Abstract
As COVID-19 vaccinations begin, dermatologists must be aware of the cutaneous adverse events reported in the clinical trials, including injection site and hypersensitivity reactions, and emerging evidence of dermal filler injection reactions after vaccination. The filler reaction may represent the development of a delayed-type hypersensitivity in the setting of another immunologic trigger (i.e., the vaccine). After conducting a literature review of similar reactions, their pathophysiology, and management, we present a set of timely clinical considerations for counseling, prevention, and management of possible cutaneous sequelae of the COVID-19 vaccine. We encourage documentation of vaccine-related reactions to aid the safety data collection in the Vaccine Adverse Event Reporting System and the American Academy of Dermatology COVID-19 Registry.
Keywords: Prevention, Vaccines, Adverse reactions, Hypersensitivity, Dermal filler
As physicians, our role of stewardship in promoting public health guidelines and vaccination is clear, but we must remain prepared to prevent and manage the possible effects on the skin. From the COVID-19 vaccine trial data available, we present an overview of the current cutaneous reactions reported with an analysis of similar reactions from the literature to guide dermatologists’ considerations for vaccine-related counseling, prevention, and management.
As seen with other vaccines, cutaneous reactions after COVID-19 vaccination have included localized redness and swelling (Fig. 1) among the mild adverse reactions in all trials reaching phase 3 (Table 1). Thus far, other skin reactions have been reported with the Moderna vaccine (e.g., injection site reactions, injection site urticaria, and “maculo-papular rash”; Table 2; U.S. Food and Drug Administration [FDA], 2020). Suspected allergic reactions to polyethylene glycol, a known excipient in the vaccines, suggest care be taken in those who are patch-test positive or have a history of allergy to polyethylene glycol–containing products (penicillin, laxatives, makeup, injected corticosteroids, antacids, chemotherapy drugs, certain fillers; Damiano Monticelli et al., 2019, Garvey and Nasser, 2020).
Fig. 1.
Local injection site reaction on the arm of a dermatologist colleague who received the vaccine and provided this photograph as a courtesy.
Table 1.
Frequency of solicited local cutaneous adverse reactions within 7 days after each vaccination: A comparison of the vaccines reaching phase 3 trials.
| Adverse reaction (solicited) | Pfizer vaccine dose 1 | Placebo dose 1 | Pfizer vaccine dose 2 | Placebo dose 2 | Moderna vaccine dose 1 | Placebo dose 1 | Moderna vaccine dose 2 | Placebo dose 2 | AstraZeneca dose 1 | AstraZeneca dose 2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Redness | 104/2238 (4.5) | 26/2248 (1.1) | 123/2045 (5.9) | 14/2053 (0.7) | 345/11401 (3.0) | 46/11404 (0.4) | 928/10357 (9.0) | 42/10317 (0.4) | 1/128 (0.7) | 2/128 (1.5) |
| Swelling | 132/2238 (5.8) | 11/2248 (0.5) | 132/2045 (6.3) | 5/2053 (0.2) | 768/11401 (6.7) | 33/11404 (0.3) | 1309/10357 (12.6) | 35/10317 (0.3) | 2/128 (1.5) | 2/128 (1.5) |
Sources: Ramasamy et al., 2021; U.S. Food and Drug Administration briefing documents for Pfizer-BioNTech and Moderna COVID-19 vaccines.
Table 2.
Unsolicited cutaneous adverse reactions reported in Moderna phase 3 trial.
| Adverse reaction (unsolicited) | Moderna vaccine (N = 15,185), n (%) | Placebo (N = 15,166), n (%) |
|---|---|---|
| Dermatitis contact | 21 (0.1) | 29 (0.2) |
| Exfoliative rash | 1 (<0.1) | 0 (0) |
| Hand dermatitis | 2 (<0.1) | 0 (0) |
| Injection site rash | 37 (0.2) | 1 (<0.1) |
| Injection site urticarial | 15 (<0.1) | 0 (0) |
| Maculopapular rash | 11 (<0.1) | 2 (<0.1) |
| Vesicular rash | 3 (<0.1) | 0 (0) |
| Facial swelling | 4 (<0.1) | 2 (<0.1) |
| Urticaria | 27 (0.2) | 23 (0.2) |
Source: Baden et al., 2020.
The FDA brief on the Moderna vaccine also reported reactions to dermal filler after vaccination in three patients in the experimental arm of the trial. Two of these patients had facial swelling, with one reporting filler injection 6 months prior and the other 2 weeks prior to vaccination. In the third patient who had lip swelling only, the timing of the last filler injection was unknown, and a similar reaction occurred after an influenza vaccination in the past (Fig. 2; U.S. Food and Drug Administration, 2020, Zhang, 2020). All reactions resolved. Two patients in the placebo group of this trial also reported facial swelling, indicating that other possible triggers apart from the vaccine or filler could have confounded the cases in the experimental vaccine group.
Fig. 2.
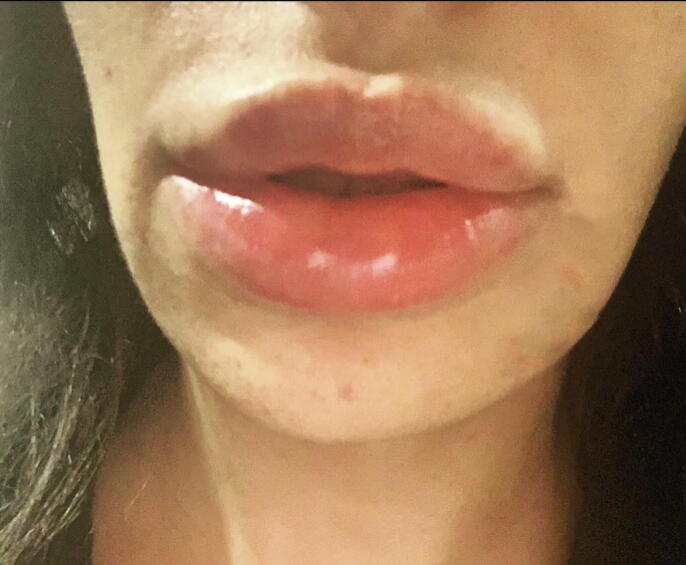
Example of lip angioedema following filler injection.
To our knowledge, no similar reactions have yet been reported with the Pfizer BNT162b2 mRNA or other COVID-19 vaccines (Polack et al., 2020); however, the information available is evolving and the estimation of risk and prevalence of a vaccine-filler cross reactivity is limited because the types of filler used in patients with reactions was not reported and a query of history of filler injection is not part of routine screening. Thus, the true number of patients with a history of filler injections who have already received the vaccine without reactions is yet unknown.
Immunogenic dermal filler reactions are rare, with both immediate and delayed type hypersensitivity reactions (DTR) reported and a global incidence rate of 0.8% for hyaluronic acid (HA) fillers (Beleznay et al., 2015). Immediate hypersensitivity reactions, within minutes of injection, occur via immunoglobulin E–mediated histamine release from mast cells and manifest as urticaria, angioedema, and anaphylaxis. Although DTRs (incidence of 0.42%), mediated by macrophage and T-cell interactions, typically develop 48 to 72 hours after injection, they can also occur weeks to months after, with swelling and erythema at the filler site (Lowe et al., 2001) or granuloma formation at the site months or even years later (Alijotas-Reig et al., 2013).
Among the existing theories for why DTRs are seen with dermal filler after certain immunogenic triggers (e.g., COVID-19 or other vaccines) is that fillers may act as adjuvants rather than direct T-cell activators, enhancing the antigen-specific immune response without triggering one on their own. Thus, in genetically predisposed individuals, there is a lower threshold for vaccines, infections, or other inciting factors to trigger inflammatory reactions (Alijotas-Reig et al., 2018). A recent report showed a higher risk of DTRs to filler in patients with HLA subtypes B*08 and DRB1*03, with linkage to a predisposition for autoimmune and/or granulomatous disorders (Decates et al., 2020). Abnormalities in acute phase reactants, C-reactive protein, fibrinogen, and low complement levels may also play a role, possibly through an autoimmune mechanism (Alijotas-Reig and Garcia-Gimenez, 2008). Additionally, HA begins to degrade 3 to 5 months after injection, which may lead to breakdown products and exposure to unknown antigens that can stimulate the immune system when paired with additional triggers (Beleznay et al., 2015).
Certain predisposing factors may cause susceptibility to HA filler DTRs, including injury, dental procedures, medications, and illnesses (e.g., facial erythema and edema after influenza-like illness; Beleznay et al., 2015, Turkmani et al., 2019). Two reports document swelling and nodules in the setting of acute sinusitis, 2 to 4 months after the last HA filler injection (Humphrey et al., 2020). Pathmanathan and Dzienis (2019) describe a case of DTR to HA progressing over the course of 48 hours after chemotherapy with cetuximab (an epidermal growth factor receptor inhibitor), with the patient developing cheek nodules and edema at areas injected with filler 6 months prior. In addition to HA, permanent fillers such as polymethylmethacrylate have also caused immunogenic reactions in susceptible individuals, with granuloma formation reported after viral infections or facial injury (Fischer et al., 2007, Lemperle et al., 2003). Even in those with no known history of immunologic risk factors, facial edema and nodule formation have occurred in the setting of respiratory infection 3 weeks after filler injection, suggesting that the window for immunogenic triggers can be at least 3 weeks (Homsy et al., 2017).
Cutaneous reactions to the COVID-19 vaccine in clinical trials, although few at this early stage, may signal dermatologic sequelae to come as larger segments of the world’s population become vaccinated. Questions may arise as patients hear of potential reactions, raising suspicion against vaccination or seeing skin reactions in themselves or others. Dermatologists must be informed and prepared to address these situations as they arise, assisting patients through the vaccination process at this critical juncture for public health in our society.
In caring for certain groups, pre-vaccine counseling may become especially relevant. For example, patients with allergies, a history of injection site reactions, or urticaria may benefit from instructions and management with antihistamines and topical medications. Cosmetic patients may now benefit from a discussion on COVID-19 vaccine–related planning, along with screening for dental procedures and the herpes simplex virus, as a part of pre-procedure counseling for dermal fillers. As cutaneous implications of both COVID-19 infection and vaccination emerge, such discussions may become part of standard medical counseling prior to esthetic procedures. Patients seeking filler injection may be counseled regarding vaccine options not associated with adverse reactions; especially for patients with risk factors discussed, procedures could be planned with a time window to minimize the risk of reactions. Although the data may be rapidly evolving, given what is known at this time, providers may consider a 4- to 8-week window between filler injections and vaccination for the general population, and potentially longer for those with risk factors autoimmune or immunologic disorders, chemotherapy or immunomodulatory medications, and those with a history of sensitivity to dermal fillers (i.e., pronounced and delayed swelling as compared to that expected for a given filler).
In cases of facial swelling, studies have shown resolution with short courses of oral steroids (<2 weeks), which do not appear to alter the effectiveness of vaccines and, if necessary, hyaluronidase for residual or prolonged edema (Beleznay et al., 2015, Pathmanathan and Dzienis, 2019, Turkmani et al., 2019). Patients should be counseled to contact their physicians for treatment if facial swelling or nodules develop, or present to the emergency room if more serious reactions occur. Additionally, although the specific fillers used by the patients in the COVID-19 vaccination trial were not reported, prior studies suggest that fillers containing HA and polymethylmethacrylate may be more likely to cause reactions. If a patient is considering a multi-modal plan to rejuvenation, other filler options (e.g., calcium hydroxyapatite, poly-L-lactic acid, or laser resurfacing) may be prioritized, especially during the months surrounding the vaccination. Dilution of filler is another reasonable consideration, with evidence showing that the dilution of both polylactic acid and HA with saline, sterile water, or lidocaine can reduce the risk of adverse events and DTRs (Lambros, 2011, Lowe et al., 2005).
With the development of novel drugs and vaccines, much of our knowledge of side effects, especially rare ones, is discovered after these medications come to market and are implemented in the population at magnitudes much larger than the clinical trial. Even a few cases in a clinical trial can foreshadow a phenomenon that may become a more common reaction in the larger population and should not be neglected, especially with estimates of >2.6 million visits for filler injections annually in the United States alone and demand continuing even during the pandemic (American Society of Plastic Surgeons, 2018, Rice et al., 2020).
Of note, the risks of the vaccine are far outweighed by the benefits of the vaccine in preventing potentially deadly illness, and dermatologists should work to encourage vaccination and dispel misconceptions. We encourage physicians to document any adverse vaccine-related reactions and report them to the Vaccine Adverse Event Reporting System (https://vaers.hhs.gov/index.html). This will aid in the ongoing safety data collection for all COVID-19 vaccinations. Additionally, we recommend reporting cutaneous reactions to any COVID-19 vaccine to the American Academy of Dermatology COVID-19 Registry (https://www.aad.org/member/practice/coronavirus/registry) to aid in the efforts of our dermatology community to better understand the specific implications of the vaccine in the skin and serve our patients.
Funding
None.
Study approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Conflicts of Interest
None.
Footnotes
Financial disclosures: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest: None.
References
- American Society of Plastic Surgeons. 2018 plastic surgery statistics [Internet]. 2018 [cited December 22, 2020]. Available from: https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf.
- Alijotas-Reig J., Esteve-Valverde E., Gil-Aliberas N., Garcia-Gimenez V. Autoimmune/inflammatory syndrome induced by adjuvants—ASIA—related to biomaterials: analysis of 45 cases and comprehensive review of the literature. Immunol Res. 2018;66(1):120–140. doi: 10.1007/s12026-017-8980-5. [DOI] [PubMed] [Google Scholar]
- Alijotas-Reig J., Fernández-Figueras M.T., Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45(1):97–108. doi: 10.1007/s12016-012-8348-5. [DOI] [PubMed] [Google Scholar]
- Alijotas-Reig J., Garcia-Gimenez V. Delayed immune-mediated adverse effects related to hyaluronic acid and acrylic hydrogel dermal fillers: clinical findings, long-term follow-up and review of the literature. J Eur Acad Dermatol Venereol. 2008;22(2):150–161. doi: 10.1111/j.1468-3083.2007.02354.x. [DOI] [PubMed] [Google Scholar]
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020 doi: 10.1056/NEJMoa2035389. NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beleznay K., Carruthers J.D.A., Carruthers A., Mummert M.E., Humphrey S. Delayed-onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41(8):929–939. doi: 10.1097/DSS.0000000000000418. [DOI] [PubMed] [Google Scholar]
- Monticelli D., Martina V., Mocchi R., Rauso R., Zerbinati U., Cipolla G. Chemical characterization of hydrogels crosslinked with polyethylene glycol for soft tissue augmentation. Open Access Maced J Med Sci. 2019;7(7):1077. doi: 10.3889/oamjms.2019.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decates T.S., Velthuis P.J., Schelke L.W., Lardy N., Palou E., Schwartz S. Increased risk of late-onset, immune-mediated, adverse reactions related to dermal fillers in patients bearing HLA-B* 08 and DRB1* 03 haplotypes. Dermatol Ther. 2020 doi: 10.1111/dth.14644. [DOI] [PubMed] [Google Scholar]
- Fischer J., Metzler G., Schaller M. Cosmetic permanent fillers for soft tissue augmentation: a new contraindication for interferon therapies. Arch Dermatol. 2007;143(4):507–510. doi: 10.1001/archderm.143.4.507. [DOI] [PubMed] [Google Scholar]
- Garvey L.H., Nasser S. Allergic reactions to the first COVID-19 vaccine: Is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2020 doi: 10.1016/j.bja.2020.12.020. S0007-0912(20)31009–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsy A., Rüegg E.M., Jandus P., Pittet-Cuénod B., Modarressi A. Immunological reaction after facial hyaluronic acid injection. Case Reports Plast Surg Hand Surg. 2017;4(1):68–72. doi: 10.1080/23320885.2017.1356202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey S., Jones D.H., Carruthers J.D., Carruthers A., Beleznay K., Wesley N. Retrospective review of delayed adverse events secondary to treatment with a smooth, cohesive 20-mg/mL hyaluronic acid filler in 4500 patients. J Am Acad Dermatol. 2020;83(1):86–95. doi: 10.1016/j.jaad.2020.01.066. [DOI] [PubMed] [Google Scholar]
- Lambros V. A technique for filling the temples with highly diluted hyaluronic acid: The “dilution solution”. Aesthet Surg J. 2011;31(1):89–94. doi: 10.1177/1090820X10391214. [DOI] [PubMed] [Google Scholar]
- Lemperle G., Romano J.J., Busso M. Soft tissue augmentation with Artecoll: 10-year history, indications, techniques, and complications. Dermatol Surg. 2003;29(6):573–587. doi: 10.1046/j.1524-4725.2003.29140.x. [DOI] [PubMed] [Google Scholar]
- Lowe N.J., Maxwell C.A., Lowe P., Duick M.G., Shah K. Hyaluronic acid skin fillers: adverse reactions and skin testing. J Am Acad Dermatol. 2001;45(6):930–933. doi: 10.1067/mjd.2001.117381. [DOI] [PubMed] [Google Scholar]
- Lowe N.J., Maxwell C.A., Patnaik R. Adverse reactions to dermal fillers. Dermatol Surg. 2005;31:1626–1633. [PubMed] [Google Scholar]
- Pathmanathan S., Dzienis M. Cetuximab-associated dermal filler reaction. BMJ Case Rep. 2019;12(8) doi: 10.1136/bcr-2018-228882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S.M., Graber E., Kourosh A.S. A pandemic of dysmorphia: “Zooming” into the perception of our appearance. Facial Plast Surg Aesthet Med. 2020;22(6):401–402. doi: 10.1089/fpsam.2020.0454. [DOI] [PubMed] [Google Scholar]
- Turkmani M.G., De Boulle K., Philipp-Dormston W.G. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clin Cosmet Investig Dermatol. 2019;12:277. doi: 10.2147/CCID.S198081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Moderna COVID-19 vaccine emergency use authorization [Internet]. 2020 [cited December 22, 2020]. Available from: https://www.fda.gov/media/144434/download
- Zhang R. FDA presentation and voting questions. U.S. Food and Drug Administration, Center for Biologics Evaluation and Research, 163rd Meeting of the Vaccines and Related Biological Products, Advisory Committee. Silver Spring, MD; 2020.



