Abstract
Acupuncture is an emerging alternative therapy that has been beneficial for the pain of osteoarthritis (OA). However, the underlying mechanism of protective effect remains unclear. MCP1/CCR2 axis can be stimulated in various periods of OA, and we hypothesize that acupuncture may treat OA by regulating the MCP1/CCR2 axis. This study aimed to explore the effect of acupuncture at points ST35 and ST36 on the effects of hyperalgesia and cartilage in OA rats including the expression of chemokines, nerve growth factor (NGF), and inflammatory-related proteins. OA was induced in male Sprague–Dawley rats by anterior cruciate ligament transection at the right knee. The first acupuncture intervention was performed on the seventh day after surgery and once a day for seven weeks. The knee-pain-related behaviors, histology, and related protein were examined in this study. We have found that electroacupuncture at ST35 and ST36 can significantly alleviate the hyperalgesia and cartilage degeneration as well as reducing nerve sprouting in OA knee joint. Moreover, acupuncture treatment may inhibit the MCP1/CCR2 axis as well as down-regulate inflaming factor and NGF in cartilage and synovial tissue. The data presented here indicate that acupuncture exerts a protective effect against hyperalgesia and cartilage degeneration, and the mechanism might involve in chemokines and NGF pathway.
Keywords: acupuncture, osteoarthritis, MCP1, CCR2, pain
Impact statement
Osteoarthritis (OA) is the most common form of arthritis, affecting an estimated 302 million people worldwide, but the mechanism of OA is far away understood. Acupuncture has been widely used in treating with chronic pain, but how does acupuncture work is still unclear. In this essay, we investigated the molecular target of acupuncture and found new mechanism of the pathogenesis in knee OA. Our results could provide new analgesic mechanisms and find new targets for acupuncture analgesia in OA. In a word, the study can help further understanding and provides more evidence of the clinical use of acupuncture in treating OA.
Introduction
Osteoarthritis (OA) is characterized by degeneration of articular cartilage and subchondral bone accompanied by joint pain, swelling, and stiffness and often leads to joint dysfunction.1,2 Although OA is not regarded as an inflammatory primary disease, it is often presented with low-grade synovitis and enhanced levels of inflammatory cytokines in synovial fluid.3 Chronic inflammation in which the innate immune systems involved is critical pathogenesis of OA.4 As a lack of innervation in cartilage tissue,5 the pain of OA is mainly sensed by sensory nerve fibers distributed in synovial membrane. Therefore, degeneration of cartilage can be uncoupled from joint hyperalgesia which mostly comes from the inflammation in synovia.6,7 In addition to structural defects and inflammation, some evidence suggested that approximately 30% of patients with OA have neuropathic pain.8,9
Physical approaches, exercise, and oral anti-inflammatory drugs are recommended to treat knee osteoarthritis (KOA) in the recent guideline.10 However, to date, oral anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs and opioids11 are hard to prevent, slow, or halt OA structural changes. The development of therapies to block inflammation, neuropathy, and pain is sorely needed.
Chemokines is a subset of cytokines that induce the recruitment and trafficking of inflammatory cells and mesenchymal progenitors.12 Many chemokines are found within the OA joint and might facilitate the onset and progression of OA. Chemokines may induce the release of matrix-degrading enzymes,13 driving and maintaining leukocyte infiltration,14 and promote fibroblast-like synoviocytes (FLS) interleukin (IL)-6 production15 and monocyte adhesion16 to cause synovial reactions and joint destruction. MCP-1 (also known as CCL2) is a chemokine highly increased in cartilage, synovium, and synovial fluids from OA patients.14 The MCP1/CCR2 axis, which is considered to play a vital role in KOA, acts as pro-mediators in inflammation pain and can activate the recruitment of monocytes and macrophages which secrete inflammatory cytokines and nerve growth factors (NGFs) to aggravate OA progression.4,17,18 The effects that down-regulation of the MCP1/CCR2 axis can protect OA knee joint from hyperalgesia have been confirmed in recent research,3 but the mechanism underlying is not completely understood. The recent research found that the role of MCP1/CCR2 axis can be more important than CCL5/CCR5 axis in the monocyte recruitment, inflammation, and cartilage destruction in OA. So, MCP-1/CCR2 axis can be a potential therapeutic target capable of reducing both pain and structural changes.4
Acupuncture has been used for preventing and treating various diseases for centuries.19 It has been validated in reducing the pain intensity of OA patients20 and down-regulating inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and IL-1β in inflammation lesions21,22 and MCP123 because of its potential role in modulating several endogenous biological mediators. Despite being mature technology, the exact analgesia mechanism of acupuncture is still unclear. In our study, we used an experimental OA model induced to causing destabilization of the joint mechanics (anterior cruciate ligament transection [ACLT]) and intend to explore the possible molecular mechanism of acupuncture improvement in OA.
Materials and methods
Ethics statement
All animal experiments were performed following the Chinese Guidelines of Animal Care and Welfare, and the present study was approved by the Animal Care and Use Committee of Hubei University of Chinese medicine (Wuhan, China).
Animals and treatment
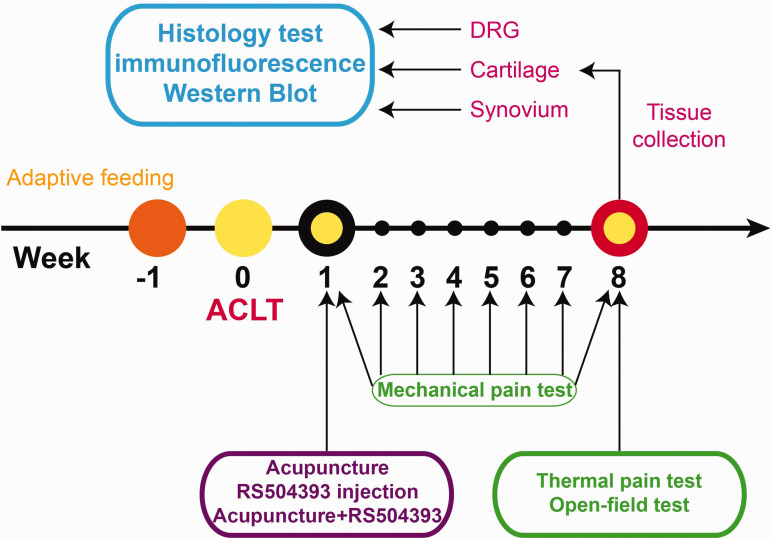
Fifty-five male eight-week-old Sprague–Dawley rats weighing 180 to 220 g (8 weeks old) were housed under a reversed 12-h light–dark cycle with temperature and humidity (24 ± 2°C and 50 ± 5%) controlled, and food and water were freely available. After seven days of adaptive feeding, five rats were prepared for retrograde labeling. The rest 50 rats were randomly divided into five groups: control group, model group, RS504393 group, manual acupuncture (MA) group, and MA+RS504393 group. Acupuncture was performed into acupoints Dubi (ST35, 2 mm deep) and Zusanli (ST36, 5 mm deep). ST35 located at lateral depression between the patella and the patellar ligament and ST36 located 3u lower than ST35. (The distance between the knee joint and the external ankle pointed is 16u in standard acupuncture measurement.) Needles (0.25 mm diameter × 40mm length) were inserted into the above two acupoints in groups MA and MA+RS504393 at the seventh day after ACLT modeling. The needles were manually twisted for 1 min with a frequency of about 2 Hz and then left still for four minutes. This pattern is repeated for six times (30 min) during the manipulation session. Rats in RS504393 and MA+RS504393 group received articular injection of 25 μL RS504393 (Abcam) at a concentration of 50 ng/mL using a 27-gauge, 0.5-inch needle.24 The first injection was given on the seventh day after ACLT surgery and once a week for seven weeks. Rats in control and model groups did not receive any intervention. The right knee was experimental, while the left knee was treated as a control with articular injection of 25 µL physiological saline. The experimental timeline was shown in Figure 1.
Figure 1.
Fifty eight-week-old Sprague–Dawley rats received an ACLT surgery to build KOA model after seven days of adaptive feeding. Then, experimental groups underwent following treatments: acupuncture, RS504393, and combination of acupuncture+RS504393 seven days after the surgery, and the mechanical pain was tested once every seven days. After seven weeks of intervention, all rats received behavioral tests (open field test and thermal pain test) and then sacrificed. Cartilage and synovium from right knee joint and DRGs from L3-5 were collected to conduct histology test and molecular biology experiment (immunofluorescence labeling and Western blot). DRG: dorsal root ganglion; ACLT: anterior cruciate ligament transection. (A color version of this figure is available in the online journal.)
Induction of OA in rats
The KOA model was induced via ACLT method. A 2-cm-long medial longitudinal incision at the right knee joint was taken under general anesthesia by pentobarbital sodium, the joint capsule was opened, medial ligament was exposed, and a lateral dislocation of the knee caused. The anterior cruciate ligament was cut off. To prevent postoperative infection, antibiotics (1.0–1.3 mg/cefotiam hydrochloride) were used for three days. All rats were sacrificed eight weeks after surgery.
Motor test
Open-field tests were performed on rats in each group to test spontaneous locomotor activity for each group. Rats were acclimated to the test apparatus for 30 min before the test. The experiment rats were placed at the center of a 1 × 1 × 0.5 m chamber and allowed to free exploration. This experiment began the day before sacrificed. Average velocity, total travel distance, and rest duration in 10 min were recorded by camera and measured by a computer.
Pain threshold test
The thermal withdrawal latency (TWL) and mechanical withdrawal threshold (MWT) were evaluated by the von Frey test (Stoelting, Wood Dale, IL) and a thermal Plantar Test (Ugo Basile, Italy), respectively. After acclimated to the test apparatus for 30 min, rats received a gradually increased perpendicular pressure at the plantar surface of the hind paw. The corresponding pressure value was recorded while withdrawing the paw . For testing the thermal hyperalgesia, a focused beam of radiant heat (up to 35°C) was irradiated to the plantar surface of the hind paws for more than three times and held for maximal 20 s. The imposed value was recorded when the paw withdrawing. Results of the mechanical pain threshold test were expressed in grams (g/F) and duration (s) for the thermal hyperalgesia test.
Western blot
The rat tissue was lysed in RIPA buffer (Beyotime, Haimen, China), and protein concentrations were measured using the BCA protein assay kit (Beyotime, Haimen, China). Protein samples were separated on an 8% SDS-PAGE gel and then transferred onto polyvinylidene fluoride membranes (Merck Millipore, Billerica, MA). After blocking with 5% BSA for 2 h at room temperature, the membranes were incubated overnight at 4°C with primary antibodies anti-MCP1 (1.33 µg/mL, Proteintech) and anti-CCR2 (1 µg/mL, Aviva). Finally, the membranes were hybridized with horseradish peroxidase-conjugated secondary antibodies. After incubation of the membranes with enhanced chemiluminescence reagent (Millipore), the protein band images were collected and analyzed by Molecular Image, ChemiDoc XRS Image System (Bio-Rad Laboratories). The density value was counted by scanning with Image Lab software, GADPH as internal marker, namely, target protein gray value/internal reference overall gray value.
Histology, immunofluorescence labeling, and histological analysis
Rats were euthanized with CO2 and perfused from the heart with saline and followed by 4% paraformaldehyde. Cartilage was harvested, postfixed 24 h in 4% paraformaldehyde, and then demineralized in 10% EDTA for four weeks followed by specimens’ dehydration and embedding in paraffin. Longitudinal-oriented sections (4 µm) of the knee joint were cut on a paraffin microtome (Finesse 325, Thermo) and processed Safranin O and fast green staining.
L3–L5 dorsal root ganglions (DRGs) and synovia were harvested, postfixed 6 h in 4% paraformaldehyde, and dehydrated in 30% sucrose at 4°C. After the tissue sink to the bottom of the vessel, transverse DRG, cartilage, and synovia sections (cartilage for fluorescence staining and synovia in PGP9.5 staining was 40 µm, others were 10 µm) were cut on a freezing microtome (CM1860, Leica). Primary antibodies used were anti-PGP9.5 (10 µg/mL, Abcam), anti-NGF (10 µg/mL, Abcam), anti-MCP1 (13.3 µg/mL, Proteintech), anti-CCR2 (10 µg/mL, Aviva), anti-IL-1β (20 µg/mL, Abcam), and anti-TrkA (5 µg/mL, Abcam). Methods of immunofluorescence labeling were applied. After blocking with 10% goat serum for 30 min at room temperature, sections were incubated with primary antibodies in the immunofluorescent antibody dilution buffer (Beyotime, China) for 12 h at 4°C. Sections were then incubated with Alexa Fluor 488 and Alexa Fluor 594 secondary antibodies (10 µg/mL, Abcam) for 2 h at room temperature. Nucleus were then stained by mounting medium with DAPI (Abcam) and observed with a fluorescence microscope (AX10, Carl Zeiss).
Five sections were taken from each group to observe the changes in the density of nerve sprouting in the knee joint, and images were analyzed with Image J software. The observation was located at synovium adjacent to the medial meniscus where the nerve sprouting consistently appeared. Three images per section were acquired in medial synovium and analyzed for quantitative histomorphometric analyses. PGP9.5+ nerve fibers were calculated as the density of nerve fibers are divided by the total area examined (µm2/µm2). All sections were analyzed in three different fields. For fluorescence intensity measurement, values from rats were measured, and the results were presented in arbitrary units. Positive cells were determined as the percentage of total counted DRG neurons and chondrocyte from an average of three fields in each slide using Image J. Immunostained images obtained at 400× magnification (n = 5 per group) were used for histomorphometric analyses. A negative control replaced the primary antibody by phosphate-buffered saline was included for each immunostaining experiment.
For histological analysis, one representative slice was chosen from each knee and evaluated. The histopathological changes were quantified by the Osteoarthritis Research Society International (OARSI) scoring system.23,25 The grade and stage score were assessed by the microscopic pathological changes, and the OARSI score is calculated by the formula: score = grade × stage. The OARSI score ranges from 0 to 24, with higher values indicating more advanced cartilage degeneration.
Statistical analysis
All data were expressed as mean ± SD and analyzed by the software GraphPad Prism version 8.0 for Windows (GraphPad Software, USA). One-way and two-way analysis of variance (ANOVA) were used for statistical comparisons between the different groups. Bonferroni’s post hoc tests were used for multiple comparisons. P < 0.05 was considered to be statistically significant.
Results
MA protects rats from cartilage degeneration and hyperalgesia
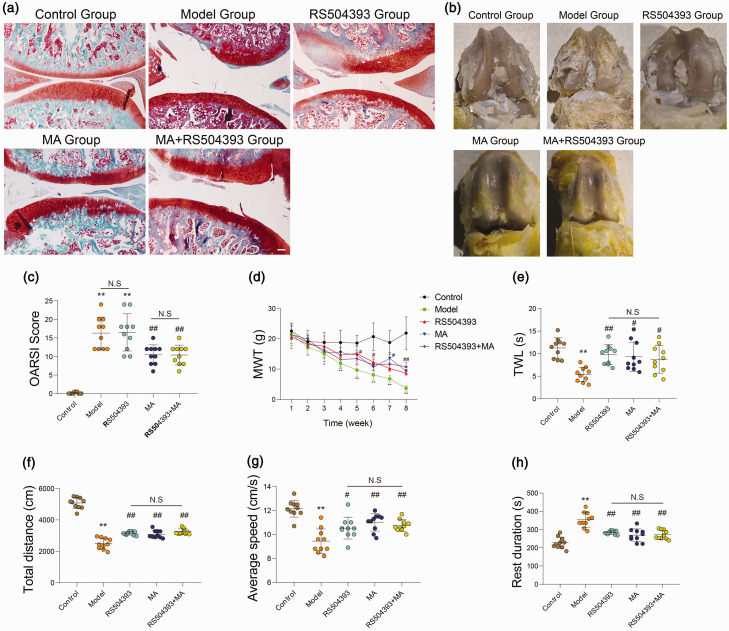
We firstly tested histopathological changes (Figure 2(a)), gross microscopy (Figure 2(b)), and histopathology grading (Figure 2(c)) in the knee joint of rats after surgery. The results indicated that rats in groups MA and MA+RS504393 showed obvious cartilage protective effect, but rats treated with RS504393 did not show a significant difference in cartilage pathological change while compared with rats in group model (P > 0.05). There was no significant difference among rats in groups RS504393, MA, and MA+RS504393 (P > 0.05). Following this, we performed a mechanical pain test and thermal pain test (Figure 2(d) and (e)). We detected the paw withdrawal threshold of mechanical and thermal stimulation of the plantar to evaluate the degree of central and thermal hypersensitization pain. In the mechanical pain test, we found that the pain threshold in group model was significantly lower than the normal value from the third week after surgery (P < 0.05). And from the fifth week, the pain threshold of the treatment group was significantly higher than that of the model group (P < 0.05), but there was no significant difference between the treatment groups (P > 0.05).
Figure 2.
Both MA and RS504393 can induce hyperalgesia in osteoarthritis, but only MA can protect rats from cartilage degeneration. OA was induced by surgery in eight-week-old Sprague–Dawley rats. All knee joints were harvested at eight weeks after surgery. (a) Histological assessment was shown by Safranin-O fast green staining (40×, each group: n = 10, Scale bar = 200 μm, ANOVA). (b) Macroscopic view of articular cartilage damage surface. (c) OARSI scoring system was used to quantify the severity of cartilage damage (each group: n = 10, ANOVA). (d and e) MWT was tested by Von Frey filament at every seven days after ACLT, and TWL was tested after last treating (each group: n = 10, two-way ANOVA for MWT and one-way ANOVA for TWL). (f to h) The open-field experiment was used to test spontaneous activity of rats in each group. Total distance, average speed, and rest duration were recorded of activity of rats in a bright free field in 10 min (each group: n = 10, ANOVA). All values were expressed as the mean ± SD. *P < 0.05, **P < 0.01: compared with control group and #P < 0.05, ##P < 0.01: compared with model group. Bonferroni’s post hoc tests was used for multiple comparisons. NS: no significant; OARSI: Osteoarthritis Research Society International. (A color version of this figure is available in the online journal.)
We then detected spontaneous activity of rats by open field test 1 day before sacrifice (Figure 2(f) to (h)). We selected total distance, average speed, and rest duration of rats to assess the mobility, and the motion trail was also recorded (Supplementary Figure 1). The results indicated that the pain thresholds and spontaneous activity reduced significantly in rats after ACLT (P < 0.05) while compared with rats in control group. The pain thresholds reduce apparently (P < 0.05) in rats of MA group and inhibitor group while compared with rats in model group. Neither paw withdrawal threshold test nor spontaneous activity test showed significant differences among rats in groups MA, inhibitor, and MA+inhibitor.
These results showed that both MA and RS504393 can protect rats from hyperpathia from eight weeks after ACLT, but only MA showed a certain resistance effect to cartilage degeneration.
MA reduced knee joint hyperalgesia by the decreased density of synovial membrane knee joint nerves through the NGF signal pathway
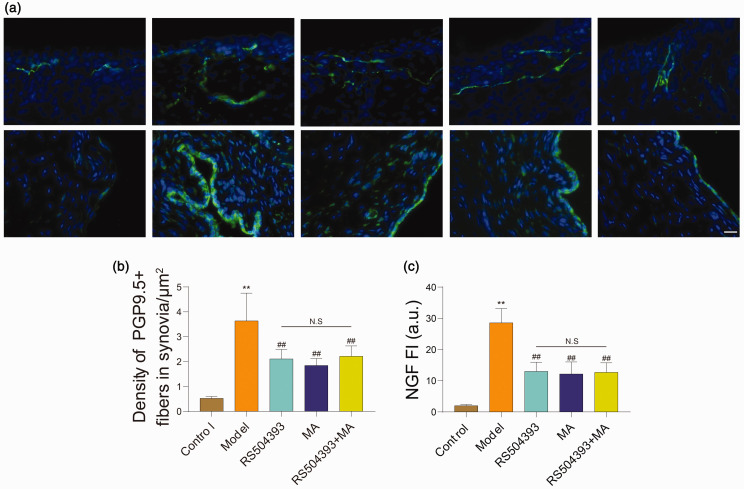
Sensory nerve sprouting in OA synovium played a vital role in local hyperalgesia. We observed nerve density (Figure 3(a)) by immunofluorescence staining with PGP9.5 antibody and found that rats in model group were significantly higher than normal rats in PGP9.5-positive structure (P < 0.01), and MA and RS504393 treatment can reduce density of PGP9.5-positive structure (P < 0.05) while compared to rats in model group, but the effects of MA+RS504393 did not exhibit better efficacy than MA (P > 0.05) (Figure 3(b)).
Figure 3.
Both MA and RS504393 can reduce expression of NGF and density of PGP9.5 positive structure in synovia of rats per group. (a) Representative images of immunofluorescence staining for PGP9.5 and NGF in synovia of rats in each group. PGP9.5 and NGF positive structure was shown by immunohistochemical green. (b) Quantitative analysis of PGP9.5 positive nerve fibers (each group: n = 5, ANOVA). (c) Quantitative analysis of NGF expression level in synovium (each group: n = 5, ANOVA). Scale bar = 20 μm. Values are mean ± SD. *P < 0.05, **P < 0.01: compared with control group. #P < 0.05, ##P < 0.01: compared with model group. Bonferroni’s post hoc tests were used for multiple comparisons. NS: no significant; FI: fluorescence intensity; NGF: nerve growth factor. (A color version of this figure is available in the online journal.)
NGF is an important factor that can stimulate neuronal sprouting and highly expressed in OA knee joint.26 We investigated the expression level of NGF in the synovium (Figure 3(a)). The results suggested that NGF was up-regulated in synovia of all rats after surgery (P < 0.05). Compared with group model, the expression of NGF in groups MA, RS504393, and MA+RS504393 were reduced, and the difference was statistically significant (P < 0.05). There was no statistically significant difference among groups MA, RS504393, and MA+RS504393 (P > 0.05) (Figure 3(c)). Taken together, both MA and RS504393 could decrease the expression of NGF to reduce neuronal sprouting to alleviate hyperalgesia in OA knee joint. But consistent with the results of PGP9.5 staining, MA+RS504393 did not show a better therapeutic effect.
MA regulate NGF through MCP1/CCR2 axis
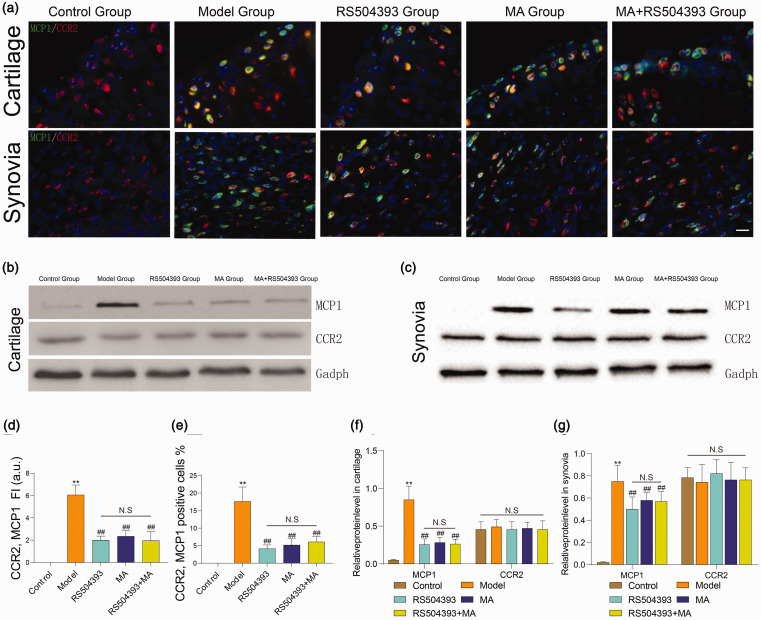
MCP1/CCR2 axis is an important pathway for chemokines in the development of OA.4 Similar to the function of NGF chemotactic neurons, MCP1 can induce aggregation of monocytes and macrophages, and these inflammatory cells may be a major source of NGF in OA knee.18 We applied RS504393 to inhibit CCR2 seven days after ACLT for once a week until sacrificed and observed the expression of MCP1 and CCR2 in cartilage and synovia (Figure 4(a) to (c)). We found that CCR2-positive cells existed at both normal and treated groups, and the expression level did not increase apparently in development of OA (Figure 4(f) and (g), P > 0.05).
Figure 4.
Both MA and RS504393 can reduce expression of MCP1 and co-expression of MCP1/CCR2. (a) Representative images of immunofluorescence staining for MCP1 (green) and CCR2 (red) in synovia and cartilage. White arrows indicated the co-localization of MCP1 and CCR2. (b and c) The protein levels of MCP1 and CCR2 were analyzed by Western blot. (d and e) Quantitative analysis of MCP1 and CCR2 co-expression in both cartilage and synovia (each group: n = 5, ANOVA). (f and g) Quantitative analysis of expression level of MCP1 and CCR2 in cartilage and synovia. Scale bar = 20 μm. Values are mean ± SD. *P < 0.05, **P < 0.01: compared with group control. #P < 0.05, ##P < 0.01: compared with group model. Bonferroni’s post hoc tests were used for multiple comparisons. NS: no significant; . (A color version of this figure is available in the online journal.)
The expression of MCP1 increased in all rats eight weeks after surgery (P < 0.01). The expression level of MCP1 deceased in rats of groups MA, RS504393, and MA+RS504393 (P < 0.01), and there was no significant difference between groups MA and MA+RS504393. We investigated the co-expression of CCR2/MCP1, and the trend was consistent with the expression of MCP1 (Figure 4(d) and (e)). The results suggested that the effects of MA to reduce NGF in the OA knee joint are related to CCR2/MCP1 pathway.
MA reduces production of inflammatory cytokines in OA
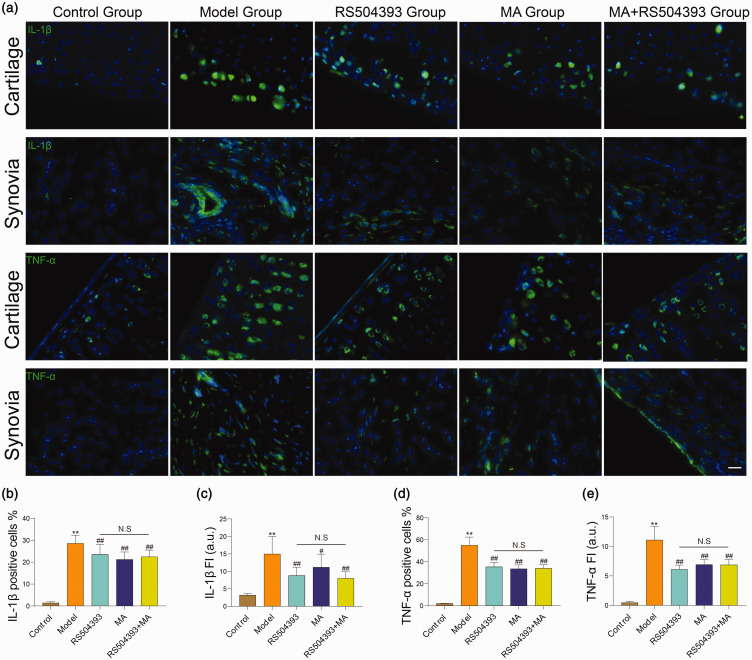
Pro-inflammatory cytokines secreted by inflammatory cells promote the progression of OA. IL-1β can stimulate MCP1/CCR2 axis and promote secretion of NGF in cartilage and synovium.18,27 To assess the effects of MA with pro-inflammatory cytokines, we gathered whole knee joint at eight weeks after surgery and examined the expression level of IL-1β and TNF-α both in cartilage and synovium (Figure 5(a)). The same as the results in chemotactic factor, the expression level of IL-1β and TNF-α was greatly elevated in the joints of rats at eight weeks after surgery (P < 0.01). Both MA and RS504393 can protect cartilage and synovia from inflammatory injury (P < 0.05), but there were no significant differences in the joints of MA-, RS504393-, and MA+RS504393-treated rats (P > 0.05) (Figure 5(b) to (e)).
Figure 5.
Both MA and RS504393 can reduce expression of IL-1β and TNF-α in cartilage and synovia. (a) Immunofluorescence staining images for IL-1β and TNF-α (green) in cartilage and synovia. (b and c) Quantitative analysis of IL-1β positive cells counting in cartilage and fluorescence intensity in synovia (each group: n = 5, ANOVA). (d and e) Quantitative analysis of TNF-α positive cells counting in cartilage and fluorescence intensity in synovia (each group: n = 5, ANOVA). Statistical analysis: one-way ANOVA with Bonferroni’s post hoc tests (n = 5). Scale bar = 20 μm in synovia images. Values are mean ± SD. *P < 0.05, **P < 0.01: compared with the control group. #P < 0.05, ##P < 0.01: compared with the model group. Bonferroni’s post hoc tests were used for multiple comparisons. NS: no significant; IL-1β: interleukin-1β; TNF-α: tumor necrosis factor-α; FI: fluorescence intensity. (A color version of this figure is available in the online journal.)
MA inhibits NGF/TrkA axis in DRG neurons
In addition to pain caused by local factors, central sensitization is also one of the factors causing OA hyperalgesia and related to NGF retrograde transmission.28 To confirm this mechanism, we collected isolated ipsilateral L3-5 DRGs and preformed immunofluorescence staining. Supplementary Figure 4(a) shows the NGF and TrkA levels in DRGs. We used NeuN (a neuron-specific marker) to display neuron cells, and it was obvious that NGF/TrkA axis was activated in DRG neurons strikingly. The expression levels of both NGF and TrkA were greatly increased in rats after ACLT (P < 0.01). This activation effect was decreased in groups MA, RS504393, and MA+RS504393 (P < 0.05) which was consistent with our expectation. But, there was no significant difference among the above three treating groups. MA+RS504393 did not exhibit a better effect on inhibiting NGF/TrkA axis in DRGs (P > 0.05) (Supplementary Figure 4(b) and (c)). The results were consistent with mechanical pain test and spontaneous activity and indicated that NGF/TrkA pathway stimulated in the progression of OA can be inhibited by MA and RS504393.
Discussion
OA is the most common joint disorder in a large number of people older than 65 years. KOA is more commonly associated with disability than OA of the other joints. In this study, we choose ACLT model to mimic features of human KOA. ACLT can cause an instability in knee joint to induce chronic KOA. Previous studies have found that pathological changes such as inflammatory synovitis,29 synovial pannus-like tissue,30 changes of proteoglycan content and collagen structure, cartilage erosions,31 late osteophytosis, subchondral bone remodelling, and chondrocytic apoptosis32 with caspase 3 activation33 and OA relevant pain34 can appear after ACLT. Thus, this model can simulate the pathophysiological process of chronic KOA and reinforcing the clinical relevance of our experimental approach.
Despite the effect of acupuncture treatment on OA is often attributed to placebo effects, the mechanism has not been fully understood. Previous research suggested that acupuncture showed an obvious effect on analgesia and cartilage protection.35,36 Our results from experimental OA rats provided a new explanation on the mechanism of acupuncture in treating OA, and the reduced hyperalgesia was related to inhibition of MCP1/CCR2 pathway. We considered that stimulated MCP1/CCR2 axis might up-regulate NGF/TrkA axis which plays an important role in OA pain and take effects through increasing sensory nerve density. Our research also suggested that MA therapy at ST35 and ST36 can inhibit MCP1/CCR2 axis which mediates monocytes/macrophage chemotaxis and reduce expression of NGF in cartilage, synovial tissues, and DRGs to reduce hyperalgesia of experimental OA in rats apparently. Understanding the effect of acupuncture and chemotaxis to neuronal signaling pathways may provide new evidence for clinical treatment of pain-related symptoms in OA.
Monocytes and macrophage are the main participants involved in inflammation reaction and resident in various organizations. Cartilage injury can activate resident macrophages and augment expression of MCP1 in knee joint tissue in OA.37,38 MCP1/CCR2 axis is an important monocyte chemotaxis signaling pathway that promotes inflammatory response and mediating peripheral nerve injury-induced neuropathic pain. In this experiment, we found MCP1 was significantly increased in articular cartilage, synovial tissue response to cartilage injury, but CCR2 was not apparently regulated which was consistent with the previous report.38,39
Inflammatory factors which highly expressed in macrophages such as IL-1β can promote local inflammation and induced hyperalgesia in knee joint.40 We found that MA and RS504393 could reduce the expression of IL-1β in OA synovial tissue significantly. Hence, the macrophage may be involved in hyperalgesia suppression effects. But, only MA can protect cartilage from degeneration, indicating that MA may protect cartilage through other pathways.
The effect that inhibition of the MCP1/CCR2 pathway on cartilage protection is indefinite. Positive results reported by Raghu et al. suggested that CCR2 inhibitor could reduce synovitis and osteophyte formation significantly with an obvious protective effect on OA cartilage,4 but a study on CCR2 null mice showed that there was only a slight trend toward cartilage protection at 12, 16, and 20 weeks.3 Similarly, Longobardi et al. found that targeting the MCP1/CCR2 axis at early OA delayed OA progression.37 We windowed CCR2 blockage and MA from 4 to 8 weeks post-surgery, and results suggested that only MA could diminish cartilage damage significantly. The protective effect of MA did not affected by RS504393.
There is no doubt that the NGF/TrkA axis plays an important role in OA pain. Anti-NGF treatment shows an encouraging efficacy of relieving OA pain, but adverse effects appeared in the clinical trial that the NGF antibody has the risk of aggravating cartilage degeneration.26 The relationship between NGF and cartilage destruction is still unclear. NGF binding to TrkA located at plasma membrane of axon and form neurotrophin dimer which transported into cell through endocytosis. Then, NGF/TrkA dimer associated with signaling molecules are retrograde transported to the soma to regulate growth and differentiation of nerve cells.41 Inhibition of NGF signaling pathways in cartilage and synovia results in reduced NGF retrograde to DRGs in OA mice.42 Our results showed that NGF expressed in healthy synovia, which indicated that NGF/TrkA axis may involve in synovia homeostasis. Rats treated with CCR2 inhibitor showed lower pain thresholds and higher spontaneous activity which related to NGF suppression in synovia. The origin of NGF in OA tissues has been controversial. Recent research found that depleted synovial macrophages could reduce NGF expression in synovia.40 Our results also showed that NGF was highly expressed in synovial tissue, and inhibition of macrophage chemotaxis could reduce NGF expression in the synovium. The results indicated that macrophage in synovia may play an important role in the production of NGF.
The mechanism of acupuncture analgesia is considered relative to nervous system. MA can activate all types of afferent fibers via inserting the needle into the acupoint and twisting of the needle up and down by hand, and diverse signal molecules participate in this process.43 We performed a retrograde neural tracer labeling (Supplementary Figure 2) and studied the expression of NGF and TrkA in DRGs after CCR2 inhibitor injected and MA treatment. The results showed that both MA and RS504393 injection decreased the expression of NGF and TrkA in DRGs. Therefore, acupuncture therapy at ST35 and ST36 inhibit NGF retrograding in DRGs.
There are several limitations in our study. Firstly, we found that the inhibition of macrophage chemokines could reduce NGF expression in the synovium and deduced that macrophages in synovia may play an important role in the production of NGF. But, we did not do the double-labeling immunofluorescence of NGF and marker of macrophage to provide direct evidence. Secondly, the subchondral bone is reported to play a vital role in the pathogenesis of OA and is commonly associated with articular cartilage defects.44 The sensory nerve fibers can innervate subchondral bone and is important in skeletal pain transmission.45 But, in this study, we only examined changes in cartilage and synovium. Further research is needed to conduct on subchondral bone.
In summary, we show that MCP1/CCR2 pathway is associated with NGF/TrkA axis. Acupuncture can inhibit the MCP1/CCR2 axis and reduce the NGF expression. The HE staining showed the pathological changes of cartilage(Supplementary figure 3). This may be a new analgesic mechanism for acupuncture analgesia in OA.
Footnotes
Authors’ contributions: All authors participated in the review of the manuscript. Li Jia and CG conceived and designed the experiments. BL, CJ, HZ, ZX, and TQ performed the experiments. Li Jia analyzed the data and critically reviewed the manuscript. CG contributed reagents/materials/analysis tools. BL wrote the paper. BL and L Jing contributed equally to this work.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Natural Science Foundation of China (nos: 81774410, 81403485, and 81804165) and the Project of Hubei Provincial Health Committee (no: ZY2019M041).
ORCID iD: Bocun Li https://orcid.org/0000-0002-3684-0234
SupplementAL MATERIAL: Supplemental material for this article is available online.
References
- 1.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73:1323–30 [DOI] [PubMed] [Google Scholar]
- 2.Aicher WK, Rolauffs B. The spatial organisation of joint surface chondrocytes: review of its potential roles in tissue functioning, disease and early, preclinical diagnosis of osteoarthritis. Ann Rheum Dis 2014; 73:645–53 [DOI] [PubMed] [Google Scholar]
- 3.Miotla Zarebska J, Chanalaris A, Driscoll C, Burleigh A, Miller RE, Malfait AM, Stott B, Vincent TL. CCL2 and CCR2 regulate pain-related behaviour and early gene expression in post-traumatic murine osteoarthritis but contribute little to chondropathy. Osteoarthr Cartil 2017; 25:406–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghu H, Lepus CM, Wang Q, Wong HH, Lingampalli N, Oliviero F, Punzi L, Giori NJ, Goodman SB, Chu CR, Sokolove JB, Robinson WH. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis 2017; 76:914–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konttinen YT, Kemppinen P, Segerberg M, Hukkanen M, Rees R, Santavirta S, Sorsa T, Pertovaara A, Polak JM. Peripheral and spinal neural mechanisms in arthritis, with particular reference to treatment of inflammation and pain. Arthritis Rheum 1994; 37:965–82 [DOI] [PubMed] [Google Scholar]
- 6.Daheshia M, Yao JQ. The bone marrow lesion in osteoarthritis. Rheumatol Int 2011; 31:143–8 [DOI] [PubMed] [Google Scholar]
- 7.Haywood L, McWilliams DF, Pearson CI, Gill SE, Ganesan A, Wilson D, Walsh DA. Inflammation and angiogenesis in osteoarthritis. Arthritis Rheum 2003; 48:2173–7 [DOI] [PubMed] [Google Scholar]
- 8.Schomberg D, Ahmed M, Miranpuri G, Olson J, Resnick DK. Neuropathic pain: role of inflammation, immune response, and ion channel activity in central injury mechanisms. Ann Neurosci 2012; 19:125–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed S, Magan T, Vargas M, Harrison A, Sofat N. Use of the painDETECT tool in rheumatoid arthritis suggests neuropathic and sensitization components in pain reporting. Journal of Pain Research 2014; 7:579–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D, Gellar K, Harvey WF, Hawker G, Herzig E, Kwoh CK, Nelson AE, Samuels J, Scanzello C, White D, Wise B, Altman RD, DiRenzo D, Fontanarosa J, Giradi G, Ishimori M, Misra D, Shah AA, Shmagel AK, Thoma LM, Turgunbaev M, Turner AS, Reston J. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2020; 72:149–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kielly J, Davis EM, Marra C. Practice guidelines for pharmacists: the management of osteoarthritis. Can Pharm J (Ott) 2017; 150:156–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2016; 12:580–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 2012; 51:249–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanzello CR. Chemokines and inflammation in osteoarthritis: insights from patients and animal models. J Orthop Res 2017; 35:735–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang C-H, Hsu C-J, Fong Y-C. The CCL5/CCR5 axis promotes interleukin-6 production in human synovial fibroblasts. Arthritis Rheum 2010; 62:3615–24 [DOI] [PubMed] [Google Scholar]
- 16.Lin Y-M, Hsu C-J, Liao Y-Y, Chou M-C, Tang C-H. The CCL2/CCR2 axis enhances vascular cell adhesion molecule-1 expression in human synovial fibroblasts. PLoS One 2012; 7:e49999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A 2005; 102:14092–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takano S, Uchida K, Inoue G, Miyagi M, Aikawa J, Iwase D, Iwabuchi K, Matsumoto T, Satoh M, Mukai M, Minatani A, Takaso M. Nerve growth factor regulation and production by macrophages in osteoarthritic synovium. Clin Exp Immunol 2017; 190:235–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manni L, Albanesi M, Guaragna M, Barbaro Paparo S, Aloe L. Neurotrophins and acupuncture. Auton Neurosci 2010; 157:9–17 [DOI] [PubMed] [Google Scholar]
- 20.Mavrommatis CI, Argyra E, Vadalouka A, Vasilakos DG. Acupuncture as an adjunctive therapy to pharmacological treatment in patients with chronic pain due to osteoarthritis of the knee: a 3-armed, randomized, placebo-controlled trial. Pain 2012; 153:1720–6 [DOI] [PubMed] [Google Scholar]
- 21.Su TF, Zhao YQ, Zhang LH, Peng M, Wu CH, Pei L, Tian B, Zhang J, Shi J, Pan HL, Li M. Electroacupuncture reduces the expression of proinflammatory cytokines in inflamed skin tissues through activation of cannabinoid CB2 receptors. Eur J Pain 2012; 16:624–35 [DOI] [PubMed] [Google Scholar]
- 22.Gondim DV, Costa JL, Rocha SS, Brito GA, Ribeiro Rde A, Vale ML. Antinociceptive and anti-inflammatory effects of electroacupuncture on experimental arthritis of the rat temporomandibular joint. Can J Physiol Pharmacol 2012; 90:395–405 [DOI] [PubMed] [Google Scholar]
- 23.Lee JY, Choi DC, Oh TH, Yune TY. Analgesic effect of acupuncture is mediated via inhibition of JNK activation in astrocytes after spinal cord injury. PLoS One 2013; 8:e73948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu YK, Ke Y, Wang B, Lin JH. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biol Res 2015; 48:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 2006; 14:13–29 [DOI] [PubMed] [Google Scholar]
- 26.Lane NE, Corr M. Osteoarthritis in 2016: anti-NGF treatments for pain—two steps forward, one step back? Nat Rev Rheumatol 2017; 13:76–8 [DOI] [PubMed] [Google Scholar]
- 27.Rana AK, Li Y, Dang Q, Yang F. Monocytes in rheumatoid arthritis: circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int Immunopharmacol 2018; 65:348–59 [DOI] [PubMed] [Google Scholar]
- 28.Schmelz M, Mantyh P, Malfait AM, Farrar J, Yaksh T, Tive L, Viktrup L. Nerve growth factor antibody for the treatment of osteoarthritis pain and chronic low-back pain: mechanism of action in the context of efficacy and safety. Pain 2019; 160:2210–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 2001; 44:1237–47 [DOI] [PubMed] [Google Scholar]
- 30.Shibakawa A, Aoki H, Masuko-Hongo K, Kato T, Tanaka M, Nishioka K, Nakamura H. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthr Cartil 2003; 11:133–40 [DOI] [PubMed] [Google Scholar]
- 31.Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat 1971; 109:411–21 [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum 1998; 41:1632–8 [DOI] [PubMed] [Google Scholar]
- 33.Matsuo M, Nishida K, Yoshida A, Murakami T, Inoue H. Expression of caspase-3 and -9 relevant to cartilage destruction and chondrocyte apoptosis in human osteoarthritic cartilage. Acta Med Okayama 2001; 55:333–40 [DOI] [PubMed] [Google Scholar]
- 34.Jeon OH, Kim C, Laberge R-M, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 2017; 23:775–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo BK, Sung WS, Park YC, Baek YH. The electroacupuncture-induced analgesic effect mediated by 5-HT1, 5-HT3 receptor and muscarinic cholinergic receptors in rat model of collagenase-induced osteoarthritis. BMC Complement Altern Med 2016; 16:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Zhong P, Liao Y, Liu J, Liao Y, Xie H, Li N, Li X, Sun G, Zeng Y. Electroacupuncture ameliorates subchondral bone deterioration and inhibits cartilage degeneration in ovariectomised rats. Acupunct Med 2018; 36:37–43 [DOI] [PubMed] [Google Scholar]
- 37.Xie J, Huang Z, Yu X, Zhou L, Pei F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev 2019; 46:36–44 [DOI] [PubMed] [Google Scholar]
- 38.Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, Kovacs A, Epelman S, Artyomov M, Kreisel D, Lavine KJ. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 2019; 124:263–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Longobardi L, Temple JD, Tagliafierro L, Willcockson H, Esposito A, D'Onofrio N, Stein E, Li T, Myers TJ, Ozkan H, Balestrieri ML, Ulici V, Loeser RF, Spagnoli A. Role of the C-C chemokine receptor-2 in a murine model of injury-induced osteoarthritis. Osteoarthr Cartil 2017; 25:914–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakurai Y, Fujita M, Kawasaki S, Sanaki T, Yoshioka T, Higashino K, Tofukuji S, Yoneda S, Takahashi T, Koda K, Asaki T, Hasegawa M, Morioka Y. Contribution of synovial macrophages to rat advanced osteoarthritis pain resistant to cyclooxygenase inhibitors. Pain 2019; 160:895–907 [DOI] [PubMed] [Google Scholar]
- 41.Marlin MC, Li G. Biogenesis and function of the NGF/TrkA signaling endosome. Int Rev Cell Mol Biol 2015; 314:239–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kc R, Li X, Kroin JS, Liu Z, Chen D, Xiao G, Levine B, Li J, Hamilton JL, van Wijnen AJ, Piel M, Shelly DA, Brass D, Kolb E, Im HJ. PKCδ null mutations in a mouse model of osteoarthritis alter osteoarthritic pain independently of joint pathology by augmenting NGF/TrkA-induced axonal outgrowth. Ann Rheum Dis 2016; 75:2133–41 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol 2008; 85:355–75 [DOI] [PubMed] [Google Scholar]
- 44.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone 2012; 51:204–11 [DOI] [PubMed] [Google Scholar]
- 45.Grässel SG. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther 2014; 16:485. [DOI] [PMC free article] [PubMed] [Google Scholar]