Abstract
Background:
Chronic pain is a significant public health problem in the United States, affecting approximately 100 million people. Yet there is a lack of robust biomarkers for clinical use in chronic pain conditions. Downstream effects of environmental, genomic, and proteomic variations in individuals with chronic pain conditions can be identified and quantified using a metabolomic approach.
Aim/Design:
The purpose of this systematic review was to examine the literature for reports of potential metabolomic signatures associated with chronic pain conditions.
Methods:
We searched relevant electronic databases for published studies that used various metabolomic approaches to investigate chronic pain conditions among subjects of all ages.
Results:
Our search identified a total of 586 articles, 18 of which are included in this review. The reviewed studies used metabolomics to investigate fibromyalgia (n = 5), osteoarthritis (n = 4), migraine (n = 3), musculoskeletal pain (n = 2), and other chronic pain conditions (n = 1/condition). Results show that several known and newly identified metabolites differ in individuals with chronic pain conditions compared to those without these conditions. These include amino acids (e.g., glutamine, serine, and phenylalanine) and intermediate products (e.g., succinate, citrate, acetylcarnitine, and N-acetylornithine) of pathways that metabolize various macromolecules.
Conclusion:
Though more high-quality research is needed, this review provides insights into potential biomarkers for future metabolomics studies in people with chronic pain conditions.
Keywords: metabolomics, chronic pain, systematic review, metabolomic signatures, osteoarthritis, fibromyalgia
Chronic pain is a significant public health problem in the United States, affecting approximately 100 million people (Institute of Medicine, 2011). The economic burden of chronic pain is substantial, with an estimated $635 billion annually in lost wages and health care costs (Gaskin & Richard, 2012). Chronic pain is one of the leading causes of visits to primary care providers (Finley et al., 2018). Yet in spite of these significant impacts, the molecular mechanisms that underlie the pathogenesis of various chronic pain conditions are not fully understood (Institute of Medicine, 2011).
Pain is frequently classified into three overlapping categories: nociceptive, neuropathic, and inflammatory (Institute of Medicine, 2011). Clinically, the differences among chronic pain conditions are not always clearly defined, and as a result, a single patient may present with more than one type of pain. These overlapping presentations and our lack of knowledge regarding the etiology of chronic pain conditions support the recent increase in research to enhance our understanding of the pathogenesis of chronic pain conditions. Evidence suggests that a variety of biological (e.g., genetic, immune response, age, sex), psychological (e.g., depression, anxiety, stress), and social (e.g., lack of support, discrimination, violence) factors are involved in the pathophysiology of chronic pain conditions (Fillingim, 2015).
Genetic, epigenetic, and biopsychosocial alterations result in downstream changes in small molecules known as metabolites, which can be identified and quantified (Dinis-Oliveira, 2019; Wei, 2011). The type and quantity of metabolites in a sample provide information about biological processes that reflect the cellular state in an individual. The characteristics of detectable metabolites can thus help us to identify biochemical molecules or biomarkers that distinguish physiologic and disease states (Clish, 2015; Wei, 2011).
Metabolomics is the use of high-throughput technology to comprehensively characterize and quantify metabolites in biological specimens (cells, fluids, tissues, or organisms; Clish, 2015). Researchers have measured metabolites in diverse body fluids and tissues including, but not limited to, serum (Hackshaw et al., 2019), urine (Forouzan et al., 2018), cerebrospinal fluid (Zielman et al., 2013), and synovial tissue (De Jong et al., 2016) and have used metabolomic methods to investigate several chronic health conditions such as diabetes and depression (Guasch-Ferré et al., 2016; MacDonald et al., 2019). Primary advantages of these methodologies include the diversity (invasive or noninvasive methods of collection) of the types of specimens that can be explored and strong associations between metabolite characteristics and the phenotype of the cell or organism. Given that the metabolome is the downstream product of the genome/epigenome, this close association between metabolite characteristics and the functional phenotype of the cell or organism makes sense (Wei, 2011). Due to the responsiveness of metabolites to disease states, metabolomics has the potential to enhance our understanding of the pathophysiology of chronic pain conditions as well as identify biomarkers for use ine diagnosing these conditions and monitoring therapeutic effectiveness to optimize patient outcomes (Finco et al., 2016; Hackshaw et al., 2019; Malatji et al., 2019). Metabolomics could also help identify pathways for new and more-precise therapeutic targets.
Over the last decade, numerous studies have assessed the metabolic changes associated with chronic pain conditions such as fibromyalgia (Caboni et al., 2014; Hackshaw et al., 2019), migraine (Zielman et al., 2016), and osteoarthritis (Adams et al., 2012; De Jong et al., 2016). In the present systematic review, we examined the literature to identify potential metabolomic signatures associated with various chronic pain conditions, explore the different metabolomic approaches used, and determine whether researchers also included measures of pain in their studies.
Method
Search Strategy
In completing this review, we followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines for conducting and reporting a systematic review (Moher et al., 2015). We performed a systematic literature search of the following databases, with no time limitation: MEDLINE (via PubMed), Embase, Scopus, Cochrane, and Cumulative Index of Nursing and Allied Health Literature (CINAHL). We used the following medical subject headings (MeSH) and text words in the search: “metabolome,” “metabolomics,” and “pain.” The search string for PubMed was (“Metabolome”[Majr] OR “Metabolomics”[Majr]) AND “Pain”[Majr]. We supplemented electronic searches with manual searching of bibliographic references.
The results were limited to studies published in the English language. Our inclusion criteria included original studies published in peer-reviewed journals, the use of human participants, either inclusion of a pain assessment or diagnosis of a chronic pain condition, and assessment of metabolomics. We did not set any a-priori limitations on age or metabolomic approaches. When studies compared participants with chronic pain conditions with those with other non-pain symptoms, we only included the parts of the study that involved the chronic pain condition.
Study Selection and Analysis
Each reviewer imported all literature search results into their EndNote library. After we removed duplicates, two of the authors independently screened titles and abstracts for inclusion. If disagreement remained after abstract screening, we included the paper for further consideration. A total of 18 articles met the inclusion criteria and were reviewed and extracted.
We used the matrix method to summarize the full-text articles according to these predetermined evaluation criteria: author, year, title, purpose, type of pain, sample size, tissue, metabolomics approach, significant findings, pain assessment tool, and comments (primarily strengths and limitations). Given the heterogeneity in study design, pain conditions, and metabolomics approach as well as the lack of randomized control studies, we did not complete the quality-appraisal part of the present systematic review.
Results
Study Characteristics
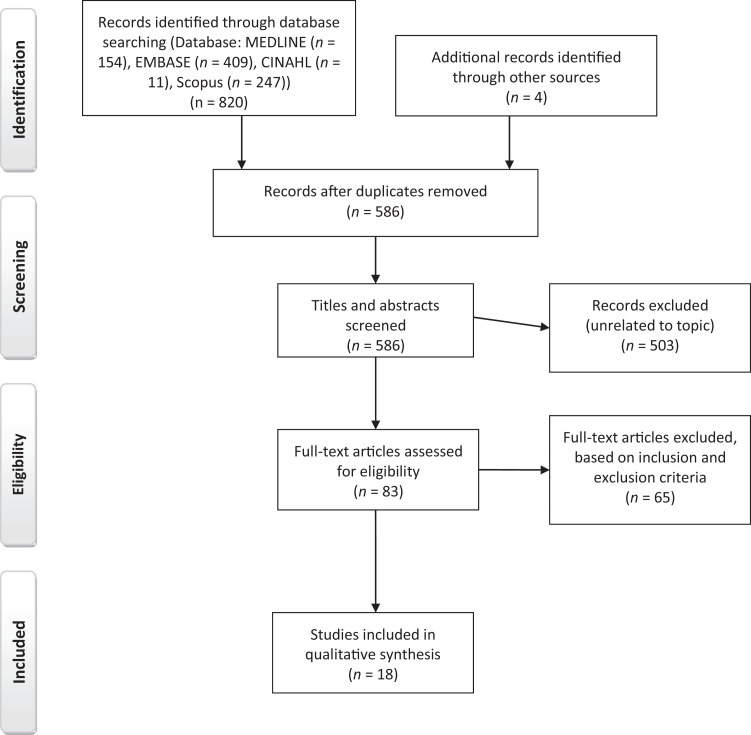
Our search yielded 821 articles: 154 from MEDLINE, 409 from Embase, 247 from Scopus, and 11 from CINAHL. After removing duplicates, we were left with 586 unique articles. Figure 1 summarizes the screening and study selection process. Of the 586 unique articles we screened, we included 18 in the systematic review. The study designs and quality were heterogeneous across the reviewed studies, and the number of participants ranged from 40 to more than 10,000. The studies were conducted in 11 countries, with most of the studies coming from the United States (n = 5), the Netherlands (n = 4), and Italy (n = 3) and one a multinational study (Italy, Belgium, and Croatia). Most of the studies did not report race or ethnicity of the participants. Table 1 summarizes the reviewed studies.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Flowchart Diagram for the Systematic Review of the Literature on the Metabolomics of Chronic Pain Conditions.
Table 1.
Selected Characteristics of Reviewed Studies on the Metabolomics of Chronic Pain Conditions (N = 19).
| Authors (Year) | Pain Type | Purpose | Tissue | Sample | Metabolomic Approach | Main Findings | Pain Assessment |
|---|---|---|---|---|---|---|---|
| Adams et al. (2012) | OA | To use global metabolic profiling to identify a distinct metabolic profile for cultured human synovial tissue from patients with end-stage OA compared to patients with little or no evidence of disease | Synovial tissue | 11 patients with late OA, 11 with little or no evidence of OA | UHLC/MS/MS, UHLC/MS/MS, & GC/MS | 105 compounds detected across all samples, with11 differing significantly between late-OA and no-OA groups. Levels of seven metabolites (pro-hydroxyproline, acetylcarnitine, myo-inositol, N-acetylornithine, succinate, glutamine, and urea) where higher and levels of 4 (gamma-glutamylleucine, 4-methyl-2-oxopentanonoate, 5-oxoproline, and phenylacetylglycine) were lower in late-OA compared with the no-OA group. | None |
| Caboni et al. (2014) | FM | To investigate the metabolic profile of patients with FM. Focused on plasma lipidome | Blood | 22 females with FM, 21 healthy controls | LC-Q-TOF/MS, GC/MS | 7 molecules (PC[14:0/0:0], PC[16:0/0:0], 1-[9E-hexadecenoyl]-sn-glycero-3-phosphocholine, 1-heptadecanoyl-sn-glycero-3-phosphocholine, 1-[9Z-octadecenoyl]-sn-glycero-3-phosphocholine, 1-[2E,4E-octadecadienoyl]-sn-glycero-3-phosphocholine, and 1-[4Z,7Z,10Z,13Z, 16Z,19Z-docosahexaenoyl]-sn-glycero-3-phosphocholine) showed significant loading differences between FM and control groups. Docking analysis revealed that lysophosphatidylcholine and platelet-activating factor-like factors may play a role on FMs | None |
| Mickiewicz et al. (2015) | OA | To use an integrated metabolomics approach to describe a broader and more detailed metabolic pattern for potential use in early OA diagnosis | Knee synovial fluid | 55 patients with OA, synovial fluid from 13 normal cadaveric knee joints | 1H NMR, GC/MS | 11 metabolites distinguished OA samples from controls. Two metabolites (fructose and citrate) were increased, and 9 metabolites (malate, methionine, O-acetylcarnitine, N-phenylacetylglycine, ethanol, creatine, ethanolamine, 3-hydroxybutyrate and hexanoylcarnitine) were decreased in OA samples compared to controls. | None |
| De Jong et al. (2016) | OA | To characterize the immune cells present in synovium from the knee of OA patients and their association with pain | Synovial tissue | 40 patients with OA of the knee undergoing total knee-replacement surgery | LC/MS | Fatty acids released by joint adipocytes enhanced the release of CD4+ T cells, which were abundant in synovial tissue and associated with VAS pain in patients with end-stage knee OA. CD4+ T cells also correlated with BMI. | VAS |
| Finco et al. (2016) | NP/ NC | To assess whether urinary metabolomic profiles can differentiate between neuropathic and nociceptive pain, as diagnosed using current clinical protocols | Urine | 12 patients with NC, 25 patients with NP, and 37 healthy controls | 1H NMR | Results showed an upregulation of phosphocholine and sphingomyelin associated with NP. Also, the urine of patients with NP had higher levels of choline and phosphocholine, citrate, alanine and taurine when compared with those with NC. | None |
| Parker et al. (2016) | IC, BPS, CP, CPPS | To discover urinary biomarkers in females with IC/BPS who underwent extensive urologic and non-urologic phenotyping in the MAPP Research Network’s central clinical protocol, the Trans-MAPP Epidemiology, and Phenotyping Study | Urine | 40 female patients with IC/BPS, 40 age-matched healthy female controls | LC/MS | Six biomarkers were capable of discriminating between patients with IC/BPS and controls, including Etio-S, a sulfoconjugated 5-β-reduced isomer of testosterone. Etio-S was highly associated with IC/BPS, predicting IC/PBS with a specificity of 87.4% and sensitivity of 91.2% (p < 0.0001). | GPI, BPI |
| Smolenska et al. (2016) | RA | To explore differences in levels of amino acids and nicotinamide metabolites between patients with RA and controls and examine the effects of age, disease activity and duration, and treatment affect these levels. | Blood | 46 adults with RA, 19 healthy controls | LC/MS | Plasma concentrations of six metabolites (arginine, aspartic acid, glutamic acid, serine, phenylalanine, and threonine) were higher and of one (lysine) was lower in RA patients. VAS score was positively correlated with levels of four metabolites (alanine, arginine, proline, and serine) and negatively correlated with two (glutamic acid and histidine). | VAS |
| Zielman et al. (2016) | Mig | To identify differences in biochemical characteristics of different subtypes of Mig (i.e., Mig with aura, Mig without aura, and hemiplegic Mig) | CSF | 91 patients with Mig (19hemiplegic, 39 with aura, and 33 without aura), 45 healthy controls | 1H NMR | 2 metabolites, 2-hydroxybutyrate and 2-hydroxyisovalerate, discriminated between patients with hemiplegic Mig and healthy controls. | None |
| Malatji et al. (2017) | FM | To identify biomarkers for objective diagnosis of FM | Urine | 18 women with FM, 41controls (11 1st-degree relative of subjects, 10 unrelated and age matched, 20 randomly selected, young, healthy subjects) | 1H NMR | Of the 21 endogenous and exogenous metabolites identified and quantified, taurine, TMAO, and succinic acid were significantly increased in patients with FM compared with controls. | FIQR |
| Mantyselka et al. (2017) | MSP | To examine whether ornithine levels differ among subjects with persistent musculoskeletal pain compared with other subjects in the population. | Blood | 76 patients with PP, 221 with NPP, and 61 no-pain controls | UHLC, LC/MS | Ornithine levels were significantly elevated in men and women with PP compared to those with NPP and no-pain controls. The concentration of ornithine was highest in women with PP. Differences in citrulline levels trended toward significance in women, with higher levels in those with PP compared to no-pain controls and those with NPP (lowest levels). | None |
| Shin et al. (2017) | Mig | To identify metabolites that could be used as potential biomarkers for the diagnosis of Mig | Serum | 11 patients with Mig and 9 controls | NMR | Compared with controls, patients with Mig had significantly lower levels of 2-hydroxybutyrate, phenylalanine, isocitrate and citrate were significantly lower, and significantly higher levels of lactate and valine. These 5 metabolites may have diagnostic power for Mig. Model validation demonstrated good diagnostic accuracy with an area under the receiver operating characteristic (ROC) curve of 94.1%. | None |
| Xu et al. (2017) | OA | To explore potential mechanisms of osteophyte formation by detecting metabolic variations between extracts of osteophyte cartilage tissues and uninvolved control cartilage tissues | Cartilage | Cartilage from osteophytes in 32 patients with OA and cartilage from lateral posterior femoral condyle in 34 controls without OA | UHLC-MS/MS | 28 endogenous metabolite variations including amino acids (phenylalanine, proline, arginine, and leucine), sulfonic acids (taurine and hypotaurine) glycerophospholipids (phosphatidyl choline and phosphatidyl ethanolamine), and fatty acids (vaccenyl carnitine and muconic acid) differentiated patients in the osteophyte group from those in the control group. Among these metabolites, 25 were elevated and 3 were decreased in the osteophyte group. Pathway analysis revealed that phenylalanine, proline, arginine, taurine, and hypotaurine metabolism were associated with osteophyte formation. | None |
| Livshits et al. (2018) | MSP | To use omics approaches to identify molecular genetic factors underlying the heritability of frailty and its genetic correlation with CWP | Blood | 3626 female twins | Multi-omics | Genomic regions involved in neurological pathways were associated with frailty and its covariation with CWP. After correction for multiple testing, FI score was associated with 51 metabolites, including amino acids (glutamate, proline, and N-acetyl glycine), carbohydrates (mannose), and lipids (glycerol, 2-linoleoylglycero-phosphocholine, and epiandrosterone sulphate). Also, levels of epiandrosterone sulphate and uridine were significantly associated with CWP. | None |
| Trbojevic-Akmacic et al. (2018) | CLBP | To identify changes in total plasma N-glycosylation pattern connected with CLBP to provide potential insight into the pathogenic mechanisms of the disease | Blood | 1128 patients with CLBP, 760 healthy controls | HILIC/UHLC | Changes in plasma N-glycome level in patients with CLBP compared to controls were consistent with changes observed in N-linked glycosylation, which are usually in chronic inflammation. Among patients with CLBP, chromatographic peaks corresponding to high-branching glycans increased and those corresponding to low-branching glycans decreased compared with controls. | None |
| Hackshaw et al. (2019) | FM | To develop simple, rapid, sensitive, robust methods for diagnosis of FM based on highly characteristic mid-IR and Raman “fingerprint” from peripheral blood samples combined with supervised pattern recognition techniques |
Blood | 30 patients with FM, 20 patients with RA, 23 patients with SLE, 19 patients with OA | Vibrational (IR and Raman) spectroscopy, UHLC | FT-IR spectral data dominated by strong vibration modes for water, glucose, polysaccharides, lipids and proteins correlated with pain intensity in FM (log-transformed FIQR scores), while the Raman spectral data dominated by vibration modes for aromatic amino acids, glycans, collagens and minerals correlated with disease severity in FM (log-transformed FIQR scores). Findings show that vibrational spectroscopy can distinguish among FM, RA, and SLE. UHLC findings distinguished FM from RA and SLE, however, known compounds or fragments were not identified. | MPI |
| Malatji et al. (2019) | FM | To perform an exploratory metabolomics study to (1) elucidate the global urinary metabolite profile of patients with FM, and (2) explore the potential of metabolite biomarkers for diagnosis of FM | Urine | 18 women with FMS, 42 controls (11 1st-degree relatives of subjects, 10 unrelated, age-matched women, 20 randomly selected, young, healthy controls) | 1H NMR | Levels of 14 metabolites (sorbose, phosphoric acid, glutaric acid, threonic acid, tagatose, oxalic acid, erythropentonic acid, rhamnose, arabinose, 4-BHA, 2,3,4-trihydroxybutyl-L, 2-keto-1-gluconic acid, 2-D-3,5-DHPL, and 3-D-ribohexonic acid) were significantly increased in the urine of patients with FM compared with controls. | FIQ, IHCQ |
| Menzies et al. (2020) | FM | To identify differences in levels of plasma metabolites between women with and without FM | Blood | 20 women with FM, 20 age-matched healthy controls | LC/MS | 1,462 known metabolites and unknown spectral features differed significantly between groups. PCA of the known metabolites revealed 71 significant metabolites with FC > 2 (n = 48) or FC < 0.5 (n = 23). Significant metabolites are involved in energy, lipid, and amino acid metabolism. | None |
| Onderwater et al. (2019) | Mig | To identify a plasma metabolomic signature for Mig | Blood | 2800 patients with Mig, 7353 healthy controls | 1H NMR | Levels of apoA1 and the ratio of free cholesterol to HDL were decreased in Mig patients compared with controls. Overall, apoA1 levels were lower in males than females, and global analysis showed a general decrease in HDL-associated metabolites in females with Mig compared with males with Mig. Males with Mig had decreased levels of omega-3 fatty acids, compared with male controls. | None |
Note. 1H NMR = proton nuclear magnetic resonance; apoA1 = apolipoprotein A1; BMI = body mass index; BPI = Brief Pain Inventory; BPS = bladder pain syndrome; CLBP = chronic low-back pain; CP = chronic prostatitis; CPPS = chronic pelvic pain syndrome; CSF = cerebrospinal fluid; CWP = chronic widespread pain; Etio-S = etiocholan3α-ol-17-one sulfate; FC = fold-change; FI = Frailty Index; FIQR = Fibromyalgia Impact Questionnaire; FT-IR = Fourier transform infrared; FM = fibromyalgia; GC = gas chromatography; GPI = Genitourinary Pain Index; HDL = high-density lipoprotein cholesterol; HILIC = hydrophilic interaction liquid chromatography; IC = interstitial cystitis; IHCQ = in-house clinical questionnaire; IR = infrared vibrational spectroscopy; LC = liquid chromatography; MAPP = Multidisciplinary Approach to the Study of Chronic Pelvic Pain; Mig = migraine; MPI = McGill Pain Index; MS = mass spectrometry; MSP = musculoskeletal pain; NC = nociceptive pain; NMR = nuclear magnetic resonance; NP = neuropathic pain; NPP/PP = nonpersistent pain/persistent pain; OA = osteoarthritis; PCA = principal component analysis; RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; TMAO = trimethylamine N-oxide; TOF-MS = time-of-flight mass spectrometry; UHLC = ultrahigh liquid chromatography; VAS = visual analog scale.
The studies varied in the type of samples used to assess metabolite levels. Many reported levels of metabolites in blood samples (n = 10). Of the studies that investigated metabolite levels in individuals with fibromyalgia, two used urine samples (Malatji et al., 2017, 2019) and three used blood samples (Caboni et al., 2014; Hackshaw et al., 2019; Menzies et al., 2020). The studies that used urine samples came from the same lab and research participants. Using blood (Onderwater et al., 2019; Shin et al., 2017) and cerebrospinal fluid (Zielman et al., 2016), several investigators examined the metabolites associated with migraine (). Other investigators targeted metabolomic differences in the synovial tissue (Adams et al., 2012; De Jong et al., 2016) or fluid (Mickiewicz et al., 2015) of patients with osteoarthritis.
Metabolomic Approach
Researchers used various metabolomics approaches to characterize the metabolites associated with chronic pain conditions, including proton nuclear magnetic resonance (1H NMR), liquid chromatography (LC), ultra-high-performance liquid chromatography (UPLC), gas chromatography in tandem with mass spectrometry (GC/MS), liquid chromatography in tandem with mass spectrometry (LC/MS), tandem mass spectrometry (MS/MS), and vibrational (infrared [IR] and Raman) spectroscopy. The majority of the reviewed studies utilized LC (n = 10), MS (n = 9), or 1H NMR (n = 7) to determine metabolite types and levels. To increase the quality of the assessment, researchers in one study coupled GC/MS with 1H NMR. Table 2 summarizes the metabolomic approaches used in the reviewed studies.
Table 2.
Summary of Metabolomic Approaches Used in Reviewed Studies by Chronic Pain Condition.
| Chronic Pain Condition | 1H NMR | UHLC/LC | GC | MS |
|---|---|---|---|---|
| Fibromyalgia* | 2 | 3 | 1 | 2 |
| Migraine | 3 | |||
| OA | 1 | 3 | 2 | 4 |
| RA | 1 | 1 | ||
| Chronic low-back pain | 1 | |||
| Nociceptive and neuropathic pain | 1 | |||
| IC/BPS and CP/CPPS | 1 | 1 | ||
| MSP | 1 | 1 |
Note. Most studies combined approaches, e.g. LC/MS, GC/MS, UHLC and MS/MS.
1H NMR = proton nuclear magnetic resonance; BPS = bladder pain syndrome; CP = chronic prostatitis; CPPS = chronic pelvic pain syndrome; GC = gas chromatography; IC = interstitial cystitis; LC = liquid chromatography; MS = mass spectrometry; OA = osteoarthritis; RA = rheumatoid arthritis; UHLC = ultra-high-performance liquid chromatography.
*One study used vibrational spectroscopy. Some studies used multi-omics approaches.
Chronic Pain Conditions
The reviewed studies investigated metabolite types and concentrations in patients with various types of chronic pain conditions, including neuropathic/nociceptive pain (n = 1), musculoskeletal pain (n = 2), fibromyalgia (n = 5), migraine (n = 3), rheumatoid arthritis (n = 1) and osteoarthritis (n = 4), chronic low-back pain (CLBP; n = 1), and interstitial cystitis/bladder pain syndrome (n = 1). While all studies compared metabolite levels in individuals with chronic pain conditions against levels in healthy controls, three also compared metabolite levels between first-degree relatives (Malatji et al., 2017, 2019), including one twin study (Livshits et al., 2018).
Fibromyalgia and Musculoskeletal Pain
In the reviewed studies, researchers identified more than 1500 metabolites that were associated with musculoskeletal pain or fibromyalgia. Mantyselka et al. (2017) reported that musculoskeletal pain was associated with elevated levels of ornithine, while Livshits et al. (2018) found associations among chronic widespread musculoskeletal pain, the Frailty Index (FI), and levels of epiandrosterone sulfate (EAS). Specifically, these researchers found that ornithine levels increased with persistent pain, while circulating levels of EAS decreased as chronic widespread pain scores increased (Livshits et al., 2018; Mantyselka et al., 2017). In other studies, researchers reported that fibromyalgia was associated with increased levels of lysophosphocholines (lysoPCs), phosphocholines, and ceramides (Caboni et al., 2014); altered brain function via microbiota in the gut–brain axis and levels of succinic acid, taurine, and creatinine (Malatji et al., 2017, 2019). These findings suggest that the intermediate metabolites of carbohydrates, proteins, and lipid pathways may be involved in the pathogenesis of fibromyalgia. Menzies et al. (2020) reported that 1,462 known metabolites and unknown spectral features significantly distinguished women with fibromyalgia from age-matched healthy controls. Their findings corroborated the involvement of energy metabolism pathways and increased oxidative stress, tryptophan degradation, and inflammation among individuals with fibromyalgia (Menzies et al., 2020). Using vibrational spectroscopy, Hackshaw et al. (2019) found that protein backbones and pyridine-carboxylic acids might serve as potential biomarkers to discriminate fibromyalgia from osteoarthritis, rheumatoid arthritis, and systemic lupus erythematosus (SLE).
Osteoarthritis
The reviewed studies reported that at least 50 metabolites differed significantly between individuals with osteoarthritis and healthy controls. Of the four studies that investigated metabolomics in individuals with osteoarthritis, one sampled synovial fluid (Mickiewicz et al., 2015) and three sampled cartilage (Adams et al., 2012; De Jong et al., 2016; Xu et al., 2017). Researchers reported that the metabolites that were increased in individuals with osteoarthritis include breakdown products of collagen (propyl-hydroxyproline), carbohydrates (succinate, malate), proteins (acetyl-carnitine, glutamine, phenylalanine, branched-chain amino acid catabolism, 4-methoxy-2-oxopentanoate, 3-methyl-2-oxobutyrate), and lipids (sulfonic acids, glycerophospholipids, and fatty acids), among others (Adams et al., 2012; Xu et al., 2017). Other researchers identified biomarkers that were decreased in samples from participants with osteoarthritis, including O-acetyl-carnitine, N-phenylacetyl-glycine, methionine, ethanol, ethanolamine, creatine, 3-hydroxybutyrate, malate, and hexanoylcarnitine (Mickiewicz et al., 2015). De Jong et al. (2016) characterized the immune cells present in the synovial tissue and reported that osteoarthritis was associated with fatty-acid release, T-lymphocyte CD4+ proliferation, and higher body mass index (BMI).
Rheumatoid arthritis and chronic low-back pain
Two of the reviewed studies examined the arthritis-related conditions of rheumatoid arthritis and CLBP using metabolomics approaches. Smolenska et al. (2016) reported that rheumatoid arthritis was associated with increased levels of six amino acid metabolites (aspartic acid, glutamic acid, threonine, phenylalanine, histidine, and tryptophan) and decreased levels of one amino acid (lysine). Among individuals with RA, amino acid levels correlated with the swollen-joint count, joint-pain count, and Disease Activity Score 28 (DAS28; Smolenska et al., 2016). Trbojevic-Akmacic et al. (2018) reported that a significant increase in high-branched N-glycan structures and high levels of mannose and glycans containing N-acetylglucosamine were associated with decreased CLBP. The metabolite changes authors reported in this multinational study are consistent with those previously reported in chronic inflammation (Arnold et al., 2008; Mackiewicz & Mackiewicz, 1995).
Migraine
Researchers identified at least eight metabolites across four studies that were associated with the presence of migraine. Zielman et al. (2016) reported that the levels of two metabolites (2-hydroxybutyrate and 2-hydroxyisovalerate) in cerebrospinal fluid significantly discriminated between people with migraine from healthy controls. Their findings suggest that individuals with hemiplegic migraines have lower levels of 2-hydroxybutyrate and higher levels of 2-hydroxyisovalerate than healthy controls. In addition to 2-hydroxybutyrate, Shin et al. (2017) also found lower levels of phenylalanine, isocitrate, and citrate in the blood samples of individuals with migraines compared to those healthy controls. They also reported higher levels of lactate and valine in the blood samples from those with migraines compared to healthy controls.
In addition, researchers in a large cohort study found a significant relationship between altered lipid metabolism and migraine (Onderwater et al., 2019). They reported that a diagnosis of migraine was associated with decreased levels of apolipoprotein A1 and high-density lipoprotein and ratio of free cholesterol to total lipids, and levels. Onderwater et al. (2019) also identified a sex-specific association between dyslipidemia and migraine, with males with migraine having significantly decreased levels of omega-3 fatty acids compared to healthy male controls. Authors did not observe this association in females.
Other pain conditions
Other researchers studied metabolite differences in neuropathic and nociceptive pain and interstitial cystitis/bladder pain syndrome/chronic pelvic pain syndrome/chronic prostatitis. Urine profiles of metabolites such as choline, phosphocholine, taurine, and alanine discriminated between patients with neuropathic pain and those with nociceptive pain (Finco et al., 2016). Similarly, Parker et al. (2016) reported that six metabolites were capable of discriminating between patients with interstitial cystitis/bladder pain syndrome and age-matched controls in a longitudinal study. Of these, etiocholan-3-α-ol-17-one sulfate (Etio-S), a sulfoconjugated 5-β-reduced isomer of testosterone, was significantly associated with interstitial cystitis/bladder pain syndrome. Specifically, researchers reported that Etio-S levels increased with the severity of symptoms (pelvic pain and number of painful body sites) that persisted for 3–6 months.
Methods of Pain Assessment
Only seven of the studies reported on methods used to assess pain. Of these, three used pain subscales measuring intensity, frequency, and duration (Malatji et al., 2017, 2019; Parker et al., 2016); two used a visual analog scale (VAS; De Jong et al., 2016; Smolenska et al., 2016); one assessed migraine type (Zielman et al., 2016); and one used self-reports of pain intensity and reports of medication use (Mantyselka et al., 2017). Besides the VAS, pain scores were derived from subscales in the Brief Pain Inventory (BPI), Genitourinary Pain Index (GPI), Fibromyalgia Impact Questionnaire (FIQ), and an in-house clinical questionnaire (INCQ; Hackshaw et al., 2019; Malatji et al., 2017, 2019). All of the studies that included measures to assess pain examined associations of clinical characteristics of pain with metabolomic features to reveal potential biomarkers for the diagnosis and management of chronic pain syndromes.
Discussion
Despite the increase in research over the past several years on the metabolomics of chronic pain conditions, few metabolites have been validated as biomarkers for pain management. Given the high prevalence of chronic pain, we are in need of metabolic biomarkers to predict, diagnose, manage, and monitor various chronic pain conditions (Gerra et al., 2017). In the present systematic review we summarized the current knowledge in the field of metabolomics in chronic pain conditions in humans. Preliminary evidence shows that individuals with various chronic pain conditions may have altered levels of the metabolites of one or more of four macromolecules whose metabolic states influence cellular processes and health status (Adams et al., 2012; Malatji et al., 2017, 2019; Mantyselka et al., 2017; Mickiewicz et al., 2015; Smolenska et al., 2016). These macromolecules include carbohydrate, lipids, proteins, and nucleic acids. Carbohydrates, lipids, and proteins are vital sources of energy produced through the citric acid cycle, oxidative phosphorylation, and the urea cycle pathways, respectively (Komoda & Matsunaga, 2015b).
Proteins
Several studies, including several included in the present review, have reported alterations in the levels of the breakdown products of proteins, such as amino acids and metabolites of amino acids. Smolenska et al. (2016) identified six amino acids (arginine, aspartic acid, glutamic acid, serine, phenylalanine, threonine) whose levels were higher and one (lysine) whose level was lower in patients with rheumatoid arthritis compared to healthy controls. The authors also reported significant correlations between scores on the pain visual analog scale and levels of four amino acids: alanine, arginine, proline, and serine showed positive correlations and glutamic acid and histidine negative. Other investigators have reported altered levels of amino acids among patients with osteoarthritis (Adams et al., 2012; Costello et al., 2020; Mickiewicz et al., 2015; Xu et al., 2017), migraine (Onderwater et al., 2019; Zielman et al., 2016), musculoskeletal pain (Livshits et al., 2018; Mantyselka et al., 2017), fibromyalgia (Hackshaw et al., 2019; Malatji et al., 2017, 2019; Menzies et al., 2020), and complex regional pain syndrome (Meissner et al., 2014). As fundamental building blocks of proteins such as neurotransmitters and enzymes, amino acids play essential roles in cellular structure and functions, including pain transmission (Komoda & Matsunaga, 2015b). For instance, research has identified associations between the amino acid glutamate in the glutaminergic system and pain transmission and management (Inquimbert et al., 2018; Medeiros et al., 2019). Additional studies are needed to examine and validate various amino acids as potential biomarkers of chronic pain conditions.
Carbohydrates
Research has also shown alterations in the metabolites of carbohydrate to be associated with chronic pain conditions. Among patients with osteoarthritis, Mickiewicz et al. (2015) reported an increase in primary intermediates of the citric acid cycle (e.g., succinate, fructose, citrate). Researchers also reported alterations in the levels of intermediates of carbohydrate metabolism in patients with several chronic pain conditions (Malatji et al., 2017, 2019; Meissner et al., 2014; Menzies et al., 2020; Onderwater et al., 2019; Zielman et al., 2016). It is unclear, however, whether the changes in these metabolites result in pain or the body’s response to pain results in the metabolite alterations. While additional studies are needed to explicate the relationship between carbohydrate metabolites and chronic pain conditions, evidence does show that altered levels of many intermediates of carbohydrate metabolism result in energy impairment (Giorgi-Coll et al., 2017; Quansah et al., 2018).
Lipids
Researchers reported an association between chronic pain conditions and metabolites of lipid metabolism pathways, suggesting that altered lipid metabolism may play a role in the etiology of chronic pain conditions (Adams et al., 2012; De Jong et al., 2016; Onderwater et al., 2019; Parker et al., 2016; Xu et al., 2017). Lipids play essential roles in neuronal transmission and energy storage. Regarding neuronal (nociceptive or pain) transmission, lipids are principal components of cell membranes and myelin sheaths, which influence the speed of impulse transmission (Komoda & Matsunaga, 2015a). Thus the reported increase in phosphatidylcholine levels in individuals with osteoarthritis may play a role in pain transmission and perception (Costello et al., 2020).
Excess body fat may play a role in weight-bearing and non-weight-bearing chronic pain conditions. De Jong et al.’s (2016) findings that fatty acids enhance the proliferation of CD4+ T cells whose levels correlate with pain intensity in patients with osteoarthritis are consistent with those of other studies, all of which have demonstrated a positive relationship between body weight and chronic pain conditions, including migraine, osteoarthritis, and CLBP (Chin et al., 2019). The reported relationship between lipid dysfunction and migraine status suggests that the BMI–pain link may be independent of the effect of weight on joints and the axial skeleton (Onderwater et al., 2019). Regarding chronic pain associated with weight bearing, Adams et al. (2012) reported that metabolites that distinguished end-stage knee osteoarthritis from early/no osteoarthritis included acetylcarnitine and myo-inositol, which are intermediate metabolites in the beta-oxidation of lipids. Other researchers have reported that levels of plasma acetylcarnitine and myo-inositol were correlated with reduced mitochondrial beta-oxidation function and high-sucrose-induced accumulation of triglycerides in the livers of rats, respectively (Bjørndal et al., 2018; Shimada et al., 2017). Further studies are needed to clarify the interrelationship between body weight and chronic pain conditions.
Limitations
This systematic review has several limitations. First, the studies did not all use the same metabolomic approach, which hindered our ability to combine and synthesize findings from various studies by examining overlapping metabolites. Second, studies were limited to articles published in the English language. As a result, impactful studies published in other languages are not included. Finally, we counted different articles from the same sample (by the same investigators) as individual papers. This may have artificially inflated the number of papers included in the systematic review. We recommend that future systematic reviews should include meta-analysis to quantify the combined effect of different studies.
Implications for Nursing Research
This systematic review has brought to light gaps in knowledge that have significant implications for nurses. First, though most of the studies did identify metabolites that are associated with pain conditions, many did not include an examination of pain as a symptom. There is a need for research studies that investigate the biological bases of pain characteristics such as pain severity, interference, and sensitivity. Nurse scientists are well positioned to design studies using targeted and untargeted metabolomics methods to examine the underlying mechanisms of the characteristics of pain in chronic pain conditions.
Given that metabolites have the potential to serve as biomarkers, more research on the metabolomics of chronic pain conditions, including large-scale and replication studies, is essential. Future studies should emphasize strategies to enhance the validity and reproducibility of findings to facilitate translation into clinical practice. Methodological rigor such as accurate and consistent phenotyping across studies is essential during the planning and implementation phases. Since small sample sizes can result in inconsistent results, researchers should consider utilizing one of several recommended approaches for estimating the sample size necessary for a metabolomics study, such as data-driven sample size determination (DSD) and MetSizeR (Billoir et al., 2015; Nyamundanda et al., 2013). Also, it is essential to examine correlations between invasive (e.g., blood, synovial fluid, and cerebrospinal fluid) and noninvasive (e.g., saliva and urine) specimens. Establishing relationships between different specimens is essential because specimen heterogeneity can result in inconsistent samples (Smith et al., 2020).
Conclusion
Alterations in the intermediate metabolites of carbohydrates, proteins, and other macromolecules are associated with chronic pain conditions such as fibromyalgia, osteoarthritis, and migraine. Unfortunately, many studies in the present review did not quantify the amount of pain experienced by participants. Further investigations are warranted to identify complete metabolomic profiles of various chronic pain conditions. Also, studies are needed to examine whether multiple metabolomic profiles correlate with pain outcomes such as pain severity and quality of life. These studies may lead to the identification of biomarkers and individualized strategies for the prevention, diagnosis, and management of chronic pain. Nurse scientists and other investigators should consider using standardized measurements to phenotype pain to facilitate comparisons across pain conditions and patient populations.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Edwin Aroke is supported by a National Institutes of Health/National Institute on Minority Health and Health Disparities (NIH/NIMHD) Administrative Supplement Grant 3R01MD010441-03S1. Keesha Roach is supported by the Integrative and Multidisciplinary Pain and Aging Research Training grant (T32AG049673), Pain Research and Intervention Center of Excellence (P30AG059297-01), and Center for Palliative Care Research & Education grant (U54CA233444).
ORCID iDs: Edwin N. Aroke  https://orcid.org/0000-0002-8355-0870
https://orcid.org/0000-0002-8355-0870
Keesha L. Powell-Roach  https://orcid.org/0000-0001-8117-3445
https://orcid.org/0000-0001-8117-3445
References
- Adams S. B., Setton L. A., Kensicki E., Bolognesi M. P., Toth A. P., Nettles D. L. (2012). Global metabolic profiling of human osteoarthritic synovium. Osteoarthritis and Cartilage, 20(1), 64–67. 10.1016/j.joca.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold J. N., Saldova R., Hamid U. M. A., Rudd P. M. (2008). Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics, 8(16), 3284–3293. 10.1002/pmic.200800163 [DOI] [PubMed] [Google Scholar]
- Billoir E., Navratil V., Blaise B. J. (2015). Sample size calculation in metabolic phenotyping studies. Briefings in Bioinformatics, 16(5), 813–819. 10.1093/bib/bbu052 [DOI] [PubMed] [Google Scholar]
- Bjørndal B., Alterås E. K., Lindquist C., Svardal A., Skorve J., Berge R. K. (2018). Associations between fatty acid oxidation, hepatic mitochondrial function, and plasma acylcarnitine levels in mice. Nutrition & Metabolism, 15(10). 10.1186/s12986-018-0241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboni P., Liori B., Kumar A., Santoru M. L., Asthana S., Pieroni E., Fais A., Era B., Cacace E., Ruggiero V., Atzori L. (2014). Metabolomics analysis and modeling suggest a lysophosphocholines-PAF receptor interaction in fibromyalgia. PLoS One, 9(9), e107626 10.1371/journal.pone.0107626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin S.-H., Huang W.-L., Akter S., Binks M. (2019). Obesity and pain: A systematic review. International Journal of Obesity, 44, 969–979. 10.1038/s41366-019-0505-y [DOI] [PubMed] [Google Scholar]
- Clish C. B. (2015). Metabolomics: An emerging but powerful tool for precision medicine. Molecular Case Studies, 1(1), a000588 10.1101/mcs.a000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello C. A., Hu T., Liu M., Zhang W., Furey A., Fan Z., Rahman P., Randell E. W., Zhai G. (2020). Metabolomics signature for non-responders to total joint replacement surgery in primary osteoarthritis patients: The Newfoundland Osteoarthritis Study. Journal of Orthopaedic Research, 38(4), 793–802. 10.1002/jor.24529 [DOI] [PubMed] [Google Scholar]
- De Jong A. J., De Lange-Brokaar B. J., Klein-Wieringa I. R., Heijink M., Hoekstra A., Everts B., Kwekkeboom J. C., Berkers C. R., Giera M., Huizinga T. W., Kloppenburg M., Toes R. E., Ioan-Facsinay A. (2016). Synovial CD4+T cells associate with pain in osteoarthritis: Is there a role for fatty acids? Osteoarthritis and Cartilage, 24, S321. [Google Scholar]
- Dinis-Oliveira R. J. (2019). Metabolism and metabolomics of opiates: A long way of forensic implications to unravel. Journal of Forensic and Legal Medicine, 61, 128–140. 10.1016/j.jflm.2018.12.005 [DOI] [PubMed] [Google Scholar]
- Fillingim R. (2015). Biopsychosocial contributions to sex differences in pain. Royal College of Obstetricians and Gynaecologists, 122(6), 769–769. 10.1111/1471-0528.13337 [DOI] [PubMed] [Google Scholar]
- Finco G., Locci E., Mura P., Massa R., Noto A., Musu M., Landoni G., d’Aloja E., De-Giorgio F., Scano P., Evangelista M. (2016). Can urine metabolomics be helpful in differentiating neuropathic and nociceptive pain? A proof-of-concept study. PLoS One, 11(3), e0150476 10.1371/journal.pone.0150476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley C. R., Chan D. S., Garrison S., Korownyk C., Kolber M. R., Campbell S., Eurich D. T., Lindblad A. J., Vandermeer B., Allan G. M. (2018). What are the most common conditions in primary care? Systematic review. Canadian Family Physician, 64(11), 832–840. [PMC free article] [PubMed] [Google Scholar]
- Forouzan A., Masoumi K., Rahim F., Moezzi M., Khavanin A., Ranjbari N., Amal Saki M., Fallah Amoli A., Akhiani N., Ghourchian F. (2018). Diagnostic accuracy of serum and urine S100A8/A9 and serum amyloid A in probable acute abdominal pain at emergency department. Disease Markers, 2018, 6457347 10.1155/2018/6457347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin D. J., Richard P. (2012). The economic costs of pain in the United States. Journal of Pain, 13(8), 715–724. 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Gerra M. C., Dagostino C., D’Agnelli S., Boggiani L., Rizza V., Marchesini M., Allegri M., Fanelli G. (2017). Omics as a potential tool to identify biomarkers and to clarify the mechanism of chronic pain development. Scandinavian Journal of Pain, 16(1), 187 10.1016/j.sjpain.2017.04.064 [DOI] [Google Scholar]
- Giorgi-Coll S., Amaral A. I., Hutchinson P. J. A., Kotter M. R., Carpenter K. L. H. (2017). Succinate supplementation improves metabolic performance of mixed glial cell cultures with mitochondrial dysfunction. Scientific Reports, 7(1), 1003 10.1038/s41598-017-01149-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch-Ferré M., Hruby A., Toledo E., Clish C. B., Martínez-González M. A., Salas-Salvadó J., Hu F. B. (2016). Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care, 39(5), 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw K. V., Aykas D. P., Sigurdson G. T., Plans M., Madiai F., Yu L., Buffington C. A. T., Giusti M. M., Rodriguez-Saona L. (2019). Metabolic fingerprinting for diagnosis of fibromyalgia and other rheumatologic disorders. Journal of Biological Chemistry, 294(7), 2555–2568. 10.1074/jbc.RA118.005816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inquimbert P., Moll M., Latremoliere A., Tong C. K., Whang J., Sheehan G. F., Smith B. M., Korb E., Athié M. C. P., Babaniyi O., Ghasemlou N., Yanagawa Y., Allis C. D., Hof P. R., Scholz J. (2018). NMDA receptor activation underlies the loss of spinal dorsal horn neurons and the transition to persistent pain after peripheral nerve injury. Cell Reports, 23(9), 2678–2689. 10.1016/j.celrep.2018.04.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. (2011). Relieving pain in America: A blueprint for transforming prevention, care, education, and research. National Academies Press; 10.17226/13172 [DOI] [PubMed] [Google Scholar]
- Komoda T., Matsunaga T. (2015. a). Constituents of the human body In Komoda T., Matsunaga T. (Eds.), Biochemistry for medical professionals (pp. 7–24). Academic Press; 10.1016/B978-0-12-801918-4.00003-7 [DOI] [Google Scholar]
- Komoda T., Matsunaga T. (2015. b). Metabolic pathways in the human body In Komoda T., Matsunaga T. (Eds.), Biochemistry for medical professionals (pp. 25–63). Academic Press; 10.1016/B978-0-12-801918-4.00004-9 [DOI] [Google Scholar]
- Livshits G., Malkin I., Bowyer R. C. E., Verdi S., Bell J. T., Menni C., Williams F. M. K., Steves C. J. (2018). Multi-OMICS analyses of frailty and chronic widespread musculoskeletal pain suggest involvement of shared neurological pathways. Pain, 159(12), 2565–2572. 10.1097/j.pain.0000000000001364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K., Krishnan A., Cervenka E., Hu G., Guadagno E., Trakadis Y. (2019). Biomarkers for major depressive and bipolar disorders using metabolomics: A systematic review. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 180(2), 122–137. 10.1002/ajmg.b.32680 [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Mackiewicz K. (1995). Glycoforms of serum α1-acid glycoprotein as markers of inflammation and cancer. Glycoconjugate Journal, 12(3), 241–247. 10.1007/bf00731326 [DOI] [PubMed] [Google Scholar]
- Malatji B. G., Mason S., Mienie L. J., Wevers R. A., Meyer H., van Reenen M., Reinecke C. J. (2019). The GC–MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics, 15 10.1007/s11306-019-1513-6 [DOI] [PubMed] [Google Scholar]
- Malatji B. G., Meyer H., Mason S., Engelke U. F. H., Wevers R. A., Reenen M., Reinecke C. J. (2017). A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurology, 17 10.1186/s12883-017-0863-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyselka P., Ali-Sisto T., Kautiainen H., Niskanen L., Viinamaki H., Velagapudi V., Lehto S. M. (2017). The association between musculoskeletal pain and circulating ornithine: A population-based study. Pain Medicine, 18(6), 1145–1151. 10.1093/pm/pnw285 [DOI] [PubMed] [Google Scholar]
- Medeiros P., Negrini-Ferrari S. E., Palazzo E., Maione S., Ferreira S. H., de Freitas R. L., Coimbra N. C. (2019). N-methyl-D-aspartate receptors in the prelimbic cortex are critical for the maintenance of neuropathic pain. Neurochemistry Research, 44(9), 2068–2080. 10.1007/s11064-019-02843-z [DOI] [PubMed] [Google Scholar]
- Meissner A., van der Plas A. A., van Dasselaar N. T., Deelder A. M., van Hilten J. J., Mayboroda O. A. (2014). 1H-NMR metabolic profiling of cerebrospinal fluid in patients with complex regional pain syndrome-related dystonia. Pain, 155(1), 190–196. 10.1016/j.pain.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Menzies V., Starkweather A., Yao Y., Thacker L. R., Garrett T. J., Swift-Scanlan T., Kelly D. L., Patel P., Lyon D. E. (2020). Metabolomic differentials in women with and without fibromyalgia. Clinical and Translational Science, 13(1), 67–77. 10.1111/cts.12679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickiewicz B., Kelly J. J., Ludwig T. E., Weljie A. M., Wiley J. P., Schmidt T. A., Vogel H. J. (2015). Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. Journal of Orthopaedic Research, 33(11), 1631–1638. 10.1002/jor.22949 [DOI] [PubMed] [Google Scholar]
- Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L. A. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4, 1 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyamundanda G., Gormley I. C., Fan Y., Gallagher W. M., Brennan L. (2013). MetSizeR: Selecting the optimal sample size for metabolomic studies using an analysis based approach. BMC Bioinformatics, 14, 338–338. 10.1186/1471-2105-14-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderwater G. L. J. Ligthart L. Bot M. Demirkan A. Fu J. van der Kallen C. J. H. Vijfhuizen L. S. Pool R. Liu J. Vanmolkot F. H. M. Beekman M. Wen K.-X. Amin N. Thesing C. S. Pijpers J. A. Kies D. A. Zielman R. de Boer I. van Greevenbroek M. M. J…van den Maagdenberg A. M. J. M. (on behalf of the BBMRI Metabolomics Consortium). (2019). Large-scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology, 92(16), e1899–e1911. 10.1212/WNL.0000000000007313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. S., Crowley J. R., Stephens-Shields A. J., van Bokhoven A., Lucia M. S., Lai H. H., Andriole G. L., Hooton T. M., Mullins C., Henderson J. P. (2016). Urinary metabolomics identifies a molecular correlate of interstitial cystitis/bladder pain syndrome in a Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network cohort. EBioMedicine, 7, 167–174. 10.1016/j.ebiom.2016.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quansah E., Peelaerts W., Langston J. W., Simon D. K., Colca J., Brundin P. (2018). Targeting energy metabolism via the mitochondrial pyruvate carrier as a novel approach to attenuate neurodegeneration. Molecular Neurodegeneration, 13(1), 28 10.1186/s13024-018-0260-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M., Hibino M., Takeshita A. (2017). Dietary supplementation with myo-inositol reduces hepatic triglyceride accumulation and expression of both fructolytic and lipogenic genes in rats fed a high-fructose diet. Nutrition Research, 47, 21–27. 10.1016/j.nutres.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Shin D. J., Shin D. H., Kim H. (2017). Metabolic signatures for migraine using NMRbased metabolomics. Neurology, 88(16). https://n.neurology.org/content/88/16_Supplement/P2.151 [Google Scholar]
- Smith L., Villaret-Cazadamont J., Claus S. P., Canlet C., Guillou H., Cabaton N. J., Ellero-Simatos S. (2020). Important considerations for sample collection in metabolomics studies with a special focus on applications to liver functions. Metabolites, 10(3), 104 10.3390/metabo10030104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolenska Z., Smolenski R. T., Zdrojewski Z. (2016). Plasma concentrations of amino acid and nicotinamide metabolites in rheumatoid arthritis—potential biomarkers of disease activity and drug treatment. Biomarkers, 21(3), 218–224. 10.3109/1354750X.2015.1130746 [DOI] [PubMed] [Google Scholar]
- Trbojevic-Akmacic I. Vuckovic F. Vilaj M. Skelin A. Karssen L. C. Kristic J. Juric J. Momcilovic A. Simunovic J. Mangino M. De Gregori M. Marchesini M. Dagostino C. Stambuk J. Novokmet M. Rauck R. Aulchenko Y. S. Primorac D. Kapural L.…Lauc G (2018). Plasma N-glycome composition associates with chronic low back pain. Biochimica et Biophysica Acta (BBA) - General Subjects, 1862(10), 2124–2133. 10.1016/j.bbagen.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Wei R. (2011). Metabolomics and its practical value in pharmaceutical industry. Current Drug Metabolism, 12(4), 345–358. 10.2174/138920011795202947 [DOI] [PubMed] [Google Scholar]
- Xu Z., Chen T., Luo J., Ding S., Gao S., Zhang J. (2017). Cartilaginous metabolomic study reveals potential mechanisms of osteophyte formation in osteoarthritis. Journal of Proteome Research, 16(4), 1425–1435. 10.1021/acs.jproteome.6b00676 [DOI] [PubMed] [Google Scholar]
- Zielman R., Postma R., Bakels F., Van Oosterhout W. P. J., Van Der Sar S. A., Terwindt G. M., Van Der Maagdenberg A. M. J. M., Deelder A. M., Mayboroda O. A., Meissner A., Ferrar M. D. (2013). Metabolomics of migraine: H-NMR study of cerebrospinal fluid [Conference Abstract]. Cephalalgia, 33, 150–151. 10.1177/0333102413490487 [DOI] [Google Scholar]
- Zielman R., Postma R., Verhoeven A., Bakels F., van Oosterhout W. P., Meissner A., van den Maagdenberg A. M., Terwindt G. M., Mayboroda O. A., Ferrari M. D. (2016). Metabolomic changes in CSF of migraine patients measured with 1H-NMR spectroscopy. Molecular BioSystems, 12(12), 3674–3682. 10.1039/c6mb00424e [DOI] [PubMed] [Google Scholar]