Key Points
Question
Is treatment with oral antibiotics alone noninferior to a combination of intravenous and oral antibiotics for treatment of computed tomography–confirmed uncomplicated acute appendicitis?
Findings
This multicenter randomized clinical trial included 583 adults with uncomplicated acute appendicitis who were treated with either 7 days of oral moxifloxacin or 2 days intravenous ertapenem followed by 5 days of levofloxacin and metronidazole. Treatment success (discharge from hospital without need for surgery and no recurrent appendicitis within 1 year) occurred in 70.2% who received oral antibiotics alone vs 73.8% of patients who received intravenous followed by oral antibiotics, with the confidence limit of the difference exceeding the noninferiority margin of 6%.
Meaning
Patients with acute, uncomplicated appendicitis treated with oral antibiotics alone met the prespecified threshold for treatment success, but failed to demonstrate noninferiority relative to systemic antibiotics followed by oral antibiotics.
Abstract
Importance
Antibiotics are an effective and safe alternative to appendectomy for managing uncomplicated acute appendicitis, but the optimal antibiotic regimen is not known.
Objective
To compare oral antibiotics with combined intravenous followed by oral antibiotics in the management of computed tomography–confirmed uncomplicated acute appendicitis.
Design, Setting, and Participants
The Appendicitis Acuta (APPAC) II multicenter, open-label, noninferiority randomized clinical trial was conducted from April 2017 until November 2018 in 9 Finnish hospitals. A total of 599 patients aged 18 to 60 years with computed tomography–confirmed uncomplicated acute appendicitis were enrolled in the trial. The last date of follow-up was November 29, 2019.
Interventions
Patients randomized to receive oral monotherapy (n = 295) received oral moxifloxacin (400 mg/d) for 7 days. Patients randomized to receive intravenous antibiotics followed by oral antibiotics (n = 288) received intravenous ertapenem (1 g/d) for 2 days followed by oral levofloxacin (500 mg/d) and metronidazole (500 mg 3 times/d) for 5 days.
Main Outcomes and Measures
The primary end point was treatment success (≥65%) for both groups, defined as discharge from hospital without surgery and no recurrent appendicitis during 1-year follow-up, and to determine whether oral antibiotics alone were noninferior to intravenous and oral antibiotics, with a margin of 6% for difference.
Results
Among 599 patients who were randomized (mean [SD] age, 36 [12] years; 263 [44%] women), 581 (99.7%) were available for the 1-year follow-up. The treatment success rate at 1 year was 70.2% (1-sided 95% CI, 65.8% to ∞) for patients treated with oral antibiotics and 73.8% (1-sided 95% CI, 69.5% to ∞) for patients treated with intravenous followed by oral antibiotics. The difference was −3.6% ([1-sided 95% CI, −9.7% to ∞]; P = .26 for noninferiority), with the confidence limit exceeding the noninferiority margin.
Conclusion and Relevance
Among adults with uncomplicated acute appendicitis, treatment with 7 days of oral moxifloxacin compared with 2 days of intravenous ertapenem followed by 5 days of levofloxacin and metronidazole resulted in treatment success rates greater than 65% in both groups, but failed to demonstrate noninferiority for treatment success of oral antibiotics compared with intravenous followed by oral antibiotics.
Trial Registration
ClinicalTrials.gov Identifier: NCT03236961; EudraCT Identifier: 2015-003633-10
This noninferiority trial compares the effects of 2 antibiotic strategies, oral moxifloxacin vs intravenous ertapenem followed by oral levofloxacin, on hospital discharge without surgery and recurrent appendicitis over 1 year among adults presenting to the emergency department with uncomplicated acute appendicitis.
Introduction
Antibiotic therapy is a safe, efficient, feasible, and cost-effective alternative to appendectomy for patients with computed tomography (CT)–confirmed uncomplicated acute appendicitis at short-term and long-term follow-up.1,2,3,4,5 The World Society of Emergency Surgery 2020 guideline recommended discussing antibiotics as a safe alternative to surgery for uncomplicated acute appendicitis without appendicolith (high quality of evidence; strong recommendation).6 During the coronavirus disease 2019 (COVID-19) pandemic, this was also acknowledged by the American College of Surgeons (COVID-19 Guideline for Triage of Emergency General Surgery Patients).7
In the first APPAC trial, at the 5-year follow-up, 61% of 256 patients who initially presented with uncomplicated acute appendicitis were successfully treated with antibiotics, and those who ultimately developed recurrent appendicitis had no adverse outcomes related to the delay in appendectomy.2 Quality of life was also similar after these 2 treatment alternatives.8,9 In previous trials, the length of hospital stay for both antibiotics and appendectomy has been similar,10 but for antibiotics alone, hospitalization was required to administer broad-spectrum intravenous antibiotics to ensure patient safety.1,9,11 Successful outpatient treatment has since been reported.12 Despite prolonged hospitalizations, antibiotic therapy is associated with significantly lower treatment costs compared with appendectomy.3,4 A shorter hospital stay for antibiotic treatment could further enhance cost savings, patient satisfaction, and quality of life. Avoidance of hospitalizations during the COVID-19 pandemic is also a desirable potential benefit for the management of appendicitis using oral rather than intravenous antibiotics.
The randomized, clinical, multicenter Appendicitis Acuta II (APPAC II) trial was designed to compare oral antibiotic monotherapy with a combination of similar-spectrum intravenous antibiotics followed by oral antibiotics in the management of CT-confirmed uncomplicated acute appendicitis. Given that nonoperative treatment for appendicitis is now well established based on outcomes from several clinical trials,1,2,13,14 the primary objective of this study was to demonstrate (1) the ability of oral antibiotics alone to manage acute appendicitis and (2) the noninferiority of oral antibiotics compared with intravenous followed by oral antibiotics.
Methods
Trial Design
This was a randomized, open-label, noninferiority multicenter trial performed at 9 Finnish hospitals (4 university hospitals [Turku, Oulu, Tampere, and Kuopio] and 5 central hospitals [Pori, Seinäjoki, Jyväskylä, Mikkeli, and Rovaniemi]). The trial protocol is available in Supplement 1. All patients gave written informed consent. The trial protocol was approved by the ethics committee at the Hospital District of Southwest Finland and by institutional research boards at each participating site. The trial was performed in accordance with the Declaration of Helsinki.
It is well established that nonoperative management of appendicitis with intravenous antibiotics is a suitable alternative to appendectomy. The aim of the current study was to avoid the use of intravenous antibiotics by replacing them with oral antibiotics. The hypothesis of the current study was that oral antibiotics were (1) effective alone and (2) noninferior to intravenous antibiotics for the management of uncomplicated acute appendicitis as first-line therapy in a large prospective patient cohort. Any potential worse outcomes attributable to the use of oral antibiotics might offset the need for hospitalization and the use of intravenous antibiotics for the management of uncomplicated appendicitis.1,15
Patients
Patients aged 18 to 60 years admitted to the emergency department with a clinical suspicion of acute appendicitis and uncomplicated acute appendicitis confirmed by CT imaging were evaluated for study enrollment. Based on the research hospital CT device, intravenous contrast-enhanced CT imaging was performed either by the standard 120 kV imaging (in patients with body mass index >30), the optimized 100 kV low-dose protocol (in patients with body mass index <30), or using a CT scanner with tube current modulation (Tampere University Hospital). Uncomplicated acute appendicitis was defined as having an appendiceal diameter larger than 6 mm with a thickened, contrast-enhanced wall along with periappendiceal edema and/or minor fluid collection and the absence of the criteria of complicated acute appendicitis (presence of appendicolith, perforation, abscess, or suspicion of tumor). A standardized CT imaging report was used. The trial inclusion criteria were age 18 to 60 years, diagnosis of uncomplicated acute appendicitis confirmed by CT imaging, and absence of the criteria of complicated appendicitis. The exclusion criteria were age younger than 18 or older than 60 years, pregnancy or lactation, allergy to contrast media or iodine, allergy or contraindication to antibiotic therapy, kidney insufficiency or serum creatinine value exceeding the upper reference limit, type 2 diabetes and use of metformin medication, severe systemic illness (eg, malignancy, medical condition requiring immunosuppressant medication), inability to cooperate and give informed consent, or complicated acute appendicitis based on CT findings.
All patients with acute appendicitis at CT imaging who were evaluated for enrollment were recorded in the database, and the aim was to also record all patients undergoing CT imaging for suspected acute appendicitis.
Randomization and Interventions
Participants were randomized in a 1:1 ratio with random permuted blocks of 10 (SAS system for Windows, version 9.4) to receive either oral moxifloxacin (400 mg once daily) for 7 days or intravenous ertapenem sodium (1 g once daily) for 2 days followed by oral levofloxacin (500 mg a day) and metronidazole (500 mg 3 times daily) for 5 days, with the first doses of the randomized treatment administered in the emergency department. The intravenous followed by oral antibiotic therapy was selected based on its efficacy and safety in the previous APPAC trial.2 To evaluate a broad-spectrum antibiotic without the need for intravenous administration, once-daily administered and commonly accessible moxifloxacin was found to be a practical choice for the oral antibiotic with proven efficacy in intra-abdominal infections16,17 and inclusion in current practical guidelines.18 Randomization was stratified by center by a study statistician.
Minimum follow-up at the hospital was 20 to 24 hours, depending on the time of day of admission and patient status, allowing for administration of 2 intravenous doses. The clinical condition of the patient was evaluated twice daily, approximately 12 hours after admission by the on-call surgeon. If the patient was suspected of not responding to the antibiotic therapy, the patient underwent laparoscopic appendectomy based on the surgeon’s decision and the reasons for proceeding to appendectomy were recorded.
Follow-up
Patient outcomes were assessed daily during hospitalization and after discharge by telephone interviews at 1 week, 2 months, and 1 year. For patients not reached for telephone follow-up, electronic hospital records were searched for information on possible appendectomy or other additional intervention-associated visits or hospitalizations.
To validate the accuracy of the differential diagnosis between uncomplicated and complicated acute appendicitis, all patients were assessed using clinical data and CT findings for patients with uncomplicated acute appendicitis treated with antibiotics or additionally for patients undergoing appendectomy using surgical and histopathological findings. All clinical diagnoses were assessed in a blinded manner by 2 investigators unaware of the other’s evaluation (S.S. and J.H.). In cases of disagreement, the clinical diagnosis was reviewed by a third investigator (P.S.).
Outcomes
The primary end point was treatment success at 1 year, defined as resolution of acute appendicitis resulting in discharge from the hospital without the need for surgical intervention and no recurrent appendicitis during the 1-year follow-up.
The predefined secondary end points included postintervention adverse events related to antibiotics or appendectomy, with possible postoperative adverse events classified using the Clavien-Dindo classification (grades 1 to 4),19 abdominal symptoms, duration of hospital stay, visual analog scale scores for pain (range, 0-10), and length of sick leave. All adverse events or symptoms related to antibiotic treatment comparable with grade 1 or higher in the Clavien-Dindo classification19 for postoperative complications and any symptoms resulting in discontinuation of the randomized treatment were classified as antibiotic-associated adverse events. Cost and quality of life are not reported in this article. Based on the actual clinical diagnosis, the true primary failure rate (nonresponders with complicated acute appendicitis based on surgical findings during the initial hospitalization), true recurrence rate (acute appendicitis based on histopathology findings after appendectomy for suspected recurrence), and primary nonresponder rate (ie, all patients who underwent appendectomy during the initial hospitalization) are reported.
Recurrent appendicitis was diagnosed clinically as determined by the surgeon on-call without repeated CT imaging or predefined clinical criteria. Patients with suspected appendicitis recurrence underwent laparoscopic appendectomy based on the study protocol. Surgical finding and histopathological examination (appendicitis defined as transmural neutrophil invasion involving the appendiceal muscularis layer) of the removed appendix were used to confirm the diagnosis.
Statistical Analysis
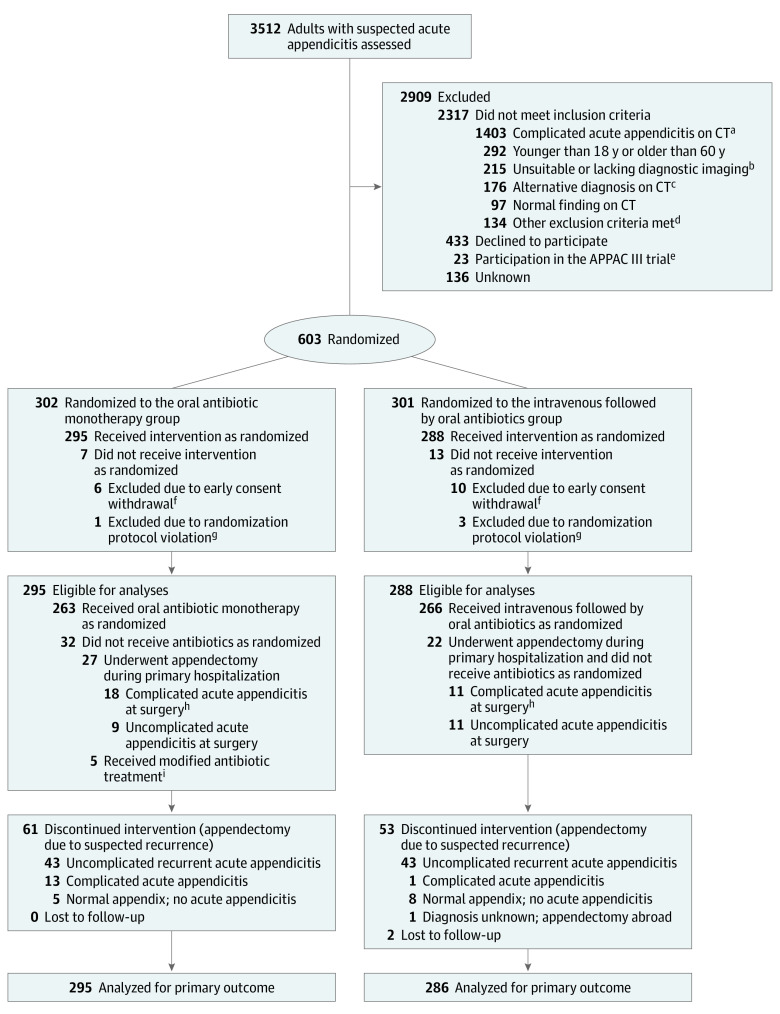
Sample size calculations were based on noninferiority tests for binomial proportion. The success rate was estimated to be 73% for the intravenous followed by oral antibiotic group during the 1-year follow-up based on the results of the APPAC trial.1 The difference between the groups (oral antibiotic monotherapy − intravenous followed by oral antibiotics) was set to zero and the noninferiority margin was set to −6%. The −6% noninferiority margin was set based on the results of the APPAC trial (ie, difference between the 1-year treatment success of 73% and the lower 95% CI of 67%).1 We estimated that a total of 469 patients would yield a power of 0.9 (1 − β) to establish whether oral antibiotic monotherapy was noninferior to intravenous followed by oral antibiotics using a one-sided significance level of α = .05. With an estimated dropout rate of 15%, a total of 552 patients (276 patients per group) needed to be enrolled. The analyses included all randomized patients (according to their randomization group), with the exclusion of erroneously randomized patients with CT-confirmed complicated acute appendicitis and early dropouts (Figure 1).
Figure 1. Flow of Patients in a Study of the Effect of Oral Moxifloxacin vs Intravenous Ertapenem Plus Oral Levofloxacin for Uncomplicated Acute Appendicitis .
aIncludes appendicolith, perforation, abscess, or suspicion of tumor.
bThe majority of these patients underwent ultrasonography examination, magnetic resonance imaging, or non–contrast-enhanced computed tomography (CT). Twelve patients underwent an operation without diagnostic imaging.
cThe alternative diagnoses were as follows: diverticulitis (n = 36), ovarian mass/cyst (n = 25), colitis (n = 24), pelvic inflammatory disease (n = 12), mesenterial lymphadenitis (n = 11), pyelonephritis (n = 8), kidney/ureteral stone (n = 4), bowel obstruction (n = 1), and miscellaneous pathological findings or suspicion of such (n = 55).
dAdditional exclusion criteria were pregnancy, lactation, allergy to contrast media, kidney insufficiency, use of metformin, systemic illness, and inability to consent.
eA randomized, placebo-controlled, double-blind, multicenter trial comparing antibiotic therapy with placebo in the treatment of uncomplicated acute appendicitis.20
fPatients who withdrew consent within 24 hours of randomization who received a maximum of 1 dose of the randomized treatment were excluded from the analyses. Two patients, who withdrew their consent 5 and 7 days after randomization, were included in the analyses.
gPatients erroneously randomized despite a finding of complicated acute appendicitis initially seen on CT imaging were excluded from the analyses according to the study protocol.
hOperative or histopathological findings of appendicolith, gangrene, perforation, abscess, or tumor were classified as complicated acute appendicitis.
iOne patient in the oral antibiotic monotherapy group mistakenly received the treatment intended for the intravenous followed by oral antibiotics group. Four patients received 1 dose of moxifloxacin and thereafter cephalexin and metronidazole (2 due to suspected reactions to moxifloxacin, 1 due to lactation, and 1 to prevent a possible adverse interaction with the patient’s antidepressant).
All main statistical analyses were planned in the study protocol and performed according to statistical analysis plan (Supplement 2). To explore the possibility of site effects, a post hoc analysis was conducted using a binomial generalized linear model with study group as a fixed effect and study center as a random effect. In addition, a post hoc assessment of the time to appendectomy was performed using a Kaplan-Meier analysis.
The primary outcome of treatment success was evaluated in 2 stages: (1) rates of success for treatments are greater than or equal to 65%, judged with the lower limit of 95% CI and (2) the difference of rates for success (oral antibiotic – combined antibiotics) is less than 6% (noninferiority margin) based on 1-sided 95% CIs. CIs were calculated using the Wald method. A survival analysis with a Wilcoxon test was used to compare the time from randomization to appendectomy between the groups. Only 2 patients were lost to follow-up and were excluded from the primary analyses.
Secondary outcome comparisons between the treatment groups were performed using Fisher exact tests for categorical variables and independent samples t tests or Mann-Whitney U tests for continuous variables. Patients with missing data were excluded from the secondary outcome comparisons. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary endpoints should be interpreted as exploratory. All continuous variables are presented as mean with SD when normally distributed and otherwise as median with lower and upper quartile or range. Assumptions for t tests were checked with studentized residuals. Two-tailed P values less than .05 were considered statistically significant for secondary outcomes. For group differences, 2-sided 95% CIs were calculated. Statistical analyses were performed using SAS system for Windows, version 9.4 (SAS Institute, Inc).
Results
From April 2017 through November 2018, a total of 3512 patients with clinically suspected acute appendicitis were screened and 603 were randomly assigned to receive either oral moxifloxacin or intravenous ertapenem followed by oral levofloxacin and metronidazole (Figure 1). After randomization, 20 patients were excluded from analysis because complicated acute appendicitis was stated on CT findings prior to randomization (randomization protocol violation; n = 4) or because of early patient withdrawal of consent (n = 16) before receiving actual allocated treatment, leaving 583 patients included in the primary analyses (295 in the oral antibiotic monotherapy group and 288 in the intravenous followed by oral antibiotics group). The intended initial sample size (n = 552)21 was exceeded based on active recruitment in all centers. The number of patients randomized at each center is shown in eTable 1 in Supplement 3. Baseline characteristics were similar in the groups (Table 1). The mean (SD) age of the patients was 36 (12) years, and 263 (43.9%) were women.
Table 1. Baseline Patient Characteristics in a Study of the Effect of Oral Moxifloxacin vs Intravenous Ertapenem Plus Oral Levofloxacin for Treatment of Uncomplicated Acute Appendicitisa.
| Characteristic | Oral antibiotic monotherapy group (n = 301) | Intravenous followed by oral antibiotics group (n = 298) |
|---|---|---|
| Sex, No. (%) | ||
| Women | 137 (45.5) | 126 (42.3) |
| Men | 164 (54.5) | 172 (57.7) |
| Age, median (IQR), y | 34 (26-45) | 33 (26-43) |
| Visual analog scale score for pain on admission, mean (SD)b | 5.2 (2.3) | 5.3 (2.4) |
| Body temperature, mean (SD), °C | 37.2 (0.6) | 37.2 (0.6) |
| Leukocyte count, median (IQR), ×109/Lc | 12.5 (9.4-14.9) | 12.2 (9.1-14.9) |
| C-reactive protein, median (IQR), mg/Lc | 29.9 (11.0-61.0) | 34.0 (13.0-62.6) |
| Neutrophil count, median (IQR), ×109/Lc | 9.4 (6.6-11.9) | 9.4 (6.1-11.9) |
| BMI, median (IQR) | 26.8 (24.2-30.1) | 26.4 (23.6-30.2) |
| Appendiceal diameter on CT imaging, mean (SD), mmd | 10.9 (2.6) | 10.7 (2.4) |
| Duration of symptoms on admission, median (IQR), h | 18.0 (10.0-30.0) | 22.0 (12.0-30.0) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CT, computed tomography; IQR, interquartile range.
SI conversion factor: To convert C-reactive protein to mg/dL, divide by 10.
Includes all randomized patients excluding erroneously randomized patients with complicated appendicitis initially seen on CT imaging.
Score range, 0 to 10; a score of 0 indicates no pain and 10 indicates the worst possible pain. A total of 324 of 599 patients (54.1%) received some form of analgesic prior to pain scale score assessment.
Reference range for leukocyte count is 3.4-8.2 ×109/L; C-reactive protein, <10 mg/L; and neutrophil count, 1.5-6.7 ×109/L.
Defined as the outer to outer surface appendiceal diameter measured from the widest part of the appendix on the axial plane (ie, perpendicular to the longitudinal axis). A diameter of 6 mm or smaller was considered normal, whereas a diameter exceeding 6 mm with signs of acute inflammation (thickened and enhancing wall and periappendiceal edema and/or minor fluid collection) was considered pathological.
Of the 1195 patients with CT-confirmed uncomplicated acute appendicitis, 569 patients who met all inclusion criteria either declined to participate or were not evaluated for study enrollment and 23 patients took part in a concurrent appendicitis trial (the Appendicitis Acuta III double-blind randomized clinical trial comparing antibiotic treatment with placebo in the management of uncomplicated acute appendicitis)20 (ie, 603 of 1172 eligible patients [51.5%] agreed to be randomized). The baseline characteristics for the nonrandomized eligible population are presented in eTable 2 in Supplement 3.
The 1-year follow-up rate for the primary outcome was 99.7% (581 of 583 patients); 2 patients moved abroad and were lost to follow-up and subsequently excluded from the primary outcome analysis. A total of 72 patients could not be reached by telephone and information about possible appendectomy for the primary outcome analysis was obtained from hospital district electronic medical records. The follow-up rates regarding secondary outcomes are presented in Table 2.
Table 2. Outcomes in a Study of the Effect of Oral Moxifloxacin vs Intravenous Ertapenem Plus Oral Levofloxacin for Treatment of Uncomplicated Acute Appendicitis.
| Outcome | Oral antibiotic monotherapy group (n = 295) | Intravenous followed by oral antibiotics group (n = 288) | Absolute mean difference (95% CI) | P value |
|---|---|---|---|---|
| Primary | ||||
| Treatment success at 1 year, %a | 70.2 | 73.8 (n = 286) | −3.6% (1-sided 95% CI, −9.7% to ∞) | .26b |
| Secondary | ||||
| Length of primary hospital stay, median (IQR), h | 28.9 (23.0 to 41.9) | 29.9 (23.3 to 43.2) | −0.77 (−3.9 to 2.4) | .38 |
| Length of overall hospital stay during 1-y follow-up, median (IQR), h | 36.5 (24.0 to 63.1) | 35.7 (24.7 to 58.6) [n = 286] | 0.68 (−4.2 to 5.5) | .91 |
| Visual analog scale score for pain, median (IQR)c | ||||
| Discharge | 1.0 (0.0 to 2.0) [n = 265] | 1.0 (0.0 to 2.0) [n = 263] | NAd | .91 |
| 1 wk | 0.0 (0.0 to 0.0) [n = 265] | 0.0 (0.0 to 0.5) [n = 252] | NAd | .84 |
| 2 mo | 0.0 (0.0 to 0.0) [n = 262] | 0.0 (0.0 to 0.0) [n = 248] | NAd | .38 |
| Length of sick leave, median (IQR), d | 7.0 (3.0 to 8.0) | 7.0 (3.0 to 9.0) | 0 (−0.70 to 0.70) | .42 |
Abbreviations: IQR, interquartile range; NA, not applicable.
Treatment success was defined as resolution of acute appendicitis, discharge from hospital, no need for surgical intervention, and no recurrent appendicitis through 1 year of follow-up. Treatment success was evaluated using noninferiority analysis.
For noninferiority.
Score range of 0 to 10; a score of 0 indicates no pain and 10 indicates the worst possible pain.
Due to similarity of the values in the 2 groups, the absolute differences for visual analog scale score for pain at discharge, 1 week, and at 2 months could not be presented.
Primary Outcome
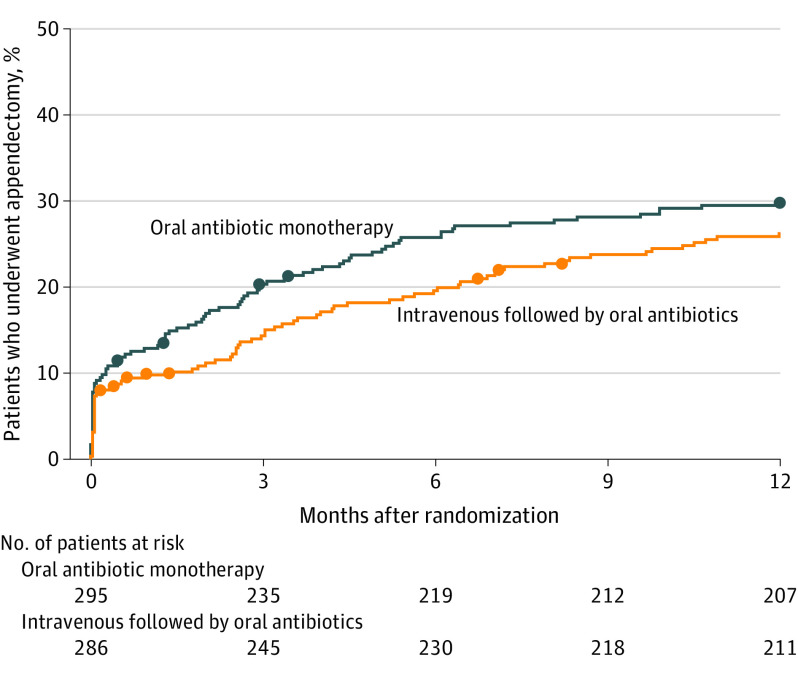
Among 295 patients randomized to receive oral antibiotic monotherapy, 27 patients (9.2%) underwent an appendectomy during the primary hospitalization and an additional 61 patients (20.7%) underwent an appendectomy during the 1-year follow-up, resulting in a treatment success rate of 70.2% (1-sided 95% CI, 65.8% to ∞) at 1 year. Of the 288 patients randomized to receive intravenous followed by oral antibiotics, 22 patients (7.6%) underwent appendectomy during the primary hospitalization and additional 53 patients (18.5%) underwent appendectomy during the 1-year follow-up for suspected appendicitis recurrence, resulting in a treatment success rate of 73.8% (1-sided 95% CI, 69.5% to ∞) at 1 year. The treatment success rates in both groups were greater than the predefined margin of 65%. For the primary outcome of treatment success between the groups at 1 year, the analysis yielded a difference of −3.6% ([1-sided 95% CI, −9.7% to ∞]; P = .26 for noninferiority), with the CI of the difference exceeding the predefined noninferiority definition of a lower limit of −6%. In a post hoc analysis, center effect was not statistically significant (P = .32) nor did it change conclusions for treatment comparison. Figure 2 shows the cumulative incidence of appendectomy during the 1-year follow-up. Among patients who underwent a surgical procedure for suspected recurrent appendicitis, the median (interquartile range) time to appendectomy was 87 (39-154) days in the oral antibiotic monotherapy group and 120 (76-211) days in the intravenous followed by oral antibiotics group.
Figure 2. Time to Appendectomy After Initial Treatment in a Study of the Effect of Oral Moxifloxacin vs Intravenous Ertapenem Plus Oral Levofloxacin for Treatment of Uncomplicated Acute Appendicitis.
A total of 581 of 583 patients (99.7%) were followed up to achievement of the primary outcome or to 1 year and included in this post hoc analysis. The solid dots represent appendectomies of histologically normal appendixes.
Of the 49 patients who underwent an appendectomy during the initial hospitalization, 29 patients (18 in the oral antibiotic monotherapy group and 11 in the intravenous followed by oral antibiotics group) were found to have complicated appendicitis at the time of the procedure. This resulted in true primary failure (ie, complicated acute appendicitis at surgery during primary hospitalization) rates of 6.1% in the oral antibiotic monotherapy group and 3.8% in the intravenous followed by oral antibiotics group, yielding a difference of 2.3% ([90% CI, −0.7% to 5.2%]; P = .25). In a blinded retrospective radiological evaluation, 18 of the 29 patients (62%) with complicated acute appendicitis found at the time of surgery during the primary hospitalization had their initial CT findings reclassified as showing complicated, rather than uncomplicated, appendicitis. Of the additional 61 patients in the oral monotherapy group and 53 patients in the intravenous followed by oral antibiotics group who underwent appendectomy during the 1-year follow-up due to clinical suspicion of recurrent appendicitis, 5 patients (8.2%) in the oral monotherapy group and 8 (15.1%) in the intravenous followed by oral antibiotics group did not have appendicitis when the appendectomy specimen was evaluated by histopathology. This resulted in a true recurrence rate of 20.9% in the oral monotherapy group and 16.7% in the intravenous followed by oral antibiotics group (difference, 4.2% [90% CI, −1.4% to 9.7%]; P = .22) after initial successful antibiotic treatment. The median time to appendectomy was 101 (95% CI, 82-127) days in patients with suspected recurrent appendicitis and 104 (95% CI, 84-132) days in patients with true recurrence.
Secondary Outcomes
The secondary outcomes are summarized in Table 2. There was no statistically significant difference between treatment groups in the length of hospital stay or sick leave or in visual analog scale scores at discharge, 1 week, and 2 months. Of all 163 patients who underwent appendectomy, 4 patients (2.6%) were diagnosed with an appendiceal tumor (eTable 3 in Supplement 3).
Adverse Events
There was no mortality during the 1-year follow-up. The overall complication rate was 4.8% (95% CI, 2.3%-7.2%) in the oral antibiotic monotherapy group and 7.3% (95% CI, 4.3%-10.4%) in the intravenous followed by oral antibiotics group (Table 3). The randomized treatment was discontinued due to suspected potential antibiotic-related adverse effects in 2 patients, both of whom were in the oral antibiotic monotherapy group (1 patients with skin eczema with facial swelling and 1 with blurred vision). Five patients, all in the intravenous followed by oral antibiotics group, reported prolonged diarrhea at 2 months of follow-up but none at 1 year of follow-up.
Table 3. Adverse Events in a Study of the Effect of Oral Moxifloxacin vs Intravenous Ertapenem Plus Oral Levofloxacin for Treatment of Uncomplicated Acute Appendicitis.
| Adverse event | No. | |
|---|---|---|
| Oral antibiotic monotherapy group (n = 295) | Intravenous followed by oral antibiotics group (n = 288) | |
| Related to antibiotic treatmenta | 6 | 14 |
| Skin eczema | 3 | 3 |
| Other allergic reaction | 1 | 2 |
| Tendinitis | 1 | 1 |
| Blurred vision | 1 | 0 |
| Prolonged diarrheab | 0 | 5 |
| Candidiasis (oral or vaginal) | 0 | 3 |
| Tendon rupture | 0 | 0 |
| Related to operative treatmenta | 9 | 10 |
| Abdominal pain, incisional pain, or obstructive symptoms | 7 | 7 |
| Surgical site infection | 2 | 3 |
| Incisional hernias | 0 | 0 |
| Other miscellaneous symptoms related to antibiotic treatment | ||
| Nausea | 23 | 40 |
| Diarrhea | 11 | 36 |
| Metallic taste sensation | 1 | 23 |
| Patients with at least 1 adverse event, No./total No. (%) [95% CI]c | 14/295 (4.8) [2.3-7.2] | 21/286 (7.3) [4.3-10.4] |
The following numbers of patients in the oral antibiotic monotherapy group and intravenous followed by oral antibiotics group were available for adverse event assessment at each time point: 295 (100%) and 288 (100%) at discharge, 273 (92.5%) and 255 (89.5%) at 1 week, 265 (89.8%) and 253 (88.8%) at 2 months, and 256 (86.8%) and 239 (83.0%) at 1 year.
Patient still reported diarrhea at 2 months.
Includes adverse events reported at any follow-up point (at discharge, 1 week, 2 months, and 1 year) and during possible rehospitalization.
Discussion
In this randomized trial of adults with uncomplicated acute appendicitis, treatment success was defined as discharge from hospital without surgery and no recurrent appendicitis within 1 year. Treatment with 7 days of oral moxifloxacin compared with 2 days of intravenous ertapenem followed by 5 days of levofloxacin and metronidazole resulted in success rates greater than 65% in both groups, but failed to demonstrate noninferiority of the oral regimen.
Although this study was not able to demonstrate noninferiority of oral antibiotic relative to combined intravenous and oral antibiotics for appendicitis, the majority of patients avoided appendectomy in both groups and 207 of 295 patients (70.2%) with uncomplicated acute appendicitis were successfully treated with oral moxifloxacin monotherapy and did not experience major complications attributable to a trial of oral-only antibiotics. In addition, the treatment outcomes of this large prospective cohort of patients with uncomplicated acute appendicitis treated with antibiotics alone corroborate the initial APPAC trial results with antibiotic treatment success of 72.7% at 1 year.1
The safety and efficacy of antibiotic treatment compared with appendectomy for the management of appendicitis is now well established by results of clinical trials,1,11,12,14,22,23 meta-analyses,5,24,25,26,27 and guidelines,6,7,28 and is confirmed at long-term follow-up2 with both substantial cost savings3,4 and similar quality of life.8 Consequently, this trial focused on optimizing antibiotic treatment of acute appendicitis. Because of the high prevalence of acute appendicitis, the feasibility, efficacy, and safety of treatment options that avoid hospitalization during the COVID-19 pandemic has become an imperative.7,28 This study was designed prior to the pandemic. Given the major risks associated with hospitalization during the pandemic, a larger noninferiority margin might have been used for this study because there is a larger benefit of avoiding hospitalization now than before the pandemic, resulting in accepting a larger treatment failure rate attributable to oral antibiotics to offset higher pandemic-related hospitalization risks. Avoiding hospitalization for the 70.2% of patients with acute appendicitis who were successfully treated with oral antibiotics might reduce the risk of disease exposure to COVID-19 for these patients and free hospital resources at a time of bed capacity shortages. These current conditions may warrant what is considered treatment success for oral antibiotic treatment of acute appendicitis.
Previous trials of uncomplicated acute appendicitis have reported up to 3-day hospitalization for antibiotics. Extended hospitalization occurred in early trials of antibiotic treatment of appendicitis in an era when appendectomy was the standard approach and there was considerable skepticism regarding the safety on nonoperative management of appendicitis.1,11,12,22 Amoxicillin-clavulanic acid was used in the trial conducted by Vons et al.23 That trial failed to demonstrate the efficacy of antibiotic treatment of appendicitis mainly because of the inclusion of patients with appendicoliths, but the antibiotic choice was also suboptimal because of possible nonsusceptibility for Escherichia coli. To avoid this limitation, oral moxifloxacin was used in the current trial. Moxifloxacin is a potent broad-spectrum oral antibiotic with efficacy for managing intraabdominal infections16,18 and an advantage of once-daily administration. Given several years of experience with managing appendicitis nonoperatively, the median hospitalization in this trial was shorter than in previous trials.1,23 A US-based pilot randomized clinical trial that compared antibiotics with appendectomy in an outpatient setting showed that 87% of patients who received antibiotics were successfully treated with antibiotics alone, and no major adverse events occurred in either group at 1 year.12 These findings were corroborated recently by 90-day results from the CODA trial, with 47% of the patients in the antibiotic group being discharged from the emergency department without hospitalization.14 Uncomplicated acute appendicitis appears to be similar to uncomplicated acute diverticulitis, for which recent studies have demonstrated no benefit of antibiotics compared with symptomatic treatment alone.29,30,31,32,33,34 Nonoperative management of uncomplicated appendicitis may succeed even without antibiotics,35 which is an area of active research.20
In a pediatric study by Minneci et al,13 patients and their families were given a choice between appendectomy and antibiotic treatment for uncomplicated acute appendicitis after receiving information on these treatments based on a script. Antibiotics were chosen by 370 of 1068 participants (35%) and treatment with antibiotics was successful in 245 participants (67%) at 1-year follow-up, which corresponds to the results of the current study in an adult population.
Primary nonresponsiveness to antibiotics and recurrence are factors that need to be both discussed with the patient and taken into consideration when choosing the optimal treatment alternative. With accurate patient selection using CT imaging, one of the most important findings associated with antibiotic therapy is the absence of major complications attributable to delaying surgery for patients who eventually needed an operation for up to 5 years.2 The CODA trial was a pragmatic trial with 90-day follow-up of patients randomized to receive antibiotics or appendectomy.14 The CODA trial showed that patients presenting with an appendicolith had a higher risk for both appendectomy (41% for patients with appendicolith and 25% for patients without) and complications (20% for patients with appendicolith and 4% for patients without), confirming the findings of earlier trials,23 and reinforced appendicolith as a finding associated with complicated appendicitis, although the nature of appendicoliths (eg, large vs small) and how it influences nonoperative management of appendicitis remains unknown.14
Complicated and uncomplicated appendicitis may be different diseases.36 Future trials investigating nonoperative management of appendicitis will need to refine the diagnosis of appendicitis severity using uniform and standardized criteria. If nonoperative therapy is considered, complicated appendicitis should be ruled out, requiring that the sensitivity and negative predictive value for tests establishing the diagnosis for appendicitis must be high, which is the case for CT imaging. Preintervention potential findings of complicated appendicitis, such as the presence of an appendicolith,14,23 are factors predictive of primary antibiotic treatment failure. Optimizing the patient selection for antibiotic treatment of uncomplicated appendicitis requires standardized imaging and identifying CT finding features for complicated appendicitis, such as contrast enhancement defect of the appendiceal wall and larger appendiceal diameter.37
Limitations
This trial has several limitations. First, moxifloxacin is a broad-spectrum antibiotic that risks the development of antibiotic resistance. Second, some patients (n = 4) were incorrectly enrolled in the study despite meeting exclusion criteria on primary CT findings, and 136 eligible patients who should have been evaluated for enrollment were not. Third, both the predefined difference of 0% and the noninferiority margin for clinical importance of 6% in the sample size calculations between the study groups were set somewhat arbitrarily because no previous trials were available comparing oral and intravenous antibiotics for uncomplicated acute appendicitis.
Conclusions
Among adults with uncomplicated acute appendicitis, treatment with 7 days of oral moxifloxacin compared with 2 days of intravenous ertapenem followed by 5 days of levofloxacin and metronidazole resulted in treatment success rates greater than 65% in both groups, but failed to demonstrate noninferiority for treatment success of oral antibiotics compared with intravenous followed by oral antibiotics.
Trial protocol
Statistical analysis plan
eTable 1. Number of randomized patients by study hospital.
eTable 2. Baseline characteristics comparison for APPAC II baseline population and non-randomized eligible patients.
eTable 3. Tumor findings in the APPAC II trial.
Data sharing statement
References
- 1.Salminen P, Paajanen H, Rautio T, et al. Antibiotic therapy vs appendectomy for treatment of uncomplicated acute appendicitis: the APPAC randomized clinical trial. JAMA. 2015;313(23):2340-2348. doi: 10.1001/jama.2015.6154 [DOI] [PubMed] [Google Scholar]
- 2.Salminen P, Tuominen R, Paajanen H, et al. Five-year follow-up of antibiotic therapy for uncomplicated acute appendicitis in the APPAC randomized clinical trial. JAMA. 2018;320(12):1259-1265. doi: 10.1001/jama.2018.13201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sippola S, Grönroos J, Tuominen R, et al. Economic evaluation of antibiotic therapy versus appendicectomy for the treatment of uncomplicated acute appendicitis from the APPAC randomized clinical trial. Br J Surg. 2017;104(10):1355-1361. doi: 10.1002/bjs.10575 [DOI] [PubMed] [Google Scholar]
- 4.Haijanen J, Sippola S, Tuominen R, et al. Cost analysis of antibiotic therapy versus appendectomy for treatment of uncomplicated acute appendicitis: 5-year results of the APPAC randomized clinical trial. PLoS One. 2019;14(7):e0220202. doi: 10.1371/journal.pone.0220202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talan DA, Saltzman DJ, DeUgarte DA, Moran GJ. Methods of conservative antibiotic treatment of acute uncomplicated appendicitis: A systematic review. J Trauma Acute Care Surg. 2019;86(4):722-736. doi: 10.1097/TA.0000000000002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15(1):27. doi: 10.1186/s13017-020-00306-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID 19: Elective Case Triage Guidelines for Surgical Care. American College of Surgeons; 2020. Updated December 8, 2020. https://www.facs.org/covid-19/clinical-guidance/elective-case/emergency-surgery [Google Scholar]
- 8.Sippola S, Haijanen J, Viinikainen L, et al. Quality of life and patient satisfaction at 7-year follow-up of antibiotic therapy vs appendectomy for uncomplicated acute appendicitis: a secondary analysis of a randomized clinical trial. JAMA Surg. 2020;155(4):283-289. doi: 10.1001/jamasurg.2019.6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minneci PC, Sulkowski JP, Nacion KM, et al. Feasibility of a nonoperative management strategy for uncomplicated acute appendicitis in children. J Am Coll Surg. 2014;219(2):272-279. doi: 10.1016/j.jamcollsurg.2014.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehlers AP, Talan DA, Moran GJ, Flum DR, Davidson GH. Evidence for an antibiotics-first strategy for uncomplicated appendicitis in adults: a systematic review and gap analysis. J Am Coll Surg. 2016;222(3):309-314. doi: 10.1016/j.jamcollsurg.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson J, Körner U, Khorram-Manesh A, Solberg A, Lundholm K. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg. 2009;96(5):473-481. doi: 10.1002/bjs.6482 [DOI] [PubMed] [Google Scholar]
- 12.Talan DA, Saltzman DJ, Mower WR, et al. Antibiotics-first versus surgery for appendicitis: a US pilot randomized controlled trial allowing outpatient antibiotic management. Ann Emerg Med. 2017;70(1):1-11. doi: 10.1016/j.annemergmed.2016.08.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minneci PC, Hade EM, Lawrence AE, et al. ; Midwest Pediatric Surgery Consortium . Association of nonoperative management using antibiotic therapy vs laparoscopic appendectomy with treatment success and disability days in children with uncomplicated appendicitis. JAMA. 2020;324(6):581-593. doi: 10.1001/jama.2020.10888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flum DR, Davidson GH, Monsell SE, et al. ; CODA Collaborative . A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383(20):1907-1919. doi: 10.1056/NEJMoa2014320 [DOI] [PubMed] [Google Scholar]
- 15.Kaji AH, Lewis RJ. Noninferiority trials: is a new treatment almost as effective as another? JAMA. 2015;313(23):2371-2372. doi: 10.1001/jama.2015.6645 [DOI] [PubMed] [Google Scholar]
- 16.De Waele JJ, Tellado JM, Alder J, et al. Randomised clinical trial of moxifloxacin versus ertapenem in complicated intra-abdominal infections: results of the PROMISE study. Int J Antimicrob Agents. 2013;41(1):57-64. doi: 10.1016/j.ijantimicag.2012.08.013 [DOI] [PubMed] [Google Scholar]
- 17.Goldstein EJ, Solomkin JS, Citron DM, Alder JD. Clinical efficacy and correlation of clinical outcomes with in vitro susceptibility for anaerobic bacteria in patients with complicated intra-abdominal infections treated with moxifloxacin. Clin Infect Dis. 2011;53(11):1074-1080. doi: 10.1093/cid/cir664 [DOI] [PubMed] [Google Scholar]
- 18.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt). 2010;11(1):79-109. doi: 10.1089/sur.2009.9930 [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sippola S, Grönroos J, Sallinen V, et al. A randomised placebo-controlled double-blind multicentre trial comparing antibiotic therapy with placebo in the treatment of uncomplicated acute appendicitis: APPAC III trial study protocol. BMJ Open. 2018;8(11):e023623. doi: 10.1136/bmjopen-2018-023623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haijanen J, Sippola S, Grönroos J, et al. ; APPAC study group . Optimising the antibiotic treatment of uncomplicated acute appendicitis: a protocol for a multicentre randomised clinical trial (APPAC II trial). BMC Surg. 2018;18(1):117. doi: 10.1186/s12893-018-0451-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Styrud J, Eriksson S, Nilsson I, et al. Appendectomy versus antibiotic treatment in acute appendicitis. a prospective multicenter randomized controlled trial. World J Surg. 2006;30(6):1033-1037. doi: 10.1007/s00268-005-0304-6 [DOI] [PubMed] [Google Scholar]
- 23.Vons C, Barry C, Maitre S, et al. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377(9777):1573-1579. doi: 10.1016/S0140-6736(11)60410-8 [DOI] [PubMed] [Google Scholar]
- 24.Harnoss JC, Probst P, Büchler MW, Diener MK. Antibiotics versus appendicectomy for the treatment of uncomplicated acute appendicitis: an updated meta-analysis of randomised controlled trials by Rollins et al. World J Surg. 2017;41(9):2411. doi: 10.1007/s00268-016-3864-8 [DOI] [PubMed] [Google Scholar]
- 25.Sallinen V, Akl EA, You JJ, et al. Meta-analysis of antibiotics versus appendicectomy for non-perforated acute appendicitis. Br J Surg. 2016;103(6):656-667. doi: 10.1002/bjs.10147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakran JV, Mylonas KS, Gryparis A, et al. Operation versus antibiotics—the “appendicitis conundrum” continues: a meta-analysis. J Trauma Acute Care Surg. 2017;82(6):1129-1137. doi: 10.1097/TA.0000000000001450 [DOI] [PubMed] [Google Scholar]
- 27.Podda M, Gerardi C, Cillara N, et al. Antibiotic treatment and appendectomy for uncomplicated acute appendicitis in adults and children: a systematic review and meta-analysis. Ann Surg. 2019;270(6):1028-1040. doi: 10.1097/SLA.0000000000003225 [DOI] [PubMed] [Google Scholar]
- 28.Ielpo B, Podda M, Pellino G, et al. ; ACIE Appy Study Collaborative . Global attitudes in the management of acute appendicitis during COVID-19 pandemic: ACIE Appy Study. Br J Surg. Published online October 8, 2020. doi: 10.1002/bjs.11999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chabok A, Påhlman L, Hjern F, Haapaniemi S, Smedh K, Group AS; AVOD Study Group . Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. Br J Surg. 2012;99(4):532-539. doi: 10.1002/bjs.8688 [DOI] [PubMed] [Google Scholar]
- 30.Daniels L, Ünlü Ç, de Korte N, et al. ; Dutch Diverticular Disease (3D) Collaborative Study Group . Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. Br J Surg. 2017;104(1):52-61. doi: 10.1002/bjs.10309 [DOI] [PubMed] [Google Scholar]
- 31.de Korte N, Kuyvenhoven JP, van der Peet DL, Felt-Bersma RJ, Cuesta MA, Stockmann HB. Mild colonic diverticulitis can be treated without antibiotics. A case-control study. Colorectal Dis. 2012;14(3):325-330. doi: 10.1111/j.1463-1318.2011.02609.x [DOI] [PubMed] [Google Scholar]
- 32.Isacson D, Smedh K, Nikberg M, Chabok A. Long-term follow-up of the AVOD randomized trial of antibiotic avoidance in uncomplicated diverticulitis. Br J Surg. 2019;106(11):1542-1548. doi: 10.1002/bjs.11239 [DOI] [PubMed] [Google Scholar]
- 33.Isacson D, Thorisson A, Andreasson K, Nikberg M, Smedh K, Chabok A. Outpatient, non-antibiotic management in acute uncomplicated diverticulitis: a prospective study. Int J Colorectal Dis. 2015;30(9):1229-1234. doi: 10.1007/s00384-015-2258-y [DOI] [PubMed] [Google Scholar]
- 34.Mali JP, Mentula PJ, Leppäniemi AK, Sallinen VJ. Symptomatic treatment for uncomplicated acute diverticulitis: a prospective cohort study. Dis Colon Rectum. 2016;59(6):529-534. doi: 10.1097/DCR.0000000000000579 [DOI] [PubMed] [Google Scholar]
- 35.Park HC, Kim MJ, Lee BH. Randomized clinical trial of antibiotic therapy for uncomplicated appendicitis. Br J Surg. 2017;104(13):1785-1790. doi: 10.1002/bjs.10660 [DOI] [PubMed] [Google Scholar]
- 36.Livingston EH, Woodward WA, Sarosi GA, Haley RW. Disconnect between incidence of nonperforated and perforated appendicitis: implications for pathophysiology and management. Ann Surg. 2007;245(6):886-892. doi: 10.1097/01.sla.0000256391.05233.aa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HY, Park JH, Lee SS, Jeon JJ, Yoon CJ, Lee KH. Differentiation between complicated and uncomplicated appendicitis: diagnostic model development and validation study. Abdom Radiol (NY). Published online September 10, 2020. doi: 10.1007/s00261-020-02737-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eTable 1. Number of randomized patients by study hospital.
eTable 2. Baseline characteristics comparison for APPAC II baseline population and non-randomized eligible patients.
eTable 3. Tumor findings in the APPAC II trial.
Data sharing statement