Abstract
Purpose
Retinoblastoma presents most commonly as advanced unilateral disease, particularly in developing countries for which primary enucleation has been the preferred method of treatment. However, with the evolution of newer treatment modalities including intravitreal chemotherapy, intra-arterial chemotherapy and newer chemotherapeutic combinations, a trend towards more conservative approaches is being observed. Our aim is to evaluate outcomes of group D eyes following conservative and non-conservative treatment options.
Patients and Methods
The ocular oncology database was used to identify eyes with unilateral retinoblastoma that fulfilled the International Intraocular Retinoblastoma Classification (IIRC) group D criteria from August 2010 to August 2018 and these were retrospectively reviewed. Overall, 39 eyes were identified.
Results
Nineteen (49%) eyes underwent primary enucleation and 20 (51%) received eye-conserving treatment. Eye salvage was possible in 15 (75%) eyes in the attempted salvage group. None of the patient revealed signs of metastasis. All eyes received conventional chemotherapy (carboplatin, vincristine, etoposide) and focal laser therapy. Additional treatment modalities offered included intravitreal chemotherapy, intra-arterial chemotherapy and topotecan. Three (11%) eyes in the primary enucleation group showed high-risk features on histopathology and none developed metastasis.
Conclusion
The results of the study seem promising and conservative measures can be adopted in selected unilateral group D eyes.
Keywords: ocular oncology, chemotherapy, enucleation, eye-conserving treatment
Introduction
Retinoblastoma accounts for about 2% of all pediatric cancers1 and 90% of all pediatric ocular cancers.2 Most cases are advanced (group D or E) at presentation. Contrary to most pediatric cancers, treatment modality often involves enucleation therefore treatment options focus not only on the overall survival but also on globe survival where possible. This is what makes retinoblastoma a bigger challenge.
With the advent of targeted chemotherapeutic regimes, the treatment of advanced retinoblastoma has greatly evolved with the intent of globe salvage, however, there is no universally adopted guideline to meet the challenges of such cases.3 Approach to such eyes is highly customized based on the status of the patient, tumor stage expertise of the ocular oncologist and the available treatment options in a particular setup.4,5,35
Despite the evolution of new treatment modalities, the management of unilateral group D retinoblastoma has led to several controversies. Firstly, treatment is not free of side effects (systemic and local), and these side effects may not be justified if the visual prognosis for the treated eye is poor, while the patient has a normal contralateral eye. Secondly, the discrepancy in the classification systems used by different groups,6 cause lack of uniformity in the data available in literature. Also, there is diversity among retinoblastoma specialists in experimenting with various treatment options,7 especially intra-arterial chemotherapy. Moreover, despite the potential for globe salvage, the risk of metastatic spread cannot be overruled.
At our setup at King Hussain cancer center (KHCC), we have also evolved and implemented various strategies to improve ocular survival for advanced cases of retinoblastoma.
The aim of this study is to analyze the outcome of group D eyes and the impact of the available treatment options for unilateral group D retinoblastoma.
Patients and Methods
This study was approved by the Institutional EthicalReview Board at KHCC. It was a retrospective review ofthe retinoblastoma database which complied with relevant data protection and privacy regulations. Data included each patient’s demographics, tumorlaterality, age at diagnosis, IIRC group, type of seeds(vitreous/subretinal), presence of retinal detachment, presence of tumor touching the lens, primary treatment, type of treatments offered, systemic and/or local chemotherapy, type of focal therapy (laser or cryotherapy), type of radiation therapy (plaque/EBRT), complications, eye salvage,mortality, histopathology features where applicable, any evidence of metastases and secondary malignancies. The main outcome measures were globe salvage and event-free survival. All parents underwent detailed counselling, and were provided with conservative and non-conservative treatment options and their possible outcomes. All patients from August 2010 to August 2018 who were clinically diagnosed with unilateral group D intraocular retinoblastomabased on the findings of indirect ophthalmoscopy and Retcam images were filtered according to the inclusion and exclusion criteria.
Inclusion Criteria
Patients with unilateral intraocular retinoblastoma, whomet the International Intraocular RetinoblastomaClassification (IIRC) criteria for group D8 tumor (Table 1) and had a minimum follow-up of 1 year, were included in the study.
Table 1.
International Intraocular Retinoblastoma Classification (IIRC)
| Group A (very low-risk) | Small (<3mm) Discrete tumor at least 3 mm from foveola and 1.5 mm from optic nerve |
| GROUP B (low-risk) | Eyes with no vitreous or subretinal seeding and discrete retinal tumor of any size and location |
| GROUP C (moderate risk) | Eyes with only focal vitreous or subretinal seeding and discrete retinal tumor of any size and location. Minimal subretinal fluid (<1 quadrant) |
| GROUP D (high-risk) | Eyes with diffuse subretinal vitreous or subretinal seeding and/or massive non-discrete endophytic or exophytic disease. Eyes with more extensive seeding than Group C. Massive and/or diffuse intraocular disseminated disease may consist of fine or greasy vitreous seeding or avascular masses. Subretinal seeding may be plaque like. Included exophytic disease and more than one quadrant of retinal detachment |
| GROUP E (very high-risk) | Tumor touching the lens. Tumor anterior to anterior vitreous face. Diffuse infiltrating retinoblastoma. Irreversible neovascular glaucoma. Massive intraocular hemorrhage. Aseptic orbital cellulitis. Tumor necrosis. Phthisis bulbi or pre-phthisis |
Notes: Reproduced from Fabian ID, Reddy A, Sagoo MS. Classification and staging of retinoblastoma. Community Eye Health. 2018;31(101):11-13.8
Exclusion Criteria
All patients with bilateral retinoblastoma, treated elsewhere,had less than 1 year follow -up, or did not fulfillthe criteria of IIRC group D were excluded from the study (Table 1).
Clinical Characteristics and Definitions
Tumors in IIRC group D (Table 1) were defined as eyes with diffuse vitreous or subretinal seeding and/or massive non-discrete endophytic or exophytic disease or exudative retinal detachment involving more than one quadrant of the retina. According to the 8th edition TNM staging all group D eyes are cT2b eyes.36
Group D was further classified into four categories. (1) Eyes with predominantly subretinal seeds; (2) Eyes with predominantly vitreous seeds; (3) Eyes with >2 quadrants retinal detachment; (4) Eyes with tumor occupying >50% of the globe. Globe salvage was defined as the absence of tumor activity or recurrence, and absence of active vitreous or subretinal seeds after a minimum of 1 year follow-up visit with no evidence of metastasis without the need for enucleation or external beam radiation therapy (EBRT).
Event-free survival was defined as any patient who had undergone enucleation after attempt of globe salvage or presence of high-risk features on histopathology, or distant metastasis. High-risk features were defined as disease involving the anterior chamber, ciliary body, >3 mm of choroidal invasion, tumor invading the optic nerve, beyond the lamina cribrosa, scleral and extrascleral extension.
Treatment
All patients treated with the intent of globe salvage received at least 6–8 cycles of first-line chemotherapy (CVE protocol) 3–4 weeks apart that included carboplatin, etoposide, and vincristine (CVE). Three cycles of topotecan systemic chemotherapy was given in cases of partial reduction by CVE chemotherapy, not enough for focal therapy to fully consolidate the disease.
Examination under anesthesia (EUA) was repeated 3 weeks after each cycle of chemotherapy. Fundus images were taken with RetCam II (Clarity Medical System, Pleasanton, CA, USA) and indirect ophthalmoscopy with 360-degree indentation was performed.
Focal therapy was given using 810 diode or cryotherapy depending on the location of the tumor, and repeated with every EUA until tumor control was fully achieved.
Intravitreal melphalan was given for persistent vitreous seeding that did not respond to a full course of CVE chemotherapy. The injection dose was 20 mcg (0–12 months), 25 mcg (1–3 years) or 30 mcg (>3 years). The injection was repeated every 1–2 weeks and the number of injections depended upon the response.
Iodine125 plaque therapy was used in cases of localized recurrence of tumor activity where parents refused enucleation. Criteria for plaque therapy included active tumor, size limited to 15 mm, and where local therapy would be insufficient to achieve consolidation.
Intra-arterial chemotherapy (IAC) was used in cases of extensive subretinal seeding, or for massive tumor recurrence. Selective arterial injection of melphalan was administered every 3–4 weeks for a total of 3 injections. The dose was calculated according to body weight (0.35–0.42 mg/kg). The choice of further treatment type after 3 injections was left to the treating physician.
Statistical Analysis
All data were entered and analyzed using SPSS version 21.0. Patients were divided into three groups, i.e. primary enucleation group, attempted salvage group and treatment failure group, and were then analyzed for descriptive statistics.
Results
Forty-five eyes were identified, 4 were lost to follow-up, and 2 children presented with unilateral group D but developed lesions in the fellow eye, and were excluded. All partly treated patients referred from elsewhere were also excluded from the study.
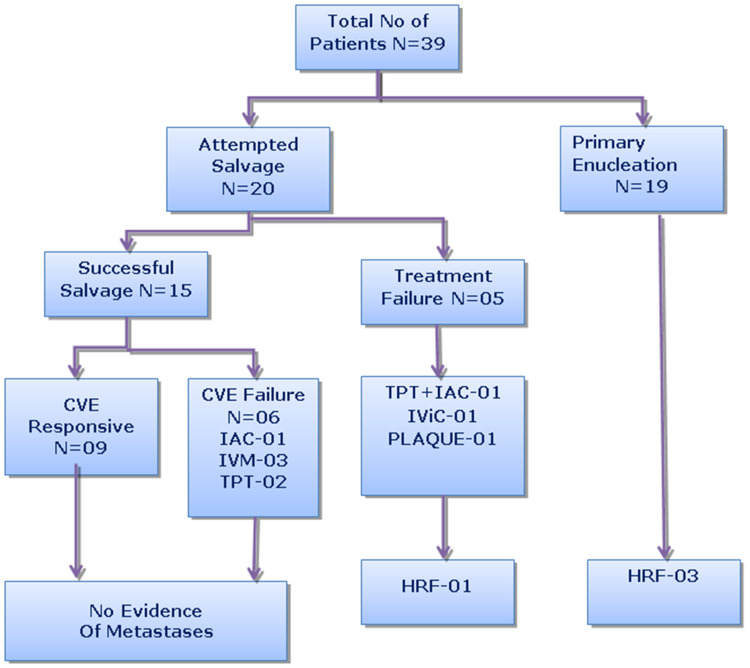
Thirty-nine eyes of 39 patients met the inclusion and exclusion criteria. Presenting features of IIRC group D eyes are summarized in Table 2. Overall mean age at presentation was 39 months (standard deviation 14.1). One (2%) of these patients had a positive family history. Leukocoria was the most common presenting sign in 28 (72%) patients followed by squint in 9 (23%) patients, and 2 (5%) patients presented with suspected decrease in vision. The affected eye was the right eye in 17 (44%) cases and the left in 22 (56%) cases. There were 21 males (54%) and 18 (46%) females. Management and treatment outcomes were categorized as follows (Figure 1).
Table 2.
Presenting Features of IIRC Group D Eyes (N=39)
| Predominant Tumor Feature | Eye Salvage Group N=15 | Treatment Failure Group N=05 | Primary Enucleation Group N=19 |
|---|---|---|---|
| Vitreous seeds | 07(47%) | 1(20%) | 2(10%) |
| Subretinal seeds | 4(27%) | 1(20%) | 3(16%) |
| Exudative RD | 4(27%) | 2(40%) | 2(11%) |
| Massive non-discrete tumor >50% of the globe | 0(0%) | 1(20%) | 12(63%) |
Abbreviations: IIRC, International Intraocular Retinoblastoma Classification; RD,retinal detachment.
Figure 1.
Summary of management and outcome for 39 patients with unilateral retinoblastoma.
Abbreviations: CVE, Carboplatin + Vincristine + Etoposide chemotherapy protocol; IAC, Intra-Arterial Chemotherapy; IVM, Intravitreal Melphalan; TPT, Topotecan chemotherapy; IViC, Intravitreal Chemotherapy; HRF, High-risk pathological features.
Primary Enucleation Group
Out of 39 eyes, primary enucleation was performed in 19 (49%) patients after detailed discussion with the family comparing pros and cons of conservative therapy vs enucleation. The average time span between diagnosis and surgery was 10 days (range 3–14 days). Three (11%) eyes showed high-risk features on histopathology. Two eyes had massive choroidal invasion and one had post-laminar invasion but not to the cut end. Both patients underwent adjuvant chemotherapy after enucleation, and no single case developed distant metastasis.
Attempted Salvage Group
Conservative treatment was offered for 20 (51%) eyes. Nine (45%) eyes were salvaged with CVE protocol combined with focal therapy. Three (15%) eyes received an additional 3 cycles of topotecan (2 for incomplete regression of tumor and 1 for recurrence of diffuse subretinal seeds), out of which 2 (10%) eyes were successfully salvaged. Overall salvage rate with systemic chemotherapy was possible in 11 (55%) eyes.
Intravitreal melphalan (IViC) was given in a total of 4 (20%) eyes that developed resistant active vitreous seeds out of which 3 were salvaged with an average number of 4 injections. Intra-arterial chemotherapy (IAC) was given to 2 patients, out of which 1 eye was salvaged. Summary of eyes that failed attempted salvage with combined chemotherapy and focal consolidation therapy is shown in Table 3.
Table 3.
Summary of Eyes That Failed Attempted Salvage with Combined Chemotherapy and Focal Consolidation Therapy (N=11)
| Feature | No. of Eyes | Treatment Modality | No. of Eyes Salvaged |
|---|---|---|---|
| Stage migration | 01(9%) | IAC (1) | 01(100%) |
| Incomplete response | 03(27%) | TPT (02), Plaque (01) | 01(33%) |
| Disease recurrence | 03(27%) | TPT (01), IAC (01) | 01(33%) |
| Resistant vitreous seeds | 04(36%) | IVM (04) | 03(75%) |
Abbreviations: IAC, intra-arterial chemotherapy; TPT, topotecan systemic chemotherapy; IVM, intravitreal melphalan injection.
Treatment Failure Group
Five (25%) eyes underwent secondary enucleation. Three eyes had incomplete tumor regression, 1 eye developed stage migration during chemotherapy, and 1 eye developed massive recurrence of subretinal seeds immediately after the last cycle of chemotherapy. In 2 cases the family decided to proceed for enucleation, and refused further treatment. Further treatment was offered in 3 (15%) eyes. One eye was treated with systemic topotecan for massive subretinal seeds and showed unfavorable response, thereafter eye salvage was attempted with 3 cycles of IAC but failed because of recurrence of subretinal seeds and development of new retinal tumors on follow-up at 6 weeks. One eye showed an initial incomplete response to treatment and subsequent progression of main tumor activity 6 weeks after completion of the CVE protocol. Radioactive iodine125 plaque therapy was carried out. Follow-up EUA showed tumor recurrence with massive recurrent subretinal seeds on subsequent follow-up, with further tumor progression. Repeat MRI showed possible extrascleral extension. Right enucleation was done, and the histopathology revealed a concomitant choroidal hemangioma at the base of the retinoblastoma that showed trans-scleral extension. No other high-risk features were present. One eye received 4 injections of intravitreal melphalan after 6 cycles of CVE due to persistent active vitreous seeds. This eye developed massive recurrence of diffuse subretinal and vitreous seeds and tumor activity at the edge of the main lesion 10 weeks after the last injection. The parents were given choice for IAC vs enucleation, and the latter was chosen. Only 1 eye in this group showed high-risk features on histopathology (massive choroidal invasion >3 mm) and underwent adjuvant chemotherapy.
Eye Salvage Group
Of the 39 eyes that were included in the study, the overall globe salvage rate was 39%. Among the eyes that were attempted to be salvaged, eye salvage inclusive of all treatment modalities used was possible in 15 of 20 eyes (75%). None of the patients revealed any evidence of metastasis.
Discussion
IIRC group D retinoblastoma includes a spectrum of different clinical features,8 but in summary refers to disseminated intraocular disease before any destructive event has occurred, and predicts high risk of treatment failure.9 Therefore enucleation has been a widely adopted treatment protocol for advanced unilateral cases,4,10 where further aggressive therapies may not be justified when the other eye is normal. However not all features of group D tumors carry the same prognosis,11 therefore physicians may justify conservative treatment in selected patients. Around two-thirds of retinoblastoma patients have unilateral disease12 and owing to lack of early detection by first-contact physicians,13 the disease is usually advanced at presentation. Despite the uncertainty of the treatment outcomes, most parents still opt for a trial to salvage the globe as the psychosocial impact of enucleation is distressing particularly in developing countries due to lack of social acceptance.
The evolution towards a more conservative approach in the management of unilateral eyes is clear in literature. Shields in 2002 advocated salvage of unilateral eyes but raised concerns regarding advanced cases as all RE group V (group D IIRC) eyes required external beam radiation for salvage.14 Similarly Mallipatna et al. in his study of unilateral retinoblastoma in 2009 reported a 100% salvage rate of group A eyes, and a 100% rate of primary enucleation for all group D tumors.10 This was before the introduction of safer additional modalities to meet the challenges of Group D eyes. In our series we noticed a similar declining trend towards primary enucleation as new modalities were introduced.
Retinoblastoma has a unique way of disseminating through seeds, a trademark of group D tumors, and is considered to be the main cause of treatment failure.7 Seeding of tumor can be present primarily or may occur at any stage of treatment, in the hyaloid space, retro hyaloid space, subretinal or in the anterior segment. This feature was highlighted by Munier et al.,15 who described distinctive features of each category. The introduction of intravitreal melphalan as a safe treatment strategy in 2012,16 has greatly improved the overall survival of group D eyes. In our series, 2 eyes (11%) in the primary enucleation group were enucleated due to predominant vitreous seeds before 2012. The success rate of intravitreal melphalan was 75% (3/4 eyes) which raised the overall salvage rate by 15%. Most studies favoring primary enucleation in eyes with disseminated disease were published before this era, however, resistant or secondary subretinal seeds are still a treatment challenge to date,9,11 especially if present in the underlying retinal folds in settling exudative retinal detachment after chemotherapy. In such a situation focal therapy may not be effective. In our study 2 eyes that were a treatment failure had exudative retinal detachment with subretinal seeds. In addition, 1 eye developed diffuse disseminated subretinal seeds after induction of chemotherapy even before initiation of focal therapy (stage migration).
An international survey of classification for treatment choices of group D tumors by Shield and associates also pointed towards the discrepancy in the classification systems used, impacting the overall result.6 Therefore the Philadelphia version using a cut-off tumor size of <50% had the highest salvage rates. In our study, the primary enucleation group included 12 (63%) eyes with tumor occupying >50% of globe, 1 (20%) in the treatment failure group and none in the eye salvage group. Tumor dimension (basal diameter and thickness) is known to be a poor prognostic factor for globe salvage17 and culprit for recurrences.11 There is emerging evidence for a superior role of IAC in this regard but recurrence continues to be a point of concern.18
Histopathology of eyes in the primary enucleation group revealed high-risk features in 3 eyes (11%) and required adjuvant chemotherapy. All three tumors were poorly differentiated. Our previous study demonstrated high-risk features in group D eyes to be 21%.19 The discrepancy may be due to a smaller sample size. These findings were seen in contrast to the histopathological finding of the treatment failure group. This group included 5 eyes out of which only 1 showed high-risk features requiring adjuvant chemotherapy. The most likely explanation is the possible down-staging of histopathology due to chemotherapy. In a retrospective study Zhao et al. showed concern regarding the possible risk of metastases secondary to down-staging for group E eyes.20 In our follow-up however of 1–7 years, out of 20 group D eyes that we attempted to salvage we found no case of metastatic disease.
CVE protocol for chemoreduction with focal therapy continues to be the conventional first-line treatment worldwide.21–23 Studies report salvage rates with this regimen for group D or V (R-E classification) to be 11–48%.9,23,24 The salvage rate in our study was found to be consistent with the previous literature with a salvage rate of 45%. Second-line chemotherapy was instituted with topotecan in 2 eyes with incomplete response and 1 eye with recurrence. In our previous report of 14 eyes with refractory tumors that received systemic topotecan chemotherapy, eye salvage was 64% (9 eyes) with limited toxicity profile.25 In this study 2 eyes (67%) achieved adequate chemoreduction with topotecan, to be controlled with focal therapy consistent with a similar report by Chantada et al.26
Intra-arterial chemotherapy has recently gained wide acceptance in the treatment of unilateral retinoblastoma with variable success rates for group D eyes and reported salvage rates from 66.6–100%,27–29 depending on the type and location of the seeds. In this series, 2 eyes received IAC, out of which the eye that did not respond to IAC was also resistant to systemic topotecan and IAC was used as a final salvage strategy. The other eye achieved chemoreduction of the main tumor but developed more dissemination of tumor with vitreous and subretinal seeds during chemotherapy and was salvaged with IAC. Most studies also support that the salvage rate of IAC is higher when used as primary treatment than in chemo-resistant eyes.30,31 A meta-analysis conducted to analyze the outcomes of intra-arterial chemotherapy concluded this treatment strategy as a promising option, but cautioned against uncertain rates of metastases for advanced cases.32
The reported success rate with IVitC for diffuse vitreous seeds is very promising, reporting salvage of 75–100% with extremely rare reported incidence of metastasis.31,33 One of the concerns regarding this regimen is ocular toxicity. In our 4 eyes we failed to identify any ocular toxicity although the sample size is too small. Our success rate was 75% but more importantly it raised the overall salvage rate by 15%.
In this study we analyzed the outcomes of 39 eyes with unilateral group D tumors over a span of 8 years. It is accepted that management of group D eyes is the biggest challenge33 and treatment of unilateral cases is debatable. Currently, there is no standard protocol for the treatment of unilateral group D eyes and management is multifactorial. The results of our study are encouraging and report no case of metastasis. Therefore, we support to attempt salvage of selected eyes and customize the treatment accordingly. However, our study focused on outcomes of unilateral group D cases and failed to identify poor predictors in this spectrum of disease. Most eyes, with tumors occupying more than 50% of the globe, were enucleated and there was no control group for comparison of outcomes.
Conclusion
While primary treatment with intra-arterial chemotherapy seems to be a more promising modality, it has limited availability and requires expertise of the treating oncologist and the interventional radiologist; therefore, considering the potentially fatal complications of IAC, institutes that lack experience in this field can consider other modalities with comparable outcomes. Most group D eyes will give at least navigational vision34 and any attempt to save the smallest potential of vision should not be disparaged. Moreover, the need of adopting one classification system cannot be over-emphasized.
Acknowledgments
We acknowledge the support of the Eye Cancer Foundation Inc. (New York, NY USA, http://eyecancerfoundation.net) and The International Council of Ophthalmology (ICO) for Dr. Saima Amin for the Ocular Oncology Fellowship.
Funding Statement
This research received no external funding.
Ethics
The authors confirm that the revised manuscript had a database that complied with relevant data protection and privacy regulations.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Castillo BV Jr, Kaufman L. Pediatric tumors of the eye and orbit. Pediatr Clin North Am. 2003;50(1):149–172. doi: 10.1016/S0031-3955(02)00115-3 [DOI] [PubMed] [Google Scholar]
- 3.Chantada G, Luna-Fineman S, Sitorus RS, et al. SIOP-PODC recommendations for graduated-intensity treatment of retinoblastoma in developing countries. Pediatr Blood Cancer. 2013;60(5):719–727. doi: 10.1002/pbc.24468 [DOI] [PubMed] [Google Scholar]
- 4.Canadian Retinoblastoma S. National retinoblastoma strategy canadian guidelines for care: strategie therapeutique du retinoblastome guide clinique canadien. Can J Ophthalmol. 2009;44(Suppl 2):S1–S88. [DOI] [PubMed] [Google Scholar]
- 5.Honavar SG, Singh AD. Management of advanced retinoblastoma. Ophthalmol Clin North Am. 2005;18(1):65–73, viii. doi: 10.1016/j.ohc.2004.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Scelfo C, Francis JH, Khetan V, et al. An international survey of classification and treatment choices for group D retinoblastoma. Int J Ophthalmol. 2017;10(6):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDaid C, Hartley S, Bagnall AM, Ritchie G, Light K, Riemsma R. Systematic review of effectiveness of different treatments for childhood retinoblastoma. Health Technol Assess. 2005;9(48):iii, ix–x, 1–145. doi: 10.3310/hta9480 [DOI] [PubMed] [Google Scholar]
- 8.Fabian ID, Reddy A, Sagoo MS.Classification and staging of retinoblastoma. Community Eye Health.2018;31(101):11–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Shields CL, Mashayekhi A, Au AK, et al. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–2280. doi: 10.1016/j.ophtha.2006.06.018 [DOI] [PubMed] [Google Scholar]
- 10.Mallipatna AC, Sutherland JE, Gallie BL, Chan H, Heon E. Management and outcome of unilateral retinoblastoma. J AAPOS. 2009;13(6):546–550. doi: 10.1016/j.jaapos.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Shields CL, Honavar SG, Shields JA, Demirci H, Meadows AT, Naduvilath TJ. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma. Arch Ophthalmol. 2002;120(4):460–464. doi: 10.1001/archopht.120.4.460 [DOI] [PubMed] [Google Scholar]
- 12.Ghassemi F, Chams H, Sabour S, et al. Characteristics of germline and non-germline retinoblastomas. J Ophthalmic Vis Res. 2014;9(2):188–194. [PMC free article] [PubMed] [Google Scholar]
- 13.Yousef YA, AlNawaiseh T, AlJabari R, Muhsen S, Al-Nawaiseh I. Retinoblastoma awareness among first contact physicians in Jordan. Ophthalmic Genet. 2019;40(3):191–195. doi: 10.1080/13816810.2019.1605387 [DOI] [PubMed] [Google Scholar]
- 14.Shields CL, Honavar SG, Meadows AT, Shields JA, Demirci H, Naduvilath TJ. Chemoreduction for unilateral retinoblastoma. Arch Ophthalmol. 2002;120(12):1653–1658. doi: 10.1001/archopht.120.12.1653 [DOI] [PubMed] [Google Scholar]
- 15.Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35(4):193–207. doi: 10.3109/13816810.2014.973045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munier FL, Soliman S, Moulin AP, Gaillard MC, Balmer A, Beck-Popovic M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilisation of the needle track. Br J Ophthalmol. 2012;96(8):1084–1087. doi: 10.1136/bjophthalmol-2011-301016 [DOI] [PubMed] [Google Scholar]
- 17.Rubin CM, Robison LL, Cameron JD, et al. Intraocular retinoblastoma group V: an analysis of prognostic factors. J Clin Oncol. 1985;3(5):680–685. doi: 10.1200/JCO.1985.3.5.680 [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Zhang B, Dong Y, et al. Comparison between intravenous chemotherapy and intra-arterial chemotherapy for retinoblastoma: a meta- analysis. BMC Cancer. 2018;18(1):486. doi: 10.1186/s12885-018-4406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousef YA, Hajja Y, Nawaiseh I, et al. A histopathologic analysis of 50 eyes primarily enucleated for retinoblastoma in a tertiary cancer center in Jordan. Turk Patoloji Derg. 2014;30(3):171–177. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Dimaras H, Massey C, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol. 2011;29(7):845–851. doi: 10.1200/JCO.2010.32.5332 [DOI] [PubMed] [Google Scholar]
- 21.Meel R, Radhakrishnan V, Bakhshi S. Current therapy and recent advances in the management of retinoblastoma. Indian J Med Paediatr Oncol. 2012;33(2):80–88. doi: 10.4103/0971-5851.99731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houston SK, Murray TG, Wolfe SQ, Fernandes CE. Current update on retinoblastoma. Int Ophthalmol Clin. 2011;51(1):77–91. doi: 10.1097/IIO.0b013e3182010f29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen VM, Kingston J, Hungerford JL. The success of primary chemotherapy for group D heritable retinoblastoma. Br J Ophthalmol. 2009;93(7):887–890. doi: 10.1136/bjo.2008.142679 [DOI] [PubMed] [Google Scholar]
- 24.Shields CL, Mashayekhi A, Cater J, Shelil A, Meadows AT, Shields JA. Chemoreduction for retinoblastoma. Analysis of tumor control and risks for recurrence in 457 tumors. Am J Ophthalmol. 2004;138(3):329–337. doi: 10.1016/j.ajo.2004.04.032 [DOI] [PubMed] [Google Scholar]
- 25.Sultan I, Hajja Y, Nawaiseh I, et al. Chemoreduction of progressive intraocular retinoblastoma by systemic topotecan. Ophthalmic Genet. 2016;37(2):209–213. doi: 10.3109/13816810.2015.1039138 [DOI] [PubMed] [Google Scholar]
- 26.Chantada GL, Fandino AC, Casak SJ, Mato G, Manzitti J, Schvartzman E. Activity of topotecan in retinoblastoma. Ophthalmic Genet. 2004;25(1):37–43. doi: 10.1076/opge.25.1.37.28996 [DOI] [PubMed] [Google Scholar]
- 27.Kaliki S, Shields CL. Retinoblastoma: achieving new standards with methods of chemotherapy. Indian J Ophthalmol. 2015;63(2):103–109. doi: 10.4103/0301-4738.154369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abramson DH, Marr BP, Dunkel IJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol. 2012;96(4):499–502. doi: 10.1136/bjophthalmol-2011-300498 [DOI] [PubMed] [Google Scholar]
- 29.Shields CL, Bianciotto CG, Jabbour P, et al. Intra-arterial chemotherapy for retinoblastoma: report No. 1, control of retinal tumors, subretinal seeds, and vitreous seeds. Arch Ophthalmol. 2011;129(11):1399–1406. doi: 10.1001/archophthalmol.2011.150 [DOI] [PubMed] [Google Scholar]
- 30.Shields CL, Meadows AT, Leahey AM, Shields JA. Continuing challenges in the management of retinoblastoma with chemotherapy. Retina. 2004;24(6):849–862. doi: 10.1097/00006982-200412000-00003 [DOI] [PubMed] [Google Scholar]
- 31.Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2013;97(10):1231–1236. doi: 10.1136/bjophthalmol-2013-303188 [DOI] [PubMed] [Google Scholar]
- 32.Yousef YA, Soliman SE, Astudillo PPP, et al. Intra-arterial chemotherapy for retinoblastoma: a systematic review. JAMA Ophthalmol. 2016;134(5):584–591. doi: 10.1001/jamaophthalmol.2016.0244 [DOI] [PubMed] [Google Scholar]
- 33.Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268–1271. doi: 10.1001/archophthalmol.2012.1983 [DOI] [PubMed] [Google Scholar]
- 34.Demirci H, Shields CL, Meadows AT, Shields JA. Long-term visual outcome following chemoreduction for retinoblastoma. Arch Ophthalmol. 2005;123(11):1525–1530. doi: 10.1001/archopht.123.11.1525 [DOI] [PubMed] [Google Scholar]
- 35.Yousef YA, Al-Nawaiseh I, Mehyar M, et al. How telemedicine and centralized care changed the natural history of retinoblastoma in a developing country: analysis of 478 patients. Ophthalmology. 2020;S0161-6420(20)30689–8. doi: 10.1016/j.ophtha.2020.07.026 [DOI] [PubMed] [Google Scholar]
- 36.Mallipatna AC, Gallie BL, Chévez-Barrios P, et al. Retinoblastoma In: Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, editors. AJCC Cancer Staging Manual. 8th New York: Springer; 2017:819–831. [Google Scholar]