Abstract
Background
Multidrug-resistant tuberculosis (MDR-TB) in HIV infected individuals is a serious threat to global efforts to combat tuberculosis. Inconsistent findings on the association between HIV infection and MDR-TB were present in many studies. We aimed to review existing data on the relationship between HIV infection and MDR-TB systematically to assess the contribution of HIV on MDR-TB worldwide. We also investigated the patterns of MDR-TB by age, country-wise income, study designs, and global regions.
Methods
We utilized PubMed, Google Scholar, and ScienceDirect databases to select eligible studies for meta-analysis that were published between January 1, 2010, and July 30, 2020. The random-effects model was used to obtain the pooled odds ratio of the crude association between HIV and MDR-TB with a 95% confidence interval. We investigated the potential publication-bias by checking funnel plot asymmetry and using the Egger’s test. Moreover, we assessed the heterogeneity using the I2 statistic. Sensitivity analysis was performed based on sample size and adjustment factors. The protocol was registered with PROSPERO-CRD42019132752.
Results
We identified 1603 studies through a database search, and after subsequent eliminations we selected 54 studies including 430,534 TB patients. The pooled odds of MDR-TB was 1.42 times higher in HIV-positive patients than HIV-negative patients (OR=1.42,CI=1.17–1.71, I2=75.8%). Subgroup analysis revealed that the estimated pooled odds for South-East Asian countries was 1.86, which is the highest in WHO regions (OR=1.86,CI=1.30–2.67, I2=0.00%), followed by Europe and Africa. The effect estimate was found to be higher for primary MDR-TB (OR=2.76,CI=1.70–4.46, I2=0.00%). There was also a trend towards increased odds of MDR-TB for HIV patients older than 40 years (OR=1.56,CI=1.17–2.06). The association was found to be significant in high-burden TB countries (OR=1.75, CI=1.39–2.19) and in high-income countries (OR=1.55, CI=1.06–2.27).
Conclusion
Such findings indicate that HIV infection raises the risk of MDR-TB, and after contrasting it with the results of the earlier pooled study, it appeared to be an upward risk trend. Moreover, we found that the risk is the highest in the South-East Asian region. A balanced allocation of resources is needed to halt both primary and secondary MDR-TB, particularly in HIV infected people with 40 years of age and older.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-020-05749-2.
Keywords: Multidrug resistant, Drug-resistant, Tuberculosis, MDR-TB, HIV, Meta-analysis
Background
Tuberculosis is a global public health problem causing illness among millions each year. In 2018, there were an estimated 10 million new TB cases, and 8.6% were living with HIV [1]. The care and control of tuberculosis are threatened by the emergence and amplification of multi-drug resistant tuberculosis (MDR-TB). It was declared as a public health crisis by WHO in 2013 [2]. Globally, there were an estimated 484,000 incident cases of MDR/Rifampicin-resistant tuberculosis (RR-TB). Of them, about 39% (186772) were notified, and 32% (156071) enrolled for treatment in 2018 [1]. The large gap in diagnosis and treatment increases the likelihood of transmission of TB plus MDR-TB [3]. Poor TB treatment outcome is also reported in TB-HIV co-infected patients, which further ignites MDR-TB development [4–6]. A WHO report shows that 3.4% of new cases and 18% of previously treated TB cases are estimated to have MDR/RR-TB [1]. However, HIV positive individuals are 20 times more likely to develop active TB than those without HIV [7]. Despite enormous concerted measures taken to control tuberculosis and reduce HIV-associated deaths, it ranks among the top ten causes of death worldwide [1]. About 0.25 million deaths were attributed to TB associated with HIV, and nearly 15% of all global tuberculosis deaths were contributed by MDR-TB in 2018 [1].
Drug-resistant tuberculosis (DR-TB) is defined as a case of one or more anti-TB drugs resistant to bacteria that cause TB. There are various forms of DR-TB: mono-drug-resistant TB (mono-DR-TB), polydrug-resistant TB (poly-DR-TB), rifampicin-resistant TB (RR-TB), multidrug-resistant TB (MDR-TB), and extensively drug-resistant TB (XDR-TB). MDR-TB is a form of TB that does not respond to at least isoniazid and rifampicin, the two most potent anti-TB drugs. The XDR-TB is also a form of MDR-TB resistant to isoniazid and rifampicin, plus any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin). RR-TB is classified as a case of TB, which shows either rifampicin (R) resistance only or accompanied by resistance to other anti-TB drugs in different forms. About half a million people worldwide acquired MDR-TB in 2017, and a further 161,000 people with RR-resistant TB were newly diagnosed with MDR-TB [1]. China, India, and Russia were the countries with the largest number of MDR / RR-TB cases (47% of the global total). Approximately 6.2% of these cases were XDR-TB [1].
Global tuberculosis burden study 2015 suggests taking the necessary steps to prevent HIV for reducing the burden of tuberculosis [8]. Estimates reveal that around 1.7 billion of the world’s population has latent tuberculosis infection [1]. Of them, 5–15% of cases are at risk of developing active diseases during their lifetime, and people living with HIV have a higher risk of falling ill [9, 10]. Alarmingly, it appeared from a mathematical model that there were approximately 19 million people globally with latent MDR tuberculosis infection (10% were at risk of active disease) in 2015 [11]. Undoubtedly, latency is affected by HIV infection and is one of the most potent risk factors for converting into active disease, drug-susceptible, or drug-resistance [10–12].
The surge of MDR-TB occurrence in HIV-prevalent settings is of great public health importance. An updated understanding of the magnitude of association is needed with the accumulation of recent evidence that supports a positive association between HIV and MDR-TB [13, 14]. Additionally, two systematic reviews revealed an increased risk of transmission-associated MDR-TB (primary) among HIV positive individuals [15, 16]. Mesfin et al. conducted the latest systematic review and meta-analysis on the relationship between HIV infection and MDR-TB in 2014, which included research from 1994 to 2011. Even after the latest meta-analysis, conflicting results on the association were found in numerous studies conducted during the last decade. The objective was to provide an up-to-date pooled risk estimate on the relationship between HIV infection and MDR-TB growth and compare the risk pattern of MDR-TB with previous systematic reviews.
Methods
Search strategy
We researched on PubMed, Google Scholar, and Science Direct databases to select eligible peer-reviewed articles for our systematic review and meta-analysis. Relevant observational studies (cross-sectional, case-control, and cohort) were screened to assess the association between HIV infection and MDR-TB, published from January 1, 2010, to July 30, 2020 (date of the last search). The screening language was restricted to English. The particular keywords used for searching articles were “Multidrug-resistant tuberculosis” or “MDR-TB” or “Drug-resistant TB” or “Risk factors of MDR-TB” or “Predictors of MDR-TB” and “HIV” or “Human Immunodeficiency Virus”. A summary of search terms is provided in Appendix A. Search results were compiled using a citation management software Zotero. In addition to databases used, we explored references of selected studies to incorporate all potential pertinent articles to construct our summary estimates. The study adheres to the Preferred Reporting Items for Systematic Review and meta-analysis (PRISMA) guidelines [17]. The protocol was registered in the PROSPERO database-CRD42019132752.
Selection criteria
The primary outcome of the meta-analysis was an unadjusted odds ratio exhibiting the crude association between HIV infection and MDR-TB. The articles that reported or provided adequate data to calculate the frequency of MDR-TB and non-MDR-TB, subdivided by HIV status (positive or negative), were included in our systematic review. We included studies where MDR-TB is the outcome of interest. We contacted the author for the insufficient information given in any study. If the author didn’t reply, we excluded the article. To be eligible for inclusion, any drug susceptibility testing method (culture on solid and/or liquid media, molecular techniques, clinical records, Table 1) were accepted for the diagnosis of multidrug-resistant tuberculosis (Mycobacterium tuberculosis strain resistant at least to rifampicin and isoniazid). The comparison group (non-MDR-TB) consisted of drug-susceptible tuberculosis patients and/or resistant to any single drug. HIV status was ascertained by HIV test reports, clinical/hospital records, databases/registers, or directly from patients. Studies reported on non-tuberculous mycobacterium, case reports, case studies, systematic review, meta-analysis, a duplicate publication of the same study, and studies with only abstract were excluded. We also didn’t include grey literature (theses and dissertations). Studies with less than three HIV/MDR-TB participants were excluded because these studies cause the analysis to be heterogeneous. Titles and abstracts of the studies obtained from database searches were screened independently by two reviewers ZZS and FH. Full text of potential articles was further reviewed for eligibility regardless of the study base (hospital or population). Articles might have been eliminated for more than one rationale. Any disagreements were discussed with senior authors (JB or AH) until a consensus was reached or by the arbitration of AH alone.
Table 1.
Characteristics of the included studies in the meta-analysis (according to the order of year)
| Author [Ref] | Year | Study design | Study period | Country | Number of TB patients | MDR-TB cases | % of male (MDR-TB cases) | Mean/ Median age (MDR-TB cases), years | TB form | % of EPTB (MDR-TB cases) | MDR-TB type | % of PTC (MDR-TB cases) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brito et al. [18] | 2010 | Cross-sectional | 2004–2006 | Brazil | 595 | 44 | 72.73 | 40.1 | PTB, EPTB | NA | 1o,2o | 61.36 |
| Andrew et al. [19] | 2010 | Case-control | 2005–2007 | South Africa | 378 | 123 | 50 | ~ 34 | PTB, EPTB | 28.63 | 1o,2o | 71.75 |
| Sangare et al. [20] | 2010 | Cross-sectional | 2005–2006 | Burkina Faso | 550 | 47 | NA | NA | PTB | NA | 1o,2o | 81.03 |
| Sangare et al. [21] | 2011 | Cross-sectional | 2005–2006 | Burkina Faso | 316 | 42 | 87.66 | NA | NA | NA | 1o,2o | 85.71 |
| Gudo et al. [22] | 2011 | Cross-sectional | 2007–2008 | Mozambique | 1102 | 27 | 61.8 | ~ 35 | PTB | NA | 1o | 0 |
| Vadwai et al. [23] | 2011 | Cross-sectional | 2009 | India | 250 | 184 | 61.95 | 33.06 | PTB | NA | 1o,2o | 62.5 |
| Macedo et al. [24] | 2012 | Cross-sectional | 2008–2010 | Portugal | 2093 | 50 | 78 | ~ 42 | PTB, EPTB | 8 | 1o,2o | 30 |
| Padilla et al. [25] | 2012 | Cross-sectional | 2009–2010 | Swaziland | 840 | 122 | 37.70 | ~ 33 | PTB | NA | 1o,2o | 77.87 |
| van Halsema et al. [26] | 2012 | Cross-sectional | 2002–2008 | South Africa | 2431 | 168 | NA | NA | NA | NA | 1o,2o | 82.74 |
| Ricks et al. [27] | 2012 | Case-control | 2007–2009 | Namibia | 368 | 117 | 56 | 36 | PTB, EPTB | 2 | 1o,2o | 97 |
| Tessema et al. [28] | 2012 | Cross-sectional | 2009 | Ethiopia | 260 | 13 | 53.85 | NA | PTB | NA | 1o,2o | 61.54 |
| Coelho et al. [29] | 2012 | Cross-sectional | 2000–2004 | Brazil | 671 | 32 | NA | 39.2 | PTB | NA | 1o,2o | 68.7 |
| Ulmasova et al. [30] | 2013 | Cross-sectional | 2010–2011 | Uzbekistan | 1037 | 372 | 57.07 | ~ 45 | PTB | NA | 1o,2o | NA |
| Minion et al. [31] | 2013 | Surveillance | 1997–2008 | Canada | 15,993 | 177 | 59.35 | 30 | PTB | NA | 1o,2o | 31.10 |
| Sethi et al. [32] | 2013 | Cross-sectional | 2006–2010 | India | 219 | 39 | 66.67 | 36.6 | PTB | NA | 1o,2o | 69.23 |
| Van Den Hof et al. [33] | 2013 | Cross-sectional | 2007–2011 | Kazakhstan | 146,461 | 18,338 | 65.81 | NA | PTB, EPTB | 1.41 | 1o,2o | 85.9 |
| Lukoye et al. [34] | 2013 | Cross-sectional | 2009–2011 | Uganda | 1537 | 31 | 67.74 | ~ 35 | PTB | NA | 1o,2o | 45.16 |
| Hang et al. [35] | 2013 | Cross-sectional | 2007–2009 | Vietnam | 546 | 22 | NA | 38.6 | PTB | NA | 1o | 0 |
| Skrahina et al. [36] | 2013 | Cross-sectional | 2010–2011 | Belarus | 1420 | 612 | 80 | ~ 46 | PTB | NA | 1o,2o | 75.60 |
| Satti et al. [37] | 2013 | Cohort | 2007–2011 | Lesotho | 148 | 47 | NA | NA | PTB | NA | 2o | 100 |
| Hirpa et al. [38] | 2013 | Case-control | 2011–2012 | Ethiopia | 268 | 134 | 60.50 | 25.1 | PTB, EPTB | 3 | 2o | 100 |
| Mor et al. [39] | 2014 | Cross-sectional | 1999–2010 | Israel | 3552 | 207 | 75.40 | 43 | PTB, EPTB | 3.90 | 2o | 100 |
| Post et al. [40] | 2014 | Cross-sectional | 2004–2006 | Belarus, Latvia, Russia, Romania, Ukraine | 144 | 55 | 74.6 | 30.2 | PTB. EPTB | 3.6 | 1o,2o | 16.4 |
| Metcalfe et al. [41] | 2014 | Cross-sectional | 2011–2012 | Zimbabwe | 129 | 25 | 52 | ~ 34 | PTB | NA | 1o,2o | 12 |
| Shariff et al. [42] | 2015 | Case-control | 2013–2014 | Malaysia | 150 | 30 | 66.70 | 51 | PTB | NA | 1o,2o | 66.70 |
| Jitmuang et al. [43] | 2015 | Case-control | 2010–2012 | Thailand | 188 | 47 | 57.40 | 48.9 | PTB, EPTB | 10.60 | 1o,2o | 53.20 |
| Chuchottaworn et al. [44] | 2015 | Case-control | 2007–2013 | Thailand | 290 | 145 | 65.50 | 46.2 | PTB | NA | 1o,2o | 96.60 |
| Elmi et al. [45] | 2015 | Case-control | 2010–2014 | Malaysia | 314 | 105 | 66.70 | 51 | PTB | NA | 1o,2o | 50.50 |
| Mulisa et al. [46] | 2015 | Case-control | 2013–2014 | Ethiopia | 265 | 88 | 56.80 | ~ 33 | PTB | NA | 1o,2o | 90.90 |
| Mulu et al. [47] | 2015 | Case-control | 2013–2014 | Ethiopia | 306 | 153 | 57.50 | 35 | PTB, EPTB | 7.80 | 1o,2o | 96.10 |
| Gunther et al. [48] | 2015 | Case-control | 2010–2011 | 23 TBNET sites in 16 countries in Europe | 756 | 380 | 62.89 | ~ 36 | PTB | NA | 1o,2o | 35 |
| Ershova et al. [49] | 2015 | Cross-sectional | 2012 | Russia | 229 | 44 | 77.30 | ~ 36 | PTB | NA | 1o | 0 |
| Abdella et al. [50] | 2015 | Cross-sectional | 2012–2013 | Ethiopia | 70 | 22 | NA | ~ 32 | PTB | 0 | 2o | 100 |
| Tadasse [51] | 2015 | Cross-sectional | 2008–2011 | Ethiopia | 439 | 113 | 64.60 | 29 | PTB, EPTB | 11.50 | 1o,2o | 99.11 |
| Salindri et al. [52] | 2016 | Cohort | 2011–2014 | Georgia | 318 | 52 | 71.15 | ~ 50 | PTB | NA | 1o | 0 |
| Lee et al. [53] | 2016 | Case-control | 2006–2014 | South Korea | 1606 | NA | NA | PTB,EPTB | 4.5 | 1o,2o | 39.85 | |
| Assefa et al. [54] | 2017 | Case-control | 2013 | Ethiopia | 710 | 229 | 47.6 | 31.7 | PTB | NA | 1o,2o | NA |
| Workicho et al. [55] | 2017 | Case-control | 2011 | Ethiopia | 180 | 90 | 45.60 | 29.4 | PTB | NA | 1o,2o | 91.10 |
| Sinha et al. [56] | 2017 | Cross-sectional | 2012–2014 | India | 235 | 124 | 45.16 | 35.7 | PTB, EPTB | 9.68 | 1o,2o | 25.80 |
| Mesfin et al. [57] | 2018 | Cross-sectional | 2015–2016 | Ethiopia | 226 | 89 | 41.60 | 34.4 | PTB | NA | 1o,2o | 82 |
| Gobena et al. [58] | 2018 | Case-control | 2016–2017 | Ethiopia | 132 | 59 | 61 | 30.2 | PTB, EPTB | 3.40 | 1o,2o | 64 |
| Kusumawati et al. [59] | 2018 | Cross-sectional | 2010–2013 | Indonesia | 842 | 98 | 72.40 | 44.5 | PTB | NA | 1o,2o | 64.30 |
| Pavlenko et al. [60] | 2018 | Cross-sectional | 2013–2014 | Ukraine | 1658 | 474 | 75 | ~ 43 | PTB | NA | 1o,2o | 37.92 |
| Desissa et al. [61] | 2018 | Case-control | 2016 | Ethiopia | 219 | 73 | 45.20 | 32.69 | PTB, EPTB | 26 | 1o,2o | 65.80 |
| Gaborit et al. [62] | 2018 | Case-control | 2002–2013 | France | 134 | 44 | 75 | ~ 33 | PTB, EPTB | 36.36 | 1o,2o | 52.27 |
| Alene et al. [63] | 2019 | Case-control | 2010–2015 | Ethiopia | 452 | 242 | 60.70 | ~ 31 | PTB, EPTB | 6.20 | 1o,2o | 93 |
| Baya et al. [64] | 2019 | Cross-sectional | 2007–2016 | Mali | 214 | 134 | 76.12 | 39.3 | PTB | NA | 2o | 100 |
| Zurcher et al. [65] | 2019 | Cross-sectional | 2013–2016 | Cote d’Ivoire, Demo graphic Republic of the Congo, Kenya, Nigeria, South Africa, Peru, Thailand | 871 | 163 | 60 | 33.2 | PTB | NA | 2o | 100 |
| Okethwangu et al. [66] | 2019 | Case-control | 2013–2017 | Uganda | 125 | 33 | NA | ~ 42 | PTB | NA | 2o | 100 |
| Fikre et al. [67] | 2019 | Case-control | 2016–2018 | Ethiopia | 204 | 102 | 67.70 | 35.6 | PTB, EPTB | 8.80 | 1o,2o | 72.50 |
| Elduma et al. [68] | 2019 | Case-control | 2017–2019 | Sudan | 1290 | 430 | 69.77 | 37.3 | PTB | NA | 1o,2o | 67.90 |
| Chan et al. [69] | 2020 | Surveillance | 2011–2016 | USA | 45,209 | 615 | 52.2 | ~ 45 | NA | NA | 1o,2o | 18.04 |
| Arroyo et al. [70] | 2020 | Cohort | 2006–2016 | Brazil | 167,726 | 866 | 70.7 | ~ 45 | PTB, EPTB | 3.4 | 1o,2o | 54.70 |
| Hirama et al. [71] | 2020 | Cohort | 2010–2016 | Canada | 402 | 46 | 47.8 | ~ 45 | PTB, EPTB | 26.08 | 1o,2o | 34.80 |
MDR-TB Multidrug-resistant tuberculosis, PTB Pulmonary tuberculosis, EPTB Extra-pulmonary tuberculosis, PTC Previously treated cases
Data extraction
A pre-specified and standardized form was used for data abstraction. For the estimation of the crude odds ratios, key data on the number of MDR-TB HIV positive, number of MDR-TB HIV negative, number of non-MDR-TB HIV positive, and number of non-MDR-TB HIV negative patients were extracted from each study. Additionally, data on the name of the first author, country, year of publication, years of recruitment, study design (cross-sectional, case-control or cohort), source of data (hospital/medical records, lab reports, database/register, or direct from the patients), methods carried out to determine drug resistance pattern, number of enrolled TB patients, the mean or median age of the MDR-TB cases, proportion of male patients among MDR-TB cases (%), type of MDR-TB (primary, secondary or both), form of tuberculosis (pulmonary, both pulmonary and extra-pulmonary or not defined), and proportion of the patients with extra-pulmonary tuberculosis among MDR-TB cases (%) were entered in the spreadsheet. We further stratified the articles based on global regions (defined by World Health Organization) income level (World Bank classification by income, GNI per capita) and burden for MDR-TB (list used by WHO 2016–2020) of the countries [1, 72]. Data abstraction from individual studies was executed by two trios of investigators (AI, JB & ZZS and HRK, DHH & FH), from March 2019 to June 2020. The presence of any inconsistency in data extraction was verified by a seventh investigator (AH).
Quality assessment
Two trios of investigators (AI, JB & ZZS and HRK, DHH & FH) scored each study to ascertain methodological quality independently. The corresponding author (AH) subsequently examined all the assessments. Furthermore, contentions aroused in the course of quality scoring were resolved through discussions between investigators. It was evaluated using the Newcastle-Ottawa Scale (NOS). It consists of three domains: selection, comparability, and exposure or outcome of interest. Few points on the NOS related to appropriate methods for evaluating exposure variables and outcomes were designed for the relevance to our research question (Supplementary file 1). Two outcome groups were considered comparable if the HIV status was adjusted for the previous history of diagnosis with tuberculosis and/or any other sociodemographic variable (e.g., age, sex, etc.). These studies also reflect high or medium NOS scores (Supplementary file 2). Articles were given scores to reflect methodological stringency, lucidity, and transparency in reporting. Nevertheless, we did not exclude any articles based on quality scoring. It may exclude studies coming from resource-limited settings. Therefore, we did not provide insight into this particular cluster of studies. Moreover, the PRISMA statement consists of a checklist of 27 items and is given in Supplementary file 3.
Statistical analysis
We performed data analysis using meta and metafor packages in the R statistical software (version 3.5.1). We calculated crude odds ratio with a 95% confidence interval (CI) for individual study from the abstracted frequencies (numerators and denominators). After that, we estimated the pooled odds ratio (overall) using the random-effects model, allowing the true effect size to vary from study to study. The summary estimate (OR) for the association between HIV infection and MDR-TB were reported with 95% CI. The calculated odds ratio of each study and the combined effect estimate with 95% CI were graphically represented by forest plots. Publication bias was assessed by observing the symmetry of funnel plots visually. Further confirmation was conducted using Egger’s test (weighted regression with multiplicative dispersion model), while the p-value < 0.10 was suggestive of statistical significance. Heterogeneity across the selected studies was assessed by I2 statistic (> 75% signifies substantial, 50–75% moderate and 25–50% low heterogeneity). The I2 statistic represents the percentage of total variation across studies due to heterogeneity rather than chance. We also conducted a sensitivity analysis that removed the study contributing to the highest weight to evaluate the robustness of the findings.
Analysis of the subgroups was carried out to determine the pooled odds ratio for each group and to look for potential explanations of the heterogeneity. Pre-determined subgroups were WHO global regions (Africa, Europe, South-east Asia, America, Pacific, and Eastern Mediterranean), the income level of the country (high, upper-middle, lower-middle and low), the burden for MDR-TB on the country (high and low), design of the study (cross-sectional, case-control and cohort), type of MDR-TB (primary, secondary and both), mean or median age 40 years and older, and diagnostic method used for MDR-TB (culture and culture and/or molecular technique). Funnel plot asymmetry and egger’s tests were done to assess the presence of publication bias in each subgroup.
Results
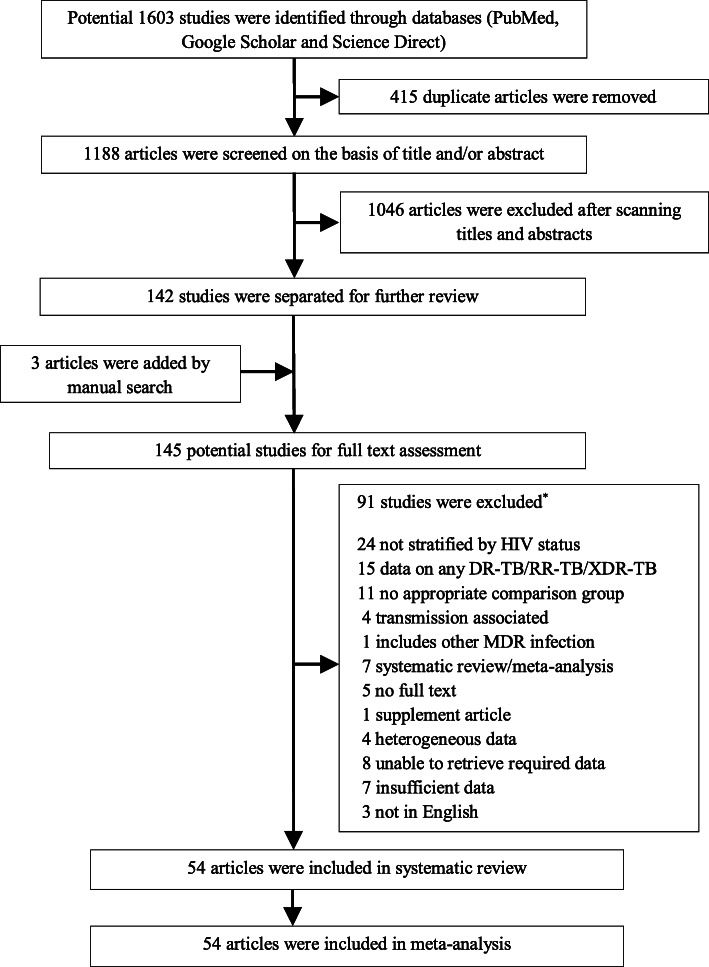
Our systematic review identified 1603 studies through a database search. After eliminating the duplicates, titles and abstracts of 1188 articles were scanned by the investigators to retrieve a set of relevant studies for further review. We subsequently narrowed down to 145 possible studies, and three were added for full-text evaluation through manual search from the reference lists of included studies. Finally, 54 studies were selected in our systematic review and meta-analysis, including 430,534 TB patients (Fig. 1). The characteristics of the selected studies can be found in Table 1.
Fig. 1.
PRISMA flow diagram for Study selection
(*Studies were excluded because of more than one rationale and the references of excluded studies are given in Supplementary file 4.)
The retrieved articles represented 36 countries from all WHO global regions (Africa, Europe, South-east Asia, America, Pacific, and Eastern Mediterranean). Of the 54 studies, three were conducted in multiple centers in different countries (Table 1). Nearly half of studies were reported from the African region. Many of the studies were performed in Ethiopia and from high MDR-TB burden countries.
The systematic review included 20 case-control studies and 31 studies considering tuberculosis as a pulmonary form. Of the 54 studies, 42 investigated both primary and secondary MDR-TB. The proportion of MDR-TB cases with extra-pulmonary tuberculosis varied from 2 to 36.36%. The proportion of previously treated tuberculosis among multidrug-resistant cases ranged from 12.00 to 99.11% (39 studies). It shows from Table 1 that only culture-confirmed MDR-TB cases were reported in 37 of the studies.
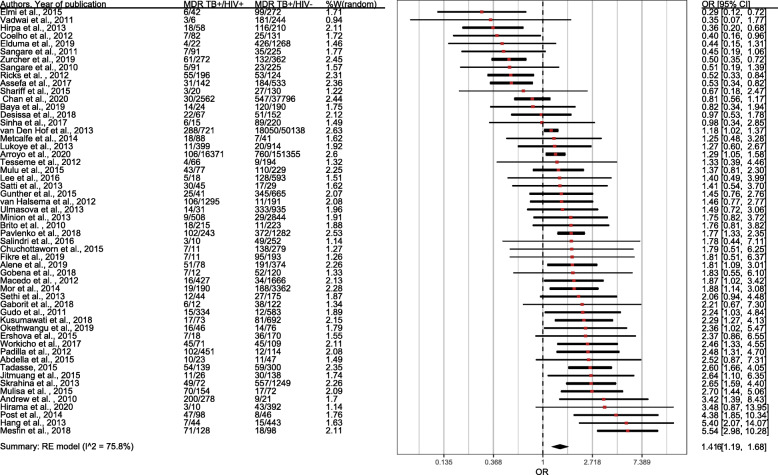
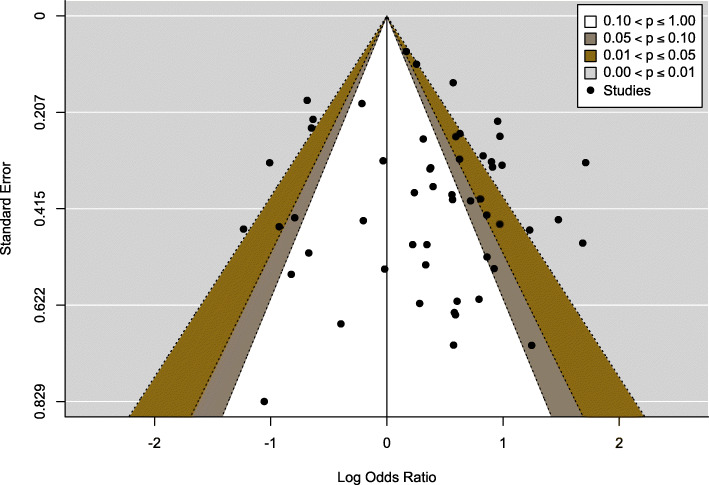
It appears from Fig. 2 that the overall pooled odds ratio is 1.42 (95% CI= 1.17–1.71), which suggests that the odds of developing MDR-TB in HIV-infected patients was 42% higher than those of HIV-negative individuals. The evidence of publication bias was tested by visual examination of funnel plot symmetry, and further, the absence of the publication bias was supported by the Egger test (p=0.36) and is shown in Fig. 3.
Fig. 2.
Forest plot exhibiting OR of each study and combined OR of the crude association between HIV infection and MDR-TB
Fig. 3.
The funnel plot reveals existence of publication bias though most points fall within the 95% confidence region. Each point represents a study; the y-axis represents standard error, and the x-axis displays the ratio of the log odds of the study
For the South-East Asian countries, the pooled odds of MDR-TB was 1.86 times higher for HIV positives than HIV negative individuals (OR=1.86, 95% CI=1.30–2.67). Most studies obtained from the African region had a pooled odds ratio of 1.41 (95% CI= 1.06–1.89). Results from WHO regions indicate that MDR-TB among HIV-persons is more in South-East Asian countries compared to other regions. The pooled ORs show lower odds of developing MDR-TB among HIV patients in upper-middle-income countries (OR=1.26, 95% CI=0.86–1.86) and low-income countries (OR=1.40, 95% CI=0.99–1.98) compared to high-income countries. The pooled odds ratio in the high TB-burden countries (OR=1.75, 95% CI= 1.39–2.19) was found to be significantly higher compared to the low TB-burden countries (OR=1.0, 95% CI= 0.72–1.39).
Findings from subgroup analysis in Table 2 shows the pooled odds ratio of the cross-sectional studies was 1.53 (95% CI= 1.20–1.96). Cohort studies provides odds ratio of 1.33 (95% CI=1.09–1.62), and case-control studies provides odds ratio of 1.22 (95% CI=0.87–1.71). Cross-sectional studies contribute about 70% of the weight of the overall meta-analysis research. For primary MDR-TB, the estimate was higher than secondary MDR-TB (OR=2.76, 95% CI= 1.70–4.46) with no heterogeneity among the studies. The majority of studies included both primary and secondary MDR-TB (42 studies), and subgroup analysis reveals that the odds of developing both primary and secondary MDR-TB in people infected with HIV were 1.42 times more compared to the individuals without HIV (OR=1.42, 95% CI= 1.16–1.74). Furthermore, the trend towards the development of MDR-TB in HIV-positive people increased with age (i.e., mean or median age of cases 40 years and older, OR=1.56, 95% CI= 1.17–2.06, and for below 40 years of age is OR=1.45, 95% CI= 1.11–1.91). In HIV infected patients, the pooled odds ratio of culturally confirmed MDR-TB cases (OR=1.52, 95% CI=1.20–1.93) was slightly higher than the mixed (i.e., culture and molecular technique) (OR=1.30, 95% CI=0.93–1.81). Only one study (OR=1.27, 95% CI= 0.6–2.17) considered the diagnosis of MDR-TB by molecular technique.
Table 2.
Summary results of subgroup analysis
| Subgroup | No of studies (TB patients) | Summary OR | 95% CI | I2 (%) | p-value* |
|---|---|---|---|---|---|
| WHO Region | |||||
| South-East Asia | 6 (1947) | 1.86 | 1.30–2.67 | 0.00 | 0.07 |
| Europe | 11 (154031) | 1.79 | 1.42–2.27 | 52.90 | 0.01 |
| Eastern Mediterranean | 1 (634) | 0.44 | 0.15–1.31 | – | – |
| Africa | 25 (14723) | 1.41 | 1.06–1.89 | 76.65 | 0.53 |
| America | 6 (256640) | 1.17 | 0.75–1.84 | 74.15 | 0.98 |
| Western Pacific | 4 (2559) | 1.11 | 0.32–3.89 | 82.89 | 0.97 |
| TB Burden countries | |||||
| Low | 18 | 1.0 | 0.72–1.39 | 63.91 | 0.71 |
| High | 30 | 1.75 | 1.39–2.19 | 76.68 | 0.09 |
| Countries by income level | |||||
| High | 7 | 1.55 | 1.06–2.27 | 50.85 | 0.09 |
| Upper-middle | 14 | 1.26 | 0.86–1.86 | 87.0 | 0.85 |
| Low-middle | 11 | 1.66 | 1.23–2.26 | 43.91 | 0.31 |
| Low | 19 | 1.40 | 0.99–1.98 | 78.44 | 0.97 |
| Study design | |||||
| Cross-sectionala | 30 | 1.53 | 1.20–1.96 | 71.6 | 0.27 |
| Case-control | 20 | 1.22 | 0.87–1.71 | 75.77 | 0.26 |
| Cohort | 4 | 1.33 | 1.09–1.62 | 0.00 | 0.20 |
| Outcome MDR-TB type | |||||
| Primary | 4 | 2.76 | 1.70–4.46 | 0.00 | 0.87 |
| Both | 42 | 1.42 | 1.16–1.74 | 80.63 | 0.50 |
| Secondary | 8 | 1.08 | 0.64–1.82 | 76.26 | 0.18 |
| Mean/Median Age (MDR-TB) | |||||
| > 40 | 15 | 1.56 | 1.17–2.06 | 71.66 | 0.57 |
| <=40 | 32 | 1.45 | 1.11–1.91 | 78.85 | 0.09 |
| Diagnosis of MDR-TB by | |||||
| Culture | 37 | 1.52 | 1.20–1.93 | 80.56 | 0.40 |
| Mixed | 14 | 1.30 | 0.93–1.81 | 76.97 | 0.48 |
| Molecular technique | 1 | 1.27 | 0.6–2.17 | – | – |
| Overall | 54 | 1.42 | 1.17–1.71 | 75.8 | 0.36 |
MDR-TB Multidrug resistant tuberculosis, HIV Human Immunodeficiency Virus, CI Confidence Interval
a two surveillance were included into cross-sectional studies
* p value for Egger’s test for publication bias
Sensitivity analysis
We conducted sensitivity analyses that excluded each of the following types of studies: studies with fewer than 1000 participants; studies from countries in Africa; and studies published in 2015 or earlier. Forest plots are reported in Supplementary file 5. When considering studies of more than 1000 participants, the OR of MDR-TB among HIV infected individuals tends to be 1.41 (OR=1.41, CI=1.13–1.76). The OR is 1.38 (CI=1.01–1.88) considering studies published after 2015. The OR is also 1.46 when studies outside African studies (OR=1.46, CI=1.14–1.87) are considered. The findings are, therefore, similar to the meta-analysis with 54 studies.
Discussion
About half a million TB patients were included from 54 studies in our systematic review and meta-analysis. Based on the findings of this meta-analysis, the odds of MDR-TB among HIV-positive cases were 1.42 times higher, and this was statistically significant. An earlier pooled study by Mesfin et al. (OR=1.24, 95% CI= 1.04–1.43) included 24 studies published from 1994 to 2011, and our finding appears to indicate an upward trend of odds ratio after being compared to it. Moreover, the 18 cross-sectional studies from Mesfin et al. gave pooled effect estimate for MDR- TB and HIV was 1.26 (OR=1.26, 95% CI= 1.02–1.49) while the result from our meta-analysis (30 studies) is 1.55 (OR=1.55, 95% CI= 1.26–1.95). Another meta-analysis (1988–2007) did not report a pooled effect estimate due to high heterogeneity among the studies [15]. Comparing with Mesfin et al. meta-analysis, it suggests over the last 10 years that people diagnosed with HIV are more likely to have MDR-TB.
Subgroup analysis by the WHO global regions reveals that the OR of South-East Asia was found the highest, followed by Europe and Africa. The WHO South-East Asia region is a home for a quarter of the world ‘s population, with a 44% TB burden, and the second-highest HIV prevalence [1]. Additionally, one-third of the global MDR-TB lies in this region [1]. In Europe, the proportion of MDR-TB among HIV-infected people increased sharply between 2008 and 2017, from 3 to 12%. The aforementioned data ratifies how our results reflect a major risk of MDR-TB among HIV patients.
Furthermore, the estimated pooled OR was found to be significant for the high TB burden countries. In our meta-analysis, with the age of 40 years and older, the pooled odds ratio of developing MDR-TB among HIV infected individuals continues to increase significantly. Findings from the subgroup study also showed that the pooled odds ratio of the cross-sectional studies was higher than that of the cohort studies. Similar findings were also seen in the previous meta-analysis [16].
Another notable finding was the significant association between HIV infection and primary MDR-TB. This finding corresponds to the previous two systematic reviews [15, 16]. In many instances, despite being infected primarily with drug-resistant strains and subsequent development of MDR-TB (primary), it will initially be reported as drug-susceptible tuberculosis. Drug susceptibility test is not routinely done in some settings and performed only after the failure of initial treatment, which will normally be classified as secondary MDR-TB [73]. We found an insignificant pooled odds ratio between HIV and MDR-TB in low-income countries, especially in Africa. It highlights the need to develop effective drug-resistance diagnosis in many resource-limited settings. Among the high-income countries, the combined estimate was found higher than that of the low-income countries. It is important to gain a clear understanding of these mechanisms to build effective strategies to control the expansion of MDR-TB in HIV patients.
In our systematic review, we found most studies considered the diagnosis of MDR-TB as a phenotypic approach that confirmed by culture. The pooled odds ratio of MDR-TB was considerably higher for studies when a diagnosis for MDR-TB was made by culture-confirmed than the mixed or molecular technique. Such culture-confirmed traditional approaches take months to confirm the diagnosis of MDR-TB and ultimately lead to delay in treatment, increased transmission, and poor outcome [74]. However, it should also be noted that the diagnosis of tuberculosis in patients infected with HIV is difficult due to reduced bacterial load and cavitation, as well as poor performance of the standard diagnostic tools [75–77]. Additionally, HIV-associated superinfection may be a motivating factor for drug-susceptible conversion to resistant tuberculosis [78]. Research also shows that multiple tuberculosis strain infections frequently interfere in HIV patients [79, 80]. It may also show misleading phenotypic drug-resistance diagnosis [81]. As such, the extent of spread when confirming MDR-TB by a traditional method is far more deadly than is illustrated, especially in HIV-endemic settings.
We acknowledge that our systematic review and meta-analysis has limitations. There was disproportionate allocation of studies among the six WHO regions. Additionally, the included studies were mostly from Africa (i.e., 25 of 54 studies from Africa). Moreover, the largest nation, China, is not included in the study. Besides, the database search language was restricted to English. Therefore, the above limitations might curb the generalizability of our findings. Moreover, substantial heterogeneity was observed between the studies, although it was expected to be from the differences in MDR-TB and HIV ascertainment, study design, and data collection methods between the selected studies. Mild or no heterogeneity was also observed in a different subgroup analysis. We included only the observational studies which are susceptible to selection bias.
Conclusion
The meta-analysis clearly shows a growing trend in MDR-TB risk among HIV-infected people. Balanced resource allocation for Asian, European and African countries should be considered to halt both primary and secondary MDR-TB, especially among those with increasing age. As such, the enhancement of the diagnosis and proper overall management of MDR-TB among HIV-positive individuals has become crucial in achieving WHO’s goals of ‘End TB’ by 2035.
Supplementary Information
Additional file 1 : Table S1. Study quality assessment details for case-control, cohort, and cross-sectional studies.
Additional file 2 : Table S2. Quality Assessment of Included Studies by New-Castle Ottawa Scale.
Additional file 3 : Table S3. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist.
Additional file 4. References of studies excluded after full-text review.
Additional file 5. Sensitivity analysis.
Acknowledgements
The authors would like to thank Abrar Wahab for English editing. We would also like to thank the two anonymous reviewers and the editor for insightful comments that improved the presentation and clarity of our manuscript.
Abbreviations
- MDR-TB
Multidrug-resistant tuberculosis
- HIV
Human immunodeficiency virus
- WHO
World Health Organization
- OR
Odds Ratio
- CI
Confidence interval of odds ratio
Appendix
Table 3.
Search strategy
| A. PubMed | ||
| #1 | “Multidrug resistant tuberculosis” [All Fields] OR “MDR-TB” [All Fields] OR “Drug resistant TB” [All Fields] OR “Risk factors of MDR-TB” [All Fields] OR “Predictors of MDR-TB” [All Fields] | 21,717 |
| #2 | “HIV” [All Fields] OR “Human Immunodeficiency Virus” [All Fields] | 287,526 |
| #3 | #1 AND #2 | 9423 |
| Searching date starting from 01/01/2010 to 30/07/2020 | ||
| B. Google scholar | ||
| #1 | “Multidrug resistant tuberculosis” OR “MDR-TB” OR “Drug resistant TB” OR “Risk factors of MDR-TB” OR “Predictors of MDR-TB” | 226,850 |
| #2 | “HIV” OR “Human Immunodeficiency Virus” | 1,570,000 |
| #3 | #1 AND #2 | 154,780 |
| Searching date starting from 01/01/2010 to 30/07/2020 | ||
| C. Science direct | ||
| #1 | “Multidrug resistant tuberculosis” OR “MDR-TB” OR “Drug resistant TB” OR “Risk factors of MDR-TB” OR “Predictors of MDR-TB” | 25,448 |
| #2 | “HIV” OR “Human Immunodeficiency Virus” | 192,835 |
| #3 | #1 AND #2 | 20,070 |
| Searching date starting from 01/01/2010 to 30/07/2020 | ||
Authors’ contributions
ZZS, FH, and AH conceived the study and, together with ZZS and FH developed the protocol. ZZS and FH did the literature search and selected the studies. Along with ZZS and FH, AI, HRK, JB and DHH ascertained the methodological quality of the study and extracted the relevant information. ZZS and FH synthesized the data. AH completed the analysis and maintained the database. ZZS, FH, and AH wrote the first draft of the paper. JB, SA, and AH critically revised successive drafts of the paper and approved its final version. AH is the guarantor of the study.
Funding
There was a student seed fund from North South University to conduct the study. The funders had no role in study design, collection, analysis, interpretation of the data, writing of the report, or decision to submit the work for publication. The corresponding author had full access to all study data and the authorized person for the final submission of the paper for publication.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not required.
Consent for publication
Not applicable.
Competing interests
None of the authors in this study have any conflict of interest regarding the publication of the paper.
Footnotes
The authors identified a typo in the Abstract, Methods section: it is written January 12,010, but it should be January 1, 2010. The original article has been corrected.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/20/2021
An amendment to this paper has been published and can be accessed via the original article.
References
- 1.Global tuberculosis report 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (Accessed 23 July 2020).
- 2.World Health Organization. Global tuberculosis report 2013. Geneva: World Health Organization; 2013. https://apps.who.int/iris/handle/10665/91355 (Accessed 23 July 2020). [Google Scholar]
- 3.TB CARE I. International standards for tuberculosis care. 3rd ed. The Hague: TB CARE I; 2014. https://www.who.int/tb/publications/ISTC_3rdEd.pdf?ua=1 (Accessed 23 July 2020). [Google Scholar]
- 4.Mekonnen D, Derbie A, Desalegn E. TB/HIV co-infections and associated factors among patients on directly observed treatment short course in Northeastern Ethiopia: a 4 years retrospective study. BMC Res Notes. 2015;8:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tweya H, Feldacker C, Phiri S, et al. Comparison of treatment outcomes of new smear-positive pulmonary tuberculosis patients by HIV and antiretroviral status in a TB/HIV clinic, Malawi. PLoS ONE. 2013;8:e56248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rifat M, Hall J, Oldmeadow C, Husain A, Hinderaker SG, Milton AH. Factors related to previous tuberculosis treatment of patients with multidrug-resistant tuberculosis in Bangladesh. BMJ Open. 2015;5:e008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolin PJ, Raviglione MC, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull World Health Organ. 1994;72:213–20. [PMC free article] [PubMed] [Google Scholar]
- 8.Kyu HH, Maddison ER, Henry NJ, et al. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2018;18:261–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaron L, Saadoun D, Calatroni I, et al. Tuberculosis in HIV-infected patients: a comprehensive review. Clin Microbiol Infect. 2004;10:388–98. [DOI] [PubMed] [Google Scholar]
- 10.Singh A, Prasad R, Balasubramanian V, Gupta N. Drug-resistant tuberculosis and HIV infection: current perspectives. HIV AIDS. 2020;12:9–31. 10.2147/HIV.S193059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight GM, McQuaid CF, Dodd PJ, Houben RMGJ. Global burden of latent multidrug-resistant tuberculosis: trends and estimates based on mathematical modelling. Lancet Infect Dis. 2019;19:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Lalwani J, Pandey P, Thakur A. Factors associated with the development of secondary multidrug-resistant tuberculosis. Int J Prev Med. 2019;10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan PY, Yates TA, Osman M, et al. Transmission of drug-resistant tuberculosis in HIV-endemic settings. Lancet Infect Dis. 2019;19:e77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suchindran S, Brouwer ES, Van Rie A. Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLoS ONE. 2009;4:e5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesfin YM, Hailemariam D, Biadgilign S, Biadglign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2014;9:e82235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [PMC free article] [PubMed] [Google Scholar]
- 18.Brito RC, Mello FCQ, Andrade MK, et al. Drug-resistant tuberculosis in six hospitals in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2010;14:24–33. [PubMed] [Google Scholar]
- 19.Andrews JR, Shah NS, Weissman D, Moll AP, Friedland G, Gandhi NR. Predictors of multidrug- and extensively drug-resistant tuberculosis in a high HIV prevalence community. PLoS ONE. 2010;5:e15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangaré L, Diandé S, Badoum G, Dingtoumda B, Traoré AS. Anti-tuberculosis drug resistance in new and previously treated pulmonary tuberculosis cases in Burkina Faso. Int J Tuberc Lung Dis. 2010;14:1424–9. [PubMed] [Google Scholar]
- 21.Sangaré L, Diandé S, Ouédraogo G, Traoré AS. HIV infection and Mycobacterium tuberculosis drug-resistance among tuberculosis patients in Burkina Faso, West Africa. Afr J Clin Exp Microbiol. 2011;12(1). 10.4314/ajcem.v12i1.61047.
- 22.Gudo PS, Cuna Z, Coelho E, et al. Is multidrug-resistant tuberculosis on the rise in Mozambique? Results of a national drug resistance survey. Eur Respir J. 2011;38:222–4. [DOI] [PubMed] [Google Scholar]
- 23.Vadwai V, Shetty A, Soman RN, Rodrigues CC. Determination of risk factors for isoniazid monoresistance and multidrug-resistant tuberculosis in treatment failure patients. Scand J Infect Dis. 2012;44:48–50. [DOI] [PubMed] [Google Scholar]
- 24.Macedo R, Antunes AF, Villar M, Portugal I. Multidrug and extensively drug-resistant tuberculosis in Lisbon and Vale do Tejo, Portugal, from 2008 to 2010. Int J Mycobacteriol. 2012;1:131–6. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Padilla E, Dlamini T, Ascorra A, et al. High prevalence of multidrug-resistant tuberculosis, Swaziland, 2009–2010. Emerg Infect Dis. 2012;18:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Halsema CL, Fielding KL, Chihota VN, Lewis JJ, Churchyard GJ, Grant AD. Trends in drug-resistant tuberculosis in a gold-mining workforce in South Africa, 2002–2008. Int J Tuberc Lung Dis. 2012;16:967–73. [DOI] [PubMed] [Google Scholar]
- 27.Ricks PM, Mavhunga F, Modi S, et al. Characteristics of multidrug-resistant tuberculosis in Namibia. BMC Infect Dis. 2012;12:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tessema B, Beer J, Emmrich F, Sack U, Rodloff AC. First- and second-line anti-tuberculosis drug resistance in Northwest Ethiopia. Int J Tuberc Lung Dis. 2012;16:805–11. [DOI] [PubMed] [Google Scholar]
- 29.Coelho AGV, Zamarioli LA, Telles MA, Ferrazoli L, Waldman EA. A study of multidrug-resistant tuberculosis in risk groups in the city of Santos, São Paulo, Brazil. Mem Inst Oswaldo Cruz. 2012;107:760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulmasova DJ, Uzakova G, Tillyashayhov MN, Turaev L, van Gemert W, Hoffmann H, Zignol M, Kremer K, Gombogaram T, Gadoev J, du Cros P, Muslimova N, Jalolov A, Dadu A, de Colombani P, Telnov O, Slizkiy A, Kholikulov B, Dara M, Falzon D. Multidrug-resistant tuberculosis in Uzbekistan:results of a nationwide survey, 2010 to 2011. Euro Surveill. 2013;18(42):20609. 10.2807/1560-7917.es2013.18.42.20609. PMID: 24176581. [DOI] [PubMed]
- 31.Minion J, Gallant V, Wolfe J, Jamieson F, Long R. Multidrug and extensively drug-resistant tuberculosis in Canada 1997–2008: demographic and disease characteristics. PLoS ONE. 2013;8:e53466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sethi S, Mewara A, Dhatwalia SK, et al. Prevalence of multidrug resistance in Mycobacterium tuberculosis isolates from HIV seropositive and seronegative patients with pulmonary tuberculosis in north India. BMC Infect Dis. 2013;13:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Hof S, Tursynbayeva A, Abildaev T, et al. Converging risk factors but no association between HIV infection and multidrug-resistant tuberculosis in Kazakhstan. Int J Tuberc Lung Dis. 2013;17:526–31. [DOI] [PubMed] [Google Scholar]
- 34.Lukoye D, Adatu F, Musisi K, Kasule GW, Were W, Odeke R, Kalamya JN, Awor A, Date A, Joloba ML. Anti-tuberculosis drug resistance among new and previously treated sputum smear-positive tuberculosis patients in Uganda: results of the first national survey. PLoS One. 2013;8(8):e70763. 10.1371/journal.pone.0070763. PMID: 23936467; PMCID: PMC3731251. [DOI] [PMC free article] [PubMed]
- 35.Hang NT, Maeda S, Lien LT, Thuong PH, Hung NV, Thuy TB, Nanri A, Mizoue T, Hoang NP, Cuong VC, Ngoc KT, Sakurada S, Endo H, Keicho N. Primary drug-resistant tuberculosis in Hanoi, Viet Nam: present status and risk factors. PLoS One. 2013;8(8):e71867. 10.1371/journal.pone.0071867. PMID: 23967255; PMCID: PMC3742467. [DOI] [PMC free article] [PubMed]
- 36.Skrahina A, Hurevich H, Zalutskaya A, et al. Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull World Health Organ. 2013;91:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satti H, McLaughlin MM, Seung KJ, Becerra MC, Keshavjee S. High risk of drug-resistant tuberculosis when first-line therapy fails in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2013;17:100–6. [DOI] [PubMed] [Google Scholar]
- 38.Hirpa S, Medhin G, Girma B, et al. Determinants of multidrug-resistant tuberculosis in patients who underwent first-line treatment in Addis Ababa: a case control study. BMC Public Health. 2013;13:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mor Z, Goldblatt D, Kaidar-Shwartz H, Cedar N, Rorman E, Chemtob D. Drug-resistant tuberculosis in Israel: risk factors and treatment outcomes. Int J Tuberc Lung Dis. 2014;18:1195–201. [DOI] [PubMed] [Google Scholar]
- 40.Post FA, Grint D, Werlinrud AM, et al. Multi-drug-resistant tuberculosis in HIV positive patients in Eastern Europe. J Infect. 2014;68:259–63. [DOI] [PubMed] [Google Scholar]
- 41.Metcalfe JZ, Makumbirofa S, Makamure B, et al. Drug-resistant tuberculosis in high-risk groups, Zimbabwe. Emerg Infect Dis. 2014;20:135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohd Shariff N, Shah SA, Kamaludin F. Previous treatment, sputum-smear nonconversion, and suburban living: the risk factors of multidrug-resistant tuberculosis among Malaysians. Int J Mycobacteriol. 2016;5:51–8. [DOI] [PubMed] [Google Scholar]
- 43.Jitmuang A, Munjit P, Foongladda S. Prevalence and factors associated with multidrug-resistant tuberculosis at Siriraj Hospital, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2015;46(4):697–706 PMID: 26867390. [PubMed] [Google Scholar]
- 44.Chuchottaworn C, Thanachartwet V, Sangsayunh P, Than TZ, Sahassananda D, Surabotsophon M, Desakorn V. Risk Factors for Multidrug-Resistant Tuberculosis among Patients with Pulmonary Tuberculosis at the Central Chest Institute of Thailand. PLoS One. 2015;10(10):e0139986. 10.1371/journal.pone.0139986. PMID: 26444421; PMCID: PMC4596622. [DOI] [PMC free article] [PubMed]
- 45.Elmi OS, Hasan H, Abdullah S, Mat Jeab MZ, Bin Alwi Z, Naing NN. Multidrug-resistant tuberculosis and risk factors associated with its development: a retrospective study. J Infect Dev Ctries. 2015;9:1076–85. [DOI] [PubMed] [Google Scholar]
- 46.Mulisa G, Workneh T, Hordofa N, Suaudi M, Abebe G, Jarso G. Multidrug-resistant Mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int J Infect Dis. 2015;39:57–61. [DOI] [PubMed] [Google Scholar]
- 47.Mulu W, Mekonnen D, Yimer M, Admassu A, Abera B. Risk factors for multidrug resistant tuberculosis patients in Amhara National Regional State. Afr Health Sci. 2015;15:368–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Günther G, van Leth F, Alexandru S, et al. Multidrug-resistant tuberculosis in Europe, 2010–2011. Emerg Infect Dis. 2015;21:409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ershova JV, Volchenkov GV, Kaminski DA, et al. Epidemiology of primary multidrug-resistant tuberculosis, Vladimir Region, Russia. Emerg Infect Dis. 2015;21:2048–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdella K, Abdissa K, Kebede W, Abebe G. Drug resistance patterns of Mycobacterium tuberculosis complex and associated factors among retreatment cases around Jimma, Southwest Ethiopia. BMC Public Health. 2015;15:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tadesse F. Risk factors for multi-drug resistant tuberculosis in Addis Ababa, Ethiopia; 2015. [Google Scholar]
- 52.Salindri AD, Kipiani M, Kempker RR, et al. Diabetes reduces the rate of sputum culture conversion in patients with newly diagnosed multidrug-resistant tuberculosis. Open Forum Infect Dis. 2016;3:ofw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S, Lee SH, Mok JH, et al. Is Multi-drug resistant tuberculosis more prevalent in HIV-infected patients in Korea? Yonsei Med J. 2016;57:1508–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Assefa D, Seyoum B, Oljira L. Determinants of multidrug-resistant tuberculosis in Addis Ababa, Ethiopia. Infect Drug Resist. 2017;10:209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Workicho A, Kassahun W, Alemseged F. Risk factors for multidrug-resistant tuberculosis among tuberculosis patients: a case-control study. Infect Drug Resist. 2017;10:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha P, Srivastava GN, Gupta A, Anupurba S. Association of Risk Factors and Drug Resistance Pattern in Tuberculosis Patients in North India. J Global Infect Dis. 2017;9:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mesfin EA, Beyene D, Tesfaye A, et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS ONE. 2018;13:e0197737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gobena D, Ameya G, Haile K, Abreha G, Worku Y, Debela T. Predictor of multidrug resistant tuberculosis in southwestern part of Ethiopia: a case control study. Ann Clin Microbiol Antimicrob. 2018;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kusumawati RL, Tania T, Mcneil E, Chongsuvivatwong V. Predictors of multidrug resistance among pulmonary tuberculosis patients in a tertiary Hospital in North Sumatera, Indonesia; 2018. 10.15562/bmj.v7i1.813. [Google Scholar]
- 60.Pavlenko E, Barbova A, Hovhannesyan A, et al. Alarming levels of multidrug-resistant tuberculosis in Ukraine: results from the first national survey. Int J Tuberc Lung Dis. 2018;22:197–205. [DOI] [PubMed] [Google Scholar]
- 61.Desissa F, Workineh T, Beyene T. Risk factors for the occurrence of multidrug-resistant tuberculosis among patients undergoing multidrug-resistant tuberculosis treatment in East Shoa, Ethiopia. BMC Public Health. 2018;18:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaborit BJ, Revest M, Roblot F, et al. Characteristics and outcome of multidrug-resistant tuberculosis in a low-incidence area. Med Mal Infect. 2018;48:457–64. [DOI] [PubMed] [Google Scholar]
- 63.Alene KA, Viney K, McBryde ES, Gray DJ, Melku M, Clements ACA. Risk factors for multidrug-resistant tuberculosis in northwest Ethiopia: a case-control study. Transbound Emerg Dis. 2019;66:1611–8. [DOI] [PubMed] [Google Scholar]
- 64.Baya B, Achenbach CJ, Kone B, et al. Clinical risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in Mali. Int J Infect Dis. 2019;81:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zürcher K, Ballif M, Fenner L, et al. Drug susceptibility testing and mortality in patients treated for tuberculosis in high-burden countries: a multicentre cohort study. Lancet Infect Dis. 2019;19:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okethwangu D, Birungi D, Biribawa C, et al. Multidrug-resistant tuberculosis outbreak associated with poor treatment adherence and delayed treatment: Arua District, Uganda, 2013–2017. BMC Infect Dis. 2019;19:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fikre A, Tewelde T, Shaweno T. Determinants of multi-drug resistant tuberculosis among tuberculosis patients in Southern Ethiopia: a case control study. J Med Bacteriol. 2019;8:1–12. [Google Scholar]
- 68.Elduma AH, Mansournia MA, Foroushani AR, et al. Assessment of the risk factors associated with multidrug-resistant tuberculosis in Sudan: a case-control study. Epidemiol Health. 2019;41:e2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen MP, Miramontes R, Kammerer JS. Multidrug-resistant tuberculosis in the United States, 2011–2016: patient characteristics and risk factors. Int J Tuberc Lung Dis. 2020;24(1):92–9. 10.5588/ijtld.19.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arroyo LH, Yamamura M, Ramos ACV, et al. Determinants of multidrug-resistant tuberculosis in São Paulo-Brazil: a multilevel Bayesian analysis of factors associated with individual, community and access to health services. Tropical Med Int Health. 2020;25(7):839–49. 10.1111/tmi.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirama T, Sabur N, Derkach P, et al. Risk factors for drug-resistant tuberculosis at a referral centre in Toronto, Ontario, Canada: 2010–2016. Can Commun Dis Rep. 2020;46(4):84–92. Published 2020 Apr 2. 10.14745/ccdr.v46i04a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.World Bank Country and Lending Groups – World Bank data help desk. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (Accessed 23 Oct 2019).
- 73.Kendall EA, Fofana MO, Dowdy DW. The burden of transmitted multi-drug resistance among epidemics of tuberculosis: a transmission model. Lancet Respir Med. 2015;3:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Yuan Z, Shen X, Wu J, Wu Z, Xu B. Time to multidrug-resistant tuberculosis treatment initiation in association with treatment outcomes in Shanghai, China. Antimicrob Agents Chemother. 2018;62:e02259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oni T, Burke R, Tsekela R, et al. High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax. 2011;66:669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwan CK, Ernst JDHIV. Tuberculosis: a deadly human syndemic. Clin Microbiol Rev. 2011;24:351–76. 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El-Sadr WM, Tsiouris SJ. HIV-associated tuberculosis: diagnostic and treatment challenges. Semin Respir Crit Care Med. 2008;29:525–31. [DOI] [PubMed] [Google Scholar]
- 78.van Rie A, Victor TC, Richardson M, et al. Reinfection and mixed infection cause changing Mycobacterium tuberculosis drug-resistance patterns. Am J Respir Crit Care Med. 2005;172:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanekom M, Streicher EM, de Berg DV, et al. Population structure of mixed Mycobacterium tuberculosis infection is strain genotype and culture medium dependent. PLoS ONE. 2013;8:e70178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen T, Chindelevitch L, Misra R, et al. Within-host heterogeneity of Mycobacterium tuberculosis infection is associated with poor early treatment response: a prospective cohort study. J Infect Dis. 2016;213:1796–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Makhado NA, Matabane E, Faccin M, et al. Outbreak of multidrug-resistant tuberculosis in South Africa undetected by WHO-endorsed commercial tests: an observational study. Lancet Infect Dis. 2018;18:1350–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 : Table S1. Study quality assessment details for case-control, cohort, and cross-sectional studies.
Additional file 2 : Table S2. Quality Assessment of Included Studies by New-Castle Ottawa Scale.
Additional file 3 : Table S3. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist.
Additional file 4. References of studies excluded after full-text review.
Additional file 5. Sensitivity analysis.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.