Abstract
Phenotypic and biological characterization of rare monogenic disorders represents 1 of the most important avenues toward understanding the mechanisms of human disease. Among patients with SH3 and multiple ankyrin repeat domains 3 (SHANK3) mutations, a subset will manifest neurologic regression, psychosis, and mood disorders. However, which patients will be affected, when, and why are important unresolved questions. Authors of recent studies suggest neuronal SHANK3 expression is modulated by both inflammatory and hormonal stimuli. In this case series, we describe 4 independent clinical observations of an immunotherapy responsive phenotype of peripubertal-onset neuropsychiatric regression in 4 girls with pathogenic SHANK3 mutations. Each child exhibited a history of stable, mild-to-moderate lifelong developmental disability until 12 to 14 years of age, at which time each manifested a similar, subacute-onset neurobehavioral syndrome. Symptoms included mutism, hallucinations, insomnia, inconsolable crying, obsessive-compulsive behaviors, loss of self-care, and urinary retention and/or incontinence. Symptoms were relatively refractory to antipsychotic medication but improved after immunomodulatory treatment. All 4 patients exhibited chronic relapsing courses during a period of treatment and follow-up ranging from 3 to 6 years. Two of the 4 girls recovered their premorbid level of functioning. We briefly review the scientific literature to offer a conceptual and molecular framework for understanding these clinical observations. Future clinical and translational investigations in this realm may offer insights into mechanisms and therapies bridging immune function and human behavior.
Characterization of monogenic variants represents an important avenue toward understanding human behavior and disease. Deletions or point mutations affecting SH3 and multiple ankyrin repeat domains 3 (SHANK3), which encodes a postsynaptic scaffolding protein, have been linked to autism spectrum disorder (ASD), Phelan-McDermid syndrome, and schizophrenia.1–3 Peripubertal neurobehavioral regression, sometimes with mania and catatonia, has been reported in some patients with Phelan-McDermid syndrome, but the etiology is unknown.4–6 Emerging literature reveals the ability of physiologic signals (eg, inflammatory stimuli, sex hormones) to regulate SHANK3 expression.7–9
In this report, we describe 4 peripubertal girls with previously stable developmental disabilities who exhibited subacute disabling symptoms that included mutism, hallucinations, insomnia, inconsolable crying, diminished self-care, and urinary retention and/or incontinence. Each child was evaluated by independent teams for suspected autoimmune encephalopathy before detection of SHANK3 point mutation. The families of these patients verbally consented to inclusion in this study.
CASE 1
A 13-year-old girl had a history of mild-to-moderate developmental delay without ASD and autoimmune thyroiditis on levothyroxine. Her family history was notable for autoimmune disease in the father and brother. At 13 years, over several weeks, her symptoms progressed from irritability to audiovisual hallucinations, insomnia, compulsivity, aggression, catatonia, mutism, and urinary retention. Her symptoms were refractory to antipsychotics and benzodiazepines.
Serology and thyroid function studies prompted consideration of Hashimoto encephalopathy. At 14 years, 40 mg of oral prednisone taken daily for 2 weeks yielded no improvement, but 2 g/kg of intravenous immunoglobulin (IVIG) led to sustained improvement in language, restlessness, and agitation within 2 days. Symptoms recurred 3 weeks later prompting monthly IVIG, which demonstrated the same pattern of response. Attempts to increase treatment intervals led to symptom reemergence.
At 16 years, she continues on IVIG (1 g/kg) and methylprednisolone every 4 to 6 weeks. She has regained 2 of 6 independent activities of daily living (ADLs) from nadir and weaned off daily psychotropic agents but remains substantially below her premorbid baseline. Whole-exome sequencing was pursued for unknown etiology of lifelong developmental delay and identified a pathogenic de novo frameshift mutation in exon 21 of SHANK3.
CASE 2
A 14-year-old girl had a history of moderate intellectual disability without ASD. Her maternal grandmother had psychosis. At age 14 years, she developed 10-day episodes of mania with psychotic features coinciding with menstruation with poor response to antipsychotics. After her third event, she developed depression with catatonic features and developmental regression. Daily oral contraceptives to suppress menses, lamotrigine, and a switch from aripiprazole to olanzapine correlated with a gradual return to her neuropsychiatric baseline over 8 months. At age 15 years, she developed myoclonic jerks, athetoid, and choreiform movements. At age 16 years, she developed overnight onset of severe anxiety, compulsions, insomnia, urinary incontinence, facial tics, sobbing, and loss of self-care and academic skills. After 2 months of symptoms and an unrevealing workup, her symptoms prompted treatment of seronegative autoimmune encephalitis using 2 g/kg of IVIG, which was temporally associated with symptom resolution within 3 weeks. She was maintained on olanzapine, lamotrigine, N-acetyl cysteine, methylated B vitamins, and oral contraceptives. After 5 months, neuropsychiatric testing matched her premorbid baseline.
Two manic relapses occurred 6 months and 1 year later, both improving in conjunction with IVIG. The latter event was notable for a seizure, echolalia, and anxiety. Within 1 hour, 0.5 mg of lorazepam for suspected catatonia yielded dramatic improvement. Mania and catatonia relapsed 1 month later but resolved with daily lorazepam.
At age 18 years, she maintains all ADLs on a daily regimen of lorazepam, olanzapine, lamotrigine, oral contraceptive, N-acetyl cysteine, vitamin D, folate, omega-3 fatty acids, and monthly IVIG (1 g/kg). Whole-exome sequencing was pursued for unknown etiology of complex neuropsychiatric illness and revealed a pathogenic frameshift mutation in exon 21 of SHANK3; the inheritance of this mutation is unknown.
CASE 3
A 12-year-old girl had a history of mild intellectual disability, ASD, tics, and attention-deficit/hyperactivity disorder. At age 12 years, 3 months after menarche, she developed abrupt onset of hyperactivity, paranoia, mutism, akathisia, audiovisual hallucinations, inconsolable screaming, and periodic staring spells. Her workup was notable for elevated antinuclear antibody and slight elevation of white blood cell count in cerebrospinal fluid. The symptoms were refractory to guanfacine and risperidone but improved with 30 mg/kg of intravenous methylprednisolone within days but reemerged over weeks. IVIG (2 g/kg) yielded significant improvement in language, social engagement, sleep, agitation, and concentration, but the effect consistently waned at approximately 3 weeks. Mycophenolate and rituximab were added. Over the next 2 years, she maintained stable premorbid language, cognition, and ADLs but relapsed within 6 months of weaning immunomodulation at 14 years, prompting reinitiation of a similar regimen. She maintained her premorbid baseline for 3 years until at age 17 years, weaning of mycophenolate was associated with neuropsychiatric decline, prompting a 3-week ICU admission with reinitiation of IVIG, rituximab, mycophenolate, high-dose lorazepam, and ultimately electroconvulsive therapy for catatonia. Within a month, she reapproximated her baseline.
At 18 years, her current treatment includes monthly IVIG, mycophenolate, and rituximab for immunotherapy in addition to lorazepam, methylphenidate, trazodone, lithium, and clonidine. Whole-exome sequencing was pursued for unknown etiology of complex autoimmune encephalitis and lifelong developmental delay and identified a pathogenic de novo frameshift mutation in exon 21 of SHANK3.
CASE 4
A 13-year-old girl who had a history of mild cognitive delay and learning disabilities without ASD developed severe acute anxiety within 4 days of starting minocycline for facial acne; the medication was discontinued. One month later, she developed overnight onset of anxiety, obsessiveness, insomnia, inability to chew or swallow, aphasia, agraphia, inconsolable screaming, impulsivity, and urinary incontinence. She was diagnosed with bipolar disorder and hospitalized 6 times in the next 6 months but was poorly responsive to antipsychotics, benzodiazepines, antidepressants, or anticonvulsants. Lithium conferred a moderate, mood-stabilizing effect. After 1 year of symptoms, an autoimmune evaluation was notable for elevated antinuclear and histone antibodies and low complement component 4, which led to treatment with IV methylprednisolone. Within 3 days, she manifested improved expressive language, transitioned to daily prednisone, and returned to her neuropsychiatric baseline for several months. However, symptoms recurred with steroid weaning. After transition to 2 g/kg of IVIG, mycophenolate, and rituximab, she maintained her premorbid baseline for the next 2 years despite weaning all psychiatric medications. However, when her immunosuppression was weaned off at 16 years, severe psychiatric symptoms returned. Despite reinitiating intravenous methylprednisolone, IVIG, mycophenolate, and rituximab, and a trial of plasmapheresis, her symptoms persisted. She was subsequently administered a 6-month course of cyclophosphamide and clozapine. Her symptoms began to improve only after her white blood cell counts began to fall.
At 20 years of age, she remains on rituximab and prednisone and has tolerated weaning of mycophenolate. She maintains a relatively stable baseline independent in all ADLs with intermittent “flares” of obsessive-compulsive/psychotic symptoms, often in the setting of a systemic infection; these symptoms often self-resolve after treating the infection, sometimes with the addition of steroids. Whole-exome sequencing was pursued because of unknown etiology of psychosis and revealed a pathogenic de novo frameshift mutation in exon 14 of SHANK3.
DISCUSSION
We describe 4 peripubertal girls with stable, lifelong developmental disabilities with and without comorbid ASD caused by pathogenic mutations in SHANK3 who developed a subacute stereotyped, profoundly disabling neuropsychiatric syndrome that prompted 4 independent clinical teams to initiate treatment of suspected autoimmune encephalopathy. Behavioral symptoms were chronic and/or relapsing but often improved with immunomodulatory therapies administered intermittently over several years (Table 1). Two patients recovered to their premorbid function.
TABLE 1.
Clinical Case Summaries
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Baseline clinical features | ||||
| Age, sex | 13-y-old girl | 14-y-old girl | 12-y-old girl | 13-y-old girl |
| SHANK3 mutation | c.3679dupG, p.A1227Gfs*69 (pathogenic de novo frameshift in exon 21) | c.3424_3425delCT, p.Leu1142Valfs*153 (pathogenic unknown inheritance frameshift in exon 21) | c.4116delC; p.Thr1373Glnfs*13 (pathogenic de novo frameshift in exon 21) | c.1864_1865delinsA, p.A622fs*XX (pathogenic de novo frameshift in exon 14) |
| Duration of follow-up | 4 y | 4 y | 5 y | 6 y |
| Neurocognitive baseline | Moderate intellectual disability (verbal IQ 78; performance IQ 53) | Moderate intellectual disability (IQ 40) | Moderate intellectual disability (IQ 55) | Mild cognitive and learning disabilities (IQ 78), delayed acquisition of speech, auditory processing disorder, and fine motor issues |
| Neuropsychiatric baseline and history | Happy, socially engaged child with no previous psychiatric illnesses | History of cyclic perimenstrual mania, catatonia, and depressive episodes beginning 2 y before intractable, subacute encephalopathy ADHD tics | ADHD tics | Happy, socially engaged, high-functioning child with no previous psychiatric illnesses or mood instability |
| Other medical history | History of autoimmune thyroiditis (elevated thyroid-stimulating hormone, elevated Abs against thyroglobulin and thyroid peroxidase) | Premature puberty at age 8 y treated with monthly Lupron injections from ages 8–12 y | Generalized tonic-clonic seizure (once) at 10 y | — |
| Family history | Father with multiple sclerosis and inflammatory bowel disease brother with lupus | Maternal grandmother with bipolar disorder | No relevant conditions | Maternal aunt with bipolar and maternal grandmother with mood disorder |
| Age at menarche | 11 y 4 mo | 12 y | 11 y 10 mo | 12 y |
| Subacute neuropsychiatric symptoms at presentation | ||||
| Age at subacute onset | 13 y 10 mo | 14 y 4 mo | 12 y 1 mo | 13 y 5 mo |
| Time from onset to symptomatic nadir | 3-wk progressive loss of bowel and bladder control followed by overnight onset of psychosis | Overnight onset of severe obsessive-compulsive symptoms | Overnight onset of agitation, psychosis, and insomnia | Overnight onset of anxiety, severe sleep disruption, eating restriction, and eventual obsessive-compulsive symptoms |
| Audiovisual hallucinations | Yes | Yes | Yes | No |
| Inconsolable screaming and/or crying | Yes | Yes | Yes | Yes |
| Aphasia or mutism | Yes, complete aphasia | No | Yes, complete aphasia | Yes, near complete aphasia |
| Severe anxiety | Yes | Yes | Yes | Yes |
| Obsessive thinking | Yes | Yes | Yes | Yes |
| Insomnia | Yes | Yes | Yes | Yes |
| Loss of self-care | Yes | Yes | Yes | Yes |
| Urinary retention or incontinence | Yes: profound urinary retention and incontinence | Yes: sporadic episodes of daytime urinary incontinence during illness | Yes: sporadic incontinence | Yes; profound polyuria followed by urinary incontinence |
| Hyperkinetic | Yes: akathisia movements | Yes: athetosis 2–3 mo before neuropsychiatric onset; tremor; facial tics | Yes: akathisia | Yes: chorea; tremor |
| Catatonia | Yes | Yes | Yes | No |
| Other | Aggression, bowel incontinence, pica | Exaggerated startle | Paranoia | Agraphia |
| Diagnostics studies | ||||
| Neurologic examination at first evaluation | Nonfocal | Nonfocal, choreiform movements of toes | Nonfocal | Choreiform hand movements and tremor |
| Neuroimaging | MRI of the brain with mild ventricular prominence | MRI of the brain unrevealing | MRI/MRA brain, CTA of the head unrevealing | MRI of the brain unrevealing |
| Elevated Ab titers | Antinuclear Ab + 1:80 antithyroperoxidase Ab (>830 U/mL; normal <4) antithyroglobulin Ab (208 IU/mL; normal <2) | Elevation in anti–calmodulin-dependent kinase II serology | Antinuclear Ab + 1:160 | Antinuclear Ab + 1:320, positive histone Abs |
| Other abnormal study results | Complement C4 (low) persistent monocytosis C1q binding (elevated) thyroid-stimulating hormone (elevated) T4 alternately high and low at different intervals | Borderline (+) titer for Bartonella | EEG with diffuse slowing | Low complement C4 (subsequently normalized) |
| Cerebrospinal studies | White cells, red cells, glucose, protein, IgG index, and oligoclonal bands all normal | White cells, red cells, glucose, and protein all normal | 9 white cells (67% neutrophils, 33% lymphocytes; normal range 0–5 white cells) protein, glucose, IgG index, oligoclonal bands, lactate, Cryptococcus antigen, India ink, Gram-stain, enterovirus PCR, human herpesvirus 6 PCR, mycoplasma PCR, rubeola IgG and IgM, herpes simplex 1 and 2 PCR, HIV PCR, NMDA receptor Ab, and paraneoplastic Ab panel were all normal | White cells, red cells, glucose, protein, IgG index, and oligoclonal bands all normal |
| Other normal or negative study results | Normal Ab serologies: Mayo autoimmune encephalopathy panel including NMDA receptor, antistreptolysin 0 B-cell and T-cell immunophenotyping panel, quantitative immunoglobulins Normal genetic and/or metabolic: single nucleotide polymorphism array, fragile X, urine organic acids, plasma amino acids, carnitine, ammonia, zinc, methylmalonic acid, acylcarnitine profile, urine glycosaminoglycans EEG unrevealing Pelvic ultrasound normal Abdominal MRI notable for mild splenic enlargement |
NMDA receptor Ab EEG unrevealing Abdominal US: negative results for teratoma |
Sedimentation rate, C-reactive protein, thyroid-stimulating hormone, antithyroglobulin Ab, serum immunoglobulins, C4, C3, paraneoplastic Ab panel, serum NMDA receptor Abs, angiotensin-converting enzyme, ANCA, Bartonella IgG and IgM, lysosomal and peroxisomal panel, urine oligosaccharide and mucopolysaccharide screen, and porphyrins Full-body PET; pelvic ultrasound |
Normal Ab serologies: NMDA receptor, thyroperoxidase, thyroglobulin, double-stranded DNA, voltage-gated potassium channels, anti-Ro, anti-La, phospholipid, cardiolipin, and β-2-glycoprotein I, neutrophils, paraneoplastic antigens Infectious studies: Bartonella species, Ehrlichia chaffeensis, Babesia microti, Leishmania, Lyme panel, West Nile virus, herpes simplex viruses 1 and 2, Epstein-Barr virus, syphilis, and enterovirus Genetic and/or metabolic: lactate, pyruvate, ammonia, fatty acid profile, acylcarnitine profiles, amino acid profiles of urine, serum and CSF, mucopolysaccharide and oligo- and polysaccharide profiles, cytogenetic fluorescence in situ hybridization, fragile X, array comparative genomic hybridization, and mercury |
| Therapeutic interventions | ||||
| Immunotherapy regimens and response | Daily oral prednisone 2 times per wk: no improvement IVIG 2 g/kg monthly for 4 mo: consistent, temporally correlated improvement in neuropsychiatric symptoms with waning effect before next dose IVIG 1 g/kg and IV methylprednisolone 1 g coadministration monthly (ongoing): similar to above |
IVIG 2 g/kg twice accompanied by azithromycin: correlated with complete resolution of obsessive-compulsive behaviors within 4 wk; improvement beyond premorbid baseline over 2 mo Second dose of IVIG during “manic relapse” correlated with resolution of mood symptoms and return to baseline |
IV methylprednisolone 30 mg/kg per d for 3 d, then monthly for 3 mo IVIG 2 g/kg once followed by IVIG 1 g/kg monthly Mycophenolate 750 mg BID with improvement in many symptoms and multiple less severe relapses when weaned off above regimen |
IV methylprednisolone 1000 mg daily for 3 d: language improved within 3 d IVIG: temporally correlated with symptom improvement Rituximab and mycophenolate: neuropsychiatric symptoms generally maintained at baseline while on rituximab and mycophenolate but relapsed when weaned Cyclophosphamide was used to treat refractory relapse. Rituximab and prednisone: near baseline but with mild psychotic exacerbations while on maintenance |
| Other therapeutic modifications during immunotherapy course | Weaned off risperidone and lorazepam during immunotherapy Addition of levonorgestrel-ethinyl after 1 y of immunotherapy Also received trazodone, vitamin D, propranolol, and melatonin for discrete periods |
Lorazepam: coadministered with a course of IVIG with immediate, marked improvement Clonazepam: initiated as maintenance therapy after lorazepam course Olanzapine: variably stopped and started during treatment course Daily oral contraceptive: maintained through most of course Metformin (for olanzapine associated wt gain) Clonidine: variably stopped and started to treat agitation Lamotrigine: started after seizure |
Sertraline: used for obsessive-compulsive features and anxiety Aripiprazole: started for irritability and/or repetitive behaviors Methylphenidate: for ADHD, continues to take Clonidine: used intermittently for sleep, ADHD symptoms Lorazepam: used for agitation and catatonia, currently weaning off this medication Lithium: started during most recent relapse to treat manic symptoms Metformin: for aripiprazole associated wt gain |
Multiple trials of antipsychotics, antidepressants, sleep medications, and benzodiazepines Slowly weaned off all psychiatric medications after initiation of immunotherapy |
| Basic ADL measures at baseline, nadir, and last follow-up | ||||
| At premorbid baseline | Independent: 5 (transferring, dressing, eating, toileting, continence) Dependent: 1 (bathing) |
Independent: 6 (transferring, bathing, dressing, eating, toileting, continence) Dependent: 0 |
Independent: 6 (transferring, bathing, dressing, eating, toileting, continence) Dependent: 0 |
Independent: 6 (transferring, bathing, dressing, eating, toileting, continence) Dependent: 0 |
| At symptomatic nadir | Independent: 0 Dependent: 6 (transferring, bathing, dressing, eating, toileting, continence) |
Independent: 2 (transferring, toileting) Dependent: 4 (bathing, dressing, eating, continence) |
Independent: 0 Dependent: 6 (transferring, bathing, dressing, eating, toileting, continence) |
Independent: 0 Dependent: 6 (transferring, bathing, dressing, eating, toileting, continence) |
| Most recent | Independent: 2 (transferring, eating) Dependent: 4 (bathing, dressing, toileting, continence) |
Independent: 6 (transferring, bathing, dressing, eating, toileting, continence) Dependent: 0 |
Independent: 6 (transferring, bathing, dressing, eating, toileting, continence) but impulsivity warrants supervision Dependent: 0 |
Independent: 6 (transferring, bathing, dressing, eating, toileting, continence) Dependent: 0 |
Ab, antibody; ADHD, attention-deficit/hyperactivity disorder; ANCA, antineutrophil cytoplasmic antibodies; BID, bis in die; C1q, complement component 1q; C3, complement component 3; C4, complement component 4; CSF, cerebrospinal fluid; CT, computed tomography; IgG, immunoglobulin G; IgM, immunoglobulin M; La, anti-La/SSB antibody; MRA, magnetic resonance angiography; PCR, polymerase chain reaction; PET, positron emission tomography; Ro,; anti-Ro/SSA antibody; T4, thyroxine T4; US, ultrasound; —, not applicable.
Each patient demonstrated symptom improvement typically within days of IVIG followed in some by a waning effect within the following weeks. This pattern approximates a theragnostic rule for seronegative autoimmune encephalopathies.10 Although the short-term treatment effect was reproducible within and across cases, the degree to which immunomodulatory therapy was responsible for long-term improvements in these patients can be debated. A precise comparison is confounded by the heterogeneity of each course including fluctuating symptoms, variation in immunomodulatory and psychotropic regimens, and incomplete return to baseline in 2 individuals. Although all patients were considered relatively refractory to antidepressant and antipsychotic medications, patients reported variable responses to lorazepam, lithium, and/or hormonal contraceptives.
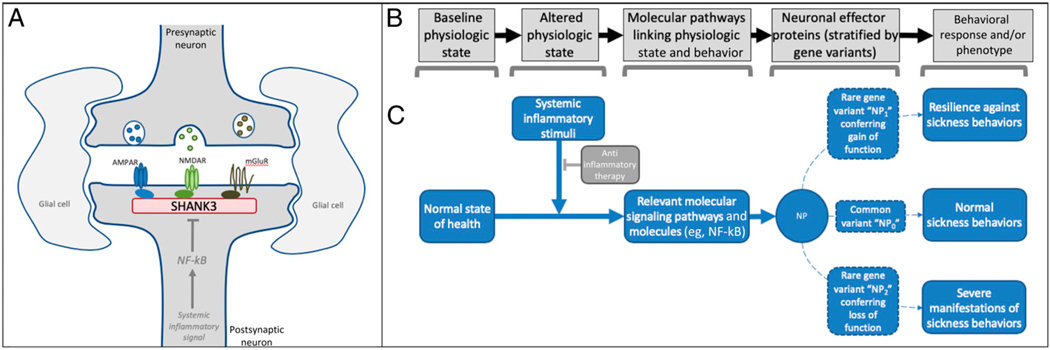
Experimental data offer possible molecular and conceptual mechanisms supporting the notion of immune-mediated behavioral modulation via SHANK3. At the molecular level, SHANK3 functions as a master scaffolding protein at the synapse, supporting several neurotransmitter receptors (Fig 1). Three cases (cases 1–3) had truncating mutations in exon 21 encoding the proline-rich domain, which mediates contacts with the metabotropic glutamate receptor, and 1 (case 4) had a truncating mutation in a well-characterized PDZ domain that interfaces with N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors.11
FIGURE 1.
Sickness behavior as a hypothetical model for conceptualizing SHANK3’s interface with the immune system. SHANK3 functions as an important scaffolding protein for neurotransmitter receptors (see 1). A, In experimental models, systemic inflammatory stimuli can decrease SHANK3 expression via nuclear factor κ–light-chain enhancer of activated B cells (NF-kB)-related signaling pathways (see 8). B, A variety of stereotyped behaviors occur as codified responses to nonhomeostatic physiologic states (eg, acute illness), each using unique molecular pathways to manifest adaptive, stimuli-specific behavioral responses. C, Alteration of proteins within these pathways, such as via genetic variants that confer a gain or loss of function, would be hypothesized to alter the associated behavioral response. The diverse behaviors that accompany states of acute infection, known as sickness behaviors, which include irritability, depressed mood, and social withdrawal, offer a template for conceptualizing the molecular and behavioral phenomena that may result from systemic inflammation and, conversely, be modified by its resolution (see 17). Considered together with our case histories, the combined observations offer a reasonable hypothesis implicating SHANK3 as a candidate protein involved in 1 or more molecular pathways regulating clinically relevant sickness behaviors in humans. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; mGluR, metabotropic glutamate receptor; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NMDAR, N-methyl-D-aspartate receptor; NP0, common variant of a hypothetical neurobehavioral protein; NP1, gain of function variant of neurobehavioral protein; NP2, loss of function of neurobehavioral protein; NP, neurobehavioral protein.
Exons 14 and 21 are subject to alternative splicing and intragenic promoters, such that mutations in these regions are predicted to result in isoform-specific disruption of SHANK3 in the brain.11–13 Autoantibodies against the synaptic proteins supported by SHANK3 scaffolding have been implicated in the onset of classic autoimmune encephalitides.14,15 Experimental models demonstrate neuronally mediated inflammatory signals capable of reducing SHANK3 expression even in the absence of overt neuroinflammation.7,8 Sex hormones modulate SHANK3 expression in a similar fashion.9
The modulation of behavior via inflammatory signaling fits within the conceptual framework of “sickness behaviors” (Fig 1), which constitute a diverse suite of evolutionarily conserved responses (eg, irritability, mild encephalopathy, depressed mood, diminished self-care, social withdrawal) that occurs to varying degrees within the setting of systemic illness.16–18 Links between sickness behaviors and psychiatric disease have been widely speculated.19 The molecular mechanisms underlying sickness behaviors have been studied in animal models, which reveal a variety of molecular pathways linking systemic inflammatory stimuli with neuronal and behavioral modulation. Genetic variants conferring a gain or loss of function of key regulatory molecules within these pathways would be expected to yield a phenotypic change in behavior conferring resilience or vulnerability to inflammation. SHANK3 perturbations, such as the putative loss of function mutations seen in these 4 cases, have been implicated in autism, schizophrenia, and bipolar disorder, all of which have been linked to immune dysregulation in genome-wide investigations, but the genetic complexity warrants further investigation because mania or autism has been seen in patients with gain and loss of function mutations, and SHANK3 has immense transcriptional complexity.20,21 Thus, although experimental models7,8 and a previous case22 have implicated SHANK3 in immune-behavior connections, the 4 cases described in this series may highlight the real-world clinical implications of this phenomenon.
Our cases, and others like them,5–7,22 support targeted genetic sequencing of SHANK3 in individuals with acute neuropsychiatric decline or catatonia in the context of preexisting intellectual disability, with or without comorbid ASD given the heterogeneous presentations of patients with SHANK3 mutations. The experience of these 4 patients lends support to additional diagnostic evaluations, structured clinical trials, and further laboratory work to investigate new mechanisms and therapies for acute neuropsychiatric events in patients with SHANK3 mutations. Immunomodulatory therapy carries risks and should only be pursued in accord with existing guidelines.10 Insights gleaned from individuals with well-characterized molecular perturbations may clarify our understanding of the neurobiological basis of human behavior and highlight new therapeutic strategies.
ACKNOWLEDGMENTS
We acknowledge Elise Brimble, MSc, MS, and Heather Van Mater, MD, MSc, for assistance reading and revising the article. We thank David Goldstein, Vandana Shashi, and Rebecca C. Spillmann for the whole-exome sequencing study of case 3 that was enrolled in protocol 32301: Genomic Study of Medical, Developmental, or Congenital Problems of Unknown Etiology. This study was approved by the Duke University Health System Institutional Review Board for Clinical Investigators (FWA 00009025), supported by the Duke University Health system, and partially funded by Union Chimique Belge Celltech. Dr Jiang is supported by National Institutes of Health grants MH117289, HD087795, and MH098114.
ABBREVIATIONS
- ADL
activity of daily living
- ASD
autism spectrum disorder
- IVIG
intravenous immunoglobulin
- NMDA
N-methyl-D-aspartate
- SHANK3
SH3 and multiple ankyrin repeat domains 3
Footnotes
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Durand CM, Betancur C, Boeckers TM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39(1):25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauthier J, Champagne N, Lafrenière RG, et al. ; S2D Team. De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci USA. 2010;107(17): 7863–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leblond CS, Nava C, Polge A, et al. Meta-analysis of SHANK mutations in autism spectrum disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10(9):e1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denayer A, Van Esch H, de Ravel T, et al. Neuropsychopathology in 7 patients with the 22q13 deletion syndrome: presence of bipolar disorder and progressive loss of skills. Mol Syndromol. 2012;3(1): 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serret S, Thümmler S, Dor E, Vesperini S, Santos A, Askenazy F. Lithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reports. BMC Psychiatry. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwanenburg RJ, Ruiter SA, van den Heuvel ER, Flapper BC, Van Ravenswaaij-Arts CM. Developmental phenotype in Phelan-McDermid (22q13.3 deletion) syndrome: a systematic and prospective study in 34 children. J Neurodev Disord. 2016;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabrucker AM. Environmental factors in autism. Front Psychiatry. 2013;3:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Lukiw WJ. Bacteroidetes neurotoxins and inflammatory neurodegeneration. Mol Neurobiol. 2018;55(12):9100–9107 [DOI] [PubMed] [Google Scholar]
- 9.Berkel S, Eltokhi A, Fröhlich H, et al. Sex hormones regulate SHANK expression. Front Mol Neurosci. 2018;11:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Xu Q, Bey AL, Lee Y, Jiang YH. Transcriptional and functional complexity of SHANK3 provides a molecular framework to understand the phenotypic heterogeneity of SHANK3 causing autism and SHANK3 mutant mice. Mol Autism. 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L, Wang X, Li XL, et al. Epigenetic dysregulation of SHANK3 in brain tissues from individuals with autism spectrum disorders. Hum Mol Genet. 2014;23(6):1563–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Rubeis S, Siper PM, Durkin A, et al. Delineation of the genetic and clinical spectrum of Phelan-McDermid syndrome caused by SHANK3 point mutations. Mol Autism. 2018;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armangue T, Petit-Pedrol M, Dalmau J. Autoimmune encephalitis in children. J Child Neurol. 2012;27(11): 1460–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78(1):8–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science. 1975;188(4184): 166–168 [PubMed] [Google Scholar]
- 17.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pecchi E, Dallaporta M, Jean A, Thirion S, Troadec JD. Prostaglandins and sickness behavior: old story, new insights. Physiol Behav. 2009;97(3–4): 279–292 [DOI] [PubMed] [Google Scholar]
- 19.Maes M, Berk M, Goehler L, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandrov PN, Zhao Y, Jaber V, Cong L, Lukiw WJ. Deficits in the proline-rich synapse-associated Shank3 protein in multiple neuropsychiatric disorders. Front Neurol. 2017;8:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandal MJ, Haney JR, Parikshak NN, et al. ; CommonMind Consortium; PsychENCODE Consortium; iPSYCH-BROAD Working Group. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018; 359(6376):693–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jungová P, Čumová A, Kramarová V, et al. Phelan-McDermid syndrome in adult patient with atypical bipolar psychosis repeatedly triggered by febrility. Neurocase. 2018;24(4):227–230 [DOI] [PubMed] [Google Scholar]