Abstract
Background and aim
Peripheral cytopaenias and dyspoiesis are common in cirrhosis; however, the prevalence of dyspoiesis and its contribution in cirrhosis-related cytopaenias has not been studied. We aimed to study the bone marrow (BM) dyspoiesis and its impact on peripheral blood cell counts and refractory anaemia in patients with cirrhosis.
Patients and methods
We reviewed all the BM aspirates and biopsies of cirrhotic cases, done from 2011 to 2018 for clinical indications. Dyspoiesis was considered if >5% of the precursor cells of any of the three lineages showed dyspoietic changes. Primary haematological or non-haematological malignancies, chronic kidney disease, drug intake, acute and chronic hepatitis and granulomatous disease were excluded.
Results
Of 608 these, 82 cases (13.5%) showed dyspoiesis in the BM precursors. There was no difference in age (p=0.16), gender (p=0.58) and spleen size (p=0.35) in cases with or without dyspoiesis. Majority of the cases had dyspoiesis in erythroid series (62, 75.6%) and megakaryocytes (15, 18.2%). Dyspoiesis was more prominent in alcoholics 44 cases (53.6%) and autoimmune diseases 13 cases (15.8%). Erythroid hyperplasia (47.7±14.4 vs 40±11.1; p<0.001) was more in cases with dyserythropoiesis, indicating ineffective erythropoiesis. Patients with dyspoiesis had lower haemoglobin (7.5±1.9 vs 9.3±2.2 g/dL, p<0.001). 54 (8.07%) had refractory anaemia with dyspoiesis present in 48 (88.8%) (p<0.01). Dyspoiesis was independently associated with refractory anaemia when adjusted for age, gender, aetiology and liver disease severity.
Conclusions
BM dyspoiesis, especially dyserythropoiesis, is associated with severe refractory anaemia in patients with cirrhosis and requires new therapeutic approaches.
Keywords: liver cirrhosis, anaemia, alcoholic liver disease
Significance of this study.
What is already known on this topic
Blood cytopaenia and anaemia are commonly associated in chronic liver disease.
There are several mechanisms postulated for cytopaenias in cirrhosis.
What this study adds
In this study, we identified that severe anaemia and refractory anaemia in patients with cirrhosis is associated with ineffective erythropoiesis at the level of source (bone marrow).
Ineffective erythropoiesis mainly occurs in alcoholic and autoimmune hepatitis-related cirrhosis.
How might it impact on clinical practice in the foreseeable future
These patients with severe refractory anaemia do not recover with nutritional supplementation and requires alternative therapy.
Introduction
Liver cirrhosis can lead to various haematological abnormalities. Anaemia secondary to various aetiologies occurs in about 75% of patients with chronic liver disease (CLD).1 Patients with CLD and end-stage cirrhosis have varying grades of cytopaenia and other haematological derangements either due to the compromised synthetic capacity of the diseased liver itself or resulting from medical or surgical insults.2–5
Some patients with CLD do not respond to appropriate treatment for severe anaemia, including nutritional supplementation. These patients are usually classified as those with ‘refractory anaemia’. Whether bone marrow abnormalities contribute to haematological derangement in such patients is hitherto unstudied. In this study, we aim to find out the prevalence of dyspoiesis in patients with cirrhosis and its relationship with refractory anaemia and various other parameters.
Materials and methods
Study design
This is an observational, cross-sectional study on a retrospectively enrolled cohort of patients with liver cirrhosis who underwent bone marrow examination during January 2010–December 2018.
Patient population
We included all patients with cirrhosis who had undergone a bone marrow examination at our institute between January 2010 and July 2018. The indication for bone marrow examination included various abnormalities such as anaemia, bicytopaenia, pancytopaenia, thrombocytopaenia, regenerative therapy and others. We excluded patients with haematological/hepatic malignancies, history of chemotherapeutic drugs that are known to cause myelodysplasia (alkylating agents (nitrogen mustard, cyclophosphamide, melphalan, busuflan and chlorambucil), nitrosoureas, procarbazines, topoisomerase II inhibitors (like etoposide and teniposide), anthracyclines (daunomycin, epirubicin and doxorubicin) and mitoxantrone).6 Cases of dialysis-dependent chronic kidney disease (CKD) were excluded, because haemodialysis itself can induce dyspoiesis in patients with CKD.7 Since we aimed to see the cirrhosis-associated acquired myelodysplasia, acute and chronic hepatitis and granulomatous disease cases were also not included. Apart from these, the cases with missing records were omitted. Study cohort with exclusion criteria and included patients have been summarised in figure 1.
Figure 1.
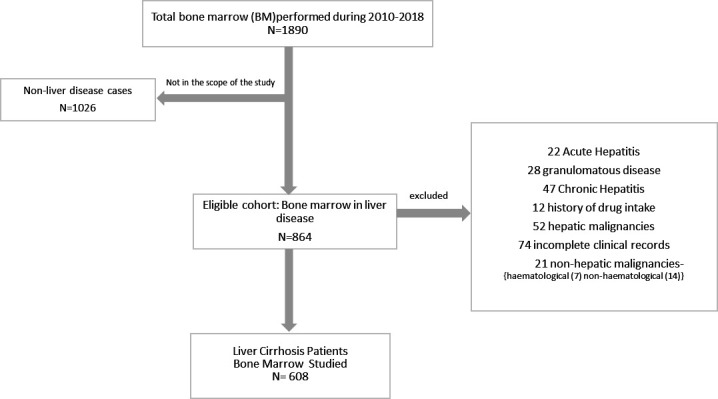
Inclusion and exclusion of the patients for analysis.
Clinical and laboratory data
Patient case records were reviewed to collect the demographic data, diagnosis and aetiology of liver dysfunction. As a protocol, all patients with CLD routinely underwent venous blood sampling for complete blood counts, prothrombin time/international normalised ratio, liver function tests, and serum urea and creatinine levels. The haematological counts were assayed using EDTA-anticoagulated blood on LH750 (Beckman Coulter Inc, Brea, California, USA) and Horiba ABX Pentra DX 120 (Horiba Medical, Montpellier, France). Citrated blood sample (with sodium citrate as anticoagulant in a ratio of 9:1) was used for studying the coagulation tests performed on fully automated coagulometer (Sysmex CA 1500; Sysmex Corporation, Kobe, Japan).The biochemical tests were performed onDXC600 Pro (Beckman Coulter Inc). Serum ferritin levels were analysed by nephelometry (Dade Behring BN ProSpec; Siemens Healthcare Diagnostics, Marburg, Germany). The aetiologies for liver diseases were classified into five different categories: ethanol related, viral (hepatitis B virus (HBV) and hepatitis C virus (HCV)), non-alcoholic steatohepatitis (NASH), cryptogenic and autoimmune hepatitis (AIH).
Dyspoiesis was considered if >5% of the precursor cells of any of the three lineages showed dyspoietic changes-dyserythropoietic features such as nuclear budding, internuclear bridging, nuclear membrane irregularity, bilobulation or multilobulation of the nucleus of erythroid cells, nuclear fragmentation, karyorhexis and uneven distribution of chromatin. Dysmegakaryopoiesis was assessed by counting 30 megakaryocytes showing features such as monolobulated megakaryocytes, abnormal hypogranulation and multiple widely separated nuclei.8 Dysmyelopoietic features such as hypogranulation of neutrophils, nuclear hyposegmentation (pseudo Pelger-Huet), nuclear hypersegmentation, and maturation arrest at myelocyte stage and presence of Auer rods were considered. A lower threshold (>5%) for dyspoiesis was taken since majority of liver disease patients exhibit megaloblastoid changes that would falsely increase the percentage of cells showing dyserythropoiesis. Cells showing megaloblastoid change were excluded in the assessment of dyserythropoiesis. Patients were classified into two groups based on the presence or absence of dyspoiesis: group A (dyspoietic group) and group B (with normal haematopoiesis). Model for End-Stage Liver Disease (MELD) and child-turcot-pugh (CTP) scores were used to assess the severity of liver disease.
Statistical analysis
Data in Excel (Microsoft, Redmond, Washington, USA) sheet were imported into and analysed using STATA (V.12.1). To begin, baseline characteristics were summarised using frequency, proportion, mean (SD) and median (IQR). The difference of distribution (unadjusted analysis) between numeric variables among groups was analysed using the test or one-way analysis of variance. Mann-Whitney or Kruskal-Wallis test was used for non-parametric data. Multivariate logistic regression analysis was performed to predict risk factors associated with refractory anaemia.
Results
Patient characteristics
We identified 608 patients with cirrhosis who fulfilled the inclusion and exclusion criteria. Out of these, 82 patients who had dyspoiesis were included in group A (dyspoietic group) and the rest 526 were in group B. The prevalence of bone marrow dyspoiesis was 13.5% (82/608). The mean age in group A was 37 years as compared with group B which was 40 years. Both groups had preponderance of male patients, and the M:F ratio was 4.4 and 3.6, respectively. Basic characteristics are being summarised in table 1.
Table 1.
Comparison of baseline parameters between the two groups
| Parameter | Total (n=608) | Dyspoietic group (n=82) 13.5% |
Normal haematopoiesis (n=526) 86.5% |
P value |
| Age, years | 39±13 | 37±14 | 40±13 | 0.166 |
| Male:female | 479:129 | 67:15 | 412:114 | 0.582 |
| Spleen size, cm | 15±1.0 | 14.9±1.1 | 15.1±1 | 0.355 |
| Haemoglobin (g/dL) | 9.0±2.2 | 7.5±1.9 | 9.3±2.2 | <0.05 |
| Total leucocyte count (×109/L) | 4.6 (2.9–7.8) | 4.3 (2.7–6.3) | 4.7 (3.0–7.8) | 0.195 |
| Platelet count (×109/L) | 61.5 (40.0–101) | 55.5 (31.2–94.5) | 64 (40–101.7) | 0.079 |
| Model of end-stage liver disease | 15.7 (11.5–20.3) | 15.14 (11.0–19.0) | 15.7 (11.6–20.5) | 0.398 |
| Lactate dehydrogenase, IU/L | 521 (415–680.2), (n=406) | 569 (419.5–924), (n=59) | 518(412-664) (n=347) | 0.111 |
| Serum ferritin, ng/mL | 203 (58.2–675) (n=325) | 116 (31.6–223) (n=39) | 228.5 (66.4–719.5) (n=286) | 0.011 |
| Total iron binding capacity, µg/dL | 216 (152–353.5) (n=159) | 268(121-765) (n=53) | 212.5 (166.5–313) (n=106) | |
| Erythroid hyperplasia, % | 41.2±11.9 | 47.7±14.4 | 40±11.1 | <0.05 |
| Aetiology, n Alcoholic/viral hepatitis/ NASH/cryptogenic/autoimmune hepatitis |
272/122/82/70/62 | 44/14/5/6/13 | 228/108/77/64/49 | 0.033 |
| Dyserythropoiesis/dysmyelopoiesis/ dysmegakaryopoiesis |
62/5/15 | |||
Cytopaenias and other blood parameters
As expected, patient in the dyspoietic group (group A) had significantly low haemoglobin compared with group B. However, the total leucocyte count (TLC) and platelet count were comparable between the two groups. There was no statistically significant difference noted in other parameters such as serum bilirubin, ferritin levels and MELD score. However, patients in the dyspoietic group had significantly higher total iron binding capacity (TIBC). These findings are tabulated in table 1.
Bone marrow findings
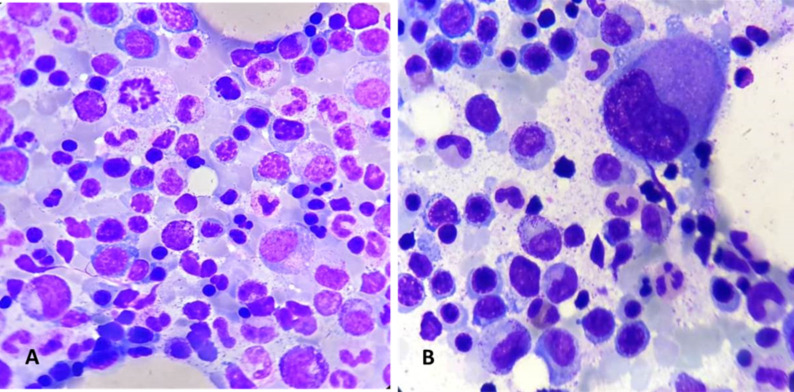
Of the 82 patients who belonged to group A, 62 patients (74%) had dyserythropoiesis (figure 2A), followed by 15 patients (18%) having dysmegakaryopiesis (figure 2B) and 5 patients (6 %) having dysmyelopoiesis. Also, the average erythroid hyperplasia was 47.8% in group A as compared with 39.3% in group B. The dyspoiesis mainly involved the red cell lineage followed by dysmegakaryopoiesis. This was supported by the higher rate of erythroid hyperplasia in patients with dyserythropoiesis. Majority of patients in the dyspoietic group had normal TLC and platelet count. The results indicated that ineffective erythropoiesis is the main cause of dyspoiesis in patients with cirrhosis.
Figure 2.
(A) Bone marrow aspirate smear showing dyserythropoiesis in the form of nuclear budding, karyorhexis in erythroid precurosors, 200× Giemsa. (B) Bone marrow aspirate smear showing a monolobated megakaryocyte-dysmegakaryopoiesis, 200×, Giemsa.
Factors associated with refractory anaemia
The aetiology of liver disease among the study group is shown in table 1. Alcoholic liver disease is the leading cause in both the groups. Dyspoiesis was more prominent in alcoholics (44; p=0.033) and autoimmune diseases (13; p=0.03) than other aetiologies. A total of 54 patients had refractory anaemia defined as Hb <7 g/dL for 6 months after nutritional supplementation and required ≥3 transfusions. Out of these, 48 belonged to group A (dyspoietic group) and six were in group B. Analysis was performed to find out the association of refractory anaemia to dyspoiesis, and other findings are tabulated in table 2. Iron deficiency as evidenced by low ferritin and high TIBC might be a contributing factor to refractory anaemia. Analysis after adjusting for age, gender, aetiology, serum iron and MELD score; dyspoiesis (OR 0.007 (95% CI 0.002 to 0.017)) was found to be independently associated with severe refractory anaemia.
Table 2.
Comparison between refractory anaemia group and normal group
| Refractory anaemia group (n=54) | Normal group (n=554) |
P value | |
| Age (mean±SD) | 48.9±14.1 | 46.6±13.6 | 0.50 |
| Spleen size, cm | 15.1±1.1 | 15±1.0 | 0.87 |
| Haemoglobin, g/dL | 7.6±2 | 9.1±2.2 | 0.05 |
| Total leucocyte counts, 106/L | 6.7±6 | 6.7±7.7 | 0.78 |
| Platelet count, 109/L | 71.7±58.9 | 83.5±72.3 | 0.08 |
| Model of end-stage liver disease | 14.1±7.1 | 15.8±9 | 0.43 |
| Lactate dehydrogenase, IU/L | 1059.3±167 | 843.3±148.8 | 0.04 |
| Ferritin, ng/mL | 610.8±356.3 | 649.9±366.3 | 0.55 |
| Total iron binding capacity, µg/dL | 548.1±229 | 778.8±514 | 0.76 |
| Erythroid, % | 46.8±16 | 39.9±12.2 | 0.05 |
| CD34 cell count, cells/20× | 12.3±6.8 | 12.5±7.4 | 0.65 |
| Dyspoiesis, n | 48/8 | 34/520 | 0.001 |
| Mortality or transplant requirement at 6 months | 28 (51.8%) | 96 (17.3%) | <0.001 |
Additionally, 28/54 (51.8%) patients died or required living-donor liver transplant (23 died and 5 underwent liver transplant) at 6 months as compared with non-refractory anaemia group (17.3%) with p=0.02, after adjusting for age, gender, aetiology and MELD score.
Discussion
In this study, we found that the severe refractory anaemia in patients with cirrhosis is related to dyserythropoiesis. A subset of patients, especially alcohol and autoimmune aetiology, can induce the defects in bone marrow cells differentiation and maturation (dypoiesis). In turn, those cirrhotic patients with severe refractory anaemia and dyspoiesis have poor prognosis.
Although haematological abnormalities such as anaemia and thrombocytopaenia are common among patients with CLD, majority of these are attributed to liver dysfunction.9 The prevalence of bone marrow abnormalities that may be the underlying pathology is largely understudied. Several authors had studied the bone marrow findings in patients with hepatitis C.10–12 Anwar et al 13 studied the utility of bone marrow examination in patients with CLD and found this test to be quite useful especially in patients with severe pancytopaenia and those with sudden alterations in peripheral cell counts. However, their series included just 75 patients, and all of them had hepatitis C-induced CLD. Also, they did not report prevalence of dyspoiesis in their study group. Ours is the first study to include various aetiologies and focus on dyspoiesis. Our study reports a high prevalence of 13% dyspoiesis in CLD, which would justify the need for bone marrow examination in patients with CLD. Klco et al 14 reported dyserythropoiesis in 19% of patients while studying the bone marrow biopsy in Hepatitis C virus infection. However, they included haematological malignancies such as acute myeloid leukemia (AML), and their study group had only 47 patients.
Stein et al had reported that iron deficiency secondary to gastrointestinal haemorrhage and antiviral therapy may be a predominant cause of anaemia in CLD.15 16 Refractory anaemic patients also had higher mortality or transplant requirement. This is a novel finding and would direct further research that could potentially identify a newer therapy for refractory anaemia focusing on addressing bone marrow dyserthropoiesis. The refractory anaemia in cirrhotics can be managed by supportive care, drug therapy and ultimately by liver transplant. Drug therapy includes (azacitidine, decitabine, lenalidomide and deferasirox) as being tried in myelodysplastic syndrome.17 It helps in controlling the symptoms and can be useful to reduce disease-related mortality in transplant-ineligible patients or as a bridging therapy and require further studies in patients with liver cirrhosis.
While patients with alcoholic liver disease are known to have low haemoglobin, the cause was primarily attributed to associated malnutrition and the toxic effects of alcohol on liver, stomach and intestine leading to malabsorption.1 17–19 Jarrold et al 20 in 1967 studied the bone marrow erthroid morphology in 26 male alcoholic individuals and found that individuals with normoblastic morphology were primarily beer drinkers and those with megaloblastic or megaloblastoid morphology were essentially wine and whiskey imbibers. However, the association between bone marrow dyspoiesis and alcoholic liver disease is hardly reported. Further prospective studies are warranted to confirm this association.
Our study has certain limitations. This is a retrospective review of prospectively collected data, and the deficiencies of a retrospective design hold true for this study. We had used a cut-off value of >5% cells demonstrating dyspoietic changes, while some authors had used a cut-off value of >10%.16 However, we report one of the largest series of bone marrow findings in patiens with CLD until. To our knowledge, this is the first study to specifically focus on dyspoiesis in CLD, and the findings of our study have clinical relevance in the management of refractory anaemia.
We would like to conclude by reporting that bone marrow dyspoiesis, especially dyserthropoiesis, is associated with severe refractory anaemia in patients with cirrhosis. Patients with refractory anaemia have higher mortality or transplant requirement and needs new therapeutic approaches as bridging therapy.
Acknowledgments
We would like to thank Ms Rekha, who helped in acquisition of the data.
Footnotes
Contributors: AV: acquired the data and drafted the manuscript; DL and RK: acquired the data from clinical records; CB: designed the study, collected data and drafted and involved in the critical revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol 2009;15:4653–8. 10.3748/wjg.15.4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jandl JH. The anemia of liver disease: observations on its mechanism 1. J Clin Invest 1955;34:390–404. 10.1172/JCI103087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abou El Azm AR, El-Bate H, Abo-Ali L, et al. Correlation of viral load with bone marrow and hematological changes in pale patients with chronic hepatitis C virus. Arch Virol 2012;157:1579–86. 10.1007/s00705-012-1321-z [DOI] [PubMed] [Google Scholar]
- 4. Deller DJ, Kimber CL, Ibbotson RN. Folic acid deficiency in cirrhosis of the liver. Am J Dig Dis 1965;10:35–42. 10.1007/BF02235073 [DOI] [PubMed] [Google Scholar]
- 5. Klipstein FA, Lindenbaum J. Folate deficiency in chronic liver disease. Blood 1965;25:443–56. 10.1182/blood.V25.4.443.443 [DOI] [PubMed] [Google Scholar]
- 6. Mintzer DM, Billet SN, Chmielewski L. Drug-Induced hematologic syndromes. Adv Hematol 2009;2009:11 10.1155/2009/495863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayari H, Pasquier F, El Karoui K, et al. Myelodysplastic syndrome in hemodialysis patients. Kidney International Reports 2019;4:1175–8. 10.1016/j.ekir.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goasguen JE, Bennett JM, Bain BJ, et al. Dyserythropoiesis in the diagnosis of the myelodysplastic syndromes and other myeloid neoplasms: problem areas. Br J Haematol 2018;182:526–33. 10.1111/bjh.15435 [DOI] [PubMed] [Google Scholar]
- 9. Sheikh M, Raoufi R, Atla P, et al. Prevalence of cirrhosis in patients with thrombocytopenia who receive bone marrow biopsy. Saudi Journal of Gastroenterology 2012;18:257 10.4103/1319-3767.98431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boone JM, Cui W. Spectrum of bone marrow morphologic findings in hepatitis C patients with and without prior liver transplantation. Int J Lab Hematol 2016;38:694–702. 10.1111/ijlh.12559 [DOI] [PubMed] [Google Scholar]
- 11. Anwar B, Asif N, Hassan K. Haematological malignancies in chronic hepatitis C patients referred for bone marrow biopsy. J Islamabad Med Dent Coll 2013;2:17–20. [Google Scholar]
- 12. Mousa SM. Hepatitis C among Egyptian patients referred for bone marrow examination: seroprevalence and analysis of hematological findings. Bone Marrow Res 2014;2014:1–4. 10.1155/2014/549716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anwar B, Hassan K, Asif N. Diagnostic utility of bone marrow examination in chronic liver disease patients referred for evaluation of hematological derangements. Int J Pathol 2012;10:13–16. [Google Scholar]
- 14. Klco JM, Geng B, Brunt EM, et al. Bone marrow biopsy in patients with hepatitis C virus infection: spectrum of findings and diagnostic utility. Am J Hematol 2010;85:106–10. 10.1002/ajh.21600 [DOI] [PubMed] [Google Scholar]
- 15. Stein J, Connor S, Virgin G, et al. Anemia and iron deficiency in gastrointestinal and liver conditions. World J Gastroenterol 2016;22:7908 10.3748/wjg.v22.i35.7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moreno Otero R, Cortés JR. [Nutrition and chronic alcohol abuse]. Nutr Hosp 2008;23:3–7. [PubMed] [Google Scholar]
- 17. Tefferi A. Myelodysplastic Syndromes—Many new drugs, little therapeutic progress. Mayo Clin Proc 2010;85:1042–5. 10.4065/mcp.2010.0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Waters AH, Morley AA, Rankin JG. Effect of alcohol on haemopoiesis. Br Med J 1966;2:1565–8. 10.1136/bmj.2.5529.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balcells A, Ingelmo M, Vivancos J, et al. [Macrocytosis in chronic alcoholism (author's transl)]. Med Clin 1979;73:312–4. [PubMed] [Google Scholar]
- 20. Jarrold T, Will JJ, Davies AR, et al. Bone marrow-erythroid morphology in alcoholic patients. Am J Clin Nutr 1967;20:716–22. 10.1093/ajcn/20.7.716 [DOI] [PubMed] [Google Scholar]