Diet is an important modulator of systemic and central nervous system inflammation, with profound effects in metabolism, immunity, and the gut microbiome. Fontana et al. discuss recent findings on the relationship between calorie intake and the mechanisms underlying neuroinflammation and neurodegeneration.
Abstract
Recent and accumulating work in experimental animal models and humans shows that diet has a much more pervasive and prominent role than previously thought in modulating neuroinflammatory and neurodegenerative mechanisms leading to some of the most common chronic central nervous system (CNS) diseases. Chronic or intermittent food restriction has profound effects in shaping brain and peripheral metabolism, immunity, and gut microbiome biology. Interactions among calorie intake, meal frequency, diet quality, and the gut microbiome modulate specific metabolic and molecular pathways that regulate cellular, tissue, and organ homeostasis as well as inflammation during normal brain aging and CNS neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis, among others. This review discusses these findings and their potential application to the prevention and treatment of CNS neuroinflammatory diseases and the promotion of healthy brain aging.
Introduction
Dietary restriction (DR) is defined as a chronic or intermittent reduction of food intake without malnutrition. It is the most robust experimental intervention to delay the onset of a wide range of age-associated pathologies and to extend lifespan, as shown in a variety of species (Fontana and Partridge, 2015). Evidence from experimental studies indicates that age-associated accumulation of molecular and cellular damage can be prevented or greatly delayed by dietary, genetic, and pharmacological manipulations that down-regulate key cellular nutrient-sensing and inflammatory pathways (Fontana et al., 2010b). Health-promoting actions of DR include metabolic, antioxidant, and immunomodulatory adaptations that could potentially influence the initiation, progression, and prognosis of a range of neurological and neuroinflammatory disorders.
Neuroinflammation is a coordinated response of the central nervous system (CNS) to harmful stimuli and injuries, including those occurring during infections, traumatic brain injury, or other neurological diseases. It is characterized by activation and proliferation of the two major CNS glial cells, microglia and astrocytes, which undergo morphological changes and release proinflammatory mediators (cytokines, chemokines, and complement proteins; Matias et al., 2019; Wolf et al., 2017). Often, neuroinflammation is associated with increased blood–brain barrier permeability, allowing immune cell trafficking and soluble proinflammatory factors to enter the CNS, where they can directly interact with glial and neuronal cells and contribute to the inflammatory process. The goal of inflammatory and immune responses is to clear the hazard. When this is achieved, activation of anti-inflammatory pathways then offsets the inflammatory process to restore tissue integrity and function. If the precipitating factors are persistent (as in some neurodegenerative diseases), chronic neuroinflammation may ensue. Chronic neuroinflammation has been proposed as a potential deleterious mediator of aging, as well as other pathological conditions (Di Benedetto et al., 2017). Increasing evidence supports the involvement of chronic neuroinflammation in the pathogenesis of neurological disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS), as well as in multiple sclerosis (MS), a more classic neuroinflammatory disease (Stephenson et al., 2018).
Recent advances are highlighting the complex relationship between nutrient metabolism and the activation of inflammatory pathways in different tissues including the brain. Notably, disruption of brain energy metabolism with reduced glucose consumption, increased central insulin resistance, and impaired mitochondrial function have been linked to the mechanisms leading to neuroinflammatory and age-related neurodegenerative diseases (Cunnane et al., 2020; Zilberter and Zilberter, 2017). This evidence prompted the hypothesis that reestablishing metabolic balance could be a key intervention to counteract underlying disease processes. DR could potentially serve this purpose by exerting its effects on metabolic and anti-inflammatory pathways. This review article aims to summarize current evidence regarding the effects of DR on neuroinflammation and the potential central and systemic underlying mechanisms. Major studies that tested the effects of DR in experimental models, epidemiological studies, and clinical trials for various neurodegenerative disorders in which neuroinflammation may play a role are discussed.
Methods of DR
DR can be achieved by chronically reducing food intake or by changing meal frequency and timing (Fig. 1). In chronic DR, daily food intake is reduced by 20–50%, but meal frequency is unchanged. In contrast, with intermittent fasting (IF), food intake is completely eliminated or greatly reduced intermittently, for example, every other day. In humans, moderate 20–30% chronic DR has been tested long-term in clinical trials and observational studies (Most et al., 2017) or during episodes of involuntary energy intake reduction (Hindhede, 1920; Strom and Jensen, 1951). IF in experimental models usually refers to every-other-day 24-h complete fasting, while in humans it refers to a variety of approaches including fasting or calorie restriction (e.g., 500 calories per day) on alternate days or 2 d per week (5:2 diet), or skipping breakfast and lunch ≥2 d each week (Patterson et al., 2015). Another form of intermittent DR is time-restricted feeding, which involves consuming all daily food in a 4- to 6-h time window and fasting for the remainder of the day (Cienfuegos et al., 2020; Fig. 1). Evidence from clinical and animal studies suggests that chronic DR and IF affect lifespan and age-associated diseases via similar metabolic and molecular mechanisms (Hadem et al., 2019; Mattson et al., 2014).
Figure 1.
Types of DR in preclinical and clinical studies. Description of the main characteristics of chronic and intermittent DR regimens used in preclinical animal studies and clinical studies in humans.
Mechanisms mediating anti-inflammatory effects of DR
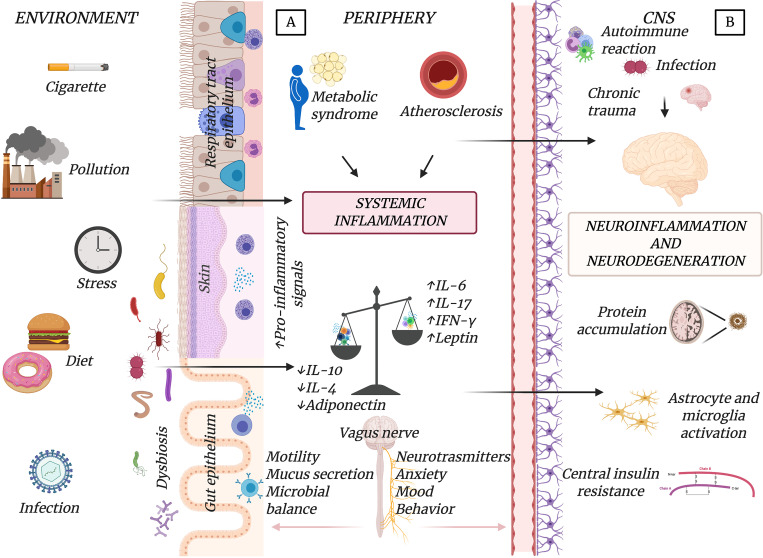
Central and systemic effector mechanisms contribute to CNS neuroinflammation. Central mechanisms are driven by primary CNS insults (e.g., brain injury, protein deposition, or accumulation of senescent cells), inducing inflammatory responses involving resident or infiltrating immune cells. CNS inflammation can also be induced or potentiated by peripheral factors such as systemic inflammatory mediators reaching the CNS, insulin resistance, and other metabolic conditions (e.g., obesity or dyslipidemia), leading to activated microglia and astrocytes (Lee and Mattson, 2014; Mattson and Arumugam, 2018). Environmental factors (e.g., diet, physical inactivity, smoke/pollution, or mental stress) can play a key role in triggering central or systemic mechanisms leading to CNS inflammation. Recent advances have recognized a prominent role in the connections between the periphery and the brain of the gut–brain axis, which is the bidirectional communication between the central and the enteric nervous systems linking gut functions with emotional and cognitive centers in the brain. A primary role in this complex cross-talk is played by the gut microbiota through neural, endocrine, immune, and humoral mechanisms partially mediated by the vagus nerve and the parasympathetic nervous system (Fig. 2). In this scenario, DR elicits protective changes in the gut microbiota composition, with metabolic and molecular adaptations in nearly all tissues and organs including the brain. Thus, DR can potentially modulate both central and peripheral factors contributing to neuroinflammation. The main adaptations induced by DR on metabolic and neuroinflammatory pathways are discussed in this section and summarized in Fig. 3.
Figure 2.
Central and peripheral mechanisms leading to neuroinflammation. (A and B) Neuroinflammation with activation of microglia and astrocytes in the CNS could be induced by peripheral (A) or central (B) effector mechanisms. Environmental factors (diet, infections, pollution, etc.) can have effects on the gut microbiota (e.g., gut dysbiosis or increased permeability) or other epithelial barriers, and then cause peripheral metabolic and immune-inflammatory responses (e.g., metabolic syndrome or atherosclerosis) responsible for systemic inflammation. This contributes to CNS inflammation, which could also be evoked by central processes (e.g., chronic trauma, autoimmune attacks, or infections). The parasympathetic system through the vagus nerve mediates the cross-talk between the periphery and the CNS and may modulate neuroinflammation.
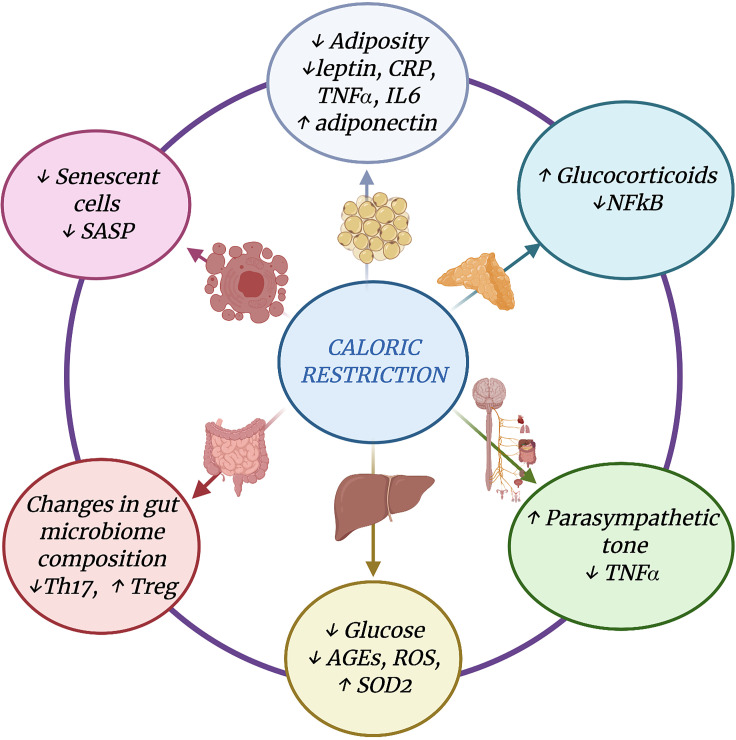
Figure 3.
Mechanisms mediating DR anti-inflammatory effects. Main adaptations induced by DR on metabolic, hormonal, gut microbiota, and immune/inflammatory pathways. AGEs, advanced glycation end products; CRP, C-reactive protein; iNOS, inducible nitric oxide synthase; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype; SOD2, superoxide dismutase 2.
Reduced adiposity
Adipose tissue is a major source of cytokines and hormones, collectively known as “adipokines,” which can modulate multiple immune and metabolic responses (Fontana et al., 2007; Lee et al., 2019). DR powerfully reduces adiposity, especially visceral adiposity (Das et al., 2017; Racette et al., 2006), resulting in diminution of fat-derived cytokines and other proinflammatory mediators and increase of anti-inflammatory molecules such as IL-10 (Ma et al., 2020; Willette et al., 2013). In experimental models, DR reduces the proinflammatory adipokine leptin and increases adiponectin, which has insulin-sensitizing and anti-inflammatory effects (Higami et al., 2004). In humans, DR induces similar changes in serum adipokines (Fontana et al., 2010a). Reduction of abdominal fat in humans was associated with lower levels of C-reactive protein, TNF-α, and F2-isoprostanes, markers of systemic inflammation and oxidative stress (Il’yasova et al., 2018; Meydani et al., 2016). Mechanistically, adipokines are important nodes integrating immunometabolic pathways and controlling T cell effector functions. Leptin, as well as insulin signaling, supports T cell metabolic (nutrient uptake and glycolytic and respiratory capacities) and immune responses leading to the production of proinflammatory cytokines IFN-γ and TNF-α (Saucillo et al., 2014; Tsai et al., 2018) and the response to pathogens (Ganeshan and Chawla, 2014).
Improved insulin sensitivity and reduced advanced glycation end-product (AGE) formation
Brain aging, AD, and other neuroinflammatory diseases are associated with insulin resistance, microgliosis, and elevated inflammatory markers (IL-6, TNF-α, and IL-1β) in the brain, cerebrospinal fluid, and plasma (Akiyama et al., 2000; Craft and Watson, 2004; Pekkala et al., 2020). In the Rotterdam study, a twofold increase in baseline insulin or insulin resistance corresponded to a 40% greater likelihood of AD conversion among participants without type 2 diabetes and doubling of the risk in diabetic patients (Schrijvers et al., 2010). Chronic DR in rodents and humans improves insulin sensitivity and glucose tolerance (Kraus et al., 2019; Weiss et al., 2006; Yoshino et al., 2020), thus reducing formation of AGEs that interfere with normal cell function (Cefalu et al., 1995; Fontana et al., 2010a; Kraus et al., 2019; Masoro et al., 1989; Weiss et al., 2006). AGEs exert their detrimental actions by binding specific receptors called RAGEs (receptors for AGEs), widely expressed on many cells including neural and immune cells. RAGE activation elicits up-regulation of proinflammatory cytokines (e.g., TNF-α and IL-1) and rapid generation of reactive oxygen species (Jiang et al., 2018; Vlassara et al., 2002), and it has been implicated in the pathogenesis of diabetes, atherosclerosis, and neuroinflammation and potentially in many CNS diseases (Ashraf et al., 2015; Basta, 2008; Wang et al., 2020).
Increased glucocorticoid production and antistress pathways
In rodents, chronic DR and IF have been consistently associated with increased circulating levels of endogenous corticosteroids, hormones with potent anti-inflammatory and immunomodulatory activities. As in animals, long-term DR in humans is associated with sustained rises in serum cortisol (Fontana et al., 2016). In people strictly practicing 30% DR for 3–15 yr, serum cortisol was ∼30% higher than in age-matched sedentary controls and endurance athletes, and inversely correlated with serum levels of TNF-α (Yang et al., 2016). In the latter study, DR was associated with reduced transcripts of several inflammatory mediators in skeletal muscle, including NF-κB, STAT5, inducible nitric oxide synthase, TNF-α, IL-6, and IL-8. Furthermore, DR was associated with a significant increase in key stress-related molecular chaperones (HSP-70 and Grp78) and autophagic (LC3 and beclin-1) mediators involved in cellular protein quality control and removal of dysfunctional proteins and organelles (Yang et al., 2016).
Reduction of senescent cells and senescence-associated secretory phenotype (SASP)
Another mechanism through which DR may inhibit inflammation is by reducing the accumulation of senescent cells during aging (Fontana et al., 2010a). Cellular senescence is a potent tumor-suppressive mechanism that inhibits the proliferation of cells at risk for malignant transformation, but at the same time induces the secretion of a range of growth factors, metalloproteinases, proinflammatory cytokines, and chemokines, collectively named SASPs (Chinta et al., 2015; Coppé et al., 2010). Cell senescence can be prematurely induced by several insults, including cellular damage caused by metabolic dysfunction, oxidative stress, genotoxic stress, and overactivation of oncogenes or loss of some tumor suppressor genes (Borghesan et al., 2020; McHugh and Gil, 2018). Accumulating evidence suggests that the chronic inflammatory SASP environment contributes to brain aging and to the development of age-associated diseases, including AD (Ovadya and Krizhanovsky, 2014). Recent studies have linked tau-dependent pathology to cellular senescence and failure to clear senescent glial cells (Bussian et al., 2018; Musi et al., 2018). Astrocytes help to maintain glutamate homeostasis in the brain by removing extracellular glutamate via excitatory amino acid transporters. Astrocyte senescence may provoke glutamate toxicity in cortical neurons, which causes and enhances neurodegeneration and neuroinflammation (Limbad et al., 2020). Several DR animal studies as well as long-term DR in humans have shown that DR was associated with reduced senescent cell markers in multiple tissues (Fontana et al., 2010a).
Activation of the parasympathetic anti-inflammatory pathway
The autonomic nervous system regulates important body functions such as heart rate, blood pressure, and gastrointestinal motility, but it also modulates systemic inflammation (Pavlov and Tracey, 2012; Salama et al., 2020). Stimulation of the parasympathetic nervous system (efferent vagus nerve) inhibits TNF-α synthesis by macrophages, reduces serum TNF-α during endotoxemia, and prevents development of shock (Borovikova et al., 2000). In contrast, vagotomy intensifies TNF-α activity and amplifies the response to endotoxemia. By binding to the macrophage α7 nicotinic acetylcholine receptor, the neurotransmitter acetylcholine (released by the efferent vagus nerve endings) is responsible for the reduced secretion of TNF-α by human macrophages (Wang et al., 2003). Rodent studies have shown that DR increases the high-frequency component of the heart rate variability spectrum, a marker of parasympathetic activity (Mager et al., 2006). Similarly, long-term DR without malnutrition in humans increased parasympathetic activity as measured by heart rate variability (Stein et al., 2012).
Enhanced gut microbiota–dependent anti-inflammatory pathways
Another mechanism through which DR may induce beneficial metabolic and anti-inflammatory effects involves alterations of gut microbiota composition and function (Estrada and Contreras, 2019). The gut microbiota plays a crucial role in maintaining a symbiotic relationship with the host and regulates several important functions, including host metabolism and intestinal and systemic immune inflammatory responses (Rooks and Garrett, 2016). Life-long DR in mice is known to change the gut microbiota structure with enrichment of anti-inflammatory bacteria strains such as the genus Lactobacillus, accompanied by reduced serum proinflammatory endotoxin load from the gut (Zhang et al., 2013). In a different study in naive mice, every-other-day fasting altered the gut microbiota composition by increasing levels of Firmicutes and the production of short-chain fatty acids, which are known to have immunomodulatory and anti-inflammatory effects (Li et al., 2017). We showed that 1 mo of IF led to increased gut bacteria diversity, with enrichment of the Lactobacillaceae, Bacteroidaceae, and Prevotellaceae families and associated enhancement of several antioxidative microbial metabolic pathways. These changes were associated with increased frequencies of gut-associated regulatory T cells and decrease of IL-17–producing T cells (Th17; Cignarella et al., 2018).
In humans, long-term DR was shown to increase gut microbiota richness and diversity, characteristics referring to the number of bacterial species (based on total bacterial gene counts) in the gut and of individual bacteria within each species, respectively. High microbiota richness is associated with improved metabolic health (reduced adiposity; lower leptin, insulin, and triglycerides; and higher adiponectin and HDL cholesterol levels) and reduced inflammation (lower C-reactive protein and white blood cell counts; Le Chatelier et al., 2013). One study showed that the gut microbiota of individuals practicing chronic DR were significantly more diverse when compared with individuals on a typical Western diet (Griffin et al., 2017). Another study showed that an energy-restricted diet increased gut bacteria richness in individuals classified as having “low gene counts” and was associated with reduced adiposity and improved insulin sensitivity, as well as a trend toward a reduction of systemic inflammation, as evidenced by decreased C-reactive protein levels (Cotillard et al., 2013).
Effects of DR on normal aging and neurodegenerative diseases
Numerous studies have explored DR effects in normal brain aging as well as chronic neuroinflammatory and neurodegenerative diseases. Most of the long-term studies were conducted in rodents and monkeys. Human studies have mainly been relatively short-term trials testing the effects of the diet primarily on systemic metabolic conditions. In the following section, we review the main published studies on the effects of DR on normal aging and neurodegenerative diseases (summarized in Table 1) in which systemic and brain inflammation plays a prominent role.
Table 1. Preclinical and clinical studies on DR in aging and neurodegenerative diseases.
| Model | Reference | Type of DR | DR effects on inflammatory or other brain pathology markers | DR effects on cognitive or motor functions | DR effects on imaging measures |
|---|---|---|---|---|---|
| Aging | |||||
| Rodents | Morgan et al., 1999; Lee et al., 2000 | 30–40% DR | ↓ Age-related increased of activation markers on microglia (e.g., MHCII) or astrocytes (e.g., GFAP); ↓ inflammatory genes | ||
| Kaur et al., 2008 | IF | ↓ Age-related changes in brain expression of NCAM, PSA-NCAM, and GFAP | |||
| Singh et al., 2012 | IF | ↓ Protein oxidative damage; ↑ markers of synaptic plasticity in the hippocampus | Ameliorates motor coordination, cognitive skills | ||
| Nonhuman primates | Qin et al., 2006 | 30% DR | ↓ Aβ deposition | ||
| Willette et al., 2010 | 30% DR | Attenuates the relation between IL-6 and brain volume loss | Attenuates the relation between IL-6 and brain volume loss | ||
| Willette et al., 2012 | 30% DR | Attenuates the negative correlation between homocysteine and global gray matter volume | Attenuates the negative correlation between homocysteine and global gray matter volume | ||
| Colman et al., 2009; Kastman et al., 2012; Sridharan et al., 2012; Sridharan et al., 2013 | 30% DR (University of Wisconsin study) | ↓ Age-related astrogliosis (↓ GFAP in hippocampus and entorhinal cortex) | Preserves motor performance | No effect on corpus callosum integrity; ↑ FA in several white matter regions; ↓ GM volume loss; ↓ brain iron accumulation | |
| Humans | Leclerc et al., 2020; Witte et al., 2009 | 25–30% DR | Ameliorates memory performance | ↓ C reactive protein and insulin levels | |
| Blumenthal et al., 2010; Smith et al., 2010 | DASH diet (Appel et al., 1997) and DR | Improves cognitive function | |||
| AD | |||||
| Rodents | Patel et al., 2005; Wang et al., 2005; Wu et al., 2008; Schafer et al., 2015 | 30–40% DR | ↓ Aβ and phospho-tau deposition; ↓ astrocyte activation | Improved performance in cognitive tests | |
| Halagappa et al., 2007; Brownlow et al., 2014 | Different DR regimens based on individual calorie consumption; 35–40% DR | No effects | Improved age-related behavioral impairments; rescued associative memory deficits | ||
| Humans | Horie et al., 2016 | DASH diet + DR 500 kcal/d or 25% DR | Improvement in cognitive functions | ||
| PD | |||||
| Rodents | Duan and Mattson, 1999 | IF | ↓ Damage to SN neurons | ↓ Motor deficits | |
| Maswood et al., 2004 | 30% DR | ↑ Levels of dopamine and dopamine metabolites in the striatal region | ↑ Locomotor activity | No differences in presynaptic dopaminergic activity in vivo | |
| Armentero et al., 2008 | IF | No effect on nigrostriatal degeneration | |||
| Griffioen et al., 2013 | IF | NA | Ameliorates autonomic function | NA | |
| Nonhuman primates | Maswood et al., 2004 | 30% DR | ↑ Levels of dopamine and dopamine metabolites in the striatal region | ↑ Locomotor activity | No differences in presynaptic dopaminergic activity in vivo |
| ALS | |||||
| Rodents | Pedersen and Mattson, 1999 | IF | Accelerates disease onset and shortens disease duration | ||
| Hamadeh et al., 2005 | 40% DR | Accelerates disease onset | |||
| MS | |||||
| Rodents | Esquifino et al., 2007 | 66% | Alters lymphocytes composition in lymphoid organs, ↓ IFN-γ production | Prevents EAE | |
| Piccio et al., 2008 | 40% DR | ↑ Corticosterone and adiponectin; ↓ leptin and IL-6 | Ameliorates EAE clinical course | ||
| Kafami et al., 2010 | IF | Ameliorates EAE clinical course and reduces incidence of disease | |||
| Cignarella et al., 2018 | IF | ↓ Th17 cells, ↑ T regulatory cells in small intestine lamina propria, altered gut microbiota | Ameliorates EAE clinical course and reduces incidence of disease | ||
| Jordan et al., 2019 | IF | ↓ Monocyte infiltration in the spinal cord, ↓ TNFα, IL-1β, CXCL2, and CXCL10 | Ameliorates EAE clinical course and reduces incidence of disease | ||
| Humans | Saadatnia et al., 2009 | Ramadan fasting | Well tolerated, no differences in relapse rate | ||
| Etemadifar et al., 2016 | Ramadan fasting | Improves physical health and mental health composites of QOL | |||
| Choi et al., 2016 | FMD + Mediterranean or ketogenic diet | Improves QOL | |||
| Fitzgerald et al., 2018 | 22% DR or IF | Improves mood | |||
| Cignarella et al., 2018 | IF | ↓ Leptin | |||
Summary of the main studies on the effects of DR on aging and neurodegenerative and neuroinflammatory diseases. FA, fractional anisotropy. GM, gray matter; NA, not applicable; NCAM, neural cell adhesion molecule; PSA, polysialylated; QOL, quality of life; SN, substantia nigra.
Normal aging
Aging is associated with systemic low-grade chronic inflammation (called “inflammaging”) that also involves the brain (Franceschi et al., 2018). Neuroinflammation accompanies the age-related decline of brain function (Di Benedetto et al., 2017) and involves activated brain glial cells. Upon aging, MHCII expression by microglia and glial fibrillary acidic protein (GFAP) expression by astrocytes are increased (Morgan et al., 1999; Wong, 2013). Acquisition of a senescent phenotype by microglia may also contribute to the development of age-associated neurodegenerative diseases (Streit, 2004; Wong, 2013).
Several lines of evidence have shown that DR exerts beneficial effects against age-driven neuroinflammation. In rodents, DR attenuated age-dependent astrocyte and microglia activation in the brain (Kaur et al., 2008; Morgan et al., 1999; Yin et al., 2018). Long-term DR in aged mice inhibited a number of inflammatory genes in the neocortex and cerebellum by inducing a type I interferon response and suppressing NF-κB signaling and oxidative stress pathways (Lee et al., 2000). Similarly, short-term IF ameliorated age-associated decrease in motor and cognitive performance in rats by enhancing mitochondrial complex IV activity and reducing oxidative molecular damage (Singh et al., 2012). The effects of DR on brain aging in rodents are discussed in more detail in a recent review by Hadem et al. (2019).
Data on DR effects on neuroinflammation in nonhuman primates are accumulating. Most published reports have examined DR neuroprotective effects on neuroimaging biomarkers or brain pathology. Results from the Wisconsin National Primate Research Center (WNPRC) study showed that moderate chronic DR (30% reduced intake from individualized baseline), initiated in young adulthood, reduces age-associated gray matter brain atrophy in several key cerebral regions of rhesus macaques compared with animals fed ad libitum (Colman et al., 2009). In the same WNPRC study, chronic DR led to a significant reduction of astrogliosis (measured as levels of GFAP expression) in the hippocampus and iron accumulation (measured by brain magnetic resonance imaging [MRI]) in the basal ganglia and cortical areas (Kastman et al., 2012; Sridharan et al., 2013); these changes were associated with improved performance on executive and motor function tests in the DR, but not in the control group (Sridharan et al., 2012). Moreover, chronic DR in nonhuman primates improved insulin sensitivity and lowered blood proinflammatory cytokine concentrations, which were associated with more gray matter volume in the hippocampus and more white matter volume primarily in visual areas and the dorsal prefrontal cortex (Willette et al., 2010, 2012). Another lifelong study in squirrel monkeys found that 30% DR reduced levels of amyloid β (Aβ)1–40 and Aβ1–42 peptides in the temporal cortex in association with elevation of α-secretase activity (Qin et al., 2006).
Chronic metabolic disorders (e.g., obesity, dyslipidemia, and insulin resistance) associated with systemic inflammation accelerate brain aging (Cunnane et al., 2020; Mattson and Arumugam, 2018). Cognitive performance of metabolically morbid individuals is poorer than their age-matched healthy counterparts (Kullmann et al., 2016). Several epidemiological studies have reported that obesity and metabolic syndrome in midlife are associated with impaired cognitive function (Debette et al., 2011). A population-based cohort study of cognitively healthy participants suggested that high visceral adiposity and systemic inflammation were associated with deep white matter brain hyperintensities, reduced gray matter volume (measured by MRI), and potentially with reduced executive functions (Lampe et al., 2019).
In humans, studies of chronic DR have reported beneficial effects on metabolic factors and cognitive functioning in healthy volunteers and obese and hypertensive patients. A 3-mo interventional study of 30% DR in healthy, normal-weight to overweight elderly subjects showed significant improvement in memory performance, with concomitant reductions in fasting insulin and C-reactive protein levels (Witte et al., 2009). Similar results have been reported for a younger population of nonobese (body mass index [BMI] 22–28 kg/m2) men and women enrolled in the large National Institute on Aging–funded multicenter randomized trial Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE). At the end of the second year, the volunteers randomized to 25% DR experienced a better performance in working memory tests than the control group (Leclerc et al., 2020). In the Exercise and Nutrition Intervention for Cardiovascular Health (ENCORE) trial, 3 mo of exercise training coupled with an energy-restricted Dietary Approaches to Stop Hypertension (DASH) diet (high in low-fat dairy products, fruits, vegetables, and fiber and low in fats; Appel et al., 1997) resulted in a significant improvement of cognitive function in hypertensive individuals (Blumenthal et al., 2010; Smith et al., 2010).
AD
AD brain pathology is characterized by abnormal accumulation of Aβ plaques, aggregated hyperphosphorylated tau in neurofibrillary tangles, and neuroinflammation. These pathological hallmarks are also observed in normal brain aging, but in AD they progress much faster and to a greater extent. Systemic inflammation and insulin resistance are emerging as important drivers of AD progression (Heneka et al., 2015; Heppner et al., 2015).
Several studies have demonstrated beneficial effects of DR or IF on brain pathology and functional outcomes in AD transgenic rodent models (Table 1). Short- and long-term DR decreased or prevented Aβ accumulation in the hippocampus and cerebral cortex of transgenic mice carrying familial AD amyloid-precursor protein mutations (Patel et al., 2005; Schafer et al., 2015; Wang et al., 2005) and attenuated Aβ plaque–associated astrogliosis (Patel et al., 2005; Wu et al., 2008). In mouse models with tau deposition (alone or combined with Aβ), long-term DR improved cognitive and behavioral performance, with inconsistent effects on Aβ and phospho-tau levels in the hippocampus (Brownlow et al., 2014; Halagappa et al., 2007). In triple-transgenic mouse models of AD, an IF regimen has been associated with increased neuronal differentiation in the hippocampus (Li et al., 2020). A comprehensive review of these studies has been recently published (Bok et al., 2019).
Epidemiological data suggest a relationship between calorie intake, obesity, and dementia risk. A prospective study of a large cohort of elderly individuals free of dementia at baseline provided evidence that those with a lower calorie intake had a reduced risk of developing AD (Luchsinger et al., 2002). Obesity at midlife increases the risk of AD, as shown in a population-based cohort study with an average 21-yr follow-up. In this study, clustering of vascular risk factors (high total cholesterol and high blood pressure) increased the risk in an additive manner (Kivipelto et al., 2005). Other epidemiological studies suggest a strong association between high blood pressure and late-life dementia (Ding et al., 2020), and the SPRINT MIND trial demonstrated that reducing systolic blood pressure to <120 mm Hg compared with <140 mm Hg significantly reduced the risk for mild cognitive impairment (MCI) and dementia (Williamson et al., 2019). In an 18-yr follow-up study of nondemented women, being overweight at age 70 was associated with a higher risk of developing dementia later in life; for every 1.0 unit of BMI increment in women aged 70–75 yr, AD risk increased by 36% at 79–88 yr of age (Gustafson et al., 2003). Preliminary data from randomized controlled trials are supportive of a cause–effect relationship. In one small 12-mo trial of 80 obese patients with MCI, aged ≥60 yr, weight loss was associated with improvements in verbal memory, verbal fluency, executive function, and global cognition (Horie et al., 2016). In this study, improvements in insulin resistance and inflammation were associated with better cognitive tests. More randomized trials on the effects of DR in individuals with subjective memory complaints and MCI are currently ongoing.
PD
PD, the second most common human CNS neurodegenerative disease, is characterized by progressive debilitating motor and nonmotor symptoms. Accumulation of α-synuclein aggregates within neurons causes neuronal loss primarily in the substantia nigra (Braak et al., 2003; Mendoza-Velásquez et al., 2019). Postmortem brain pathology of PD patients reveals inflammatory changes in microglia (McGeer et al., 1988). Activation of microglia by α-synuclein aggregates resulting in inflammatory and oxidative damage of neurons is one of the main culprits in PD pathogenesis (Hoenen et al., 2016; Hoffmann et al., 2016). One novel compelling hypothesis is that α-synuclein pathology originates in the gastrointestinal tract and is transmitted to the brain via the vagus nerve (Braak et al., 2003; Kim et al., 2019; Van Den Berge et al., 2019).
Preclinical data suggest a potential role of diet and DR in the progression of PD. High-fat diet–induced obesity can increase the vulnerability of dopaminergic neurons of the substantia nigra in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)–induced mouse model of PD (Choi et al., 2005), whereas IF and 30% DR attenuated MPTP-induced neurotoxicity in both mouse and nonhuman primate models (Duan and Mattson, 1999; Maswood et al., 2004). These results were not confirmed in a 6-hydroxydopamine model of PD, in which IF did not prevent nigrostriatal degeneration (Armentero et al., 2008). IF can ameliorate autonomic dysfunction in a PD transgenic mouse model (Griffioen et al., 2013), and growing evidence suggests that modifications of gut microbiota may be involved in the development of motor symptoms and α-synuclein inclusions in the CNS of PD mouse models. Remarkably, transplantation of microbiota from PD-affected patients into α-synuclein–overexpressing mice enhanced motor dysfunction, suggesting that dietary manipulation of the human gut microbiome may play a role in the treatment of PD (Sampson et al., 2016).
Little is known on the effects of diet and DR on PD development and progression in humans. Some epidemiological studies suggest a relationship between BMI and PD risk (Hu et al., 2006), but others did not confirm this association (Roos et al., 2018). Being underweight might even have a negative effect by increasing the risk of PD, especially in patients with diabetes mellitus (Jeong et al., 2020). No randomized clinical trials of the effects of DR or fasting on PD progression have been published so far. However, it is interesting to note that people with PD display lower levels of ghrelin compared with healthy controls, even when matched by BMI (Fiszer et al., 2010). In a mouse model of PD, ghrelin exerted neuroprotective effects during 30% DR through the attenuation of MPTP-induced nigrostriatal damage and loss of dopaminergic neurons (Bayliss et al., 2016). Prolonged DR and fasting powerfully increases plasma concentrations of ghrelin (Hoddy et al., 2016), a “hunger hormone” with anti-inflammatory functions (Dixit et al., 2004).
ALS
ALS is a fatal neurodegenerative disease, causing motor neuron death and progressive muscle paralysis. Approximately 85–90% of cases are sporadic; the remaining 10–15% are classified as familial, with Cu/Zn superoxide dismutase 1 (SOD1) mutations accounting for ∼30% of them (Rosen et al., 1993). CNS pathology has been extensively studied in SOD1 mutation carriers and SOD1 mutant animal models. Although neuroinflammation may accelerate neuronal death (Boillée et al., 2006), preclinical data suggest a detrimental effect of DR on ALS. An early study from the late 1990s reported that Cu/Zn SOD mutant mice undergoing IF experienced earlier disease onset and shorter disease duration compared with mice fed ad libitum (Pedersen and Mattson, 1999). Another study found that 40% DR accelerates disease onset and progression (Hamadeh et al., 2005). Although the exact mechanism is not known, it seems that DR in the SOD1G93A animal model of ALS increases lipid peroxidation, inflammation, and apoptosis in the skeletal muscle, probably by decreasing mitochondrial bioenergetic efficiency and impairing stress response (Patel et al., 2010). To the best of our knowledge, no human studies on the effects of DR on ALS exist.
MS
MS is an inflammatory demyelinating human disease, presumed to be autoimmune, with varying degrees of axonal and neuronal damage (Wallin et al., 2019). Several studies have shown that obesity during childhood/young adulthood is a risk factor for MS development (Hedström et al., 2012; Langer-Gould et al., 2013; Munger et al., 2009, 2013; Wesnes et al., 2015) and could influence the response to therapy (Huppke et al., 2019). A protective effect of DR in the main animal model of MS, experimental autoimmune encephalomyelitis (EAE), was reported in 2004. Severe DR, 66% below ad libitum intake, for 15 d before immunization prevented clinical manifestations in the rat EAE model. In this study, DR caused major reductions in circulating growth hormone and increased prolactin levels; depressed lymphocyte responses, such as to the mitogen concanavalin A (Esquifino et al., 2004), and reduced IFN-γ production in lymphoid tissues were also reported (Esquifino et al., 2007). Similarly, our group has shown that 40% chronic DR greatly reduced murine EAE severity, in concert with increased endogenous serum corticosterone (the main glucocorticoid hormone in mice) and adiponectin, and decreased serum leptin levels (Piccio et al., 2008). An IF regimen started before disease induction showed similar reductions of murine EAE incidence and severity (Cignarella et al., 2018; Kafami et al., 2010). Additional studies suggested a potential role of the gut microbiome in mediating some of these protective effects. We observed Lactobacilli species enrichment, with reduced Th17 cells and increased regulatory T cells in the small intestine lamina propria of mice undergoing IF (Cignarella et al., 2018). Transfer of gut microbiota from these mice inhibited EAE in recipients immunized to induce EAE (Cignarella et al., 2018).
Jordan et al. (2019) have independently confirmed a protective role of IF in the murine EAE model and demonstrated decreased mobilization of monocytes from bone marrow with reduced CNS monocytes and expression of TNF-α, IL-1β, MMP9, CXCL10, and CXCL2 inflammatory mediators. Notably, IF did not inhibit the ability of monocytes to mobilize and function during acute infection or wound healing, suggesting that this strategy might not compromise responses to infection or tissue repair. Indeed, a randomized clinical trial in nonobese men and women demonstrated that prolonged DR markedly reduced inflammation without impairing cell-mediated immunity (Meydani et al., 2016).
Preliminary data suggest that other forms of fasting might have an effect in the EAE model. Three cycles of a fasting mimicking diet (FMD), involving a very-low-calorie and protein diet for 3 consecutive days per week, initiated after clinical EAE onset, increased serum corticosterone levels, reduced proinflammatory cytokines, and improved the clinical course of the disease. FMD also promoted oligodendrocyte differentiation and remyelination in the cuprizone-induced model of CNS demyelination (Choi et al., 2016).
Several studies have focused on potential beneficial effects of DR in MS patients. DR was demonstrated to be safe in an observational study conducted in 40 adult MS patients with mild disability who were followed for 6 mo after fasting during the Ramadan month (Saadatnia et al., 2009). Another study of >200 relapsing-remitting MS patients with mild disability reported that Ramadan fasting improved mean physical and mental health composites of quality of life (Etemadifar et al., 2016).
In an 8-wk randomized feeding study, DR (22% daily calorie reduction) and intermittent DR (75% caloric reduction 2 d/wk) were each compared with a control diet and found to be safe and feasible in people with MS. DR of either type improved mood, as indicated by the Functional Assessment of MS Emotional Well-being subcomponent, while not altering fatigue or sleep quality (Fitzgerald et al., 2018). A study enrolling 60 relapsing-remitting MS patients revealed that 1 wk of FMD followed by 6 mo of Mediterranean diet or ketogenic diet improved some quality of life measures (Choi et al., 2016).
Our group performed a randomized trial of 15 d of intermittent DR versus normal diet in 16 MS patients being treated with corticosteroids for acute MS relapse. IF was well tolerated and reduced leptin without altering adiponectin levels. Enrichment of specific gut bacteria similar to that observed in EAE mice undergoing IF was seen (Cignarella et al., 2018). We are currently performing a 12-wk randomized controlled pilot study of intermittent DR (2 d/wk) compared with a Western diet in 40 MS patients (NCT03539094) to investigate its effects on peripheral blood inflammatory markers and gut microbiota. Also currently underway is an 18-mo, three-arm study in 111 relapsing MS patients comparing ketogenic diet versus IF (1 wk of fasting every 6 mo, plus fasting ≥14 h per day) versus a vegetarian-focused diet, and with new MRI lesions as the primary outcome measure (NCT03508414).
Conclusions and future directions
Neurodegenerative diseases, among many other chronic conditions, are on the rise. The epidemic of obesity and unhealthy aging that is rapidly spreading from industrialized to developing countries likely has a role in this. Excessive calorie intake coupled with a sedentary lifestyle are major players in this extremely costly, and soon unsustainable, pandemic of unhealthy lifestyle–driven chronic diseases. People are living longer, but not healthier. More than 65% of people >65 yr old have two or more chronic diseases, such as heart disease, stroke, vascular dementia, type 2 diabetes, fatty liver disease, cancer, and kidney disease (Atella et al., 2019; Hung et al., 2011), which often share a common metabolic and molecular substrate (Fontana, 2018). As we have illustrated in this review, accumulating data suggest that targeting well-characterized nutrient-sensing and inflammatory pathways can reduce the accumulation of cellular and tissue damage and influence the clinical progression of neurological diseases such as PD, AD, and MS. Specific dietary manipulations play a significant role in modulating pathophysiological mechanisms leading to metabolic and inflammatory changes that characterize several neurodegenerative disorders (Fig. 2). For example, recent findings indicate that altering meal timing can improve brain energy metabolism and function in the absence of changes in overall energy intake. Lowered consumption of particular nutrients is also key in mediating some of the effects of DR, with protein and specific amino acids and nutritional modulation of the gut microbiome playing prominent roles (Fontana and Partridge, 2015). For example ketogenic diets can alter neuronal metabolic and electrical activities and are a proven effective treatment for children with drug-resistant epilepsy (Neal et al., 2008).
Based on our current knowledge, mechanism-based research on interventions to change diet, exercise, and other lifestyle patterns may be a safe and effective solution to slow brain aging and multiple neurodegenerative diseases before the onset of clinical symptoms (Cunnane et al., 2020). We base this comment on the reports that peripheral and central insulin resistance, systemic inflammation, and disruption of brain energy metabolism are linked to the pathological processes of microglia activation, neurotoxic protein accumulation, axonal and synaptic dysfunction, and neuronal death observed in neurodegeneration (Aldana, 2019; Tups et al., 2017; Zilberter and Zilberter, 2017). As outlined in this review, DR and other dietary manipulations might be an effective approach to address metabolic and immune-inflammatory responses associated with impaired brain metabolism. Several clinical trials are underway to test the effects, efficacy, and safety of intermittent or chronic DR in subjects with AD or MS. The results of these trials will be extremely helpful to elucidate the importance of specific dietary manipulations and their potential side effects and risks, such as the potential long-term consequences of mild DR-induced hypercortisolism on learning and memory (Qiu et al., 2012), as well as other DR side effects (e.g., on bone mass and sex hormones), which will depend on the level of restriction (Most et al., 2017). However, more animal and human studies are warranted to understand the interactions among energy expenditure and calorie intake, meal frequency and timing, diet quality, and other factors such as the gut microbiome in slowing molecular damage leading to brain aging and CNS neurodegenerative diseases. Finally, an important practical consideration in choosing different approaches for DR is also its feasibility for the individual. Various types of intermittent DR (e.g., 5:2 diet or time-restricted feeding) are relatively easy ways to incorporate DR into the daily routine compared with chronic calorie restriction. Thus, continued investigation of the intersection of nutrition, metabolism, and neuroinflammation holds immense promise to prevent and potentially treat several chronic neurodegenerative diseases.
Acknowledgments
We thank members of our laboratories for helpful discussions. The authors apologize for the omission of relevant citations owing to space constraints. We thank Dr. Celeste Karch for assisting with Figs. 2 and 3, created with BioRender.com.
The work performed by our group and cited in this review was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (grant R01 NS102633-01 to L. Piccio); the Office of the Assistant Secretary of Defense for Health Affairs, through the Multiple Sclerosis Research Program under award no. W81XWH-14-1-0156; the Fondazione Italiana Sclerosi Multipla (FISM 2014/R/15), cofinanced with “5 per mille” public funding; and the Leon and Harriet Felman Fund for Human MS Research (to L. Piccio and A.H. Cross). L. Fontana is supported by grants from an Australian National Health and Medical Research Council Investigator Grant (APP1177797), Australian Youth and Health Foundation, and Bakewell Foundation. L. Ghezzi was supported by a Fondazione Italiana Sclerosi Multipla research fellowship (FISM 2018/B/1) and a National Multiple Sclerosis Society Post-Doctoral Fellowship (FG-1907-34474).
Author contributions: L. Fontana wrote the initial draft and revised the paper. L. Ghezzi wrote the initial draft and prepared the table and the figures. A.H. Cross wrote the initial draft and revised the paper. L. Piccio wrote the initial draft, provided oversight and leadership responsibility for this paper, and edited the manuscript.
References
- Akiyama, H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., et al. 2000. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 21:383–421. 10.1016/S0197-4580(00)00124-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldana, B.I. 2019. Microglia-Specific Metabolic Changes in Neurodegeneration. J. Mol. Biol. 431:1830–1842. 10.1016/j.jmb.2019.03.006 [DOI] [PubMed] [Google Scholar]
- Appel, L.J., Moore T.J., Obarzanek E., Vollmer W.M., Svetkey L.P., Sacks F.M., Bray G.A., Vogt T.M., Cutler J.A., Windhauser M.M., et al. DASH Collaborative Research Group . 1997. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 336:1117–1124. 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- Armentero, M.T., Levandis G., Bramanti P., Nappi G., and Blandini F.. 2008. Dietary restriction does not prevent nigrostriatal degeneration in the 6-hydroxydopamine model of Parkinson’s disease. Exp. Neurol. 212:548–551. 10.1016/j.expneurol.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Ashraf, J.M., Ahmad S., Choi I., Ahmad N., Farhan M., Tatyana G., and Shahab U.. 2015. Recent advances in detection of AGEs: Immunochemical, bioanalytical and biochemical approaches. IUBMB Life. 67:897–913. 10.1002/iub.1450 [DOI] [PubMed] [Google Scholar]
- Atella, V., Piano Mortari A., Kopinska J., Belotti F., Lapi F., Cricelli C., and Fontana L.. 2019. Trends in age-related disease burden and healthcare utilization. Aging Cell. 18:e12861 10.1111/acel.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basta, G. 2008. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis. 196:9–21. 10.1016/j.atherosclerosis.2007.07.025 [DOI] [PubMed] [Google Scholar]
- Bayliss, J.A., Lemus M.B., Stark R., Santos V.V., Thompson A., Rees D.J., Galic S., Elsworth J.D., Kemp B.E., Davies J.S., and Andrews Z.B.. 2016. Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson’s Disease. J. Neurosci. 36:3049–3063. 10.1523/JNEUROSCI.4373-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal, J.A., Babyak M.A., Hinderliter A., Watkins L.L., Craighead L., Lin P.H., Caccia C., Johnson J., Waugh R., and Sherwood A.. 2010. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch. Intern. Med. 170:126–135. 10.1001/archinternmed.2009.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillée, S., Yamanaka K., Lobsiger C.S., Copeland N.G., Jenkins N.A., Kassiotis G., Kollias G., and Cleveland D.W.. 2006. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 312:1389–1392. 10.1126/science.1123511 [DOI] [PubMed] [Google Scholar]
- Bok, E., Jo M., Lee S., Lee B.R., Kim J., and Kim H.J.. 2019. Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link. Int. J. Mol. Sci. 20:464 10.3390/ijms20030464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesan, M., Hoogaars W.M.H., Varela-Eirin M., Talma N., and Demaria M.. 2020. A Senescence-Centric View of Aging: Implications for Longevity and Disease. Trends Cell Biol. 30:777–791. 10.1016/j.tcb.2020.07.002 [DOI] [PubMed] [Google Scholar]
- Borovikova, L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., and Tracey K.J.. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 405:458–462. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- Braak, H., Rüb U., Gai W.P., and Del Tredici K.. 2003. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. (Vienna). 110:517–536. 10.1007/s00702-002-0808-2 [DOI] [PubMed] [Google Scholar]
- Brownlow, M.L., Joly-Amado A., Azam S., Elza M., Selenica M.L., Pappas C., Small B., Engelman R., Gordon M.N., and Morgan D.. 2014. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Res. 271:79–88. 10.1016/j.bbr.2014.06.001 [DOI] [PubMed] [Google Scholar]
- Bussian, T.J., Aziz A., Meyer C.F., Swenson B.L., van Deursen J.M., and Baker D.J.. 2018. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 562:578–582. 10.1038/s41586-018-0543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cefalu, W.T., Bell-Farrow A.D., Wang Z.Q., Sonntag W.E., Fu M.X., Baynes J.W., and Thorpe S.R.. 1995. Caloric restriction decreases age-dependent accumulation of the glycoxidation products, N epsilon-(carboxymethyl)lysine and pentosidine, in rat skin collagen. J. Gerontol. A Biol. Sci. Med. Sci. 50:B337–B341. 10.1093/gerona/50A.6.B337 [DOI] [PubMed] [Google Scholar]
- Chinta, S.J., Woods G., Rane A., Demaria M., Campisi J., and Andersen J.K.. 2015. Cellular senescence and the aging brain. Exp. Gerontol. 68:3–7. 10.1016/j.exger.2014.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, I.Y., Piccio L., Childress P., Bollman B., Ghosh A., Brandhorst S., Suarez J., Michalsen A., Cross A.H., Morgan T.E., et al. 2016. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep. 15:2136–2146. 10.1016/j.celrep.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J.Y., Jang E.H., Park C.S., and Kang J.H.. 2005. Enhanced susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in high-fat diet-induced obesity. Free Radic. Biol. Med. 38:806–816. 10.1016/j.freeradbiomed.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Cienfuegos, S., Gabel K., Kalam F., Ezpeleta M., Wiseman E., Pavlou V., Lin S., Oliveira M.L., and Varady K.A.. 2020. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 32:366–378.e3. 10.1016/j.cmet.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarella, F., Cantoni C., Ghezzi L., Salter A., Dorsett Y., Chen L., Phillips D., Weinstock G.M., Fontana L., Cross A.H., et al. 2018. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 27:1222–1235.e6. 10.1016/j.cmet.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman, R.J., Anderson R.M., Johnson S.C., Kastman E.K., Kosmatka K.J., Beasley T.M., Allison D.B., Cruzen C., Simmons H.A., Kemnitz J.W., and Weindruch R.. 2009. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 325:201–204. 10.1126/science.1173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé, J.P., Desprez P.Y., Krtolica A., and Campisi J.. 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5:99–118. 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotillard, A., Kennedy S.P., Kong L.C., Prifti E., Pons N., Le Chatelier E., Almeida M., Quinquis B., Levenez F., Galleron N., et al. ANR MicroObes consortium . 2013. Dietary intervention impact on gut microbial gene richness. Nature. 500:585–588. 10.1038/nature12480 [DOI] [PubMed] [Google Scholar]
- Craft, S., and Watson G.S.. 2004. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 3:169–178. 10.1016/S1474-4422(04)00681-7 [DOI] [PubMed] [Google Scholar]
- Cunnane, S.C., Trushina E., Morland C., Prigione A., Casadesus G., Andrews Z.B., Beal M.F., Bergersen L.H., Brinton R.D., de la Monte S., et al. 2020. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 19:609–633. 10.1038/s41573-020-0072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, S.K., Roberts S.B., Bhapkar M.V., Villareal D.T., Fontana L., Martin C.K., Racette S.B., Fuss P.J., Kraus W.E., Wong W.W., et al. CALERIE-2 Study Group . 2017. Body-composition changes in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE)-2 study: a 2-y randomized controlled trial of calorie restriction in nonobese humans. Am. J. Clin. Nutr. 105:913–927. 10.3945/ajcn.116.137232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette, S., Seshadri S., Beiser A., Au R., Himali J.J., Palumbo C., Wolf P.A., and DeCarli C.. 2011. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 77:461–468. 10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto, S., Müller L., Wenger E., Düzel S., and Pawelec G.. 2017. Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci. Biobehav. Rev. 75:114–128. 10.1016/j.neubiorev.2017.01.044 [DOI] [PubMed] [Google Scholar]
- Ding, J., Davis-Plourde K.L., Sedaghat S., Tully P.J., Wang W., Phillips C., Pase M.P., Himali J.J., Gwen Windham B., Griswold M., et al. 2020. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 19:61–70. 10.1016/S1474-4422(19)30393-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit, V.D., Schaffer E.M., Pyle R.S., Collins G.D., Sakthivel S.K., Palaniappan R., Lillard J.W. Jr., and Taub D.D.. 2004. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J. Clin. Invest. 114:57–66. 10.1172/JCI200421134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, W., and Mattson M.P.. 1999. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s disease. J. Neurosci. Res. 57:195–206. [DOI] [PubMed] [Google Scholar]
- Esquifino, A.I., Cano P., Jiménez V., Cutrera R.A., and Cardinali D.P.. 2004. Experimental allergic encephalomyelitis in male Lewis rats subjected to calorie restriction. J. Physiol. Biochem. 60:245–252. 10.1007/BF03167069 [DOI] [PubMed] [Google Scholar]
- Esquifino, A.I., Cano P., Jimenez-Ortega V., Fernández-Mateos M.P., and Cardinali D.P.. 2007. Immune response after experimental allergic encephalomyelitis in rats subjected to calorie restriction. J. Neuroinflammation. 4:6 10.1186/1742-2094-4-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada, J.A., and Contreras I.. 2019. Nutritional Modulation of Immune and Central Nervous System Homeostasis: The Role of Diet in Development of Neuroinflammation and Neurological Disease. Nutrients. 11:1076 10.3390/nu11051076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemadifar, M., Sayahi F., Alroughani R., Toghianifar N., Akbari M., and Nasr Z.. 2016. Effects of prolonged fasting on fatigue and quality of life in patients with multiple sclerosis. Neurol. Sci. 37:929–933. 10.1007/s10072-016-2518-9 [DOI] [PubMed] [Google Scholar]
- Fiszer, U., Michałowska M., Baranowska B., Wolińska-Witort E., Jeske W., Jethon M., Piaścik-Gromada M., and Marcinowska-Suchowierska E.. 2010. Leptin and ghrelin concentrations and weight loss in Parkinson’s disease. Acta Neurol. Scand. 121:230–236. 10.1111/j.1600-0404.2009.01185.x [DOI] [PubMed] [Google Scholar]
- Fitzgerald, K.C., Tyry T., Salter A., Cofield S.S., Cutter G., Fox R.J., and Marrie R.A.. 2018. A survey of dietary characteristics in a large population of people with multiple sclerosis. Mult. Scler. Relat. Disord. 22:12–18. 10.1016/j.msard.2018.02.019 [DOI] [PubMed] [Google Scholar]
- Fontana, L. 2018. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol. 15:566–577. 10.1038/s41569-018-0026-8 [DOI] [PubMed] [Google Scholar]
- Fontana, L., and Partridge L.. 2015. Promoting health and longevity through diet: from model organisms to humans. Cell. 161:106–118. 10.1016/j.cell.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana, L., Eagon J.C., Trujillo M.E., Scherer P.E., and Klein S.. 2007. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 56:1010–1013. 10.2337/db06-1656 [DOI] [PubMed] [Google Scholar]
- Fontana, L., Klein S., and Holloszy J.O.. 2010a Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr.). 32:97–108. 10.1007/s11357-009-9118-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana, L., Partridge L., and Longo V.D.. 2010b Extending healthy life span--from yeast to humans. Science. 328:321–326. 10.1126/science.1172539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana, L., Villareal D.T., Das S.K., Smith S.R., Meydani S.N., Pittas A.G., Klein S., Bhapkar M., Rochon J., Ravussin E., and Holloszy J.O.. CALERIE Study Group . 2016. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 15:22–27. 10.1111/acel.12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi, C., Garagnani P., Parini P., Giuliani C., and Santoro A.. 2018. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14:576–590. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- Ganeshan, K., and Chawla A.. 2014. Metabolic regulation of immune responses. Annu. Rev. Immunol. 32:609–634. 10.1146/annurev-immunol-032713-120236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, N.W., Ahern P.P., Cheng J., Heath A.C., Ilkayeva O., Newgard C.B., Fontana L., and Gordon J.I.. 2017. Prior Dietary Practices and Connections to a Human Gut Microbial Metacommunity Alter Responses to Diet Interventions. Cell Host Microbe. 21:84–96. 10.1016/j.chom.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen, K.J., Rothman S.M., Ladenheim B., Wan R., Vranis N., Hutchison E., Okun E., Cadet J.L., and Mattson M.P.. 2013. Dietary energy intake modifies brainstem autonomic dysfunction caused by mutant α-synuclein. Neurobiol. Aging. 34:928–935. 10.1016/j.neurobiolaging.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson, D., Rothenberg E., Blennow K., Steen B., and Skoog I.. 2003. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med. 163:1524–1528. 10.1001/archinte.163.13.1524 [DOI] [PubMed] [Google Scholar]
- Hadem, I.K.H., Majaw T., Kharbuli B., and Sharma R.. 2019. Beneficial effects of dietary restriction in aging brain. J. Chem. Neuroanat. 95:123–133. 10.1016/j.jchemneu.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Halagappa, V.K., Guo Z., Pearson M., Matsuoka Y., Cutler R.G., Laferla F.M., and Mattson M.P.. 2007. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 26:212–220. 10.1016/j.nbd.2006.12.019 [DOI] [PubMed] [Google Scholar]
- Hamadeh, M.J., Rodriguez M.C., Kaczor J.J., and Tarnopolsky M.A.. 2005. Caloric restriction transiently improves motor performance but hastens clinical onset of disease in the Cu/Zn-superoxide dismutase mutant G93A mouse. Muscle Nerve. 31:214–220. 10.1002/mus.20255 [DOI] [PubMed] [Google Scholar]
- Hedström, A.K., Olsson T., and Alfredsson L.. 2012. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult. Scler. 18:1334–1336. 10.1177/1352458512436596 [DOI] [PubMed] [Google Scholar]
- Heneka, M.T., Carson M.J., El Khoury J., Landreth G.E., Brosseron F., Feinstein D.L., Jacobs A.H., Wyss-Coray T., Vitorica J., Ransohoff R.M., et al. 2015. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14:388–405. 10.1016/S1474-4422(15)70016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner, F.L., Ransohoff R.M., and Becher B.. 2015. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16:358–372. 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- Higami, Y., Pugh T.D., Page G.P., Allison D.B., Prolla T.A., and Weindruch R.. 2004. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 18:415–417. 10.1096/fj.03-0678fje [DOI] [PubMed] [Google Scholar]
- Hindhede, M. 1920. The effects of food restriction during war on mortality in Copenhagen. JAMA. 74:381–382. 10.1001/jama.1920.02620060015005 [DOI] [Google Scholar]
- Hoddy, K.K., Gibbons C., Kroeger C.M., Trepanowski J.F., Barnosky A., Bhutani S., Gabel K., Finlayson G., and Varady K.A.. 2016. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin. Nutr. 35:1380–1385. 10.1016/j.clnu.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Hoenen, C., Gustin A., Birck C., Kirchmeyer M., Beaume N., Felten P., Grandbarbe L., Heuschling P., and Heurtaux T.. 2016. Alpha-Synuclein Proteins Promote Pro-Inflammatory Cascades in Microglia: Stronger Effects of the A53T Mutant. PLoS One. 11:e0162717 10.1371/journal.pone.0162717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A., Ettle B., Bruno A., Kulinich A., Hoffmann A.C., von Wittgenstein J., Winkler J., Xiang W., and Schlachetzki J.C.M.. 2016. Alpha-synuclein activates BV2 microglia dependent on its aggregation state. Biochem. Biophys. Res. Commun. 479:881–886. 10.1016/j.bbrc.2016.09.109 [DOI] [PubMed] [Google Scholar]
- Horie, N.C., Serrao V.T., Simon S.S., Gascon M.R., Dos Santos A.X., Zambone M.A., Del Bigio de Freitas M.M., Cunha-Neto E., Marques E.L., Halpern A., et al. 2016. Cognitive Effects of Intentional Weight Loss in Elderly Obese Individuals With Mild Cognitive Impairment. J. Clin. Endocrinol. Metab. 101:1104–1112. 10.1210/jc.2015-2315 [DOI] [PubMed] [Google Scholar]
- Hu, G., Jousilahti P., Nissinen A., Antikainen R., Kivipelto M., and Tuomilehto J.. 2006. Body mass index and the risk of Parkinson disease. Neurology. 67:1955–1959. 10.1212/01.wnl.0000247052.18422.e5 [DOI] [PubMed] [Google Scholar]
- Hung, W.W., Ross J.S., Boockvar K.S., and Siu A.L.. 2011. Recent trends in chronic disease, impairment and disability among older adults in the United States. BMC Geriatr. 11:47 10.1186/1471-2318-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppke, B., Ellenberger D., Hummel H., Stark W., Röbl M., Gärtner J., and Huppke P.. 2019. Association of Obesity With Multiple Sclerosis Risk and Response to First-line Disease Modifying Drugs in Children. JAMA Neurol. 76:1157 10.1001/jamaneurol.2019.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il’yasova, D., Fontana L., Bhapkar M., Pieper C.F., Spasojevic I., Redman L.M., Das S.K., Huffman K.M., and Kraus W.E.. CALERIE Study Investigators . 2018. Effects of 2 years of caloric restriction on oxidative status assessed by urinary F2-isoprostanes: The CALERIE 2 randomized clinical trial. Aging Cell. 17:e12719 10.1111/acel.12719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, S.M., Han K., Kim D., Rhee S.Y., Jang W., and Shin D.W.. 2020. Body mass index, diabetes, and the risk of Parkinson’s disease. Mov. Disord. 35:236–244. 10.1002/mds.27922 [DOI] [PubMed] [Google Scholar]
- Jiang, X., Wang X., Tuo M., Ma J., and Xie A.. 2018. RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci. Lett. 672:65–69. 10.1016/j.neulet.2018.02.049 [DOI] [PubMed] [Google Scholar]
- Jordan, S., Tung N., Casanova-Acebes M., Chang C., Cantoni C., Zhang D., Wirtz T.H., Naik S., Rose S.A., Brocker C.N., et al. 2019. Dietary Intake Regulates the Circulating Inflammatory Monocyte Pool. Cell. 178:1102–1114.e17. 10.1016/j.cell.2019.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafami, L., Raza M., Razavi A., Mirshafiey A., Movahedian M., and Khorramizadeh M.R.. 2010. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicenna J. Med. Biotechnol. 2:47–52. [PMC free article] [PubMed] [Google Scholar]
- Kastman, E.K., Willette A.A., Coe C.L., Bendlin B.B., Kosmatka K.J., McLaren D.G., Xu G., Canu E., Field A.S., Alexander A.L., et al. 2012. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J. Neurosci. 32:11897–11904. 10.1523/JNEUROSCI.2553-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, M., Sharma S., and Kaur G.. 2008. Age-related impairments in neuronal plasticity markers and astrocytic GFAP and their reversal by late-onset short term dietary restriction. Biogerontology. 9:441–454. 10.1007/s10522-008-9168-0 [DOI] [PubMed] [Google Scholar]
- Kim, S., Kwon S.H., Kam T.I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A., et al. 2019. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron. 103:627–641.e7. 10.1016/j.neuron.2019.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto, M., Solomon A., and Winblad B.. 2005. Statin therapy in Alzheimer’s disease. Lancet Neurol. 4:521–522. 10.1016/S1474-4422(05)70150-2 [DOI] [PubMed] [Google Scholar]
- Kraus, W.E., Bhapkar M., Huffman K.M., Pieper C.F., Krupa Das S., Redman L.M., Villareal D.T., Rochon J., Roberts S.B., Ravussin E., et al. CALERIE Investigators . 2019. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 7:673–683. 10.1016/S2213-8587(19)30151-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann, S., Heni M., Hallschmid M., Fritsche A., Preissl H., and Häring H.U.. 2016. Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol. Rev. 96:1169–1209. 10.1152/physrev.00032.2015 [DOI] [PubMed] [Google Scholar]
- Lampe, L., Zhang R., Beyer F., Huhn S., Kharabian Masouleh S., Preusser S., Bazin P.L., Schroeter M.L., Villringer A., and Witte A.V.. 2019. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann. Neurol. 85:194–203. 10.1002/ana.25396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Gould, A., Brara S.M., Beaber B.E., and Koebnick C.. 2013. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 80:548–552. 10.1212/WNL.0b013e31828154f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Chatelier, E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G., Almeida M., Arumugam M., Batto J.M., Kennedy S., et al. MetaHIT consortium . 2013. Richness of human gut microbiome correlates with metabolic markers. Nature. 500:541–546. 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- Leclerc, E., Trevizol A.P., Grigolon R.B., Subramaniapillai M., McIntyre R.S., Brietzke E., and Mansur R.B.. 2020. The effect of caloric restriction on working memory in healthy non-obese adults. CNS Spectr. 25:2–8. 10.1017/S1092852918001566 [DOI] [PubMed] [Google Scholar]
- Lee, C.K., Weindruch R., and Prolla T.A.. 2000. Gene-expression profile of the ageing brain in mice. Nat. Genet. 25:294–297. 10.1038/77046 [DOI] [PubMed] [Google Scholar]
- Lee, E.B., and Mattson M.P.. 2014. The neuropathology of obesity: insights from human disease. Acta Neuropathol. 127:3–28. 10.1007/s00401-013-1190-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T.H., Cheng K.K., Hoo R.L., Siu P.M., and Yau S.Y.. 2019. The Novel Perspectives of Adipokines on Brain Health. Int. J. Mol. Sci. 20:5638 10.3390/ijms20225638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G., Xie C., Lu S., Nichols R.G., Tian Y., Li L., Patel D., Ma Y., Brocker C.N., Yan T., et al. 2017. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 26:672–685.e4. 10.1016/j.cmet.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W., Wu M., Zhang Y., Wei X., Zang J., Liu Y., Wang Y., Gong C.X., and Wei W.. 2020. Intermittent fasting promotes adult hippocampal neuronal differentiation by activating GSK-3β in 3xTg-AD mice. J. Neurochem.:jnc.15105 10.1111/jnc.15105 [DOI] [PubMed] [Google Scholar]
- Limbad, C., Oron T.R., Alimirah F., Davalos A.R., Tracy T.E., Gan L., Desprez P.Y., and Campisi J.. 2020. Astrocyte senescence promotes glutamate toxicity in cortical neurons. PLoS One. 15:e0227887 10.1371/journal.pone.0227887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger, J.A., Tang M.X., Shea S., and Mayeux R.. 2002. Caloric intake and the risk of Alzheimer disease. Arch. Neurol. 59:1258–1263. 10.1001/archneur.59.8.1258 [DOI] [PubMed] [Google Scholar]
- Ma, S., Sun S., Geng L., Song M., Wang W., Ye Y., Ji Q., Zou Z., Wang S., He X., et al. 2020. Caloric Restriction Reprograms the Single-Cell Transcriptional Landscape of Rattus Norvegicus Aging. Cell. 180:984–1001.e22. 10.1016/j.cell.2020.02.008 [DOI] [PubMed] [Google Scholar]
- Mager, D.E., Wan R., Brown M., Cheng A., Wareski P., Abernethy D.R., and Mattson M.P.. 2006. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 20:631–637. 10.1096/fj.05-5263com [DOI] [PubMed] [Google Scholar]
- Masoro, E.J., Katz M.S., and McMahan C.A.. 1989. Evidence for the glycation hypothesis of aging from the food-restricted rodent model. J. Gerontol. 44:B20–B22. 10.1093/geronj/44.1.B20 [DOI] [PubMed] [Google Scholar]
- Maswood, N., Young J., Tilmont E., Zhang Z., Gash D.M., Gerhardt G.A., Grondin R., Roth G.S., Mattison J., Lane M.A., et al. 2004. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 101:18171–18176. 10.1073/pnas.0405831102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias, I., Morgado J., and Gomes F.C.A.. 2019. Astrocyte Heterogeneity: Impact to Brain Aging and Disease. Front. Aging Neurosci. 11:59 10.3389/fnagi.2019.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, M.P., and Arumugam T.V.. 2018. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 27:1176–1199. 10.1016/j.cmet.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson, M.P., Allison D.B., Fontana L., Harvie M., Longo V.D., Malaisse W.J., Mosley M., Notterpek L., Ravussin E., Scheer F.A., et al. 2014. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA. 111:16647–16653. 10.1073/pnas.1413965111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer, P.L., Itagaki S., Boyes B.E., and McGeer E.G.. 1988. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 38:1285–1291. 10.1212/WNL.38.8.1285 [DOI] [PubMed] [Google Scholar]
- McHugh, D., and Gil J.. 2018. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 217:65–77. 10.1083/jcb.201708092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Velásquez, J.J., Flores-Vázquez J.F., Barrón-Velázquez E., Sosa-Ortiz A.L., Illigens B.W., and Siepmann T.. 2019. Autonomic Dysfunction in α-Synucleinopathies. Front. Neurol. 10:363 10.3389/fneur.2019.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydani, S.N., Das S.K., Pieper C.F., Lewis M.R., Klein S., Dixit V.D., Gupta A.K., Villareal D.T., Bhapkar M., Huang M., et al. 2016. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging (Albany NY). 8:1416–1431. 10.18632/aging.100994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, T.E., Xie Z., Goldsmith S., Yoshida T., Lanzrein A.S., Stone D., Rozovsky I., Perry G., Smith M.A., and Finch C.E.. 1999. The mosaic of brain glial hyperactivity during normal ageing and its attenuation by food restriction. Neuroscience. 89:687–699. 10.1016/S0306-4522(98)00334-0 [DOI] [PubMed] [Google Scholar]
- Most, J., Tosti V., Redman L.M., and Fontana L.. 2017. Calorie restriction in humans: An update. Ageing Res. Rev. 39:36–45. 10.1016/j.arr.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, K.L., Bentzen J., Laursen B., Stenager E., Koch-Henriksen N., Sørensen T.I., and Baker J.L.. 2013. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult. Scler. 19:1323–1329. 10.1177/1352458513483889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, K.L., Chitnis T., and Ascherio A.. 2009. Body size and risk of MS in two cohorts of US women. Neurology. 73:1543–1550. 10.1212/WNL.0b013e3181c0d6e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musi, N., Valentine J.M., Sickora K.R., Baeuerle E., Thompson C.S., Shen Q., and Orr M.E.. 2018. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 17:e12840 10.1111/acel.12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal, E.G., Chaffe H., Schwartz R.H., Lawson M.S., Edwards N., Fitzsimmons G., Whitney A., and Cross J.H.. 2008. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 7:500–506. 10.1016/S1474-4422(08)70092-9 [DOI] [PubMed] [Google Scholar]
- Ovadya, Y., and Krizhanovsky V.. 2014. Senescent cells: SASPected drivers of age-related pathologies. Biogerontology. 15:627–642. 10.1007/s10522-014-9529-9 [DOI] [PubMed] [Google Scholar]
- Patel, B.P., Safdar A., Raha S., Tarnopolsky M.A., and Hamadeh M.J.. 2010. Caloric restriction shortens lifespan through an increase in lipid peroxidation, inflammation and apoptosis in the G93A mouse, an animal model of ALS. PLoS One. 5:e9386 10.1371/journal.pone.0009386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, N.V., Gordon M.N., Connor K.E., Good R.A., Engelman R.W., Mason J., Morgan D.G., Morgan T.E., and Finch C.E.. 2005. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiol. Aging. 26:995–1000. 10.1016/j.neurobiolaging.2004.09.014 [DOI] [PubMed] [Google Scholar]
- Patterson, R.E., Laughlin G.A., LaCroix A.Z., Hartman S.J., Natarajan L., Senger C.M., Martínez M.E., Villaseñor A., Sears D.D., Marinac C.R., and Gallo L.C.. 2015. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 115:1203–1212. 10.1016/j.jand.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov, V.A., and Tracey K.J.. 2012. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 8:743–754. 10.1038/nrendo.2012.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, W.A., and Mattson M.P.. 1999. No benefit of dietary restriction on disease onset or progression in amyotrophic lateral sclerosis Cu/Zn-superoxide dismutase mutant mice. Brain Res. 833:117–120. 10.1016/S0006-8993(99)01471-7 [DOI] [PubMed] [Google Scholar]
- Pekkala, T., Hall A., Mangialasche F., Kemppainen N., Mecocci P., Ngandu T., Rinne J.O., Soininen H., Tuomilehto J., Kivipelto M., and Solomon A.. 2020. Association of Peripheral Insulin Resistance and Other Markers of Type 2 Diabetes Mellitus with Brain Amyloid Deposition in Healthy Individuals at Risk of Dementia. J. Alzheimers Dis. 76:1243–1248. 10.3233/JAD-200145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccio, L., Stark J.L., and Cross A.H.. 2008. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J. Leukoc. Biol. 84:940–948. 10.1189/jlb.0208133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, W., Chachich M., Lane M., Roth G., Bryant M., de Cabo R., Ottinger M.A., Mattison J., Ingram D., Gandy S., and Pasinetti G.M.. 2006. Calorie restriction attenuates Alzheimer’s disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus). J. Alzheimers Dis. 10:417–422. 10.3233/JAD-2006-10411 [DOI] [PubMed] [Google Scholar]
- Qiu, G., Spangler E.L., Wan R., Miller M., Mattson M.P., So K.F., de Cabo R., Zou S., and Ingram D.K.. 2012. Neuroprotection provided by dietary restriction in rats is further enhanced by reducing glucocortocoids. Neurobiol. Aging. 33:2398–2410. 10.1016/j.neurobiolaging.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette, S.B., Weiss E.P., Villareal D.T., Arif H., Steger-May K., Schechtman K.B., Fontana L., Klein S., and Holloszy J.O.. Washington University School of Medicine CALERIE Group . 2006. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J. Gerontol. A Biol. Sci. Med. Sci. 61:943–950. 10.1093/gerona/61.9.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks, M.G., and Garrett W.S.. 2016. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16:341–352. 10.1038/nri.2016.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, E., Grotta A., Yang F., Bellocco R., Ye W., Adami H.O., Wirdefeldt K., and Trolle Lagerros Y.. 2018. Body mass index, sitting time, and risk of Parkinson disease. Neurology. 90:e1413–e1417. 10.1212/WNL.0000000000005328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X., et al. 1993. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 362:59–62. 10.1038/362059a0 [DOI] [PubMed] [Google Scholar]
- Saadatnia, M., Etemadifar M., Fatehi F., Ashtari F., Shaygannejad V., Chitsaz A., and Maghzi A.H.. 2009. Short-term effects of prolonged fasting on multiple sclerosis. Eur. Neurol. 61:230–232. 10.1159/000197108 [DOI] [PubMed] [Google Scholar]
- Salama, M., Akan A., and Mueller M.R.. 2020. Transcutaneous Stimulation of Auricular Branch of the Vagus Nerve Attenuates the Acute Inflammatory Response After Lung Lobectomy. World J. Surg. 44:3167–3174. 10.1007/s00268-020-05543-w [DOI] [PubMed] [Google Scholar]
- Sampson, T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. 2016. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 167:1469–1480.e12. 10.1016/j.cell.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucillo, D.C., Gerriets V.A., Sheng J., Rathmell J.C., and Maciver N.J.. 2014. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 192:136–144. 10.4049/jimmunol.1301158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, M.J., Alldred M.J., Lee S.H., Calhoun M.E., Petkova E., Mathews P.M., and Ginsberg S.D.. 2015. Reduction of β-amyloid and γ-secretase by calorie restriction in female Tg2576 mice. Neurobiol. Aging. 36:1293–1302. 10.1016/j.neurobiolaging.2014.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers, E.M., Witteman J.C., Sijbrands E.J., Hofman A., Koudstaal P.J., and Breteler M.M.. 2010. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 75:1982–1987. 10.1212/WNL.0b013e3181ffe4f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R., Lakhanpal D., Kumar S., Sharma S., Kataria H., Kaur M., and Kaur G.. 2012. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr.). 34:917–933. 10.1007/s11357-011-9289-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P.J., Blumenthal J.A., Babyak M.A., Craighead L., Welsh-Bohmer K.A., Browndyke J.N., Strauman T.A., and Sherwood A.. 2010. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 55:1331–1338. 10.1161/HYPERTENSIONAHA.109.146795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, J.D., Pajewski N.M., Auchus A.P., Bryan R.N., Chelune G., Cheung A.K., Cleveland M.L., Coker L.H., Crowe M.G., Cushman W.C., et al. SPRINT MIND Investigators for the SPRINT Research Group . 2019. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 321:553–561. 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan, A., Pehar M., Salamat M.S., Pugh T.D., Bendlin B.B., Willette A.A., Anderson R.M., Kemnitz J.W., Colman R.J., Weindruch R.H., et al. 2013. Calorie restriction attenuates astrogliosis but not amyloid plaque load in aged rhesus macaques: a preliminary quantitative imaging study. Brain Res. 1508:1–8. 10.1016/j.brainres.2013.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan, A., Willette A.A., Bendlin B.B., Alexander A.L., Coe C.L., Voytko M.L., Colman R.J., Kemnitz J.W., Weindruch R.H., and Johnson S.C.. 2012. Brain volumetric and microstructural correlates of executive and motor performance in aged rhesus monkeys. Front. Aging Neurosci. 4:31 10.3389/fnagi.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein, P.K., Soare A., Meyer T.E., Cangemi R., Holloszy J.O., and Fontana L.. 2012. Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell. 11:644–650. 10.1111/j.1474-9726.2012.00825.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson, J., Nutma E., van der Valk P., and Amor S.. 2018. Inflammation in CNS neurodegenerative diseases. Immunology. 154:204–219. 10.1111/imm.12922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit, W.J. 2004. Microglia and Alzheimer’s disease pathogenesis. J. Neurosci. Res. 77:1–8. 10.1002/jnr.20093 [DOI] [PubMed] [Google Scholar]
- Strom, A., and Jensen R.A.. 1951. Mortality from circulatory diseases in Norway 1940-1945. Lancet. 1:126–129. 10.1016/S0140-6736(51)91210-X [DOI] [PubMed] [Google Scholar]
- Tsai, S., Clemente-Casares X., Zhou A.C., Lei H., Ahn J.J., Chan Y.T., Choi O., Luck H., Woo M., Dunn S.E., et al. 2018. Insulin Receptor-Mediated Stimulation Boosts T Cell Immunity during Inflammation and Infection. Cell Metab. 28:922–934.e4. 10.1016/j.cmet.2018.08.003 [DOI] [PubMed] [Google Scholar]
- Tups, A., Benzler J., Sergi D., Ladyman S.R., and Williams L.M.. 2017. Central Regulation of Glucose Homeostasis. Compr. Physiol. 7:741–764. 10.1002/cphy.c160015 [DOI] [PubMed] [Google Scholar]
- Van Den Berge, N., Ferreira N., Gram H., Mikkelsen T.W., Alstrup A.K.O., Casadei N., Tsung-Pin P., Riess O., Nyengaard J.R., Tamgüney G., et al. 2019. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 138:535–550. 10.1007/s00401-019-02040-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara, H., Cai W., Crandall J., Goldberg T., Oberstein R., Dardaine V., Peppa M., and Rayfield E.J.. 2002. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc. Natl. Acad. Sci. USA. 99:15596–15601. 10.1073/pnas.242407999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin, M.T., Culpepper W.J., Campbell J.D., Nelson L.M., Langer-Gould A., Marrie R.A., Cutter G.R., Kaye W.E., Wagner L., Tremlett H., et al. US Multiple Sclerosis Prevalence Workgroup . 2019. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology. 92:e1029–e1040. 10.1212/WNL.0000000000007035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., et al. 2003. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 421:384–388. 10.1038/nature01339 [DOI] [PubMed] [Google Scholar]
- Wang, J., Ho L., Qin W., Rocher A.B., Seror I., Humala N., Maniar K., Dolios G., Wang R., Hof P.R., and Pasinetti G.M.. 2005. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 19:659–661. 10.1096/fj.04-3182fje [DOI] [PubMed] [Google Scholar]
- Wang, X., Sun X., Niu M., Zhang X., Wang J., Zhou C., and Xie A.. 2020. RAGE Silencing Ameliorates Neuroinflammation by Inhibition of p38-NF-κB Signaling Pathway in Mouse Model of Parkinson’s Disease. Front. Neurosci. 14:353 10.3389/fnins.2020.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, E.P., Racette S.B., Villareal D.T., Fontana L., Steger-May K., Schechtman K.B., Klein S., and Holloszy J.O.. Washington University School of Medicine CALERIE Group . 2006. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am. J. Clin. Nutr. 84:1033–1042. 10.1093/ajcn/84.5.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesnes, K., Riise T., Casetta I., Drulovic J., Granieri E., Holmøy T., Kampman M.T., Landtblom A.M., Lauer K., Lossius A., et al. 2015. Body size and the risk of multiple sclerosis in Norway and Italy: the EnvIMS study. Mult. Scler. 21:388–395. 10.1177/1352458514546785 [DOI] [PubMed] [Google Scholar]
- Willette, A.A., Bendlin B.B., McLaren D.G., Canu E., Kastman E.K., Kosmatka K.J., Xu G., Field A.S., Alexander A.L., Colman R.J., et al. 2010. Age-related changes in neural volume and microstructure associated with interleukin-6 are ameliorated by a calorie-restricted diet in old rhesus monkeys. Neuroimage. 51:987–994. 10.1016/j.neuroimage.2010.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette, A.A., Bendlin B.B., Colman R.J., Kastman E.K., Field A.S., Alexander A.L., Sridharan A., Allison D.B., Anderson R., Voytko M.L., et al. 2012. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes. 61:1036–1042. 10.2337/db11-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette, A.A., Coe C.L., Birdsill A.C., Bendlin B.B., Colman R.J., Alexander A.L., Allison D.B., Weindruch R.H., and Johnson S.C.. 2013. Interleukin-8 and interleukin-10, brain volume and microstructure, and the influence of calorie restriction in old rhesus macaques. Age (Dordr.). 35:2215–2227. 10.1007/s11357-013-9518-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte, A.V., Fobker M., Gellner R., Knecht S., and Flöel A.. 2009. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. USA. 106:1255–1260. 10.1073/pnas.0808587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, S.A., Boddeke H.W., and Kettenmann H.. 2017. Microglia in Physiology and Disease. Annu. Rev. Physiol. 79:619–643. 10.1146/annurev-physiol-022516-034406 [DOI] [PubMed] [Google Scholar]
- Wong, W.T. 2013. Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Front. Cell. Neurosci. 7:22 10.3389/fncel.2013.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, P., Shen Q., Dong S., Xu Z., Tsien J.Z., and Hu Y.. 2008. Calorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout mice. Neurobiol. Aging. 29:1502–1511. 10.1016/j.neurobiolaging.2007.03.028 [DOI] [PubMed] [Google Scholar]
- Yang, L., Licastro D., Cava E., Veronese N., Spelta F., Rizza W., Bertozzi B., Villareal D.T., Hotamisligil G.S., Holloszy J.O., and Fontana L.. 2016. Long-Term Calorie Restriction Enhances Cellular Quality-Control Processes in Human Skeletal Muscle. Cell Rep. 14:422–428. 10.1016/j.celrep.2015.12.042 [DOI] [PubMed] [Google Scholar]
- Yin, Z., Raj D.D., Schaafsma W., van der Heijden R.A., Kooistra S.M., Reijne A.C., Zhang X., Moser J., Brouwer N., Heeringa P., et al. 2018. Low-Fat Diet With Caloric Restriction Reduces White Matter Microglia Activation During Aging. Front. Mol. Neurosci. 11:65 10.3389/fnmol.2018.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino, M., Kayser B.D., Yoshino J., Stein R.I., Reeds D., Eagon J.C., Eckhouse S.R., Watrous J.D., Jain M., Knight R., et al. 2020. Effects of Diet versus Gastric Bypass on Metabolic Function in Diabetes. N. Engl. J. Med. 383:721–732. 10.1056/NEJMoa2003697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C., Li S., Yang L., Huang P., Li W., Wang S., Zhao G., Zhang M., Pang X., Yan Z., et al. 2013. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat. Commun. 4:2163 10.1038/ncomms3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter, Y., and Zilberter M.. 2017. The vicious circle of hypometabolism in neurodegenerative diseases: Ways and mechanisms of metabolic correction. J. Neurosci. Res. 95:2217–2235. 10.1002/jnr.24064 [DOI] [PubMed] [Google Scholar]