Key Points
Question
Is early neurological deterioration of ischemic origin (ENDi) predictable in patients with minor stroke and large vessel occlusion (LVO) treated with intravenous thrombolysis (IVT)?
Findings
In this cohort of 729 patients with minor stroke (National Institutes of Health Stroke Scale score of 5 or less) and LVO intended for IVT alone, an easily applicable score based on occlusion site and thrombus length—2 independent predictors of ENDi—showed good discriminative power for ENDi risk prediction and was successfully validated in an independent cohort of 347 patients.
Meaning
ENDi can be reliably predicted in patients with IVT-treated minor strokes and LVO, which may help to select the best candidates for direct transfer for additional thrombectomy.
This cohort study aims to develop and validate an easily applicable predictive score of early neurological deterioration of presumed ischemic origin following intravenous thrombolysis in patients with minor stroke and large vessel occlusion (LVO).
Abstract
Importance
The best reperfusion strategy in patients with acute minor stroke and large vessel occlusion (LVO) is unknown. Accurately predicting early neurological deterioration of presumed ischemic origin (ENDi) following intravenous thrombolysis (IVT) in this population may help to select candidates for immediate transfer for additional thrombectomy.
Objective
To develop and validate an easily applicable predictive score of ENDi following IVT in patients with minor stroke and LVO.
Design, Setting, and Participants
This multicentric retrospective cohort included 729 consecutive patients with minor stroke (National Institutes of Health Stroke Scale [NIHSS] score of 5 or less) and LVO (basilar artery, internal carotid artery, first [M1] or second [M2] segment of middle cerebral artery) intended for IVT alone in 45 French stroke centers, ie, including those who eventually received rescue thrombectomy because of ENDi. For external validation, another cohort of 347 patients with similar inclusion criteria was collected from 9 additional centers. Data were collected from January 2018 to September 2019.
Main Outcomes and Measures
ENDi, defined as 4 or more points’ deterioration on NIHSS score within the first 24 hours without parenchymal hemorrhage on follow-up imaging or another identified cause.
Results
Of the 729 patients in the derivation cohort, 335 (46.0%) were male, and the mean (SD) age was 70 (15) years; of the 347 patients in the validation cohort, 190 (54.8%) were male, and the mean (SD) age was 69 (15) years. In the derivation cohort, the median (interquartile range) NIHSS score was 3 (1-4), and the occlusion site was the internal carotid artery in 97 patients (13.3%), M1 in 207 (28.4%), M2 in 395 (54.2%), and basilar artery in 30 (4.1%). ENDi occurred in 88 patients (12.1%; 95% CI, 9.7-14.4) and was strongly associated with poorer 3-month outcomes, even in patients who underwent rescue thrombectomy. In multivariable analysis, a more proximal occlusion site and a longer thrombus were independently associated with ENDi. A 4-point score derived from these variables—1 point for thrombus length and 3 points for occlusion site—showed good discriminative power for ENDi (C statistic = 0.76; 95% CI, 0.70-0.82) and was successfully validated in the validation cohort (ENDi rate, 11.0% [38 of 347]; C statistic = 0.78; 95% CI, 0.70-0.86). In both cohorts, ENDi probability was approximately 3%, 7%, 20%, and 35% for scores of 0, 1, 2 and 3 to 4, respectively.
Conclusions and Relevance
The substantial ENDi rates observed in these cohorts highlights the current debate regarding whether to directly transfer patients with IVT-treated minor stroke and LVO for additional thrombectomy. Based on the strong associations observed, an easily applicable score for ENDi risk prediction that may assist decision-making was derived and externally validated.
Introduction
Likely owing to good collaterals, a sizeable fraction of patients with acute stroke and large vessel occlusion (LVO) present with only mild neurological deficit. Although intravenous thrombolysis (IVT) is standard-of-care in patients with minor but disabling stroke and LVO,1 the substantial risk of early neurological deterioration (END) and poor 3-month outcome has been repeatedly underlined.2,3,4 Given that END is rarely due to intracranial hemorrhage in this population, it is presumed to be of ischemic origin (ENDi) in most patients, yet the precise underlying mechanisms are incompletely deciphered.2,5 Considering the strong association between lack of recanalization and ENDi in unselected stroke populations,6,7 bridging therapy (ie, IVT followed by mechanical thrombectomy [MT]) may be an attractive option for patients with minor stroke and high ENDi risk. However, although bridging therapy is currently recommended in patients with nonminor LVO eligible for IVT (ie, with National Institutes of Health Stroke Scale [NIHSS] score greater than 5), whether it is superior to IVT alone in those with minor stroke and LVO is unknown because few such patients were enrolled in the pivotal thrombectomy trials.8
Here, we aimed to identify the incidence and predictors of ENDi in a large sample of patients with IVT-treated minor stroke and LVO and to develop and externally validate a prediction score intended to assist clinicians in assessing the risk of ENDi in this population and, in turn, in selecting the most appropriate candidates for bridging therapy.
Methods
This report was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. In accordance with local legislation, as this study only implied retrospective analysis of anonymized data collected as part of routine care, formal approval by an ethics committee and informed consent were not required.
Study Design and Data Sources
Derivation Cohort
The MINOR-STROKE collaboration9 retrospectively collected data from all consecutive patients with acute stroke admitted to 45 French stroke centers between 2006 and 2018 (inclusion dates varied among centers) (eTable 1 in the Supplement) who fulfilled the following criteria: (1) baseline admission NIHSS score of 5 or less; (2) LVO on pretreatment vascular imaging (internal carotid artery [ICA], first [M1] or second [M2] segment of middle cerebral artery [MCA], basilar artery); and (3) treated with IVT (alteplase only), with or without additional MT. In all the participating centers, vascular imaging (magnetic resonance angiography or computed tomography angiography [CTA]) was systematically performed on admission for patients with acute stroke admitted within a reperfusion time window during the inclusion time period, irrespective of symptom severity. All patients were informed of their participation in the study.
In the present study, we focused on patients with MCA or basilar artery occlusion intended for IVT alone, ie, including those who eventually received rescue MT because of ENDi. Therefore, we excluded the patients (1) directly intended for additional MT or (2) with isolated ICA occlusion (ie, without MCA occlusion).
Validation Cohort
We used data from the prospectively gathered databases of 9 additional stroke centers (6 from France and 3 from Switzerland) (eTable 1 in the Supplement) to construct the validation cohort. The same inclusion criteria as above were applied.
Clinical and Radiological Data
Clinical variables routinely recorded in the acute stroke setting were collected (eMethods in the Supplement). All included patients underwent either computed tomography (CT) with CTA or magnetic resonance imaging (MRI) with magnetic resonance angiography before IVT start and follow-up MRI or CT within approximately 24 hours following admission. Perfusion imaging (CT or MR perfusion) was part of routine admission protocol in some centers. Additional MRI or CT was also obtained in case of END. In the derivation cohort, to ensure homogeneity in radiological evaluation, 1 stroke neurologist (P. S.) reviewed all pre-IVT and follow-up imaging and MT procedures of all included patients, blinded to clinical outcomes. The following variables were collected: (1) occlusion site, divided into the following categories: intracranial T or L ICA (ICA-T/L), proximal M1, distal M1, M2, tandem cervical ICA and M1 or M2, and basilar artery; (2) thrombus length measured either on MRI (ie, susceptibility vessel sign), CT (hyperdense MCA sign), or CTA10,11,12; (3) infarct core extent; and (4) whenever perfusion imaging was available, severity of hypoperfusion, assessed using the hypoperfusion intensity ratio (HIR) (eMethods in the Supplement).13 For patients receiving groin puncture (ie, rescue MT), recanalization was evaluated on the final intracranial run using the modified Thrombolysis in Cerebral Infarction (mTICI) scale. mTICI grade of 2b to 3 was considered as successful recanalization. The same methods were applied for the validation cohort, with central imaging reading for 4 of 9 participating centers.
Definition of ENDi
ENDi refers to neurological deterioration of presumed ischemic origin, ie, the only END category that may be prevented by MT. It was defined as an NIHSS score increase of 4 points or more within the first 24 hours after IVT5,6 without evidence of a parenchymal hemorrhage according to the European Cooperative Acute Stroke Study definition on follow-up imaging or with no identified alternative cause (eg, poststroke seizure) after careful review of the medical records by a local stroke physician.7
Statistical Analysis
Continuous variables were described as means and standard deviations or medians and interquartile ranges (IQRs), as appropriate, and categorical variables as counts and percentages. In the derivation cohort, we modeled the probability of a worse 3-month functional outcome in patients with and without ENDi via an ordinal logistic regression, providing a common odds ratio (OR) and 95% CI across the whole range of the modified Rankin Scale. The procedures used to identify predictors of ENDi and derive and externally validate a predictive score are detailed in the eMethods in the Supplement. Briefly, univariable associations between baseline variables and ENDi were assessed in the derivation cohort using t test or Mann-Whitney U test for continuous variables and χ2 or Fisher exact test for categorical variables, as appropriate. Then stepwise multivariable binary logistic regression analysis was conducted with ENDi as a dependent variable. Two different models were derived, with and without thrombus length, as this variable was not available for all patients. The ENDi score was derived based on the multivariable model above with the highest C statistic. Discrimination of the score to predict ENDi was assessed using C statistic. Internal cross-validation was performed using the bootstrap method, and external validation was performed on the validation cohort. Statistical analyses were performed using SAS version 9.4 (SAS Institute) and SPSS version 16.0 (SPSS Inc). Two-tailed P < .05 was considered statistically significant.
Results
Study Population
A total of 729 and 347 patients were included in the derivation and validation cohorts, respectively (eFigure in the Supplement). Of the 729 patients in the derivation cohort, 335 (46.0%) were male, the mean (SD) age was 70 (15) years, the median (IQR) NIHSS score was 3 (1-4), and occlusion sites were ICA, M1, M2, and basilar in 97 patients (13.3%), 207 patients (28.4%), 395 patients (54.2%), and 30 patients (4.1%), respectively. Of the 347 patients in the validation cohort, 190 (54.8%) were male, the mean (SD) age was 69 (15) years, the median (IQR) NIHSS score was 3 (2-4), and occlusion sites were ICA, M1, M2, and basilar in 31 patients (8.9%), 60 patients (17.3%), 244 patients (70.3%), and 12 patients (3.5%), respectively. Baseline characteristics of patients from the derivation and validation cohorts are provided in eTable 2 in the Supplement.
END Incidence, Causes, and Characteristics
In the derivation cohort, END occurred in 96 patients (13.2%; 95% CI, 10.7-15.7), and ENDi occurred in 88 (12.1%; 95% CI, 9.7-14.4) (eTable 2 in the Supplement). The median (IQR) NIHSS score increase in patients with ENDi was 8 (5-14), and the timing of ENDi after IVT start was within 2 hours in 42 patients (48%), 2 to 6 hours in 11 (13%), 6 to 12 hours in 7 (8%), and 12 to 24 hours 28 (32%). Among patients with ENDi, 49 of 88 (56%) underwent rescue MT, with groin puncture occurring at a median (IQR) delay of 95 (70-150) minutes following ENDi and successful reperfusion (mTICI grade of 2b to 3) obtained in 40 of 49 patients (82%). Further details regarding the timeline and MT procedures and baseline comparison of patients with ENDi treated with or without rescue MT are provided in eTable 3 in the Supplement. Compared with patients with ENDi without rescue MT, those with rescue MT were more frequently treated after 2014 and had shorter IVT-to-ENDi delays. In the validation cohort, ENDi occurred in 38 patients (11.0%; 95% CI, 7.7-14.3) (eTable 2 in the Supplement).
Outcome According to ENDi Status in the Derivation Cohort
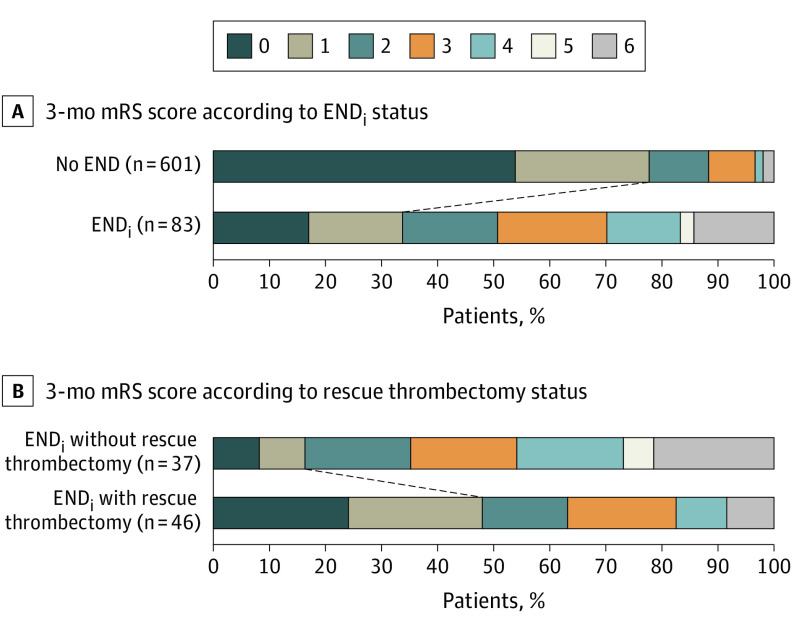
Patients with ENDi had significantly poorer 3-month outcome than those without (common OR, 7.37; 95% CI, 4.79-11.35; P < .001) (Figure 1A). The rate of excellent functional outcome (mRS score less than 2) was 34% (28 of 83) and 77.5% (466 of 601) in patients with and without ENDi, respectively (P < .001). Patients with ENDi treated with rescue MT had better outcome than those without (common OR, 3.72; 95% CI, 1.67-8.32; P = .001) (Figure 1B). The rate of excellent functional outcome was 48% (22 of 46) and 16% (6 of 37) in patients with ENDi with and without rescue MT, respectively (P = .002). Patients with ENDi with rescue MT had lower rates of excellent functional outcome than patients without ENDi (48% [22 of 46] vs 77.5% [466 of 601], respectively; P < .001).
Figure 1. Three-Month Modified Rankin Scale (mRS) Scores According to Early Neurological Deterioration of Presumed Ischemic Origin (ENDi) Status and Rescue Thrombectomy Status in the ENDi Subgroup in the Derivation Cohort.
mRS score was not available for 37 patients (5 patients with ENDi and 32 with no early neurological deterioration [END]).
Predictors of ENDi in the Derivation Cohort
Univariable Analysis
The baseline characteristics of patients with and without ENDi and the results of the univariable analyses are presented in Table 1. Patients experiencing ENDi were more frequently men and had more proximal occlusion and longer thrombus. Perfusion parameters, including HIR, were not associated with ENDi. The association of thrombus length with ENDi was similar for patients with baseline MRI (unadjusted OR per 1-mm increase, 1.11; 95% CI, 1.06-1.16) and CT/CTA (unadjusted OR per 1-mm increase, 1.12; 95% CI, 0.98-1.29) (P for interaction = .83). Figure 2 illustrates the ENDi rate according to occlusion site and the ENDi predicted probability as a function of thrombus length.
Table 1. Univariate Associations Between Baseline Variables and Early Neurological Deterioration of Presumed Ischemic Origin (ENDi) in the Derivation Cohort.
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| ENDi (n = 88) | No END (n = 633)a | ||
| Patient history | |||
| Age, mean (SD), y | 69 (15) | 70 (15) | .25 |
| Male | 50 (57) | 282 (44.5) | .03 |
| Hypertension | 50 (58) | 361 (57.0) | .94 |
| Diabetes | 12 (14) | 100 (15.8) | .63 |
| Current smoking | 18 (21) | 111 (17.7) | .49 |
| Antiplatelets | 28 (32) | 181 (28.6) | .50 |
| Pre-IVT characteristics | |||
| NIHSS score, median (IQR) | 3.5 (2-5) | 3 (1-4) | .08 |
| Treatment after 2014 | 55 (63) | 367 (58.0) | .42 |
| Blood pressure, median (IQR), mm Hgb | |||
| Systolic | 154 (139-170) | 150 (135-164) | .08 |
| Diastolic | 83 (75-90) | 80 (70-90) | .21 |
| Onset-to-IVT time, median (IQR), min | 173 (129-215) | 160 (130- 203) | .23 |
| On-site endovascular facility | 62 (71) | 401 (63.3) | .19 |
| Pre-IVT imaging | |||
| MRI | 80 (91) | 569 (89.9) | .77 |
| Left hemispheric stroke, No./total No. (%) | 40/80 (50) | 303/611 (49.6) | .95 |
| Occlusion site | |||
| ICA-T/L | 12 (14) | 10 (1.6) | <.001 |
| Tandem | 17 (19) | 58 (9.2) | |
| M1 | |||
| Proximal | 10 (11) | 42 (6.6) | |
| Distal | 20 (23) | 133 (21.0) | |
| M2 | 21 (24) | 368 (58.1) | |
| Basilar | 8 (9) | 22 (3.5) | |
| DWI-ASPECTS, median (IQR)c | 9 (8-9) | 9 (8-9) | .66 |
| Thrombus visible | 76 (86) | 538 (85.0) | .74 |
| Thrombus length, median (IQR), mmd | 11.5 (8.8-14.8) | 8.3 (5.8-11.0) | <.001 |
| MRIe | 11.0 (6.0-14.0) | 8.3 (6.0-11.0) | <.001 |
| CT/CTAf | 13.4 (10.4-15.7) | 8.2 (5.0-11.3) | .05 |
| Tmax volume, median (IQR), mLg | |||
| >6 s | 41 (25-73) | 34 (18-55) | .23 |
| >8 s | 19 (8-35) | 16 (6-31) | .38 |
| >10 s | 10 (0-19) | 9 (0-17) | .67 |
| HIR, median (IQR), %g | |||
| 10/6 | 22 (0-32) | 21 (0-37) | .99 |
| 8/6 | 47 (41-60) | 47 (26-60) | .53 |
Abbreviations: CT, computed tomography; CTA, computed tomography angiography; DWI-ASPECTS, diffusion-weighted imaging Alberta Stroke Program Early CT Score; END, early neurological deterioration; HIR, hypoperfusion intensity ratio; ICA-T/L, T or L intracranial internal carotid artery; IQR, interquartile range; IVT, intravenous thrombolysis; M1, first segment of the middle cerebral artery; M2, second segment of the middle cerebral artery; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; Tmax, time to maximum.
A total of 8 patients with END due to intracranial hemorrhage or clear alternative cause were excluded.
A total of 23 patients had missing data (ENDi, n = 3; no END, n = 20).
A total of 649 patients with baseline MRI were included (ENDi, n = 80; no END, n = 569).
Patients without visible thrombus were excluded. A total of 614 patients with visible thrombus were included (ENDi, n = 76; no END, n = 538).
Patients without visible thrombus were excluded. A total of 561 patients with visible thrombus were included (ENDi, n = 69; no END, n = 492).
Patients without visible thrombus were excluded. A total of 53 patients with visible thrombus were included (ENDi, n = 7; no END, n = 46).
Patients without perfusion imaging were excluded. A total of 186 patients with perfusion imaging were included (ENDi, n = 28; no END, n = 158).
Figure 2. Early Neurological Deterioration of Presumed Ischemic Origin (ENDi) as a Function of Occlusion Site and Thrombus Length in the Derivation Cohort.

A, ENDi rates according to each occlusion site. Error bars indicate 95% CIs. B, The regression curve estimates the probability of ENDi according to thrombus length. The shaded area depicts the 95% CIs (logistic regression model). END indicates early neurological deterioration; ICA-T/L, T or L intracranial internal carotid artery; M1, first segment of the middle cerebral artery; M2, second segment of the middle cerebral artery.
Multivariable Analysis
After stepwise variable selection into the multivariable model using the variables with P < .20 from Table 1 except for thrombus length, occlusion site was the sole variable associated with ENDi (model 1) (Table 2). The alternative multivariable model including thrombus length as a continuous variable revealed that both occlusion site and thrombus length were independently associated with ENDi. We then dichotomized thrombus length using the Youden index for ENDi prediction as a cutoff (namely, thrombus length less than 9 mm and 9 mm or greater). This multivariable model is presented in Table 2 (model 2). There was no interaction between thrombus length and occlusion site for ENDi prediction, ie, the association of thrombus length with ENDi did not differ across occlusion sites. The C statistic of model 2 was significantly higher than model 1 (model 2: C statistic = 0.77; 95% CI, 0.71-0.83; model 1: C statistic = 0.72; 95% CI, 0.66-0.78; P = .006 for comparison).
Table 2. Variables Independently Associated With Early Neurological Deterioration of Presumed Ischemic Origin (ENDi) in Multivariable Logistic Regression, Including or Excluding Thrombus Length (Derivation Cohort).
| Variable | Adjusted OR (95% CI) | P value |
|---|---|---|
| Model 1, excluding thrombus length (n = 721)a | ||
| Occlusion site | ||
| M2 | 1 [Reference] | <.001 |
| Distal M1 | 2.6 (1.4-5.0) | |
| Proximal M1 | 4.2 (1.8-9.4) | |
| Tandem | 5.1 (2.6-10.3) | |
| ICA-T/L | 21.0 (8.2-54.2) | |
| Basilar | 6.4 (2.5-16.0) | |
| Model 2, including thrombus length (n = 614 with visible thrombus)a | ||
| Occlusion site | ||
| M2 | 1 [Reference] | <.001 |
| Distal M1 | 2.5 (1.2-5.1) | |
| Proximal M1 | 5.2 (2.1-13.1) | |
| Tandem | 4.5 (2.1-9.7) | |
| ICA-T/L | 16.0 (5.7-44.9) | |
| Basilar | 7.2 (2.6-20.0) | |
| Thrombus length, mm | ||
| <9 | 1 [Reference] | .002 |
| ≥9 | 3.2 (1.8-5.7) | |
Abbreviations: ICA-T/L, T or L intracranial internal carotid artery; M1, first segment of the middle cerebral artery; M2, second segment of the middle cerebral artery; OR, odds ratio.
Variables not retained in the model: National Institutes of Health Stroke Scale score, sex, on-site endovascular facility, and systolic blood pressure.
The sensitivity analyses of the 2 models for patients treated before and after 2015 are presented in eTable 4 in the Supplement. The results were similar for each time period.
ENDi Prediction Score
Derivation of the Score
We used model 2 for the derivation of the ENDi score because it was associated with the highest C statistic. The integer-based score (range, 0-4 points) was constructed according to the magnitude of the regression coefficients observed in model 2 (Figure 3). The probability of ENDi per incremental point of the score is shown in Figure 3; ENDi probability was less than 7% for scores of 0 or 1, which represents two-thirds of the overall sample, but was greater than 18% for scores of 2 to 4, reaching 35% for scores of 3 or 4. The C statistic of the score was 0.76 (95% CI, 0.70-0.82).
Figure 3. The ENDi Score for Prediction of Early Neurological Deterioration of Presumed Ischemic Origin (ENDi) in Patients With Minor Stroke Due to Intracranial Large Vessel Occlusion.
A, ENDi risk prediction score. B, Probability of ENDi according to incremental points on the ENDi score applied to the derivation and validation cohorts. Incremental points are presented on the x-axis and probability of ENDi on the y-axis. Error bars indicate the 95% CIs. ICA-T/L indicates T or L intracranial internal carotid artery; M1, first segment of the middle cerebral artery; M2, second segment of the middle cerebral artery.
Score Validation
The internal cross-validation based on 2000 bootstrap replicates showed a similar C statistic (0.75; 95% CI, 0.69-0.82). In the external validation cohort, the ENDi score showed good discrimination (C statistic = 0.78; 95% CI, 0.70-0.86) and calibration (Hosmer-Lemeshow test P = .78) to predict ENDi (Figure 3).
Discussion
Based on 2 large multicentric cohorts of patients with minor stroke and LVO, this study disclosed 4 key findings: (1) ENDi affected approximately 12% of patients and accounted for approximately 90% of END cases; (2) 3-month functional outcome following ENDi improved with rescue MT but remained poor overall; (3) in multivariable analysis, more proximal occlusion and longer thrombus were independently associated with ENDi; and (4) the derived ENDi score had good discriminative power and was successfully validated in an independent cohort.
Cause of END
In both cohorts, ENDi accounted for approximately 90% of patients with END following IVT. Indeed, symptomatic intracranial hemorrhage occurred in less than 7% of END cases—which is expected considering the mild baseline symptoms and small baseline infarct core—and a clear alternative cause was identified in only 3% to 7% of all END cases, in line with previous studies.2,3,5 As our ultimate aim here was to help physicians select the most appropriate candidates for immediate transfer for thrombectomy following IVT, we will focus on ENDi in what follows.
Incidence of ENDi and Associations With Outcome
Thus far, to our knowledge, a single previous study has focused on ENDi in patients with IVT-treated minor stroke and LVO, reporting a slightly lower incidence rate than in the present study (9% vs 12%, respectively).2 This difference is likely explained by the a priori exclusion in this previous study of patients receiving rescue MT (ie, MT performed because of ENDi).2 Importantly, consistent with earlier studies,2,7,14 ENDi was found here to be strongly associated with poor functional outcome. Although excellent functional outcome was more frequent in patients receiving rescue MT vs no rescue MT (48% vs 16%, respectively), the former subset had significantly poorer outcome than patients without ENDi (78%) despite successful reperfusion obtained in 82% of cases. This observation is consistent with a previous study in a similar population.15 Note that, because of noninvasive brain imaging being performed on site to rule out intracranial hemorrhage, the time elapsed between ENDi and groin puncture was substantial in our study (median [IQR] delay, 95 [70-150] minutes), which may in part explain the poor outcomes observed despite rescue MT. Considering that END is of presumed ischemic origin in 90% of patients in this population, direct transfer to the angiosuite, ie, bypassing noninvasive brain imaging, might therefore be an option to reduce this delay, particularly in mothership patients.
The substantial incidence of post-IVT ENDi together with its strong association with poor outcome despite rescue MT sheds new light on current debates regarding treatment of patients with minor stroke and LVO, particularly whether immediate transfer for MT following IVT should be considered in these patients. As minor strokes were excluded a priori from the pivotal thrombectomy trials,8 the benefits from bridging therapy remain unknown in this population for which current guidelines regarding thrombectomy are somewhat vague (“may be reasonable”).1,8 Randomized trials testing MT added on to best medical management vs best medical management alone in this population are under way (Endovascular Therapy for Low NIHSS Ischemic Strokes16 and Minor Stroke Therapy Evaluation17). However, because of the mild baseline clinical severity and overall good 3-month outcome following IVT alone in this population, large samples will be required to show significant superiority of bridging therapy, a major challenge considering the relative rarity of minor stroke with LVO. As patients at higher ENDi risk may benefit from direct transfer for additional thrombectomy, our next aim was to identify independent predictors of ENDi.
Independent Predictors of ENDi
Two independent predictors of ENDi emerged from the present study. The first was more proximal occlusion site, in line with the single previous comparable study2 as well as with earlier studies in patients with minor stroke not treated with IVT18,19 and patients with nonminor stroke treated with IVT.6,7,20 The second independent predictor of ENDi was thrombus length, such that the longer the thrombus, the higher the odds of ENDi. To our knowledge, this is the first study to report such an association. Interestingly, perfusion parameters—including HIR, a surrogate marker of collateral flow13—were not associated with ENDi in our cohort, consistent with a smaller-scaled study of a similar population apart from a lower use of IVT.14
Longer thrombus and more proximal occlusion have been previously found to be the main predictors of lack of early post-IVT recanalization, both in minor stroke21 and nonminor stroke.22,23,24,25,26 In turn, lack of early recanalization may be one pathophysiological link explaining the association observed here between ENDi and these variables.7 Although the precise mechanisms underlying ENDi are still uncertain, one influential hypothesis is extension of symptomatic ischemic tissue (ie, infarct core and/or penumbra) into the surrounding asymptomatic tissue27,28 as a result of secondary hemodynamic, thrombotic, and/or metabolic events in the context of persistent LVO.5,27,28 Considering the apparent major role of hemodynamic or thrombotic factors, ensuring early recanalization with additional MT would appear a logical approach to prevent ENDi.8,29 However, the relatively small rate of ENDi observed in our study may explain the lack of clear benefit derived from direct transfer for additional MT found in most observational studies in similar populations.4,9,30,31,32,33,34,35 However, interestingly, we recently reported that occlusion site is a strong modifier of the association of additional MT with outcome, with additional MT being associated with higher odds of excellent outcome in M1 occlusions (ie, involving a high risk of post-IVT ENDi) but not in more distal occlusions (ie, with low ENDi risk).9
ENDi Prediction Score
Based on the above results, we derived and validated a clinical score intended to predict ENDi after admission imaging, the aim being to assist physicians in assessing the risk of ENDi and in turn making a decision whether or not to immediately transfer the patient for additional MT. In the derivation cohort, patients with scores of 0 and 1, which includes two-thirds of our sample, had very low rates of ENDi (less than 7%), while patients with a score of 2 (22% of our sample) had a substantial risk (1 in 5) and those with scores of 3 or 4 (15% of the sample) had an even larger risk (roughly 1 in 3). Importantly, our clinical score was externally validated, with very similar figures found in the validation cohort. Of note, the generalizability of our prediction score would, if anything, be strengthened by the differences in clinical-radiological variables present between the derivation and validation cohorts (eTable 2 in the Supplement).
Limitations
This study has several limitations. First, although it brings up important new data regarding the risk of ENDi following IVT alone in patients with minor stroke and LVO, our study does not directly inform whether bridging therapy is superior to IVT alone, even in the subgroups at highest risk of ENDi. However, in line with the present results, another observational study from our large cohort suggests that bridging therapy compared with IVT alone may be beneficial only in patients with proximal LVO, ie, those patients at higher risk of post-IVT ENDi.9 This will need to be confirmed in randomized trials. Second, 262 patients with minor stroke and LVO treated with first-line bridging therapy were excluded from the derivation cohort (eFigure in the Supplement), which might have induced a selection bias. However, more than 95% of these 262 patients were treated after 2015, and sensitivity analyses showed very similar results of prediction models regardless of whether patients were treated before or after this date. Third, clinical fluctuations before admission or on hospital arrival—either spontaneous or provoked by hemodynamic maneuvers, such as the head-up position14—may have relevance in ENDi prediction14 but could not be retrospectively collected in a reliable way. Additionally, thrombus length was evaluated using 2 distinct imaging modalities. However, in our cohorts, median thrombus length was similar regardless of imaging modality, and furthermore, the association of thrombus length with incidence of ENDi was quite similar across the 2 modalities.
Conclusions
In conclusion, our study documents a substantial rate of ENDi in patients with minor stroke and LVO treated with IVT, fueling the current debate on whether bridging therapy should be carried out in this population. Second, we demonstrate that the odds of post-IVT ENDi are strongly determined by occlusion site and thrombus length. Lastly, the straightforward score derived from these associations and successfully validated in an independent cohort affords good discriminative power for ENDi prediction, which may eventually help clinicians for decision-making.
eMethods.
eTable 1. List of participating centers and dates of inclusion in the derivation and validation cohorts.
eTable 2. Characteristics of and comparison between the 2 cohorts.
eTable 3. Comparison of ENDi patients with or without rescue mechanical thrombectomy in the derivation cohort.
eTable 4. Variables independently associated with early neurological deterioration in multivariable logistic regression in sensitivity analysis including only patients treated before or since 2015 (derivation cohort).
eFigure. Study flowchart.
Nonauthor Collaborators. MINOR-STROKE Collaborators.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 2.Mazya MV, Cooray C, Lees KR, et al. Minor stroke due to large artery occlusion. when is intravenous thrombolysis not enough? results from the SITS International Stroke Thrombolysis Register. Eur Stroke J. 2018;3(1):29-38. doi: 10.1177/2396987317746003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heldner MR, Jung S, Zubler C, et al. Outcome of patients with occlusions of the internal carotid artery or the main stem of the middle cerebral artery with NIHSS score of less than 5: comparison between thrombolysed and non-thrombolysed patients. J Neurol Neurosurg Psychiatry. 2015;86(7):755-760. doi: 10.1136/jnnp-2014-308401 [DOI] [PubMed] [Google Scholar]
- 4.Heldner MR, Chaloulos-Iakovidis P, Panos L, et al. Outcome of patients with large vessel occlusion in the anterior circulation and low NIHSS score. J Neurol. 2020;267(6):1651-1662. doi: 10.1007/s00415-020-09744-0 [DOI] [PubMed] [Google Scholar]
- 5.Seners P, Baron JC. Revisiting ‘progressive stroke’: incidence, predictors, pathophysiology, and management of unexplained early neurological deterioration following acute ischemic stroke. J Neurol. 2018;265(1):216-225. doi: 10.1007/s00415-017-8490-3 [DOI] [PubMed] [Google Scholar]
- 6.Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015;86(1):87-94. doi: 10.1136/jnnp-2014-308327 [DOI] [PubMed] [Google Scholar]
- 7.Seners P, Turc G, Tisserand M, et al. Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke. 2014;45(7):2004-2009. doi: 10.1161/STROKEAHA.114.005426 [DOI] [PubMed] [Google Scholar]
- 8.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)–European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019;11(6):535-538. doi: 10.1136/neurintsurg-2018-014568 [DOI] [PubMed] [Google Scholar]
- 9.Seners P, Perrin C, Lapergue B, et al. ; MINOR-STROKE Collaborators . Bridging therapy or IV thrombolysis in minor stroke with large vessel occlusion. Ann Neurol. 2020;88(1):160-169. doi: 10.1002/ana.25756 [DOI] [PubMed] [Google Scholar]
- 10.Naggara O, Raymond J, Domingo Ayllon M, et al. T2* “susceptibility vessel sign” demonstrates clot location and length in acute ischemic stroke. PLoS One. 2013;8(10):e76727. doi: 10.1371/journal.pone.0076727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohan V, Baxa J, Tupy R, et al. Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke. 2014;45(7):2010-2017. doi: 10.1161/STROKEAHA.114.005731 [DOI] [PubMed] [Google Scholar]
- 12.Riedel CH, Jensen U, Rohr A, et al. Assessment of thrombus in acute middle cerebral artery occlusion using thin-slice nonenhanced computed tomography reconstructions. Stroke. 2010;41(8):1659-1664. doi: 10.1161/STROKEAHA.110.580662 [DOI] [PubMed] [Google Scholar]
- 13.Olivot JM, Mlynash M, Inoue M, et al. ; DEFUSE 2 Investigators . Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 cohort. Stroke. 2014;45(4):1018-1023. doi: 10.1161/STROKEAHA.113.003857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saleem Y, Nogueira RG, Rodrigues GM, et al. Acute neurological deterioration in large vessel occlusions and mild symptoms managed medically. Stroke. 2020;51(5):1428-1434. doi: 10.1161/STROKEAHA.119.027011 [DOI] [PubMed] [Google Scholar]
- 15.Kim JT, Heo SH, Yoon W, et al. Clinical outcomes of patients with acute minor stroke receiving rescue IA therapy following early neurological deterioration. J Neurointerv Surg. 2016;8(5):461-465. doi: 10.1136/neurintsurg-2015-011690 [DOI] [PubMed] [Google Scholar]
- 16.Endovascular Therapy for Low NIHSS Ischemic Strokes (ENDOLOW) . ClinicalTrials.gov identifier: NCT04167527. Updated September 11, 2020. Accessed September 20, 2020. https://clinicaltrials.gov/ct2/show/NCT04167527
- 17.Minor Stroke Therapy Evaluation (MOSTE) . ClinicalTrials.gov identifier: NCT03796468. Updated September 17, 2020. Accessed September 20, 2020. https://clinicaltrials.gov/ct2/show/NCT03796468
- 18.Rajajee V, Kidwell C, Starkman S, et al. Early MRI and outcomes of untreated patients with mild or improving ischemic stroke. Neurology. 2006;67(6):980-984. doi: 10.1212/01.wnl.0000237520.88777.71 [DOI] [PubMed] [Google Scholar]
- 19.Kim JT, Park MS, Chang J, Lee JS, Choi KH, Cho KH. Proximal arterial occlusion in acute ischemic stroke with low NIHSS scores should not be considered as mild stroke. PLoS One. 2013;8(8):e70996. doi: 10.1371/journal.pone.0070996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nacu A, Bringeland GH, Khanevski A, Thomassen L, Waje-Andreassen U, Naess H. Early neurological worsening in acute ischaemic stroke patients. Acta Neurol Scand. 2016;133(1):25-29. doi: 10.1111/ane.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seners P, Delepierre J, Turc G, et al. ; PREDICT-RECANAL Collaborators . Thrombus length predicts lack of post-thrombolysis early recanalization in minor stroke with large vessel occlusion. Stroke. 2019;50(3):761-764. doi: 10.1161/STROKEAHA.118.023455 [DOI] [PubMed] [Google Scholar]
- 22.Seners P, Turc G, Naggara O, et al. ; PREDICT-RECANAL Collaborators . Post-thrombolysis recanalization in stroke referrals for thrombectomy: incidence, predictors, and prediction scores. Stroke. 2018;49(12):2975-2982. doi: 10.1161/STROKEAHA.118.022335 [DOI] [PubMed] [Google Scholar]
- 23.Menon BK, Al-Ajlan FS, Najm M, et al. ; INTERRSeCT Study Investigators . Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. 2018;320(10):1017-1026. doi: 10.1001/jama.2018.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seners P, Turc G, Maïer B, Mas JL, Oppenheim C, Baron JC. Incidence and predictors of early recanalization after intravenous thrombolysis: a systematic review and meta-analysis. Stroke. 2016;47(9):2409-2412. doi: 10.1161/STROKEAHA.116.014181 [DOI] [PubMed] [Google Scholar]
- 25.Kaesmacher J, Giarrusso M, Zibold F, et al. Rates and quality of preinterventional reperfusion in patients with direct access to endovascular treatment. Stroke. 2018;49(8):1924-1932. doi: 10.1161/STROKEAHA.118.021579 [DOI] [PubMed] [Google Scholar]
- 26.Vanacker P, Heldner MR, Seiffge D, et al. ASTRAL-R score predicts non-recanalisation after intravenous thrombolysis in acute ischaemic stroke. Thromb Haemost. 2015;113(5):1121-1126. doi: 10.1160/TH14-06-0482 [DOI] [PubMed] [Google Scholar]
- 27.Tisserand M, Seners P, Turc G, et al. Mechanisms of unexplained neurological deterioration after intravenous thrombolysis. Stroke. 2014;45(12):3527-3534. doi: 10.1161/STROKEAHA.114.006745 [DOI] [PubMed] [Google Scholar]
- 28.Fu J, Zhou Y, Li Q, et al. Perfusion changes of unexplained early neurological deterioration after reperfusion therapy. Transl Stroke Res. 2020;11(2):195-203. doi: 10.1007/s12975-019-00723-w [DOI] [PubMed] [Google Scholar]
- 29.Dargazanli C, Consoli A, Gory B, et al. ; ETIS investigators . Is reperfusion useful in ischaemic stroke patients presenting with a low National Institutes of Health Stroke Scale and a proximal large vessel occlusion of the anterior circulation? Cerebrovasc Dis. 2017;43(5-6):305-312. doi: 10.1159/000468995 [DOI] [PubMed] [Google Scholar]
- 30.Goyal N, Tsivgoulis G, Malhotra K, et al. Medical management vs mechanical thrombectomy for mild strokes: an international multicenter study and systematic review and meta-analysis. JAMA Neurol. 2020;77(1):16-24. doi: 10.1001/jamaneurol.2019.3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manno C, Disanto G, Bianco G, et al. Outcome of endovascular therapy in stroke with large vessel occlusion and mild symptoms. Neurology. 2019;93(17):e1618-e1626. doi: 10.1212/WNL.0000000000008362 [DOI] [PubMed] [Google Scholar]
- 32.Nagel S, Bouslama M, Krause LU, et al. Mechanical thrombectomy in patients with milder strokes and large vessel occlusions. Stroke. 2018;49(10):2391-2397. doi: 10.1161/STROKEAHA.118.021106 [DOI] [PubMed] [Google Scholar]
- 33.Dargazanli C, Arquizan C, Gory B, et al. ; ETIS REGISTRY Investigators . Mechanical thrombectomy for minor and mild stroke patients harboring large vessel occlusion in the anterior circulation: a multicenter cohort study. Stroke. 2017;48(12):3274-3281. doi: 10.1161/STROKEAHA.117.018113 [DOI] [PubMed] [Google Scholar]
- 34.Urra X, San Román L, Gil F, et al. ; Catalan Stroke Code and Reperfusion Consortium (Cat-SCR) . Medical and endovascular treatment of patients with large vessel occlusion presenting with mild symptoms: an observational multicenter study. Cerebrovasc Dis. 2014;38(6):418-424. doi: 10.1159/000369121 [DOI] [PubMed] [Google Scholar]
- 35.Sarraj A, Hassan A, Savitz SI, et al. Endovascular thrombectomy for mild strokes: how low should we go? Stroke. 2018;49(10):2398-2405. doi: 10.1161/STROKEAHA.118.022114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. List of participating centers and dates of inclusion in the derivation and validation cohorts.
eTable 2. Characteristics of and comparison between the 2 cohorts.
eTable 3. Comparison of ENDi patients with or without rescue mechanical thrombectomy in the derivation cohort.
eTable 4. Variables independently associated with early neurological deterioration in multivariable logistic regression in sensitivity analysis including only patients treated before or since 2015 (derivation cohort).
eFigure. Study flowchart.
Nonauthor Collaborators. MINOR-STROKE Collaborators.