Abstract
This study aimed to elucidate the molecular mechanisms, whereby hyaluronic acid, a main extracellular matrix component of articular cartilage, promotes the chondrogenic differentiation of human amniotic mesenchymal stem cells (hAMSCs). Our previous findings indicated that hyaluronic acid combined with hAMSCs showed a marked therapeutic effect against rat osteoarthritis. In the present study, hyaluronic acid markedly enhanced the expression of chondrocyte-specific markers including Col2α1, Acan, and Sox9 in hAMSCs, with strong synergistic effects on chondrogenic differentiation, in combination with the commonly used inducer, transforming growth factor β3 (TGF-β3). Microarray analysis showed that Ras-like protein family member 11B (RASL11B) played a pivotal role in the process of hyaluronic acid-mediated chondrogenesis of hAMSCs. This directional differentiation was significantly inhibited by RASL11B knockdown, but RASL11B overexpression dramatically promoted the expression of Sox9, a master chondrogenesis transcriptional factor, at the levels of transcription and translation. Increased Sox9 expression subsequently resulted in high expression levels of Col2α1 and Acan and the accumulation of cartilage-specific matrix components, such as type 2 collagen and glycosaminoglycans. Moreover, we observed that RASL11B activated the signal molecules such as ERK1/2, and Smad2/3 in the presence of hyaluronic acid during TGF-β3-induced chondrogenesis of hAMSCs. Taken together, these findings suggest that hyaluronic acid activates the RASL11B gene to potentiate the chondrogenic differentiation of hAMSCs via the activation of Sox9 and ERK/Smad signaling, thus providing a new strategy for cartilage defect repairing by hyaluronic acid-based stem cell therapy.
Keywords: RASL11B, hyaluronic acid, human amniotic mesenchymal stem cells, chondrogenic differentiation, Smad signaling, ERK signaling
Impact statement
RASL11B, a member of the small GTPase superfamily, has high similarity to RAS proteins, and involves some pathophysiological processes, such as inflammation, arteriosclerosis, and cancer. However, there is no available information regarding the role of RASL11B in chondrogenic differentiation. We show that RASL11B is activated in the process of HA-mediated chondrogenesis of hAMSCs, and RASL11B regulates the differentiation of hAMSCs into chondrocytes through the activation of Sox9 and ERK/Smad signals. The results of this study support that RASL11B may be used as a target in the chondroinductive differentiation of hAMSCs in the presence of HA and even in the cartilage defect repairing by HA-based stem cell therapy.
Introduction
Articular cartilage, a type of hyaline cartilage, has no blood vessels or nerves. Thus, the self-repair or regeneration of articular cartilage damage is quite limited.1,2 Articular cartilage damage most commonly occurs in the knee due to trauma, osteoporosis, or degenerative osteoarthritis. As the disease advances, the patient's activity is gradually limited and due to the loss of joint function leads to disability. At present, the most commonly recommended treatment for cartilage damage is pharmacotherapy with non-steroidal anti-inflammatory agents, which can delay the progression of disease, and improve the symptoms of patients, but they do not reverse the pathological process and most of these drugs have obvious side effects. Therefore, an alternative therapeutic strategy is urgently required. Cartilage transplantation technique such as autologous chondrocyte implantation, and osteochondral allograft is a very promising therapeutic approach. However, some disadvantages including an insufficient supply of cells, adverse effects of donor site, and immunological rejection have deeply impacted the efficacy. Mesenchymal stem cells (MSCs) may offer an improved starting material for generating the large numbers of functional chondrocytes required.3
In the past few decades, as multifunctional cells, MSCs have become well known for their ability to maintain an undifferentiated status and be induced to differentiate into desired cell types for the purpose of cell replacement under the corresponding culture conditions.4 Among these cells, human amniotic MSCs (hAMSCs) derived from placental tissue have significant advantages, including the absence of ethical issues, low immunogenicity, potent paracrine activity, and non-tumorigenic properties. Thus, hAMSCs are recognized as an ideal seed cell source with broad clinical application prospects for regenerative medicine,5,6 especially for cartilage damage.7,8 However, the optimal technique to directly and efficiently regulate their differentiation into chondrocyte requires further investigation. Numerous previous studies have shown that some growth factors including insulin-like growth factor (IGF), fibroblast growth factors (FGF), transforming growth factor β (TGF-β), and bone morphogenetic proteins (BMP) have the potential to induce cartilage formation.9–11 These factors can regulate the expression of chondrogenic-associated genes by activating intracellular protein kinase A (PKA), TGF-β, protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and other signals, thus inducing the differentiation of MSCs into chondrocytes.12 Nevertheless, these induction approaches are not sufficient to enable MSCs to efficiently generate functional chondrocytes.
The extracellular matrix plays a vital role in maintaining the function of chondrocytes and signal transmission between chondrocytes and the organism.13–15 Hyaluronic acid (HA) is one of the main extracellular matrix components,16 and it is recognized as a functional ligand influencing cell migration, proliferation, and differentiation.17,18 Furthermore, HA is commonly used as a scaffold material in tissue engineering of cartilage, where it plays a key role in cartilage regeneration and in attenuating cartilage degeneration in early osteoarthritis.19,20 In our previous study, a cocktail of HA and hAMSCs transplantation showed a significant therapeutic effect against rat knee osteoarthritis.21Also, HA displayed a similar function of routine chondroindutive chemicals in hAMSCs and a synergistic effect on the chondrogenic differentiation of hAMSCs in combination with chondroinductive chemicals.21However, how does HA modulate the chondrogenic differentiation of hAMSCs, and its underlying molecular mechanism remains to be elucidated.
The sex-determining region Y-box (Sox9), a transcription factor with a high mobility group DNA-binding domain, is required for chondrocyte differentiation of MSCs and cartilage formation, in which Sox9 binds to chondrocyte-specific enhancer elements in the collagen type 2 gene (Col2α1) and activates Col2α1 expression after chondrogenic mesenchymal condensations.22 Thereby, Sox 9 is expressed in all prechondrocytic and chondrocytic cells, while its expression is dramatically downregulated in hypertrophic chondrocytes. Furthermore, the cartilage formation is a multi-step cell differentiation process that is modulated by a complex signaling network. Here members of the TGF-β superfamily play an indispensable role in almost every aspect of cartilage formation and maintenance, including coagulation, proliferation, terminal differentiation, and the maintenance of chondrocytes.23–25Many previous studies showed that TGF-β stimulates chondrogenesis through the downstream small mothers against decapentaplegic (Smad) signaling.26,27 Smad3 is involved in enhanced transcriptional activity of Sox9 in human MSCs.27 In addition, an increasing evidence suggests that the MAPK signaling pathway plays an essential role in MSCs-differentiated intracellular signaling, in which extracellular signal-regulated kinase (ERK) mainly participate in chondrogenesis.28,29 ERK showed either inhibitory or promotive effects on chondrocytes differentiation.30 Furthermore, our recent study has shown that Smad and ERK signals co-regulate the expression of type II collagen and aggrecan in CD44-induced chondrogenic differentiation.31In addition, the ERK signal can regulate the Sox9 expression in MSCs and chondrocytes.30Therefore, Sox9/ERK/Smad signals may involve chondroinductive differentiation in hAMSCs by the combination of HA and TGF-β3.
In the present study, the gene targeted by HA to promote the chondrogenic differentiation of hAMSCs was identified by microarray analysis and the downstream signaling pathway was also investigated for the first time.
Materials and methods
Reagents
HA (300 kD) was obtained from the Seebio Biotech Inc. (Shanghai, China). Dulbecco's modified Eagle medium-low/high glucose (LG/HG-DMEM), fetal bovine serum (FBS), non-essential amino acids (NEAAs), GlutaMAX, trypsin, and β-mercaptoethanol were purchased from GIBCO Industries Inc. (Los Angeles, CA, USA). Dexamethasone (Dex), β-glycerol phosphate disodium (β-Gly), L-ascorbic acid (Asc), and collagenase II, DNase I were all purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Dulbecco’s phosphate-buffered saline (D-PBS), penicillin, and streptomycin were purchased from Sino-American Biotech Inc. (Shanghai, China).
The isolation and culture of hAMSCs
After gaining informed consent, we conducted 15 term placentas obtained from healthy individuals undergoing caesarean delivery in accordance with the guidelines of the Ethical Committee of Affiliated Hospital of Zunyi Medical University (Zunyi, China). In brief, the amniotic membrane was stripped from the placental tissue using medical forceps, repeatedly washed with D-PBS containing 100 U/mL of penicillin and 100 μg/mL of streptomycin to remove residual blood, and the amniotic tissue was shredded. To the amniotic membrane pieces, the mixture (0.05% trypsin containing 0.02% EDTA-2Na) was added twice to its volume. Samples were shaken and digested at 190 r/min for 45 min at 37°C in a shaking incubator, and then were filtered using a 300-mesh sieve. Subsequently, the supernatant was discarded and the above procedure mentioned was repeated once for the digestion of amniotic membrane pieces. Next, the above amniotic membrane pieces were then washed once with D-PBS containing antibiotics. An equal volume to amniotic membrane pieces of type II collagenase solution (0.5 mg/mL) containing 0.05 mg/mL DNase I was added and the mixture was digested at 37°C, 190 r/min for 1 h in a shaking incubator. Finally, after the amniotic membrane pieces were completely digested until flocculent, samples were filtered through a 300-mesh sieve. The filtrates were collected and centrifuged at 1500 r/min for 11 min to obtain hAMSCs. The harvested cells were plated in 25-cm2 flasks at a density of 2 × 105 cells/flask in the LG-DMEM supplemented with 10% (v/v) FBS, 1% (v/v) NEAA, 1% (v/v) L-GlutaMAX, and 10 ng/mL human bFGF. The cells were then maintained at 37°C in a humid atmosphere consisting of 5% CO2 and 95% air. When the degree of cell confluence reaches 80–90%, subculture is carried out after digestion. Cells at passage 2 (P2) were used for further experiments.
Phenotypic analysis of hAMSCs by flow cytometry
hAMSC-specific surface markers were identified by flow cytometry. For the flow cytometry analysis, all primary antibodies, including PE-conjugated anti human monoclonal CD29, CD11b, CD34, CD44, CD45, and HLA-DR antibodies; FITC-conjugated anti human CD90 and CD45 antibodies; APC-conjugated anti human CD 73 antibody; Cy5.5-conjugated anti human CD 105 antibody; or their corresponding isotype controls were purchased from BD Pharmingen (San Diego, CA, USA). In brief, the third passage of cells in the logarithmic phase was collected and suspended at a density of 2 × 106 cells/mL in D-PBS containing 0.1% (m/v) bovine serum albumin (BSA). After adding the appropriate antibody, approximately 4 × 105 cells were co-incubated for 25 min. The labeled cells were then washed with D-PBS and analyzed on a BD FACSCalibur system using the CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
Screen for target genes involved in hyaluronic acid-induced chondrogenic differentiation of hAMSCs
The microarray technique was employed to screen possible target genes. Briefly, total RNA was extracted from the following groups tested, including a negative control group (NC), a positive drug TGF-β3 control group (PG), and a combined group with PG+HA (CG). Referring to the RNAiso Plus extraction kit (Takara Bio, Dalian, China) instructions, RNA was extracted from hAMSCs at day 21 after induction. Subsequently, A NanoDrop ND-1000 instrument (Thermo Fisher Scientific, Waltham, MA, USA) was employed to assess the quantity and quality of RNA. The collection and analysis of microarray data were performed by Shanghai Kangcheng Bioengineering Co., Ltd (Shanghai, China). Microarray data were analyzed to identify differentially expressed mRNAs and were screened for genes involved in the chondrogenic differentiation of hAMSCs using an Arraystar Human array. A comparative analysis of whole genome expression profiles was performed for the NC, PG, and CG groups (Figures S1 and 2).
Analysis of the effects of RASL11B overexpression and knockdown
Recombinant adenovirus expression vectors, including HBAD-GFP, HBAD-h-RASL11B-1–3*flag-GFP, HBAD-h-RASL11B-shRNA1-GFP, HBAD-RA SL11B-shRNA2-GFP, and HBAD-RASL11B-shRNA3-GFP were constructed by Shanghai Hanheng Biotechnology Co., Ltd (Shanghai, China). The map of the selected interference vector, pHBAd-U6-MCS-CMV-GFP, is shown in Figure S4.
To determine the optimal multiplicity of infection (MOI), P2 hAMSCs were seeded at a density of 2 × 105 per well in 6-well plates (Corning, NY, USA) in basal medium. Different concentrations of adenovirus were then added in the logarithmic growth phase, at MOI values of 10, 30, 50, 100, and 200 and the medium was replaced with the fresh medium after 6 h of incubation. Finally, green fluorescent protein expression was measured after 72 h. The optimal MOI was chosen based on the fluorescence reaching 80% and a minimum amount of virus required (Figure S5).
To analyze the effects of overexpression and knockdown of the RASL11B gene, P2 hAMSCs were seeded at a density of 2 × 105 per well in 6-well plates and were then transfected with the appropriate vector in the logarithmic growth phase. A normal control group (NC, no virus solution) and a null-vector negative control group (vector-NC; transfected with no-load virus, HBAD-GFP) were included along with an RASL11B-overexpression group (h-RASL11B, transfected with HBAD-h-RASL11B -1–3*flag-GFP) and an RASL11B-knockdown group (sh-RASL11B, transfected with HBAD-RASL11B shRNA3-GFP). After 4–8 h, virus-containing media were changed to normal media and total RNA was extracted on days 7, 14, and 21 after induction. RT-qPCR was then used to analyze the overexpression and knockdown of the RASL11B gene.
Directional differentiation of hAMSCs into chondrocytes
For chondrocyte differentiation, P2 hAMSCs were plated at a cell density of 2 × 105 per well in 6-well plates and incubated at 37°C until reaching 80% confluence, the medium was then replaced with the fresh medium, with or without the adenovirus vector, for 4–8 h. Chondrogenic differentiation experiments not involving adenoviral vectors included a negative control group (NC), an HA treatment group (HG), a positive drug control group (PG), and a PG + HG combined group (CG). For the NC group, hAMSCs were grown only in the basal media that consisted of HG-DMEM supplemented with 10% (v/v) FBS, 1% (v/v) L-GlutaMAX, and 1% (v/v) NEAA. The HG group was added with 0.05 mg/mL HA, the PG group was added with a commonly used inducer, TGF-β3, at 10 ng/L, and the CG group was added with 0.05 mg/mL HA and 10 ng/L TGF-β3. Chondrogenic differentiation experiments involving adenovirus vectors included a null-vector negative control group (vector), an RASL11B-overexpression group (h-RASL11B), an RASL11B-knockdown group (sh-RASL11B). Upon successful transfection, each of the above groups included PG and CG samples. For long-term chondrogenic differentiation, the above active ingredients were added to each group when the media was changed, which occurred every three days.
Toluidine blue staining
After culturing in different chondrogenic induction media for 21 days, hAMSCs were washed with PBS for three times. They were then fixed at 24–28°C for 30 min with 4% paraformaldehyde. After washing with PBS for three times, cells were stained with 1% toluidine blue in the dark for 30 min at 24–28°C. Finally, the cells were washed three times with PBS again, dried, and observed under a microscope.
Immunohistochemical staining
P3 hAMSCs or chondrogenically differentiated cells were fixed at 24–28°C for 30 min with 4% paraformaldehyde and then washed three times with PBS. For immunostaining, the cells were permeabilized at 24–28°C for 15 min with 0.3% (v/v) Triton X-100 and then washed with PBS. Subsequently, they were blocked at 24–28°C for 1 h with 5% BSA containing 0.1% Triton X-100, and were then incubated with the following primary antibodies, anti-CK19 (Sigma, St Louis, MO, USA), anti-vimentin (Sigma), or anti- type II collagen (ab34712; Abcam, Cambridge, UK) overnight at 4°C. After washing off unbound primary antibody with PBS, the cells were re-incubated at 37°C for 30 min, and were then treated in the dark for 1 h at 37°C with horseradish peroxidase (HRP)-conjugated secondary antibodies. Finally, the cells were washed with PBS again, and were then counterstained with hematoxylin to visualize the nuclei. The images were taken under a microscope.
Quantitative reverse transcription polymerase chain reaction assay
Following the manufacturer’s protocols, total RNA was extracted in hAMSCs at days 7, 14, and 21 after induction using an RNAiso Plus Kit and cDNA was synthesized using a PrimeScript™ RT Reagent Kit (TaKaRa, Dalian, China). qRT-PCR was performed using a standard SYBR Green PCR Kit (Qiagen, Hilden, Germany) and was processed on a CFX96 Touch real-time PCR detection system with CFX Manager Software (Bio-Rad, Hercules, CA, USA). List of the primers for all genes tested in the study is shown in Table 1. The comparative cycle threshold (Ct) method was used to calculate the relative expression level of the genes tested. β-actin was used as an internal control for normalization.
Table 1.
Primer sequences of target genes.
| Gene | Sequence (5′→3′) | GenBank ID | Length of product (bp) |
|---|---|---|---|
| RASL11B | For: AAGCGAAGAACCTCCCTCATTRev: AGGGCTTGCTTAAACCTCCTC | NM_023940.2 | 77 |
| Sox9 | For: GCGGAGGAAGTCGGTGAAGARev: GAAGATGGCGTTGGGGGAGA | NM_000346.3 | 82 |
| Col2α1 | For: CAACACTGCCAACGTCCAGATRev: TCTTGCAGTGGGCTGCCTTAT | NM_001844.4NM_033150.2 | 121 |
| Acan | For: GTGCCTATCAGGACAAGGTCTRev: GATGCCTTTCACCACGACTTC | NM_001135.3NM_013227.3 | 167 |
| ERK1 | For: CTACACGCAGTTGCAGTACATRev: CAGCAGGATCTGGATCTCCC | NM_002746.2 | 157 |
| ERK2 | For: TCTGGAGCAGTATTACGACCCRev: CTGGCTGGAATCTAGCAGTCT | NM_138957.3 | 134 |
| Smad1 | For: CAGCCTCTTAGCTCAGTTCCGRev: CTCCCAGGAGAGTTTGGGTAA | NM_005900.2 | 144 |
| Smad2 | For: CCGACACACCGAGATCCTAACRev: GAGGTGGCGTTTCTGGAATATAA | NM_005901.5 | 125 |
| Smad3 | For: TGGACGCAGGTTCTCCAAACRev: CCGGCTCGCAGTAGGTAAC | NM_001145102.1 | 90 |
| Smad5 | For: TCTCCAAACAGCCCTTATCCCRev: GCAGGAGGAGGCGTATCAG | NM_001001420.2 | 113 |
| Smad8 | For: ATGTGATTTACTGTCGCGTGTRev: GGCGGTAGTGGTAAGGGTTAAT | NM_005905.5 | 132 |
| β-actin | For: TGGCACCCAGCACAATGAARev: CTAAGTCATAGTCCGCCTAGAAG | NM_001101.3 | 186 |
Western blotting analysis
The protein expression of Sox9, Col2α1, and Acan on the 7th, 14th and 21th day of differentiation, and ERK1/2, p-ERK1/2, Smad2/3, p-Smad2/3 on the 7th day of differentiation were analyzed by Western blotting assay. Total protein was extracted from cultured hAMSCs using a total protein extraction kit (Applygen, Beijing, China). Protein concentration was quantified using a BCA kit, according to the manufacturer's recommendations. After denaturing, the proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transfer, membranes were incubated overnight at 4°C with the following primary antibodies including mouse anti-human Sox9, mouse anti-human Col2α1, mouse anti-human Acan, mouse anti-human β-actin, mouse anti-human ERK1/2, rabbit anti-human p-ERK1/2, mouse anti-human smad2/3, and mouse anti-human p-smad2/3. After washing for three times with tris-buffered saline/Tween 20 (TBST), HRP-labeled rabbit anti-mouse IgG or goat anti-rabbit IgG was added to the membranes and they were incubated at 24–28°C for 1 h. The membranes were then washed with TBST again and visualized using an enhanced chemiluminescence (ECL) luminescent solution (Beyotime, Shanghai, China). Finally, the membranes were exposed in the Bio-Rad ChemiDoc™ MP Imaging System (Bio-Rad, Hercules, CA, USA) and the resulting images were preserved. ImageJ 1.46a software (NIH, Bethesda, MD, USA) was employed to analyze the protein bands. β-Actin was used as an internal reference in the study.
Statistics
All experimental data are presented as mean ± SD and were obtained from at least three individual experiments. The experimental data were analyzed statistically by SPSS v. 13.0 software (IBM, Armonk, NY, USA) and significance of difference between two groups was determined by one-way ANOVA and/or Student’s t test. A value of P < 0.05 was considered statistically significant.
Results
Characterization of hAMSCs
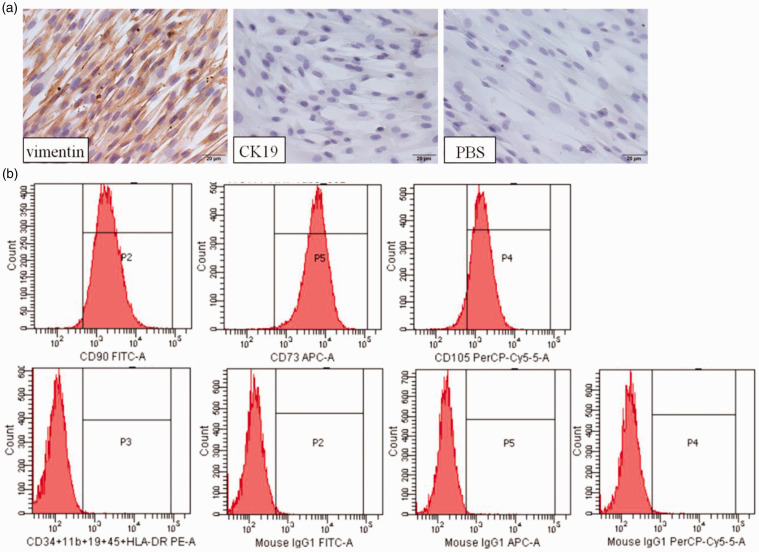
To ensure the quality of cultured cells, we identified their phenotypic characteristics. As shown in Figure 1(a), hAMSCs were stained strongly for the mesenchymal cell marker vimentin, but were negative for the epithelial cell marker cytokeratin 19 (CK19), by immunohistochemical staining. Furthermore, flow cytometric analysis showed that hAMSCs highly expressed CD90 (91.30% ± 5.31%), CD73 (91.22% ± 5.19%), CD105 (96.88% ± 3.28%), and CD44 (98.12% ± 3.49%), but did not express CD34, CD45, CD11b, CD19 and the MHC class II antigen molecule, HLA-DR. In addition, these hAMSCs can differentiate into osteoblasts, adipocytes, and chondrocytes as described in our previous report.5 Therefore, these results suggested that the isolated cells of mesenchymal origin met the definition and minimum criteria of mesenchymal stromal cells, as proposed by the International Society for Cellular Therapy.31
Figure 1.
Characterization of hAMSCs. (a) The expression of vimentin and cytokeratin (CK19) was measured in hAMSCs. Brown staining denotes the positive expression for vimentin and/or CK19. PBS: negative control. The nuclei were counterstained with DAPI (blue). Scale bar = 100 µm. (b) Flow cytometry analysis of hAMSCs, including samples and homotypic controls. (A color version of this figure is available in the online journal.)
HA promoted chondrogenic differentiation of hAMSCs
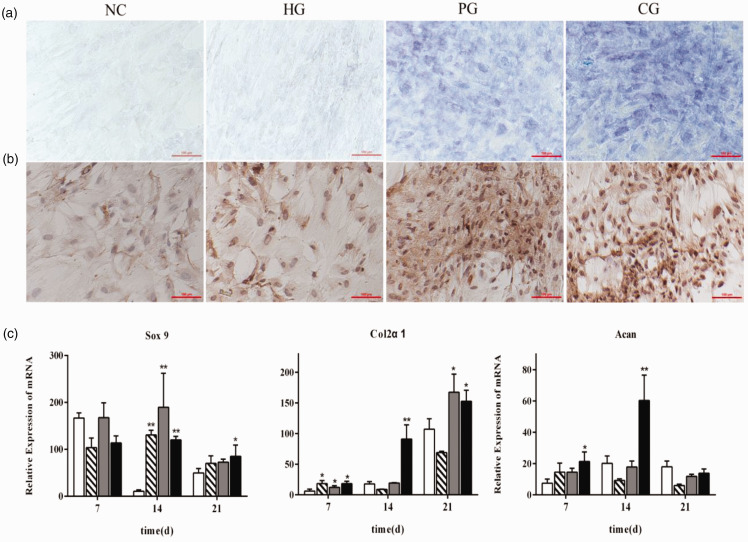
Differentiated chondrocytes can secrete structural extracellular matrix components, including type II collagen and glycosaminoglycan, to form cartilage tissue. hAMSCs were cultured under different chondroinductive conditions for 21 days and the deposition of cartilage-specific matrix components, such as glycosaminoglycan and type II collagen was observed to determine the effect of HA on the chondrogenic differentiation of hAMSCs. Glycosaminoglycan synthesis in the extracellular matrix was examined by toluidine blue staining and type II collagen synthesis was analyzed by immunohistochemical staining. As shown in Figure 2(a) and (b), HA alone had no significant effect on chondrogenic differentiation at a dose of 0.05 mg/mL, but when combined with TGF-β3, could significantly increase the accumulation of the cartilage-specific matrix components, glycosaminoglycan, and type II collagen, compared with PG cells. Similar to the TGF-β3, further study showed that HA could give rise to a dramatic upregulation of Sox 9, a master chondrogenesis transcription factor, on day 14 after induction of chondrogenic differentiation, as well as chondrocyte-specific gene Col2α1 on day 7 at transcriptional level (Figure 2(c)). Furthermore, CG group exerted a very strong synergistic effect on up-regulation of chondrogenic genes, such as Col2α1 and Acan, on day 14 as shown in Figure 2(c).
Figure 2.
Effect of hyaluronic acid on the chondrogenic differentiation of hAMSCs. (a) Glycosaminoglycan detected by toluidine blue staining. (b) Type II collagen assessed by immunohistochemical staining. (c) Expression of chondrocyte-specific genes, such as Sox9, Col2α1, and Acan was detected by qRT-PCR on days 7, 14, and 21 after the induction of chondrogenic differentiation. NG: negative control group (white bar); HG: hyaluronic acid treatment group (hatched bar); PG: positive drug control group (gray bar); CG: PG + HG combined group (dark bar). *P < 0.05, **P < 0.01, vs. NC. (A color version of this figure is available in the online journal.)
RASL11B regulated the chondrogenic differentiation of hAMSCs during synergistic induction with HA and TGF-β3
To clarify the underlying mechanism of HA synergism with TGF-β3 in the promotion of the chondrogenesis of hAMSCs, we screened for genes involved in the chondrogenic differentiation of hAMSCs using an Arraystar Human mRNA array. A comparative analysis of whole genome expression profiles was performed between groups. A total of 113 differentially expressed mRNAs were identified by whole-genome expression profiling, of which 8 showed large differences, and thus were possibly related to chondrogenic differentiation (Tables S1 and 2). Here these genes were screened using the NCBI Gene database. However, the validation of eight candidate genes by qRT-PCR did not show complete consensus with the results of whole-genome microarray, only both RASL11B and DLX5 indicated a slightly better consistent trend (Figure S2). Further study presented that overexpression of DLX5 did not give rise to a marked change of cartilage-specific markers, including glycosaminoglycan, and collagen type II in hAMSCs (Figure S3). By contrast, overexpression or knockdown of RASL11B could significantly alter the expression of chondrogenic markers as shown in Figure 3(c) and (d). Therefore, RASL11B was possibly associated with HA-mediated chondrogenic differentiation. Based on this finding, this study mainly focused on the effects and mechanisms of the target gene, RASL11B, on the chondrogenic differentiation of hAMSCs.
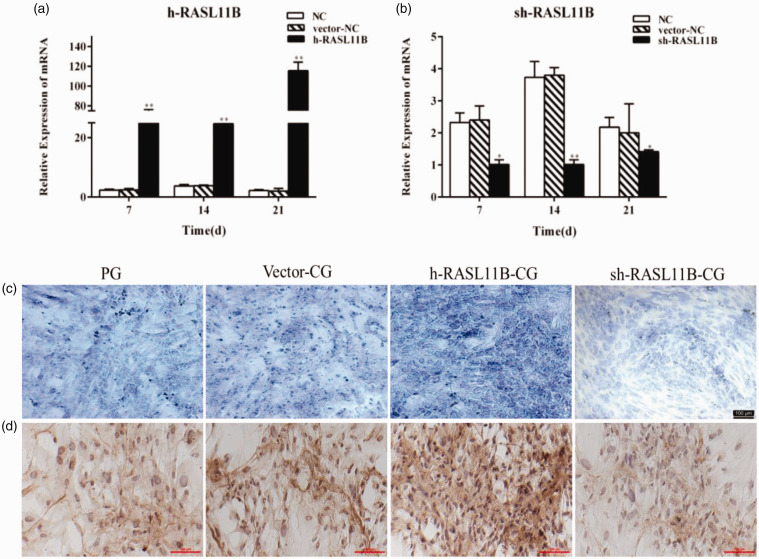
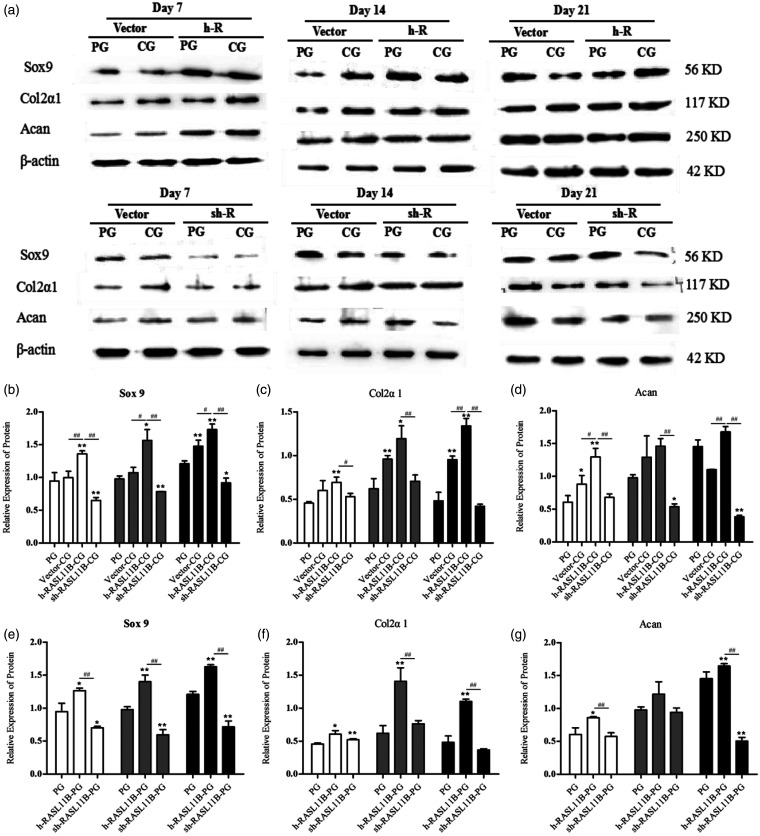
Figure 3.
Effect of RASL11B on the chondrogenic differentiation of hAMSCs on day 21 after induction. (a and b) Analysis of the effects of RASL11B gene overexpression and knockdown. (c) Glycosaminoglycan detected by toluidine blue staining. (d) Type II collagen detected by immunohistochemical staining. NC: normal control group; vector-NC: null-vector negative control group; h-RASL11B: overexpression of RASL11B group; sh-RASL11B: knockdown of RASL11B group; PG: positive drug control group; vector-CG: null-vector CG group; h-RASL11B-CG: CG group with overexpression of RASL11B; sh-RASL11B-CG: CG group with knockdown of RASL11B. *P < 0.05, **P < 0.01, vs. NC. (A color version of this figure is available in the online journal.)
The adenovirus vectors, HBAD-h-RASL11B-1–3*flag-GFP and HBAD-RASL11B shRNA3-GFP, were transfected into hAMSCs to enhance or inhibit RASL11B expression, respectively. Before performing the chondrogenic differentiation experiments, we determined that the optimal multiplicity of infection (MOI) was 100, when the fluorescence intensity was 80% and virus usage was at its lowest (Figure S5). Upon successful transfection, RASL11B mRNA was measured in hAMSCs at days 7, 14, and 21 after induction. As shown in Figure 3(a) and (b), the RASL11B mRNA expression was significantly up-regulated in HBAD-h-RASL11B-1–3*flag-GFP-transfected hAMSCs and knocked down in HBAD-RASL11B shRNA3-GFP-transfected hAMSCs. Moreover, these effects persisted for more than 21 days. The hAMSCs transfected with adenoviruses at different experimental groups were then induced to undergo chondrocyte differentiation, as shown in Figure 3(c) and (d). Knockdown of the RASL11B gene in the CG group (sh-RASL11B-CG) resulted in a significant decrease in the secretion of glycosaminoglycan and the expression of type II collagen, compared with the vector-CG group on day 21 after the induction of chondrogenic differentiation. Meanwhile, overexpression of the RASL11B gene in the CG group (h-RASL11B-CG group) led to a significant increase in the accumulation of cartilage-specific extracellular matrix components in hAMSCs, including glycosaminoglycan and collagen type II.
Effect of RASL11B on the expression of chondrocyte-specific genes and proteins in hAMSCs during the synergistic induction of differentiation by HA and TGF-β3
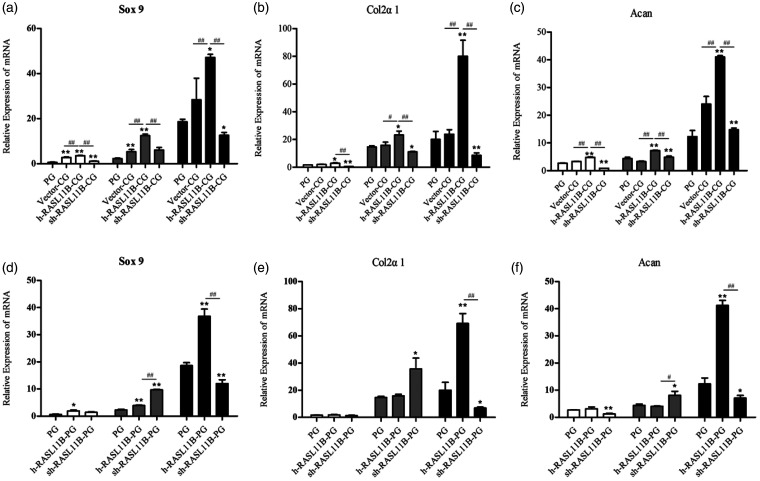
After a preliminary confirmation of the modulatory effect of RASL11B on chondrogenic differentiation, we further investigated the effect of RASL11B on chondrogenic differentiation-related genes and proteins on days 7, 14, and 21 after induction with HA. During the chondroinductive differentiation of hAMSCs, the expression levels of all three of the chondrogenesis-related genes, Sox9, Col2α1, and Acan were increased in a time-dependent manner in each different CG group, as shown in Figure 4(a) to (c). Furthermore, overexpression of RASL11B in the CG group (h-RASL11B-CG group) remarkably up-regulated all chondrogenesis-related genes tested, i.e. Sox9 (Figure 4(a)), Col2α1 (Figure 4(b)), and Acan (Figure 4(c)), in a time-dependent manner, when compared with the PG and vector-CG groups (P < 0.05 or P < 0.01). The relative expression of these genes reached their highest levels on day 21. In comparison with the PG and vector-CG groups, knockdown of RASL11B in the CG group (sh-RASL11B-CG group) led to a significant decrease in the expression levels of all genes tested (Figure 4(a) to (c); P < 0.05 or P < 0.01), in which only Sox9 expression on day 14 had no difference compared with the vector-CG group (Figure 4(a)). In addition, a significant change for all genes tested at the transcriptional level was shown between the h-RASL11B-CG group and the sh-RASL11B-CG group (Figure 4(a) to (c), P < 0.01).
Figure 4.
Effect of RASL11B on the transcriptional level of chondrogenesis-related genes in hAMSCs. Gene expression was evaluated by qRT-PCR on days 7 (white bar), 14 (gray bar), and 21 (dark bar) after the induction of chondrogenic differentiation. (a and d) Relative mRNA expression level of Sox9; (b and e) relative mRNA expression level of Col2α1; (c and f) relative mRNA expression level of Acan. PG: positive drug control group; vector-CG: null-vector CG group; h-RASL11B-CG: CG group with overexpression of RASL11B; sh-RASL11B-CG: CG group with knockdown of RASL11B; h-RASL11B-PG: PG group with overexpression of RASL11B; sh-RASL11B-PG: PG group with knockdown of RASL11B. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. PG; #P < 0.05, ##P < 0.01, vs. vector-CG or sh-RASL11B-CG.
Similar to the CG group, the PG also gave rise to a time-dependent up-regulation for the expression level of three chondrogenic-related genes (Figure 4(d) to (f)). In contrast to the aforementioned CG groups, however, overexpression or knockdown of RASL11B did not result in a time-dependent expression in the PG group. For example, the transcriptional levels of Col2α1 and Acan on day 14 were higher than those on day 21 in the sh-RASL11B-PG group (Figure 4(e) and (f)). Interestingly, overexpression of RASL11B did not result in a significant increase in the transcriptional levels of Col2α1 or Acan on days 7 or 14, compared with the PG group. By contrast, RASL11B knockdown significantly increased the relative expression levels of all three genes, Sox9, Col2α1, and Acan, on day 14, when compared with both other groups (Figure 4(d) to (f)). However, the changes in expression observed for all three genes on day 21 were similar to those in the corresponding CG groups.
At the translational level, all three chondrogenesis-related genes, Sox9, Col2α1, and Acan showed similar responses to their transcriptional level responses for all CG groups, in that overexpression of RASL11B in the CG group (h-RASL11B-CG group) resulted in a significant increase in the levels of Sox9 (Figure 5(b)), Col2α1 (Figure 5(c)), and Acan (Figure 5(d)) proteins in a time-dependent manner on day 7, 14, and 21 after synergistic induction with HA, compared with the PG and vector-CG groups (P < 0.05 or P < 0.01). Moreover, the relative expression of these proteins reached their highest levels on day 21. Meanwhile, knockdown of RASL11B resulted in a significant decrease in Sox9 (Figure 5(b)) and Acan (Figure 5(d)) protein levels in comparison with the PG group during chondrogenic differentiation of hAMSCs, with the exception of Acan levels on day 7. However, RASL11B knockdown had no effect on Col2α1 protein levels, as shown in Figure 5(c). Similar to the results of their transcriptional levels (Figure 4(a) to (c)), a significant change in the levels of all proteins tested was shown between the h-RASL11B-CG group and the sh-RASL11B-CG group (Figure 5(b) to (d), P < 0.05 or P < 0.01). As shown in Figure 5, we also examined the effect of RASL11B on the chondrogenesis-related markers, Sox9, Col2α1, and Acan after induction by TGF-β3 (PG group, Figure 5(e) to (g)). Unlike the transcriptional level results (Figure 4(d) to (f)), all three proteins in each different PG group (Figure 5(e) to (g)) showed a similar trend to their corresponding CG group (Figure 5(b) to (d)) during chondrogenic differentiation, in which h-RASL11B promoted the expression of all three proteins tested and sh-RASL11B suppressed the expression of Sox9 on days 7, 14, and 21 and Acan on day 21 in comparison with the PG group alone. Moreover, sh-RASL11B did not affect the relative expression of Col2α1 on days 7, 14, or 21 (Figure 5(f)) or Acan on days 7 or 14 (Figure 5(g)), after differentiation induction by TGF-β3.
Figure 5.
Effect of RASL11B on the protein levels of chondrogenesis-related factors in hAMSCs. These factors were detected by Western blotting on days 7 (white bar), 14 (gray bar), and 21 (dark bar) after induction of chondrogenic differentiation. (a) Western blotting results; (b and e) relative expression level of Sox9 protein; (c and f) relative expression level of Col2α1 protein; (d and g) relative expression level of Acan protein. PG: positive drug control group; vector-CG: null-vector CG group; h-RASL11B-CG: CG group with overexpression of RASL11B; sh-RASL11B-CG: CG group with knockdown of RASL11B; h-RASL11B-PG: PG group with overexpression of RASL11B; sh-RASL11B-PG: PG group with knockdown of RASL11B. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, vs. PG; #P < 0.05, ##P < 0.01, vs. vector-CG or sh-RASL11B-CG.
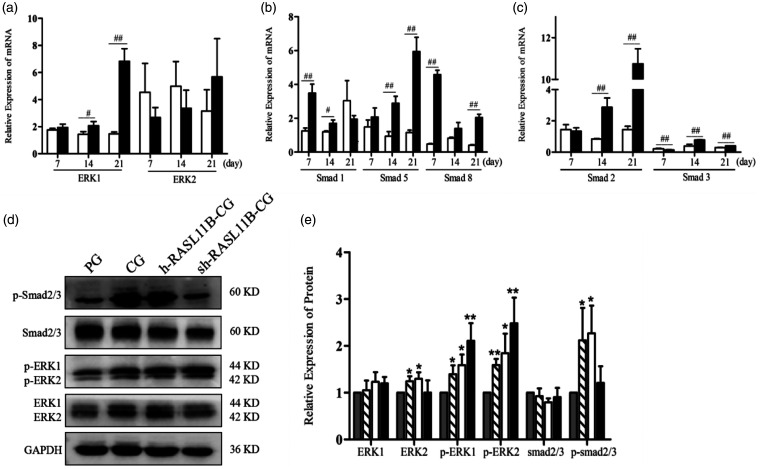
Smad and ERK signals are involved in the synergistic induction of chondrocyte differentiation by HA and TGF-β3 in hAMSCs
To further determine the possible mechanism underlying the synergistic effect of chondroinductive differentiation in hAMSCs by HA and TGF-β3, we first investigated key genes involved in the TGF-β/BMP and ERK1/2 signaling pathways by RT-qPCR on days 7, 14, and 21 after the overexpression or knockdown of RASL11B (Figure 6(a) to (c)). There was no change in ERK1 expression at different time points when RASL11B was overexpressed and the expression level of ERK1 was higher in the sh-RASL11B-CG group than in the h-RASL11B-CG group, as shown in Figure 6(a). Furthermore, RASL11B knockdown led to the up-regulation of ERK1 expression in a time-dependent manner, reaching a highly significant difference on day 21 compared with the h-RASL11B-CG group. RASL11B overexpression resulted in a trend toward the up-regulation of ERK2, but without statistical significance, on days 7 and 14 compared with RASL11B knockdown. However, on day 21, the expression level of ERK2 was lower in the h-RASL11B-CG group than in the sh-RASL11B-CG group (Figure 6(a)).
Figure 6.
Relative expression of ERK/Smad pathway-related genes and proteins. (a) Relative mRNA expression levels of ERK1 and ERK2; (b) relative mRNA expression levels of Smad1, Smad5, and Smad8; (c) relative mRNA expression levels of Smad2 and Smad3. (d) Western blotting bands for Smad2/3, ERK1/2, p-Smad 2/3, and p-ERK1/2 at day 7 after differentiation. (e) Quantitative analysis of Western blots at day 7 after differentiation. PG (grey bar); CG (hatched bar); h-RASL11B-CG (white bar); sh-RASL11B-CG (dark bar). Data are presented as mean ± SD (n = 3). PG: positive drug control group; CG: PG + hyaluronic acid (concentration: 0.05 mg/mL). *P < 0.05, **P < 0.01, vs. PG; #P < 0.05, ##P < 0.01, vs. h-RASL11B-CG.
Major Smad family members were also investigated, as shown in Figure 6(b) and (c). sh-RASL11B up-regulated the expression of all Smads tested, compared with h-RASL11B, during the chondrogenic differentiation of hAMSCs, with the exception of Smad1 on day 21 and Smad2 and Smad3 on day 7. Smad1 and Smad8 showed the highest transcriptional expression levels on day 7 in the sh-RASL11B-CG group, while Smad5 showed the highest level of on day 21. The sh-RASL11B gave rise to a highly significant change in the expression level of Smad2, in a time-dependent manner, compared with h-RASL11B in the CG group (Figure 6(c)). Smad2 expression reached its highest level on day 21, whereas the highest level of Smad3 expression was seen on day 14. Similar to the ERK expression results, overexpression of RASL11B could not significantly alter the expression level of any of the Smads tested at different time points.
Subsequently, we focused further on the changes in expression of ERK1/2 and Smad2/3 at the translational level and their phosphorylation levels on day 7 after the chondrogenic differentiation of hAMSCs. As shown in Figure 6(d) and (e), the expression of ERK1/2 and Smad2/3 proteins was consistent with the result at the mRNA level, with no evident difference in expression levels between h-RASL11B-CG and sh-RASL11B-CG groups. However, the phosphorylation levels of ERK1/2 were higher in the groups with abnormal RASL11B expression than in the PG and CG groups. The order of ERK1/2 phosphorylation levels was found to be PG < CG < h-RASL11B-CG < sh-RASL11B-CG. For Smad2/3 phosphorylation, the CG group had higher levels than the PG group and the sh-RASL11B-CG showed significantly lower levels than the CG and h-RASL11B-CG groups However, Smad2/3 phosphorylation levels were only slightly higher in the h-RASL11B-CG group than in the CG group. Accordingly, these results showed that RASL11B activated the ERK/Smad signaling pathway during HA-induced chondrogenic differentiation.
Discussion
HA is one of the main extracellular matrix components in hyaline cartilage as well as in mesenchyme during the early stage of chondrogenesis. Thus, HA and HA-based biomaterials have been used to modulate stem cell chondrogenesis and stem-cell based cartilage tissue engineering.32 Our previous study showed that HA alone or in combination with routine inducer exerted a positive effect on the chondroinductive differentiation of hAMSCs, and in the hAMSCs transplantation against rat knee osteoarthritis as well.21 In the study, we found that HA facilitated the expression of chondrocyte-specific genes including Sox9, Col2α1, and Acan to some extent. In particular, the combination of HA and TGF-β3 showed a potent synergistic effect for the expression of Col2α1 and Acan on day 14 after chondrogenic induction (Figure 2(c)). Here the expression of Col2α1 reached the highest value on day 21 for all groups tested, while the expression of Sox9 and Acan was decreased. As we know, the transition of chondrocytes to hypertrophy and terminal differentiation is a common phenomenon during the late stage of chondrogenic differentiation. It is unclear whether these cells still retained a chondrocyte phenotype but not a hypertrophic chondrocyte one in this study. As described previously, the duration of common protocols of in vitro chondroinductive differentiation was 21–28 days.5,32–34 Furthermore, the hypertrophy of chondrocytes was inhibited in the presence of TGF-β.35 In the study, a common protocol of TGF-β3-based in vitro chondroinductive differentiation was employed in hAMSCs. In addition, the addition of HA was conducive to the maintenance of stemness during the proliferation of differentiation of hAMSCs.5,6 To achieve a more stringent control of hAMSCs differentiation to chondrocyte, however, the effect of HA on hypertrophic chondrocyte during induction of chondrogenesis in hAMSCs and possible mechanism should be investigated in further study.
Induction of the chondrogenic differentiation in stem/progenitor cells is accurately regulated by essential transcription factors and complex signals, such as TGF-β, BMPs, Wnt/β-catenin, and MAPK.12,15,22,23,27,32 However, the molecular mechanisms underlying this process remain largely unclear. Here we used whole-genome microarray analysis and qRT-PCR validation to investigate the mechanisms of HA-mediated regulation of chondrogenic differentiation induced by TGF-β3. The expression of differential gene RASL11B was significantly changed after addition of HA during the process of chondrogenic differentiation of hAMSCs. Furthermore, using gene ontology information, the RASL11B gene was predicted to be involved in HA-mediated chondrogenesis.
The microarray is an ideal research tool for comparative analysis of whole-genome gene expression changes due to its high-throughput. However, the simultaneous measurement of thousands of gene transcripts coupled with each step in the experimental process can introduce experimental errors that easily introduce a certain level of false-positive result. Thus, false-positive results for gene microarray are always present. In the study, eight differential expressed genes with high fold changes did not get a desired result in the qRT-PCR validation test (Figure S2). Also, the candidate DLX5 that indicated a relatively concordance between qRT-PCR and microarray data could not significantly alter the HA-mediated chondrogenesis in hAMSCs (Figure S3). Only RASL11B was well correlated with the HA-mediated chondrogenic differentiation of hAMSCs.
RASL11B, a member of the small GTPase protein superfamily, has high similarity to RAS proteins. It is expressed in many tissues, but especially in the placenta and primary macrophages.36 The protein encoded by the RASL11B gene does not anchor to the cell membrane, as it lacks the characteristics of other RAS proteins, such as the presence of prenylation sites.36 It has demonstrated that RASL11B is involved in TGF-β1-mediated pathophysiological processes including arteriosclerosis inflammation and cancer.36,37 However, nothing is known about the physiological function of RASL11B in developmental processes or in pathophysiologies.36,37 A recent study showed that RASL11B negatively regulates the proliferation of retinal precursor cells in the developing zebrafish eye.38 Another research group found that the long non-coding RNA, maternally expressed gene 3, up-regulates RASL11B to inhibit cell proliferation, invasion, and migration, induce cell cycle arrest, and promote apoptosis, by suppressing miR-7 in clear cell renal cell carcinoma.39 However, there is no information available on RASL11B only and no experimental data regarding its role in chondrogenic differentiation.
Subsequent functional studies showed that the overexpression of RASL11B increased the expression level of chondrogenic markers, including Col2α1 and Acan, at the mRNA and protein levels and the accumulation of cartilage-specific matrix components, such as type 2 collagen and glycosaminoglycan. Thus, RASL11B overexpression promoted the chondrogenic differentiation of hAMSCs. By contrast, knockdown of RASL11B decreased the expression of chondrogenic markers and the accumulation of cartilage-specific matrix components. Therefore, we next aimed to determine the mechanism whereby RASL11B regulated the HA-mediated chondrogenic differentiation of hAMSCs induced by TGF-β3.
Growth factors, such as TGF-βs, BMPs, and IGF-1, play crucial roles in the in vitro chondrogenic differentiation of MSCs. Among these growth factors, TGF-βs (TGF-β3 or TGF-β1) are widely known to efficiently induce the chondrogenic differentiation of MSCs.40–42 The underlying mechanism of TGF-β-mediated chondrogenesis generally involves TGF-β first binding to the TGF-β type 2 receptor (TGFβR2) on the plasma membrane. A heteromeric complex of TGFβR1, also known as anaplastic lymphoma kinase 5, is subsequently formed and phosphorylated following the activation of TGFβR2.40,41 Activated TGFβR1 initiates the phosphorylation of Smad2/3 and phosphorylated Smad2/3 then combines with co-Smad (Smad4). The resulting Smad heteromeric complex translocates into the nucleus where it regulates the transcription of downstream target genes through directly binding to Smad-binding elements.40,41 Among these target genes, Sox9 is a master transcriptional factor. Smad signaling activates the Sox9 transcription factor, which increases the expression of chondrocyte-specific markers, such as Col2α1 and Acan.40,43 In the study, Sox9 expression was significantly up-regulated, in a time-dependent manner, at the mRNA and protein level, after continuous overexpression of RASL11B in the CG group (HA plus TGF-β3 induction). Consistent with the changes of Sox9 expression, chondrocyte-specific markers, such as Col2α1 and Acan, also exhibited a time-dependent increase in expression (Figures 4 and 5). Finally, the accumulation of cartilage-specific matrix components, such as glycosaminoglycans and type 2 collagen, was markedly elevated on day 21 after the induction of differentiation, in comparison to the null-vector CG group (Figure 3). By contrast, knockdown of RASL11B in hAMSCs resulted in significantly lower expression levels of Sox9, Col2α1, and Acan. Interestingly, continuous overexpression of RASL11B in the PG group (TGF-β3 induction) did not lead to high Sox9 mRNA expression levels at the early stage of chondrogenic differentiation (days 7 and 14) or affect the expression of Col2α1 or Acan mRNA. Therefore, high levels of RASL11B expression were closely associated with the addition of HA during the TGF-β3-induced chondrogenic differentiation of hAMSCs. As previously mentioned, Smad signaling activates the transcription of the downstream target gene, Sox9, which eventually triggers the expression of Col2α1 and Acan during the TGF-β3-induced chondrogenic differentiation of MSCs.40,43 Therefore, we further assessed the effect of the RASL11B gene on Smad signaling in hAMSCs induced to differentiate by the combined treatment of HA and TGF-β3. Our results showed that Smad signaling was significantly different between CG groups with overexpression or knockdown of RASL11B, at all differentiation stages (Figure 6). In comparison to overexpression of RASL11B, knockdown of RASL11B was shown to markedly increase the expression of Smad1/5/8 at the transcriptional level during the chondroinductive differentiation. Furthermore, RASL11B knockdown also gave rise to a significant increase of Smad2 and Smad3 at the mRNA level on days 14 and 21 after induction differentiation. However, the transcriptional level of Smad3 on day 7 was evidently higher in the group overexpressing RASL11B than in the group with RASL11B knockdown, while the transcriptional level of Smad2 has no difference between both the groups. Consistent with the transcriptional level of Smad 3, RASL11B overexpression significantly increased the phosphorylation levels of Smad2/3 on day 7 after chondrogenic differentiation of hAMSCs. As described previously, Smad2/3 phosphorylation is known to block terminal differentiation and promote the continual deposition of type 2 collagen and generation of stable hyaline-like cartilage, whereas Smad1/5/8 phosphorylation is associated with terminal differentiation, hypertrophic differentiation, and mineralization during the chondrogenic differentiation of bone marrow MSCs (BMSCs).26,42 Therefore, Smad2/3 phosphorylation is the dominant signaling pathway for the chondrogenic differentiation of BMSCs. This suggests that blockage of Smad1/5/8 activation not only prevents the hypertrophic differentiation of BMSCs, but also results in a more stable cartilage construct without mineralization. Therefore, in the study, RASL11B overexpression was found to inhibit the expression of Smad1/5/8 during all stages of chondrogenic differentiation (Figure 6(b)). This indicated that the addition of HA was conducive to the eventual formation of stable hyaline-like cartilage during the TGF-β3-induced chondrogenic differentiation of hAMSCs, instead of inducing the terminal differentiation of the chondrocytes. In addition, Smad2 and Smad3 play differential roles in the TGF-β-induced chondrogenic differentiation in BMSCs. Recent studies have indicated that both knockdown and overexpression of Smad2 have a minor inhibitory effect on the TGF-β1-induced chondrogenic differentiation in human BMSCs, while knockdown and overexpression of Smad3 strongly inhibit chondrogenic differentiation.27 Thus, Smad3 has a more dominant role than Smad 2 in the TGF-β1-induced chondrogenic differentiation in human BMSCs and chondrogenic differentiation may rely on a sufficient quantity of Smad3. However, regardless of the overexpression or knockdown of RASL11B, Smad3 mRNA levels were markedly lower than Smad2 mRNA levels during the entire differentiation process in the present study (Figure 6(c)). Therefore, the pro-chondrogenic differentiation effects of RASL11B overexpression in the CG group may not be completely attributable to the activation of Smad2/3. Thus, HA-mediated chondrogenic differentiation via RASL11B is not limited to TGF-β signaling.
The small GTPase protein, RAS, is an upstream protein of the RAS/ERK signaling pathway,44 while RASL11B has also high similarity to RAS. Thus, RASL11B may trigger the RAS/ERK signaling pathway during the induction of the chondrogenic differentiation of hAMSCs by the combined treatment with HA plus TGF-β3. Our results showed that ERK1 mRNA levels were markedly different between CG groups with the overexpression or knockdown of RASL11B, in the mid-to-late stage of chondrogenic differentiation. At the early stage of differentiation (day 7), ERK1/2 phosphorylation levels were increased in all CG groups tested, compared with the PG group, most notably after RASL11B knockdown. Recent studies have demonstrated that ERK signaling has either stimulatory or inhibitory effect on chondrogenic differentiation.32,45 For example, Wu et al.32 reported that ERK signaling promotes Sox9 expression and chondrogenic differentiation in the 2000 kDa HA-induced chondrogenesis in adipose-derived MSCs, while Han et al.45 found that the inhibition of ERK signaling enhances the simvastatin-induced differentiation of chondrocytes. However, further investigation is required to understand whether ERK signaling regulates Smad signaling or directly activates downstream target genes, such as Sox9, Col2α1, and Acan during the chondrogenic differentiation of hAMSCs.
In summary, our study demonstrated that HA enhanced the TGF-β3-induced chondrogenic differentiation in hAMSCs by targeting RASL11B. The overexpression of RASL11B triggered the expression of chondrocyte-specific markers, such as Col2α1 and Acan, through the activation of Sox9 and ERK/Smad signaling. This eventually resulted in chondrogenic differentiation of hAMSCs, with the accumulation of cartilage-specific matrix components, such as glycosaminoglycans and type 2 collagen. Collectively, our findings suggest that RASL11B plays a key role in HA-mediated chondrogenic differentiation and that this occurs via ERK and Smad signaling pathways during early chondrogenesis.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220944375 for RASL11B gene enhances hyaluronic acid-mediated chondrogenic differentiation in human amniotic mesenchymal stem cells via the activation of Sox9/ERK/smad signals by Yi Luo, Ai-Tong Wang, Qing-Fang Zhang, Ru-Ming Liu and Jian-Hui Xiao in Experimental Biology and Medicine
Footnotes
Authors’ contributions: YL performed partial experiments and drafted the manuscript. A-TW and Q-F Zhang collected partial experimental data. R-ML participated in the experimental design. J-H Xiao made substantial intellectual contributions to conception and design, analysis and interpretation of data, and edited the manuscript. All authors read and approved the final manuscript.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The protocol of this study was approved by the Ethics Committee of Affiliated Hospital of Zunyi Medical University (Zunyi, China) (License number granted: KLLY-2017–003) and the declaration of Helsinki was strictly abode by the investigators. The informed consent was obtained from the donor prior to collection of human placenta.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant numbers 81260278, 81660363); the S&T Innovation Leading Academics of National High-level Personnel of Special Support Program [grant number GKFZ-2018–29]; the Guizhou High-Level Innovative Talent Support Program [grant number QKH-RC-20154028]; and the S&T Foundation of Guizhou [grant number QKH-2017–1422].
ORCID iD: Jian-Hui Xiao https://orcid.org/0000-0003-2563-9153
Supplemental material: Supplemental material for this article is available online.
References
- 1.Cancedda R, Dozin B, Giannoni P, Quarto R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol 2003; 22:81–91 [DOI] [PubMed] [Google Scholar]
- 2.Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev 2008; 60:243–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narakornsak S, Poovachiranon N, Peerapapong L, Pothacharoen P, Aungsuchawan S. Mesenchymal stem cells differentiated into chondrocyte-like cells. Acta Histochem 2016; 118:418–29 [DOI] [PubMed] [Google Scholar]
- 4.Wang YQ, Wang NX, Luo Y, Yu CY, Xiao JH. Ganoderal A effectively induces the osteogenic differentiation of human amniotic mesenchymal stem cells via wnt/β-catenin and BMPs/SMADs signaling pathways. Biomed Pharmacother 2020; 123C:109807. [DOI] [PubMed] [Google Scholar]
- 5.Liu RM, Sun RG, Zhang LT, Zhang QF, Chen DX, Zhong JJ, Xiao JH. Hyaluronic acid enhances proliferation of human amniotic mesenchymal stem cells through activation of Wnt/β-catenin signaling pathway. Exp Cell Res 2016; 345:218–29 [DOI] [PubMed] [Google Scholar]
- 6.Zhang LT, Liu RM, Luo Y, Zhao YJ, Chen DX, Yu CY, Xiao JH. Hyaluronic acid promotes osteogenic differentiation of human amniotic mesenchymal stem cells through the TGF-β/Smad signaling pathway. Life Sci 2019; 232:116669. [DOI] [PubMed] [Google Scholar]
- 7.Topoluk N, Hawkins R, Tokish J, Mercuri J. Amniotic mesenchymal stromal cells exhibit preferential osteogenic and chondrogenic differentiation and enhanced matrix production compared with adipose mesenchymal stromal cells. Am J Sports Med 2017; 45:2637–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muinos-López E, Hermida-Gómez T, Fuentes-Boquete I, de Toro-Santos FJ, blanco FJ, Díaz-Prado SM. Human amniotic mesenchymal stromal cells as favorable source for cartilage repair. Tissue Eng Part A 2017; 23:901–12 [DOI] [PubMed] [Google Scholar]
- 9.Karl A, Olbrich N, Pfeifer C, Berner A, Zellner J, Kujat R, Angele P, Nerlich M, Mueller MB. Thyroid hormone-induced hypertrophy in mesenchymal stem cell chondrogenesis is mediated by bone morphogenetic protein-4. Tissue Eng Part A 2014; 20:178–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner EF, Karsenty G. Genetic control of skeletal development. Curr Opin Genet Dev 2001; 11:527–32 [DOI] [PubMed] [Google Scholar]
- 11.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthr Cartil 2000; 8:309–34 [DOI] [PubMed] [Google Scholar]
- 12.Xu T, Wu M, Feng J, Lin X, Gu Z. RhoA/rho kinase signaling regulates transforming growth factor-beta1-induced chondrogenesis and actin organization of synovium-derived mesenchymal stem cells through interaction with the smad pathway. Int J Mol Med 2012; 30:1119–25 [DOI] [PubMed] [Google Scholar]
- 13.Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem 2006; 97:33–44 [DOI] [PubMed] [Google Scholar]
- 14.de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol 2000; 19:389–94 [DOI] [PubMed] [Google Scholar]
- 15.Chang SH, Oh CD, Yang MS, Kang SS, Lee YS, Sonn JK, Chun JS. Protein kinase C regulates chondrogenesis of mesenchymes via mitogen-activated protein kinase signaling. J Biol Chem 1998; 273:19213–9 [DOI] [PubMed] [Google Scholar]
- 16.Zamboni F, Vieira S, Reis RL, Miguel Oliveira J, Collins MN. The potential of hyaluronic acid in immunoprotection and immunomodulation: chemistry, processing and function. Prog Mater Sci 2018; 97:97–122 [Google Scholar]
- 17.Lei Y, Gojgini S, Lam J, Segura T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials 2011; 32:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulsom R. CD44 and hyaluronan help mesenchymal stem cells move to a neighborhood in need of regeneration. Kidney Int 2007; 72:389–90 [DOI] [PubMed] [Google Scholar]
- 19.Hwang HD, Cho HJ, Balakrishnan P, Chung CW, Yoon IS, Oh YK, Byun Y, Kim DD. Cross-linked hyaluronic acid-based flexible cell delivery system: application for chondrogenic differentiation. Colloids Surf B Biointerfaces 2012; 91:106–13 [DOI] [PubMed] [Google Scholar]
- 20.Cai Z, Feng Y, Li C, Yang K, Sun T, Xu L, Chen Y, Yan CH, Lu WW, Chiu KY. Magnoflorine with hyaluronic acid gel promotes subchondral bone regeneration and attenuates cartilage degeneration in early osteoarthritis. Bone 2018; 116:266–78 [DOI] [PubMed] [Google Scholar]
- 21.Wang AT, Zhang QF, Wang NX, Yu CY, Liu RM, Luo Y, Zhao YJ, Xiao JH. Cocktail of hyaluronic acid and human amniotic mesenchymal cells effectively repairs cartilage injuries in sodium iodoacetate-induced osteoarthritis rats. Front Bioeng Biotechnol 2020; 8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami S, Kan M, McKeehan WL, de Crombrugghe B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc Natl Acad Sci U S A 2000; 97:1113–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li TF, O’Keefe RJ, Chen D. TGF-beta signaling in chondrocytes. Front Biosci 2005; 10:681–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serra R, Chang C. TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res C Embryo Today 2003; 69:333–51 [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Rigueur D, Lyons KM. TGF-β signaling in cartilage development and maintenance. Birth Defects Res C Embryo Today 2014; 102:37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellingman CA, Davidson ENB, Koevoet W, Vitters EL, van den Berg WB, van Osch GJVM, van der Kraan PM. Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A 2011; 17:1157–67 [DOI] [PubMed] [Google Scholar]
- 27.de Kroon LMG, Narcisi R, van den Akker GGH, Vitters EL, Blaney Davidson EN, van Osch GJVM, van der Kraan PM. SMAD3 and SMAD4 have a more dominant role than SMAD2 in TGFβ-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Sci Rep 2017; 7:43164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chun JS. Expression, activity, and regulation of MAP kinases in cultured chondrocytes. Methods Mol Med 2004; 100:291–306 [DOI] [PubMed] [Google Scholar]
- 29.Stanton LA, Underhill TM, Beier F. MAP kinases in chondrocyte differentiation. Dev Biol 2003; 263:165–75 [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Wang YQ, Wang AT, Yu CY, Luo Y, Liu RM, Zhao YJ, Xiao JH. Effect of CD44 on differentiation of human amniotic mesenchymal stem cells into chondrocytes via smad and ERK signaling pathways. Mol Med Rep 2020; 21:2357–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006; 8:315–7 [DOI] [PubMed] [Google Scholar]
- 32.Wu SC, Chen CH, Wang JY, Lin YS, Chang JK, Ho ML. Hyaluronan size alters chondrogenesis of adipose-derived stem cells via the CD44/ERK/SOX-9 pathway. Acta Biomater 2018; 66:224–37 [DOI] [PubMed] [Google Scholar]
- 33.O’Conor CJ, Case N, Guilak F. Mechanical regulation of chondrogenesis. Stem Cell Res Ther 2013; 4:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy MK, Huey DJ, Hu JC, Athanasiou KA. TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 2015; 33:762–73 [DOI] [PubMed] [Google Scholar]
- 35.Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. TGF-β1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol 1993; 158:414–29 [DOI] [PubMed] [Google Scholar]
- 36.Stolle K, Schnoor M, Fuellen G, Spitzer M, Cullen P, Lorkowski S. Cloning, genomic organization, and tissue-specific expression of the RASL11B gene. Biochim Biophys Acta 2007; 1769:514–24 [DOI] [PubMed] [Google Scholar]
- 37.Pézeron G, Lambert G, Dickmeis T, Strähle U, Rosa FM, Mourrain P. Rasl11b knock down in zebrafish suppresses one-eyed-pinhead mutant phenotype. PLoS One 2008; 3:e1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emerson SE, St. Clair RM, Waldron AL, Bruno SR, Duong A, Driscoll HE, Ballif BA, McFarlane S, Ebert AM. Identification of target genes downstream of semaphorin6A/plexinA2 signaling in zebrafish. Dev Dyn 2017; 246:539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He H, Dai J, Zhuo R, Zhao J, Wang H, Sun F, Zhu Y, Xu D. Study on the mechanism behind lncRNA MEG3 affecting clear cell renal cell carcinoma by regulating miR-7/RASL11B signaling. J Cell Physiol 2018; 233:9503–15 [DOI] [PubMed] [Google Scholar]
- 40.Kim YI, Ryu JS, Yeo JE, Choi YJ, Kim YS, Ko K, Koh YG. Overexpression of TGF-β1 enhances chondrogenic differentiation and proliferation of human synovium-derived stem cells. Biochem Biophys Res Commun 2014; 450:1593–9 [DOI] [PubMed] [Google Scholar]
- 41.Lee HL, Yu B, Deng P, Wang CY, Hong C. Transforming growth factor-β-induced KDM4B promotes chondrogenic differentiation of human mesenchymal stem cells. Stem Cells 2016; 34:711–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroon LMG, Davidson ENB, Narcisi R, Farrell E, Kraan PMV, van Osch GJVM. Activin and nodal are not suitable alternatives to TGF-β for chondrogenic differentiation of mesenchymal stem cells. Cartilage 2017; 8:432–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kondo M, Yamaoka K, Sonomoto K, Fukuyo S, Oshita K, Okada Y, Tanaka Y. IL-17 inhibits chondrogenic differentiation of human mesenchymal stem cells. PLoS One 2013; 8:e79463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox AD, Der CJ. Ras history. Small GTPases 2010; 1:2–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han Y, Kim SJ. Simvastatin induces differentiation of rabbit articular chondrocytes via the ERK-1/2 and p38 kinase pathways. Exp Cell Res 2016; 346:198–205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220944375 for RASL11B gene enhances hyaluronic acid-mediated chondrogenic differentiation in human amniotic mesenchymal stem cells via the activation of Sox9/ERK/smad signals by Yi Luo, Ai-Tong Wang, Qing-Fang Zhang, Ru-Ming Liu and Jian-Hui Xiao in Experimental Biology and Medicine