis of COVID-19 showed the absence of interaction between anticancer treatments and COVID-19 morbidity or mortality, leading to the conclusion that the standard oncologic care should be offered, if feasible, including chemotherapy [16].
A survey conducted by the European Society for Radiotherapy and Oncology (ESTRO) [17] revealed that 60% of its members, including Italy, saw a decline in patient volume by an average of 25%, mainly due to delays/deferral and reduced referral, especially regarding early-stage BC and prostate cancer. At the IEO hub, the overall RT patient volume remained basically unchanged and even increased for BC treatments, primarily due to a greater stream of patients coming from other cities across Lombardy. The main reason was that their closest RT facility was located in a hospital devoted to treating COVID-19 patients and they feared being exposed. Of note, the overall proportion of BC patients living outside Lombardy was only slightly decreased compared to that registered in 2019, despite restrictions of mobility. For BC patients, the deferral was 13.6%, less than the figure of 40% reported by the ESTRO survey for the general RT population in Italy [17], and in 15/21 cases it was attributed to COVID-19-related issues (fear of contracting the virus, travel ban, difficulty in finding sleeping accommodation).
Treatment prioritization and recommendations [1, 2, 3, 4, 5, 18, 19, 20] were issued to ensure that high-quality therapy continued under difficult circumstances, especially in the curative setting such as in frontline hospitals, where Oncology and Radiotherapy Divisions were hard-pressed to keep their services up and running [14, 17]. Treatment prioritization was adopted by more than 80% of the centers in Lombardy, favoring short treatments and home assistance, if feasible [13]. Medical Oncology, Radiotherapy and Breast Surgery Societies joined forces to release recommendations specific for BC [1, 5]. The tier of elevated priority included ongoing treatments and biologically aggressive tumors. Hypofractionated schemes were highly recommended [2, 3, 5].
BC patients represent the ideal category to perform forms of de-escalation, which are supported by high-level evidence [21]. However, a number of RT centers are still attached to conventional approaches [22] and it can be hard under extreme circumstances to change attitudes and clinical protocols. An international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST) [15] on BC management during the pandemic showed that only a minority of the responding centers changed their RT protocols, turning to forms of moderate (15.9%) or extreme (7.4%) hypofractionation regimens. Efforts to make de-escalation approaches were documented in almost three-quarters of Italian RT centers [14] during the pandemic and hopefully they will become part of the clinical routine. Currently, all the radiation regimens used in our Institute are hypofractionated, except for chest wall re-irradiation. Intraoperative RT (IORT) has been considered an option under situations of externally elevated risk [23]. However, at the IEO, IORT has not been available since the beginning of 2019, pending a machine replacement, otherwise full-dose treatment would have been a valid alternative for at least 21% of patients undergoing RT during the pandemic peak according to the APBI guidelines [24]. The simultaneous integrated boost delivered with the TomoTherapy® Hi-Art System (Tomotherapy Inc., Madison, WI, USA), which does not impact on treatment duration, was prescribed less frequently in patients over 60 years and without high-risk factors [25] to decrease the complexity of treatment, in keeping with international guidelines [2, 3]. Delays in delivering RT, which were allowed until 5 months following surgery by the multidisciplinary Italian guidelines [5] in selected patients, were handled with caution. Besides the fact that physicians and patients were reported to be uncomfortable with both the options of omission and deferral [18, 25], caution was due to the largely unknown pandemic trajectory in the early phase of the outbreak. As a matter of fact, while priority for high-risk patients was always fulfilled, low-risk patients experienced a statistically significant, although clinically negligible, delay in commencing RT, which was well within the range allowed by the national recommendations [5].
The privileged position of the IEO as a hub for cancer care allowed RT treatments to continue at a virtually unchanged pace during the pandemic peak. On one hand, the hub eased the strain on frontline COVID-19 hospitals, enhancing their capacity to meet the growing demand for medical assistance. On the other hand, the hub assured the continuity of essential oncologic therapies. The structure of the hub embedded in the regional healthcare network can be seen as a model of healthcare provision under challenging conditions.
Statement of Ethics
All procedures performed in the present study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. This study has been approved by the institutional Ethical Committee of the IRCCS European Institute of Oncology as part of the research project entitled “Adjuvant Radiation Treatments with Intensity-Modulated Radiotherapy and/or Hypofractionated Schedules for Breast Cancer” (May 26, 2016, Milan, Italy). All patients gave written informed consent for the treatment and anonymous use of their data for educational and research purposes.
Conflict of Interest Statement
M.C.L., F.C., B.A.J.-F. received honorarium fees from Accuray Inc. outside the current work. The remaining authors declare no conflicts of interest.
Funding Sources
There were no sources of funding associated with this work.
Author Contributions
M.C.L., E.M., V.E.G., M.Z., D.P.R., S.D., M.A.Z., A.M., F.M., R.O., and B.A.J.-F. were responsible for the conception and design of the study and wrote the first draft of the paper. C.S., S.G., M.C., P.V., M.A.G., D.A., R.L., M.S.F., G.B., and F.C. were responsible for data collection and analysis and wrote sections of the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
Supplementary Material
Supplementary data
Acknowledgements
The institution of some authors (IEO, European Institute of Oncology IRCCS, Milan, Italy) was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5 × 1,000 funds.
References
- 1.de Azambuja E, Trapani D, Loibl S, Delaloge S, Senkus E, Criscitiello C, et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: breast Cancer. ESMO Open. 2020 May;5(Suppl 3):1–12. doi: 10.1136/esmoopen-2020-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coles CE, Aristei C, Bliss J, Boersma L, Brunt AM, Chatterjee S, et al. International Guidelines on Radiation Therapy for Breast Cancer During the COVID-19 Pandemic. Clin Oncol (R Coll Radiol) 2020 May;32((5)):279–81. doi: 10.1016/j.clon.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunstein LZ, Gillespie EF, Hong L, Xu A, Bakhoum SF, Cuaron J, et al. Breast Radiation Therapy Under COVID-19 Pandemic Resource Constraints-Approaches to Defer or Shorten Treatment From a Comprehensive Cancer Center in the United States. Adv Radiat Oncol. 2020 Apr;5((4)):582–8. doi: 10.1016/j.adro.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ASTRO guidelines available at https://www.astro.org/Daily-Practice/COVID-19-Recommendations-and-Information/Clinical-GuidanceAccessed July 13th, 2020. [Google Scholar]
- 5.AIOM-AIRO-SICO The treatment of cancer patients during COVID19 pandemia. 2020 27th March; Available at https://www.aiom.it/en/the-treatment-of-cancer-patients-during-covid19-pandemia/Accessed July 13th, 2020. [Google Scholar]
- 6.World Health Organization Coronavirus disease (COVID-19) Pandemic. Available at https://www.who.int/emergencies/diseases/novelcoronavirus-2019Accessed July 12th, 2020) [Google Scholar]
- 7.Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020 May;323((18)):1775–6. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 8.Coronavirus Regione Lombardia Available at https://www.regione.lombardia.it/wps/portal/istituzionale/HP/coronavirusAccessed July 13th, 2020. [Google Scholar]
- 9.Alterio D, Volpe S, Marvaso G, Turturici I, Ferrari A, Leonardi MC, et al. Head and neck cancer radiotherapy amid COVID-19 pandemic: Report from Milan, Italy. Head Neck. 2020:1–9. doi: 10.1002/hed.26319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei W, Zheng D, Lei Y, Wu S, Verma V, Liu Y, et al. Radiotherapy workflow and protection procedures during the Coronavirus Disease 2019 (COVID-19) outbreak: Experience of the Hubei Cancer Hospital in Wuhan, China. Radiother Oncol. 2020 Jul;148:203–10. doi: 10.1016/j.radonc.2020.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meattini I, Franco P, Belgioia L, Boldrini L, Botticella A, De Santis MC, et al. Radiation therapy during the coronavirus disease 2019 (covid-19) pandemic in Italy: a view of the nation's young oncologists. ESMO Open. 2020 Apr;5((2)):8–10. doi: 10.1136/esmoopen-2020-000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curigliano G. How to Guarantee the Best of Care to Patients with Cancer During the COVID-19 Epidemic: the Italian Experience. Oncologist. 2020 Jun;25((6)):463–7. doi: 10.1634/theoncologist.2020-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jereczek-Fossa BA, Palazzi MF, Soatti CP, Cazzaniga LF, Ivaldi GB, Pepa M, et al. CODRAL (Board of Directors of Radiation Oncology Departments in Lombardy) network COVID-19 Outbreak and Cancer Radiotherapy Disruption in Lombardy, Northern Italy. Clin Oncol (R Coll Radiol) 2020 Jul;32((7)):e160–1. doi: 10.1016/j.clon.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jereczek-Fossa BA, Pepa M, Marvaso G, Bruni A, Buglione di Monale E Bastia M, Catalano G, et al. AIRO (Italian Association of Radiotherapy and Clinical Oncology) COVID-19 outbreak and cancer radiotherapy disruption in Italy: Survey endorsed by the Italian Association of Radiotherapy and Clinical Oncology (AIRO) Radiother Oncol. 2020 Aug;149:89–93. doi: 10.1016/j.radonc.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasparri ML, Gentilini OD, Lueftner D, Kuehn T, Kaidar-Person O, Poortmans P. Changes in breast cancer management during the Corona Virus Disease 19 pandemic: An international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST) Breast. 2020 Aug;52:110–5. doi: 10.1016/j.breast.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poortmans PM, Guarneri V, Cardoso MJ. Cancer and COVID-19: what do we really know? Lancet. 2020 Jun;395((10241)):1884–5. doi: 10.1016/S0140-6736(20)31240-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slotman BJ, Lievens Y, Poortmans P, Cremades V, Eichler T, Wakefield DV, et al. Effect of COVID-19 pandemic on practice in European radiation oncology centers. Radiother Oncol. 2020 Sep;150:40–2. doi: 10.1016/j.radonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Rashdan A, Roumeliotis M, Quirk S, Grendarova P, Phan T, Cao J, et al. Adapting Radiation Therapy Treatments for Patients with Breast Cancer During the COVID-19 Pandemic: Hypo-Fractionation and Accelerated Partial Breast Irradiation to Address World Health Organization Recommendations. Adv Radiat Oncol. 2020 Apr;5((4)):1–2. doi: 10.1016/j.adro.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherny NI, Dafni U, Bogaerts J, Latino NJ, Pentheroudakis G, Douillard JY, et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017 Oct;28((10)):2340–66. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 20.Curigliano G, Cardoso MJ, Poortmans P, Gentilini O, Pravettoni G, Mazzocco K, et al. editorial board of The Breast Recommendations for triage, prioritization and treatment of breast cancer patients during the COVID-19 pandemic. Breast. 2020 Aug;52:8–16. doi: 10.1016/j.breast.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith BD, Bellon JR, Blitzblau R, Freedman G, Haffty B, Hahn C, et al. Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018 May - Jun;8((3)):145–52. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Gregucci F, Fozza A, Falivene S, Smaniotto D, Morra A, Daidone A, et al. Italian Society of Radiotherapy and Clinical Oncology (AIRO) Breast Group Present clinical practice of breast cancer radiotherapy in Italy: a nationwide survey by the Italian Society of Radiotherapy and Clinical Oncology (AIRO) Breast Group. Radiol Med (Torino) 2020 Jul;125((7)):674–82. doi: 10.1007/s11547-020-01147-5. [DOI] [PubMed] [Google Scholar]
- 23.Simcock R, Thomas TV, Estes C, Filippi AR, Katz MA, Pereira IJ, et al. COVID-19: global radiation oncology's targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020 Mar;22((22)):55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Correa C, Harris EE, Leonardi MC, Smith BD, Taghian AG, Thompson AM, et al. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol. 2017 Mar - Apr;7((2)):73–9. doi: 10.1016/j.prro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Omarini C, Maur M, Luppi G, Narni F, Luppi M, Dominici M, et al. Cancer treatment during the coronavirus disease 2019 pandemic: do not postpone, do it! Eur J Cancer. 2020 Jul;133:29–32. doi: 10.1016/j.ejca.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data