Abstract
A 60-year-old male liver transplant recipient presented to his local hospital with left-sided partial seizures following a few days of generalised headache. He had undergone transplantation for primary sclerosing cholangitis 4 years earlier and maintained on tacrolimus monotherapy immunosuppression. He had no other comorbidities of note and worked as an arable farmer. At last follow-up, he had been well with preserved graft function and afternoon trough tacrolimus levels of 2–4 ng/mL. Over the preceding 4 weeks, he had been investigated locally for weight loss and a productive cough, where CT of the chest showed calcified mediastinal and hilar lymphadenopathy. Bronchoscopy samples were negative for acid-fast bacilli and he had been empirically treated for assumed community acquired pneumonia. Initial seizure management was with intravenous diazepam and phenytoin. On transfer to our centre, he was noted to be dysarthric with persisting 4/5 left upper limb weakness and nystagmus to all extremes of gaze. Blood tests were significant for mild anaemia (haemoglobin 90 g/L) and elevated C reactive protein (134 mg/L). The peripheral white cell count was 6.6×109/L. Biochemical liver graft function was normal and the 8am trough tacrolimus level was low at 2 ng/mL.
CT head revealed bilateral ring enhancing cerebral lesions with surrounding vasogenic oedema but no mass effect. On MRI these exhibited restricted diffusion and marked perilesional oedema, suggestive of infection. Cerebrospinal fluid (CSF) analysis was as follows: white cell count <1/mm3, protein 0.57 g/L (normal range <45 g/L) and glucose 3 mmol/L (paired plasma glucose 4.8 mmol/L). Testing for virological causes via PCR, toxoplasma serology and blood and CSF cultures, including for tuberculosis, were all negative. Whole body positron emission tomography-CT demonstrated uptake in numerous peritoneal and intramuscular lesions as well as right-sided cervical lymphadenopathy, which was sampled with fine needle aspiration. Microscopy revealed a filamentous, beading and branching Gram-positive bacillus that was partially acid-fast, subsequently speciated as Nocardia farcinica.
Keywords: liver, orthotopic liver transplantation, infectious disease
Case report
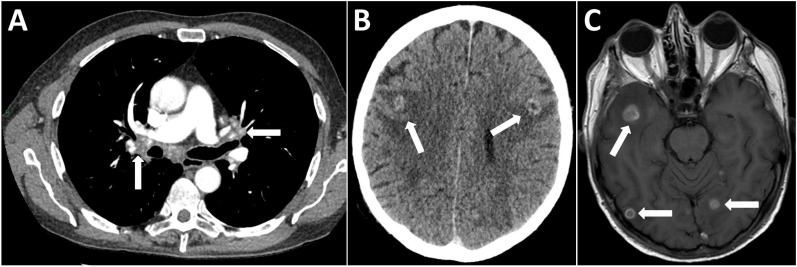
A 60-year-old male liver transplant recipient presented to his local hospital with left-sided partial seizures following a few days of generalised headache. He had undergone transplantation for primary sclerosing cholangitis 4 years earlier and maintained on tacrolimus monotherapy immunosuppression. He had no other comorbidities of note and worked as an arable farmer. At last follow-up, he had been well with preserved graft function and afternoon trough tacrolimus levels of 2–4 ng/mL. Over the preceding 4 weeks, he had been investigated locally for weight loss and a productive cough, where CT of the chest (figure 1A) showed calcified mediastinal and hilar lymphadenopathy. Bronchoscopy samples were negative for acid-fast bacilli and he had been empirically treated for assumed community acquired pneumonia.
Figure 1.
(A) CT chest demonstrating calcified enlarged mediastinal and hilar lymphadenopathy (white arrows). (B) Contrast-enhanced CT head revealed multiple ring enhancing lesions in both cerebral hemispheres with surrounding vasogenic oedema but no gross mass effect (white arrows). (C) MRI head with contrast demonstrating multiple ring enhancing lesions associated with restricted diffusion and marked perilesional oedema suggestive of opportunistic infection (white arrows).
Initial seizure management was with intravenous diazepam and phenytoin. On transfer to our centre, he was noted to be dysarthric with persisting 4/5 left upper limb weakness and nystagmus to all extremes of gaze. Blood tests were significant for mild anaemia (haemoglobin 90 g/L) and elevated C-reactive protein (134 mg/L). The peripheral white cell count was 6.6×109/L. Liver graft function was normal and the 8am trough tacrolimus level was low at 2 ng/mL.
CT head revealed bilateral ring enhancing cerebral lesions with surrounding vasogenic oedema but no mass effect (figure 1B). On MRI, these exhibited restricted diffusion and marked perilesional oedema, suggestive of infection (figure 1C). Cerebrospinal fluid (CSF) analysis was as follows: white cell count <1/mm3, protein 0.57 g/L (normal range<45 g/L) and glucose 3 mmol/L (paired plasma glucose 4.8 mmol/L). Testing for virological causes via PCR, toxoplasma serology and blood and CSF cultures, including for tuberculosis, were all negative.
What is your differential diagnosis? How would you manage this patient?
The differential diagnosis of enhancing cerebral lesions in a transplant recipient is broad but principally falls between infectious causes (including fungi, bacterial abscesses, tuberculosis, toxoplasmosis and parasitic infections) versus malignancy, particularly cerebral lymphoma.
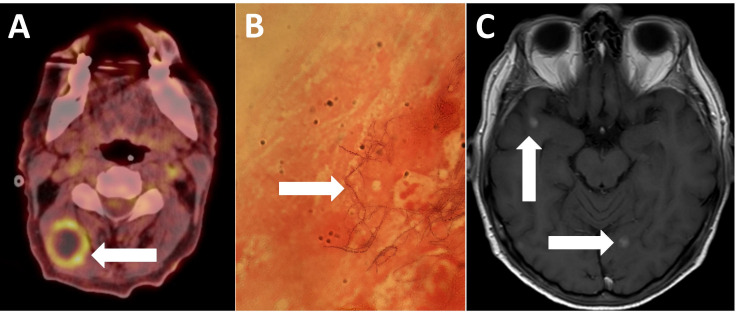
Whole body positron emission tomography (PET)-CT demonstrated uptake in numerous peritoneal and intramuscular lesions as well as right-sided cervical lymphadenopathy (figure 2A), which was sampled with fine needle aspiration. Microscopy revealed a filamentous, beading and branching Gram-positive bacillus that was partially acid-fast (figure 2B), subsequently speciated as Nocardia farcinica.
Figure 2.
(A) PET CT demonstrating high uptake in a right cervical lymph node (white arrow). (B) Gram stain of a lymph node aspirate demonstrating the beaded and branching Gram-positive rod, Nocardia farcinica (white arrow). (C) MRI head with contrast demonstrating the decreasing size of enhancing brain lesions (white arrows). PET, positron emission tomography.
Broad-spectrum antibiotics were started (meropenem, amikacin and co-trimoxazole). Over the following weeks, his neurological symptoms resolved and C reactive protein normalised. He was discharged on intravenous ceftriaxone and, after repeat imaging at 3 months, he demonstrated marked radiological improvement (figure 2C) and then was switched to oral moxifloxacin and co-trimoxazole. He remained well for 6 months following this but then re-presented to his local hospital with a short history of diarrhoea and vague lower abdominal pain, when blood cultures confirmed recurrent Nocardia bacteraemia. During this admission, he developed severe lower back pain and radicular symptoms in both legs without focal neurological deficit. MRI demonstrated likely infective T11/T12 spondylodiscitis associated with a small epidural collection causing cord impingement (figure 3). In the absence of focal neurological deficit, conservative management was initially attempted but he became bed-bound by pain and ultimately required decompressive discectomy for symptom management. Based on updated antimicrobial sensitivities, he received a 1-year course of imipenem and minocycline. Six months after cessation of antimicrobials, he reports chronic fatigue but resolution of neurological symptoms. Inflammatory markers remain low and radiological appearances are stable.
Figure 3.
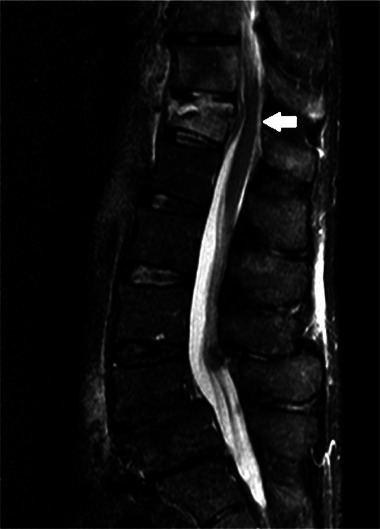
Sagittal MRI (T2-weighted short T1 inversion recovery (STIR)) showing T11/T12 spondylodiscitis associated with a small epidural collection causing cord impingement (white arrow).
Nocardia farcinica resides in soil and usually infects immunocompromised patients, manifesting as pulmonary, cutaneous or disseminated forms;1 hence, obtaining a detailed occupational history was helpful in the evaluation of this patient. The mortality in immunosuppressed patients with disseminated and central nervous system (CNS) infections is up to 50%.2 Rapid diagnosis is key and this case highlights the role for functional imaging, such as PET-CT, in the evaluation of culture-negative CNS infection to identify peripheral deposits amenable to biopsy.3 Nocardia infection can frequently relapse and consensus regarding antibiotic duration recommends 6–24 months of therapy.4
Footnotes
JF and PJS contributed equally.
Contributors: JF and PJS prepared the manuscript and figures. DY prepared the radiology images and reports. PJS, JF, JP, PMT, RJ, IC and AM initially managed the patient. PH prepared the microbiology image and legend. AM and RJ supervised the manuscript preparation. All authors approved the submitted manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc 2012;87:403–7. 10.1016/j.mayocp.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ozgenç O, Avcı M, Arı A, et al. Long-Term treatment of persistent disseminated Nocardia cyriacigeorgica infection. Braz J Infect Dis 2014;18:556–60. 10.1016/j.bjid.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tseng J-R, Chen K-Y, Lee M-H, et al. Potential usefulness of FDG PET/CT in patients with sepsis of unknown origin. PLoS One 2013;8:e66132 10.1371/journal.pone.0066132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corti ME, Villafañe-Fioti MF. Nocardiosis: a review. Int J Infect Dis 2003;7:243–50. 10.1016/S1201-9712(03)90102-0 [DOI] [PubMed] [Google Scholar]