Abstract
Background:
Gain-of-function (GOF) mutations in signal transducer and activator of transcription 1 (STAT1) cause susceptibility to a range of infections, autoimmunity, immune dysregulation, and combined immunodeficiency. Disease manifestations can be mild or severe and life-threatening. Hematopoietic stem cell transplantation (HSCT) has been used in some patients with more severe symptoms to treat and cure the disorder. However, the outcome of HSCT for this disorder is not well established.
Objective:
We sought to aggregate the worldwide experience of HSCT in patients with GOF-STAT1 mutations and to assess outcomes, including donor engraftment, overall survival, graft-versus-host disease, and transplant-related complications.
Methods:
Data were collected from an international cohort of 15 patients with GOF-STAT1 mutations who had undergone HSCT using a variety of conditioning regimens and donor sources. Retrospective data collection allowed the outcome of transplantation to be assessed. In vitro functional testing was performed to confirm that each of the identified STAT1 variants was in fact a GOF mutation.
Results:
Primary donor engraftment in this cohort of 15 patients with GOF-STAT1 mutations was 74%, and overall survival was only 40%. Secondary graft failure was common (50%), and posttransplantation event-free survival was poor (10% by 100 days). A subset of patients had hemophagocytic lymphohistiocytosis before transplant, contributing to their poor outcomes.
Conclusion:
Our data indicate that HSCT for patients with GOF-STAT1 mutations is curative but has significant risk of secondary graft failure and death.
Keywords: Hematopoietic stem cell transplantation, chronic mucocutaneous candidiasis, signal transducer and activator of transcription, Janus kinase, gain of function, graft-versus-host disease, graft rejection, hemophagocytic lymphohistiocytosis
Autosomal dominant gain-of-function (GOF) mutations in signal transducer and activator of transcription 1 (STAT1) cause susceptibility to a range of infections in human subjects, including severe viral and bacterial infections, nontuberculous mycobacterial disease, dimorphic fungal infections, and chronic mucocutaneous candidiasis (CMC), and are associated with development of autoimmunity.1–9 Some patients with GOF-STAT1 mutations present with a syndrome that clinically resembles immune dysregulation–polyendocrinopathy–enteropathy–X linked (IPEX) syndrome10 or with combined immunodeficiency (CID), with variable effects on immunoglobulin levels, antibody production, and lymphocyte development.11 However, the clinical phenotype can vary from mild to severe.1,2,4,7–9
Other than treatment of the infectious manifestations with long-term antifungal, antibacterial, and antiviral therapies, the approach to management of patients with GOF-STAT1 mutations remains challenging and controversial. Immunosuppression to treat the autoimmune phenomena can cause exacerbation of infections. Ruxolitinib, a Janus kinase (JAK) family tyrosine kinase inhibitor targeting the JAK-STAT1 pathway, has been used to successfully treat CMC and alopecia areata12 and autoimmune cytopenias and CMC13 in 2 patients with GOF-STAT1 mutations. Its mechanism is thought to include reduction of exaggerated cytokine-driven STAT1 phosphorylation,14 reduction of follicular helper T-cell responses,13 improved TH17 cell differentiation,13 and induced IL-17 production in vitro.15 Granulocyte colony-stimulating factor has also been used to successfully treat CMC in 1 patient with a GOF-STAT1 mutation.16
In patients with severe recalcitrant disease, hematopoietic stem cell transplantation (HSCT) has been attempted with mixed results. Successful transplants were reported in 1 patient with CMC and a confirmed DNA-binding domain GOF-STAT1 mutation17 and in 2 patients with severe CMC who were not evaluated for STAT1 mutations,18,19 whereas transplantations in 3 patients with confirmed GOF-STAT1 mutations were unsuccessful.20–22 Because of the need for viable treatment options for patients with GOF-STAT1 mutations who have severe disease, we set out to obtain a more complete assessment of outcomes of HSCT and the challenges and complications encountered by using this approach.
METHODS
Patients
Patients undergoing transplantation were identified from national and international sites by request to specific transplant centers and by announcements made through the European Society for Blood and Marrow Transplantation Inborn Errors Working Party and the Primary Immune Deficiency Treatment Consortium. To be included, patients were required to have a confirmed heterozygous mutation in STAT1 that confers GOF activity. Deidentified data for each case were collected by using a questionnaire/spreadsheet filled out by investigators at each institution contributing a patient. All studies involving human subjects were performed in accordance with site-specific Institutional Review Board–approved protocols, as well as the guidelines in the 1964 Declaration of Helsinki and its later amendments.
Patients were categorized based on clinical and laboratory phenotype: IPEX-like, IPEX-like and CID, CID, and severe infections. Patients with an IPEX-like phenotype had evidence of polyendocrinopathy, enteropathy, and autoimmunity; patients with CID had low T-cell and/or B-cell quantities, abnormal lymphocyte proliferation to mitogens or antigens, or both. Patients categorized as those with only severe infections had no evidence of T- or B-cell defects, or immunologic assays had not been performed.
DNA sequencing
DNA was extracted, and full-length sequencing of STAT1 in genomic DNA was performed, as previously described.10 In some patients GOF-STAT1 mutations were identified by means of whole-exome sequencing and confirmed by using Sanger sequencing.3 In one patient GOF-STAT1 mutation was identified by means of whole-genome sequencing.23
Expression and phosphorylation of GOF-STAT1 mutants determined by means of immunoblotting
Various STAT1 mutants were generated by using site-directed mutagenesis with a pcDNA3-V5–based wild-type (WT) STAT1 expression vector.24 Immunoblot analysis was performed, as previously described.4 Briefly, WT or mutant STAT1-containing plasmids were transfected into a U3C STAT1-null fibrosarcoma cell line by using Lipofectamine LTX (Thermo Fisher Scientific, Waltham, Mass), according to the manufacturer’s protocol. Twenty-four hours later, cells were stimulated with 1000 U/mL IFN-γ (R&D Systems, Minneapolis, Minn) for 20 minutes. Cells were then lysed and subjected to immunoblot analysis. Antibodies to detect Tyr701 phosphorylated STAT1 (D4A7; Cell Signaling, Danvers, Mass), STAT1 (BD Biosciences, San Jose, Calif), and β-actin (Sigma-Aldrich, St Louis, Mo) were used. Experiments were performed in triplicate to confirm reproducibility.
Luciferase reporter assay to evaluate transcriptional activation by GOF-STAT1 mutants
WT and the STAT1 mutants generated by means of site-directed mutagenesis (see above) were evaluated by using a Luciferase assay that measures luciferase activity of a reporter gene under the control of the γ-activated sequence (GAS) promoter, as previously described.3 Reporter plasmids (Cignal GAS Reporter Assay Kit; SABiosciences, Frederick, Md) and WT or mutant STAT1-containing plasmids were transferred into U3C cells by means of lipofection. Twenty-four hours after transfection, the cells were stimulated with 10, 102, or 103 U/mL IFN-γ for 16 hours. The Dual-Glo Luciferase Assay System (Promega, Madison, Wis) was used to analyze firefly and Renilla luciferase activities. Experiments were performed in triplicate, and the data were expressed in relative luciferase units.
Cytokine-induced STAT1 phosphorylation
Intracellular staining for phosphorylated STAT1 (pSTATl) was performed on thawed PBMCs (from patients 1 and 2) allowed to recover for 2 hours in complete medium. Cells were then stained with anti-human CD4 antibody (fluorescein isothiocyanate–conjugated mouse clone M-T441; Ancell Immunology Research Products, Bayport, Minn) in sodium azide–free buffer (0.1 % BSA in 1× PBS), washed twice in complete medium, and seeded at 100,000 cells per well in 96-well plates at a volume of 100 μL. After 30 minutes of incubation at 37°C, cells were stimulated with either IL-27 (200 ng/mL) or IL-6 (50 ng/mL) for 7.5, 15, or 30 minutes and fixed for 10 minutes at 37°C with prewarmed paraformaldehyde (at a final concentration of 2%). Cells were then washed twice and permeabilized with prechilled (20°C) BD Phosflow Perm Buffer III (BD Biosciences). After 30 minutes on ice, cells were washed twice with staining buffer (2% FBS in DPBS) and stained for 40 minutes on ice with anti-human phosphorylated STAT1 antibody (Alexa Fluor 647–conjugated mouse clone 4a, BD Biosciences) directed against the phosphorylated tyrosine at position 701 (Y701). Data were collected with a BD LSR II (BD Biosciences). FlowJo software (version 7.2.5/version 10.0.7 (TreeStar, Ashland, Ore) was used for data analysis.
Statistical analysis
Kaplan-Meier survival curves and significance of differences in overall survival (OS) and event-free survival (EFS) were generated and evaluated by using the Online Application for Survival Analysis (OASIS).25 EFS was determined by analysis of time to first transplant-related complication.
RESULTS
Patients
The clinical characteristics of the 15 patients with GOF-STAT1 mutations included in this retrospective study are presented in Table I. They were submitted by 12 participating centers from Canada, the United Kingdom, The Netherlands, Japan, Peru, Russia, Spain, Turkey, and the United States and met the criteria for study inclusion. The clinical course of 4 patients (P7, P11, P12, and P15) has been published previously,11,17,20–22 but those reports lack comprehensive data about HSCT, which have been included in this report. The cohort consists of 9 male and 6 female subjects who ranged in age from 13 months to 33 years at the time of transplantation (Table I). Of this cohort, 6 are alive and 9 died of complications related to HSCT (Table II and see Table El in this article’s Online Repository at www.jacionline.org).
TABLE I.
Clinical phenotype of GOF-STAT1 mutations
| No. and country of origin | Sex | cDNA mutation protein change domain | Mutation analysis done before/after transplantation | Phenotype | Pretransplantation complications |
|||
|---|---|---|---|---|---|---|---|---|
| Infections | Autoimmunity | Growth | Other | |||||
| 1. United States | Male | c. 983 A>G p.H328R CC |
Pre-HSCT Sanger sequencing |
IPEX-like | Norovirus enteritis VRE and Pseudomonas abscess Clostridium difficile enteritis Mycobacterium fortuitum mediastinal lymphadenitis |
Enteropathy Type 1 diabetes Thyroiditis |
FIT | — |
| 2. Russia | Male | c. 1154C>T p. T385M DB |
Pre-HSCT Sanger sequencing |
IPEX-like | CMC Pulmonary aspergillosis Recurrent pneumonia BCG lymphadenitis |
Enteropathy Autoimmune neutropenia Thyroiditis |
FTT | Eczema |
| 3. United States | Male | c. 1154C>T p. T385M DB |
Pre-HSCT Whole-genome sequencing |
IPEX-like | CMC | Enteropathy Thyroiditis Growth hormone deficiency + anti-GAD antibody − hyperglycemia with concurrent steroid use |
FTT | — |
| 4. Spain | Male | c. 1154C>T p. T385M DB |
Pre-HSCT Sanger sequencing |
IPEX-like CID | CMC Cryptococcal meningitis Pneumonia Pulmonary tuberculosis |
AIHA Type 1 diabetes IBD Glomerulonephritis Autoimmune hepatitis |
Normal | — |
| 5. Canada | Female | c. 1154C>T p. T385M DB |
Post-HSCT Sanger sequencing |
IPEX-like CID | VZV Systemic CMV Pneumonia |
Type 1 diabetes IBD Thyroiditis |
FTT | — |
| 6. The Netherlands | Male | c. 494A>G p.D165G CC |
Pre-HSCT Sanger sequencing |
CID | Pulmonary aspergillosis MCV |
Autoimmune hepatitis Thyroiditis |
FTT | — |
| 7. Turkey | Male | c.820G>A p.R274W CC |
Pre-HSCT Sanger sequencing |
CID | CMC Orf cutaneous infection Mycotic cerebral aneurysms Pulmonary CMV Pneumonia (Haemophilus influenzae, Pseudomonas aeruginosa, Streptococcus pneumoniae) |
AIHA Thyroiditis Autoimmune hepatitis Pernicious anemia Anti-phospholipid syndrome |
Normal | — |
| 8. Japan | Female | c. 821 G>A p.R274Q CC |
Pre-HSCT Sanger sequencing |
CID | CMC Pulmonary aspergillosis VZV Pneumonia (H influenzae, S pneumoniae) |
None | FTT | Severe gastroesophageal reflux |
| 9. Japan | Male | c.876C>A p.D292E CC |
Pre-HSCT Sanger sequencing |
CID HLH |
CMC Parvovirus B19 Recurrent sinusitis (H influenza, S pneumoniae) |
Vitiligo Pure red cell aplasia* |
FTT | Gray hair depigmentation |
| 10. Japan | Female | c.881T>C p.I294T CC |
Post-HSCT Whole-exome sequencing |
CID | CMC MCV VZV CMV Norovirus BK virus JC viremia Chronic sinusitis, otitis media, tonsillitis, bronchitis, pneumonia Bronchiectasis Cutaneous abscess |
AIHA Autoimmune Neutropenia† Autoimmune thrombocytopenia Colitis |
Normal | Malrotated kidney and pancreas divisum laryngeal edema chronic liver dysfunction |
| 11.Canada | Male | c. 1154C>T p.T385M DB |
Pre-HSCT Whole-exome sequencing |
CID | CMC Pneumonia Otitis media |
None | Short stature | — |
| 12. Canada | Female | c. 1189 A>G P.N397D DB |
Post-HSCT Sanger sequencing |
CID HLH |
CMC EBV with microabscessed spleen and kidneys Cutaneous HSV Pneumonia |
None | Normal | — |
| 13. United Kingdom | Female | c.1398 C>G p.S466R DB |
Pre-HSCT Sanger sequencing |
CID | CMC Bronchiectasis EBV and Adenovirus pneumonia and colitis VZV HHV-6 CMV Pyelonephritis Blepharitis Paronychia |
None | FTT | — |
| 14. Japan | Male | c. 1169 T>C P.M390T DB |
Post-HSCT Sanger sequencing |
Severe infections | CMC HSV VZV Chronic EBV Recurrent otitis media (Staphylococcus aureus) Recurrent pneumonia (S aureus, S pneumoniae) Sepsis (S aureus) Urinary tract infection |
Hypothyroidism | FTT | — |
| 15. Peru | Female | c. 1189 A>G p.N397D DB |
Pre-HSCT Sanger sequencing |
Severe infections | CMC CMV Severe diarrhea (Escherichia coli, Klebsiella pneumoniae) Sepsis |
None | FTT | — |
AIHA, Autoimmune hemolytic anemia; CC, coiled-coil; CMV, cytomegalovirus; DB, DNA binding; FTT, failure to thrive; GAD, glutamic acid decarboxylase; HHV-6, human herpes virus 6; HSV, herpes simplex virus; IBD, inflammatory bowel disease; MCV, molluscum contagiosum virus; VRE, vancomycin-resistant Enterococcus; VZV, varicella zoster virus.
Pure red cell aplasia likely secondary to parvovirus.
No anti-neutrophil antibody detected.
TABLE II.
Transplantation course and outcome of patients with GOF-STAT1 mutations
| Patient no. | Phenotype | Age at transplantation | Donor HLA match | CD34 dose | Conditioning |
|---|---|---|---|---|---|
| 1 | IPEX-like | 4 y | MUD, 10/10 match | 8 × 106/kg | Fludarabine, 150 mg/m2, days −8 to −4 Melphalan, 140 mg/m2, day −3 Almetuzumab, 10, 15, or 20, days −21 to −19 |
| 2 | IPEX-like | (1) 6 y | (1) T cell α/β/CD19+ depleted matched unrelated PBSCs, 10/10 match | (1) 11.21 × 106/kg | (1) Treosulfan, 42 mg/m2, days −5 to −3 Fludarabine, 150 mg/m2, days −6 to −2 ATG, 5 mg/kg, days −5 to −4 Rituximab, 375 mg/m2, day −1 |
| (2) 6 y | (2) T cell α/β/CD 19+ depleted haploidentical PBSCs | (2) 14.56 × 106/kg | (2) TBI, 6 gray Fludarabine, 30 mg/m2, days −6 to −2 ATG, 2.5 mg/kg, days −5 and −4 Cyclophosphamide, 60 mg/kg, days −3 and −2 Melphalan, 140 mg/m2, day − 1 Rituximab, 375 mg/m2, day − 1 |
||
| 3 | IPEX-like | 12 y | MUD, 10/10 match | 4.58 × 106/kg | Fludarabine, 150 mg/m2, days −8 to −4 Melphalan, 140 mg/m2, day −3 Almetuzumab, 10, 15, 20 mg; days −21 to −19 |
| 4 | IPEX-like CID |
29 y | MUD PBSCs, 10/10 match | 3.33 × 106/kg | Fludarabine, 50 mg/d, days −6 to −3 Melphalan, 140 mg/m2/d, day −3 to −2 Alemtuzumab, 12 mg/d, days −10 to −6 |
| 5 | IPEX-like CID |
7 y | MUD, 6/6 match | 6.5 × 106/kg | Cyclophosphamide, 50 mg/kg/d, days −5 to −2 Busulfan, 4 mg/kg/d, days −9 to −6 |
| 6 | CID | (1) 17 y | (1) UCB, 5/6 matched | (1) 0.14 × 106/kg | (1) Busulfan (myeloablative TDM AUC 90 mg*h*I) Fludarabine, 160 mg/m2 ATG, 10 mg/kg |
| (2) 17 y | (2) UCB, 6/6 matched | (2) 0.14 × 106/kg | (2) Treosulfan, 42 g/m2 Fludarabine, 160 mg/m2 TBI, 2 × 2 Gy | ||
| 7 | CID | 33 y | MRD, 6/6 matched | 3.84 × 106/kg | None |
| 8 | CID | 18 y | MRD, 8/8 matched | 8.14 × 106/kg | Busulfan, 4 mg/kg/d, days −9 to −6 Cyclophosphamide, 50 mg/kg/d, days −5 to −2 |
| 9 | CID HLH |
(1) 8 y (2) 8 y (3) 8 y |
(1) MMUD, 6/8 matched (2) UCB, 7/8 matched (3) Haploidentical marrow, 5/8 matched |
(1) 2.3 × 106/kg (2) 0.77 × 105/kg (3) 2.09 × 106/kg |
(1) Fludarabine, 180 mg/m2, days −9 to −6 Busulfan, 16 mg/kg, days −5 to −2 rATG, 8 mg/kg, days −9 to −6 (2) Fludarabine, 90 mg/m2, days −4 to −2 Cyclophosphamide, 1 g/m2, day −2 Etoposide, 100 mg/m2, day −3 TB1, 4 Gy; day −5 (3) No conditioning |
| 10 | CID | 12 y | UCB, 5/8 matched | 0.126 × 106/kg | Fludarabine, 180 mg/m2, days −7 to −2 Busulfan, 7.6 mg/kg, days −3 to −2 ATG, 8 mg/kg, days −9 to −6 |
| 11 | CID | 7 y | MRD, HLA identical | 5.7 × 108/kg nucleated cells | Busulfan, 16 mg/kg Cyclophosphamide, 200 mg/kg |
| 12 | CID HLH |
10 y | MUD, 10/10 matched | 6.32 × 106/kg | Busulfan, 3.6 mg/kg, days −8 to −5 Etoposide, 30 mg/kg/dose, day −4 Cyclophosphamide, 60 mg/kg, days −3 to −2 |
| 13 | CID | 7.5 y | MUD PBSCs | 31.2 × 106/kg | Treosulfan, 14 mg/m2/d, days −7 to −5 Fludarabine, 30 mg/m2/d, days −6 to −2 Alemtuzumab, 0.2 mg/kg/d, days −8 to −4 Ruxolitinib, 2-wk course (5 mg/d); stopped day −9 |
| 14 | Severe infections | 29 y | MUD, 6/6 matched | 3 × 106/kg nucleated cells | Cyclophosphamide, 200 mg/kg, days −10 to −7 Fludarabine, 125 mg/m2, days −6 to −2 ATG, 25 mg/kg, days −6 to −2 TBI, 3 Gy, day −7 |
| 15 | Severe infections | 13 mo | MRD 6/6, matched | 6.56 × 106/kg | Fludarabine, 30 mg/m2/d, days −8 to −3 Melphalan, 140 mg/m2/d, day −3 ATG, 5 mg/kg/d, days −3 to −2 |
| Cyclosporine MMF |
D+12 | EBV reactivation catheter-associated venous thrombus Supraventricular tachycardia |
None | Secondary graft loss within 30 d Mixed chimera with 2% donor myeloid cells and 39% donor lymphoid cells Recurrence of Clostridium difficile colitis Recurrent sinusitis and pneumonia (bacterial and viral) Abnormal pulmonary diffusion capacity Progressive lymphopenia Hypogammaglobulinemia On IVIG |
Alive |
| (1) Tacrolimus | (1) Day +26 (lymphocytes) | (1) Streptococcus parasanguis bacteremia | (1) None | (1) Secondary graft loss in first 30 d | Alive |
| (2) Tacrolimus | (2) Day +35 (lymphocytes) | CMV viremia (2) Drug-associated nephropathy |
(2) Grade I skin | (2) Full immune reconstitution Alive 100% donor Enteropathy resolved Improved growth |
|
| Tacrolimus MMF Methotrexate |
D+16 | None | Grade III skin | Full immune reconstitution Alive 100% donor Enteropathy resolved Improved growth |
Alive |
| Sirolimus Tacrolimus |
D+23 | Severe thrombocytopenia Reaction to almetuzumab Pneumonia |
Grade I skin | Secondary graft loss over 2 mo Continued lymphopenia Continued hypogammaglobulinemia Continued infections |
Dead 2 y after pneumonia and sepsis |
| Prednisone Cyclosporine |
No engraftment | CMV | Grade I skin | Died D+12 | Died Multiorgan failure |
| (1) Cyclosporine Prednisone | (1) Primary graft failure | (1) Fungal Pneumonia | (2) Grade III gut | (1) Primary graft failure | Died |
| (2) Cyclosporine MMF | (2) D+15 | (2) BK virus reactivation Adenovirus reactivation Cryptosporidium gut Pneumonia |
(2) Full immune reconstitution | 4 mo after pneumonia | |
| None | D+25 | Severe thrombocytopenia | None | Primary engraftment but not immune reconstituted | Died 3 mo after bleeding from mycotic aneurysms |
| Tacrolimus Methotrexate |
No engraftment | Cardiomyopathy and heart failure secondary to cyclophosphamide | None | Died D+3 from heart failure |
|
| (1) Tacrolimus Short methotrexate (2) Tacrolimus Short methotrexate (3) Cyclosporine Dexamethasone |
(1) D+19 (2) None (3) None |
Refractory HLH Streptococcus mitis sepsis Cardiac effusion Ascites Pancreatitis Adenovirus viremia and cystitis |
None | (1) Secondary graft loss in first 30 d (2) Refractory HLH (3) Refractory HLH |
Died D+109 from multiorgan failure |
| Tacrolimus Methotrexate Prednisolone |
D+33 | Acute pulmonary edema TMA Recurrent pancreatitis |
None | Full immune reconstitution 100% Donor | Alive |
| Cyclosporine Methylprednisolone |
D+12 | Hemorrhagic ulcers with massive GI bleeding Hemorrhagic cystitis Pulmonary aspergillosis |
Acute GvHD skin and GI tract | Full immune reconstitution 95% Donor in myeloid and lymphoid lines |
Alive |
| Prednisone Cyclosporine Methotrexate Almetuzumab used as salvage therapy after HSCT |
D+16 | Refractory HLH GI bleeding Pulmonary hemorrhage Toxic epidermal necrosis Renal failure |
Grade II skin | Refractory HLH | Died D+42 from multiorgan failure |
| Cyclosporine MMF |
D+16 | None | Grade II skin | Full immune reconstitution 100% Donor | Alive |
| Tacrolimus Methotrexate |
D+17 | CMV Candidiasis Sepsis |
None | Secondary graft loss in first 90 d | Died D+410 from sepsis |
| Cyclosporine Methotrexate |
D+15 | Severe thrombocytopenia Increased transaminase levels | None | Secondary graft loss within first 100 d | Died 10 mo after from fulminant lung infection |
ATG, Antithymocyte globulin; AUC, area under the blood concentration time curve; CMV, cytomegalovirus; GvHD, graft-versus-host disease; MMF, mycophenolate mofetil; IVIG, intravenous immunoglobulin; rATG, rabbit anti-thymocyte globulin; TMA, thrombotic microangiopathy; GI, gastrointestinal; TBI, total body irradiation; TDM, therapeutic drug monitoring; UCB, unrelated cord blood.
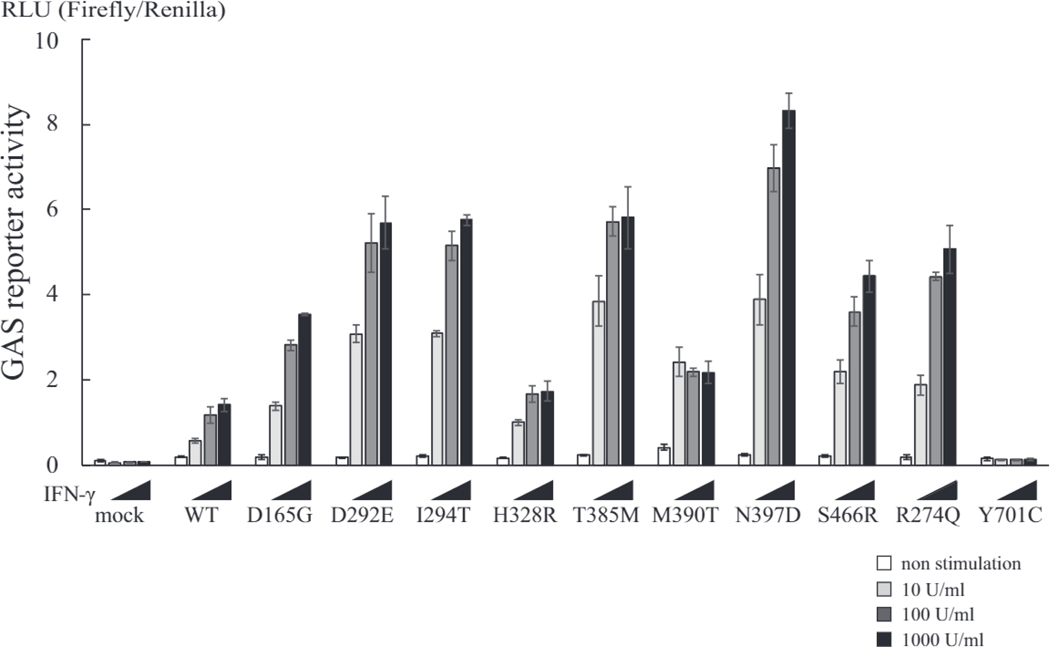
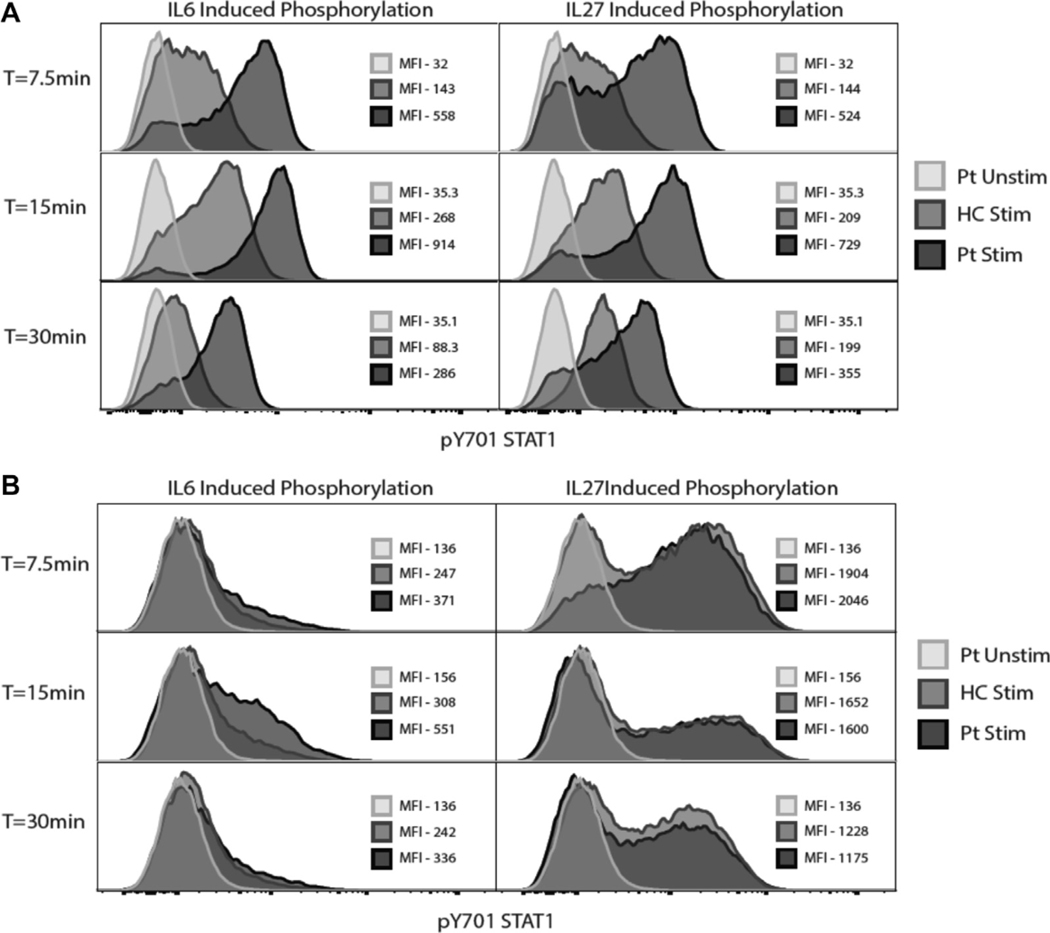
All 15 patients had heterozygous missense mutations in either the coiled-coil or DNA-binding domain of STAT1 (Table I). Five patients (P2-P5 and P11) from 4 different countries shared the same c.1154C>T, p.T385M missense mutation, and 2 patients (P12 and P14) from Canada and Peru shared the same c.1189A>G, p.N397D missense mutation, both of which were located in the DNA-binding domain. The remaining mutations were identified in only a single patient. All 10 unique mutations conferred GOF activity with enhanced STAT1 phosphorylation in response to IFN-γ (Fig 1) and showed increased GAS-dependent reporter gene transcriptional activity after stimulation with IFN-γ (Fig 2): P1, who was studied before HSCT, demonstrated enhanced and prolonged STAT1 phosphorylation after stimulation with IL-6 or IL-27 (Fig 3, A).
FIG 1.
Evaluation of expression and phosphorylation of GOF-STAT1 mutants by using immunobiotting. GOF-STAT1 mutations led to enhanced phosphorylated STAT1 (pSTAT1) expression after stimulation with IFN-γ. U3C cells were transfected with STAT1 mutants or WT and stimulated with IFN-γ for 20 minutes. Western blotting was performed with anti-phosphorylated STAT1, anti-STAT1, and anti-β-actin antibodies. Mutations affecting Y701 prevent STAT1 phosphorylation.
FIG 2.
Luciferase reporter assay to evaluate transcriptional activation by GOF-STAT1 mutants. STAT1 mutants led to enhanced luciferase GAS-induced activity. Transcriptional responses to increasing concentrations of IFN-γ (white bar, nonstimulated; light gray bar, 10 U/mL; dark gray bar, 100 U/mL; black bar, 1000 U/mL) were added to U3C cells transfected with STAT1 mutants or WT STAT1 and cultured for 16 hours before GAS-induced activity was measured. Experiments were performed in triplicate, and data are expressed in relative luciferase units (RLU). Individual reporter assays were performed 3 times to confirm reproducibility. Y701 mutation eliminated transcriptional activity of STAT1.
FIG 3.
Cytokine-induced STAT1 phosphorylation. A, Pre-HSCT PBMCs from patient 1 (H328R) led to hyperphosphorylation and delayed dephosphorylation after IL-6 and IL-27 stimulation. B, Post-HSCT PBMCs from patient 2 IT385M) show normal phosphorylation kinetics compared with healthy control subjects (HC).
Clinical phenotype and pre-HSCT complications
Infections occurred in all 15 patients before HSCT, with fungal infections being the most common. Twelve patients had CMC affecting the nails, skin, oral mucosa, and intestinal tract. Five had invasive fungal infections, including pulmonary aspergillosis in 3 patients, candidemia in 1 patient, and cryptococcal meningitis in 1 patient. Bacterial infections were also frequent, most commonly affecting the upper and lower respiratory tract, but also causing sepsis in 2 patients and gastroenteritis in 2 patients. When culture results were available, encapsulated organisms were predominant (Haemophilus influenzae, n = 3; Streptococcus pneumoniae, n = 3), followed by Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Enterococcus faecalis, and Clostridium difficile. Recurrent pneumonia led to bronchiectasis in 2 patients, one of whom has recurrent Pseudomonas species pneumonia. Varicella zoster virus and cytomegalovirus were the most common viral infections among this cohort (n = 5 each), but multiple other cutaneous and invasive viral infections occurred (EBV in 3 patients; molluscum contagiosum virus, norovirus, and herpes simplex virus each in 2 patients; and parvovirus, BK virus, JC virus, adenovirus, and human herpes virus 6 each in 1 patient). Disseminated EBV led to hemophagocytic lymphohistiocytosis (HLH) in 1 patient (P12), and parvovirus B19 induced pure red cell aplasia in 1 patient (P9). BK and JC viremia occurred in the context of immunosuppression for treatment of chronic neutropenia and thrombocytopenia in another (P10). Nontuberculous mycobacterial infections were observed in patients 1 and 2, both with lymphadenitis. One patient (P4) had pulmonary tuberculosis.
Autoimmune disorders were observed in 10 patients, 5 of whom had an IPEX-like syndrome (P1-P5). Enteropathy, the hallmark feature of those with IPEX-like syndrome, was often associated with other autoantibody/T cell-mediated manifestations, including type 1 diabetes mellitus, thyroiditis, autoimmune neutropenia, hemolytic anemia, and growth hormone deficiency. Four of the IPEX-like patients had the same missense mutation affecting the DNA binding domain (c.l 154C>T p.T385M). In 6 of the 10 patients without a typical IPEX-like syndrome, a variety of autoimmune symptoms occurred, including vitiligo, hypothyroidism, autoimmune cytopenias, antiphospholipid syndrome, pernicious anemia, and autoimmune hepatitis.
Two patients in this cohort had features consistent with HLH that were refractory to medical therapy, contributing to the decision to initiate HSCT (see Fig E1 in this article’s Online Repository at www.jacionline.org). Patient 9 had HLH associated with pneumonia at age 7 years. He was successfully treated with prednisolone and high-dose intravenous immunoglobulin, but HLH relapsed a few months later, leading to agranulocytosis unresponsive to granulocyte colony-stimulating factor, prednisone, intravenous immunoglobulin, and cyclosporine. Despite 3 attempted HSCTs, HLH never cleared, and the patient died of multiorgan failure 3 months after the third transplantation and secondary graft failure from the first transplantation.
Patient 12 had HLH at age 10 years in association with EBV viremia while also having microabscesses of the spleen and kidneys and cutaneous herpes zoster infection. She did not respond to dexamethasone, and HLH progressed to involve the central nervous system. Treatment was escalated to include etoposide, cyclosporine, and intrathecal methotrexate but unfortunately failed to induce remission, and the patient died of multiorgan failure within 1 week of transplantation.
Both patients had classic presentations of HLH with very increased inflammatory markers and ferritin and hemophagocytosis visualized in the bone marrow. Assessment of natural killer (NK) cell cytotoxicity was not performed in patient 9 and was normal in patient 12.
Antibody deficiency was present in 9 patients, prompting treatment with immunoglobulin supplementation (P4, P6–P11, P13, and P14). Nine patients (P4, P5, and P7–13) had T-cell lymphopenia, and 3 had abnormal T-cell function when tested (P5, P6, and P11). Eight patients with T-cell defects also had concurrent B-cell and/or NK cell lymphopenia (P4, P6, and P8–13). T-cell receptor excision circles (TRECs) and kappa-deleting recombination excision circles (KRECs) were quantified in 3 patients (P8–P10) and were less than the limit of detection in all 3. Defects in cellular and humoral immunity occurred independent of the presence of autoimmunity in some and coincided with autoimmunity in others.
Transplantation course and complications
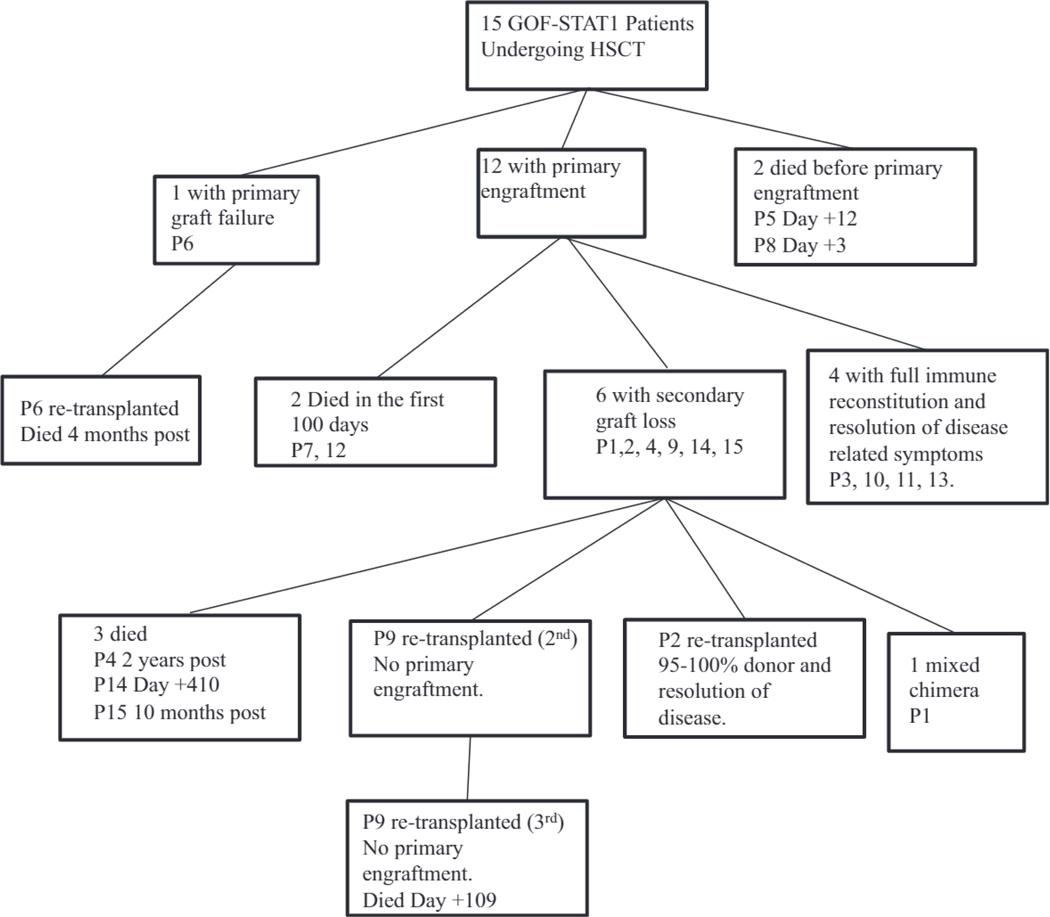
Nineteen HSCTs were performed in this cohort of 15 patients (Fig 4 and Table II). One patient (P9) received 3 transplants, and 2 (P2 and P6) received 2 transplants. Indications for HSCT were severe clinical manifestations, including recurrent infections, autoimmunity, IPEX-like symptoms refractory to medical therapy, HLH, and CID (see Fig E1). Of those patients who survived HSCT, the mean age at HSCT was 8.5 years (range, 4–12 years); of those who died, the mean age was 16.5 years (range, 13 months to 33 years) at the time of transplantation. A variety of graft sources were used, including matched related donors (MRDs; 4 HSCTs), matched unrelated donors (MUDs; 5 HSCTs), mismatched unrelated donors (MMUDs; 1 HSCT), a haploidentical donor (1 HSCT), partially matched unrelated umbilical cord blood (UCB; 3 HSCTs), and fully matched UCB (1 HSCT). Peripheral blood stem cells (PBSCs) were used in 4 transplants. Four of six survivors received unrelated grafts (2 MUD-marrow, 1 MUD-PBSCs, and 1 partially matched UCB); the fifth and sixth survivors received haploidentical PBSCs and matched related bone marrow, respectively.
FIG 4.
Outcome of patients with GOF-STAT1 mutations after HSCT. Fifteen patients with GOF-STAT1 mutations underwent HSCT. Primary engraftment occurred in 12. Six had secondary graft loss. Six are alive, and 5 have full immune reconstitution and reversal of disease manifestations.
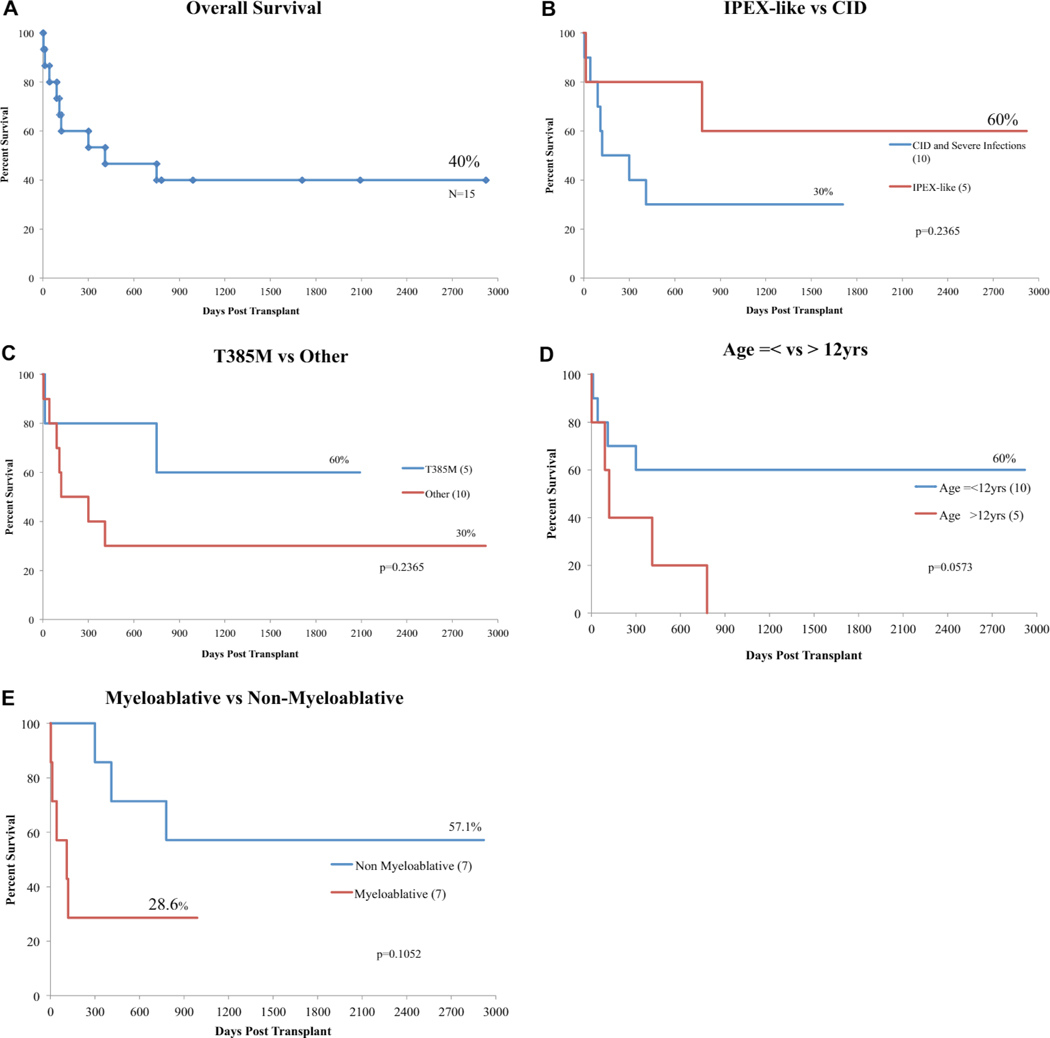
Reduced-intensity conditioning (RIC) regimens were used most frequently (P1, P2 [first HSCT], P3, P4, P6 [second HSCT], P9 [second HSCT], P10, P13, P14, and P15) and were associated with higher OS (P =.11; Fig 5, E) and EFS (P = .07; see Fig E2, E, in this article’s Online Repository at www.jacionline.org). The decision to use RIC regimens was institution specific. Patients 1, 3, and 4, 2 of whom survived, received the same RIC regimen consisting of fludarabine, melphalan, and alemtuzumab based on its previous success in patients with IPEX syndrome.26 Myeloablative regimens were used in 7 HSCTs (haploidentical PBSCs in P2, MMUD in P9, MRD in P8 and P11, partially matched UCB in P6, and MUD in P5 and P12). Five of 7 patients (P5, P6, P8, P9, and P12) who received myeloablative regimens died; however, death was complicated by chronic HLH in 2 patients (P9 and P12). Patients 5 and 8 died before primary engraftment, the latter of which was directly related to cyclophosphamide toxicity. Patient 6 had secondary graft failure and did not survive a second transplantation. Patient 2 received myeloablative conditioning before a second HSCT after secondary graft loss from the first transplantation with RIC. Patient 11 had full immune reconstitution and resolution of disease symptoms and is alive 2 years after HSCT.
FIG 5.
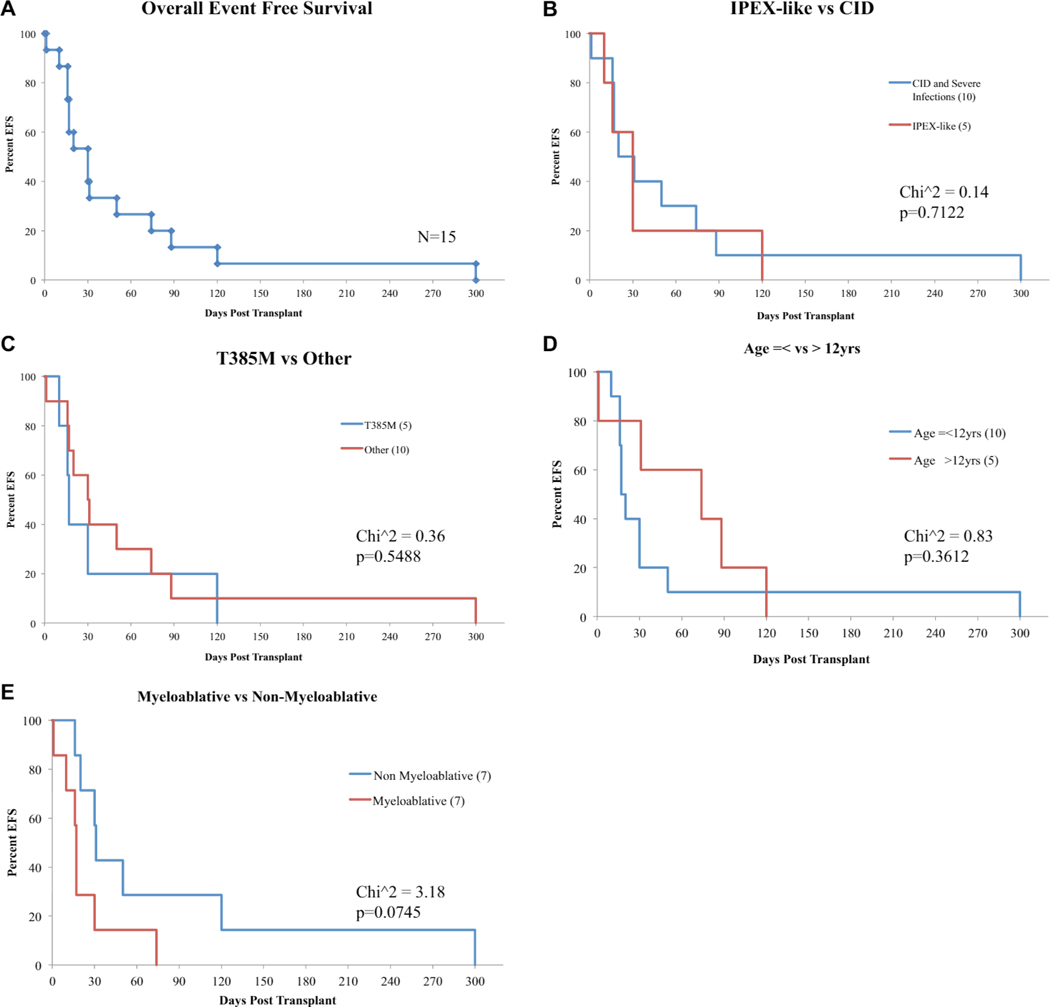
Overall posttransplantation survival and survival analysis. A, Overall posttransplantation survival in patients with GOF-STAT1 mutations. B, Survival analysis comparing patients with an IPEX-like presentation versus patients with CID, significant infections, or both. C, Survival analysis comparing patients with T385M amino acid substitution versus patients with other GOF-SLAT1 mutations. D, Survival analysis comparing patients younger or older than 12 years of age. E, Survival analysis comparing myeloablative and nonmyeloablative protocols. In Fig 5, B-E, numbers in brackets represent the number of patients in each group. In patients undergoing more than 1 HSCT, survival is calculated since the last transplantation performed.
A total of 80 posttransplantation events occurred (see Table E1), with infections consisting of viral reactivation and sepsis being the most common. Median event-free time from a posttransplantation complication was 31.5 days. There were also a high number of vascular and cardiac-related transplant complications: catheter-associated venous thrombus formation and supraventricular tachycardia in the same patient and cardiac effusion, thrombotic microangiopathy, and cyclophosphamide-induced cardiac toxicity each in 1 patient. The association of vascular aneurysms and complications in patients with GOF-STAT1 mutations8,10,27 could be a risk factor for cardiac- and vascular-related events after HSCT. Other noninfectious complications of HSCT included pancreatitis, acute pulmonary edema, gastrointestinal bleeding, and hepatitis. Overall EFS was low (10% by day +100 after HSCT) and was not affected by age at transplantation, phenotype, genotype, or conditioning regimen (see Fig E2, B–E).
Immune reconstitution
Primary engraftment, which was defined as an absolute neutrophil count of greater than 500 cells/μL for 3 consecutive days, occurred at a median of day + 18 in 14 (74%) of 19 HSCTs and in 13 (80%) of 15 patients with their first HSCT. With the exception of patients 5 and 8, who died soon after transplantation and before engraftment, 12 of 13 patients engrafted with their first transplantation. Primary graft failure occurred in patient 6.
Secondary graft failure occurred in 6 patients (within the first 4 months in 5 patients) after HSCT that had primary engraftment and survived the first 100 days (Fig 4 and Table II and see Fig E3, A, in this article’s Online Repository at www.jacionline.org). No isolated risk factors for secondary graft loss, including phenotype, genotype, age at transplantation, conditioning regimen, or donor source, were identified (see Fig E3, B). Of the 6 patients with secondary graft failure: 2 (P2 and P9) underwent subsequent transplant, with 1 (P2) surviving with full immune reconstitution. The other survivor with secondary graft failure (P1) is a mixed chimera, has experienced return of symptoms, and has not yet undergone retrains plantation (Fig 4 and Table II). All 5 survivors (P2, P3, P10, P11, and P13) with 95% to 100% donor chimerism established full immune reconstitution and complete reversal of immunodeficiency and resolution of infectious and autoimmune manifestations.
Poor thrombocyte recovery was observed in 3 patients (P4, P7, and P15), resulting in severe transfusion-dependent thrombocytopenia. Patient 7, who received an MRD transplant, engrafted without conditioning but died 3 months after HSCT from bleeding at sites of mycotic brain aneurysms; no bleeding episodes occurred in patients 4 or 15.
Graft-versus-host disease (GvHD) prophylaxis was used in all but 1 patient, P7, who was pancytopenic and received an MRD transplant. Acute GvHD was generally mild or well controlled by immunosuppression. Acute GvHD occurred after 8 of 18 evaluated HSCTs in 8 (57%) patients (P2 [second HSCT], P3, P4, P5, P6 [second HSCT], P11, P12, and P13; Table II). Seven patients had acute GvHD of the skin (grades 1–3), and in all cases there was good response to topical and systemic corticosteroids. Two patients (P6 and P11) had acute GvHD of the gastrointestinal tract.
Outcome
Overall, 6 (40%) patients survived and are presently more than 1 year after transplantation (Fig 4 and Table II). Nine patients died, 7 less than 1 year after HSCT (Fig 4 and Table II and Table El). Of the surviving patients. 5 of 6 had full immune reconstitution, and 1 is a mixed chimera. One survivor had complete secondary graft loss (P2), and 1 (P1) has split donor chimerism with 2% donor myeloid cells and 39% donor lymphoid cells. Patient 2 underwent a second HSCT and had full immune reconstitution and reversal of in vitro hyperphosphorylation of STATI (Fig 3, B). Graft loss was gradual in patient 1 and associated with return of infections, enteropathy, continued failure to thrive, and development of lymphopenia and hypogammaglobulinemia. Symptoms remain milder than those in his pre-HSCT condition.
Those patients with IPEX-like syndrome who engrafted and have 95% to 100% immune reconstitution (P2 and P3) resolved enteropathy within the first 100 days, and immunosuppression could be discontinued within 1 year. Patient 3 was able to discontinue parenteral nutrition by day +38. All surviving patients with 95% to 100% donor engraftment (P2. P3, P10, P11, and P13) have complete resolution of autoimmunity and infections and underwent catch-up growth.
Two patients with CID had HLH several months before HSCT (P9 and P12). In both patients HLH was lethal when the patients underwent HSCT in the presence of active disease. HLH-associated death accounted for 22% of the lethal outcomes of HSCT in this cohort. A CID phenotype was associated with a higher frequency of posttransplantation infections (12/22 posttransplantation infections, see Table E1) and negatively affected OS (P = .24; Fig 5, B) and EFS (P = .14; see Fig E2, B), but neither reached statistical significance.
Death occurred in all patients by the end of this study who did not have some degree of donor engraftment and immune reconstitution. Only patients with 95% to 100% immune reconstitution had resolution of infections and autoimmunity (P2, P3, P10, P11, and P13). Younger age (P = .05; Fig 5, D), a mutation leading to T385M (P = .24; Fig 5, C), lack of CID (P = .24; Fig 5, B), and use of RIC (P = .11; Fig 5, E) were associated with increased OS, but only age at transplantation reached statistical significance. IPEX-like disease and mutation leading to T385M were favorable factors; however, patients with these characteristics underwent transplantation at a younger age. Four of 5 patients with IPEX-like disease and 3 of 4 with mutations leading to T385M were 12 years or younger at the time of HSCT suggesting that age and not phenotype or genotype most strongly predict OS and EFS.
DISCUSSION
This multinational cohort of 15 patients is the largest aggregation of patients with GOF-STAT1 mutations who have undergone HSCT yet collected. In 6 patients GOF-STAT1 mutations were identified retrospectively after HSCT and postmortem in 3. In none of the patients was HSCT elective but rather intended to be lifesaving to reverse severe infections, HLH, or autoimmunity. For this reason, survival rates might have been more dismal and would likely be better in patients undergoing transplantation earlier before the development of severe manifestations.
Data from this cohort suggest that HSCT is a viable and curative treatment option for patients with GOF-STAT1 mutations; however, disease-related complications are common and can strongly affect outcomes. Numerous questions surrounding appropriate patient selection, timing, donor, and conditioning regimen for HSCT in patients with GOF-STAT1 mutations still exist. However, our data suggest that HSCT can be considered curative, particularly if performed early in patients with GOF-STAT1 mutations with severe phenotypes, including those with IPEX-like symptoms, CID, serious life-threatening infections, and severe autoimmunity but not active HLH. Younger age was the strongest positive indicator of OS, suggesting a negative effect of disease-related morbidity on EFS and a higher rate of success when undergoing early transplantation. Because of the effect of age on OS and EFS, early recognition and diagnosis of GOF-STAT1 is imperative. OS and EFS were not affected by genotype, phenotype, or conditioning regimen. When assessed, abnormal TREC and KREC numbers coincided with immunodeficiency and predisposition to infections. The role of TREC and KREC analysis in early diagnosis and prognosis of patients with GOF-STAT1 mutations needs more investigation.
Five of 6 patients in this series with IPEX-like symptoms had the common DNA binding domain mutation c.1154C>T, p.T385M, which is known to be associated with IPEX-like disease.10 Ten patients in this series had mutations (c.1154C>T, p.T385M in P2–P5 and P11; c.494A>G, p.D165G in P6; c.820G>A; R274W in P7, c.821G>A, p.R274Q in P8; and c.1189A>G, p.N397D in P12 and P15) that were previously described in other patients as associated with severe clinical manifestations, including severe infections and autoimmune disease.11 In agreement with this, these patients also had severe infections, autoimmunity, and immunodeficiency. With the exception of the common DNA binding domain mutation c.1154C>T, p.T385M, which was associated with a higher incidence of IPEX-like symptoms and overall better outcome (Fig 5, C), there was no specific genotype phenotype outcome correlation. Within this cohort, patients with GOF-STAT1 mutations were preferentially observed in the DNA-binding domain (10/15), suggesting that mutations in this region are associated with worse infections, autoimmunity, and immunodeficiency, and patients with these mutations might benefit from early consideration of transplantation.
Although HLH has not been described as a major problem in patients with GOF-STAT1 mutations,1–10 the 2 patients reported here had severe HLH without entering remission and died shortly after HSCT during active disease. The mechanism of HLH in patients with GOF-STAT1 mutations is not well elucidated, but given the proposed role played by IFN-γ in the pathogenesis of HLH, development of HLH associated with GOF-STAT1 mutations might be related to hyperactivation of IFN-γ–dependent STAT signaling pathways. Defects in NK cell cytotoxicity in patients with GOF-STAT1 mutations have recently been reported,29,30 and this might also contribute to an increased risk of HLH in these patients, particularly in the context of viral infections.
IPEX-like symptoms are conventionally treated with long-term immunosuppression, including corticosteroids, calcineurin inhibitors, and rituximab. In the 3 patient with GOF-STAT1 mutations associated IPEX-like symptoms without CID (P1-P3), various immunosuppressive agents were ineffective in correcting the autoimmune features. HSCT was well tolerated in those with isolated IPEX-like disease. There were no deaths, and two thirds had full immune reconstitution with complete reversal of clinical manifestations, suggesting that HSCT should be considered in patients with GOF-STAT1 mutations with IPEX-like symptoms, especially if the symptoms are refractory to medical therapy.
Secondary graft loss occurred frequently (6/12 with primary engraftment) and did not discriminate between phenotype, genotype, conditioning regimen, age, or donor source. The reasons behind the high rate of secondary graft loss are unclear, including what role heightened IFN-γ–induced STAT1 signaling plays in patients with GOF-STAT1 mutations.
IFN-γ receptor deficiency and primary and secondary HLH are examples of primary immunodeficiency diseases in which heightened IFN-γ expression correlates with poor engraftment rates and worse outcome after transplantation.31,32 Recently, the JAK inhibitor ruxolitinib33 and anti–IFN-γ mAbs34 have been effective at suppressing lFN-γ–induced inflammation in murine and human HLH, respectively. In vitro exposure of T lymphocytes from patients with GOF-STAT1 mutations to ruxolitinib resulted in profound suppression of IFN-α and 1FN-β STAT1 phosphorylation,12,14 and treatment of a patients with a GOF-STAT1 mutation resulted in improvement of their individual symptoms.12,13 Ruxolitinib was used as immunosuppressive therapy in 1 patient in this series (P13) for 2 weeks before transplantation. This patient was one of the 6 survivors and had full immune reconstitution with no secondary graft loss. With the exception of acute GvHD, he has no other posttransplantation complications. The success of this specific case might suggest that use of JAK inhibitors, as well as anti-IFN- γ therapies, might be an effective strategy in controlling IFN-γ–induced inflammation in patients with GOF-STAT1 mutations in the setting of transplantation and could perhaps improve engraftment rate and overall outcome.
Our retrospective data demonstrate that HSCT can be curative for patients with GOF-STAT1 mutations. With full immune reconstitution, disease manifestations are reversed quickly and permanently. Because those with a severe phenotype have a less favorable outcome, patients should be considered for transplantation earlier to prevent serious complications and improve OS. Without novel strategies to control HLH, HSCT in patients with active disease is likely to fail.
Extended Data
FIG E1.
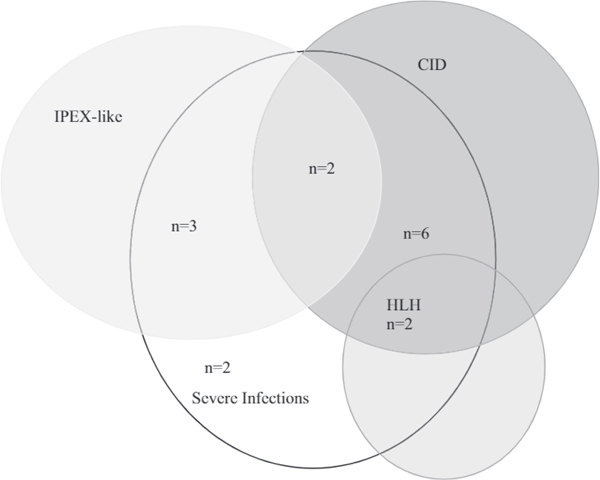
Indication for HSCT in patients with GOF-STAT1 mutations. All patients in this cohort had severe infections (n = 15). Indications for HSCT were IPEX-like symptoms (n = 3), CID (n = 6), CID with HLH (n = 2), IPEX-like symptoms and CID (n = 2), or only severe infections (n = 2).
FIG E2.
Overall posttransplantation event-free survival (EFS). A, Overall posttransplantation EFS in patients with GOF-STAT1 mutations. B, EFS analysis comparing patients with IPEX-like presentation versus patients with CID, significant infections, or both. C, EFS analysis comparing patients with T385M amino acid substitution versus patients with other GOF-STAT1 mutations. D, EFS analysis comparing patients younger or older than 12 years. E, EFS analysis comparing patients receiving myeloablative versus nonmyeloablative conditioning regimen. In Fig E2, B and D, numbers in brackets represent the number of patients in each group.
FIG E3.
Secondary graft failure data. A total of 19 HSCTs were performed in 15 patients with GOF-STAT1 mutations, including 3 patients who underwent 2 HSCTs and 1 patient who underwent 3 HSCTs. A total of 6 events of secondary graft failure were reported, 2 of which occurred in the same patient (patient no. 9). A, Survival curve showing the timing of each of the 6 events. B, Incidence of different variants in the graft failure group compared with their incidence in all HSCTs performed does not show any statistical significance. Each of the following variants was analyzed: presence of T385M amino acid substitution, clinical phenotype of IPEX-like disease, age less than or equal to 12 years at transplantation, use of a myeloablative conditioning protocol, and use of any stem cell donor other than an MRD (T385M mutation: 33.3% vs 31.58%, P = .94; IPEX-like: 50% vs 31.58%, P = .43; age < 12 years: 66.67% vs 73.68%, P = .7512; myeloablative conditioning: 16.66% vs 31.58%, P = .49; non-MRD: 100% vs 78.95%, P = .24).
TABLE E1.
Posttransplantation complications in patients with GOF-STAT1 mutations
| Day after transplantation | Reversible | Fatal | |
|---|---|---|---|
| Patient 1 | |||
| Infection | |||
| EBV reactivation | + 159 | Yes | No |
| Recurrent pneumonia | + 1464 | Yes | No |
| Bleeding (coagulopathy associated with colitis) | +2765 | Yes | No |
| CV | |||
| Catheter-associated thrombus | + 159 | Yes | No |
| SVT | + 159 | Yes | No |
| GI | |||
| Recurrence of colitis | +2756 | Yes | No |
| Heme | |||
| Lymphopenia | +2249 | No | No |
| Hypogammaglobulinemia | + 1611 | No | No |
| Secondary graft loss | + 30 | No | No |
| Patient 2 (first transplantation) | |||
| Infection | |||
| S parasanguis bacteremia | −2 | Yes | No |
| CMV viremia | + 19 | Yes | No |
| Secondary graft loss | + 33 | No | No |
| Patient 2 (second transplantation) | |||
| Renal/GU | |||
| Drug-associated nephropathy | + 28 | Yes | No |
| GvHD | + 30 | Yes | No |
| Patient 3 | |||
| GvHD | + 16 | Yes | No |
| Patient 4 | |||
| Heme | |||
| Severe thrombocytopenia | +5 | Yes (+490) | No |
| Lymphopenia | + 30 | No | no |
| Hypogammaglobulinemia | + 304 | No | No |
| Secondary graft loss | + 120 | No | No |
| Death (sepsis, pneumonia) | +779 | No | Yes |
| Patient 5 | |||
| Infection | |||
| CMV | Before transplantation | No | +7 |
| GvHD | + 10 | Yes | No |
| Death | + 12 | ||
| Patient 6 (first transplantation) | |||
| Infection | |||
| Fungal pneumonia | + 24 | Yes | No |
| BK virus reactivation | + 24 | No | No |
| Patient 6 (second transplantation) | |||
| Infection | |||
| Adenovirus reactivation | + 2 | Yes | No |
| Cryptosporidium gut | + 20 | Yes | No |
| Fungal pneumonia | + 74 | No | Yes |
| GvHD | + 17 | Yes | No |
| Death | +4 mo | No | Yes |
| Patient 7: Turkey | |||
| Infection | |||
| Mycotic aneurysm | Before transplantation | No | Yes |
| Bleeding | + 88 | No | Yes |
| Death | +3 mo | ||
| Patient 8 | |||
| CV | |||
| Cardiomyopathy | + 1 | No | Yes |
| Death | +3 | ||
| Patient 9 (first transplantation) | |||
| Infection | |||
| S mitis sepsis | + 22 | Yes | No |
| Hemophagocytosis | +25 (first) | No | No |
| Secondary graft loss | +45 | No | Yes |
| Patient 9 (second transplantation) | |||
| Infection | + 14 | No | Yes |
| Adenovirus viremia | +35 | ||
| CV | |||
| Cardiac/pleural effusion | + 21 | No | Yes |
| GI | |||
| Ascites | +21 | No | Yes |
| Renal/GU | |||
| Adenovirus cystitis | +29 | No | No |
| Secondary graft loss | +32 | No | Yes |
| Patient 9 (third transplantation) | |||
| Bleeding | + 17 | No | Yes |
| GI | |||
| Pancreatitis | + 13 | Yes | No |
| Death | + 109 | No | Yes |
| Patient 10: Japan | |||
| CV | |||
| TMA | +20 | Yes | No |
| Lung | |||
| Acute pulmonary edema | + 4 | Yes | No |
| GI | |||
| Recurrent pancreatitis | −1, +32, +42, +53, +58, +63 | Yes | No |
| Patient 11 | |||
| Infection | |||
| Pulmonary aspergillosis | + 150 | Yes | No |
| GI | |||
| Hemorrhagic bleeding | +30 | Yes | No |
| Renal/GU | |||
| Hemorrhagic cystitis | + 18, +120 | Yes | No |
| Heme | |||
| Lymphopenia | +50 | Yes | No |
| Hypogammaglobulinemia | +50 | ||
| GvHD | |||
| Skin | + 7 | Yes | No |
| GI | + 17 | Yes | No |
| Patient 12: Canada | |||
| Lung | |||
| Pulmonary hemorrhage | +20 | No | Yes |
| GI | |||
| GI bleeding | + 13 | Yes | No |
| Renal/GU | |||
| Renal failure | +21 | No | Yes |
| Other | |||
| TEN | + 16 | No | Yes |
| GvHD | + 12 | No | No |
| Death | +42 | No | Yes |
| Patient 13: United Kingdom | |||
| GvHD | +50 | Yes | No |
| Patient 14 | |||
| Infection | |||
| CMV | +31 | Yes | No |
| Candidiasis | +72 | Yes | No |
| Sepsis | +214 | Yes | No |
| Secondary graft loss | + 28, +90 | No | No |
| Death (sepsis) | +410 | No | Yes |
| Patient 15 | |||
| Infection | |||
| Pneumonia | +52, +300 | Yes, no | No, yes |
| GI | |||
| Increased transaminase levels | + 150 | No | No |
| Heme | |||
| Severe thrombocytopenia | + 150 | No | No |
| Secondary graft loss | + 130 | No | No |
| Death | +10 mo | No | Yes |
CV, Cardiovascular; CVM. cytomegalovirus; GI, gastrointestinal; GU, genitourinary; SVT, supraventricular tachycardia; TEN, toxic epidermal necrolysis; TMA, thrombotic microangiopathy.
Clinical implications:
HSCT in patients with GOF-STAT1 mutations can be curative but might be associated with complications that can lead to secondary graft failure and decreased survival.
Acknowledgments
Funding from the following grants contributed to this work: National Institutes of Health (NIH) grants R13 AI094943 and RO1DK091374 and NIH grant U54 AI082973, Grants in Aid for Scientific Research from the Japan Society for the Promotion of Science (16H05355, 25713039, and 26293244), and the Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development (AMED). The authors were also provided support from the Jeffrey Modell Foundation.
Abbreviations used
- CID
Combined immunodeficiency
- CMC
Chronic mucocutaneous candidiasis
- EFS
Event-free survival
- GAS
γ-Activated sequence
- GOF
Gain of function
- HLH
Hemophagocytic lymphohistiocytosis
- HSCT
Hematopoietic stem cell transplantation
- IPEX
Immune dysregulation–polyendocrinopathy–enteropathy–X–linked
- JAK
Janus kinase
- KREC
Kappa-deleting recombination excision circle
- MMUD
Mismatched unrelated donor
- MRD
Matched related donor
- MUD
Matched unrelated donor
- NK
Natural killer
- OS
Overall survival
- PBSC
Peripheral blood stem cell
- R1C
Reduced-intensity conditioning
- STAT
Signal transducer and activator of transcription
- TREC
T-cell receptor excision circle
- UCB
Unrelated umbilical cord blood
- WT
Wild-type
Footnotes
Disclosure of potential conflict of interest: S. Okada has received grants from the Japan Agency for Medical Research and development, AMED, and the Japan Society for the Promotion of Science (16H05355 and 25713039). S. L. Guthery has received grants from the National Institute of Diabetes and Digestive and Kidney Diseases and Regeneron Pharmaceuticals. C. Lindemans has consultant arrangements with Chimerix and has a US patent for IL-22 and F-652 as ISC growth factors (US 61/901,151). K. E. Sullivan has consultant arrangements with Immune Deficiency Foundation, ADMA Pharmaceuticals, and UpToDate; has received grants from Baxter; has received payment for lectures from the American Academy of Allergy, Asthma & Immunology; and has received royalties from Elsevier. K. Imai has consultant arrangements with CSL Behring K.K., has received grants from CSL Behring K.K. and Sony, and has received payment for lectures from CSL Behring K.K. H.D. Ochs has consultant arrangements with Grifols Pharma and has received a grant from the Jeffrey Modell Foundation. T.R. Torgerson has consultant arrangements with Baxalta Biosciences, CSL Behring, and ADMA Biosciences; has received grants from Baxalta Biosciences, CSL Behring, and the National Institutes of Health; and has received payment for lectures from Baxalta Biosciences, CSL Behring, Questcor Pharmaceuticals, and the Robert Wood Johnson Foundation. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L, et al. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol 2012;24:364–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Depner M, Fuchs S, Raabe J, Prede N, Glocker C, Doffinger R, et al. The extended clinical phenotype of 26 patients with chronic mucocutaneous candidiasis due to gain-of-function mutations in STATI. J Clin Immunol 2016;36:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STATI mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 2011;208:1635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizoguchi Y, Tsumura M, Okada S, Mirata O, Minegishi S, Imai K, et al. Simple diagnosis of STATI gain-of-function alleles in patients with chronic mucocutaneous candidiasis. J Leukoc Biol 2014;95:667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romberg N, Morbach H, Lawrence MG, Kim S, Kang I, Holland SM, et al. Gain-of-function STATI rmirations are associated with PD-LI overexpression and a defect in B-cell survival. J Allergy Clin Immunol 2013;131:1691–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol 2013;131:1624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soltesz B, Toth B, Shabashova N, Bondarenko A, Okada S, Cypowyj S, et al. New and recurrent gain-of-function STAT1 mutations in patients with chronic mucocutaneous candidiasis from Eastern and Central Europe. J Med Genet 2013;50:567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STATI gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood 2016;127:3154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 2011;365:54–61. [DOI] [PubMed] [Google Scholar]
- 10.Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, et al. Dominant gain-of-function STATI mutations in FOXP3 wild-type immune dysreguiation-poIyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol 2013;131:1611–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharfe N, Nahum A, Newell A, Dadi H, Ngan B, Pereira SL, et al. Fatal combined immunodeficiency associated with heterozygous mutation in STAT1. J Allergy Clin Immunol 2014;133:807–17. [DOI] [PubMed] [Google Scholar]
- 12.Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STATI) mutation. J Allergy Clin Immunol 2015;135:551–3. [DOI] [PubMed] [Google Scholar]
- 13.Weinacht KG, Charbonnier LM, Alroqi F, Plant A, Qiao Q, Wu H, et al. Ruxolitinib reverses dysregulated T helper cell responses and controls autoimmunity caused by a novel signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J Allergy Clin Immunol 2017; 139: 1629–40.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baris S, Alroqi F, Kiykim A, Karakoc-Aydiner E, Ogulur I, Ozen A, et al. Severe early-onset combined immunodeficiency due to heterozygous gain-of-function mutations in STAT1. J Clin Immunol 2016;36:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mossner R, Diering N, Bader O, Forkel S, Overbeck T, Gross U, et al. Ruxolitinib induces interleukin 17 and ameliorates chronic mucocutaneous candidiasis caused by STAT1 gain-of-function mutation. Clin Infect Dis 2016;62:951–3. [DOI] [PubMed] [Google Scholar]
- 16.Wildbaum G, Shahar E, Katz R, Karin N, Etzioni A, Pollack S. Continuous G-CSF therapy for isolated chronic mucocutaneous candidiasis: complete clinical remission with restoration of IL-17 secretion. J Allergy Clin Immunol 2013;132: 761–4. [DOI] [PubMed] [Google Scholar]
- 17.Grunebaum E, Kim VH, Somers GR, Shammas A, Roifman CM. Bone marrow transplantation for monoallelic signal transducer and activator of transcription 1 deficiency. J Allergy Clin Immunol 2016;138:612–5.el. [DOI] [PubMed] [Google Scholar]
- 18.Deeg HJ, Lum LG, Sanders J, Levy GJ, Sullivan KM, Beatty P, et al. Severe aplastic anemia associated with chronic mucocutaneous candidiasis. Immunologic and hematologic reconstitution after allogeneic bone marrow transplantation. Transplantation 1986;41:583–6. [DOI] [PubMed] [Google Scholar]
- 19.Hoh MC, Lin HP, Chan LL, Lam SK. Successful allogeneic bone marrow transplantation in severe chronic mucocutaneous candidiasis syndrome. Bone Marrow Transplant 1996;18:797–800. [PubMed] [Google Scholar]
- 20.Aldave JC, Cachay E, Nunez L, Chunga A, Murillo S, Cypowyj S, et al. A 1-year-old girl with a gain-of-function STAT1 mutation treated with hematopoietic stem cell transplantation. J Clin Immunol 2013;33:1273–5. [DOI] [PubMed] [Google Scholar]
- 21.Faitelson YBA, Shroff M, Grunebaum E, Roifman CR, Naqvi A. A mutation in the STAT1 DNA binding domain associated with hemophagocytic lymphohistiocytosis. Lymphosign J 2014; 1:1–9. [Google Scholar]
- 22.Kilic SS, Puel A, Casanova JL. Orf infection in a patient with Statl gain-of-function. J Clin Immunol 2015;35:80–3. [DOI] [PubMed] [Google Scholar]
- 23.Hu H, Roach JC, Coon H, Guthery SL, Voelkerding KV, Margraf RL, et al. A unified test of linkage analysis and rare-variant association for analysis of pedigree sequence data. Nat Biotechnol 2014;32:663–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong XF, Ciancanelli M, Al-Hajjar S, Alsina L, Zumwalt T, Bustamante J, et al. A novel form of human STAT1 deficiency impairing early but not late responses to interferons. Blood 2010;116:5895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang JS, Nam HJ, Seo M, Han SK, Choi Y, Nam HG, et al. OASIS: online application for the survival analysis of lifespan assays performed in aging research. PLoS One 2011;6:e23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao A, Kamani N, Filipovich A, Lee SM, Davies SM, Dalal J. et al. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood 2007;109:383–5. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka S, Muramatsu H, Okuno Y, Hayashi Y. Mizoguchi Y, Tsumura M, et al. Extrapulmonary tuberculosis mimicking Mendel ian susceptibility to mycobacterial disease in a patient with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J Allergy Clin Immunol 2016; 137: 619–22.el. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y, Xu X, Song H, Yang S, Shi S, Wei J, et al. Early diagnostic and prognostic significance of a specific Thl/Th2 cytokine pattern in children with haemophagocytic syndrome. Br J Haematol 2008;143:84–91. [DOI] [PubMed] [Google Scholar]
- 29.Tabellini G, Vairo D, Scomodon O, Tamassia N, Ferraro RM, Patrizi O, et al. Impaired NK cell functions in patients with STAT1 gain-of-function mutations. J Allergy Clin Immunol 2017. [Epub ahead of print], [DOI] [PubMed] [Google Scholar]
- 30.Vargas-Hernandez A, Mace EM, Freeman AF, Rosenzweig S, Chinn IK, Holland SM, et al. LASID Meeting 2015. J Clin Immunol 2015;35(suppl 1):1–57. [Google Scholar]
- 31.Ouachee-Chardin M, Elie C, de Saint Basile G, Le Deist F, Mahlaoui N, Picard C, et al. Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: a single-center report of 48 patients. Pediatrics 2006;117:e743–50. [DOI] [PubMed] [Google Scholar]
- 32.Roesler J, Horwitz ME, Picard C, Bordigoni P, Davies G, Koscielniak E, et al. Hematopoietic stem cell transplantation for complete IFN-gamma receptor 1 deficiency: a multi-institutional survey. J Pediatr 2004;145:806–12. [DOI] [PubMed] [Google Scholar]
- 33.Das R Guan P, Sprague L, Verbist K, Tedrick P, An QA, et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood 2016;127:1666–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan M, Locatelli F, Allen C, DeBendetti F, Grom AA, Ballabio M, et al. 2015 annual meeting abstracts. Blood 2015;126(23). [Google Scholar]