Abstract
Crohn’s disease is a severe, incurable inflammatory bowel disease. Orally administered emu oil has demonstrated anti-inflammatory properties in previous models of gastrointestinal disease. We aimed to determine whether orally administered emu oil could attenuate disease in a mouse model of Crohn’s-like colitis. Female ARC(s) mice (CD-1 equivalent, n = 10/group) were intra-rectally administered water (120 μL) or trinitrobenzene sulfonic acid (TNBS; 3 mg in 50% ethanol; 120 μL bolus) on day 0. Mice were orally administered water (80 μL) or emu oil (80 μL or 160 μL) daily for five days and euthanized on day six. Bodyweight and disease activity were recorded daily. Colonoscopy, burrowing activity, facial grimace, histological parameters (damage severity, small intestinal villus height/crypt depth and colonic crypt depth), myeloperoxidase activity and intestinal permeability were assessed. P < 0.05 was considered statistically significant. TNBS decreased bodyweight (days 1, 2, 4; P < 0.05) and increased disease activity (days 1–6; P < 0.01), compared to normal controls. Emu oil (80 μL) attenuated disease activity on days 5–6 (P < 0.05), although bodyweight loss was not significantly impacted (P > 0.05). Facial grimace and colonoscopy scores were significantly increased in TNBS-control mice; effects attenuated by both volumes of emu oil (P < 0.001). TNBS increased histological damage severity compared to normal controls (P < 0.05); an effect attenuated by 80 μL emu oil (proximal and distal colon; P < 0.05) and 160 μL emu oil (distal colon; P < 0.01). In the ileum, villus height and crypt depth were unaffected by TNBS or emu oil treatment compared to normal (P > 0.05). TNBS-induced distal colonic crypt lengthening was unaffected following emu oil administration (P > 0.05). Remaining parameters, including burrowing, myeloperoxidase activity and intestinal permeability, were unchanged across all treatment groups (P > 0.05). In normal mice, emu oil treatment did not significantly impact any parameter compared to normal controls. In conclusion, emu oil reduced overall disease severity and facial grimace scores in TNBS mice. These results suggest therapeutic potential for orally administered emu oil in the management of Crohn’s disease.
Keywords: Emu oil, inflammatory bowel disease, Crohn’s disease, mouse model, nutraceutical, gastroenterology
Impact statement
The submitted work details novel research to contribute to the field of inflammatory bowel diseases, specifically Crohn’s disease and alternative therapies. This work is important as current therapies for Crohn’s disease are variably effective and often significantly compromise patient quality of life. Emu oil, used in the current study, has the potential to alleviate disease severity and promote intestinal repair in a mouse model of Crohn’s-like colitis. The new findings from this manuscript, whereby emu oil attenuated disease severity from clinical scores and colonoscopy results, add to the literature of inflammatory bowel disease mouse models and support the therapeutic potential of emu oil. This research may advance the progression to clinical trials and ultimately the commercialization of emu oil as an adjunctive or alternative therapy for the detrimental inflammatory bowel diseases.
Introduction
Inflammatory bowel disease (IBD) is the collective term for a group of incurable inflammatory conditions of the small intestine and colon, encompassing Crohn’s disease, ulcerative colitis, and indeterminate colitis. Crohn’s disease is a chronic inflammatory condition characterized by transmural inflammation of the gastrointestinal tract occurring in alternating active and quiescent periods, distinguishing it from colitis which affects only the colonic mucosa.1 Crohn’s disease may affect all layers of the gastrointestinal tract from mucosa to serosa in a discontinuous pattern of inflamed and healthy segments.2,3
Common treatments for Crohn’s disease include corticosteroids (prednisolone, budesonide) and targeted anti-TNF-α antibody therapies (infliximab, adalimumab) with the aim to alleviate inflammation and induce remission.1 There are detrimental aspects to the use of commonly prescribed medications which limit their efficacy, particularly with prolonged use. Reported side-effects of adverse extra-intestinal manifestations include ocular and dermatological reactions, metabolic and nervous system interference, as well as increased risk of opportunistic infections resulting from immunosuppression.4,5 Surgical intervention is often required to resect severely inflamed sections of bowel, yet this is not curative and post-operative recurrence is common. The variable efficacy and side-effects of conventional IBD treatments emphasize the need for new and novel adjunctive treatment options, and naturally derived orally administered therapies (nutraceuticals) have been of recent interest.6
Emu oil is one such animal-derived product investigated for its potential therapeutic applications. Traditionally used by Indigenous Australian people as a topical anti-inflammatory treatment for wound healing and arthritis relief,7,8 emu oil is rendered from the subcutaneous and retroperitoneal adipose tissue of the emu (Dromaius novaehollandiae). Modern emu oil refinement involves heating and filtration of adipose tissue to remove solids, and centrifugation to further separate contents.9 The refined oil comprises approximately 98% fatty acids: 49% n-9 oleic acid, 24% palmitic acid, 4% n-7 palmitoleic acid, 1% n-3 α-linoleic acid, with lower levels of other fatty acids.6 The content and ratio of n-3, n-6 and n-9 fatty acids have been of particular interest. n-3 fatty acids have demonstrated anti-inflammatory potential by downregulating pro-inflammatory eicosanoid pathways and reducing levels of pro-inflammatory cytokines including TNF-α and interleukin-12 in a rodent model of colitis.10 Topically applied emu oil has exhibited greater anti-inflammatory and reparative properties than other animal-derived oils with lower n-6 and higher n-3 fatty acid content11 as well as exhibiting a greater antioxidant effect than other ratite oils with similar fatty acid profiles. Dietary fish oil has previously been investigated in IBD attributed to its high n-3 content; however, it has been ineffective at long-term maintenance of disease remission.12
Oral administration of emu oil has effectively attenuated disease parameters in preclinical models of gastrointestinal disease, including non-steroidal anti-inflammatory drug (NSAID)-induced enteropathy,13 ulcerative colitis,6,14colitis-associated colorectal cancer,15–17 and chemotherapy-induced mucositis.18–21 Although emu oil has demonstrated efficacy in rodent models of colitis, it has yet to be investigated in a Crohn’s disease-specific model. The trinitrobenzene sulfonic acid (TNBS) rodent model is a well-established IBD model that mimics the histopathology and clinical presentation of Crohn’s disease.22
In the current study, it was hypothesized that orally administered emu oil would attenuate disease severity in a mouse model of TNBS-induced Crohn’s-like colitis. The aim of the study was to determine whether emu oil could reduce the severity of TNBS-induced Crohn’s-like colitis as assessed by clinical indicators, biochemical and histological analyses.
Materials and methods
Animals
All studies were conducted in accordance with the ‘Australian code of practice for the care and use of animals for scientific purposes 8th edition (2013)’ (National Health and Medical Research Council; Canberra, 2013) under approval from the Animal Ethics Committees of the University of Adelaide and the Women’s and Children’s Health Network (approval number 1024/2/2018). Female ARC(s) mice (12 weeks of age, mean day 0 bodyweight 33.7 g) were sourced from the Animal Resource Centre (Perth, Western Australia, Australia) and housed at the Women’s and Children’s Hospital Animal Care Facility (North Adelaide, South Australia, Australia). Animals were group-housed in standard open-top cages with litter and shredded newspaper bedding at room temperature with a light:dark cycle of 14:10 h. All animals underwent a one-week acclimatization period and were provided ad libitum access to standard mouse chow and drinking water throughout the trial period.
Experimental trial
The experimental trial duration was determined from a seven-day pilot study. Female ARC(s) mice (n = 50) were assigned to five groups (n = 10/group) by random stratification based on initial bodyweight and burrowing ability: Group 1: normal control; mice administered water enema and water gavage (Water+Water), Group 2: mice administered water enema and emu oil gavage (Water+emu oil (160 µL)), Group 3: Crohn’s-like colitis control; mice administered TNBS enema and water gavage (TNBS+Water), Group 4: mice administered TNBS enema and low volume emu oil gavage (TNBS+emu oil (80 µL)), Group 5: mice administered TNBS enema and high volume emu oil gavage (TNBS+emu oil ((160 µL)). On day 0, mice were anaesthetized (1.5–2% isoflurane; AbbVie; Mascot, New South Wales, Australia) and administered an intra-rectal enema of Water (120 μL) or TNBS (3 mg in 50% ethanol; 120 µL bolus; Sigma, Castle Hill, New South Wales, Australia) while in the Trendelenburg position. Water or emu oil (100% emu oil) was administered by oral gavage once daily from day 1 to 5 of the trial (total five gavages).
Emu Oil
Commercially available pure emu oil was purchased from Emu Tracks (Marleston, South Australia, Australia; batch #09171018). Aliquots (5 mL) of emu oil were stored in darkness at 4°C. Fatty acid composition (Table 1) was analyzed by gas chromatography by the Waite Lipid Analysis Service (Urrbrae, South Australia, Australia).
Table 1.
Fatty acid composition of emu oil (Emu Tracks, Marleston, South Australia, Australia; batch #09171018) analyzed by gas chromatography.
| Analyte | Common name | Total lipids (%) |
|---|---|---|
| Total saturates | 44.0 | |
| 14:00 | Myristic acid | 5.0 |
| 16:00 | Palmitic acid | 22.7 |
| 18:00 | Stearic acid | 12.7 |
| 20:00 | Arachidonic acid | 0.2 |
| Total monounsaturated | 44.7 | |
| 16: 1n-7 Omega 7 | Palmitoleic acid | 6.3 |
| 18: 1n-9 Omega 9 | Oleic acid | 36.4 |
| 18: 1n-7 Omega 7 | Vaccenic acid | 3.0 |
| 20: 1n-9 Omega 9 | Gondoic acid | 0.4 |
| Total Omega 9 | 36.6 | |
| Total Omega 7 | 8.1 | |
| Total Omega 3 | 2.9 | |
| 18: 3n-3 | Alpha-linolenic acid | 0.9 |
| Total Omega 6 | 7.6 | |
| 18: 2n-6 | Linoleic acid | 8.0 |
| 20: 2n-6 | Eicosadienoic acid | 0.8 |
| 20: 4n-6 | Arachidonic acid | 0.8 |
Bold characters signify the total lipids for that category of Analytes. Non-bold characters listed below the bold characters represent the breakdown of the lipids within that Analyte category.
Colonoscopy
Disease progression was monitored using a high-resolution Karl Storz colonoscope (1.9 mm outer diameter; Tuttlingen, Germany). On day 0 and 5, mice were anaesthetized using isoflurane inhalant (AbbVie; Mascot, New South Wales, Australia) and colonoscopy was conducted to obtain baseline data and visualize disease progression. Colitis severity was scored by a blinded assessor using five parameters scored from 0 to 3 (maximal severity): colon wall thickening, changes in vasculature pattern, fibrin, mucosal granularity, and stool consistency.23
Disease activity index and daily monitoring
Mice were monitored daily for changes in body weight and condition throughout the experimental period. Disease activity index (DAI) was used to assess colitis severity based on four parameters scored from 0 to 3 (maximal severity): daily body weight change, rectal bleeding, stool consistency and general condition.24 Rectal bleeding was scored as 0 for not present, 1; if identified in the feces, 2; if present around the anus, and 3; when bleeding was obviously wet/smeared along the mouse’s tail. Additionally, general condition was scored by observing grooming, activity, alertness and movement of each mouse. Mice with severely impaired general condition appeared with a ruffled coat, hunched and immobile.
Behavioral analyses
Burrowing behavior was recorded pre- and post-enema as an indicator of welfare and pain due to disease progression. Burrowing studies were conducted on days −2, −1 and 4 of the experimental period. On day −2, animals were burrowed in pairs to encourage burrowing behavior and familiarize the animals with the burrowing apparatus, with subsequent burrowing conducted individually (day −1 being the baseline recording of individual burrowing ability). Burrows were pre-filled with 400 g of clay substrate gravel (cat litter, Black and Gold; Adelaide, South Australia, Australia). Mice were allowed to acclimatize in darkness for 1 h before being moved to test cages, where they remained in darkness to burrow for an additional hour. At the end of the test period, the burrow was removed and weighed to determine the volume of displaced substrate.
TNBS-induced pain was measured using the validated mouse facial grimace criteria.25 Animals were individually placed in a transparent plastic enclosure and video recorded (JVC Everio Hard Disk Camcorder GZ-MG330; Yokohama, Japan) for a 10-min period. Ten still images were retrieved from the video footage when animals were facing the camera front-on, or when a clear side-on image could be acquired. Images were cropped to show only the face, and five images were randomly selected for scoring by two treatment-blinded observers.
Tissue collection and histological analysis
On day 6, animals were euthanized by CO2 asphyxiation and whole blood was obtained by cardiac puncture. The small intestine and colon were removed, measured, emptied of contents and weighed before being separated into segments for further processing and histological analysis. Organs (thymus, heart, lungs, liver, stomach, spleen, kidneys) were removed, weighed and discarded.
Sections of the ileum and colon (4 μm) were stained with hematoxylin and eosin. Proximal and distal colonic crypt depth measurements were obtained from 40 crypts per tissue section per mouse. Histological damage severity scoring was assessed using a quantitative scoring system.26 Additionally, measurements of 10 well-orientated villi and crypts for each ileum section per mouse were recorded. Sections were analyzed in a blinded fashion using a light microscope (Olympus Corporation; Tokyo, Japan) and Olympus Soft Imaging Solutions GmbH software analysis version 5.2 (Tokyo, Japan).
Myeloperoxidase assay
Colonic myeloperoxidase (MPO) activity was determined as an indicator of acute inflammation by neutrophil infiltration using techniques previously described.13 Absorbance (450 nm) was measured at 1-min intervals for 15 min with a BioTek Synergy Mx Microplate Reader (BioTek; Winooski, Vermont, USA) and Gen5 version 2.00.18 software.
Intestinal permeability assay
Three hours prior to euthanasia, mice from Water+Water, TNBS+Water and TNBS+emu oil (160 µL) treatment groups received a 500 mg/kg dose of fluorescein isothiocyanate (FITC)-dextran (mol wt 4000, 75 mg/mL; Sigma; Castle Hill, New South Wales, Australia) by oral gavage. Blood samples were centrifuged (11,000g at 23°C) for 12 min and serum collected. Serum samples were diluted 1:3 with 0.2 M PBS and FITC-dextran was quantified using a BioTek Synergy Mx Microplate Reader (BioTek; Winooski, Vermont, USA) and Gen5 version 2.00.18 software relative to a standard curve (0.001–100 μg/mL).
Statistics and sample size justification
Sample size calculations (SigmaPlot 12.3) based upon the primary outcome of MPO activity (indicative of acute inflammation) indicated that a total of 10 mice per group would enable the detection of a 3.6-fold difference between groups with 95% power (alpha = 0.05). Statistical analyses were conducted using SPSS version 17 for Windows (SPSS Inc.; Chicago, Illinois, USA). Shapiro–Wilk test and residual plots were used to test data for normality. Bodyweight, DAI, burrowing activity and colonoscopically-assessed disease severity were analyzed using a repeated measures ANOVA with least significant difference (LSD) post hoc. Facial grimace score, intestinal villus height/crypt depth, MPO activity, FITC-dextran and organ data were analyzed by one-way ANOVA with Tukey’s post hoc test. Data were presented as mean±standard error of the mean (SEM). Histological severity score data were analyzed by non-parametric Kruskal–Wallis test with Mann–Whitney U post hoc and were presented as median and interquartile range (IQR). P < 0.05 was considered statistically significant.
Results
Bodyweight
Bodyweight was determined as a percentage change from starting weight (day 0, 100% bodyweight). TNBS administration significantly decreased bodyweight compared to normal controls (days 1, 2, and 4; maximum 4.5% bodyweight reduction [(95.5% starting bodyweight); P < 0.05; Figure 1). There was no statistically significant effect of emu oil administration (80 µL or 160 µL) on bodyweight when compared to both normal controls and TNBS-treated controls (P > 0.05; Figure 1).
Figure 1.
Daily bodyweight change of mice (n = 10/group). Data analyzed by repeated measures ANOVA with LSD post hoc, expressed as mean (% change from starting weight)+SEM. *P < 0.05 compared to Water+Water. (A color version of this figure is available in the online journal.)
Disease activity index
Compared to normal controls, emu oil treatment did not increase any DAI parameters in normal animals (P > 0.05; Figure 2). TNBS increased DAI compared to normal controls throughout the trial period (days 1–6; maximum 11-fold increase; P < 0.01; Figure 2). In TNBS-treated mice, 80 µL emu oil treatment significantly reduced DAI scores compared to controls on days 5 and 6 (maximum 55% reduction, P < 0.05; Figure 2). There was no statistically significant effect of 160 µL emu oil treatment on DAI score (P > 0.05; Figure 2).
Figure 2.
Daily disease activity index (DAI) score (n = 10/group). DAI score calculated from bodyweight loss, general condition, stool consistency, and rectal bleeding. Data analyzed by repeated measures ANOVA with LSD post hoc, expressed as mean (disease activity index total)+SEM. **P < 0.01, ***P < 0.001 compared to Water+Water. ^P < 0.05 compared to TNBS+Water. #P < 0.05 compared to TNBS+emu oil (80 µL). (A color version of this figure is available in the online journal.)
Colonoscopy
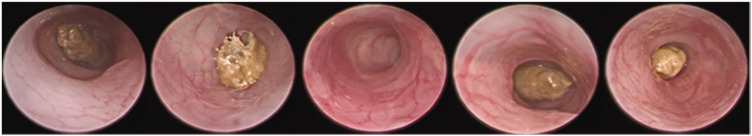
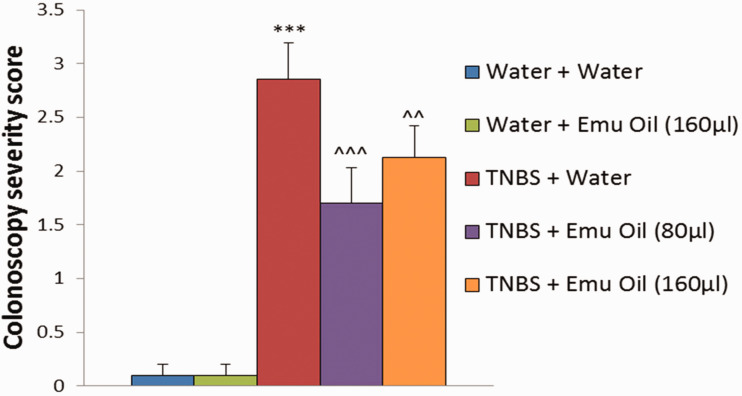
Water and emu oil (160 µL) treated normal controls did not develop Crohn’s-like colitis throughout the trial (Figure 3). TNBS administration induced colitis, increasing colonoscopically assessed disease severity scores (2.9 ± 0.3) compared to normal controls (0.1 ± 0.1; P < 0.001; Figure 4). In TNBS-treated mice, both 80 µL and 160 µL volumes of emu oil significantly reduced colonoscopically assessed disease severity (80 µL: 1.7 ± 0.3; 160 µL: 2.1 ± 0.3; P < 0.01; Figure 4) compared to TNBS-treated controls.
Figure 3.
Images of mouse colon taken from day 5 colonoscopy. From left to right: Water+Water, Water+emu oil (160 µL), TNBS+Water, TNBS+emu oil (80 µL), TNBS+emu oil (160 µL). (A color version of this figure is available in the online journal.)
Figure 4.
Colonoscopically assessed colitis severity scores (n = 10/group). Colitis severity score was calculated from parameters including; mucosal thickening, vasculature pattern, stool consistency, granularity of mucosal surface, and presence of fibrin. Data analyzed by repeated measures ANOVA with LSD post hoc, expressed as mean (colitis score)+SEM. ***P < 0.001 compared to Water+Water. ^^P < 0.01, ^^^P < 0.001 compared to TNBS+Water. (A color version of this figure is available in the online journal.)
Facial grimace score
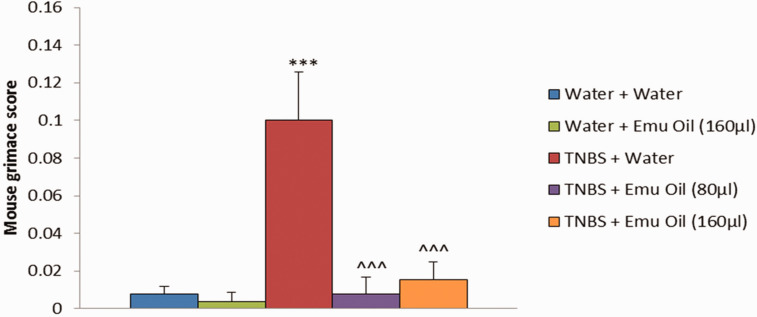
TNBS administration significantly increased facial grimace scores compared to normal controls (11.5-fold increase; P < 0.001; Figure 5). Both 80 µL and 160 µL volumes of emu oil significantly reduced facial grimace score compared to TNBS-treated controls (11.5-fold and 5.4-fold decrease respectively, P < 0.001; Figure 5). There were no statistically significant differences between the two volumes of emu oil (P > 0.05).
Figure 5.
Facial grimace score (n = 10/group). Data analyzed by one-way ANOVA with Tukey’s post hoc, expressed as mean (grimace score)+SEM. ***P < 0.001 compared to Water+Water. ^^^P < 0.001 compared to TNBS+Water. (A color version of this figure is available in the online journal.)
Visceral and gastrointestinal organ weights and lengths
Organ weights were expressed as a percentage of day 6 bodyweight. TNBS administration did not affect visceral organ weights across treatment groups, with the exception of liver and left kidney weight. Liver weight was increased in TNBS-treated mice receiving 80 µL emu oil treatment compared to both TNBS-treated controls and 160 µL emu oil treatment (P < 0.05; data not shown). Left kidney weight was increased in normal mice receiving 160 µL emu oil compared to normal controls (P < 0.05; data not shown).
TNBS administration did not significantly impact gastrointestinal lengths or weights. Emu oil administration also had no impact on gastrointestinal lengths or weights in either water- or TNBS-treated animals (P > 0.05; data not shown).
Histological analyses
Histological damage severity score was increased in TNBS-treated animals compared to normal controls (P < 0.05; Figure 6); an effect significantly attenuated by 80 µL emu oil in both the proximal and distal colon (P < 0.05), and by 160 µL emu oil in the distal colon (P < 0.01). Importantly, there was no effect of emu oil treatment on histological severity score in normal animals compared to normal controls (P > 0.05; Figure 6).
Figure 6.
Histologically assessed colitis severity score for (a) proximal and (b) distal colon (n = 10/group). Data analyzed by Kruskal–Wallis test with Mann–Whitney U post hoc, expressed as median and IQR. *P < 005, ***P < 0.001 compared to Water+Water. ^P < 0.05, ^^P < 0.01 compared to TNBS+Water. (A color version of this figure is available in the online journal.)
TNBS induced compensatory intestinal crypt lengthening in the distal colon (190.0 ± 8.6 μm) compared to normal controls (161.1 ± 3.9 μm; P < 0.05; Figure 7). Emu oil administration in TNBS-treated animals had no impact on proximal or distal colonic crypt depth compared to TNBS-treated controls (80 µL: 206.6 ± 10.7 μm; 160 µL: 202.4 ± 4.9 μm; P > 0.05).
Figure 7.
Intestinal crypt depth of the proximal and distal colon (n = 10/group). Data analyzed by one-way ANOVA with Tukey’s post hoc, expressed as mean crypt depth (µm)+SEM. *P < 0.05 compared to Water+Water. (A color version of this figure is available in the online journal.)
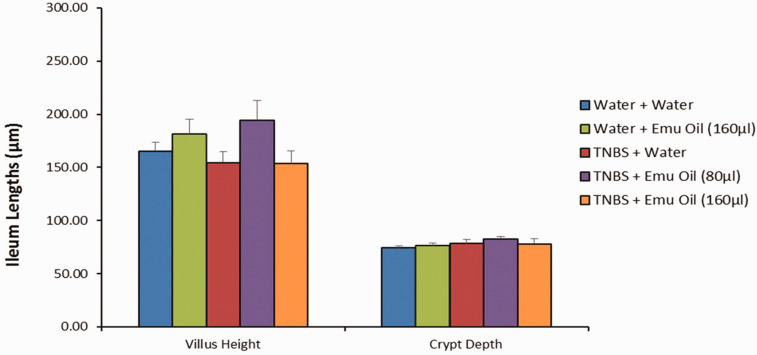
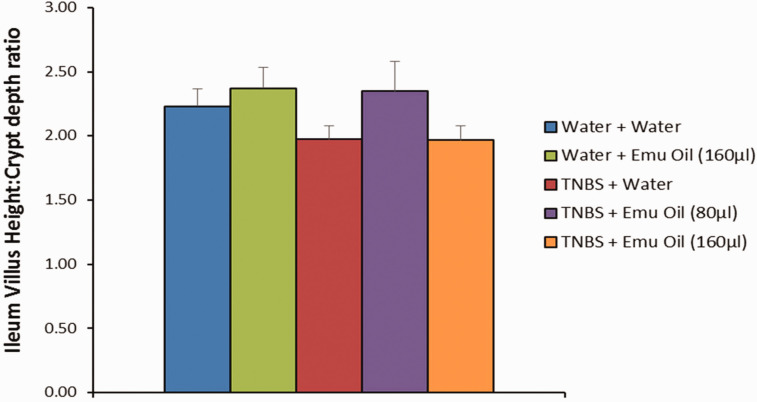
In the ileum, emu oil did not affect villus height (181 ± 14 µm), crypt depth (76 ± 2 µm), or villus height:crypt depth ratio (2 ± 0.2 µm) in normal mice compared to normal controls (165 ± 8 µm; 74 ± 2 µm; 2 ± 0.1 µm respectively; P > 0.05; Figures 8 and 9). Furthermore, TNBS did not affect villus height (154 ± 10 µm), crypt depth (78 ± 4 µm), and villus height: crypt depth ratio (2 ± 0.1 µm) compared to normal controls (P > 0.05). Moreover, emu oil (high dose: 153 ± 12 µm; low dose: 194 ± 19 µm) did not alter villus height or crypt depth of TNBS-treated animals compared to TNBS controls (P > 0.05; Figures 8 and 9). Finally, villus height: crypt depth ratio remained unchanged in TNBS-mice treated with emu oil (high dose: 2 ± 0.1 µm; low dose: 2 ± 0.2 µm) compared to TNBS controls (P > 0.05; Figure 9).
Figure 8.
Small intestinal villus height and crypt depth measurements (n = 10/group). Data analyzed by one-way ANOVA with Tukey’s post hoc, expressed as mean crypt depth (µm)+SEM. (A color version of this figure is available in the online journal.)
Figure 9.
Small intestinal villus height:crypt depth ratio (n = 10/group). Data analyzed by one-way ANOVA with Tukey’s post hoc, expressed as mean crypt depth (µm)+SEM. (A color version of this figure is available in the online journal.)
Burrowing, myeloperoxidase activity, and intestinal permeability
There was no demonstrable impact of TNBS administration or emu oil treatment on burrowing ability, tissue MPO, or serum FITC-dextran levels.
Discussion
In the present study, TNBS administration induced clinical, macroscopic and histological features of Crohn’s-like colitis. Importantly, orally administered emu oil attenuated selected disease parameters as evidenced by reduced disease activity, decreased colonoscopically- and histologically-assessed disease severity and normalized behavioral analyses. Moreover, histological measurements of villus height/crypt depth in the ileum were unaffected by TNBS or emu oil, highlighting that the current model induced damage that was confined to the colon. Therefore, the current study mimicked a Crohn’s-like colitis disease phenotype compared to a traditional Crohn’s model, in which damage can be observed in the small intestine.
The safety of emu oil for intestinal application has been confirmed in both acute and chronic models of gastrointestinal disease.6,13–15,17–21,27–29 Abimosleh et al.13 reported that emu oil administration did not adversely affect small intestinal function in normal animals, as assessed by the non-invasive 13C-sucrose breath test.6 Chartier et al.15 were the first to demonstrate the safety of emu oil for long-term use in normal mice, demonstrating consistent biological parameters in normal animals. Additionally, Mashtoub et al.28 confirmed that indicators of intestinal proliferation in rodent models of mucositis and colitis returned to normal levels following cessation of emu oil treatment. In the current study, emu oil administration in normal animals did not elicit significant biological changes across any parameters investigated, supporting these previous studies detailing the safety of emu oil for application in healthy individuals.
Previously, oral administration of emu oil was effective at attenuating clinically assessed disease severity in rodent models of gastrointestinal disease, including both bodyweight and disease activity index parameters.15,17,29 Nonetheless, Abimosleh et al.13 reported that bodyweight was not significantly affected by emu oil administration in a rat model of NSAID enteropathy.13 Similar results are reflected in the current study, whereby emu oil administration did not impact bodyweight, though significantly reduced clinically-assessed disease severity compared to TNBS controls. Moreover, in normal animals, emu oil did not significantly impact bodyweight, indicating that the observed restoration of bodyweight may not have been attributed to dietary lipid intake by emu oil ingestion,rather, a protective or reparative effect of emu oil on the intestinal barrier.20
It is well documented that communication occurs between the central and enteric nervous system; hence, inflammation and altered microbiota in the gut could impact not only intestinal physiology but also affective state and behavior.30 Burrowing is a behavior used as a measure of wellbeing in mice. In a previous study of colitis-associated colorectal cancer in mice, disease control animals displayed impaired burrowing activity during periods of peak disease; an effect which was attenuated by emu oil.15 Additionally, Jirkof et al.31 reported that a decrease in burrowing behavior coincided with an increase in colitis severity in mice. Interestingly, neither TNBS administration nor emu oil impacted burrowing activity in the current study. Animals were allocated to treatment groups by stratified randomization based upon their initial burrowing ability and bodyweight; however, increasing the number of baseline tests conducted prior to TNBS or water treatment may have benefited the current preselection criteria by allowing the animals to familiarize themselves with the burrowing apparatus. An insult to the gut microbiota can negatively impact behavior, as demonstrated by anxiety-like behaviors in a mouse model of DSS colitis during a light/dark box test30; hence, further analyses on cecal content would be useful to determine the effect of emu oil treatment on the gut microbiota in future studies.
Pain has been assessed by “rodent grimace scales” in various studies as a method of evaluating acute pain.25,32,33 In the present study, TNBS administration induced an increase in the mouse grimace scale compared to normal controls; an effect which was attenuated by both doses of emu oil. The grimace test was conducted at baseline and repeated at four days post TNBS administration, allowing time for inflammation to progress as confirmed by colonoscopy the following day. Importantly, the current study is the first to incorporate the mouse grimace scale as a measure of pain in a model of TNBS-induced inflammation, and the results indicate potential for the scale to be utilized in other models of gastrointestinal disease.
In a model of acute colitis, emu oil treatment reduced histologically assessed colonic damage severity up to threefold and induced colonic crypt lengthening compared to DSS controls.6 Chartier et al.15 reported that colonic crypt depth was increased in a chronic mouse model of colitis-associated colorectal cancer, with no further effect following long-term emu oil administration. In the current acute study, there was compensatory crypt lengthening following TNBS administration, with no further effect of emu oil treatment. In future studies, it would be useful to conduct a time-course study to determine if emu oil treatment in mice administered TNBS could result in compensatory crypt lengthening at an earlier time point than the endpoint of the current study.
In the present study, intestinal permeability, as quantified by serum FITC-dextran uptake, was unaffected by both TNBS administration and emu oil treatment. This may have been attributed to the timing of blood collection, upon which the site of FITC-dextran uptake within the gastrointestinal tract is highly dependent.34 It would be useful to employ a multi-sugar permeability assay in future studies using the TNBS model to allow separate determinations of small intestinal and colonic permeability.35
Prior studies have reported an increase in MPO activity in animal models of small intestinal disease including chemotherapy-induced mucositis18,20 and NSAID-induced enteropathy.13 Despite a reduction in ileal MPO following emu oil treatment in these models of small intestinal disease, MPO levels remained unchanged across all treatment groups in the current model of colonic disease.
TNBS colitis increases levels of circulating IL-12 and IL-17 as inflammation becomes chronic.36 Additionally, serum levels of the pro-inflammatory cytokines IL-6, IL-11 and IL-22 are known to increase in human IBD patients.37IL-6, IL-11 and IL-22 are of particular interest as they are responsible for activation of the Signal Transducer and Activator of Transcription 3 (STAT3) gene, known to be associated with IBD susceptibility.38 These interleukins have both protective and antagonistic action, as they can confer a protective effect on intestinal epithelial cells, yet may simultaneously elicit an inflammatory immune response.37 It would be beneficial in further studies to analyze tissue activity of pro-inflammatory cytokines known to be involved in both the pathogenesis of human Crohn’s disease and the inflammatory pathways associated with TNBS-induced Crohn’s-like colitis.
While the exact mechanism of emu oil action is not well understood, it has been suggested that its protective and reparative effects may be attributed to its fatty acid profile. A higher ratio of n-6 to n-3 FAs has been associated with colitis development11 Dietary n-3 FAs eicosapentanoic acid (EPA) and docosahexanoic acid (DHA) regulate multiple inflammatory pathways, inhibiting the transcription of genes responsible for production of pro-inflammatory cytokines.39 Interestingly, emu oil has exhibited greater anti-inflammatory action than other animal-derived oils with both similar FA profiles and profiles with higher n-3 content.40 This suggests that the properties of emu oil cannot be attributed to the FA profile alone. It is therefore important to consider the composition of the 2% non-triglyceride fraction as well as the ratio of unsaturated to saturated FAs. Previous studies suggest that a high unsaturated to saturated FA ratio is indicative of greater radical scavenging activity (RSA), thereby protecting against oxidative damage.40 However, Mashtoub et al.41 demonstrated that this ratio was greater for olive oil than for emu oil, despite emu oil exhibiting far greater RSA.40,41 This further supports the theory of the 2% non-triglyceride fraction of emu oil comprising compounds responsible for its antioxidant properties.
This study is the first to investigate emu oil administration in a Crohn’s-specific animal model, providing encouraging results for the potential application of emu oil in the management of Crohn’s disease. Exploring microbiome analyses, relevant cytokine profiles and intestinal permeability measures will further elucidate the mechanism of emu oil action. Moreover, although there were no overt indications of extra-intestinal effects of emu oil, future studies could include serological and urine tests to determine renal and hepatic function. Finally, it would be worthwhile investigating emu oil in a model of chronic Crohn’s disease which is more reflective of the long-term relapsing disease course.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr Alexandra Whittaker for assistance with the mouse facial grimace analysis and burrowing input.
Authors’ contributions: CJM performed all animal work and analyses detailed, as well as writing the final manuscript. GSH and SM supervised and conceptualized the study. GSH and SM also critically reviewed and edited the manuscript. DT and ICL assisted in the planning and conceptualization of the study. LCC assisted with animal work and analyses involved in the study and contributed to editing of the final manuscript. LSH contributed to data analyses. All authors have read and approved the final version of this manuscript.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lauren C Chartier https://orcid.org/0000-0001-7068-627X
References
- 1.Akobeng AK. Crohn's disease: current treatment options. Arch Dis Child 2008; 93:787–92 [DOI] [PubMed] [Google Scholar]
- 2.Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of Crohn's disease. Autoimmun Rev 2014; 13:467–71 [DOI] [PubMed] [Google Scholar]
- 3.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006; 3:390–407 [DOI] [PubMed] [Google Scholar]
- 4.Mocci G, Marzo M, Papa A, Armuzzi A, Guidi L. Dermatological adverse reactions during anti-TNF treatments: focus on inflammatory bowel disease. J Crohns Colitis 2013; 7:769–79 [DOI] [PubMed] [Google Scholar]
- 5.Vavricka SR, Schoepfer AM, Scharl M, Rogler G. Steroid use in Crohn's disease. Drugs 2014; 74:313–24 [DOI] [PubMed] [Google Scholar]
- 6.Abimosleh SM, Lindsay RJ, Butler RN, Cummins AG, Howarth GS. Emu oil increases colonic crypt depth in a rat model of ulcerative colitis. Dig Dis Sci 2012; 57:887–96 [DOI] [PubMed] [Google Scholar]
- 7.Snowden JM, Whitehouse MW. Anti-inflammatory activity of emu oils in rats. Inflammopharmacology 1997; 5:127–32 [DOI] [PubMed] [Google Scholar]
- 8.Whitehouse MW, Turner AG, Davis CK, Roberts MS. Emu oil(s): a source of non-toxic transdermal anti-inflammatory agents in aboriginal medicine. Inflammopharmacology 1998; 6:18 [DOI] [PubMed] [Google Scholar]
- 9.Beckerbauer LM, Thiel-Cooper R, Ahn DU, Sell JL, Parrish FC, Jr., Beitz DC. Influence of two dietary fats on the composition of emu oil and meat. Poult Sci 2001; 80:187–94 [DOI] [PubMed] [Google Scholar]
- 10.Whiting CV, Bland PW, Tarlton JF. Dietary n-3 polyunsaturated fatty acids reduce disease and colonic proinflammatory cytokines in a mouse model of colitis. Inflamm Bowel Dis 2005; 11:340–9 [DOI] [PubMed] [Google Scholar]
- 11.Yoganathan S, Nicolosi R, Wilson T, Handelman G, Scollin P, Tao R, Binford P, Orthoefer F. Antagonism of croton oil inflammation by topical emu oil in CD-1 mice. Lipids 2003; 38:603–7 [DOI] [PubMed] [Google Scholar]
- 12.Aberra FN. Omega-3 fatty acids for maintenance of remission of Crohn's disease. Gastroenterology 2008; 135:1005–6; discussion 06–7 [DOI] [PubMed] [Google Scholar]
- 13.Abimosleh SM, Tran CD, Howarth GS. Emu oil reduces small intestinal inflammation in the absence of clinical improvement in a rat model of indomethacin-induced enteropathy. Evid Based Complement Alternat Med 2013; 2013:429706–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safaeian R, Howarth GS, Lawrance IC, Trinder D, Mashtoub S. Emu oil reduces disease severity in a mouse model of chronic ulcerative colitis. Scand J Gastroenterol 2019; 54:273–80 [DOI] [PubMed] [Google Scholar]
- 15.Chartier LC, Howarth GS, Lawrance IC, Trinder D, Barker SJ, Mashtoub S. Emu oil improves clinical indicators of disease in a mouse model of colitis-associated colorectal cancer. Dig Dis Sci 2018; 63:135–45 [DOI] [PubMed] [Google Scholar]
- 16.Chartier LC, Maiolo KE, Howarth GS, Lawrance I, Trinder D, Barker SJ, Scherer B, Mitchell CJ, Mashtoub S. Emu oil improves clinical indicators of disease and reduces proximal colonic crypt hyperplasia in a murine model of colitis-associated colorectal cancer. Gastroenterology 2018; 154:S875–S75 [Google Scholar]
- 17.Mashtoub S, Howarth GS, Trinder D, Lawrance I. Emu oil attenuates disease severity and results in fewer large colonic tumours in a mouse model of colitis-associated colorectal cancer. Gastroenterology 2017; 152:S737–S37 [DOI] [PubMed] [Google Scholar]
- 18.Lindsay RJ, Geier MS, Yazbeck R, Butler RN, Howarth GS. Orally administered emu oil decreases acute inflammation and alters selected small intestinal parameters in a rat model of mucositis. Br J Nutr 2010; 104:513–9 [DOI] [PubMed] [Google Scholar]
- 19.Mashtoub S, Lampton LS, Eden GL, Cheah KY, Lymn KA, Bajic JE, Howarth GS. Emu oil combined with lyprinol reduces small intestinal damage in a rat model of chemotherapy-induced mucositis. Nutr Cancer 2016; 68:1171–80 [DOI] [PubMed] [Google Scholar]
- 20.Mashtoub S, Tran CD, Howarth GS. Emu oil expedites small intestinal repair following 5-fluorouracil-induced mucositis in rats. Exp Biol Med 2013; 238:1305–17 [DOI] [PubMed] [Google Scholar]
- 21.Raghu Nadhanan R, Abimosleh SM, Su YW, Scherer MA, Howarth GS, Xian CJ. Dietary emu oil supplementation suppresses 5-fluorouracil chemotherapy-induced inflammation, osteoclast formation, and bone loss. Am J Physiol Endocrinol Metab 2012; 302:E1440–9 [DOI] [PubMed] [Google Scholar]
- 22.Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol 2000; 19:51–62 [DOI] [PubMed] [Google Scholar]
- 23.Becker C, Fantini MC, Wirtz S, Nikolaev A, Kiesslich R, Lehr HA, Galle PR, Neurath MF. In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut 2005; 54:950–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howarth GS, Xian CJ, Read LC. Predisposition to colonic dysplasia is unaffected by continuous administration of insulin-like growth factor-I for twenty weeks in a rat model of chronic inflammatory bowel disease. Growth Factors 2000; 18:119–33 [DOI] [PubMed] [Google Scholar]
- 25.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 2010; 7:447–9 [DOI] [PubMed] [Google Scholar]
- 26.Yazbeck R, Howarth GS, Geier MS, Demuth HU, Abbott CA. Inhibiting dipeptidyl peptidase activity partially ameliorates colitis in mice. Front Biosci 2008; 13:6850–8 [DOI] [PubMed] [Google Scholar]
- 27.Barker SJ, Howarth GS, Scherer BL, Chartier LC, Cheah KY, Lymn KA, Mashtoub S. Mucosal thickening following oral administration of emu oil represents a process of normal intestinal growth in rats. J Gastroen Hepatol 2017; 32:15 [Google Scholar]
- 28.Mashtoub S, Cheah KY, Lymn KA, Howarth GS. Intestinal homeostasis is restored in mice following a period of intestinal growth induced by orally administered emu oil. Exp Biol Med 2018; 243:945–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mashtoub S, Ghaemi R, Lawrance I, Trinder D, Howarth GS. Emu oil attenuates disease severity in mouse models of colitis and inflammation-associated colorectal cancer. Gastroenterology 2016; 150:S1154–S54 [Google Scholar]
- 30.Emge JR, Huynh K, Miller EN, Kaur M, Reardon C, Barrett KE, Gareau MG. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 2016; 310:G989–98 [DOI] [PubMed] [Google Scholar]
- 31.Jirkof P, Leucht K, Cesarovic N, Caj M, Nicholls F, Rogler G, Arras M, Hausmann M. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Lab Anim 2013; 47:274–83 [DOI] [PubMed] [Google Scholar]
- 32.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JC, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. The rat grimace scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain 2011; 7:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whittaker AL, Leach MC, Preston FL, Lymn KA, Howarth GS. Effects of acute chemotherapy-induced mucositis on spontaneous behaviour and the grimace scale in laboratory rats. Lab Anim 2016; 50:108–18 [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods 2015; 421:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson AD, Poon P, Greenway GM, MacFie J. A simple method for the analysis of urinary sucralose for use in tests of intestinal permeability. Ann Clin Biochem 2005; 42:224–6 [DOI] [PubMed] [Google Scholar]
- 36.Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis 2009; 15:341–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen PM, Putoczki TL, Ernst M. STAT3-Activating cytokines: a therapeutic opportunity for inflammatory bowel disease? J Interferon Cytokine Res 2015; 35:340–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atreya R, Atreya I, Neurath MF. Novel signal transduction pathways: analysis of STAT-3 and rac-1 signaling in inflammatory bowel disease. Ann N Y Acad Sci 2006; 1072:98–113 [DOI] [PubMed] [Google Scholar]
- 39.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol 2003; 284:L84–9 [DOI] [PubMed] [Google Scholar]
- 40.Bennett DCC, WE, Godin David V, Cheng, Kimberly M. Comparison of the antioxidant properties of emu oil with other avian oils. Aust J Exp Agric 2008; 48:1345–50 [Google Scholar]
- 41.Abimosleh SM, Tran CD, Howarth GS. Emu oil: a novel therapeutic for disorders of the gastrointestinal tract? J Gastroenterol Hepatol 2012; 27:857–61 [DOI] [PubMed] [Google Scholar]