Abstract
Initially being considered as an environmental pollutant, nitric oxide has gained the momentum of research since its discovery as endothelial derived growth factor in 1987. Extensive researches have revealed the various pathological and physiological roles of nitric oxide such as inflammation, vascular and neurological regulation functions. Hence, the development of methods for quantifying nitric oxide concentration and its metabolites will be beneficial to well know about its biological functions and effects. This review summaries various methods for in vitro and in vivo nitric oxide detection, and introduces their merits and demerits.
Keywords: nitric oxide, detection method, nitric oxide synthase, nitric oxide therapy, colorimetric, chemiluminescence, fluorescence, electrochemical, gas chromatography, magnetic resonance imaging
INTRODUCTION
Since its discovery by Joseph Priestly in 1722 as a kind of colorless gas, nitric oxide (NO) was majorly considered as an environmental pollutant. Until 1987, NO was recognized as an important molecule that can regulate endothelial functions in the body. Ignarro et al.1 proved that endothelial derived relaxing factor was NO, thus giving a boost for in depth research on NO. This discovery paved way for later recognization of NO synthesis pathways and its various biological functions. In 1988, L-arginine was found to be the precursor for generation of NO which could be converted to L-citrulline by NO synthases (NOSs) in vivo. Three isoforms of NOSs were named according to their functions and the type of tissues in which they were firstly found. The neuronal isoform was named as neuronal NOS (nNOS, NOS1) found in neuronal cells, inducible NOS (iNOS, NOS2) was found in cells responsible for inflammation, such as macrophages and microglias, while endothelial NOS (eNOS, NOS3) was found in endothelial cells (Figure 1).2 The importance of NO was established over years of research, where NO was involved in the variety of pathological and physiological functions such as endothelial vasorelaxation, cardiovascular functions, antimicrobial action, wound healing, tissue repair, neurotransmission, immune functions, blood pressure regulation, cytotoxicity, relaxation of the human penile corpus cavernosum, etc.1,3,4
Figure 1.
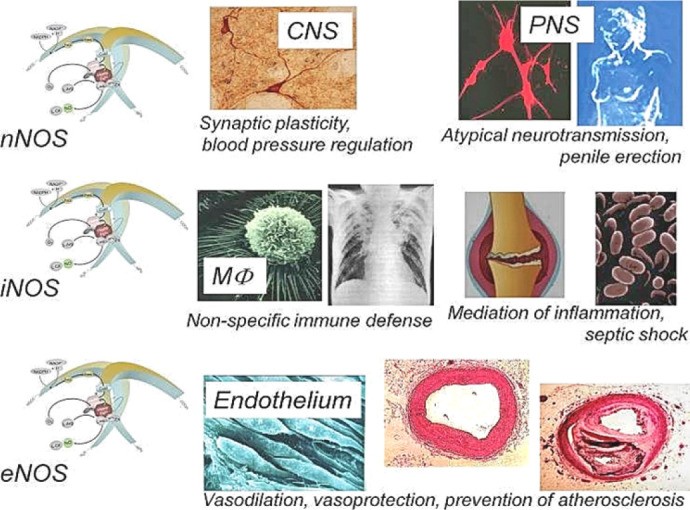
General functions of three isoforms of nitric oxide synthases.
Note: nNOS: Neuronal nitric oxide synthase; iNOS: inducible nitric oxide synthase; eNOS: endothelial nitric oxide synthase; CNS: central nervous system; PNS: peripheral nervous system; MΦ: macrophages. Adapted from Förstermann et al.8
NO is an unstable, highly lipophilic, free radical molecule made up of an unpaired electron.3 It has poor solubility (1.9 mM) in water but relatively higher solubility in lipid membranes and in nonpolar solvents.4,5,6 The diffusion rate of NO is found to be 50 μm/s in a single direction in biological systems.7 The half-life of NO in biological tissues is observed to be only 3–5 seconds, as opposed to 500 seconds in pure aqueous solutions.5 Due to the radical nature of NO, it is readily oxidized into nitrite (NO2–) or even nitrate (NO3–).5 It is difficult to directly measure NO in vivo, and it is frequent to indirectly measure its oxidative products such as NO2– and NO3–.5,7
Decrease and defective release of NO can lead to atherosclerosis generation, coronary vasopasm and restenosis after angiopathy. On the other hand, the increase in NO synthesis can cause hypotension in patients suffering from liver cirrhosis and failure, and also cause hemorrhagic and anaphylactic shock. Endogenous release of NO at basal levels maintains the low resting stage of pulmonary vasculature.3 The use of NO prodrugs and nanomaterials has proved useful to develop promising NO-based therapy. NO gas at a higher concentration range (> nM) is known to exhibit anti-Warburg effect inhibiting tumor occurrence and development. On the other hand, concentration greater than 1 mM can result in NO poisoning, whereas concentration in the range of pM to nM in the tumor cells can promote tumor cell growth.9,10 The use of nanomaterials have helped to overcome the limitations of NO having short half-life, instability during storage and possibility of toxicity. Many types of nanomaterials such as polymers, dendrimers, hydrogels, liposomes, gold and silica nanoparticles, certain quantum dots with NO donors have been exploited in the past under various conditions to release NO. Controlled release of NO from intelligent nanoparticles makes it an attractive tool for cancer therapy due to its tumoricidal effects.11 The vital roles performed by NO in regulating a variety of functions make it necessary for its rapid and accurate determination in vitro and in vivo. Over the past few years, various detection methods including colorimetry, chemiluminescence, fluorescence, electrochemical sensing, gas chromatography, electron spin resonance (ESR) spectroscopy and magnetic resonance imaging (MRI) have been developed to help to understand the pathology and to treat various diseases. The strategy involves searching each of these methods with the proper keywords and listing the various ways employed by researchers to detect and measure NO in vitro and in vivo. This review summarizes these advanced methods and discusses their advantages and shortcomings as following.
COLORIMETRIC METHOD
Colorimetric method for NO detection utilizes the quantification of color change caused by reaction between indicator and NO. NO concentration in vitro can be measured directly by the nitrosation of hemoglobin and myoglobin (Figure 2).12 This method depends on the change of multiple absorption bands and does not need standard curve, exhibiting high sensitivity and accuracy. Besides, Griess assay is one of most general colorimetric methods for in vitro NO detection. Due to its relative simplicity,7 Griess assay has been extensively used since its discovery in 1879 for analysis of biological samples like saliva, urine, serum, cerebrospinal fluid and culture media.13 Griess assay provides an indirect approach for NO detection by measuring nitrite, nitrate and nitrosating agents as an index for NO.7 The two-step diazotization reaction is illustrated in Figure 3. First, dinitrogen trioxide (N2O3) which is obtained from acidified nitrite (or from NO autoxidation) reacts with sulfanilamide to form diazonium derivative diazobenzenesulfonic acid. The derivative then interacts with N-(1-napthyl)ethylenediamine to produce purple colored diazo product azo-α-aminonaphthalene-parabenzene-sulfonic acid which exhibits a strong absorbance at 540 nm.5,7,13
Figure 2.
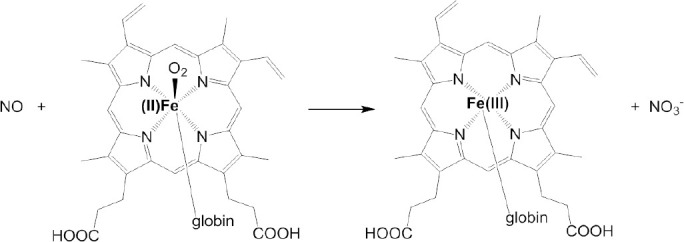
Hemoglobin/myoglobin methods for detection of nitric oxide (NO) concentration.
Figure 3.
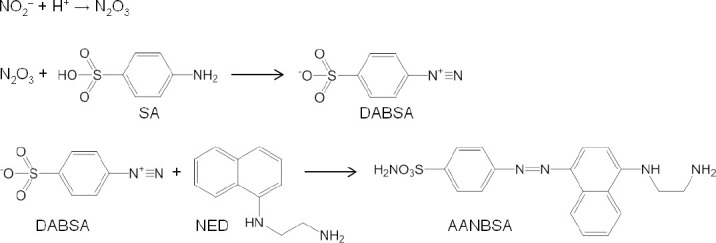
Chemical reaction process involved in Griess assay.
Note: N2O3: Dinitrogen trioxide; SA: sulfanilamide; DABSA: diazobenzenesulfonic acid; NED: N-(1-napthyl) ethylenediamine; AANBSA: azo-α-aminonaphthalene-parabenzene-sulfonic acid.
A few variants of Griess assay have been extensively employed for NO measurement. For example, in conjunction with nitrate reductase, Griess assay has proved to be an accurate, sensitive and inexpensive tool to measure nitrite and nitrate in biological fluids, tissue and serum samples.7,14 In another variant, Griess assay is coupled with microtitre plate assay to determine the kinetics of NOS enzymatic reaction in crude or purified enzyme solutions. The oxidation of ferroheme by NO causes the increase in absorbance and hence can be used to measure the generation of NO.15 Flow injection method was also used to determine NO in vivo when NOS activity affected the acute renal failure in rats by detecting NOx as the final metabolite of NO with a high sensitivity of above 0.5 µM.16 Griess assay can be employed to detect NO2– and NO3– in the extracellular fluid as an index for NO by using Aspergillus nitrate reductase (NADPH, nitrate oxidoreductase), as it can reduce very small amounts of NO3–.17 It can also be used in human plasma cells to detect nitrite and nitrate in the plasma of healthy volunteers with an automated analyzer.18,19,20 Macrophage in Drosophila melanogaster can express NOS and generate NO as an important signaling molecule during its immune response, where Griess assay was used by Ajjuri et al.21 to measure nitrites as an index of NO in the brain tissue of the organism in response to neuroinflammation. Lipopolysaccharide (LPS)-stimulated murine RAW264.7 macrophages produced NO which once readily converted to nitrite could be detected by Griess assay.22 The production of NO as an anti-inflammatory response from LPS-induced macrophages could be measured in Magnolia sieboldii extract,23 Garcinia xanthochymus extracts,24 Sambucus australis,25 Ovis canadensis and Ovis aries,26 peat moss extracts27 via Greiss assay. In addition, Griess assay can also be used to determine NO concentration in plants like cucumber and tomato.28
Colorimetric method provides measurement of NO with good sensitivity and high accuracy with inexpensive tools and is economically feasible.7 However, NO measurement in whole blood with colorimetric method seems to be not feasible because of the disturbance of nitrogen oxide species.29,30
CHEMILUMINESCENCE METHOD
The chemiluminescence method for detecting NO is considered to be a useful technique. The principle of chemiluminescence detection is based on NO-triggered chemiluminescence reactions. Chemiluminescence can be realized by the oxidization of NO with ozone (O3) into nitrogen dioxide in the excited state (NO2*), which emits a photon spontaneously when it decays back to its basal lower energy state (Figure 4A).2,3,7,31,32,33 Another route to chemiluminescence is the use of chemiluminescence agent such as luminol to be excited by highly oxidative peroxynitrite (ONOO–) which is generated by the reaction of NO with H2O2.34 The emission of photon or luminescence is measured by a photomultiplier. Photomultiplier converts this luminescence into electrical signal which is proportional to NO concentration (Figure 4A).35 The high specificity of NO detection is attributed to its unique properties, which includes its ability to exist as a gas and its quick reaction rate with ozone.32
Figure 4.
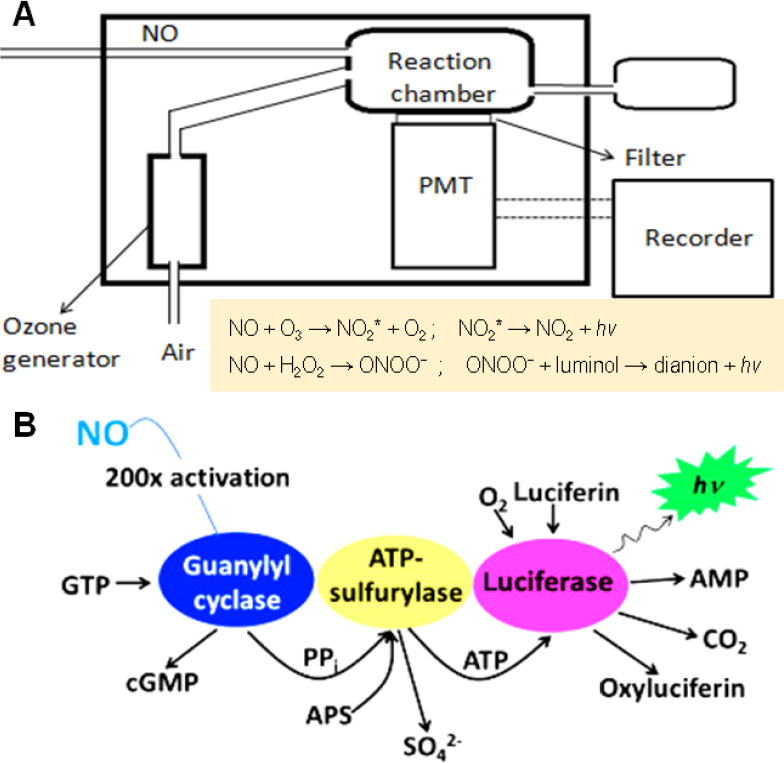
General mode of operation of the chemiluminescence method for detection of NO.
Note: (A) Working model of chemiluniescence method for NO detection. Adapted from Bates,33 Hetrick and Schoenfisch.34 (B) Luciferase-based chemiluminescence method for detection of NO concentration in cell cultures. Adapted from Woldman et al.35 NO: Nitric oxide; PMT: photomultiplier; NO2*: nitrogen dioxide in the excited state; hν: luminescence (h: Planck’s constant, ν: frequency of photon); ONOO–: highly oxidative peroxynitrite; GTP: guanosine triphosphate; cGMP: cyclic guanosine monophosphate; PPi: pyrophosphate; APS: adenosine-5′ phosphosulfate; ATP: adenosine triphosphate; AMP: adenosine monophosphate.
It is extremely difficult to directly detect the NO level in blood. Lopez et al.36 used acidic vanadium III to reduce the intracellular metabolic products of NO including nitrite and nitrate in the collected serum into NO at 98°C, which was then quantified by the chemiluminescence method. Chemiluminescence assay has also proved to be efficient in measuring NO generated by NOS in endothelial cells.7 In the treatment of persistent pulmonary hypertension in newborns and respiratory distress syndrome in adults, exhaled NO levels of asthma patients as an important indicator were also sequentially measured successfully.37 Kikuchi et al.38 measured the continuous release of NO from the isolated perfused rat kidney organ along with changes of perfusion pressure by the luminol-based chemiluminescence method, achieving a high determination limit of approximately 100 fM.
In addition, Woldman et al.35 developed a chemiluminescence method for detection of NO generation in cell cultures by a luciferin–luciferase system (Figure 4B). The activation product (pyrophosphate) of guanylyl cyclase by NO reaction was converted to adenosine triphosphate by adenosine triphosphate sulfurylase which further excited the luciferin–luciferase system to generate chemiluminescence. The luciferin-based chemiluminescence method was proved to be two orders of magnitude more sensitive than fluorescent method using 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) (mentioned in details in the subsequent section) when tested on cell cultures of bovine aortic endothelial cells and activated murine macrophages.
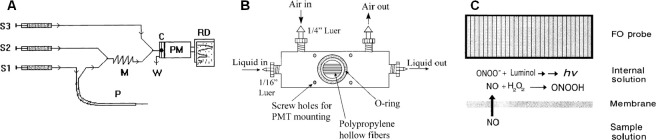
In order to increase the operability and applicable range of the chemiluminescence method, the microdialysis technique based on gas permeable porous membrane is frequently integrated. Yao et al.39 realized in vivo NO measurement with high sensitivity by combining microdialysis technique with chemiluminescence method. Special probe designed allowed the detection of NO in blood and brain tissue of rat and rabbit in spite of the influence of various physiological conditions (Figure 5A). The luminol-based chemiluminescence method was used by Robinson et al.40 to measure NO in exhaled breath. They used a kind of gas permeable porous hollow polypropylene fiber membranes to make gaseous NO react with the solution of luminol and H2O2 in the interface, successfully detecting NO with a limit of 0.3 ppb and a response time of 2 seconds (Figure 5B). Zhou and Arnold41 developed a kind of gas permeable silicone membrane to detect NO concentration in solution by the luminol-based chemiluminescence method, achieving a detection limit of 1.3 µM, a response time of 8–17 seconds, and a dynamic range from 5 to 40 μM (Figure 5C).
Figure 5.
Different ways adopted for monitoring NO levels using chemiluminescence method.
Note: (A) Experimental setup for in vivo NO monitoring. Adapted from Yao et al.39 (B) Schematic representation of hollow fiber gas-liquid exchange module. Chemiluminescence reaction occurs when the gas, which flows along the exterior diffuses through the pores in the fiber membrane and into the luminol/H2O2 solution flowing through the interiors of the fiber. Adapted from Robinson et al.40 (C) Membrane phase and reaction chemistry for the nitric oxide fiber-optics sensor. Adapted from Zhou and Arnold.41 C: Snail shell-like cell; M: mixer; P: microdialysis probe; PM: photomultiplier tube; NO: nitric oxide; S1–3: syringes for different streams; PMT: photomultiplier; ONOO–: highly oxidative peroxynitrite; hν: luminescence (h: Planck’s constant, ν: frequency of the photon).
Chemiluminescence method provides highly sensitive, real time monitoring of NO with rapid and reliable results.7 However, this method is not feasible to monitor NO in vivo. Moreover, the detection can be interfered by ozone gas stream, since the ozone generators are difficult to provide a stable, repeatable gas stream.7 Liquid phase detection was made feasible by the luminol/H2O2 assay, but has low specificity and luminol can react with free radicals causing interference.2 Measurement of exhaled breath can be achieved which can aid diagnosis of respiratory diseases, monitor efficacy of therapies for respiratory diseases.40 On the other hand, NO measurement by this method is limited by its bulky and expensive instruments, time consuming factor, low specificity and sensitivity.2,3,42
FLUORESCENCE METHOD
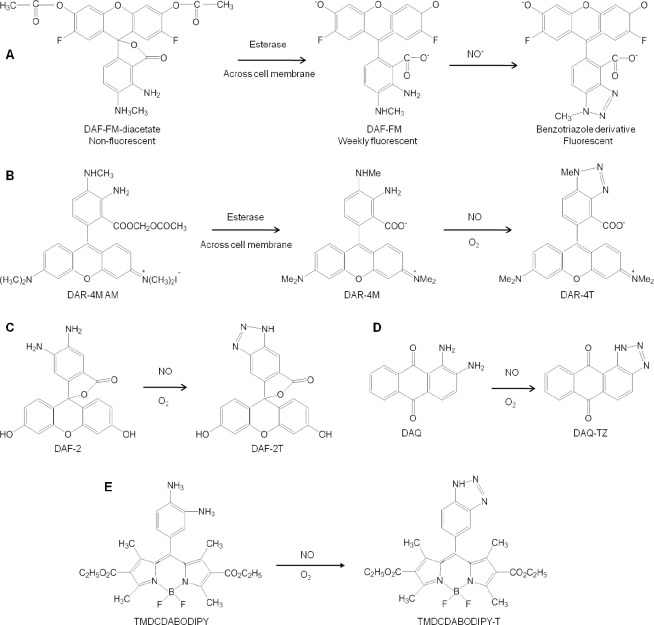
Fluorescence method has emerged as a very useful technique for detection of NO. A number of fluorometric probes and dyes have been devised to exploit the full ability of fluorescence in measurement of NO. The most commonly used fluorescence agent 2,3-diaminonaphthalene (DAN), is exploited for its ability to produce fluorescent N-nitrisating agent 2,3-naphthotriazole from NO (Figure 6A).7,34,42 Another kind of fluorescent agent diaminofluoresceins (DAF) has also proved to be equally useful for detection of NO (Figure 6B). Spatial and temporal aspects of NO production have been monitored using fluorescent probes which mainly involve DAF, DAN and some other fluorescent agents discussed below.34
Figure 6.
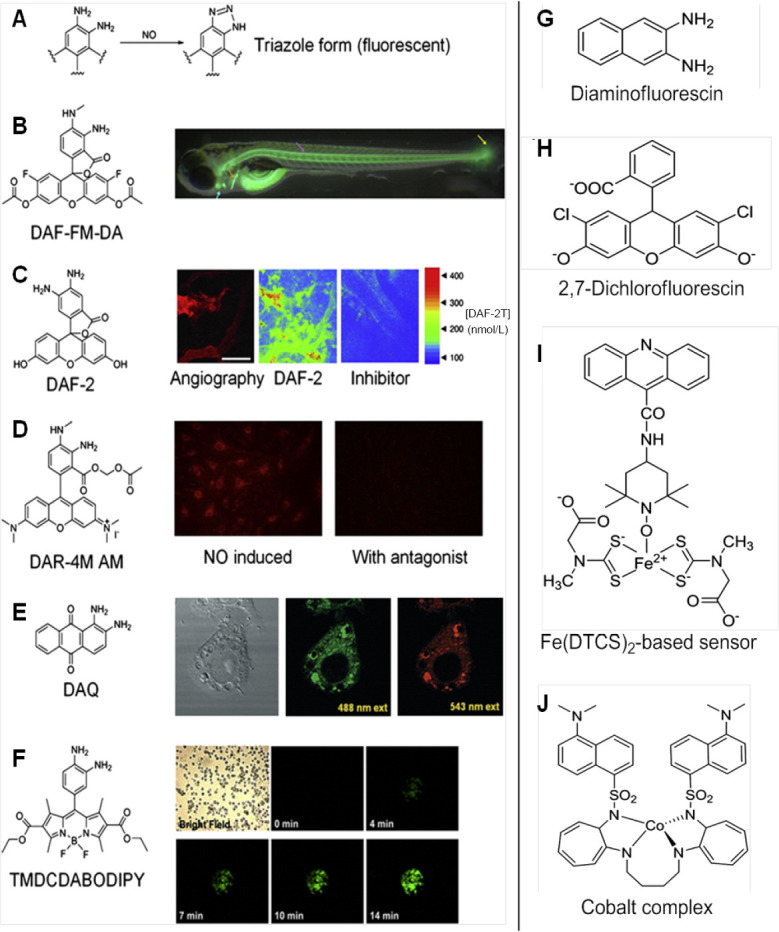
Various fluorescent compounds used for imaging of NO.
Note: (A) 2,3-Diaminonaphthalene after reaction with nitric oxide (NO) coverts into fluorescent triazole form. Adapted from Hong et al.2 (B) Overview of Danio rerio (Zebrafish model) indicates the fluorescent labeling of organs with Caudal fin (yellow), the notochord (purple), the cleithrum (orange) and the heart (blue). Adapted from Lepiller et al.53 (C) NO distribution in B16-F10 tumors grown in the cranial window in mouse, left: microangiography using tetramethylrhodamine-dextran; middle and right: pseudo-color representation of DAF-2T microfluorgraphs. Color bar represents the calibration of the fluorescence intensity with the known concentrations of DAF-2. Adapted from Kashiwagi et al.50 (D) Left: fluorescent image of DAR-4M loaded endothelial cells at 10 minutes after stimulation; right: fluorescence intensity strongly abolished by treatment with the agonist of the stimuli. Adapted from Kikuchi et al.56 (E) DAQ loaded cells and stimulated to produce NO imaged using confocal laser fluorescence microscopy, left: bright-field, middle: 488 nm excitation; right: 543 nm excitation. Adapted from Galindo et al.59 (F) Bright field and fluorescence images of activated PC12 cells loaded with 1,3,5,7-tetramethyl-2,6-dicarbethoxy-8-(3′,4′ -diaminophenyl)-difluoroboradiaza-s-indacence (TMDCDABODIPY) in the presence of arginine. Adapted from Chen et al.65 (G–J) Agents used for fluorescent based NO-sensing. Adapted from Hong et al.2
DAN has been employed as an indicator for NO detection in many studies. In 2002, Wada et al.43 developed a high performance liquid chromatography method to determine the concentration of NO in cultivated cells of plant Agavepacifica using fluorescence detection with DAN. This method successfully detected NO with the limit of 3.4 pmol/g cells.43 In 2003, Gharavi et al.44 employed similar fluorometric high performance liquid chromatography detection for one of the stable oxidation products of NO, i.e., nitrite in murine hepatoma cells. Fluorescence monitoring provided limit of detection of nitrite to be 10 pM. Similarly, DAN assay was used to determine the endothelial NO release from cultured porcine pulmonary artery endothelial cells by measuring the fluorescence from 1-(H)-naphthotriazole which was formed by the reaction between DAN and nitrite. However, DAN assay failed to detect NO in agonist stimulated cultured cells. When the stimulated pulmonary artery endothelial cells were treated with nitrate reductase, DAN assay was able to detect minor levels of NO generated by these cells. Hence, these results suggested that nitrate reduction was essential for the function of DAN.45
Other agents like 2,7-dichlorfluorescin, cobalt complex, Fe(DTCS)2-based sensor did find their application in fluorescent based NO-sensing, however were limited due to poor detection sensitivity, low specificity for NO, fluorescence from interference, etc. (Figure 6G-J).2 In order to measure NO under physiological conditions, Kim et al.46 prepared a novel set of fluorescent indicators, which are popularly known as DAFs. Since its discovery, DAF has been used in various studies for detection of NO. DAFs interact with NO in the presence of dioxygen to yield the triazole derivatives which are highly fluorescent. DAFs generate less fluorescence than the triazole derivatives by 180-fold.The probe most commonly employed uses DAF-2 form which exhibits high fluorescence due to formation of triazofluorescin upon reaction with oxidation product of NO. Given this property of fluorescence, DAF-2 has been employed to measure NO in various settings (Figure 7C). The membrane permeable form of DAF-2, DAF-diacetate was utilized to measure NO in endothelial cells by Leikert et al.47 Langendorff perfused rabbit heart was used by Patel et al.48 to monitor the change ofNO concentration using DAF-2. Similarly, Strijdom et al.49 demonstrated the use of DAF-2-DA to detect intracellular NO in fresh adult rat cardiomycetes by flow cytometry and compared the results with cellular nitrate or nitrite level. Use of DAF-2 as an NO sensitive probe revealed the release of NO around the blood vessel wall and sporadically in the extracellular space of B16 melanomas using multiphoton laser-scanning microscopy. However, in the presence of inhibitors, the fluorescence appeared abolished. Along with the immunohistochemical analysis, eNOS was found to be predominant source of NO in vascular endothelial cells, whereas iNOS was the sporadic source of NO in the stromal cells of B16 melanomas (Figure 6C).50
Figure 7.
Reaction mechanisms of different fluorescent probes for nitric oxide (NO) detection.
Note: (A) 4-amino-5-methylamino-2’,7’-difluorofluorescein diacetate (DAF-FM-DA). Adapted from Xie and Shen.69 (B) Diaminorhodamine-4M acetoxymethyl ester (DAR-4M AM). Adapted from Gomes et al.70 (C) Diaminofluorescein-2 (DAF-2). (D) 1,2-Diaminoanthrauinone (DAQ). (E) 1,3,5,7-Tetramethyl-2,6-dicarbethoxy-8-(3' ,4' -diaminophenyl)-difluoroboradiaza-s-indacence (TMDCDABODIPY).
Another form of DAF, 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM-DA) (Figure 7A) was used by Metto et al.51 to detect NO produced in single T1 lymphocytes (Jurkat cells) with the help of a microfluidic device. Standard set of cells was obtained by labeling them with 6-carboxyfluorescein diacetate. Immune cells expressed iNOS upon stimulation by LPS when compared with control set of cells, which exhibited two-fold increase in NO production.51 Recently, Agrawal et al.52 targeted overexpressing NOS isoform of HEK 293 T cell line imaging to screen the capacity of NOS inhibitors using DAF-FM-DA. Lepiller et al.53 employed DAF-FM-DA to detect NO generation sites in a living zebrafish Danio rerio model. The authors observed that NO production changed along the development in the notochord caudal fin. However, no changes were seen in the bulbus arteriosus. This method was also employed by the group to measure local changes in NO production in response to any stress. From their findings, the authors monitored changes in NO production in live zebrafish under physiological and pathophysiological conditions (Figure 6B).53
Zhou et al.54 previously showed measurements of NO in intact venules by platelet activating factor induced endothelial cells. However, the leakage of the DAF-2 dye after the washout steps compromised the accurate measurement of NO. Hence in 2011, to overcome the dye retention problem and improve the sensitivity for NO measurements, the same group used DAF-2-DA in rat venules. Continuous perfusion of DAF-2-DA in the rat venules was used to measure NO by fluorescence imaging under basal and stimulated conditions. Once DAF-2 achieved a stable state in the endothelial cells, basal and stimulated NO was quantified. This study also showed that, the measurement of fluorescence was mainly due to the hydrolyzed DAF-2 in the cells. With the help of this, NO can be assessed by subtracting non-NO-dependent intracellular DAF-2 in living tissues.
Furthermore, in order to overcome the limitations of using DAF-2 in biological applications, in 2001, Kojima et al.55 developed a fluorescent indicator on rhodamine chromophore DAR-4M AM (Figure 7B) for detection of NO. The membrane permeable property of DAR-4M AM was successfully employed by the authors to carry out bioimaging in bovine aortic endothelial cells for detection of NO with a limit of 7 nM without any pH dependency above pH 7. Human umbilical vein endothelial cells when treated with platelet-activating factor (PAF) could help to determine its effect on NO production. The produced NO was measured using DAR-4M AM and visualized using fluorescence microscopy and also revealed that in the presence of NOS inhibitor and PAF-receptor antagonist, NO production was not achieved, hence confirming the role of PAF in intracellular NO generation by activation of PAF-receptors in the human umbilical vein endothelial cells (Figure 6D).56 Recently, another rhodamine-deoxylactam based probe was developed and used to detect endogenous and exogenous NO in living HepG2 and RAW 264.7 cells. Once the probe came in contact with NO, it emitted a strong fluorescence which aided the easy detection of NO with high specificity.57
Externally stimulated and non-stimulated RAW 264.7 macrophage cells were treated with 1,2-diaminoanthraquinone (DAA or DAQ) (Figure 7D), a non-toxic probe known for its ability to visualize NO in living cells. Its reaction with intracellular NO in the presence of oxygen generated the triazole form of DAA, which could be spectrally visualized using confocal microscopy and fluorescence spectroscopy (Figure 6E).58,59 The NO reductase property of cytochrome P450 55B1 from Chlamydomonas reinhardtii was exploited by Li et al.60 in 2016 to develop a new fluorescence biosensor for detecting NO. The constructed biosensor was employed to detect NO release from L-arginine stimulated rat liver homogenate. The authors concluded that this biosensor can be successfully used for detection of NO in biological samples. Lim et al.61 synthesized a fluorescent probe with a copper Cu(II) complex and metal chelating ligand in 2006. The irreversible reduction of Cu(II) to Cu(I) along with the release of nitrosated ligand was determined to be the reaction causing NO induced fluorescence by spectroscopic and mass spectroscopic methods. The Cu(II) based complex was employed by the authors to detect NO generated in macrophages and live neurons by inducible and constitutive NOSs (iNOS and cNOS). MNIP-Cu {Copper derivative of [4-methoxy-2-(1H-napthol2-3-d]imidazol-2-yl)phenol]} was developed by Jain et al.62 to localize NO generation sites in various parts of sunflower (Helianthus annuus L.) almost without lag time which was found to be more specific than DAF probes.
Liu et al.63 designed ADNO (2-(α-(3,4-diaminophenoxy)acetyl)-6-(dimethylamino)naphthalene) a new two photon fluorescent probe in 2014 (Figure 8A). This probe detected NO on the basis of photoinduced electron transfer mechanism. The NO sensitive fluorescence modulator function of the probe is carried out by o-phenylenediamine moiety and 2-acetyl-6-(dimethylamino) naphthalene moiety functions as the two photon fluorophore. The electron-rich diamine moiety can be altered to quench the fluorescence of 2-acetyl-6-(dimethylamino) naphthalene by transferring the electron to excited fluorophore which can help to detect NO as anticipated by the authors. The experiment carried out in aqueous solution and NIH 3T3 cells presented that ADNO has rapid response rate to NO by exhibiting significant turn-on fluorescence. This probe was found to be less pH dependent and showed high selectivity and can be very useful tool in future biological applications.
Figure 8.
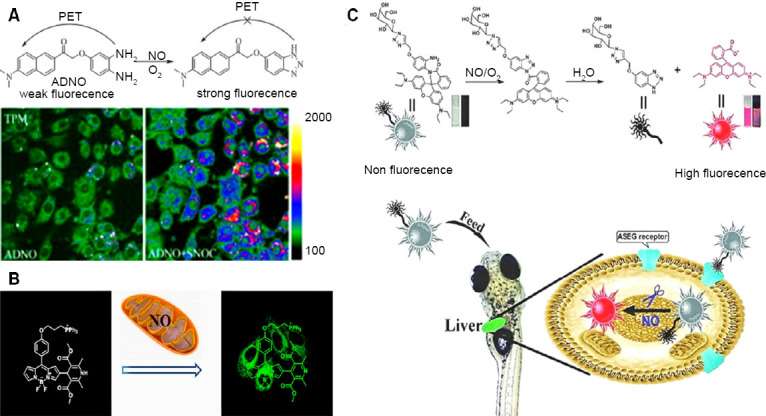
Various fluorescent probes employed with different strategies to detect NO.
Note: Above: design strategy of ADNO probe; below: two-photon microscopy image of NO in ADNO-labeled NIH3T3 cells and ADNO-labeled cells treated with SNOC (S-nitrocysteine, a NO donor). Adapted from Liu et al.63 (B) Mito-DHP probe specifically targeting mitochondria for endogenous and exogenous produced NO by stimulated RAW264.7 murine macrophage and HepG2 cells, respectively. Adapted from Gao et al.66 (C) Gal-RhB sensor yielding fluorescent image of hepatocellular NO in Zebrafish. Adapted from Zhang et al.68 ADNO: (2-(α-(3,4-diaminophenoxy)acetyl)-6-(dimethylamino)naphthalene); NO: nitric oxide; Gal-RhB: a hepatocyte targeting fluorescent sensor.
Recently, various modifications are done to develop better fluorescent probes. For example, Zhang et al.64 developed a dual turn on type BODIPY probe with no intrinsic fluorescence due to photoinduced electron transfer effect. This probe could detect NO and nitrite ion in HepG2 cells with fluorescence confocal microscope under neutral and acidic conditions respectively with DEA-NONOate as the NO donor; (which switches off the photoinduced electron transfer) in assistance with intracellular cysteine and glutathione. Similarly, Chen et al.65 developed a BODIPY probe which could simultaneously detect endogenous and exogenous NO and glutathione in macrophage cells to uncover their inter-relation for maintaining the biological system’s redox balance (Figure 6F). Another modification in the BODIPY dye was done by Gao et al.66 by adding dihydropyridine and triphenylphosphonium moieties to it which could specifically target mitochondria (Mito-DHP probe), tracking the exogenously produced NO in HepG2 cells and endogenously produced NO in stimulated RAW264.7 murine macrophage cells under anaerobic conditions (Figure 8B). 1,3,5,7-tetramethyl-2,6-dicarbethoxy-8-(3 ',4' -diaminophenyl)-difluoroboradiaza-s-indacence (TMDCDABODIPY) (Figure 7E) was developed by Huang et al.67 which allowed the real-time NO imaging using inverted fluorescence microscope in human umbilical vein endothelial cells, Sf9 cells and PC12 cells when treated with L-arginine. Thus, this combination of the probe with inverted fluorescence microscope could provide selective and sensitive detection of NO released from cells. A hepatocyte targeting fluorescent sensor (Gal-RhB) was developed by Zhang et al.68 in 2018 (Figure 8C). The cellular and in vivo imaging using HepG2 and Zebrafish using this sensor allowed detecting NO about 1.26 nm with good selectivity and sensitivity making it an obvious choice for liver diseases caused due to NO deficiency or surplus amount.
Overall, each type of fluorescent probe has its own limitations and advantages. DAN being highly specific and sensitive shows cytotoxic effects, has slow reaction time,71 requires acidic conditions, not suitable for NO imaging and may damage living cells.2 DAR has high photostability and pH independence being unsuitable for in vivo applications.71 Copper based fluorescent probes have proved to be beneficial owing to its cell membrane permeability, detection of NO under physiological conditions, independence to oxygen allowing imaging under hypoxic conditions without interference.2 However, they are poorly stable in vivo, do not allow in vivo imaging due to suboptimal emission wavelength and may cause potential toxic effects. MNIP-Cu fluorescence is independent of oxygen, and simple synthesis method allows detection of NO in whole tissues, cellular and sub-cellular levels in plants.62
ELECTROCHEMICAL METHOD
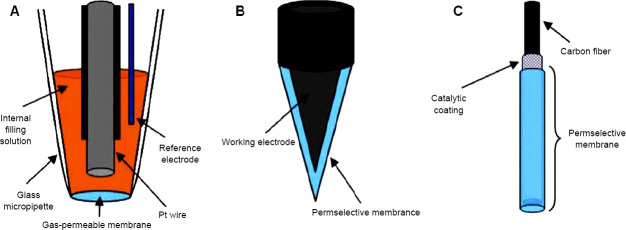
Use of various electrodes for electrochemical detection of NO in vitro and in vivo is widely employed. The choice of electrode is of great importance for detection of NO since the electrochemical process takes place at its surface and its quality influences the charge transfer process between target analyst and electrode material. Carbon and noble metal electrodes are most commonly used for NO detection. Other types of materials used for making the electrodes are described in Table 1 and Figure 9.72 Efforts are being made continuously by researchers to modify electrode for better measurement of NO in various settings. This section will focus on such electrodes and their application for NO detection.
Table 1.
Types of basic electrodes
| Type of electrode | Material |
|---|---|
| Clark | Platinum wire (working electrode, silver wire (reference electrode), inserted in glass micropipette |
| Carbon fiber nitric oxide microelectrodes | pyrrole-functionalized porphyrins, containing metals such as Ni, Pd, and Mn, immobilized on their surfaces via oxidative polymerization, or coated with iron porphyrin, with or without unmetalled porphyrin electrodes along with Nafion layer |
| Integrated nitric oxide microelectrodes | Carbon fiber electrode combined with separate integrated Ag/AgCl reference electrode, covered with proprietary gas permeable, shielded with high performance faraday layer. Platinum or Iridium wire can be used instead of carbon fiber electrode. |
Note: Adapted from Serpe and Zhang.75
Figure 9.
Types of basic electrodes.
Note: (A) Clark’s type electrode, (B) whole solid type electrode, (C) composite electrode. Adapted from Bedioui et al.74
The basic of NO detection using amperometric method is the current generated on the surface of electrode due to oxidation of NO. A typical system for NO detection includes use of working (coated with platinum or Teflon) and reference electrodes (Ag/AgCl) immersed in solution containing NO. The application of positive potential (800–900 mV) causes NO to be oxidized at the surface of working electrode by generating a redox current. The oxidation occurs via electrochemical reaction followed by chemical reaction. One electron transfer from NO molecule to electrode constitutes the electrochemical reaction which generates nitrosonium ion (NO+). This cation reacts with OH– to form nitrite (NO2–) which further gets oxidized to nitrate. NO meter measures the amount of NO oxidized which is proportional to the current flow between working and reference electrodes. An internal electrolyte is enclosed in the NO gas selective permeable membrane of the working electrode. Current due to oxidation gets generated at the surface of working electrode when NO gas passes through the membrane and the electrolyte. However, this method generates redox potential as low as 1–10 pA in biological system. This justifies the need to identify better electrodes to overcome this limitation. ISO-NO Mark II system allows the detection of redox current as low as 0.1 pA. This instrument could measure NO without the need of special electrical screening, ex.Faraday Cage. The development of optically isolated Apollo-4000 has paved the way for detection of NO and other free radicals.73
The use of NO specific electrodes has seen a successful graph in the recent past for monitoring NO in vitro and in vivo. In 1990, NO release stimulated by activation of specific neural elements in brain tissue was measured by inserting an electrochemical microprobe into the molecular level of rat cerebellar slice with a range of 8–58 nM by Shibuki.76 This was followed by detecting NO release in real time in the hemoglobin free effluent of guinea pig isolated hearts by Fujita et al.77 in 1998. The amount of released NO was measured by comparing the effects of several vasodilator drugs on altering the coronary flow. The same group used Clark type electrode with a selective permeable membrane to measure effluent NO release and bradykinin induced coronary flow in anesthetics treated crystalloid perfused guinea pig hearts. Development of microcoaxial electrode by Kitamura et al.78 permitted continuous and direct detection of NO in bovine aortic cultured endothelial cells stimulated by acetylcholine and inhibited NG-nitro-L-arginine methyl esterin the range of 0.1–1.0 µM. They suggested that future application of this electrode could allow measurement of intracellular NO in vitro and in vivo because of the coaxial arrangements of the working and reference close together.
A few of researchers applied the NO microelectrode to determine the changes in NO concentration in rat superior mesenteric artery by using certain stimulators and attenuators, acetylcholine and NG-nitro-L-arginine. The effect of the stimulator and attenuator allowed the authors to conclude that contractility of rat superior mesenteric artery is majorly regulated by endothelium derived NO.79 In a similar manner, variety of studies are carried out under various stimulators and attenuators to determine the changes in level of NO in rat superior mesenteric artery,80 renal hypertensive rat superior mesenteric artery with impaired endothelium dependent vasorelaxation.81 Platinum based NO sensor was used by Griveau et al.82 in 2006 to detect NO in tumor bearing mouse. In 2008, real-time detection of NO in tobacco cell suspension treated by cryptogein was studied by Besson-Bard et al.83 using NO sensor. The increase in NO concentration due to cryptogein was evaluated in extracellular environment given its role in intracellular environment.
Brown et al.84 studied the reaction of NO with metallophthalocyanine (MPc) surface coated electrodes which lead to initial electron transfer from NO to MPc, further resulting in electron to relay to electrode charge sink, hence producing current response. The transient NO+ is assumed to be stabilized by MPc before its oxidation to nitrite. Hence, NO signal was amplified and lowered potentials for voltammetric features. Reduced potential for NO oxidation supposedly caused amplification of sensitivity. Selectivity was improved when applied potential was lowered only with high potential interferences such as nitrites. Signal from low potential intereferents such as amino acid was found to be not affected. However, they did hamper continuous detection of NO with good selectivity. As a result, the only feasible way for MPc complexes to improve NO selectivity was via the amplification of signal sensitivity. However, by comparison to the permselective membrane, the use of MPc complexes for improved selectivity is achievable but currently limited.
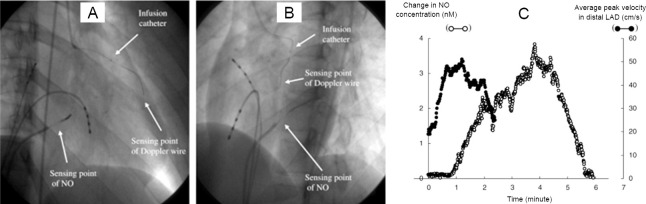
Catheter type NO sensor was employed for real-time NO measurement in the coronary circulations in humans diagnosed with dilated cardiomyopathy.85 The NO sensor was inserted in the great cardiac vein (Figure 10), and measurement of plasma NO level along with average peak velocity were carried out under the influence of acetylcholine and NG-monomethyl-L-arginine. This study demonstrated the clinical application of NO sensor for evaluating endothelial functions in patients with heart condition. They claimed that this sensor can be further employed for diagnostics and therapeutic applications in the future.
Figure 10.
X-ray pictures with the position of Doppler wire, nitric oxide sensor and infusion catheter.
Note: (A) Right anterior oblique (RAO) 30 μg acetylcholine, and (B) left anterior oblique (LAO) 60 μg acetylcholine. 7-Fr Amplatz guiding catheter was used to place the detection of nitric oxide (NO) sensor in the great cardiac vein from the femoral vein. A coronary-infusion catheter was positioned distal to the first major septal branch on the other hand Doppler guide wire was positioned distal to the infusion catheter to monitor coronary flow velocity.(C) NO concentration displayed in real-time profile and averaged peak velocity in the distal left anterior descending coronary artery (LAD). Adapted from Takarada et al.85
Scanning electrochemical microscopy was coupled with amperometric NO nanosensor by Jo et al.,86 allowing the real-time imaging of NO releasing sites in nNOS immunoreactive cells which comprise the NO releasing sites of the living mouse brain. The authors successfully provided in vivo 2-dimensional images along with 3-dimensional NO distribution data in the living brain with high sensitivity and spatial resolution. Apart from its long data acquisition time, this technique also enlightened the relationship between NO measurements, the size or location of nNOS immunoreactive cells and immunoreactive intensity. A hemin-functionalized graphene field effect transistor sensor constructed by Jiang et al.87 was used on macrophage and endothelial cells which provided rapid response time, high specificity and selectivity of generating NO signal with high spatiotemporal resolution. NO selective electrode allowed robust and reproducible detection of subtle changes in NO concentration produced by cultured pulmonary myofibroblasts of rats under wide variety of conditions.88 Recently, amperometric NO release was viewed in real time at the stimulated acupoints in rats inserted with an acupuncture microsensor needle made up of gold film and doped with iron-porphyrin functionalized graphene composite.89 Further, the authors claimed the application of the acupuncture needle to detect other vital signaling molecules (Figure 11).
Figure 11.
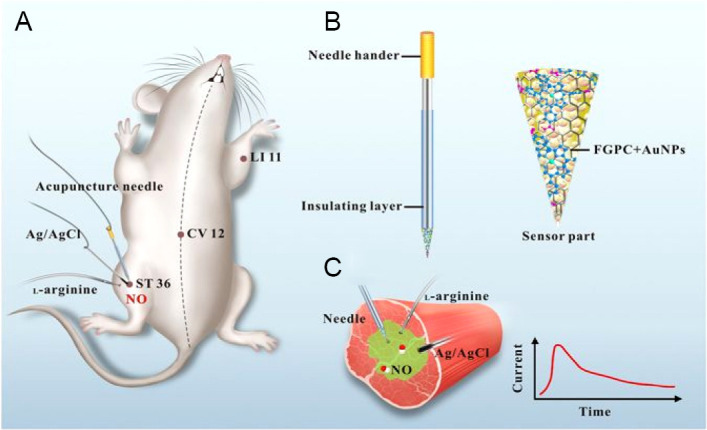
Schematic representation of real-time monitoring of NO at acupoints of rats using acupuncture microsensor needle.
Note: (A) Microsensor needle measuring NO at the acupoints of rats in real time, (B) FGPC/AuNPs/acupuncture needle, (C) real-time NO measurement in acupoint ST 36 (Zusanli) stimulated by L-arginine. Adapted from Tang et al.89 NO: Nitric oxide; FGPC: iron-porphyrin functionalized graphene composite; CV 12: Zhongwan; LI 11: Quchi.
These electrochemical sensors are known to have a response time of few seconds with good sensitivity; real-time monitoring and can measure the slight change on NO concentration.71 Electrode modification and/or the applied potential allow the enhancing of selectivity and sensitivity.34 In general, electrochemical electrodes allow detection of NO in biological samples due to their small size and at low cost.42 However, the measurement is affected by gas interference and may provide inaccurate results.3,34
GAS CHROMATOGRAPHY METHOD
The use of gas chromatography for NO detection in vitro and in vivo is currently limited. There is very less data determining NO levels using gas chromatography due to its limited life span. Hence, gas chromatography is employed to measure NO in its different converted forms. The use of nitrite and nitrate as a surrogate to measure NO is commonly employed. Gas chromatography was used to detect oxides of nitrogen in gaseous mixtures like Diesel exhaust gas using argon ionization detection cell. Samples were prepared to measure NO and argon mixture and compared their chromatogram with NO and air mixtures. With the sample size of 0.1 to 1 µL, the increase in retention time is observed with the decrease of NO concentration. Hence, retention time being the function of NO added, it was demonstrated by authors that it might be possible to use this measurement for analytical purposes.90
GC-MS method was freqxuently used to measure nitrite and nitrate for detection of NO. In vivo studies revealed that administration of paracetamol caused the temporary increase in the plasma nitrite concentration which may indicate that eNOS activity and/or expression of eNOS increased after few hours of paracetamol administration. On the other hand, proliferating in vitro adult rat hepatocytes exhibited weak inhibitory effect in response to paracetamol.91 Implications of recent research revealed that NO is responsible for cavernous arterial and trabecular smooth muscle relaxation. Hence, GC-MS was employed by Becker et al.92 to measure nitrite and nitrate as a determinant of NO metabolites in healthy men and patients with erectile dysfunction under different functional conditions of penis.
ELECTRON PARAMAGNETIC RESONANCE/ELECTRON SPIN RESONANCE SPECTROSCOPY METHOD
Electron paramagnetic resonance (EPR) or ESR has been widely employed for characterizing free radicals with unpaired electrons without need of any interference.4,7 Due to unpaired electrons, the free radicals become very reactive and unstable at room temperature. The reactive nature of free radicals and short-life make them difficult being detected. Hence, spin trapping method of EPR was developed. The basic principle of spin trap method involves the use of specific compound to react with short lived radicals to convert them to stable radicals in order to be characterized by EPR. These special compounds are termed as spin trap reagents. A variety of such reagents are currently being used to react with NO in biological systems since it also contains unpaired electron thereby converting it to a stable form to be further characterized using EPR spectroscopy.
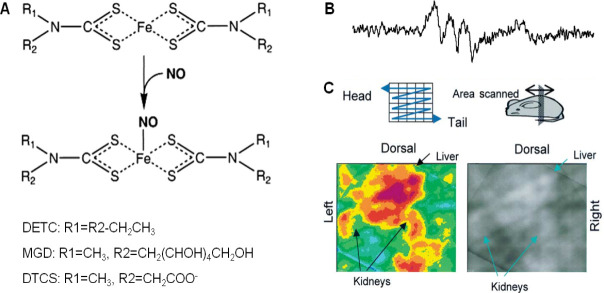
The most commonly employed spin trap reagents for NO detection are variants of iron (Fe) complex with dithiocarbamate derivates (Figure 12A) since NO has high affinity for them and the resulting stable complexes allow endogenous NO measurements in vivo. The different types of these complexes are named as diethyldithiocarbamate (DETC) (water insoluble), N-dithiocarcoxy sarcosine (DTCS) (ligand, water soluble), N-methyl-D-glucamine dithiocarbamate (MGD) (water soluble),di(N-(dithiocarboxy)-N-methyl-L-serine (MSD), etc.93,94
Figure 12.
Use of various spin traps with different strategies to detect NO.
Note: (A) Different types of iron (Fe) complex with dithiocarbamate derivates (DTC) reaction with nitric oxide (NO). Adapted from Tsuchiya et al.95,96 (B) In vivo characteristic 3-line electron paramagnetic resonance spectrum of NO-Fe(DTCS)2 from the upper abdominal area of a lipopolysaccharide (LPS) treated mouse. Adapted from Hirayama et al.97 (C) 3-Dimensional tiled image of the abdominal area from an LPS treated mouse. Adapted from Hirayama et al.97 DETC: Diethyldithiocarbamate; MGD: N-methyl-D-glucamine dithiocarbamate; DTCS: N-dithiocarcoxy sarcosine.
Hirayama et al.97 carried out NO measurement in live LPS treated mice kidney by using iron and N-dithiocarboxy sarcosine complex (Fe-DTCS)2. NO was spin trapped as NO-Fe(DTCS)2 and detected using EPR spectroscopy which resulted in characteristic 3-line EPR spectrum (Figure 12B). EPR spectroscopy was also performed on organ homogenates confirming NO distribution in upper abdominal area, renal and hepatic areas (Figure 12C). Similar spin trap was employed to detect NO in melanoma tumor in mice and live mice liver.98,99 NO was detected by EPR spectroscopy using Fe-(DETC) in upper abdomen of living septic shock mice,100 brain of septic shock mice,101 kidney of ischemia-reperfusion rats,102 forebrain of ischemia rats,103 in rabbit vascular tissue.104 NO in the blood circulation of conscious mice was detected by EPR spectroscopy using Fe-MGD complex which reacted with NO to form [(MGD)2-Fe2+-NO] complex. The S-band EPR spectrometer resulted in the characteristic 3-line spectrum in the blood circulation of mouse tail administered with sodium nitroprusside as NO donor.105 Furthermore, the same complex was also used to detect the NO distribution in mice in vivo.106 Four kinds of dithiocarbamate iron complexes were synthesized by modifying the functional group of ligand and used as a spin trap to measure NO in LPS-treated mouse liver and blood. The newly synthesized complexes proved to be good traps for NO in vivo. Low frequency ESR spectroscopy data was obtained using Fe(MSD)2, while the other two complexes [Fe(DTCP)2, Fe(DTCTP)2] along with Fe(MSD)2 were measured in blood in ex vivo experiments. A unique property was exhibited by the fourth complex Fe(DTCMP)2 by giving NO adduct only in blood, reflecting the amount of NO in the blood.107
NO performs numerous functions in plants, including pollen tube growth, root development closure of stomata, defense genes activation, etc. Being a diffusible gas, its presence in the intracellular and extracellular spaces is inevitably allowing it to react with the surrounding environment. Nodule infected tissues contain leghemoglobin which is rich in Fe2+. The Lb2+ NO nitrosyl complex formed when NO binds to the iron radical can thus be detected using EPR spectroscopy. This was performed in the nodules of soybean as a direct method to detect NO.108 Mice infused with nitrite was induced with cardiopulmonary arrest and subjected to EPR resonator. The EPR spectra achieved was confirmed to be due to the labeled infused nitrite rather than the endogenous tissue nitrite or from enzymatic reactions. The results were compared with control mice, infused with just saline. The NO generation was found to be increased in mice with cardiopulmonary arrest infused with nitrite; NO mainly found in heart, liver and lungs. This study further proved the NO generation due to tissue ischemia by direct reduction of tissue nitrite during the ischemic acidic and reduction conditions.109
However, there are certain limitations using these spin traps. MGD and DTCS need to be prepared just before being administered, since they are unstable, especially in acidic solutions.110 Also high concentration of iron complexes might be toxic, and oxidant formation of the complexes is possible which might affect the system being studied. Toxicity of the traps might be system specific depending on their concentration. Furthermore, less water soluble DETC species are unsuitable for NO trapping in vivo even though it can pass the cell membrane and blood brain barrier.110 Nonetheless, EPR technique qualifies as a valuable method to identify free radicals in complex systems along with providing important details regarding the structure, mobility, interactions and kinetics of the radical,111 but is limited due to high cost of instrumentation and time consumption to prepare sample.2
MAGNETIC RESONANCE IMAGING METHOD
There are very limited literatures on using MRI modality to detect NO. However, efforts are made to employ EPR spin trap agents which detect NO to be further imaged using MRI (Figure 13A and B). Considering this as a powerful tool, MRI enables imaging, visualizing and localizing NO generation sites with the use of specific contrast agents.
Figure 13.
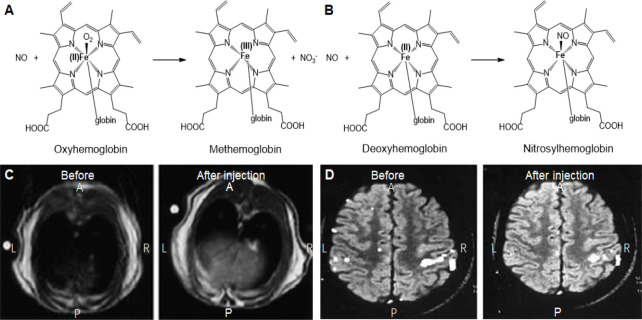
General reaction of hemoglobin and deoxyhemoglobin with NO and MR imaging of NO in liver and brain.
Note: (A, B) Reaction mechanisms of oxygenated (A) and deoxygenated (B) hemoglobin with nitric oxide (NO) for generation of paramagnetic methemoglobin and nitrosyl hemoglobin for magnetic resonance imaging. (C) T1 weighted magnetic resonance imaging of the axial pane of Wistar rat’s liver before injection and 60 minutes after injection of spin trap of (MGD)2-Fe(II)-NO. Adapted from Fujii et al.112 (D) Functional magnetic resonance imaging of healthy subject’s brain before and after intravenous administration of ascorbic acid. Adapted from Di Salle et al.113 MGD: N-methyl-D-glucamine dithiocarbamate; A: anterior; P: posterior; L: left; R: right.
Fujii et al.112 used this concept and combined NO spin traps with MRI to measure its distribution in rats with septic shock. Adult male Wistar rates were administered with (MGD)2-Fe(II)-NO spin trap and LPS was used to induce NO. EPR measurements were carried out in vivo and in vitro followed by MRI. NO signal from excised organs was highest in liver (Figure 13C) followed by heart and kidney and very little quantity in brain. The NO spin trap complex was determined to be stable in complex tissues and organs. The paramagnetic properties of the spin traps causes strong proton relaxation enhancement. The spin-spin and spin-trap relaxation of the water protons is stimulated by the magnetic moment of unpaired electrons, which causes the decreases in the T1 and T2 relaxation times, thus enabling the enhancement of signal intensity in T1 and T2 weighted MR images. The relaxivity was found to increase distinctly once NO complexes with (MGD)2-Fe(II) which can enable to view the regions of NO entrapment in vivo. The authors named this technique as nuclear magnetic resonance-spin traps and mention that this method can be extended for imaging other free radicals in vivo with suitable spin trap agents. Reaction of NO with oxygenated and deoxygenated hemoglobin generates paramagnetic methemoglobin (Figure 13A) and nitrosylhemoglobin (Figure 13B) species which are known to significantly cause concentration dependent changes in signal intensity in erythrocytes when imaged using MRI. Healthy male volunteers were administered with regional brain activator ascorbic acid specifically reduced methemoglobin (which measured by EPR) resulting in a significant decrease of functional MRI signal changes thereby suggesting the role of reductant on blood flow independent effect. These signal intensity changes can be attributed to NO generation during brain activation (Figure 13D).113
The paramagnetic chemical exchange saturation transfer (PARACEST) effect is irreversible disappearance from amide and appearance for amine when observed under MRI caused by PARACEST MRI contrast agent which converts amide to amine in the enzyme catalysis reaction. On this basis, Liu et al.114 designed a new PARACEST MRI contrast agent (Yb(III)-(1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid)-orthoaminoanilide which could measure extracellular pH within in vivo animal models to detect NO. The same group developed a similar smart PARACEST MRI agent which after reaction with NO undergoes chemical modifications resulting in the formation of hydrazine, which does not exhibit the PARACEST effect thereby enabling detection of NO.115 Chelation of lanthanides (III) such as Yb, Er and Tm with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid o-aminoanilide is used to synthesize the agent which produces protons with high shift exchange from the functional groups on amide and amine. The absence of PARACEST effect due to reaction of agent with NO is assessed by nuclear magnetic resonance in the presence of NONOate (NO donor). pH and temperature conditions were applied to assess the PARACEST effect before and after the reaction of agent and NO. The absence of the PARACEST effect is attributed to the detection of NO, as PARACEST effect at different pH was achieved from amide and amine.
Trapping of NO in biologic tissues using paramagnetic mono and dinitrosyl-iron complexes (MNIC and DNIC, respectively) which act as magnetic resonance contrast agents and utilizing their ability to enter into dynamic nuclear polarization with water protons with the help of unpaired electrons of these complexes can be imaged using proton-electron double resonance imaging. Aqueous solutions of MNIC and DNIC resulted in alterations of proton-electron double resonance imaging signal intensity. Furthermore, sodium nitroprussside treated rat liver induced a characteristic MNIC and DNIC ESR spectrum. The researchers thus concluded that exploitation of NIC for detection of NO using proton-electron double resonance imaging and MRI (could be employed for in vivo NO imaging).116,117
MRI has proved to possess advantages owing to its availability with good enough field of view. It also provides high resolution imaging along with good contrast for soft tissues and anatomical and functional details.2 However, due to its low sensitivity, it can detect NO only at high concentrations which requires high concentration of the contrast agents, which may cause toxic effects. The need for long data acquisition time for generating the MRI image increases the overall time for the detection, which may lead to question the amount of NO detected, owing to its short lifetime.2
SUMMARY AND OUTLOOK
NO plays a vital role in the body carrying out numerous pathological and physiological functions. However, future research focusing on these functions will require identifying better quantification methods for NO. To overcome the disadvantages of a single approach, multimodal approach or hybrid technology can be used to provide synergistic advantages for NO detection. The future application of these approaches in clinical research is worth exploring. NO is a difficult molecule to trap and hence causes difficulty in quantifying it. The measure of NOx as a surrogate for NO detection in various methods provides an indirect approach. The above mentioned approaches come with their own pros and cons, as listed in Table 2. However, continuous efforts are being made by researchers for real-time in vitro and in vivo monitoring of NO with reliable data by developing better methods. NO concentration, sample size and interference are some of the factors that direct the choice of method to be used. The choice also depends on the limit of detection, the merits and demerits of the method, accuracy, sensitivity, specificity, direct or indirect measurement, etc.
Table 2.
Comparison of various methods for NO detection in vitro and in vivo.
| Methods | In vitro detection | In vivo detection | ||||
|---|---|---|---|---|---|---|
| Principles | Advantages | Disadvantages | Principles | Advantages | Disadvantages | |
| Colorimetric method | HB/MB + NO → HB/MB− NO HBO2/MBO2 + NO → metHB/metMB + NO3 – NO2 – + H+ + SA + NED → AANBSA ABTS2− + NO → ABTS2— NO [Fe(CN)6]4– + NO + O2 → [Fe(CN)5(NO)]3– NO + Fe2+ + SCN– + H+ → [Fe(SCN)(NO)]+ |
High sensitivity, high accuracy, easily accessible, economical | Cannot quantify NO in whole blood, poor specificity | N/A | N/A | N/A |
| Chemiluminescence method | NO + O3 → NO2 + O2 + hν NO + H2O2 + luminol → dianion + hν | High sensitivity, rapid, real-time monitoring, low detection limit, applicable in cells and organs | Expensive, time consuming, poor specificity | NO@porousmembrane@detection agents | Direct measurement in tissues and exhaled breath, diagnosis of respiratory diseases, monitor therapeutic efficacy of respiratory diseases | Expensive calibration method, not applicable for local and systemic measurements, low sensitivity |
| Fluorescence method | DAF-FM + NO + O2 → triazole DAR-4M + NO + O2 → DAR-4T DAQ + NO + O2 → DAQ-TZ TMDCDABODIPY + NO + O2 → TMDCDABODIPY-T ADNO + NO + O2 → ADNO-SNOC Gal-RhB + NO + O2 → RhB Mito-DHP + NO + O2 → Mito-PY |
High specificity, high sensitivity, convenient, real-time imaging of living cells | Long reaction time | Similar to in vitro | Sensitive, reversible, picomolar level detection, convenient detection in whole tissue, cellular and sub-cellular levels | Poor in vivo stability, potential toxicity |
| Electrochemical method | NO → NO+ + e 2NO + 4OH− → NO3
– + 2H+ + 4e NO + 2e → N2O2 2– |
High reaction efficacy, fast response, high selectivity, high specificity | High detection limit, unreproducible, current saturation | Similar to in vitro | High reaction efficacy, high selectivity, high stability, direct, real-time monitoring | Easily affected by environment conditions, slow response, large size |
| Gas chromatography method | Derivtization procedure:PFB-Br + Nitrite/ Nitrate → PFB-NO2/PFB-ONO2 | High sensitivity | Poor sensitivity, high detection limit, easily influenced by other gases | N/A | N/A | N/A |
| Electron paramagnetic resonance method | Heme + NO → Heme−NO Fe(II) + 2L– + 2NO → [Fe(NO)2L2] Fe(II) + 2DETC– + NO → [Fe(NO)(DETC)2] NNO + NO → INO PTIOs + NO → PTIs + NO2 NOCTs + NO → NOCTs− NO | Adequate sensitivity and specificity, applicable to intracellular NO detection, low detection limit | Costly instrumentation, time consuming sample preparation, complicated operation, expertise required | Similar to in vitro | Noninvasive visualization of in vivo NO distribution, high specificity, low detection limit, high availability, high stability | Interference by metHb, reductants, O2 and reactive oxygen species |
| MRI method | N/A | N/A | N/A | HB/MB + NO → HB/MB−NO HBO2/MBO2 + NO → metHB/metMB + NO3– | High resolution, noninvasive, large field-of-view, easily available, functional and anatomical information | Low sensitivity, high detection limit, long data acquisition time, short lifetime affecting the accuracy |
Note: NO: Nitric oxide; HB: hemoglobin; MB: myoglobin; SA: sulfanilamide; NED: N-(1-napthyl)ethylenediamine; AANBSA: azo-α-aminonaphthalene-parabenzene-sulfonic acid; N/A: not applicable; hν: luminescence (h: Planck’s constant, ν: frequency of the photon); DAF-FM: 4-amino-5-methylamino-2’,7’-difluorofluorescein; DAR-4M: diaminorhodamine- 4M; DAR-4T: diaminorhodamine-4M triazole; DAQ: 1,2-diaminoanthraquinone; DAQ-TZ: 1,2-diaminoanthraquinone triazole; TMDCDABODIPY: 1,3,5,7-tetramethyl-2,6- dicarbethoxy-8-(3′,4′ -diaminophenyl)-difluoroboradiaza-s-indacence; ADNO: (2-(α-(3,4-diaminophenoxy)acetyl)-6-(dimethylamino)naphthalene); SNOC: S-nitrocystein; Gal-RhB: a hepatocyte targeting fluorescent sensor; Mito-DHP: mitochondria targeting dihydropyridine-derived BODIPY probe; Mito-PY: a probe which specifically target mitochondria; e: electron; PFB-Br: α-bromo-2,3,4,5,6-pentafluorotoluene; PFB-NO2: α-nitro-2,3,4,5,6-pentafluorotoluene; PTIO: 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide; PTI: corresponding imino nitroxides of PTIO; NOCT: nitric oxide cheletropic traps.
Footnotes
Financial support: The work was supported by the National Natural Science Foundation of China, No. 51872188, Special Funds for the Development of Strategic Emerging Industries in Shenzhen, China, No. 20180309154519685, and Center of Hydrogen Science, Shanghai Jiao Tong University, China.
Conflicts of interest
The authors have no conflicts of interests to declare.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol. 2006;45:268–276. doi: 10.1016/j.vph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Hong H, Sun J, Cai W. Multimodality imaging of nitric oxide and nitric oxide synthases. Free Radical Biol Med. 2009;47:684–698. doi: 10.1016/j.freeradbiomed.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Body SC, Hartigan PM, Shernan SK, Formanek V, Hurford WE. Nitric oxide: delivery, measurement, and clinical application. J Cardiothorac Vasc Anesth. 1995;9:748–763. doi: 10.1016/s1053-0770(05)80242-3. [DOI] [PubMed] [Google Scholar]
- 4.Kiechle FL, Malinski T. Nitric oxide Biochemistry, pathophysiology, and detection. Am J Clin Pathol. 1993;100:567–575. doi: 10.1093/ajcp/100.5.567. [DOI] [PubMed] [Google Scholar]
- 5.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radical Biol Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuto JM, Cho JY, Switzer CH. Chapter 2 - The chemical properties of nitric oxide and related nitrogen oxides. In: Ignarro LJ, editor. Nitric Oxide. San Diego: Academic Press; 2000. pp. 23–40. [Google Scholar]
- 7.Yao D, Vlessidis AG, Evmiridis NP. Determination of nitric oxide in biological samples. Mikrochim Acta. 2004;147:1–20. [Google Scholar]
- 8.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Z, Wen Y, Hu Y, et al. MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine. Nanoscale. 2017;9:3637–3645. doi: 10.1039/c7nr00231a. [DOI] [PubMed] [Google Scholar]
- 10.Fan J, He Q, Liu Y, et al. Light-responsive biodegradable nanomedicine overcomes multidrug resistance via NO-enhanced chemosensitization. ACS Appl Mater Interfaces. 2016;8:13804–13811. doi: 10.1021/acsami.6b03737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saraiva J, Marotta-Oliveira SS, Cicillini SA, Eloy Jde O, Marchetti JM. Nanocarriers for nitric oxide delivery. J Drug Deliv. 2011;2011:936438. doi: 10.1155/2011/936438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy ME, Noack E. Nitric oxide assay using hemoglobin method. Methods Enzymol. 1994;233:240–250. doi: 10.1016/s0076-6879(94)33027-1. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Zhang X, Broderick M, Fein H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors. 2003;3:276–284. [Google Scholar]
- 14.Giovannoni G, Land JM, Keir G, Thompson EJ, Heales SJ. Adaptation of the nitrate reductase and Griess reaction methods for the measurement of serum nitrate plus nitrite levels. Ann Clin Biochem. 1997;34:193–198. doi: 10.1177/000456329703400212. [DOI] [PubMed] [Google Scholar]
- 15.Gross SS. Microtiter plate assay for determining kinetics of nitric oxide synthesis. Methods Enzymol. 1996;268:159–168. doi: 10.1016/s0076-6879(96)68018-5. [DOI] [PubMed] [Google Scholar]
- 16.Komurai M, Ishii Y, Matsuoka F, et al. Role of nitric oxide synthase activity in experimental ischemic acute renal failure in rats. Mol Cell Biochem. 2003;244:129–133. [PubMed] [Google Scholar]
- 17.Grisham MB, Johnson GG, Lancaster JR., Jr Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268:237–246. doi: 10.1016/s0076-6879(96)68026-4. [DOI] [PubMed] [Google Scholar]
- 18.Giustarini D, Rossi R, Milzani A, Dalle-Donne I. Nitrite and nitrate measurement by Griess reagent in human plasma: evaluation of interferences and standardization. Methods Enzymol. 2008;440:361–380. doi: 10.1016/S0076-6879(07)00823-3. [DOI] [PubMed] [Google Scholar]
- 19.Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R. Adaptation of the Griess reaction for detection of nitrite in human plasma. Free Radical Res. 2004;38:1235–1240. doi: 10.1080/10715760400017327. [DOI] [PubMed] [Google Scholar]
- 20.Pereira RdS, Piva SJ, Tatsch E, et al. A simple, fast and inexpensive automated technique for measurement of plasma nitrite. Clin Chem Lab Med. 2010;48:1837–1839. doi: 10.1515/CCLM.2010.332. [DOI] [PubMed] [Google Scholar]
- 21.Ajjuri RR, O’Donnell JM. Novel whole-tissue quantitative assay of nitric oxide levels in Drosophila neuroinflammatory response. J Vis Exp. 2013:50892. doi: 10.3791/50892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmölz L, Wallert M, Lorkowski S. Optimized incubation regime for nitric oxide measurements in murine macrophages using the Griess assay. J Immunol Methods. 2017;449:68–70. doi: 10.1016/j.jim.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Oyungerel B, Lim H, Lee CH, Choi EH, Li GH, Choi KD. Anti-inflammatory effects of Magnolia sieboldii extract in lipopolysaccharide-stimulated RAW264 7 macrophages. Trop J Pharm Res. 2013;12:913–918. [Google Scholar]
- 24.Hamidon H, Taher M, Jaffri JM, et al. Cytotoxic and anti-inflammatory activities of Garcinia xanthochymus extracts on cell lines. Makara J Health Res. 2016;20:3. [Google Scholar]
- 25.Benevides Bahiense J, Marques FM, Figueira MM, et al. Potential anti-inflammatory, antioxidant and antimicrobial activities of Sambucus australis. Pharm Biol. 2017;55:991–997. doi: 10.1080/13880209.2017.1285324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacco RE, Waters WR, Rudolph KM, Drew ML. Comparative nitric oxide production by LPS-stimulated monocyte-derived macrophages from Ovis canadensis and Ovis aries. Comp Immunol, Microbiol Infect Dis. 2006;29:1–11. doi: 10.1016/j.cimid.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Choi WS, Jeong JW, Kim SO, et al. Anti-inflammatory potential of peat moss extracts in lipopolysaccharide-stimulated RAW 2647 macrophages. Int J Mol Med. 2014;34:1101–1109. doi: 10.3892/ijmm.2014.1881. [DOI] [PubMed] [Google Scholar]
- 28.Mur LAJ, Mandon J, Cristescu SM, Harren FJM, Prats E. Methods of nitric oxide detection in plants: a commentary. Plant Sci. 2011;181:509–519. doi: 10.1016/j.plantsci.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Hunter RA, Storm WL, Coneski PN, Schoenfisch MH. Inaccuracies of nitric oxide measurement methods in biological media. Anal Chem. 2013;85:1957–1963. doi: 10.1021/ac303787p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nims RW, Darbyshire JF, Saavedra JE, et al. Colorimetric methods for the determination of nitric oxide concentration in neutral aqueous solutions. Methods. 1995;7:48–54. [Google Scholar]
- 31.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circul Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- 32.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Bates JN. Nitric oxide measurement by chemiluminescence detection. Neuroprotocols. 1992;1:141–149. [Google Scholar]
- 34.Hetrick EM, Schoenfisch MH. Analytical chemistry of nitric oxide. Annu Rev Anal Chem (Palo Alto Calif) 2009;2:409–433. doi: 10.1146/annurev-anchem-060908-155146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woldman YY, Eubank TD, Mock AJ, et al. Detection of nitric oxide production in cell cultures by luciferin-luciferase chemiluminescence. Biochem Biophys Res Commun. 2015;465:232–238. doi: 10.1016/j.bbrc.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez BL, Davis-Moon L, Ballas SK, Ma XL. Sequential nitric oxide measurements during the emergency department treatment of acute vasoocclusive sickle cell crisis. Am J Hematol. 2000;64:15–19. doi: 10.1002/(sici)1096-8652(200005)64:1<15::aid-ajh3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 37.Piacentini GL, Suzuki Y, Bodini A. Exhaled nitric oxide levels in childhood asthma: a more reliable indicator of asthma severity than lung function measurement? BioDrugs. 2000;13:279–288. doi: 10.2165/00063030-200013040-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kikuchi K, Nagano T, Hayakawa H, Hirata Y, Hirobe M. Detection of nitric oxide production from a perfused organ by a luminol-H2O2 system. Anal Chem. 1993;65:1794–1799. doi: 10.1021/ac00061a025. [DOI] [PubMed] [Google Scholar]
- 39.Yao D, Evmiridis NP, Zhou Y, Xu S, Zhou H. New method for monitoring nitric oxide in vivo using microdialysis sampling and chemiluminescence reaction International Conference on Sensor Technology (ISTC 2001) [Google Scholar]
- 40.Robinson JK, Bollinger MJ, Birks JW. Luminol/H2O2 chemiluminescence detector for the analysis of nitric oxide in exhaled breath. Anal Chem. 1999;71:5131–5136. doi: 10.1021/ac990646d. [DOI] [PubMed] [Google Scholar]
- 41.Zhou X, Arnold MA. Response characteristics and mathematical modeling for a nitric oxide fiber-optic chemical sensor. Anal Chem. 1996;68:1748–1754. [Google Scholar]
- 42.Taha ZH. Nitric oxide measurements in biological samples. Talanta. 2003;61:3–10. doi: 10.1016/S0039-9140(03)00354-0. [DOI] [PubMed] [Google Scholar]
- 43.Wada M, Morinaka C, Ikenaga T, Kuroda N, Nakashima K. A simple HPLC-fluorescence detection of nitric oxide in cultivated plant cells by in situ derivatization with 2,3-diaminonaphthalene. Anal Sci. 2002;18:631–634. doi: 10.2116/analsci.18.631. [DOI] [PubMed] [Google Scholar]
- 44.Gharavi N, El-Kadi AOS. Measurement of nitric oxide in murine Hepatoma Hepa1c1c7 cells by reversed phase HPLC with fluorescence detection. J Pharm Pharm Sci. 2003;6:302–307. [PubMed] [Google Scholar]
- 45.Kleinhenz DJ, Fan X, Rubin J, Hart CM. Detection of endothelial nitric oxide release with the 2,3-diaminonapthalene assay. Free Radical Biol Med. 2003;34:856–861. doi: 10.1016/s0891-5849(02)01438-7. [DOI] [PubMed] [Google Scholar]
- 46.Kim W-S, Ye X, Rubakhin SS, Sweedler JV. Measuring nitric oxide in single neurons by capillary electrophoresis with laser-induced fluorescence: use of ascorbate oxidase in diaminofluorescein measurements. Anal Chem. 2006;78:1859–1865. doi: 10.1021/ac051877p. [DOI] [PubMed] [Google Scholar]
- 47.Leikert JF, Räthel TR, Müller C, Vollmar AM, Dirsch VM. Reliable in vitro measurement of nitric oxide released from endothelial cells using low concentrations of the fluorescent probe 4,5-diaminofluorescein. FEBS Lett. 2001;506:131–134. doi: 10.1016/s0014-5793(01)02901-5. [DOI] [PubMed] [Google Scholar]
- 48.Patel VH, Brack KE, Coote JH, Ng GA. A novel method of measuring nitric-oxide-dependent fluorescence using 4,5-diaminofluorescein (DAF-2) in the isolated Langendorff-perfused rabbit heart. Pflugers Archiv. 2008;456:635–645. doi: 10.1007/s00424-007-0440-y. [DOI] [PubMed] [Google Scholar]
- 49.Strijdom H, Muller C, Lochner A. Direct intracellular nitric oxide detection in isolated adult cardiomyocytes: flow cytometric analysis using the fluorescent probe, diaminofluorescein. J Mol Cell Cardiol. 2004;37:897–902. doi: 10.1016/j.yjmcc.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Kashiwagi S, Izumi Y, Gohongi T, et al. NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest. 2005;115:1816–1827. doi: 10.1172/JCI24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metto EC, Evans K, Barney P, et al. An integrated microfluidic device for monitoring changes in nitric oxide production in single T-lymphocyte (Jurkat) cells. Anal Chem. 2013;85:10188–10195. doi: 10.1021/ac401665u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agrawal S, Kumari R, Luthra PM. A reliable fluorimetric method to screen the nitric oxide synthase inhibitors in 96 well plate. Anal Biochem. 2019;577:42–44. doi: 10.1016/j.ab.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 53.Lepiller S, Laurens V, Bouchot A, Herbomel P, Solary E, Chluba J. Imaging of nitric oxide in a living vertebrate using a diamino-fluorescein probe. Free Radical Biol Med. 2007;43:619–627. doi: 10.1016/j.freeradbiomed.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Zhou X, He P. Improved measurements of intracellular nitric oxide in intact microvessels using 4,5-diaminofluorescein diacetate. Am J Physiol Heart Circ Physiol. 2011;301:H108–H114. doi: 10.1152/ajpheart.00195.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kojima H, Hirotani M, Nakatsubo N, et al. Bioimaging of nitric oxide with fluorescent indicators based on the rhodamine chromophore. Anal Chem. 2001;73:1967–1973. doi: 10.1021/ac001136i. [DOI] [PubMed] [Google Scholar]
- 56.Kikuchi M, Shirasaki H, Himi T. Platelet-activating factor (PAF) increases NO production in human endothelial cells-real-time monitoring by DAR-4M AM. Prostaglandins Leukot Essent Fatty Acids. 2008;78:305–309. doi: 10.1016/j.plefa.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Jiang WL, Li Y, Liu HW, et al. A rhodamine-deoxylactam based fluorescent probe for fast and selective detection of nitric oxide in living cells. Talanta. 2019;197:436–443. doi: 10.1016/j.talanta.2019.01.061. [DOI] [PubMed] [Google Scholar]
- 58.Marín MJ, Thomas P, Fabregat V, Luis SV, Russell DA, Galindo F. Fluorescence of 1,2-diaminoanthraquinone and its nitric oxide reaction product within macrophage cells. Chembiochem. 2011;12:2471–2477. doi: 10.1002/cbic.201100371. [DOI] [PubMed] [Google Scholar]
- 59.Galindo F, Kabir N, Gavrilovic J, Russell DA. Spectroscopic studies of 1,2-diaminoanthraquinone (DAQ) as a fluorescent probe for the imaging of nitric oxide in living cells. Photochem Photobiol Sci. 2008;7:126–130. doi: 10.1039/b707528f. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Liu Q, Liang X, Xiao Q, Fang Y, Wu Y. A new fluorescence biosensor for nitric oxide detection based on cytochrome P450 55B1. Sens Actuators B Chem. 2016;230:405–410. [Google Scholar]
- 61.Lim MH, Wong BA, Pitcock WH, Jr, Mokshagundam D, Baik MH, Lippard SJ. Direct nitric oxide detection in aqueous solution by copper(II) fluorescein complexes. J Am Chem Soc. 2006;128:14364–14373. doi: 10.1021/ja064955e. [DOI] [PubMed] [Google Scholar]
- 62.Jain P, David A, Bhatla SC. A novel protocol for detection of nitric oxide in plants. Methods Mol Biol. 2016;1424:69–79. doi: 10.1007/978-1-4939-3600-7_7. [DOI] [PubMed] [Google Scholar]
- 63.Liu Q, Xue L, Zhu DJ, Li GP, Jiang H. Highly selective two-photon fluorescent probe for imaging of nitric oxide in living cells. Chin Chem Lett. 2014;25:19–23. [Google Scholar]
- 64.Zhang J, Pan F, Jin Y, et al. A BODIPY-based dual-responsive turn-on fluorescent probe for NO and nitrite. Dyes Pigm. 2018;155:276–283. [Google Scholar]
- 65.Chen XX, Niu LY, Shao N, Yang QZ. BODIPY-based fluorescent probe for dual-channel detection of nitric oxide and glutathione: visualization of cross-talk in living cells. Anal Chem. 2019;91:4301–4306. doi: 10.1021/acs.analchem.9b00169. [DOI] [PubMed] [Google Scholar]
- 66.Gao C, Lin L, Sun W, et al. Dihydropyridine-derived BODIPY probe for detecting exogenous and endogenous nitric oxide in mitochondria. Talanta. 2018;176:382–388. doi: 10.1016/j.talanta.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 67.Huang KJ, Wang H, Ma M, Zhang X, Zhang HS. Real-time imaging of nitric oxide production in living cells with 1,3,5,7-tetramethyl-2,6-dicarbethoxy-8-(3’,4’-diaminophenyl)-difluoroboradiaza-s-indacence by invert fluorescence microscope. Nitric Oxide. 2007;16:36–43. doi: 10.1016/j.niox.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 68.Zhang P, Tian Y, Liu H, et al. In vivo imaging of hepatocellular nitric oxide using a hepatocyte-targeting fluorescent sensor. Chem Commun (Camb) 2018;54:7231–7234. doi: 10.1039/c8cc03240h. [DOI] [PubMed] [Google Scholar]
- 69.Xie YJ, Shen WB. In vivo imaging of nitric oxide and reactive oxygen species using laser scanning confocal microscopy. Methods Mol Biol. 2012;913:191–200. doi: 10.1007/978-1-61779-986-0_12. [DOI] [PubMed] [Google Scholar]
- 70.Gomes A, Fernandes E, Lima JL. Use of fluorescence probes for detection of reactive nitrogen species: a review. J Fluoresc. 2006;16:119–139. doi: 10.1007/s10895-005-0030-3. [DOI] [PubMed] [Google Scholar]
- 71.Ye X, Rubakhin SS, Sweedler JV. Detection of nitric oxide in single cells. Analyst. 2008;133:423–433. doi: 10.1039/b716174c. [DOI] [PubMed] [Google Scholar]
- 72.Ciszewski A, Milczarek G. Electrochemical detection of nitric oxide using polymer modified electrodes. Talanta. 2003;61:11–26. doi: 10.1016/S0039-9140(03)00355-2. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X. Real time and in vivo monitoring of nitric oxide by electrochemical sensors--from dream to reality. Front Biosci. 2004;9:3434–3446. doi: 10.2741/1492. [DOI] [PubMed] [Google Scholar]
- 74.Bedioui F, Ismail A, Griveau S. Electrochemical detection of nitric oxide and S-nitrosothiols in biological systems: Past, present & future. Curr Opin Electrochem. 2018;12:42–50. [Google Scholar]
- 75.Serpe MJ, Zhang X. The Principles, development and application of microelectrodes for the in vivo determination of nitric oxide. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. [PubMed] [Google Scholar]
- 76.Shibuki K. An electrochemical microprobe for detecting nitric oxide release in brain tissue. Neurosci Res. 1990;9:69–76. doi: 10.1016/0168-0102(90)90048-j. [DOI] [PubMed] [Google Scholar]
- 77.Fujita S, Roerig DL, Chung WW, Bosnjak ZJ, Stowe DF. Volatile anesthetics do not alter bradykinin-induced release of nitric oxide or L-citrulline in crystalloid perfused guinea pig hearts. Anesthesiology. 1998;89:421–433. doi: 10.1097/00000542-199808000-00019. [DOI] [PubMed] [Google Scholar]
- 78.Kitamura Y, Uzawa T, Oka K, et al. Microcoaxial electrode for in vivo nitric oxide measurement. Anal Chem. 2000;72:2957–2962. doi: 10.1021/ac000165q. [DOI] [PubMed] [Google Scholar]
- 79.Simonsen U, Wadsworth RM, Buus NH, Mulvany MJ. In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. J Physiol. 1999;516:271–282. doi: 10.1111/j.1469-7793.1999.271aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hernanz R, Alonso MJ, Zibrandtsen H, Alvarez Y, Salaices M, Simonsen U. Measurements of nitric oxide concentration and hyporeactivity in rat superior mesenteric artery exposed to endotoxin. Cardiovasc Res. 2004;62:202–211. doi: 10.1016/j.cardiores.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Stankevicius E, Martinez AC, Mulvany MJ, Simonsen U. Blunted acetylcholine relaxation and nitric oxide release in arteries from renal hypertensive rats. J Hypertens. 2002;20:1571–1579. doi: 10.1097/00004872-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 82.Griveau S, Dumézy C, Séguin J, Chabot GG, Scherman D, Bedioui F. In vivo electrochemical detection of nitric oxide in tumor-bearing mice. Anal Chem. 2007;79:1030–1033. doi: 10.1021/ac061634c. [DOI] [PubMed] [Google Scholar]
- 83.Besson-Bard A, Griveau S, Bedioui F, Wendehenne D. Real-time electrochemical detection of extracellular nitric oxide in tobacco cells exposed to cryptogein, an elicitor of defence responses. J Exp Bot. 2008;59:3407–3414. doi: 10.1093/jxb/ern189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown MD, Schoenfisch MH. Catalytic selectivity of metallophthalocyanines for electrochemical nitric oxide sensing. Electrochim Acta. 2018;273:98–104. doi: 10.1016/j.electacta.2018.03.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takarada S, Imanishi T, Goto M, et al. First evaluation of real-time nitric oxide changes in the coronary circulation in patients with non-ischaemic dilated cardiomyopathy using a catheter-type sensor. Eur Heart J. 2010;31:2862–2870. doi: 10.1093/eurheartj/ehq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jo A, Do H, Jhon GJ, Suh M, Lee Y. Electrochemical nanosensor for real-time direct imaging of nitric oxide in living brain. Anal Chem. 2011;83:8314–8319. doi: 10.1021/ac202225n. [DOI] [PubMed] [Google Scholar]
- 87.Jiang S, Cheng R, Wang X, et al. Real-time electrical detection of nitric oxide in biological systems with sub-nanomolar sensitivity. Nat Commun. 2013;4:2225. doi: 10.1038/ncomms3225. [DOI] [PubMed] [Google Scholar]
- 88.Sharma BV, Rowland NS, Clouse MM, Rice NA. An improved assay for measuring low levels of nitric oxide in cultured pulmonary myofibroblasts. Adv Biol Chem. 2014;4:214. [Google Scholar]
- 89.Tang L, Li Y, Xie H, et al. A sensitive acupuncture needle microsensor for real-time monitoring of nitric oxide in acupoints of rats. Sci Rep. 2017;7:6446. doi: 10.1038/s41598-017-06657-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kipping PJ, Jeffery PG. Detection of nitric oxide by gas-chromatography. Nature. 1963;200:1314. [Google Scholar]
- 91.Trettin A, Böhmer A, Suchy MT, et al. Effects of paracetamol on NOS, COX, and CYP activity and on oxidative stress in healthy male subjects, rat hepatocytes, and recombinant NOS. Oxid Med Cell Longev. 2014;2014:212576. doi: 10.1155/2014/212576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Becker AJ, Uckert S, Tsikas D, et al. Determination of nitric oxide metabolites by means of the Griess assay and gas chromatography-mass spectrometry in the cavernous and systemic blood of healthy males and patients with erectile dysfunction during different functional conditions of the penis. Urol Res. 2000;28:364–369. doi: 10.1007/s002400000141. [DOI] [PubMed] [Google Scholar]
- 93.Fujii S, Yoshimura T, Kamada H. Nitric oxide trapping efficiencies of water-soluble iron(iii) complexes with dithiocarbamate derivatives. Chem Lett. 1996;25:785–786. [Google Scholar]
- 94.Weaver J, Porasuphatana S, Tsai P, Budzichowski T, Rosen GM. Spin trapping nitric oxide from neuronal nitric oxide synthase: A look at several iron-dithiocarbamate complexes. Free Radical Res. 2005;39:1027–1033. doi: 10.1080/10715760500231885. [DOI] [PubMed] [Google Scholar]
- 95.Tsuchiya K, Yoshizumi M, Houchi H, Mason RP. Nitric oxide-forming reaction between the iron-N-methyl-D-glucamine dithiocarbamate complex and nitrite. J Biol Chem. 2000;275:1551–1556. doi: 10.1074/jbc.275.3.1551. [DOI] [PubMed] [Google Scholar]
- 96.Tsuchiya K, Jiang JJ, Yoshizumi M, et al. Nitric oxide-forming reactions of the water-soluble nitric oxide spin-trapping agent, MGD. Free Radical Biol Med. 1999;27:347–355. doi: 10.1016/s0891-5849(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 97.Hirayama A, Nagase S, Ueda A, et al. Electron paramagnetic resonance imaging of nitric oxide organ distribution in lipopolysuccaride treated mice. Mol Cell Biochem. 2003;244:63–67. [PubMed] [Google Scholar]
- 98.Pustelny K, Bielanska J, Plonka PM, Rosen GM, Elas M. In vivo spin trapping of nitric oxide from animal tumors. Nitric Oxide. 2007;16:202–208. doi: 10.1016/j.niox.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 99.Fujii S, Suzuki Y, Yoshimura T, Kamada H. In vivo three-dimensional EPR imaging of nitric oxide production from isosorbide dinitrate in mice. Am J Physiol. 1998;274:G857–G862. doi: 10.1152/ajpgi.1998.274.5.G857. [DOI] [PubMed] [Google Scholar]
- 100.Quaresima V, Takehara H, Tsushima K, Ferrari M, Utsumi H. In vivo detection of mouse liver nitric oxide generation by spin trapping electron paramagnetic resonance spectroscopy. Biochem Biophys Res Commun. 1996;221:729–734. doi: 10.1006/bbrc.1996.0664. [DOI] [PubMed] [Google Scholar]
- 101.Suzuki Y, Fujii S, Numagami Y, Tominaga T, Yoshimoto T, Yoshimura T. In vivo nitric oxide detection in the septic rat brain by electron paramagnetic resonance. Free Radic Res. 1998;28(3):293–299. doi: 10.3109/10715769809069281. [DOI] [PubMed] [Google Scholar]
- 102.Ren J, Fung PCW, Chang C, et al. A comparative ESR study on blood and tissue nitric oxide concentration during renal ischemia-reperfusion injury. Appl Magn Reson. 2007;32:243–255. [Google Scholar]
- 103.Tominaga T, Sato S, Ohnishi T, Ohnishi ST. Electron paramagnetic resonance (EPR) detection of nitric oxide produced during forebrain ischemia of the rat. J Cereb Blood Flow Metab. 1994;14:715–722. doi: 10.1038/jcbfm.1994.92. [DOI] [PubMed] [Google Scholar]
- 104.Kleschyov AL, Mollnau H, Oelze M, et al. Spin trapping of vascular nitric oxide using colloid Fe(II)-diethyldithiocarbamate. Biochem Biophys Res Commun. 2000;275:672–677. doi: 10.1006/bbrc.2000.3361. [DOI] [PubMed] [Google Scholar]
- 105.Komarov A, Mattson D, Jones MM, Singh PK, Lai CS. In vivo spin trapping of nitric oxide in mice. Biochem Biophys Res Commun. 1993;195:1191–1198. doi: 10.1006/bbrc.1993.2170. [DOI] [PubMed] [Google Scholar]
- 106.Komarov AM. In vivo detection of nitric oxide distribution in mice. Mol Cell Biochem. 2002;234-235:387–392. [PubMed] [Google Scholar]
- 107.Nakagawa H, Ikota N, Ozawa T, Masumizu T, Kohno M. Spin trapping for nitric oxide produced in LPS-treated mouse using various new dithiocarbamate iron complexes having substituted proline and serine moiety. Biochem Mol Biol Int. 1998;45:1129–1138. doi: 10.1080/15216549800203352. [DOI] [PubMed] [Google Scholar]
- 108.Lindermayr C, Durner J. Nitric oxide sensor proteins with revolutionary potential. J Exp Bot. 2018;69:3507–3510. doi: 10.1093/jxb/ery193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuppusamy P, Shankar RA, Roubaud VM, Zweier JL. Whole body detection and imaging of nitric oxide generation in mice following cardiopulmonary arrest: detection of intrinsic nitrosoheme complexes. Magn Reson Med. 2001;45:700–707. doi: 10.1002/mrm.1093. [DOI] [PubMed] [Google Scholar]
- 110.Fujii H, Berliner LJ. Nitric oxide: prospects and perspectives of in vivo detection by L-band EPR spectroscopy. PMB. 1998;43:1949–1956. doi: 10.1088/0031-9155/43/7/016. [DOI] [PubMed] [Google Scholar]
- 111.Hawkins CL, Davies MJ. Detection and characterisation of radicals in biological materials using EPR methodology. Biochim Biophys Acta. 2014;1840:708–721. doi: 10.1016/j.bbagen.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 112.Fujii H, Wan X, Zhong J, Berliner LJ, Yoshikawa K. In vivo imaging of spin-trapped nitric oxide in rats with septic shock: MRI spin trapping. Magn Reson Med. 1999;42:235–239. doi: 10.1002/(sici)1522-2594(199908)42:2<235::aid-mrm4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 113.Di Salle F, Barone P, Hacker H, Smaltino F, d’Ischia M. Nitric oxide-haemoglobin interaction: a new biochemical hypothesis for signal changes in fMRI. Neuroreport. 1997;8:461–464. doi: 10.1097/00001756-199701200-00017. [DOI] [PubMed] [Google Scholar]
- 114.Liu G, Li Y, Pagel M. A single PARACEST MRI contrast agent for accurate in vivo pH measurements International Society for Magnetic. Resonance in Medicine. 2007 [Google Scholar]
- 115.Liu G, Pagel M. A smart PARACEST MRI contrast agent for nitric oxide detection Proceedings of the 14th Annual Meeting of ISMRM 2006. Seattle, WA USA: [Google Scholar]
- 116.Mülsch A, Lurie DJ, Seimenis I, Fichtlscherer B, Foster MA. Detection of nitrosyl-iron complexes by proton-electron-double-resonance imaging. Free Radical Biol Med. 1999;27:636–646. doi: 10.1016/s0891-5849(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 117.Fichtlscherer B, Mülsch A. MR imaging of nitrosyl-iron complexes: experimental study in rats. Radiology. 2000;216:225–231. doi: 10.1148/radiology.216.1.r00jl10225. [DOI] [PubMed] [Google Scholar]