Hypertension, present in approximately 33% of the U.S. population, is associated with left ventricular (LV) dysfunction in patients with cancer receiving anthracycline-based chemotherapy (Anth-bC) (1). In hypertensive patients, interstitial LV myocardial fibrosis is frequently present and contributes to LV systolic and diastolic dysfunction (2). To date, the underlying pathophysiological mechanisms that contribute to LV dysfunction after receipt of Anth-bC in hypertensive patients are not firmly established. Recently, several studies have identified an increase in LV myocardial extracellular volume fraction, a cardiovascular magnetic resonance imaging marker indicative of interstitial LV myocardial fibrosis, in patients receiving Anth-bC (3). We hypothesized that the administration of doxorubicin (DOX)–a frequently used Anth-bC agent–may accelerate LV myocardial fibrosis in the presence of hypertension.
To test this hypothesis, we randomized 25-week-old spontaneously hypertensive (SHR) and age-matched Wistar Kyoto (WKY) rats to receive weekly intraperitoneal normal saline versus sequential cycles of DOX (1.5 mg DOX/kg/week for 4 weeks [total cumulative dose: 6 mg/kg]) in a pattern similar to the chronic repetitive dosing strategy used clinically in humans. Our study was approved by the Wake Forest University Animal Care and Use Committee and followed the principles of the National Institutes of Health, Guide for the Care and Use of Laboratory Animals.
At 4 weeks, we assessed systolic blood pressure (SBP) by tail-cuff, and then animals were humanely euthanized to measure: 1) histological LV myocardial fibrosis (collagen volume fraction [CVF]); and 2) myocellular injury (cell injury severity as assessed by modified Billingham score) by blinded observers.
After euthanasia, a transverse mid-section of the LV myocardium was fixed in 4% paraformaldehyde. A 5-μm section was obtained and stained with Picrosirius red to quantify interstitial myocardial fibrosis (4). Twenty random photomicrograph fields (20×, avoiding perivascular collagen) were obtained to assess CVFs using ImageJ software version 1.51q (NIH, Bethesda, Maryland) and expressed as percentage of the area. To assess the severity of myocellular injury, a second 5-μm section was obtained and stained with hematoxylin-eosin, and myocyte damage was assessed using Billingham’s scoring system on 5 random 20× fields (4).
One-way analysis of variance with Tukey’s correction was used to compare the 4 groups with data expressed as mean ± SEM; a p value <0.05 was considered significant for all analyses.
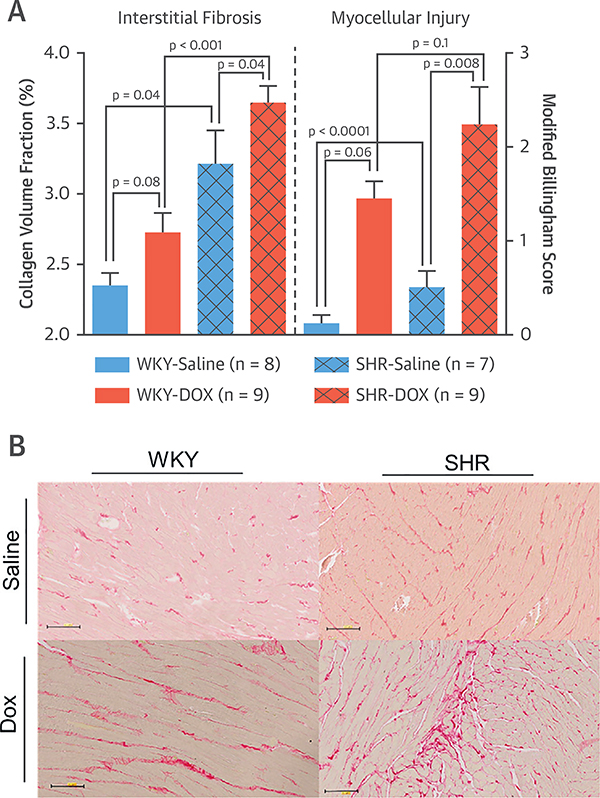
Compared with those receiving saline, animals receiving DOX exhibited more weight loss during the experimental period (p < 0.001). SHR-saline– and -DOX–treated animals exhibited elevated SBP compared with their WKY counterparts (SHR-Saline 167 ± 11 mm Hg vs. WKY-Saline 104 ± 10 mm Hg; p = 0.001; and SHR-DOX 162 ± 14 mm Hg vs. WKY-DOX 97 ± 11 mm Hg; p = 0.003). Receipt of DOX was not associated with differences in SBP between animals in the 2 SHR groups (p = 0.74). Hypertensive animals receiving DOX experienced elevated CVFs when compared with SHR-Saline and WKY hearts with or without DOX. The CVF was increased in SHR-Saline compared with WKY-Saline recipients (Figure 1). Billingham scores were elevated in SHR and WKY receiving DOX relative to controls (Figure 1) and correlated mildly with CVF (r = 0.41, p = 0.03) across all 4 groups.
FIGURE 1. Histopathological Assessment of Cardiac Fibrosis and Myocellular Injury.
(A) Collagen volume fraction (CVF) (left y-axis) and modified Billingham Score for myocardial injury (right y-axis) of WKY rats treated with saline (solid blue bars) or DOX (solid orange bars) and SHRs treated with saline (hash-marked blue bars) or DOX (hash-marked orange bars). All values are mean ± SEM. (B) Representative microphotographs (20×) of left ventricles from each treatment group stained with Picrosirius red that identifies collagen. DOX = doxorubicin; SHR= spontaneously hypertensive; WKY = Wistar Kyoto.
The results of our study indicate that during receipt of DOX, hypertensive animals experience more collagen deposition compared with animals with hypertension alone or those without hypertension receiving DOX. Because interstitial myocardial fibrosis contributes to LV dysfunction, these findings represent a plausible underlying mechanism responsible for the increased susceptibility of patients with pre-existing hypertension receiving Anth-bC to develop LV dysfunction. It remains to be determined whether the accelerated collagen deposition in the hypertensive heart is the result of enhanced cardiomyocyte injury, increased oxidative or nitrosative stress, a direct activation of cardiac fibroblasts that may be predisposed to secrete collagen due to the presence of hypertension, or a combination thereof.
Acknowledgments
Please note: This research was supported in part by National Institutes of Health grants R43 HL127878, R43 CA174261, R01CA167821, R01HL118740-01S1, R01 HL118740, and 1R01CA199167. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Kotwinski P, Smith G, Cooper J, et al. Body surface area and baseline blood pressure predict subclinical anthracycline cardiotoxicity in women treated for early breast cancer. PloS One 2016;11:e0165262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mordi IR, Singh S, Rudd A, et al. Comprehensive echocardiographic and cardiac magnetic resonance evaluation differentiates among heart failure with preserved ejection fraction patients, hypertensive patients, and healthy control subjects. J Am Coll Cardiol Img 2018;11:577–85. [DOI] [PubMed] [Google Scholar]
- 3.Meléndez GC, Jordan JH, D’Agostino RB Jr., Vasu S, Hamilton CA, Hundley WG. Progressive 3-month increase in LV myocardial ECV after anthracycline-based chemotherapy. J Am Coll Cardiol Img 2017;10:708–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong YJ, Park HS, Park JK, et al. Early detection and serial monitoring of anthracycline-induced cardiotoxicity using T1-mapping cardiac magnetic resonance imaging: an animal study. Sci Rep 2017;7:2663. [DOI] [PMC free article] [PubMed] [Google Scholar]