Key Points
Question
Does family navigation (FN), an individually tailored, culturally informed care management strategy, increase the likelihood of achieving diagnostic ascertainment among children from low-income, racial/ethnic minority families who have positive screening results for autism spectrum disorder (ASD)?
Findings
In this multisite randomized clinical trial of 249 children aged 15 to 27 months, FN decreased the time to diagnostic ascertainment and increased the likelihood of diagnostic ascertainment over the course of 1 year. However, Hispanic ethnicity and site moderated the effect of FN.
Meaning
Family navigation is a promising approach to improve early ASD diagnosis among children from low-income, racial/ethnic minority families; its effects may be contextually dependent.
Abstract
Importance
Early identification of autism spectrum disorder (ASD) is associated with improved cognitive and behavioral outcomes. Targeted strategies are needed to support equitable access to diagnostic services to ensure that children from low-income and racial/ethnic minority families receive the benefits of early ASD identification and treatment.
Objective
To test the efficacy of family navigation (FN), an individually tailored, culturally informed care management strategy, to increase the likelihood of achieving diagnostic ascertainment among young children at risk for ASD.
Design, Setting, and Participants
This randomized clinical trial of 249 families of children aged 15 to 27 months who had positive screening results for possible ASD was conducted in 11 urban primary care sites in 3 cities. Data collection occurred from February 24, 2015, through November 5, 2018. Statistical analysis was performed on an intent-to-treat basis from November 5, 2018, to July 27, 2020.
Interventions
Families were randomized to FN or conventional care management (CCM). Families receiving FN were assigned a navigator who conducted community-based outreach to families to address structural barriers to care and support engagement in recommended services. Families receiving CCM were assigned to a care manager, who did limited telephone outreach. Families received FN or CCM after positive initial screening results and for 100 days after diagnostic ascertainment.
Main Outcomes and Measures
The primary outcome, diagnostic ascertainment, was measured as the number of days from randomization to completion of the child’s clinical developmental evaluation, when a diagnosis of ASD or other developmental disorder was determined.
Results
Among 250 families randomized, 249 were included in the primary analysis (174 boys [69.9%]; mean [SD] age, 22.0 [3.5] months; 205 [82.3%] publicly insured; 233 [93.6%] non-White). Children who received FN had a greater likelihood of reaching diagnostic ascertainment over the course of 1 year (FN, 108 of 126 [85.7%]; CCM, 94 of 123 [76.4%]; unadjusted hazard ratio [HR], 1.39 [95% CI, 1.05-1.84]). Site (Boston, New Haven, and Philadelphia) and ethnicity (Hispanic vs non-Hispanic) moderated the effect of FN (treatment × site interaction; P = .03; Boston: HR, 2.07 [95% CI, 1.31-3.26]; New Haven: HR, 1.91 [95% CI, 0.94-3.89]; and Philadelphia: HR, 0.91 [95% CI, 0.60-1.37]) (treatment × ethnicity interaction; P < .001; Hispanic families: HR, 2.81 [95% CI, 2.23-3.54] vs non-Hispanic families: HR, 1.49 [95% CI, 1.45-1.53]). The magnitude of FN’s effect was significantly greater among Hispanic families than among non-Hispanic families (diagnostic ascertainment among Hispanic families: FN, 90.9% [30 of 33], and CCM, 53.3% [16 of 30]; vs non-Hispanic families: FN, 89.7% [35 of 39], and CCM, 77.5% [31 of 40]).
Conclusions and Relevance
Family navigation improved the likelihood of diagnostic ascertainment among children from racial/ethnic minority, low-income families who were detected as at risk for ASD in primary care. Results suggest differential effects of FN by site and ethnicity.
Trial Registration
ClinicalTrials.gov Identifier: NCT02359084
This randomized clinical trial tests the efficacy of family navigation, an individually tailored, culturally informed care management strategy, to increase the likelihood of achieving diagnostic ascertainment among young children at risk for autism spectrum disorder.
Introduction
Disparities exist in access to autism spectrum disorder (ASD) diagnostic and treatment services for low-income and racial/ethnic minority families.1,2,3,4 The consequences of existing disparities have become evident as both the prevalence of ASD and evidence of effectiveness of ASD-specific treatments have increased.5,6,7,8,9 Autism spectrum disorder now affects 1 in 54 children in the United States.6 High-quality studies demonstrate the effectiveness of early intensive behavioral intervention to improve skills and reduce ASD-related impairments.7,8,9,10,11 Such services are more efficacious when they are initiated at younger ages.12,13 Because a formal ASD diagnosis is typically required to qualify for ASD-specific services, decreasing disparities in the age at ASD diagnosis is a critical step to ensuring equitable early access to evidence-based services, and plausibly to better outcomes. Without explicit strategies to promote early identification and engagement with diagnostic and treatment services among low-income and racial/ethnic minority children, disparities in ASD outcomes are likely to persist.
There is growing interest and investment in evidence-based approaches to reduce disparities and address barriers to accessing ASD-specific diagnostic and treatment services.14,15 Family navigation (FN) is 1 strategy to address barriers to care.14,15,16 The goal of FN is to support families in overcoming structural and psychological obstacles (eg, transportation, language, fear, and stigma) to achieve diagnostic ascertainment and engagement in recommended services.17 Rooted in the chronic care model,18 FN shares basic tenets of patient navigation.19 Patient navigation originally targeted individual patients with cancer risk.20,21 It focused on the period from a suspicious screening result through diagnostic ascertainment and engagement in recommended services, addressing the “discovery to diagnosis disconnect.”19(p5) Its use has expanded to other conditions and currently addresses a range of barriers to care.22,23,24 Similar to the original navigation model, FN focuses on the period beginning with a suspicious screening result but expands the model to engage the entire family unit rather than just the individual. Family navigation uses community health workers, referred to as navigators, who are trained in motivational interviewing, collaborative problem solving, and psychoeducation, to accomplish family goals, navigate barriers to care, and provide cross-sector care coordination.17
As 1 of 5 studies of the National Institute of Mental Health’s ASD Pediatric Early Detection, Engagement, and Services Research Network,25 this multisite randomized clinical trial is the first, to our knowledge, to systematically assess the effect of FN on time to diagnostic ascertainment among children with an initial positive screening result for ASD.
Methods
We conducted a parallel-group, randomized trial of families of children aged 15 to 27 months identified as at risk for ASD based on screening and surveillance in primary care. The trial took place in 11 urban pediatric primary care practices, which were part of 3 integrated care networks in Boston, Massachusetts; New Haven, Connecticut; and Philadelphia, Pennsylvania, and their developmental and behavioral pediatrics (DBP) specialty clinics. Each clinic was a member of the Health Resources and Services Administration–funded DBP Research Network (DBPNet). The full trial protocol is provided in Supplement 1.16 Study procedures were approved by the Boston University Medical Center Institutional Review Board. Parents and/or guardians provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Families were recruited from February 12, 2015, to October 23, 2017; data collection occurred through November 5, 2018. After completing the baseline assessment, participants (parent-child dyads) were randomized 1:1 to FN or conventional care management (CCM) using randomly permuted blocks of 2 and 4. Participants were stratified by primary care site and receipt of prescreening educational materials. Randomization lists were generated for each site by a secure web-based data management system.26 Investigators and staff responsible for data collection were masked to study allocation.
Participants
Children were identified as being at risk for ASD based on results from the Modified Checklist for Autism in Toddlers, Revised with Follow-up (MCHAT-R/F),27 which was administered at the 18-month and 24-month well-child visits as standard care.23,25 Research staff confirmed ASD risk by readministering the MCHAT-R/F using recommended administration and scoring procedures. Readministration ensured consistency across sites and that all children received the MCHAT-R Follow-up Interview, which decreases the number of children requiring further evaluation.27 To emulate conditions in real-world practices, primary care clinicians who had high levels of concern about a child were allowed to override negative screening results and recommend study enrollment. Children who had a previous ASD diagnosis or who were in custody of child protective services were excluded. No families were excluded based on language.
Interventions
Family Navigation
Family navigation is a structured, manualized intervention consisting of 11 core components.17 Navigators were predominantly bilingual, bicultural members of the community, who received training in ASD management, community resources, motivational interviewing, and principles of patient navigation.19 Family navigation was designed to support families from the time of a positive ASD screening result through engagement in recommended services; it included a minimum of 3 standardized visits that aligned with critical time points in the child’s diagnostic process. In this report, we present only the FN components that supported families through diagnostic ascertainment. These components were a minimum of 1 structured visit conducted prior to the diagnostic evaluation to prepare the family and identify barriers to engagement. Additional in-person and remote contacts occurred based on families’ needs (eg, evaluation-specific service outreach, housing, and transportation). Navigators had cellular telephones to facilitate communication via telephone, text, or email; they also had a subscription to a car service for travel to families’ homes. Contacts were initiated by navigators or families; there was no limit to the amount of contacts.
Navigators worked closely with existing staff at primary care sites but were supervised centrally by the project team. They participated in monthly group and individual supervision conducted by Boston’s lead navigator. To assess fidelity, 20% of FN sessions between each navigator and family were randomly selected and audiotaped. A blinded assessor evaluated FN visit content fidelity using a standardized visit checklist; visits in which 80% of the items were present were considered to have met fidelity.28 Fidelity to motivational interviewing principles was assessed using the Motivational Interviewing Supervision and Training Scale.29 The threshold for motivational interviewing fidelity was set at 70% because the varied nature of FN interactions did not support the use of all motivational interviewing tenets in all visits. A second assessor cross-checked 13% of FN fidelity recordings and 15% of Motivational Interviewing Supervision and Training Scale recordings.
Conventional Care Management
Conventional care management exceeded usual care at all sites and was provided in addition to existing procedures. Usual care was enhanced by low-cost, easily implemented procedures that could be integrated into existing workflows, including a manualized protocol of outreach to families and the child’s primary care clinician and a designated direct line to reach the care manager. Outreach to families consisted of an introductory telephone call, during which care managers reminded families about their child’s DBP intake appointment, offered to answer questions about the developmental assessment and developmental services, and provided resources to community services to address social needs. If the family was not reached by telephone after 3 attempts, a letter was mailed to the family introducing the care manager and the date of the child’s appointment. Outreach to the child’s primary care clinician included a letter to introduce the role of the care manager and provide contact information. Conventional care management was delivered by designated care managers. They were existing staff who had access to all resources at their site, including interpreter services. At 2 of the 3 sites, designated care managers’ race/ethnicity mirrored that of participants. All other contacts with care managers after the initial telephone call were initiated by the family or other members of the child’s care team. All study participants, regardless of randomization group, were offered the next available DBP appointment for ASD diagnostic evaluation.
Primary Outcome
The primary outcome was time to completion of a clinical diagnostic evaluation, during which a diagnosis of ASD or other developmental condition was made. This time period was measured as the number of days from randomization to the date of diagnostic ascertainment, obtained from the child’s medical record. As a pragmatic trial, the diagnostic evaluations were completed within the context of DBP standard clinical care, which typically included a detailed history and administration of the Autism Diagnostic Observation Schedule, 2nd edition,30 to inform Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) ASD diagnostic criteria.31
Other Subgroup Analyses
We identified 2 a priori, theory-based, potential effect modifiers: site and ethnicity. Site was modeled as a state-level variable and hypothesized to be a potential effect modifier based on state differences in available services for children with ASD; Hispanic ethnicity was examined as an effect modifier based on the large body of research describing substantial barriers to care experienced by Hispanic families.32,33,34,35 We also conducted analyses comparing children enrolled based on screening results with those enrolled based on clinical concerns alone.
Sample Size
Using the threshold of clinical significance from Kraemer et al,36 we considered a 25% absolute difference in diagnostic ascertainment rates between treatment groups to be clinically meaningful and to provide evidence of the efficacy of FN compared with CCM. Based on pilot studies,14,15 assuming that 65% of children in the CCM group would achieve diagnostic ascertainment and a moderate design effect resulting from site clustering (intraclass correlation of 0.01), a sample size of 250 was estimated to detect a 25% difference in diagnostic ascertainment with 80% power at a 2-sided α of .05.
Statistical Analysis
Statistical analysis was performed on an intent-to-treat basis from November 5, 2018, to July 27, 2020. Analysis of the primary outcome included all randomized participants, with the exception of 1 postrandomization exclusion of a child who received care from a nonparticipating primary care site. Time to diagnostic ascertainment was analyzed descriptively using Kaplan-Meier curves. Children who did not complete a diagnostic evaluation within 1 year of study enrollment were censored. Cox proportional hazards regression was performed to estimate the hazard ratio (HR) and 95% CI for the difference in the likelihood of achieving diagnostic ascertainment. We assessed potential clustering by site using the method of Wei et al.37 We performed formal testing of interaction terms to assess effect modification followed by stratified analyses. We tested the proportional hazards assumption for the intervention effect using a Kolmogorov-type, simulation-based supremum test as well as graphically examining plots of cumulative Martingale residuals.38
Results
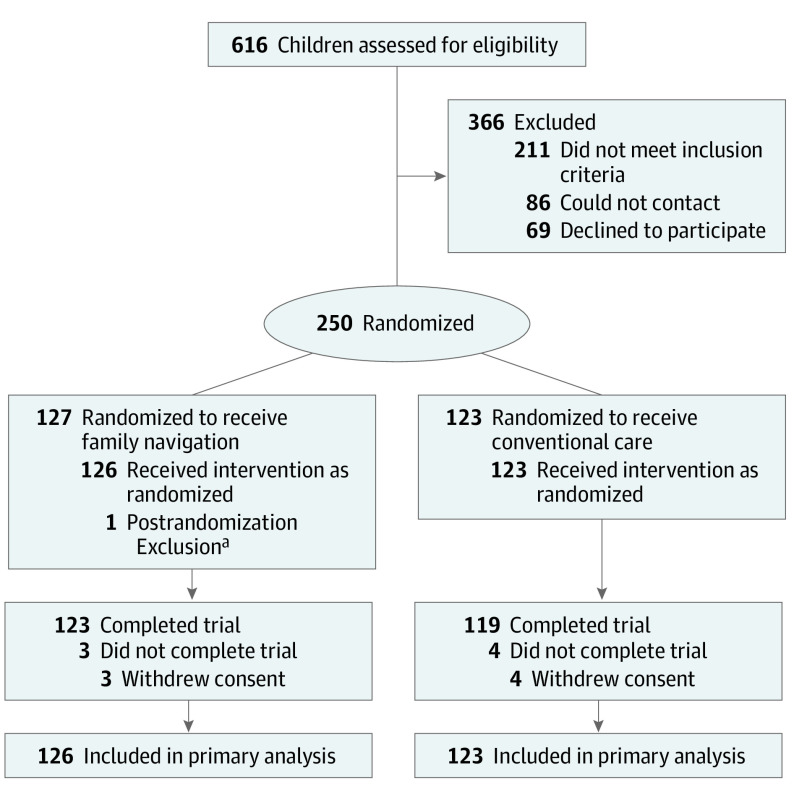
A total of 616 families were referred to the study: 211 did not meet inclusion criteria; 86 could not be contacted; 69 declined participation. A total of 250 families were randomly assigned to an intervention; 249 received the allocated intervention (126 families received FN and 123 families received CCM) (Figure 1).
Figure 1. Participant Flow Diagram.
aChild was enrolled from a primary care site not participating in the study.
Baseline Data
There were no clinically significant differences in sociodemographic variables by treatment group at baseline. The sample was racially and ethnically diverse, with 72 Hispanic participants (28.9%), 131 non-Hispanic Black participants (52.6%), 16 non-Hispanic White participants (6.4%), and 30 participants of other races/ethnicities (12.0%) (Table). The mean (SD) parent age was 31.4 (7.3) years. Ninety-eight parents (39.7%) were born outside the United States; 45 (18.1%) preferred to communicate in a language other than English. The mean (SD) child age was 22.0 (3.5) months, 174 children (69.9%) were male, 205 (82.3%) received public insurance, and 109 (43.8%) were receiving IDEA Part C39 Birth to Three developmental services at enrollment. Seven children (2.8%) were enrolled based on clinical concerns alone.
Table. Baseline Characteristics of Participants Enrolled in Project Early, by Treatment Group.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Family navigation (n = 126) | Care management (n = 123) | |
| Site | ||
| Boston, Massachusetts | 48 (38.1) | 46 (37.4) |
| New Haven, Connecticut | 24 (19.0) | 24 (19.5) |
| Philadelphia, Pennsylvania | 54 (42.9) | 53 (43.1) |
| Eligibility criteria | ||
| Positive screening result on MCHAT-R/F | 121 (96.0) | 121 (98.4) |
| Clinician concern only | 5 (4.0) | 2 (1.6) |
| Parent race/ethnicitya | ||
| Non-Hispanic | ||
| Black | 69 (54.8) | 62 (50.4) |
| White | 10 (7.9) | 6 (4.9) |
| Hispanic | 37 (29.4) | 35 (28.5) |
| Other, non-Hispanicb | 10 (7.9) | 20 (16.3) |
| Born in the United States | 81 (64.3) | 69/122 (56.6) |
| Preferred language | ||
| English | 105 (83.3) | 99 (80.5) |
| Spanish | 13 (10.3) | 18 (14.6) |
| Other | 8 (6.3) | 6 (4.9) |
| Currently working (outside home) | 69 (54.8) | 65 (52.8) |
| High school graduate | 101 (80.2) | 94/121 (77.7) |
| Married or living with partner | 61 (48.4) | 70/122 (57.4) |
| Insurance | ||
| Public (Medicaid) | 106 (84.1) | 99 (80.5) |
| Other | 20 (15.9) | 24 (19.5) |
| Parent age, mean (SD), y | 31.8 (7.3) | 31.1 (7.3) |
| No. of children per family, mean (SD) | 2.2 (1.4) | 2.4 (1.4) |
| Worry about child’s development, mean (SD)c | 6.8 (2.8) | 6.3 (3.1) |
| Child sex | ||
| Male | 91 (72.2) | 83 (67.5) |
| Female | 35 (27.8) | 40 (32.5) |
| Gestational age of <37 wk | 18 (14.3) | 18 (14.6) |
| Diagnosis | ||
| ASD | 66/108 (61.1) | 52/94 (55.3) |
| Language or other developmental disorder | 42/108 (38.9) | 42/94 (44.7) |
| MCHAT-R total score, mean (SD) | 8.7 (3.2) | 8.4 (3.0) |
| Receiving | ||
| EI | 49 (38.9) | 60 (48.8) |
| WIC | 88 (69.8) | 84 (68.3) |
| Cash assistance | 31 (24.6) | 25 (20.3) |
Abbreviations: ASD, autism spectrum disorder; EI, early intervention; MCHAT-R/F, Modified Checklist for Autism in Toddlers, Revised With Follow-up; MCHAT-R, Modified Checklist for Autism in Toddlers, Revised; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
Race/ethnicity was classified based on parental self-report.
Other race/ethnicity includes Asian and more than 1 race/ethnicity.
Scored on a scale of 0 to 10, where 0 indicates not at all worried and 10 indicates very worried.
Protocol Adherence
Prior to diagnostic ascertainment, 92.9% of families (117 of 126) assigned to FN had at least 1 face-to-face navigation encounter. The mean (SD) number of contacts with the navigator was 19.6 (12.7): 2.6 (1.3) in-person visits, 9.2 (6.8) telephone calls, 6.9 (4.8) texts, and 2.7 (4.8) emails. Seventeen of 126 families (13.5%) disengaged from the intervention, defined as no navigator contact for more than 10 weeks. Of reviewed visits, 80.0% (40 of 50) met criteria for visit content fidelity and 72.3% (34 of 47) for motivational interviewing fidelity. Within the CCM group, 95.9% of families (118 of 123) received the protocol as described; 26.8% (33 of 123) initiated outreach to the care manager. The mean (SD) number of contacts with the care manager beyond the introductory telephone call was 2.4 (2.1): 1.8 (1.8) telephone calls, 0.07 (0.3) texts, and 0.4 (0.9) emails. No associations between intervention fidelity and study outcomes were observed.
Diagnostic Ascertainment
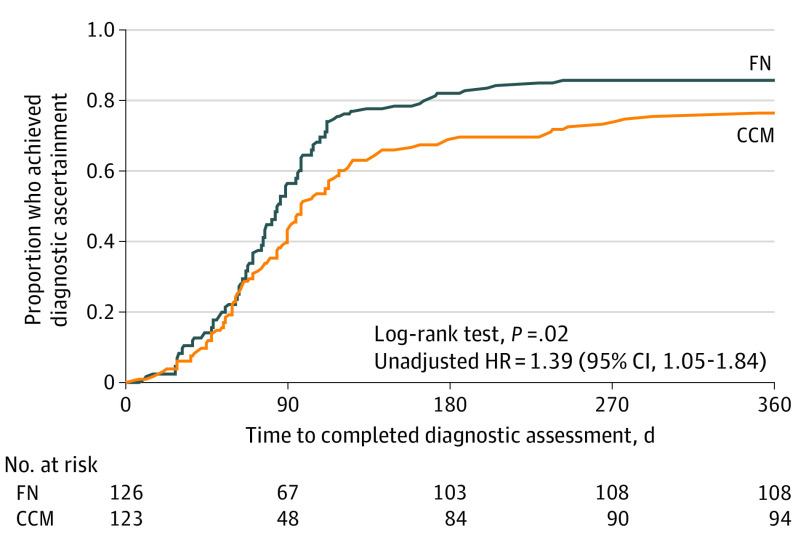
Time to diagnostic ascertainment is shown in Figure 2. The proportion who reached diagnostic ascertainment within 1 year was 85.7% (108 of 126) in the FN group and 76.4% (94 of 123) in the CCM group (unadjusted HR, 1.39 [95% CI, 1.05-1.84]; P = .02). Among 202 families who achieved diagnostic ascertainment, 118 children (58.4%) received an ASD diagnosis; differences between groups in receipt of an ASD diagnosis were not significant. All 84 children who did not receive an ASD diagnosis received a diagnosis of other developmental conditions.
Figure 2. Time to Diagnostic Ascertainment, by Treatment Group, All Sites.
CCM indicates conventional care management; FN, family navigation; and HR, hazard ratio.
Subgroup Analyses
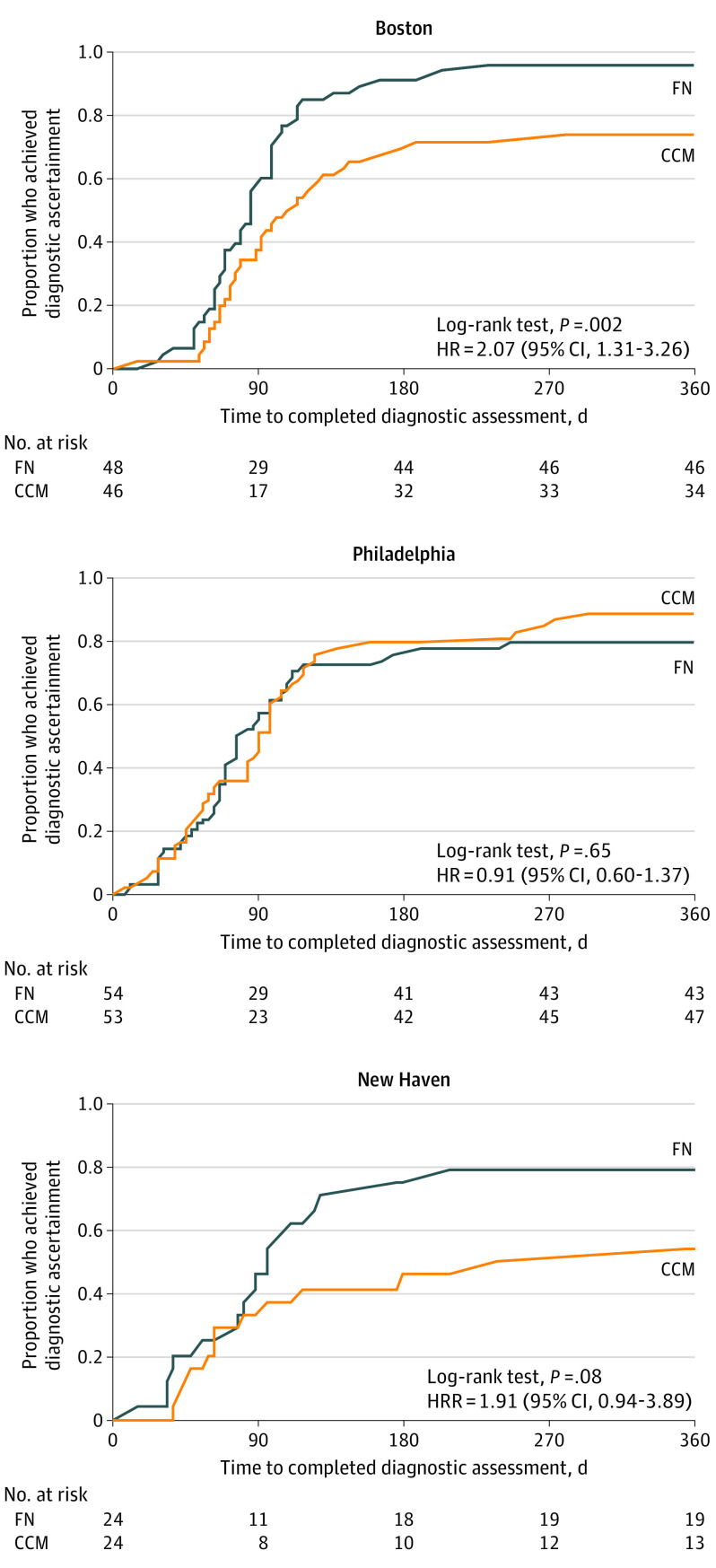
When site was included as a clustering variable in the model, the HR remained the same, but the 95% CI widened and the association was no longer statistically significant (HR, 1.39 [95% CI, 0.88-2.21]; P = .16), likely owing to loss of power related to nonindependence of events. We tested for effect modification by site and found a significant treatment × site interaction (P = .03). Site-specific Kaplan-Meier plots are shown in Figure 3. In Cox proportional hazards regression models stratified by site, children in Boston and New Haven who received FN were about twice as likely as families who received CCM to achieve diagnostic ascertainment (Boston: HR, 2.07 [95% CI, 1.31-3.26]; P = .002; and New Haven: HR, 1.91 [95% CI, 0.94-3.89]; P = .08). In Philadelphia, there was no difference in the likelihood of diagnostic ascertainment between CCM and FN (HR, 0.91 [95% CI, 0.60-1.37]; P = .65), likely a result of Philadelphia’s high ascertainment rate among CCM families compared with that of other sites.
Figure 3. Time to Diagnostic Ascertainment, by Site and Treatment Group.
CCM indicates conventional care management; FN, family navigation; and HR, hazard ratio.
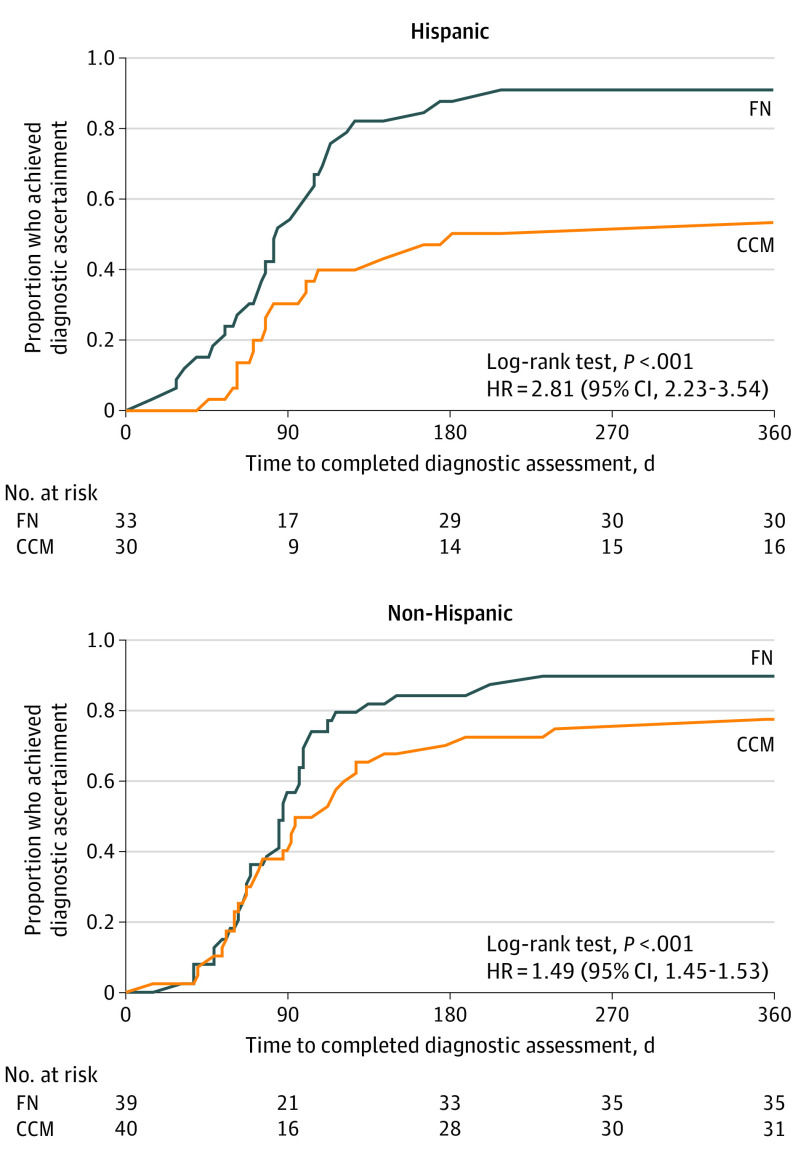
The effect of ethnicity was assessed among participants from Boston and New Haven only, as Philadelphia enrolled very few Hispanic families. Given the difference in intervention effect between Philadelphia and the other sites, its inclusion necessitated a 3-way site × ethnicity × treatment interaction term, which the sample size did not support. Figure 4 displays Kaplan-Meier plots by ethnicity for Boston and New Haven families. The difference between the proportions who achieved diagnostic resolution in FN and CCM groups was greater among Hispanic families (90.9% [30 of 33] vs 53.3% [16 of 30]) than among non-Hispanic families (89.7% [35 of 39] vs 77.5% [31 of 40]). In the Cox proportional hazards regression models, the treatment × ethnicity interaction term was significant (P < .001). In models stratified by ethnicity, the magnitude of the effect of FN was greater for Hispanic than non-Hispanic families (Hispanic families: HR, 2.81 [95% CI, 2.23-3.54]; and non-Hispanic families: HR, 1.49 [95% CI, 1.45-1.53]).
Figure 4. Time to Diagnostic Ascertainment, by Ethnicity.
This analysis included only the Boston and New Haven sites, which accounts for the loss of sample. CCM indicates conventional care management; FN, family navigation; and HR, hazard ratio.
To assess the effect of including participants based on clinician concerns alone, we compared sociodemographic characteristics and diagnostic outcomes between these 7 children and the 242 children enrolled because of screening results. We did not find differences between the 2 groups.
Discussion
To our knowledge, this is the first multisite randomized clinical trial of a primary care–based FN intervention designed specifically to improve early evaluation of low-income, racial/ethnic minority children at risk for ASD. Compared with CCM, FN increased the likelihood of achieving diagnostic ascertainment within a 1-year period. The effects varied by site and ethnicity, however, suggesting that the positive effects of FN are contextually dependent. At 2 of the 3 research sites (Boston and New Haven), children who received FN were about twice as likely to achieve diagnostic ascertainment; no difference was observed at the third site (Philadelphia), where both FN and CCM families had high rates of diagnostic ascertainment. Hispanic families benefited more from FN compared with their non-Hispanic counterparts. Although the FN main effect was below the 25% threshold that we set a priori as clinically significant, the difference between FN and CCM participants who achieved diagnostic ascertainment approached or exceeded this threshold at 2 of the 3 sites (Boston, 23% difference; and New Haven, 32% difference) and among Hispanic participants (41% difference). These findings suggest the potential of FN to affect disparities in ASD diagnostic ascertainment in many, but not all, health service settings and populations experiencing disparities.
Initially, we hypothesized that any observed effect modification would result from differences in FN efficacy. However, we found that FN outcomes were fairly consistent across sites (85.7% [108 of 126] achieved diagnostic ascertainment). Instead, the observed effect modification by site and ethnicity was better explained by variations in diagnostic ascertainment among CCM families.
A possible explanation for this finding relates to site racial/ethnic demographic characteristics. Sociodemographic characteristics were similar across sites, with the exception of race/ethnicity and variables associated with immigration status. The Philadelphia primary care sites served predominantly English-speaking Black families (76.6% [82 of 107]); the Boston and New Haven sites served more Hispanic (44.4% [63 of 142]) and ethnically diverse non-US–born families (59.6% [84 of 141]). It is possible that Hispanic and other ethnic minority participants experienced greater difficulty accessing health services than the English-speaking Black families owing to language and cultural barriers. Thus, they accrued greater benefits of FN. Common experiences, values, and other cultural influences among Hispanic families may make FN a particularly effective intervention for this subgroup. Family navigation is rooted in tenets of culturally informed care; thus, navigators were selected based on their racial/ethnic background and knowledge of the local community. The cultural concordance between families and the navigators may have had greater saliency for Hispanic than Black families. What remains unexplained is why Black families, who have historically experienced delays in ASD diagnosis,3 had a higher than expected rate of diagnostic ascertainment in Philadelphia, despite consistent implementation of CCM and a relatively similar standard of care across sites. Findings suggest that FN may be most valuable when baseline diagnostic ascertainment rates are low and may be relevant to other conditions for which disparities in diagnostic ascertainment exist.
Limitations
This study has some limitations. Analyses of moderation by site and ethnicity provided initial explanations regarding contextual factors and their effects on FN. However, we were unable to identify specific factors contributing to differential site effects, particularly those that supported engagement of Black families. Study findings may not be generalizable to other sites, as urban care networks have site-specific screening practices and exist within state-specific service systems. Regarding potential selection bias, we were unable to assess differences between study participants and the 155 parent-child dyads who could not be contacted or declined to participate. We also did not assess associations between early ASD diagnosis and functional outcomes.
Conclusions
Study findings provide evidence that a replicable model of FN increased the likelihood of ASD diagnostic ascertainment. Because diagnostic ascertainment is the first step in the pathway to receipt of evidence-based services, earlier diagnostic ascertainment has the potential to improve outcomes for vulnerable ethnically and racially diverse young children with ASD and other developmental disabilities. Future analyses of the contextual factors that affect the effectiveness of FN in various settings and populations are necessary to determine the conditions under which implementing FN is most likely to yield the strongest benefits.
Trial Protocol
Data Sharing Statement
References
- 1.Durkin MS, Maenner MJ, Baio J, et al. Autism spectrum disorder among US children (2002-2010): socioeconomic, racial, and ethnic disparities. Am J Public Health. 2017;107(11):1818-1826. doi: 10.2105/AJPH.2017.304032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu W. Child and adolescent mental disorders and health care disparities: results from the National Survey of Children’s Health, 2011–2012. J Health Care Poor Underserved. 2017;28(3):988-1011. doi: 10.1353/hpu.2017.0092 [DOI] [PubMed] [Google Scholar]
- 3.Mandell DS, Listerud J, Levy SE, Pinto-Martin JA. Race differences in the age at diagnosis among Medicaid-eligible children with autism. J Am Acad Child Adolesc Psychiatry. 2002;41(12):1447-1453. doi: 10.1097/00004583-200212000-00016 [DOI] [PubMed] [Google Scholar]
- 4.McGuire TG, Miranda J. New evidence regarding racial and ethnic disparities in mental health: policy implications. Health Aff (Millwood). 2008;27(2):393-403. doi: 10.1377/hlthaff.27.2.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baio J, Wiggins L, Christensen DL, et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1-23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maenner MJ, Shaw KA, Baio J, et al. ; EdS1; PhD-7 . Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2016. MMWR Surveill Summ. 2020;69(4):1-12. doi: 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17-e23. doi: 10.1542/peds.2009-0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landa RJ, Kalb LG. Long-term outcomes of toddlers with autism spectrum disorders exposed to short-term intervention. Pediatrics. 2012;130(suppl 2):S186-S190. doi: 10.1542/peds.2012-0900Q [DOI] [PubMed] [Google Scholar]
- 9.Kasari C, Gulsrud AC, Wong C, Kwon S, Locke J. Randomized controlled caregiver mediated joint engagement intervention for toddlers with autism. J Autism Dev Disord. 2010;40(9):1045-1056. doi: 10.1007/s10803-010-0955-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Research Council . Educating Children with Autism. National Academies Press; 2001. [Google Scholar]
- 11.Dawson G, Jones EJH, Merkle K, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51(11):1150-1159. doi: 10.1016/j.jaac.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huttenlocher PR. Neural Plasticity: The Effects of Environment on the Development of the Cerebral Cortex. Harvard University Press; 2002. [Google Scholar]
- 13.Sullivan K, Stone WL, Dawson G. Potential neural mechanisms underlying the effectiveness of early intervention for children with autism spectrum disorder. Res Dev Disabil. 2014;35(11):2921-2932. doi: 10.1016/j.ridd.2014.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feinberg E, Abufhele M, Sandler J, et al. Reducing disparities in timely autism diagnosis through family navigation: results from a randomized pilot trial. Psychiatr Serv. 2016;67(8):912-915. doi: 10.1176/appi.ps.201500162 [DOI] [PubMed] [Google Scholar]
- 15.Feinberg E, Kuhn J, Sandler Eilenberg J, et al. Improving family navigation for children with autism: a comparison of two pilot randomized controlled trials. Acad Pediatr. Published online April 24, 2020. doi: 10.1016/j.acap.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broder-Fingert S, Walls M, Augustyn M, et al. A hybrid type I randomized effectiveness-implementation trial of patient navigation to improve access to services for children with autism spectrum disorder. BMC Psychiatry. 2018;18(1):79. doi: 10.1186/s12888-018-1661-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broder-Fingert S, Stadnick NA, Hickey E, Goupil J, Diaz Lindhart Y, Feinberg E. Defining the core components of family navigation for autism spectrum disorder. Autism. 2020;24(2):526-530. doi: 10.1177/1362361319864079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glasgow RE, Orleans CT, Wagner EH. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2001;79(4):579-612, iv-v. doi: 10.1111/1468-0009.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer. 2011;117(15)(suppl):3539-3542. doi: 10.1002/cncr.26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall JK, Mbah OM, Ford JG, et al. Effect of patient navigation on breast cancer screening among African American Medicare beneficiaries: a randomized controlled trial. J Gen Intern Med. 2016;31(1):68-76. doi: 10.1007/s11606-015-3484-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKevitt E, Dingee C, Warburton R, et al. Patient navigation reduces time to care for patients with breast symptoms and abnormal screening mammograms. Am J Surg. 2018;215(5):805-811. doi: 10.1016/j.amjsurg.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 22.McBrien KA, Ivers N, Barnieh L, et al. Patient navigators for people with chronic disease: a systematic review. PLoS One. 2018;13(2):e0191980. doi: 10.1371/journal.pone.0191980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuno Y, Higa DH, Leighton CA, Roland KB, Deluca JB, Koenig LJ. Is HIV patient navigation associated with HIV care continuum outcomes? AIDS. 2018;32(17):2557-2571. doi: 10.1097/QAD.0000000000001987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley L, Capp R, Carmona JF, et al. Patient navigation to reduce emergency department (ED) utilization among Medicaid insured, frequent ED users: a randomized controlled trial. J Emerg Med. 2020;58(6):967-977. doi: 10.1016/j.jemermed.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 25.Gordon J. Towards Interventions Across the Autism Spectrum. National Institute of Mental Health; 2017. [Google Scholar]
- 26.StudyTRAX, version 3.73.0020. ScienceTRAX LLC; 2020.
- 27.Robins DL, Casagrande K, Barton M, Chen C-MA, Dumont-Mathieu T, Fein D. Validation of the Modified Checklist for Autism in Toddlers, Revised with Follow-up (M-CHAT-R/F). Pediatrics. 2014;133(1):37-45. doi: 10.1542/peds.2013-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71(s1)(suppl 1):S52-S63. doi: 10.1111/j.1752-7325.2011.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madson MB, Campbell TC, Barrett DE, Brondino MJ, Melchert TP. Development of the Motivational Interviewing Supervision and Training Scale. Psychol Addict Behav. 2005;19(3):303-310. doi: 10.1037/0893-164X.19.3.303 [DOI] [PubMed] [Google Scholar]
- 30.Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop S. Autism Diagnostic Observation Schedule, 2nd ed. Western Psychological Services; 2012. [Google Scholar]
- 31.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 32.Zuckerman KE, Sinche B, Mejia A, Cobian M, Becker T, Nicolaidis C. Latino parents’ perspectives on barriers to autism diagnosis. Acad Pediatr. 2014;14(3):301-308. doi: 10.1016/j.acap.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magaña S, Lopez K, Aguinaga A, Morton H. Access to diagnosis and treatment services among Latino children with autism spectrum disorders. Intellect Dev Disabil. 2013;51(3):141-153. doi: 10.1352/1934-9556-51.3.141 [DOI] [PubMed] [Google Scholar]
- 34.Lopez K. Sociocultural perspectives of Latino children with autism spectrum disorder. Best Practices Ment Health. 2014;10(2):15-31. [Google Scholar]
- 35.Blanche EI, Diaz J, Barretto T, Cermak SA. Caregiving experiences of Latino families with children with autism spectrum disorder. Am J Occup Ther. 2015;69(5):5010p1-11. doi: 10.5014/ajot.2015.017848 [DOI] [PubMed] [Google Scholar]
- 36.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63(5):484-489. doi: 10.1001/archpsyc.63.5.484 [DOI] [PubMed] [Google Scholar]
- 37.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84(408):1065-1073. doi: 10.1080/01621459.1989.10478873 [DOI] [Google Scholar]
- 38.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80(3):557-575. doi: 10.1093/biomet/80.3.557 [DOI] [Google Scholar]
- 39.US Department of Education. Individuals with Disabilities Education Act (IDEA) Part C—Infants and Toddlers with Disabilities, Pub L No. 108–446, §631 (2004).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement