Abstract
Nitric oxide (NO) is an endogenous gas with several physiological activities. Owing to the NO physiological functions, such as inhibition of platelet aggregation and adhesion, vascular muscle relaxation, modulation of inflammation and immune response, antibacterial and anticancer activity, increasing attensions have been paid to the development of biomaterials with the ability to release this medical gas. Nowadays, numerous prodrugs have been developed to release NO in vivo. However, due to the low payloads and non-controlled delivery of the prodrug, the NO-releasing devices do not fulfil the expectations, which restricts their widespread application. Recently, several methods have been proposed to address the issue above, including physical and chemical methods and specific designs. This review aims to briefly introduce the latest achievements with recent 3 years involving coatings which mimic the vascular endothelium to treat atherosclerosis, nanocarriers which generate NO for a sustained anticancer treatment, and a framework which modifies the prodrug as a stable cardiovascular stent or as an anticancer targeted drug.
Keywords: nitric oxide, biomaterial, controlled release, cardiovascular disease, anti-cancer, anti-bacterial
INTRODUCTION
Nitric oxide (NO) is a physiological messenger molecule, which plays a regulative role in several physiological processes and exhibits exceptional therapeutic potential in different fields. It is involved in cardiovascular homeostasis, immune response, bone metabolism, neurotransmission, and cancer.1,2,3,4,5 Physiologically, endothelial cells produce NO at a flux of 0.05–0.40 nmol/(cm2•min), which inhibits platelet adhesion, vascular smooth muscle cell proliferation, and promotes vascular vasodilation.6,7 All of these turn the endothelium to an essential tissue, which guarantees the patency and homeostasis of blood vessels. Besides this, NO is also widely applied in anti-bacterial materials which is derived from its toxicity in high concentration.
The function of NO is dose-dependent, and the concentration has to be adjusted to obtain the desired therapeutic effect. For example, NO has been applied to inhabit tumor development on the basis of the “anti-Warburg effect” at a high concentration range.8 However, excessively high concentrations of NO in the blood may lead to NO poisoning, whereas low concentrations of NO may support tumor cell growth.9,10 Likewise, low NO levels have obviously anti-apoptotic effects, and high NO levels result in necrosis and cell death.5 These results highlight the importance to control the delivery velocity and concentration of NO.
This review is based on the development of the NO-releasing materials in recent 3 years, and takes the emphasis on the means of the control of release and new methods of the response which is in order to increase drug targeting to realize the gas therapy. The advantage of these materials have been highlighted and the prospects for development have been concluded in the end.
NITRIC OXIDE DONOR
In order to obtain a sufficient amount of NO for the treatment, several donors, which release NO by means of different reactions, have been investigated. Up to date, the typical low-molecular-weight NO donors, which are widely applied in NO-releasing materials are divided into the following categories, N-diazeniumdiolate (NONOate), S-nitrosothiols (RSNO) and L-arginine (L-Arg).11,12,13,14 NONOate is well-known as its release under physiological environment dispenses with no catalyst.15,16 Different from the NONOate, RSNO is an endogenous NO donor and the physiological transporter in vivo. RSNO is sensitive to conditions of the environment, such as metal ions, pH, and light. Hence RSNO can be used to design systems with regulated NO release.17,18,19 L-Arg is a natural NO donor which produces NO by the catalysis via inducible NO synthase. Meanwhile, L-Arg also releases NO by the oxidization of ROS which has high concentrations in inflammatory tissues or cancer, and show the great potential to address the above issue.14,20,21
NEW DEVELOPMENTS IN THE NITRIC OXIDE-RELEASING BIOMATERIALS
Numerous designs have been applied to deliver NO donors or release NO directly by the biomaterials, to make use of the physiological functions of NO.
Immobilization of nitric oxide donors
The traditional NO donor which can not release for a long period and is easy to breakdown to lead to the sudden release limits the development of NO-releasing material. Hopkins et al.22 reported a method for S-nitroso-N-acetylpenicillamine (SNAP) conjugation to polydimethylsiloxane (PDMS) to achieve a long-term biocompatible silicone. As shown in Figure 1, the immobilization of SNAP to PDMS prevents the NO donor from direct reaction in the internal environment, and an NO release is maintained for over 125 days. Due to the continuous NO release, the new material possesses a high antibacterial potential and prevents platelet adhesion.
Figure 1.
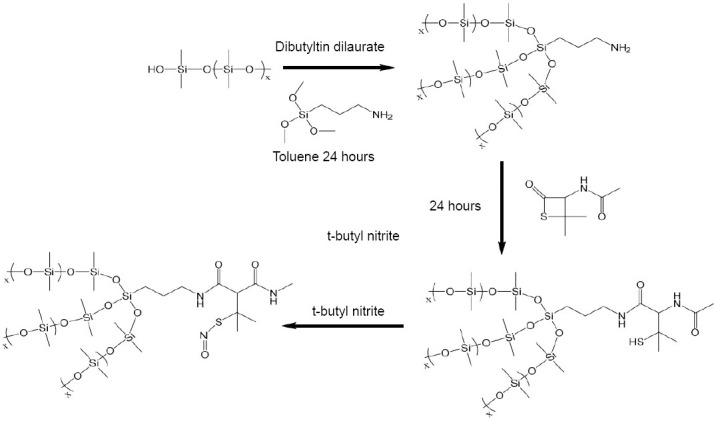
Route for covalent conjugation of the SNAP molecule to hydroxyl-terminated PDMS polymer.
Note: Reprinted with permission from Hopkins et al.22 SNAP: S-nitroso-N-acetylpenicillamine; PDMS: polydimethylsiloxane.
The release of NO is measured by chemiluminescence NO analyzer and the samples were tested in amberreaction vessels which contains 0.01 M phosphate buffer saline with ethylenediaminetetraacetic acid at 37°C using a nitrogen bubbler and sweep gas at a combined flowrate of 200 mL/min. And the result is shown in Figure 2. The NO release kinetics of SNAP-PDMS films in phosphate buffer saline with ethylenediaminetetraacetic acid at 37°C measured by chemiluminescence NO analyzer which proves that the SNAP-PDMS film habits the potential of long-term NO release.
Figure 2.
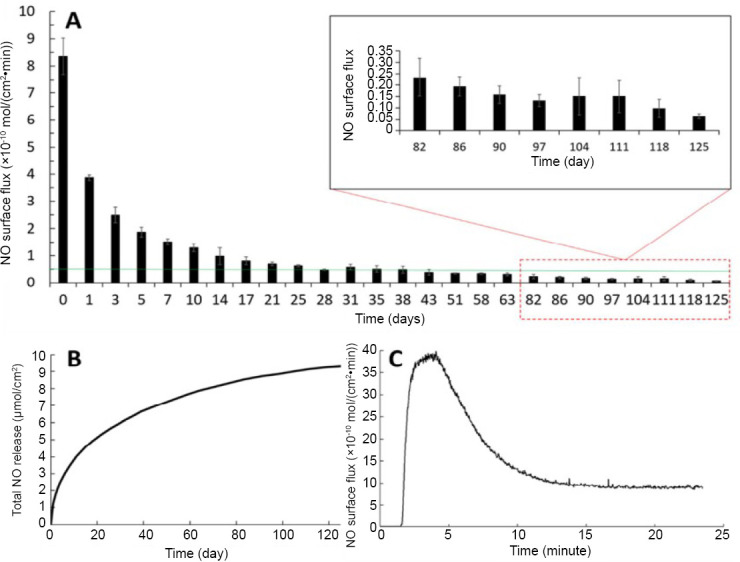
Nitric oxide (NO) release kinetics of SNAP-PDMS films.
Note: (A) The continuous NO release flux, taken at specified days while storing the films in phosphate buffer saline with ethylenediaminetetraacetic acid at 37°C (n = 4). The green line represents the minimum physiological level of NO flux (0.5 × 10-10 mol/(cm2•min)). (B) Cumulative NO release over the 125-day testing period, measured and normalized per cm2 of SNAP-PDMS. (C) Representative NO release profile on day 0 from SNAP-PDMS films when placed in phosphate buffer saline with ethylenediaminetetraacetic acid at 37°C. Reprinted with permission from Hopkins et al.22 SNAP: S-nitroso-N-acetylpenicillamine; PDMS: polydimethylsiloxane.
Tailoring re-endothelialization
A normal endothelial function desires the appropriate concentrations of NO in the blood vessel to prevent thrombosis, the proliferation and adhesion of the vascular smooth muscle cells, and leukocyte activation.23 However, vanishing of the endothelial cells in atherosclerotic areas and reduced activity also decreases the secretion of NO in these areas.24 Hence, several approaches have been pursued to mimic the normal endothelium function to treat atherosclerosis. For example, Yang et al.25 describe a NO producing coating for multifunctional vascular stents, which is functionalized with a plasma polymerized allylamine coating and then immobilized 3,3-diselenodipropionic acid (SeDPA). SeDPA has glutathione peroxidase-like catalytic activity to generate NO from RSNOs. According to the design, NO donors, such as S-nitrosoglutathione, S-nitrosocysteine, and S-nitrosoalbumin will be decomposed by the catalysis of SeDPA in vivo. A such functionalized stent implanted into the vessel continuously releases NO, simulating natural endothelium and remitting the atherosclerosis (Figure 3).
Figure 3.
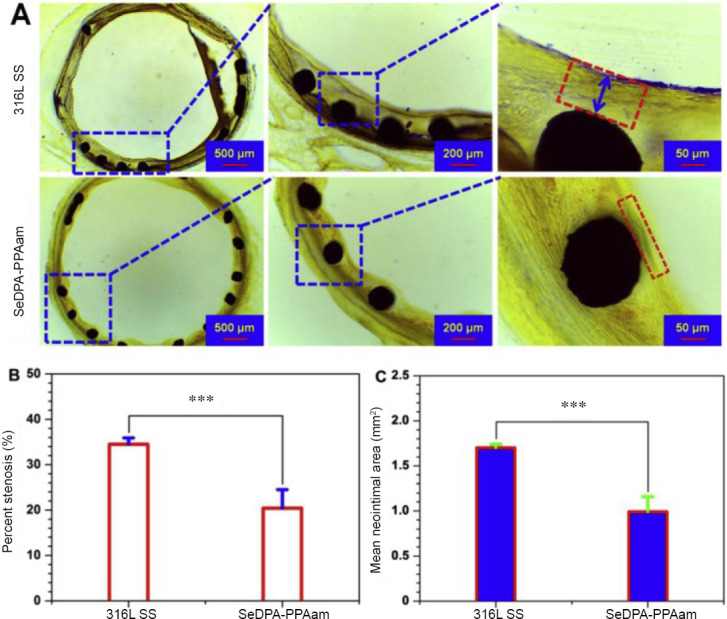
The result of in vivo stent implantation.
Note: (A) Native 316L SS stents and SeDPA-PPAam coated stents implanted in adult New Zealand white rabbits for 28 days. Histomorphometric analysis of the in-stent restenosis. (B, C) Percent neointimal stenosis (B) and mean neointimal area analysis (C) indicated significantly reduced in-stent restenosis. Data are expressed as mean ± SD. ***P < 0.001. SS: Stainless steel; SeDPA: 3,3-diselenodipropionic acid. Reprinted with permission from Yang et al.25
Based on the function of the NO, the SeDPA was immobilized to an amine which bears plasma polymerized allylamine (PPAam). Then cell and animal studies with the SeDPA-PPA coating showed a great inhibitory effect on collagen-induced platelet activation and adhesion, further on proliferation and migration of arterial smooth muscle cells. The NO-mediated effect was confirmed by analysis of intracellular cyclic guanosine monophosphate signaling pathways. The NO-catalytic bioactive coating enhanced endothelial cell migration and growth. Thus, there was cell selectivity with competitive growth of endothelial cells above smooth muscle cells. These results indicate that the NO-catalytic bioactive coating provides a new approach of an endothelium-mimetic microenvironment to promote the recovery of atherosclerosis.
L-arginine producing nitric oxygen via reactive oxygen species in tumor
As mentioned above, L-Arg releases NO by oxidization of reactive oxygen species (ROS), which provides a method to treat cancer. According to the free diffusion and penetration of the small molecule and the high concentration of ROS in the hypoxic tumor microenvironment,26 Wan et al.27 proposed a new design to achieve the gas therapy, which induces NO by means of ROS and realizes synergistic effects.
The nanoplatform is constructed on porous coordination network (PCN), and the NO donor is coated by the cancer cell membrane (Mem), which constitute the whole material (L-Arg@PCN@Mem). With the irradiation of near-infrared light (NIR), the ROS is produced to damage the cancer cell to achieve the traditional photodynamic therapy and the ROS can converse L-Arg to NO to meet the aim of gas therapy. 2′,7′-Dichlorofluorescin diacetate (DCFH-DA) is a fluorochrome which is used to measure the ROS. The production of ROS and the release of NO was measured by fluorescence spectrum under different materials and conditions and is expressed in relative fluorescence intensity, which shows that L-Arg induced NO by the ROS in Figure 4A and B, and it is also proved by Figure 4C which shows that the release of NO is prevented by vitamin C and controlled by the light irradiation. In order to exhibit the response of NO release under the control of NIR, the Figure 4D shows the condition of NO release and proves that the design meets the personal treatment requirements.
Figure 4.
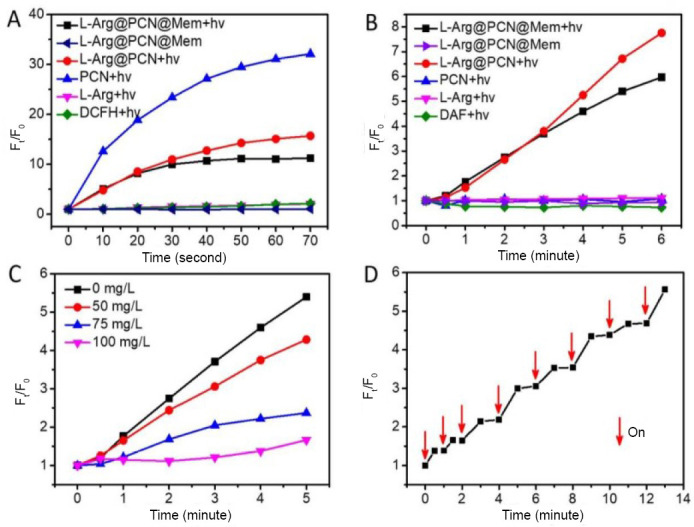
Production of reactive free radicals under different irradiation by fluorescence spectrum.
Note: (A) Reactive oxygen species generation of various samples with DCFH as the probe. (B) NO generation of various samples with 4,5-diaminofluorescein as the probe. (C) The change of NO level at different concentrations of vitamin C in L-Arg@PCN@Mem. (D) NO release of L-Arg@PCN@Mem under ON-OFF irradiation. hv: Light irradiation; Ft/F0: relative fluorescence intensity. DCFH: 2′,7′-dichlorofluorescin; L-Arg: L-arginine; PCN: porous coordination network; Mem: cancer cell membrane; NO: nitric oxide. Reprinted with permission from Wan et al.27
At 48 hours after injection of L-Arg@PCN@Mem and L-Arg@PCN, the tumor and issue condition is measured by fluorescence imaging and mean fluorescence intensity. According to the Figure 5, L-Arg@PCN@Mem with light irradiation and little tumor inhibition observed in L-Arg@PCN@Mem further confirmed biocompatibility of nanoparticles in vivo, which provided a possibility for further clinical research. The PCN group had an obvious tumor suppression when irradiated with 660 nm, which attributed to the effect of photodynamic therapy.
Figure 5.
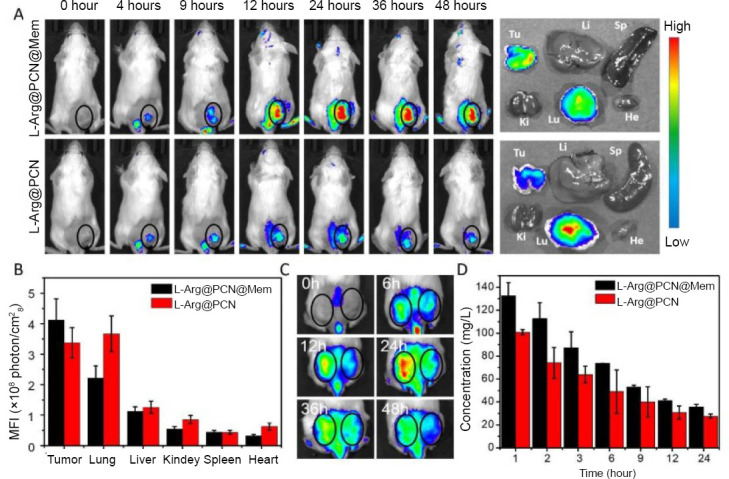
In vivo targeted imaging and pharmacokinetics.
Note: (A) In vivo fluorescence imaging of 4T1 tumor-bearing mice at 0–48 hours after intravenous injection of L-Arg@PCN@Mem and L-Arg@PCN, and the fluorescence represents the materials. Right: The corresponding ex vivo tissues 48 hours post-injection. (B) The MFI value of the major organs and tumor tissues after 48 hours intravenous injection. (C) In vivo fluorescence imaging of 4T1 and CT26 dual tumor-bearing mouse at 0–48 hours after intravenous injection of L-Arg@PCN@Mem. Left ring: 4T1 tumor, right ring: CT26 tumor. (D) Blood concentrations of L-Arg@PCN@Mem and L-Arg@PCN after intravenous injection. He: Heart; Li: liver; Sp: spleen; Lu: lung; Ki: kidney; Tu: tumor; MFI: mean fluorescence intensity; L-Arg: L-arginine; PCN: porous coordination network; Mem: cancer cell membrane. Reprinted with permission from Wan et al.27
Fe3O4 as a photoconversion agent to release nitric oxygen
Various concepts for NO releasing materials are applied in nanomedicine. As indicated above, NO has anti-cancer activity, however, it disperses randomly in the body and does not reach effective concentrations in the tumor. Besides, the therapeutic suitability of NO is also limited by its gaseous state and short half-life. In the last few years, nanomedicine has addressed the issue. Nanomedicine attains tumor-targeted drug delivery, and numerous stimuli-triggered release ways can be designed to obtain the active drug concentration within the tumor.
Polydopamine (PDA) is widely used in antibacterial design for its great biocompatibility, biodegradability, and photoconversion efficiency. Iron oxide nanoparticles (Fe3O4 NPs) is applied in bacteria treatment and separation due to its superparamagnetic property and the poly(amidoamine) (PAMAM) is a new dendrimer which can react with NO by forming NONOates. Recently, Yu et al.28 have developed antibacterial materials that exhibit the ability of controlled NO release, taking advantage of Fe3O4@PDA@PAMAM@NONOate under intermittent 808 nm laser irradiation. In order to get the ability to release NO, the three generation dendritic poly(amidoamine) (PAMAM-G3) is grafted on the surface of the PDA coated iron oxide nanocomposite (Fe3O4@PDA) which is after preparation and gets Fe3O4@PDA@PAMAM-G3, and then the NO is loaded with the formation of NONOate. The material causes leakage of intracellular components, which kills bacteria when it adheres to their surface and releases NO under laser irradiation with the rise of local temperature (Figure 6).
Figure 6.
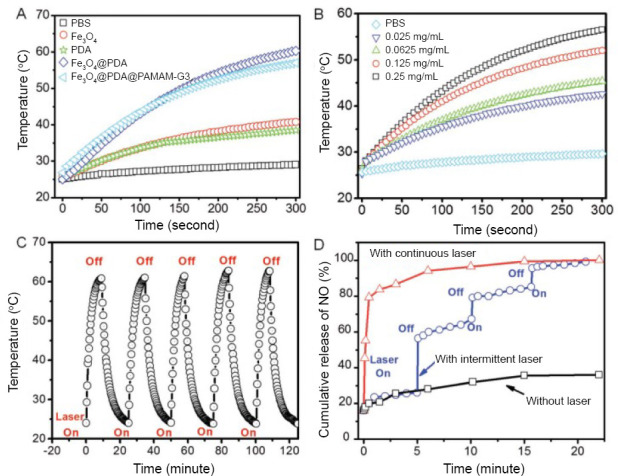
The respone of materials under the different environment.
Note: (A) Photothermal effects of different nanomaterials under laser irradiation at 808 nm with a power density of 0.5 W/cm2. (B) Concentration-dependent photothermal effect of Fe3O4@PDA@PAMAM-G3 under irradiation of 808 nm laser with a power density of 0.5 W/cm2. (C) Photothermal stability evaluation of Fe3O4@PDA@PAMAM-G3 with five laser on and off cycles. (D) NO release profile of Fe3O4@PDA@PAMAM@PAMAM@NONOate under different laser irradiation conditions. PBS: Phosphate buffer saline; PDA: polydopamine; PAMAM: poly(amidoamine); PAMAM-G3: three generation dendritic poly(amidoamine); NO: nitric oxide. Reprinted with permission from Yu et al.28
The photothermal effect, which means the continuous rise of temperature at laser irradiation, accelerates the NO release from Fe3O4@PDA@PAMAM@NONOate. NO is released in a controllable way by intermitted irradiation, which provides a method of tailored NO release flux to achieve a desired biological effect.
The synergistic actions of photothermal effect and NO treatment show a remarkable bactericidal effect (Figure 7).
Figure 7.
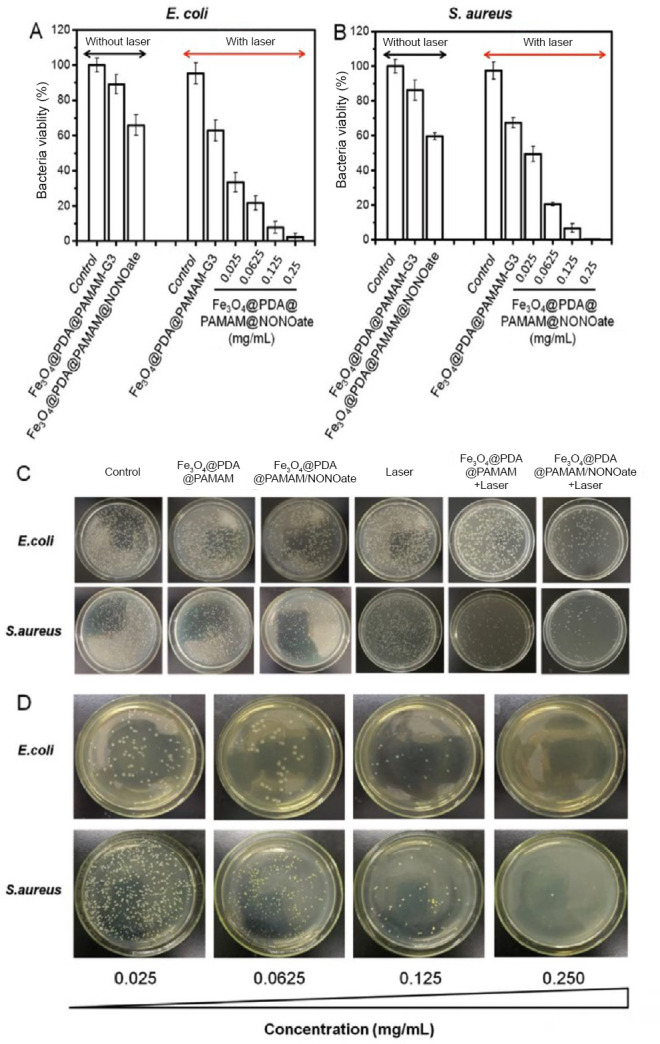
The antibacterial effect of Fe3O4@PDA@PAMAM@NONOate.
Note: (A, B) Viability of Escherichia coli (E. coli) (A) and Staphylococcus aureus (S. aureus) (B) treated with Fe3O4@PDA@PAMAM-G3 and Fe3O4@PDA@PAMAM@NONOate under different laser irradiation conditions. (C) Colony formation of E. coli and S. aureus under different treatments. (D) Colony formation of E. coli and S. aureus treated with different concentrations of Fe3O4@PDA@PAMAM@NONOate. PDA: Polydopamine; PAMAM: poly(amidoamine); NONOate: N-diazeniumdiolate. Reprinted with permission from Yu et al.28
Design of the enzyme-prodrug pair
NO is precisely controlled in vivo as a versatile endogenous messenger. In order to deliver NO to a specific site precisely and to make it available for clinical application, Hou et al.29 have proposed a bump-and-hole strategy which modifies an enzyme prodrug (pair of galactosidase galactosyl-NONOate) to accomplish a NO delivery system. In an in vivo near-infrared imaging assay, the NO function was measured in a hindlimb-ischemia rat model and an acute-kidney-injury mouse model and the NO delivery was highly targeted to enhance the therapeutic effect in tissue and function recovery, which also abolished the side effects on the whole body.
Nitric oxygen nanogenerators
The nano-gaseous-transmitters attract several researchers in the recent years to develop rapid generators of this medical gas. Guo et al.30 developed phototriggered NO nanogenerators to reverse the multidrug resistance in cancer based on the ues of NIR light and heat where the NO can in hibit the expression of P-glycoprotein. In this design, the nanocarriers combine with the heat-sensitive NO donor RSNO, and the photothermal conversion with heat sensitivity drives the nanogenerators to absorb the NIR light and utilizes the heat to breakdown the S-NO bonds.31
The preparation of material is shown in Figure 8. The nanocarriers is designed as a core–shell–shell structure (Fe3O4@PDA@mesoporous silica) and the mesoporous silica is functionalized with sulfhydryl groups which can obtain photothermal carriers.26,32 Then, RSNO reacts the -SH groups to conjugate to the shell, and thiolated transferrin is linked to the material surface in the end. In order to load the doxorubicin (DOX), the phototriggered NO nanogenerators were dispersed in DOX solution for 12 hours and got the materials for the animal texts.
Figure 8.
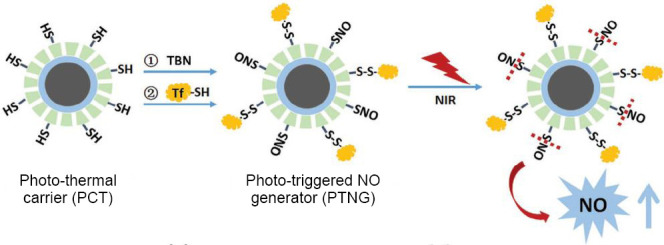
Schematic illustration of the fabrication of phototriggered NO nanogenerators.
Note: NO: Nitric oxide; TBN: tert-butyl nitrite; SNO: S-nitrosothiols; NIR: near-infrared light. Reprinted with permission from Fan et al.26 and Guo et al.32
For cancer-cell-targeting, transferrin is conjugated to the surfaces of nanoparticles and the result of in vivo chemotherapy is shown in Figure 9. Under NIR laser irradiation, the nanogenerators produce NO, which inhibits the P-glycoprotein expressing in order to reduce the drug resistance and improve the toxicity to cancer cells.
Figure 9.
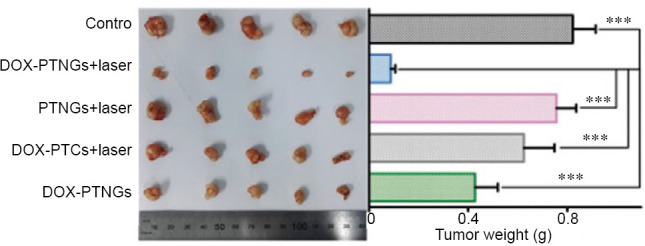
Photograph (left) and weights (right) of tumors after excision from each group.
Note: Data are expressed as mean ± SD. ***P < 0.001. DOX: Doxorubicin; PTNG: phototriggered nitric oxide nanogenerator; PTC: photothermal carrier. Reprinted with permission from Fan et al.26 and Guo et al.32
Liquid-infused nitric oxygen-releasing materials
Besides the conventional chemical fixation, other designs to exploit NO for synergistic effects have been proposed. Goudie et al.33 introduced a novel liquid-infused material, which can avoid platelet activation and acts as an antibacterial agent at the same time. This liquid-infused NO-releasing material was formed by blending the NO donor (SNAP) and silicone oil in silicone rubber tubing through a solvent swelling process. This work not only takes advantage of the liquid-infused materials, which present ultra-low fouling surfaces, but also prevents bacterial proliferation and platelet activation by means of NO release. In the Figure 10, the result shows that the liquid-infused NO-releasingn materials own a great anti-bacterial potential and excellent biocompatibility.
Figure 10.
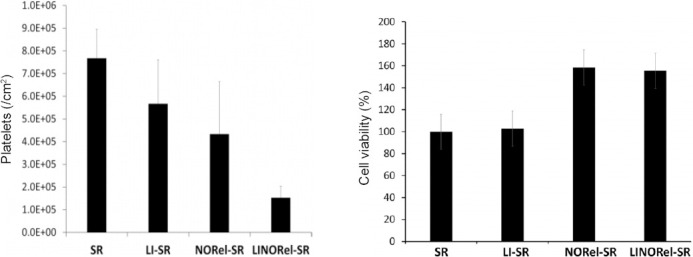
Degree of platelet adhesion on various silicone tubings after 2 hours exposure to porcine platelet rich plasma as measured using a lactate dehydrogenase quantification assay and cytocompatbility and cell growth support of various infused SR tubing towards mouse fbroblast cells in 24 hours study.
Note: Data are expressed as mean ± SD. SR: The medical grade Tygon™ 3350 silicone rubber tubing; LI-SR: liquid-infused SR; NORel-SR: nitric oxide-releasing SR; LINORel-SR: liquid-infused NO-releasing SR.
Glucose-responsive nitric oxygen-releasing biomaterials
As the key energy supplier and nutrient for tumors, glucose can be hydrolyzed by glucose oxidase and generate gluconic acid and H2O2.34,35 As described above, H2O2 has the potential to oxidize L-Arg into NO to be applied in gas therapy to treat cancer as a synergistic effect with the starving therapy.36 Fan et al.21 proposed a biocompatible/biodegradable nanocarrier of hollow mesoporous organosilica nanoparticles, which can be responsed by glucose due to the co-delivery of glucose oxidase and L-Arg. The experiment results show a remarkable H2O2 and NO cooperative anticancer effectwith minimal side effects.
According to Figure 11, after the treatment with L-Arg-hollow mesoporous organosilica nanoparticles-glucose oxidase, the tumors did shrink remarkably. It is assumed that both the elevated H2O2 concentration and the NO gas generated from L-Arg resulted in the apoptosis and necrosis of the tumor. Also a remarkable extension of survival time has been shown to prove the in vitro treatment efficacy.
Figure 11.
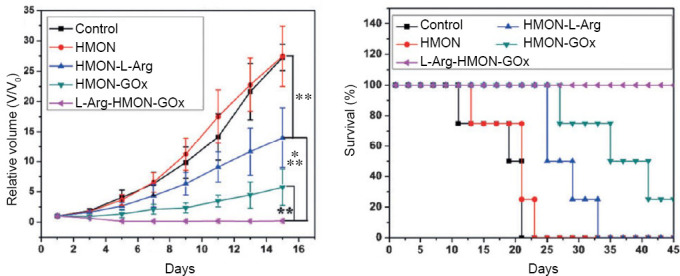
Tumor growth curve and survival curve of U87 tumor bearing mice after different treatments.
Note: Data are expressed as mean (± SD). **P < 0.01, ***P < 0.001. HMON: Hollow mesoporous organosilica nanoparticles; L-Arg: L-arginine; GOx: glucose oxidase. Reprinted with permission from Fan et al.21
CONCLUSIONS
Several NO-based therapies have been developed for antithrombotic, antibacterial, and anticancer applications. Compared with the traditional approaches, the NO-releasing biomaterials show an immense potential to address the clinical issues. Although this review has set forth the progress in the recent years, plenty of problems are urgently needed to be solved. In some cases, the timing NO release is frequently common in normal internal environment which we need to mimicking to achieve the treatment requirements, and in the other cases, the design for anticancer or antibacterial treatment and recovery of damaged tissue should consider the toxic and protective NO concentrations, which have to be kept under precise control by the material. Fruther attention is needed for the completely opposite effects that different NO concentrations in cancer may have. Besides this, the order of the substrate materials which cover vascular stents, nanocarriers and prodrug or enzyme bearing compounds, should subside the toxicity. The in vitro experiment should be designed much more carefully in order to meet the demand of clinical application. In summary, with the development of the NO-releasing biomaterials, owing to the interoperability of the design concept, more and more medical gas related materials have gotten tremendous advance, such as hydrogen sulfide, hydrogen, and carbonic oxide. As a new delivery system of medical gas, the NO-releasing biomaterials are going to advance the area of medical gas therapeutics and become the available routine in clinical application hopefully.
Footnotes
Financial support: This work was supported by the National Natural Science Foundation of China (No. 31570957), the National Key Research and Development Program of China (No. 2017YFB0702504), the International Cooperation Project by Science and Technology Department of Sichuan Province, China (No. 2019YFH0103) and the Applied Basic Research Project funded by Sichuan Provincial Science and Technology Department, China (No. 2017JY0296).
Conflicts of interest
The authors confirm that this article content has no conflict of interest.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocellin S, Bronte V, Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med Res Rev. 2007;27:317–352. doi: 10.1002/med.20092. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Wu J, Xi C, Meyerhoff ME. Diazeniumdiolate-doped poly(lactic-co-glycolic acid)-based nitric oxide releasing films as antibiofilm coatings. Biomaterials. 2012;33:7933–7944. doi: 10.1016/j.biomaterials.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–169. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 6.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 7.Khan M, Khan H, Singh I, Singh AK. Hypoxia inducible factor-1 alpha stabilization for regenerative therapy in traumatic brain injury. Neural Regen Res. 2017;12:696–701. doi: 10.4103/1673-5374.206632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caneba CA, Yang L, Baddour J, et al. Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis. 2014;5:e1302. doi: 10.1038/cddis.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter AW, Schoenfisch MH. Nitric oxide release: part II Therapeutic applications. Chem Soc Rev. 2012;41:3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brookes PS, Salinas EP, Darley-Usmar K, et al. Concentration-dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome c release. J Biol Chem. 2000;275:20474–20479. doi: 10.1074/jbc.M001077200. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DD, Ridnour LA, Isenberg JS, et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radical Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grisham MB, Jourd’Heuil D, Wink DA. Nitric oxide I Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am J Physiol. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 13.Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 14.Kudo S, Nagasaki Y. A novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(l-arginine)-based nanoparticles. J Control Release. 2015;217:256–262. doi: 10.1016/j.jconrel.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Wink DA, Darbyshire JF, Nims RW, Saavedra JE, Ford PC. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993;6:23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 16.Naghavi N, de Mel A, Alavijeh OS, Cousins BG, Seifalian AM. Nitric oxide donors for cardiovascular implant applications. Small. 2013;9:22–35. doi: 10.1002/smll.201200458. [DOI] [PubMed] [Google Scholar]
- 17.Wang PG, Xian M, Tang X, et al. Nitric oxide donors: chemical activities and biological applications. Chem Rev. 2002;102:1091–1134. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 18.Hwang S, Meyerhoff ME. Polyurethane with tethered copper(II)-cyclen complex: preparation, characterization and catalytic generation of nitric oxide from S-nitrosothiols. Biomaterials. 2008;29:2443–2452. doi: 10.1016/j.biomaterials.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joslin JM, Lantvit SM, Reynolds MM. Nitric oxide releasing Tygon materials: studies in donor leaching and localized nitric oxide release at a polymer-buffer interface. ACS Appl Mater Interfaces. 2013;5:9285–9294. doi: 10.1021/am402112y. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, Xu H, Jia X, et al. Ultrasound-triggered nitric oxide release platform based on energy transformation for targeted inhibition of pancreatic tumor. ACS Nano. 2016;10:10816–10828. doi: 10.1021/acsnano.6b04921. [DOI] [PubMed] [Google Scholar]
- 21.Fan W, Lu N, Huang P, et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew Chem Int Ed Engl. 2017;56:1229–1233. doi: 10.1002/anie.201610682. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins SP, Pant J, Goudie MJ, Schmiedt C, Handa H. Achieving long-term biocompatible silicone via covalently immobilized S-nitroso-N-acetylpenicillamine (SNAP) that exhibits 4 months of sustained nitric oxide release. ACS Appl Mater Interfaces. 2018;10:27316–27325. doi: 10.1021/acsami.8b08647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Major TC, Brant DO, Reynolds MM, et al. The attenuation of platelet and monocyte activation in a rabbit model of extracorporeal circulation by a nitric oxide releasing polymer. Biomaterials. 2010;31:2736–2745. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushwaha M, Anderson JM, Bosworth CA, et al. A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials. 2010;31:1502–1508. doi: 10.1016/j.biomaterials.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Yang Y, Xiong K, et al. Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials. 2015;63:80–92. doi: 10.1016/j.biomaterials.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Fan W, Bu W, Zhang Z, et al. X-ray radiation-controlled NO-release for on-demand depth-independent hypoxic radiosensitization. Angew Chem Int Ed Engl. 2015;54:14026–14030. doi: 10.1002/anie.201504536. [DOI] [PubMed] [Google Scholar]
- 27.Wan SS, Zeng JY, Cheng H, Zhang XZ. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials. 2018;185:51–62. doi: 10.1016/j.biomaterials.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Li G, Liu R, Ma D, Xue W. Dendritic Fe3O4@poly(dopamine)@PAMAM nanocomposite as controllable no-releasing material: a synergistic photothermal and no antibacterial study. Adv Funct Mater. 2018;28:1707440. [Google Scholar]
- 29.Hou J, Pan Y, Zhu D, et al. Targeted delivery of nitric oxide via a ‘bump-and-hole’-based enzyme-prodrug pair. Nat Chem Biol. 2019;15:151–160. doi: 10.1038/s41589-018-0190-5. [DOI] [PubMed] [Google Scholar]
- 30.Guo R, Tian Y, Wang Y, Yang W. Near-infrared laser-triggered nitric oxide nanogenerators for the reversal of multidrug resistance in cancer. Adv Funct Mater. 2017;27:1606398. [Google Scholar]
- 31.Aller SG, Yu J, Ward A, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo R, Peng H, Tian Y, Shen S, Yang W. Mitochondria-targeting magnetic composite nanoparticles for enhanced phototherapy of cancer. Small. 2016;12:4541–4552. doi: 10.1002/smll.201601094. [DOI] [PubMed] [Google Scholar]
- 33.Goudie MJ, Pant J, Handa H. Liquid-infused nitric oxide-releasing (LINORel) silicone for decreased fouling, thrombosis, and infection of medical devices. Sci Rep. 2017;7:13623. doi: 10.1038/s41598-017-14012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Yang J, Wang HF, et al. Glucose oxidase-catalyzed growth of gold nanoparticles enables quantitative detection of attomolar cancer biomarkers. Anal Chem. 2014;86:5800–5806. doi: 10.1021/ac500478g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 36.Gehring J, Trepka B, Klinkenberg N, Bronner H, Schleheck D, Polarz S. Sunlight-triggered nanoparticle synergy: teamwork of reactive oxygen species and nitric oxide released from mesoporous organosilica with advanced antibacterial activity. J Am Chem Soc. 2016;138:3076–3084. doi: 10.1021/jacs.5b12073. [DOI] [PubMed] [Google Scholar]


