Abstract
Purpose
Current treatment options for muscle-invasive bladder cancer (MIBC) are associated with substantial morbidity. Local release of doxorubicin (DOX) from phosphatidyldiglycerol-based thermosensitive liposomes (DPPG2-TSL-DOX) potentiated by hyperthermia (HT) in the bladder wall may result in bladder sparing without toxicity of systemic chemotherapy. We investigated whether this approach, compared to conventional DOX application, increases DOX concentrations in the bladder wall while limiting DOX in essential organs.
Materials and Methods
Twenty-one pigs were anaesthetized, and a urinary catheter equipped with a radiofrequency-emitting antenna for HT (60 minutes) was placed. Experimental groups consisted of iv low or full dose (20 or 60 mg/m2) DPPG2-TSL-DOX with/without HT, iv low dose (20 mg/m2) free DOX with HT, and full dose (50 mg/50 mL) intravesical DOX with/without HT. After the procedure, animals were immediately sacrificed. HPLC was used to measure DOX levels in the bladder, essential organs and serum, and fluorescence microscopy to evaluate DOX distribution in the bladder wall.
Results
Iv DPPG2-TSL-DOX with HT resulted in a significantly higher bladder wall DOX concentration which was more homogeneous distributed, than iv and intravesical free DOX administration with HT. Specifically in the detrusor, DPPG2-TSL-DOX with HT led to a >7- and 44-fold higher DOX concentration, compared to iv free DOX with HT and intravesical DOX, respectively. Organ DOX concentrations were significantly lower in heart and kidneys, and similar in liver, spleen and lungs, following iv DPPG2-TSL-DOX with HT, compared to iv free DOX. Intravesical DOX led to the lowest organ DOX concentrations.
Conclusion
Iv DPPG2-TSL-DOX combined with HT achieved higher DOX concentrations in the bladder wall including the detrusor, compared to conventional iv and intravesical DOX application. In combination with lower DOX accumulation in heart and kidneys, compared to iv free chemotherapy, DPPG2-TSL-DOX with HT has great potential to attain a role as a bladder-sparing treatment for MIBC.
Keywords: MIBC, drug delivery system, therapy, chemotherapeutic, local release, porcine model
Introduction
In Western countries, bladder cancer is the fourth most common cancer in men and the ninth in women.1 About 25% of newly diagnosed patients have muscle invasive bladder cancer (MIBC), and up to 20% of the remaining non-muscle invasive bladder cancer patients (NMIBC) progress to muscle invasive disease in 5 years.2
A key problem with the standard radical treatment for MIBC is the high morbidity and mortality. For MIBC that is confined to the bladder, current treatment guidelines advise neoadjuvant chemotherapy (NAC) followed by radical cystectomy (RC) with urinary diversion and lymph node dissection.3 About 60% of patients develop complications within 90 days after surgery, including infections.4 Late complications are also common and are linked to the type of urinary diversion, like stoma problems and impairment of renal function.5 The mortality is up to 8.0% within 90 days after surgery.6 Moreover, physical and social functioning of MIBC patients is significantly impaired after RC.7 Since most patients presenting with MIBC are older than 70 years and frequently have comorbidities, these patients are regularly unfit for or decline major surgery.
Current bladder-sparing treatment, which includes the use of systemic chemotherapy, has failed to show superiority over RC and has significant shortcomings. In this multimodality treatment (MMT) the bladder tumor is removed as radical as possible by transurethral resection (TURB) followed by chemoradiation.3 Although MMT could result in comparable survival rates as RC in highly selected patients, no RCTs comparing MMT with RC have been completed. Moreover, up to 30% of patients need salvage cystectomy. Although chemotherapy (NAC and as part of MMT) has shown efficacy in MIBC to some extent, the main drawbacks of iv chemotherapy are poor accessibility to tumor tissue and systemic toxicity,8 such as cardiomyopathy, mucosal toxicity and myelosuppression for doxorubicin (DOX).9
Chemotherapeutic drug delivery systems using liposomes could potentially lead to more specific drug delivery, improving the local effect and limiting drug-related side effects. Passive targeting can be accomplished through the enhanced permeability and retention (EPR) phenomenon, initially described by Matsumura and Maeda et al10. Nanoparticles passively accumulate in tumors based on the nanometer size range and the leaky vasculature and impaired lymphatic drainage.11
To date, liposomal formulations encapsulating chemotherapeutic agents approved for clinical use have not shown improved cancer outcome over standard chemotherapy. Doxil was approved by the FDA 25 years ago and has a role in the standard clinical management of patients with Kaposi's sarcoma, ovarian cancer and metastatic breast cancer in which prior (chemo)therapy has failed.12 Clinical studies show that Doxil leads to a more attractive toxicity profile, but does not improve anti-tumor activity compared to iv free DOX.13–17 This is largely explained by the passive targeting mechanism, which exploits tumor angiogenesis and the often impaired vasculature and leads to drug accumulation in the tumor of less than 4% of the administered dose.18 In addition, the release of the active ingredient from the liposomes is rather slow.19
Where classical liposomal drug delivery systems are dependent on passive drug release, thermosensitive liposomes (TSL) might improve efficacy by heat-triggered local release of encapsulated drug induced by hyperthermia (HT). TSL release their cargo once the temperature is higher than the threshold value which could be adjusted by the phospholipid composition.20 However, the only TSL-system that has entered clinical studies, ie, low-temperature sensitive liposomal doxorubicin (LTLD; Thermodox), did not improve progression-free survival in hepatocellular carcinoma patients when added to radiofrequency (RF) ablation.21 This was presumably due to insufficient heating of multiple liver tumors in combination with the short half-life of LTLD. LTLD combined with conductive hyperthermia has also been studied in a pig bladder model and showed improved drug accumulation and distribution in the bladder wall.22 A newly developed TSL consists of the three phospholipid excipients 1.2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1.2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1.2-dipalmitoyl-sn-glycero-3-phosphodiglycerol (DPPG2; DPPG2-TSL). DPPG2-TSL encapsulating carboxyfluorescein (to monitor drug release) or gemcitabine showed a high stability at physiologic temperatures with sustained high plasma levels for 60 minutes and a rapid drug release at 40–42°C in vitro and in vivo.23,24 Moreover, DPPG2-TSL with encapsulated DOX (DPPG2-TSL-DOX) showed improved stability in complete human serum compared to a LTLD-mimicking formulation.25 DPPG2-TSL-DOX was investigated for treatment of feline sarcoma.26
DPPG2-TSL-DOX combined with HT may have potential as treatment for MIBC. RF-induced HT can achieve sufficient and stable temperatures in the bladder wall and has already proven its efficacy in treatment of NMIBC when combined with intravesical instillation of chemotherapeutics (chemohyperthermia [CHT]).27–30 Since MIBC invades the detrusor muscle layer of the bladder, homogeneous distributed and high DOX concentrations in the full bladder wall are required. The intravascular release of DOX in the bladder wall from DPPG2-TSL-DOX by HT could provide these conditions. Additionally, HT can potentiate the effect of DOX due to increased tissue penetration and induction of an immune response.31,32
Administration of DPPG2-TSL-DOX was combined with bladder HT to investigate whether this results in higher and more homogeneous distributed DOX concentration in the bladder wall, while limiting DOX concentration in essential organs, compared to conventional DOX application (ie, iv administration, intravesical instillation and CHT). Since the physiology and urinary tract anatomy of pigs closely resembles that of humans, a pig bladder model was used.
Materials and Methods
We performed the experiments at the animal facility at the Radboud University Medical Center in Nijmegen, the Netherlands. The study was approved by the appropriate ethical committees on animal experiments (Dierexperimentencommissie and Centrale Commissie Dierproeven) under the number 2016–0066. The European Union Directive 2010–63-EU for welfare of the laboratory animals was followed.
Animals and Experimental Groups
Local vendors supplied 21 female pigs of 75–85 kg, which were randomly assigned to seven experimental groups of three pigs each.
The experimental groups consisted of: iv low dose DPPG2-TSL-DOX (20 mg/m2) with or without HT (60 minutes), iv low dose DPPG2-TSL-DOX (20 mg/m2) with extended HT (90 minutes), iv low dose free DOX (20 mg/m2) with HT, iv full dose (60 mg/m2) DPPG2-TSL-DOX with HT, and full dose intravesical DOX (50 mg/50 mL) with or without HT.
The iv “full dose” refers to the standard clinical dose of 60 mg/m2 DOX administered in cancer patients. Because it was unclear whether side effects would occur, the iv free DOX group and three DPPG2-TSL-DOX groups received one-third of the iv full dose. Once it was clear that this dose was safe, full dose was administered in one DPPG2-TSL-DOX group and in the intravesical DOX groups. The intravesical groups received bladder instillations with a corresponding full dose of 50 mg DOX dissolved in 50 mL physiologic saline, which was refreshed after 30 minutes, similar to CHT protocol for bladder cancer treatment. Intravenous DPPG2-TSL-DOX and free DOX were dosed based on the following formula: body surface area of pigs (in m2) = 0.0734 * body weight 0.656.33 The pigs received infusion of iv medication over 30 minutes.
Materials
Thermosome GmbH provided DPPG2-TSL-DOX as a dispersion in 10 mL vials of two independently prepared batches. DOX concentration in the batches was measured with HPLC and was 1.6 mg/mL and 1.9 mg/mL, respectively. Mean particle size (z average) was in the range between 110 and 130 nm with a narrow, monodisperse particle size distribution with a polydispersity index of <0.10 for both batches.
Experimental Procedure
Pigs were sedated with ketamine and midazolam and anesthetized with propofol and isoflurane. Intravenous access was obtained by placing two intravenous catheters in each ear: one for the experimental medication, one for the anesthetics. General vital signs were monitored by standard non-invasive measurements, including the body temperature with a deep nose temperature probe. The femoral artery was cannulated for continuous arterial blood pressure monitoring and blood sampling every 15 minutes. From the fourth pig that received DPPG2-TSL-DOX onwards, animals receiving DPPG2-TSL-DOX were premedicated with 10 mg dexamethasone and 2 mg clemastine to prevent nanoparticle-induced anaphylactoid infusion reactions after this was observed in the first three pigs.34 A transurethral catheter was placed for HT and/or instillation of intravesical solutions.
Subsequently, the bladder was approached through median laparotomy and nine thermocouples (TCs) with a ± 0.1°C accuracy were placed for temperature mapping at the bladder neck, side wall, and dome; in the mucosa, detrusor muscle and serosa. TCs were fixed with tissue glue (Derma+Flex®, Chemence Medical Products Inc, Alpharetta, GA, USA) and 6–0 polypropylene sutures (Prolene, Ethicon, LLC, USA). Temperatures were recorded using a Keithley Multiplexer device (CN Rood, Zoetermeer, The Netherlands).
A Synergo® system, including a three-way 20-French catheter equipped with three TCs and a 915 MHz RF waves emitting antenna, was used to achieve HT. Iv free and liposomal DOX was administered in HT groups as soon as the intravesical temperature was stable at 43°C. Relying on the three intravesical TCs, this temperature was aimed to be maintained in the bladder for 60 (HT) or 90 (extended HT) minutes, in respectively four HT groups or the one extended HT group.
Necropsy
Animals receiving HT were sacrificed immediately 60 minutes (or 90 minutes in the extended HT group) from the moment an intravesical temperature of 43°C was established, and animals that did not receive HT were sacrificed immediately 60 minutes after administration of experimental mediation, all using pentobarbital (25 mg/kg). Subsequently, the bladder was emptied and flushed twice with 50 mL physiological saline to remove any residual free DOX. The bladder vasculature was clamped and the bladder was removed and the bladder neck, side wall, and dome were biopsied. Samples were divided into mucosa, detrusor muscle and serosa. Additionally, biopsies from the heart, kidney, liver, lung and spleen were obtained by a standardized protocol. Samples harvested to determine DOX concentrations were weighed and snap-frozen, tissue for histopathology was fixed in formaldehyde and paraffin embedded, and tissue for fluorescence was snap-frozen in TissueTec.
HPLC DOX Assessment in Tissue and Plasma
DOX plasma and tissue sample levels were quantified with high-performance liquid chromatography (HPLC) following the slightly adapted method as described by Peller et al35, without distinguishing between free and DPPG2-TSL encapsulated DOX. For tissues, solid-phase extraction was performed with Strata-X columns (60 mg/3 mL, Phenomenex Ltd., Aschaffenburg, Germany) and DOX elution was achieved by aqueous washing solutions with 0–30% methanol and 2% formic acid in methanol. HPLC analysis was conducted with the limit of quantification in tissue and serum samples being 0.2 ng/mg and 0.2 ng/µL DOX, respectively.
DOX Fluorescence in the Bladder Wall
DOX autofluorescence was used for DOX imaging and immunohistochemistry (IHC) was performed to visualize cytokeratin (epithelial cells), Von Willebrand Factor (VWF; blood vessel walls), and smooth muscle actin filaments (SMA; muscle cells). Two subsequent slides of snap-frozen bladder biopsies were prepared (A and B). The slides were fixed for 10 minutes in acetone, washed three times in phosphate-buffered saline (PBS) and blocked with 10% goat serum in PBS for 30 minutes, again washed, and incubated over night at 4°C with primary antibodies (1:200 dilution with 10% bovine serum albumin [BSA]). Slide A was incubated with the combination of mouse monoclonal anti-pan cytokeratin antibody (AE1/AE3 + 5D3; ab86734, Abcam, Cambridge, UK) and rabbit polyclonal anti-human VWF (A0082, Dako Agilent, Hamburg, Germany) antibody. Slide B was incubated with rabbit polyclonal anti-SMA (ab5694, Abcam, Germany). Slides were washed with PBS and incubated with secondary antibodies for 30 minutes at room temperature under light protection. Slide A was incubated with AlexaFluor 594 goat anti-rabbit IgG H&L (A11037, Fisher Scientific GmbH, Schwerte, Germany) and AlexaFluor 488 goat anti-mouse IgG H&L (ab150113, Abcam, Germany), and slide B only with AlexaFluor 594 (all 1:500 dilution in 10% BSA). After a final wash with PBS, slides were mounted using fluorescent mounting medium (Fluoromount-G, Southern Biotechnology Associates Inc, Birmingham, USA) and imaged with a Leica DMI6000 B-inverted microscope and Leica MM AF software. ImageJ software was used to edit the images and combine the photos of slide A and B digitally.
Histopathological Assessment
For histopathological assessment, 10 µm slides of paraffin embedded material were haematoxylin and eosin (HE) stained. Images were obtained with a Leica DMD108 digital microimaging system.
Data Analysis and Statistics
Statistical analyses were performed with GraphPad Prism version 5.03. A one-way ANOVA analysis with a Dunnett or Tukey correction for multiple comparisons was performed to compare groups with corresponding doses (low or full; see Figure 1A–C and 2A–E for the comparisons), where appropriate. A students t-test was performed to compare low versus full dose DPPG2-TSL-DOX with HT groups. Temperature was evaluated using R statistical package version 3.2.4.
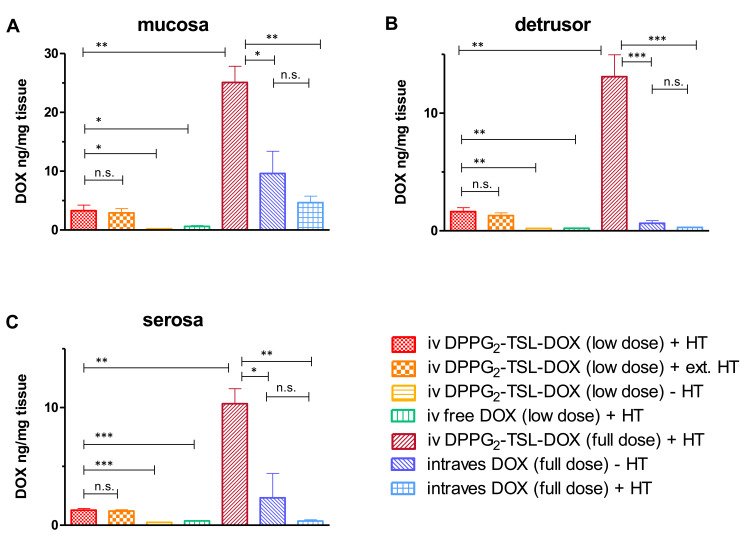
Figure 1.
DOX concentrations per group in (A) mucosa, (B) detrusor and (C) serosa. ***p<0.001; **p<0.01; *p < 0.05; n.s.: not significant. Error bars represent standard error of mean.
Abbreviations: DOX, doxorubicin; DPPG2, 1.2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; HT, hyperthermia; intraves, intravesical; iv, intravenous; TSL, thermosensitive liposomes.
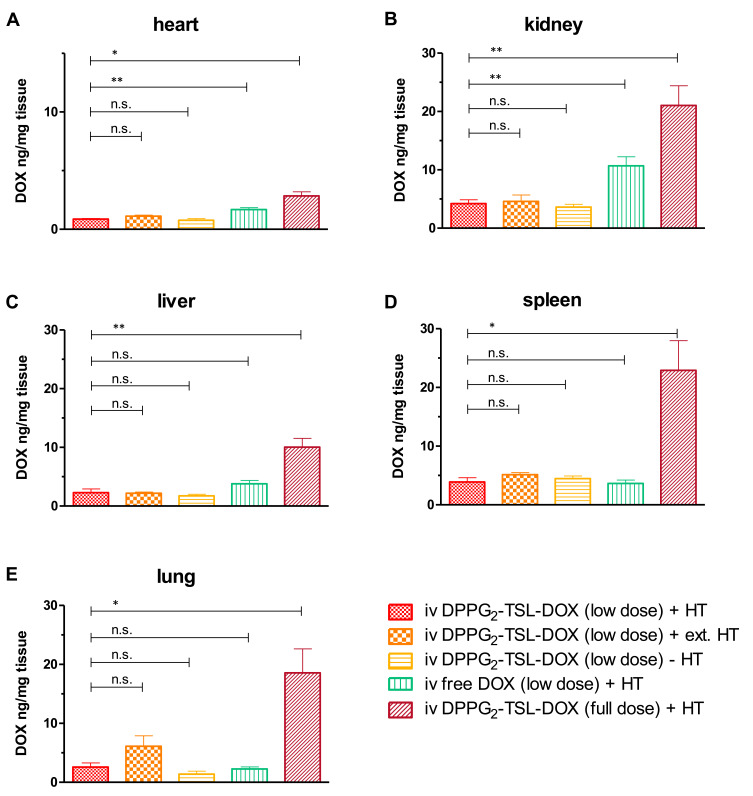
Figure 2.
DOX concentrations per group in (A) heart, (B) kidney, (C) liver, (D) spleen and (E) lung. **p<0.01; *p < 0.05. Error bars represent standard error of mean.
Abbreviations: n.s., not significant; DOX, doxorubicin; DPPG2, 1.2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; HT, hyperthermia; iv, intravenous; TSL, thermosensitive liposomes.
Results
DOX Concentration
Bladder Wall
Intravenous injection of low dose DPPG2-TSL-DOX combined with local HT resulted in a significantly higher DOX concentration in mucosa, detrusor and serosa compared to low dose iv free DOX with HT (p=0.01, p=0.002 and p<0.001, respectively; Table 1, Figure 1A–C). In the detrusor, low dose iv DPPG2-TSL-DOX combined with HT resulted in a more than 7-fold higher DOX concentration compared to a similar dose iv free DOX with HT (1.63 ± 0.34 vs 0.22 ± 0.02, p=0.002). Intravenous full dose DPPG2-TSL-DOX with HT also resulted in a higher DOX concentration than full dose intravesical free DOX with and without HT in all bladder layers (detrusor p<0.001, mucosa and serosa p=0.005). In the detrusor, the increase of DOX concentration by full dose iv DPPG2-TSL-DOX with HT was 44-fold and 21-fold in comparison to full dose intravesical free DOX with and without HT, respectively. In bladder wall biopsies of the control group that received low dose DPPG2-TSL-DOX without HT, DOX concentrations were below the limit of quantification of 0.20 ng/mg in mucosa and detrusor, and levels in the serosa were only 0.24 ± 0.04 ng/mg. A threefold increase in the DPPG2-TSL-DOX dose (to full dose) combined with HT resulted in an eight times higher DOX concentration in the mucosa, detrusor and serosa, compared to the low dose. The extension of HT from 60 to 90 minutes combined with low dose DPPG2-TSL-DOX did not significantly increase tissue DOX concentrations in any bladder layer. DOX concentrations in various bladder layers after intravesical DOX administration were independent of HT.
Table 1.
DOX Concentrations per Group and Bladder Wall Layer
| Group | Mucosa | Detrusor | Serosa |
|---|---|---|---|
| iv DPPG2-TSL-DOX (low dose) + HT | 3.29 ± 0.94 | 1.63 ± 0.34 | 1.28 ± 0.15 |
| iv DPPG2-TSL-DOX (low dose) + ext. HT | 2.91 ± 0.73 | 1.29 ± 0.25 | 1.21 ± 0.12 |
| iv DPPG2-TSL-DOX (low dose) - HT | <0.20* | <0.20* | 0.24 ± 0.04 |
| iv free DOX (low dose) + HT | 0.61 ± 0.15 | 0.22 ± 0.02 | 0.37 ± 0.03 |
| iv DPPG2-TSL-DOX (full dose) + HT | 25.10 ± 2.74 | 13.11 ± 1.86 | 10.33 ± 1.28 |
| intraves DOX (full dose) - HT | 9.62 ± 3.77 | 0.64 ± 0.23 | 2.33 ± 2.07 |
| intraves DOX (full dose) + HT | 4.66 ± 1.11 | 0.30 ± 0.03 | 0.36 ± 0.11 |
Notes: Concentrations are ± standard error of mean in ng/mg; *Represents below limit of quantification.
Abbreviations: DOX, doxorubicin; DPPG2, 1.2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; HT, hyperthermia; intraves intravesical; iv, intravenous; TSL, thermosensitive liposomes.
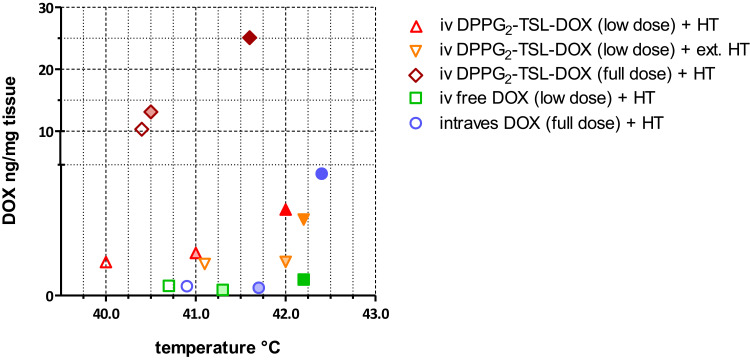
Figure 3 shows the DOX concentration in different bladder layers plotted against the corresponding temperatures for DPPG2-TSL-DOX with HT groups, showing an increase from serosa towards mucosa level, following the temperature gradient. DOX concentrations did not differ significantly between biopsies harvested from different locations in the bladder (neck, side wall and posterior wall) (Supplementary Table 1). In one pig that received DPPG2-TSL-DOX with HT, a mean temperature of only 38.7°C was reached. However, the bladder DOX concentrations of this animal were similar to the other two animals in the same group.
Figure 3.
DOX concentrations of the HT groups at mucosa (dark filling), detrusor (light filling), and serosa (clear filling).
Abbreviations: DOX, doxorubicin; DPPG2, 1.2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; HT, hyperthermia; intraves, intravesical; iv, intravenous; TSL, thermosensitive liposomes.
Organs
DOX concentrations were significantly lower in the heart and kidney after low dose iv DPPG2-TSL-DOX combined with HT, compared to low dose iv free DOX with HT (p=0.003 and p=0.004; Table 2, Figure 2A and B). In the liver, spleen and lung, after low dose DPPG2-TSL-DOX with HT, concentrations were similar to low dose iv free DOX (Table 2, Figure 2C–E). Extending HT from 60 to 90 minutes did not significantly alter DOX retention in any organ. Interestingly, after increasing the dose of DPPG2-TSL-DOX threefold (to full dose), DOX concentration in spleen and lung were six- and seven-fold higher compared to low dose, respectively. DOX concentrations in organs of animals receiving full dose intravesical DOX were below the quantification levels, except for a very low DOX concentration in the kidney (without HT 0.22 ± 0.02 ng/mg and combined with HT 0.24 ± 0.04 ng/mg).
Table 2.
DOX Concentrations per Group and Organ
| Group | Heart | Liver | Spleen | Kidney | Lung |
|---|---|---|---|---|---|
| iv DPPG2-TSL-DOX (low dose) + HT | 0.86 ± 0.06 | 2.29 ± 0.61 | 3.90 ± 0.71 | 4.21 ± 0.64 | 2.60 ± 0.69 |
| iv DPPG2-TSL-DOX (low dose) + ext. HT | 1.12 ± 0.08 | 2.19 ± 0.20 | 5.13 ± 0.36 | 4.59 ± 1.10 | 6.12 ± 1.76 |
| iv DPPG2-TSL-DOX (low dose) - HT | 0.77 ± 0.14 | 1.73 ± 0.27 | 4.47 ± 0.45 | 3.61 ± 0.47 | 1.40 ± 0.49 |
| iv free DOX (low dose) + HT | 1.68 ± 0.17 | 3.79 ± 0.58 | 3.63 ± 0.57 | 10.07 ± 1.54 | 2.26 ± 0.35 |
| iv DPPG2-TSL-DOX (full dose) + HT | 2.85 ± 0.34 | 10.03 ± 1.52 | 22.90 ± 5.06 | 21.03 ± 3.38 | 18.57 ± 4.06 |
| intraves DOX (full dose) - HT | <0.20* | <0.20* | <0.20* | 0.22 ± 0.02 | <0.20* |
| intraves DOX (full dose) + HT | <0.20* | <0.20* | <0.20* | 0.24 ± 0.04 | <0.20* |
Notes: Concentrations are ± standard error of mean in ng/mg; *Represents below limit of quantification.
Abbreviations: DOX, doxorubicin; DPPG2, 1.2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; HT, hyperthermia; intraves intravesical; iv, intravenous; TSL, thermosensitive liposomes.
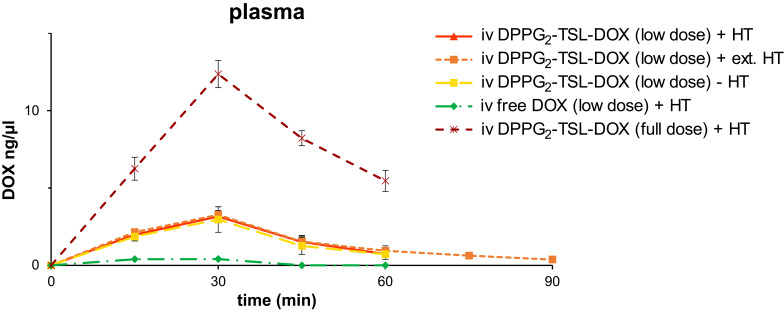
Pharmacokinetics
For pigs receiving low dose (20 mg/m2) intravenous DPPG2-TSL-DOX, the DOX peak plasma concentration was four times higher compared to a corresponding dose of iv free DOX (3.15 ng/µL vs 0.41 ng/µL; Figure 4). Plasma DOX concentration after iv free DOX did not exceed 0.4 ng/µL and was not quantifiable (thus below 0.2 ng/µL) from 45 minutes on. Pharmacokinetics could only be assessed grossly because we sampled blood only every 15 minutes. Administration of low dose DPPG2-TSL-DOX resulted in a 12 times longer beta half-life (T½ß) and an 11 times larger area under the curve (AUC) of (encapsulated and free) DOX, compared to a corresponding dose of iv free DOX. The DOX T½ß for 20 mg/m2 DPPG2-TSL-DOX was 19 min, and 25 min for 60 mg/m2 DPPG2-TSL-DOX. The AUC1-60min was 105.1 ng*min/µL and 443.4 ng*min/µL, respectively. DOX T½ß of 20 mg/m2 iv free DOX was 1.6 minutes and the AUC1-60min was 9.3 ng*min/µL. After intravesical administration, DOX could not be detected in plasma at any timepoint.
Figure 4.
DOX plasma concentrations per group. Error bars represent standard error of mean.
Abbreviations: DOX, doxorubicin; DPPG2, 1.2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; HT, hyperthermia; intraves, intravesical; iv, intravenous; TSL, thermosensitive liposomes.
DOX Distribution in the Bladder Wall
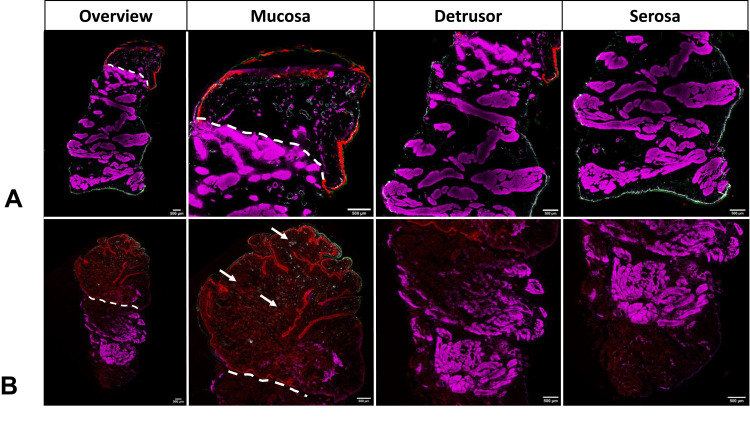
The bladder wall of pigs treated with DPPG2-TSL-DOX with HT (low- and full dose), showed the most homogeneous distribution of DOX, with DOX concentrations decreasing from mucosa to serosa, as judged by fluorescence microscopy (Figure 5B, Supplementary Figure 1 A-G). DOX was clearly visible around blood vessels in bladder biopsies of animals treated with DPPG2-TSL-DOX and HT. DOX visualisation was minimally increased when HT was increased to 90 minutes (Supplementary Figure 1B). In the intravesical DOX groups, visualisation of DOX showed intense staining at the very luminal side of the mucosa, with a penetration depth of less than 1000 µm (Figure 5A). Iv free DOX with HT and DPPG2-TSL-DOX without HT resulted in the lowest fluorescence intensity of DOX in the bladder wall (Supplementary Figure 1D and C, respectively).
Figure 5.
Fluorescence microscopy of the bladder of (A) intravesical doxorubicin (DOX) without hyperthermia (HT) (full dose); (B) intravenous DPPG2-thermosensitive liposomes containing DOX (DPPG2TSL-DOX) with HT (full dose). Red: DOX contained within the tissue; green: cytokeratin filaments of epithelial cells; white: Von-Willebrand-Factor (VWF) within blood vessel walls; and purple: actin filaments in smooth muscle fibres (SMA). White lines mark the transition from mucosa (above the line) to detrusor. The white arrows pointed at red dots show DOX accumulation around the small vessels after DPPG2-TSL-DOX with HT.
Although high variance between pigs and different biopsy locations was observed, the DOX distribution was in concordance with the tissue DOX concentration using HPLC quantification.
Adverse Effects
A severe anaphylactoid infusion reaction with life-threatening hemodynamic disturbance was seen in six of the twelve pigs that received iv DPPG2-TSL-DOX. Symptoms included an increase in pulse and a drop or increase in arterial blood pressure, decreased exhaled CO2 levels, and hyperemia, recorded three to five minutes following start of TSL infusion. After iv administration of noradrenaline and clemastine, this response was quickly reversed. Due to the unexpected anaphylactoid reactions in the first three pigs that received iv DPPG2-TSL-DOX, the other nine pigs in the iv DPPG2-TSL-DOX groups were administered antihistaminic and steroidal premedication as described in the methods section. From these subsequent nine pigs that were administered iv DPPG2-TSL-DOX, three pigs developed a similar infusion reaction requiring medical intervention. All animals survived and underwent the pre-planned procedures.
In pigs that received HT, the bladder wall showed edema and burn-spots at necropsy (posterior wall thermal reaction [PWTR]),27 which were transmural in two pigs (Supplementary Figure 2). Other essential organs had a normal macroscopic aspect.
Histopathology
Inflammatory reactions with transmural immune cell infiltration were observed in all bladder specimens, especially in the mucosa, serosa and perivascular regions. Submucosal oedema was observed in all groups that received HT (Supplementary Figure 3). No further differences between experimental groups based on morphologic tissue properties were observed. In two of the fifteen bladders that were exposed to HT, PWTR had resulted in transmural thermal necrosis.
Temperatures
In pigs that received HT, the temperature was highest at the submucosal side (42.1 ± 1.9°C), followed by the detrusor (41.3 ± 1.7°C) and serosa (40.6 ± 1.5°C) (Supplementary Figures 4A–G). Intra- and inter-individual variability in bladder wall temperature was high and ranged from 38.7°C at the submucosa level up to 44.5°C at the serosa level. Therefore, DOX concentrations were correlated to the achieved temperatures (Figure 3).
The mean body temperature of the animals was 37.3 ± 0.9°C during the experimental procedure, with the highest mean body temperature of a pig being 38.6°C, a normal body temperature for pigs. Median temperatures in the bladder wall in pigs that did not receive HT varied from 34.5°C to 37.5°C.
Discussion
We show that DPPG2-TSL-DOX combined with local heating of the bladder led to significantly higher DOX concentrations in bladder tissue, compared to iv and intravesical DOX. DOX levels in the detrusor layer were 7.5- and >20-fold higher, respectively. DOX fluorescence confirmed the objective measurements, which showed an increased and more homogeneous DOX distribution per bladder wall layer after DPPG2-TSL-DOX with HT. Compared to iv free DOX, DPPG2-TSL-DOX caused lower DOX concentrations in the heart and kidneys and similar DOX concentrations in the liver, spleen and lungs. The AUC of DOX in serum was substantially higher after DPPG2-TSL-DOX, compared to a similar dose of iv free DOX.
The significantly higher DOX concentrations in all bladder wall layers and improved DOX distribution after DPPG2-TSL-DOX compared to conventional DOX application are likely to lead to an improved anti-tumor effect, as drug concentration and distribution determine the exposure of tumor cells to a drug. A threefold increase of the DPPG2-TSL-DOX dose resulted in an eight times higher DOX concentration in the bladder wall, compared to low dose DPPG2-TSL-DOX. This increased concentration after a higher dose supports the assumption that the local HT initiated the local release of DOX from the liposomes. Moreover, HPLC and DOX fluorescence showed increased DOX levels towards the warmer mucosa side and close to small blood vessels. This observation is in line with the presumed mechanism of action of TSL formulations such as DPPG2-TSL-DOX that HT causes intravascular drug release from the liposomes.36
Administration of a low dose DPPG2-TSL-DOX (20 mg/m2) resulted in DOX concentrations of 3.29 ng/mg in the mucosa, 1.63 ng/mg in the detrusor and 1.28 ng/mg in the serosa, respectively. Based on the mean IC50 of DOX against the eight human MIBC cell lines in the online Sanger Institute dataset (0.16 ± 0.07 uM = 0.09 ± 0.04 ng/mg),37 this indicates that DPPG2-TSL-DOX combined with HT results in local DOX levels that are sufficiently high to reach an anti-tumor effect. HT might further increase the effect of DOX in tumor tissue because HT is known to increase cell sensitization and permeability for drugs, induce denaturation of cytoplasmic structures causing cell death and stimulate an immune response.38
Mikhail et al investigated LTLD combined with 60 minutes intravesical conductive HT (with water temperature of 45°C) in a pig bladder model, and also measured DOX bladder wall concentrations.22 A dose of 0.7 mg/kg DOX in LTLD achieved DOX concentrations of 9.7 ± 0.67 µg/g and 4.09 ± 0.67 µg/g in mucosal and detrusor muscle layer, respectively. Compared to LTLD, 1.4 higher dose of DPPG2-TSL-DOX used in our experiments (~1.0 mg/kg) led to DOX concentrations in the mucosa and detrusor that were 2.6- and 3.2-fold higher. The differences in DOX release from liposomes could be attributable to the liposomal composition. The hyperthermia modality presumably does not play a major role.30
When intravesical DOX was combined with HT, the bladder wall concentrations were similar to the cold intravesical DOX instillation in pigs. In contrast, in patients, intravesical mitomycin-C instillation combined with RF-induced HT (RITE/CHT) led to higher MMC bladder wall concentrations.32 Whether this difference is related to the drug examined or to instillation in a healthy versus sick bladder remains to be established.
As anticipated, DPPG2-TSL-DOX with HT resulted in a significantly lower DOX concentration in the heart and kidneys compared to iv free DOX with HT (both twice as low). Intravesical DOX led to the lowest DOX concentrations in all organs. This is particularly important since cardiotoxicity is the most prevalent and hazardous side effect of systemic DOX administration in patients.39,40 Surprisingly, DOX concentrations in spleen and lung were six- and seven-fold higher, respectively, after a three times higher dose of DPPG2-TSL-DOX, compared to a low dose. The observed increase in DOX concentration in these organs, which appears to be more prominent after a full dose, might be the result of the interaction between mononuclear phagocyte system (MPS) immune cells and liposomes.41,42 These phagocytic cells, such as macrophages, are located in the liver, spleen and kidneys, bone marrow and lymph nodes.43 In pigs, an additional and important part of the MPS is represented by large amounts of pulmonary intravascular macrophages (PIMs).44 MPS cells bind to liposomes and eliminate them from blood circulation, possibly explaining the increased DOX concentrations in these organs. Previous studies have shown that liposome-MPS interaction is not necessarily problematic for translation into a human setting: radiolabeled liposomes with the same composition as Doxil accumulate in liver and spleen in humans,18 but despite the interaction of Doxil and the MPS, little toxicity of these organs has been reported over the years.45 Moreover, humans are believed to lack PIMs46 and, therefore, the relevance of the DOX accumulation in lungs of pigs for clinical translation is uncertain. Lastly, the DOX measurements in this study could not distinguish free DOX from non-bioavailable liposome-encapsulated DOX. High concentration of encapsulated drugs do not necessarily cause increased toxicity,47 and therefore, we might overestimate the impact of the DOX on organs. Implications of organ DOX concentrations on long-term organ function warrants further research.
Pharmacokinetics could only be assessed grossly with the interval between blood sampling being 15 minutes. The DOX T½ß and AUC after 20 mg/m2 iv free DOX were 1.6 minutes and 9.3 ng*min/µL, which is similar to the pharmacokinetics of DOX in pigs of earlier studies.48,49 The T½ß of DOX in humans is about 30 hours,9 thus the hepatic clearance of DOX is remarkably faster in pigs than in humans. The DOX T½ß and AUC after 20 mg/m2 and 60 mg/m2 DPPG2-TSL-DOX were 19 min and 25 min, 105.1 ng*min/µL and 443.4 ng*min/µL, respectively. Although the T½ß and AUC following DPPG2-TSL-DOX administration were substantially longer than after iv free DOX, likely reflecting TSL-encapsulated DOX, the clearance was faster than anticipated. In cats, a species that also has resident PIMs,50 the AUC of serum DOX levels was of the same magnitude as the AUC in our pig study following DPPG2-TSL-DOX administration.25 Sixty percent of the DPPG2-TSL-DOX dose (0.6 mg/kg) of the full dose used in our pigs (~1 mg/kg) resulted in an AUC1-135min of 404 ng*min/µL in cats. The alpha T½ could not be determined due to the limited serum sampling timepoints of every 15 minutes. The faster clearance of liposomal DOX in pigs and cats than expected is likely attributable to DOX uptake by MPS including PIMs, as discussed. Also, the mean body temperature of pigs during the procedure was 37.3 ± 0.9°C, could be underestimated since it was measured in the nose, which presumably has caused systemic DOX release. Other factors that could affect the circulating DOX half-life, are the method of HT and the heating volume which influence the local DOX release from DPPG2-TSL.51 Moreover, lipid dosage52 and vesicle size24 can influence pharmacokinetics of liposomes. Our expectation is that the DOX half-life following DPPG2-TSL-DOX administration will be longer in humans, due to the absence of PIMs, the lower mean body temperature of humans and the slower DOX clearance.
Six of the twelve pigs developed a life-threatening infusion reaction including cardiopulmonary distress within minutes after DPPG2-TSL-DOX administration. This liposome-induced infusion reaction is a known phenomenon in pigs and is also referred to as complement activation-related pseudoallergy (CARPA), as it is caused by activation of the complement system.53 In addition to the complement system, PIMs may also play a role in the induction of an infusion reaction in pigs.44,54 However, no DPPG2-TSL-DOX based infusion reaction was observed in cats25 despite the presence of pulmonary resident PIMs. Infusion reactions in human have also been described for both Doxil and LTLD. However, these reactions were infrequent and less severe in humans than in pigs.53,55,56 Slow infusion protocols have proven to be successful in limiting infusion reactions and may overcome this problem.57 Future studies should address the potential infusion reaction.
All animals exposed to HT had PWTR of the bladder wall and two of the pigs showed transmural necrosis due to HT. PWTR is a common and clinically irrelevant finding in NMIBC patients treated with intravesical RF-induced HT.26 Transmural necrosis has not been objectified in humans since the bladder lumen is first evaluated at cystoscopy, six weeks after CHT treatment. Therefore, the relevance of this observation is unknown.
Intra- and inter-variability of bladder wall temperatures measured by different TCs was observed. Most likely these differences were caused by inhomogeneous heating of the RF-emitting antenna or interference of RF with the temperature measurements by TCs. The mean bladder temperature of one animal that received DPPG2-TSL-DOX with HT only reached 38.7°C. Nevertheless, the bladder DOX concentrations of this animal were similar to the other two animals in the same group, suggesting that the temperature was sufficient for DOX release from the liposomes.
A limitation of the current study is the presence of PIMs, the higher mean body temperature, and the higher susceptibility for infusion reactions in pigs compared to humans. As a consequence of our study design, direct and long-term toxicity could not be assessed. Also, DOX measurements could not distinguish free DOX from non-bioavailable liposome-encapsulated DOX.
Conclusion
Intravenous administration of DPPG2-TSL-DOX combined with RF-induced HT achieved substantially higher DOX concentrations and more homogeneous DOX distribution in the bladder wall, compared to iv free chemotherapy and intravesical instillation with DOX. DPPG2-TSL-DOX with HT provided lower DOX accumulation in the heart and kidneys, compared to iv free chemotherapy. Intravesical DOX led to the lowest organ DOX concentrations. These findings provide compelling evidence that DPPG2-TSL-DOX with HT could overcome the efficacy of conventional DOX application and has the potential to attain a role in MIBC treatment.
Acknowledgments
We would like to thank Gerard van Ooijen for his technical support with the thermocouples, and Alex Hanssen and Maikel School for their support during the implementation of the experiment.
Funding Statement
This study was partially sponsored by Thermosome. Catheters for this study were donated by Medical Enterprises Ltd (MEL). The Synergo® system was provided by MEL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
AUC, area under the curve; BSA, bovine serum albumin; CARPA, complement activation-related pseudoallergy; CHT, chemohyperthermia; DOX, doxorubicin; DPPC, 1.2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPC, 1.2-distearoyl-sn-glycero-3-phosphocholine; DPPG2, 1.2-dipalmitoyl-sn-glycero-3-phosphodiglycerol; DPPG2-TSL-DOX, phosphatidyldiglycerol-containing thermosensitive liposomes with encapsulated doxorubicin; EPR, enhanced permeability and retention; HE, haematoxylin and eosin; HPLC, high performance liquid chromatography; HT, hyperthermia; IHC, immunohistochemistry; Iv, intravenous; LTLD, low-temperature sensitive liposomal doxorubicin; MIBC, muscle-invasive bladder cancer; MMT, multimodality treatment; MPS, mononuclear phagocyte system; NAC, neoadjuvant chemotherapy; NMIBC, non-muscle invasive bladder cancer patients; PBS, phosphate-buffered saline; PIM, pulmonary intravascular macrophage; PWTR, posterior wall thermal reaction; RF, radiofrequency; RITE, radiofrequency-induced hyperthermia; RC, radical cystectomy; SEM, standard error of mean; SMA, smooth muscle actin; TC, thermocouple; TURB, transurethral resection of the bladder; TSL, thermosensitive liposomes; VWF, Von Willebrand Factor.
Disclosure
Lars H. Lindner, Pascal Schweizer and Martin Hossann have equity shares in Thermosome. Pascal Schweizer and Martin Hossann report grants from BMBF TSL-LIFU and BMBF IO-TSL, outside the submitted work. Lars H. Lindner receives research and travel support from Dr. Sennewald Medizintechnik and travel support from PharmaMar, honoraria from Novartis, Lilly, Eisai and EL Medconsult (all not related to this manuscript). J. Alfred Witjes is advisor for Sanofi Pasteur, Spectrum, Astellas (all not related to this manuscript) and is an advisor for Medical Enterprises Ltd. (Synergo®) and has no associated financial interest or conflict of interest. Egbert Oosterwijk reports Thermosome contributed financially to the animal experiments, during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 2.Cambier S, Sylvester RJ, Collette L, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus calmette-guerin. Eur Urol. 2016;69(1):60–69. doi: 10.1016/j.eururo.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Compérat E, et al. EAU guideline non-muscle-invasive bladder cancer. Edn. presented at the EAU Annual Congress Amsterdam 2020: EAU guidelines office: Arnhem, the Netherlands; 2020. Available from: http://uroweb.org/guidelines/compilations-of-all-guidelines/. Accessed August20, 2020.
- 4.Hautmann RE, de Petriconi RC, Volkmer BG. Lessons learned from 1000 neobladders: the 90-day complication rate. J Urol. 2010;184(3):990–994; quiz 1235. [DOI] [PubMed] [Google Scholar]
- 5.Hautmann RE, Abol-Enein H, Davidsson T, et al. ICUD-EAU international consultation on bladder cancer 2012: urinary diversion. Eur Urol. 2013;63(1):67–80. doi: 10.1016/j.eururo.2012.08.050 [DOI] [PubMed] [Google Scholar]
- 6.Nielsen ME, Mallin K, Weaver MA, et al. Association of hospital volume with conditional 90-day mortality after cystectomy: an analysis of the National Cancer Data Base. BJU Int. 2014;114(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith AB, Jaeger B, Pinheiro LC, et al. Impact of bladder cancer on health-related quality of life. BJU Int. 2018;121(4):549–557. [DOI] [PubMed] [Google Scholar]
- 8.Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmacotherapeutisch Kompas: doxorubicin [website]. Available from: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/d/doxorubicine. Accessed July20, 2020.
- 10.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 11.Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–1198. doi: 10.1111/jphp.13098 [DOI] [PubMed] [Google Scholar]
- 12.Administration USFaD. Doxil Highlights Of Prescribing Information. 2013.
- 13.Bhowmik S, Bhowmick S, Maiti K, et al. Two multicenter Phase I randomized trials to compare the bioequivalence and safety of a generic doxorubicin hydrochloride liposome injection with Doxil((R)) or Caelyx((R)) in advanced ovarian cancer. Cancer Chemother Pharmacol. 2018;82(3):521–532. doi: 10.1007/s00280-018-3643-3 [DOI] [PubMed] [Google Scholar]
- 14.O’Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a Phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097 [DOI] [PubMed] [Google Scholar]
- 15.Judson I, Radford JA, Harris M, et al. Randomised Phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37(7):870–877. doi: 10.1016/S0959-8049(01)00050-8 [DOI] [PubMed] [Google Scholar]
- 16.Rifkin RM, Gregory SA, Mohrbacher A, Hussein MA. Pegylated liposomal doxorubicin, vincristine, and dexamethasone provide significant reduction in toxicity compared with doxorubicin, vincristine, and dexamethasone in patients with newly diagnosed multiple myeloma: a phase III multicenter randomized trial. Cancer. 2006;106(4):848–858. doi: 10.1002/cncr.21662 [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, Pouli A, Zervas K, et al. Prospective randomized comparison of vincristine, doxorubicin and dexamethasone (VAD) administered as intravenous bolus injection and VAD with liposomal doxorubicin as first-line treatment in multiple myeloma. Ann Oncol. 2003;14(7):1039–1044. doi: 10.1093/annonc/mdg287 [DOI] [PubMed] [Google Scholar]
- 18.Harrington KJ, Mohammadtaghi S, Uster PS, et al. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin Cancer Res. 2001;7(2):243–254. [PubMed] [Google Scholar]
- 19.Seynhaeve ALB, Dicheva BM, Hoving S, Koning GA, Ten Hagen TLM. Intact Doxil is taken up intracellularly and released doxorubicin sequesters in the lysosome: evaluated by in vitro/in vivo live cell imaging. J Control Release. 2013;172(1):330–340. doi: 10.1016/j.jconrel.2013.08.034 [DOI] [PubMed] [Google Scholar]
- 20.Kneidl B, Peller M, Winter G, Lindner LH, Hossann M. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine. 2014;9:4387–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tak WY, Lin SM, Wang Y, et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin Cancer Res. 2018;24(1):73–83. doi: 10.1158/1078-0432.CCR-16-2433 [DOI] [PubMed] [Google Scholar]
- 22.Mikhail AS, Negussie AH, Pritchard WF, et al. Lyso-thermosensitive liposomal doxorubicin for treatment of bladder cancer. Int J Hyperthermia. 2017;33(7):733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindner LH, Eichhorn ME, Eibl H, et al. Novel temperature-sensitive liposomes with prolonged circulation time. Clin Cancer Res. 2004;10(6):2168–2178. doi: 10.1158/1078-0432.CCR-03-0035 [DOI] [PubMed] [Google Scholar]
- 24.Limmer S, Hahn J, Schmidt R, et al. Gemcitabine treatment of rat soft tissue sarcoma with phosphatidyldiglycerol-based thermosensitive liposomes. Pharm Res. 2014;31(9):2276–2286. doi: 10.1007/s11095-014-1322-6 [DOI] [PubMed] [Google Scholar]
- 25.Hossann M, Syunyaeva Z, Schmidt R, et al. Proteins and cholesterol lipid vesicles are mediators of drug release from thermosensitive liposomes. J Control Release. 2012;162(2):400–406. doi: 10.1016/j.jconrel.2012.06.032 [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann K, Hossann M, Hirschberger J, et al. A pilot trial of doxorubicin containing phosphatidyldiglycerol based thermosensitive liposomes in spontaneous feline soft tissue sarcoma. Int J Hyperthermia. 2017;33(2):178–190. doi: 10.1080/02656736.2016.1230233 [DOI] [PubMed] [Google Scholar]
- 27.van Valenberg H, Colombo R, Witjes F. Intravesical radiofrequency-induced hyperthermia combined with chemotherapy for non-muscle-invasive bladder cancer. Int J Hyperthermia. 2016;32(4):351–362. doi: 10.3109/02656736.2016.1140232 [DOI] [PubMed] [Google Scholar]
- 28.Arends TJ, Nativ O, Maffezzini M, et al. Results of a randomised controlled trial comparing intravesical chemohyperthermia with mitomycin C versus bacillus calmette-guerin for adjuvant treatment of patients with intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol. 2016;69(6):1046–1052. doi: 10.1016/j.eururo.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 29.Liem EI, Crezee H, de la Rosette JJ, de Reijke TM. Chemohyperthermia in non-muscle-invasive bladder cancer: an overview of the literature and recommendations. Int J Hyperthermia. 2016;32(4):363–373. doi: 10.3109/02656736.2016.1155760 [DOI] [PubMed] [Google Scholar]
- 30.van Valenberg FJP, Witjes JA, Aklan B, de Jong SF, Zegers H, Oosterwijk E. Inducing intravesical hyperthermia of the ex-vivo porcine bladder wall: radiofrequency-induction versus recirculation using a custom-made device. Int J Hyperthermia. 2018;35(1):323–329. doi: 10.1080/02656736.2018.1499046 [DOI] [PubMed] [Google Scholar]
- 31.Arends TJ, Falke J, Lammers RJ, et al. Urinary cytokines in patients treated with intravesical mitomycin-C with and without hyperthermia. World J Urol. 2015;33(10):1411–1417. doi: 10.1007/s00345-014-1458-3 [DOI] [PubMed] [Google Scholar]
- 32.van Valenberg FJP, van der Heijden AG, Lammers RJM, et al. Intravesical radiofrequency induced hyperthermia enhances mitomycin C accumulation in tumour tissue. Int J Hyperthermia. 2017;1–6. [DOI] [PubMed] [Google Scholar]
- 33.Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49(2):344–356. doi: 10.1177/0300985811402846 [DOI] [PubMed] [Google Scholar]
- 34.Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist. 2008;13(6):725–732. doi: 10.1634/theoncologist.2008-0012 [DOI] [PubMed] [Google Scholar]
- 35.Peller M, Willerding L, Limmer S, et al. Surrogate MRI markers for hyperthermia-induced release of doxorubicin from thermosensitive liposomes in tumors. J Control Release. 2016;237:138–146. doi: 10.1016/j.jconrel.2016.06.035 [DOI] [PubMed] [Google Scholar]
- 36.Manzoor AA, Lindner LH, Landon CD, et al. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012;72(21):5566–5575. doi: 10.1158/0008-5472.CAN-12-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Institute S. Genomics of Drug Sensitivity in Cancer. 2020.
- 38.Tan WS, Kelly JD. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol. 2018;15(11):667–685. doi: 10.1038/s41585-018-0092-z [DOI] [PubMed] [Google Scholar]
- 39.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115(2):155–162. doi: 10.1159/000265166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho FS, Burgeiro A, Garcia R, Moreno AJ, Carvalho RA, Oliveira PJ. Doxorubicin-induced cardiotoxicity: from bioenergetic failure and cell death to cardiomyopathy. Med Res Rev. 2014;34(1):106–135. [DOI] [PubMed] [Google Scholar]
- 41.Gabizon AA. Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001;19(4):424–436. doi: 10.1081/CNV-100103136 [DOI] [PubMed] [Google Scholar]
- 42.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315. [PMC free article] [PubMed] [Google Scholar]
- 43.Guyton and Hall. Textbook of Medical Physiology. 2011:426–428.
- 44.Csukás D, Urbanics R, Wéber G, Rosivall L, Szebeni J. Pulmonary intravascular macrophages: prime suspects as cellular mediators of porcine CARPA. Eur J Nanomed. 2015;7(1):27. doi: 10.1515/ejnm-2015-0008 [DOI] [Google Scholar]
- 45.Rafiyath SM, Rasul M, Lee B, Wei G, Lamba G, Liu D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp Hematol Oncol. 2012;1(1):10. doi: 10.1186/2162-3619-1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneberger D, Aharonson-Raz K, Singh B. Pulmonary intravascular macrophages and lung health: what are we missing? Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L498–503. doi: 10.1152/ajplung.00322.2011 [DOI] [PubMed] [Google Scholar]
- 47.Fielding RM, Singer AW, Wang LH, Babbar S, Guo LS. Relationship of pharmacokinetics and drug distribution in tissue to increased safety of amphotericin B colloidal dispersion in dogs. Antimicrob Agents Chemother. 1992;36(2):299–307. doi: 10.1128/AAC.36.2.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersson M, Domellof L, Eksborg S, et al. Pharmacokinetics and central haemodynamic effects of doxorubicin and 4ʹepi-doxorubicin in the pig. Acta Oncol. 1989;28(5):709–714. doi: 10.3109/02841868909092298 [DOI] [PubMed] [Google Scholar]
- 49.August DA, Verma N, Vaertan MA, Shah R, Brenner DE. An evaluation of hepatic extraction and clearance of doxorubicin. Br J Cancer. 1995;72(1):65–71. doi: 10.1038/bjc.1995.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkler GC. Pulmonary intravascular macrophages in domestic animal species: review of structural and functional properties. Am J Anat. 1988;181(3):217–234. [DOI] [PubMed] [Google Scholar]
- 51.Willerding L, Limmer S, Hossann M, et al. Method of hyperthermia and tumor size influence effectiveness of doxorubicin release from thermosensitive liposomes in experimental tumors. J Control Release. 2016;222:47–55. doi: 10.1016/j.jconrel.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 52.Allen TM, Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta. 1991;1068(2):133–141. [DOI] [PubMed] [Google Scholar]
- 53.Szebeni J, Muggia F, Gabizon A, Barenholz Y. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv Drug Deliv Rev. 2011;63(12):1020–1030. doi: 10.1016/j.addr.2011.06.017 [DOI] [PubMed] [Google Scholar]
- 54.Szebeni J, Simberg D, Gonzalez-Fernandez A, Barenholz Y, Dobrovolskaia MA. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat Nanotechnol. 2018;13(12):1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swenson CE, Haemmerich D, Maul DH, Knox B, Ehrhart N, Reed RA. Increased duration of heating boosts local drug deposition during radiofrequency ablation in combination with thermally sensitive liposomes (ThermoDox) in a porcine model. PLoS One. 2015;10(10):e0139752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood BJ, Poon RT, Locklin JK, et al. Phase I study of heat-deployed liposomal doxorubicin during radiofrequency ablation for hepatic malignancies. J Vasc Interv Radiol. 2012;23(2):248–255.e247. doi: 10.1016/j.jvir.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fülöp T, Kozma GT, Vashegyi I, et al. Liposome-induced hypersensitivity reactions: risk reduction by design of safe infusion protocols in pigs. J Control Release. 2019;309:333–338. [DOI] [PubMed] [Google Scholar]