Abstract
Objectives
This study aims to analyze the incidence of Post-acute COVID-19 syndrome (PCS) and its components, and to evaluate the acute infection phase associated risk factors.
Methods
A prospective cohort study of adult patients who had recovered from COVID-19 (27th February to 29th April 2020) confirmed by PCR or subsequent seroconversion, with a systematic assessment 10–14 weeks after disease onset. PCS was defined as the persistence of at least one clinically relevant symptom, or abnormalities in spirometry or chest radiology. Outcome predictors were analyzed by multiple logistic regression (OR; 95%CI).
Results
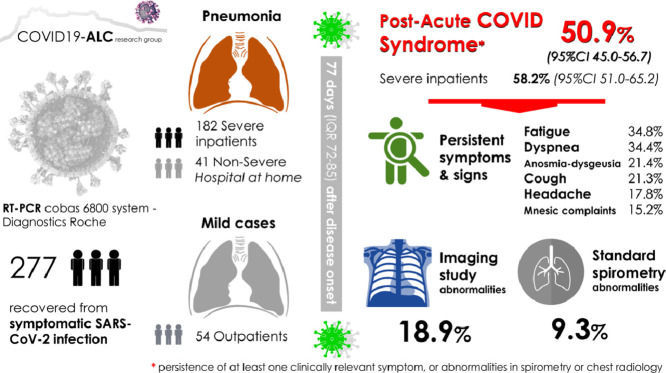
Two hundred seventy seven patients recovered from mild (34.3%) or severe (65.7%) forms of SARS-CoV-2 infection were evaluated 77 days (IQR 72–85) after disease onset. PCS was detected in 141 patients (50.9%; 95%CI 45.0–56.7%). Symptoms were mostly mild. Alterations in spirometry were noted in 25/269 (9.3%), while in radiographs in 51/277 (18.9%). No baseline clinical features behaved as independent predictors of PCS development.
Conclusions
A Post-acute COVID-19 syndrome was detected in a half of COVID19 survivors. Radiological and spirometric changes were mild and observed in less than 25% of patients. No baseline clinical features behaved as independent predictors of Post-acute COVID-19 syndrome development.
Keywords: COVID-19, Sequelae, Syndrome, Spirometry, Associated factors, Life quality, Health-related quality of life, Diagnostic imaging, Cohort study, Symptoms
Graphical abstract
Background
The battle against COVID-19 does not seem to end with screening and management of acute disease. The medium and long-term health consequences experienced by survivors of COVID-19, if any, are currently unknown.1 Series have reported the incidence of persistent symptoms ranging from 40 to 90% of patients, but the interpretation of results is hampered by non-systematic and short-term evaluations, with high heterogeneity in relation to age, severity of infection, follow-up and characteristics of the clinical evaluation.2, 3, 4, 5
The so-called “Post-COVID Syndrome” includes persistent symptoms that could be related to residual inflammation (convalescent phase), organ damage, non-specific effects from the hospitalization or prolonged ventilation (post-intensive care syndrome), social isolation6 or impact on pre-existing health conditions.
The detailed analysis of these symptoms as well as their temporary evolution is essential for the characterization of this syndrome. This study aims to analyze the medium-term persistent symptoms through a comprehensive and structured clinical assessment in patients recovered from COVID-19 and to evaluate the acute infection phase predictors of Post-acute COVID-19 syndrome (PCS).
Methods
Patients and design
This is a prospective cohort study of adult patients with COVID-19, attended in the Emergency Department from 27th February to 29th April 2020. SARS-CoV2 infection was confirmed by PCR (in nasopharyngeal swab or lower respiratory tract sample) or subsequent seroconversion. Patients were classified as hospitalized (severe pneumonia), mild pneumonia managed as hospital follow-up at home and cases without pneumonia managed by primary care.
Patients were offered an assessment by COVID-19 medical team 10–14 weeks after ambulatory COVID-19 recovery or discharge from hospital. General characteristics of the study population and acute infection phase data, including Charlson index and COVID-GRAM score,7 were extracted from the electronic medical record. A structured evaluation was performed in the same visit: clinical examination, blood test, Chest-X ray, pulmonary function test and quality of life questionnaire with EuroQol visual analogue scale (VAS).
Chest radiographs were evaluated by an experienced thoracic radiologist, unaware of the clinical status; a pathological radiograph was defined as a score ≥ 2.8 Standard spirometry was performed (MasterScreen PFT-Pro; Jaeger, Germany) according to the ATS/ERS recommendations and the Global Lung Function Initiative 2012 reference values were used.
Patients with significant alterations were referred for subsequent specialist evaluations around week 16–18.
Outcomes
The primary endpoint was PCS defined as the persistence of at least one clinically relevant symptom, spirometry disturbances or significant radiological alterations.
Secondary endpoints were the identification of risk factors for the PCS related to the baseline characteristics of the acute episode of COVID-19, and changes in quality of life related to COVID-19.
The cumulative incidence of PCS (95%CI) and its components were determined both in the global cohort and in the severe pneumonia subpopulation. Associations between disease severity, other explanatory variables and PCS or its components were evaluated by chi-2 test. Multiple logistic regression models were built to explore which risk factors present at COVID-19 diagnosis were associated with a higher incidence of PCS; odds ratios (OR) with (95%CI) were estimated. Variables were included as covariates if they showed significant associations in simple models. IBM SPSS Statistics v25 (Armonk, NY) was used for analyses. P<0.050 defined statistical significance. Written informed consent was obtained from all the participants, with approval by the institutional review board (EXP. 200,145).
Results
Of 422 patients evaluated in the emergency department, 58 (13.7%) died, eight after discharge from hospital due to progression of neoplasia (n = 2), bacterial infections not related to COVID-19 (n = 3), sudden death (n = 1) and of unknown cause (n = 2). Thirty-eight were not included in the present study: 24 by severe comorbidity, 7 followed in other health areas and 7 by being monitored by other physicians. Four patients refused to participate, 15 patients did not attend the face-to-face assessment (although a telephone interview was conducted) and 30 patients (7.1%) were lost to follow-up. Finally, 277 (76% of the survivors) were included in the study. Two hundred sixty-nine (97.1%) were PCR positive for SARS-CoV2 during admission; the remaining population presented a clinical picture with high clinical suspicion, confirmed by the presence of anti-SARS-CoV-2 antibodies measured at a median (range) of 76 days (IQR 72–83) after disease onset.
Two hundred and seventy-seven patients were included, median age 62.0 years (53.0–72.0), 52.7% males and 30.3% had a Charlson index ≥3. There were fifty-four (19.5%) patients without pneumonia, 41 (14.8%) with non-severe pneumonia and 182 (65.7%) with severe pneumonia (i.e. Hospitalized). Patients were evaluated at a median (IQR) of 77 days (72–85) after disease onset, and 182 (65.8%) reported having recovered their health status prior to infection.
Post- COVID syndrome and associated factors
The PCS was detected in 141 patients (50.9%; 95%CI 45.0–56.7). The cumulative incidence was 58.2% (95%CI 51.0–65.2), 36.6% (95%CI 23.5–51.8) and 37.0% (95%CI 25.4–50.3) in patients with severe pneumonia, mild pneumonia and without pneumonia, respectively (p = 0.003).
Table 1 shows the general characteristics of the study population and main features of the comprehensive medical review.
Table 1.
General characteristics of the study population and main features in medical assessment post-COVID infection.
| Charateristics of patients | ||
| Demographics | ||
| Age (median), years | 56.0 (42.0–67.5) | |
| Males,% | 52.7 (146/277) | |
| Comorbidities | ||
| Charlson comorbidity index | 2.0 (0.0–3.0) | |
| Charlson index ≥3,% | 30.3 (84/277) | |
| Hypertension,% | 36.5 (101/277) | |
| Diabetes,% | 11.6 (32/277) | |
| Obesity,% | 30.6 (83/271) | |
| Cardiovascular disease,% | 6.9 (18/261) | |
| Chronic respiratory disease,% | 18.1 (50/277) | |
| Immunosuppression,% | 4.7 (13/277) | |
| Admission data | ||
| ICU admission,% | 8.7 (24/277) | |
| Length-hospital stay, median | 8.5 (6.0–12.0) | |
| Length-ICU stay, median | 9.0 (4–5–13.7) | |
|
Medical assessment post-COVID infection |
||
| Global Assessment | Specialized evaluation | |
| (8–12 weeks) | (16–18 weeks) | |
| “Post-COVID syndrome “ | 50.9 (141/277) | |
| Severe pneumonia | 58.2 (106/182) | |
| Mild pneumonia | 36.6 (15 / 41) | |
| No pneumonia | 37.0 (20 /54) | |
| General Clinical Features | ||
| Fatigue,% | 34.8 (96/277) | |
| Anosmia-dysgeusia,% | 21.4 (59/277) | |
| Myalgias-arthralgias,% | 19.6 (54/277) | |
| Pneumological features | Pneumologist* | |
| Dyspnea,% | ||
| Persistence | 34.4 (95/277) | 11.1 (31/277) |
| Cough,% | ||
| Persistence | 21.3 (59/277) | 2.1 (6/277) |
| Neurological features | Neurologist* | |
| Headache,% | 17.8 (49/277) | |
| Moderate-Severe*,% | 53 (26/49) | |
| Persistence,% | 2.9 (8/277)1 | |
| De novo,% | 2.5 (7/277) | |
| Pathological CT or MR,% | 0.3 (1/277) | |
| Mnesic complaints,% | 15.2 (42/277) | |
| Clinical relevance*,% | 57.1(24/42) | |
| Persistence,% | 5.0 (14/277)2 | |
| De novo,% | 3.6 (10/277) | |
| Pathological CT or MRI,% | 1.4 (4/277) | |
| Pathological neurocognitive test, | 1.8 (5/277)3 | |
| Diarrhoea,% | 10.5 (29/277) | |
| Skin features,% | 8.3 (23/277) | |
| Visual loss,% | 5.4% (15/277) | |
| Fever,% | 0.0 (0/277) | |
| Laboratory features4 | ||
| Lymphocytes, <1500 per mm3 | 19.9 (55/277) | |
| C-reactive protein > 0.5 mg/dL | 11.6 (32/276) | |
| D-dimers > 0.5 mg/mL | 24.9 (68/273) | |
| Ferritin > 150 mg/L | 40.6 (112/276) | |
| Lactate dehydrogenase> 250 U/L | 9.9 (27/274) | |
| Troponin T, > 14 ng/L | 14.5 (40/275) | |
| CK > 170 U/L | 13.0 (34/276) | |
| Pro-BNP, | ||
| <50 years old, >450 pg/mL | 1.9 (2/104) | |
| 50–75 years old, >900 pg/mL | 2.7 (4/145) | |
| >75 years old, >1800 pg/mL | 18.5 (5/27) | |
| Spirometry5 | ||
| Global cohort | 3.7 (10/269) | |
| Restriction | 12.6 (34/269) | |
| Obstruction | 1.9 (5/269) | |
| Mixed patterns | ||
| Non previous pulmonary disease | 2.7 (6/227) | |
| Restriction,% | 9.9 (22/227) | |
| Obstruction,% | 1.4 (3/227) | |
| Mixed patterns,% | ||
| Radiological features6 | ||
| Chest X-rays score ≥2 | 18.9 (51/269) | |
| Chest X-rays score ≥5 | 4.0 (11/269) | |
| Quality of life | ||
| (EuroQol visual analog scale, VAS) | ||
| Previous COVID infection, median | 90 (80–100) | |
| Post-COVID infection, median | 83.0 (70–90) | |
Associated with anxious depressive symptoms 90% (13/14).
Associated with anxious depressive symptoms 71.4% (10/14).
One patient with previous cognitive impairment, two with severe B12 vitamin deficiency and one with demyelination lesions. Only one patient associated ansioux depressive symptoms.
Laboratory reference range are used as cut-off point for the categorization of variables; for pro-BNP, pro-natriuretic peptide type B, the cut-off point of high probability of heart failure has been used, categorized by age.
Patient underwent standard spirometry (MasterScreen PFT-Pro; Jaeger, Germany) accordingly with the ATS/ERS recommendations. Forced vital capacity (FVC) and forced expiratory volume at the first second of exhalation (FEV1) was obtained. Obstruction was considered as FEV1/FVC <0.7 and restriction when FVC <0.8 and FEV1/FVC ≥0.7 without corporal pletismography confirmation.
Chest radiographs at the time of diagnosis and in the follow-up visit were evaluated by a 25-year experienced thoracic radiologist, without knowledge of the clinical status. Lung involvement was classified as reticular, ground-glass or consolidation predominance, or as a combination of them, and extension of abnormalities was graded according to an adaptation of a previously described scoring system8 ranging from 0 to 10.
The referral criteria for pneumology were the presence of cough, dyspnea, radiographic or spirometry abnormalities; for neurology were a referred memory impairment, new-onset self-perceived cognitive impairment or previous exacerbation or new neurological deficit in the anamnesis / exploration.
The most frequent symptoms were dyspnea and fatigue. Anosmia-dysgeusia was associated with younger age (<65 yr 24.9% (48/194) vs >65 yr 13.5% (11/83), p 0.03). The most relevant laboratory findings were lymphopenia and high ferritin and D-dimer levels.
Relevant neurological symptoms (headache, memory disorders / cognitive deterioration, or both) were present in 33 patients (11.9%).
The neurological and respiratory symptoms generally improved in the evaluation by specialists, 16–18 weeks after disease onset, with persistent alterations that required medical follow-up by pneumologits in 13.3% (37/277) and neurologist in 7.5% (21/277).
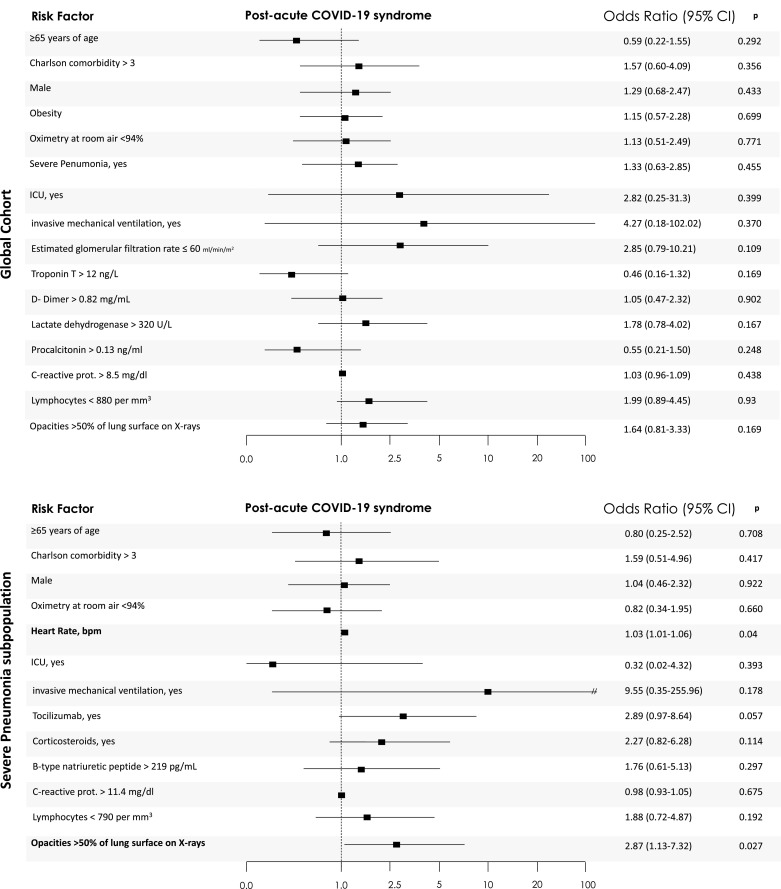
After multivariate adjustment, no baseline clinical features, neither age, sex, comorbidity, severity of acute COVID-19 infection, COVID-GRAM score, inflammatory markers, ICU-admission, hospital/ICU length of stay, or treatment behave as independent predictors of “PCS” (see Fig. 1 ).
Fig. 1.
Post-acute COVID-19 syndrome associated factors. Variables were included as covariates if shown significant associations in simple models. Some covariates could be excluded in case of been highly correlated, >20% of missing values or number of events was too small to calculate odds ratios. The 95% confidence intervals (CIs) of the odds ratios have been adjusted for multiple testing. In bold, independent predictors associated with the outcomes. For the purpose of logistic regression, variables were categorized regarding their 75-percentiles within each subpopulation, to show the impact of severe extreme values in the outcomes – except for those in which severity is defined by lowest levels, such as lymphocyte counts, where 25-percentiles were used. For the following variables, standard categorizations were followed: age ≥65 years, Oximetry at room air <94%, respectively.
In severe pneumonia, only opacities of lung surface on X-rays >50% (OR 2.87 (1.13–7.32), p = 0.027) and higher heart rate at admission (OR 1.03 (1.01–1.06, p = 0.04), were independent predictors of post-COVID syndrome.
Respiratory function test and imaging and associated factors
Standard spirometry and imaging study were performed in 269 patients (97.1% of study population). Spirometry abnormalities, after ruling out 14 patients with history of lung disease, were present in 25/269 (9.3%); with a clear predominance of the obstructive pattern (22/25), that was mild in 63.6% (14/22) of patients. Relevant imaging changes were found in 51 patients (18.9%; 95%CI 14.7–24.1), but 52.9% of them were free of respiratory symptoms. Conversely, patients with cough or dyspnea showed relevant findings on chest imaging by 20.7% or pulmonary function test by 14.3%.
In the overall cohort the predictors of abnormalities in spirometry were an estimated glomerular filtrate ≤ 60 ml/min/m2 (OR 37.17 (3.38–408.81, p = 0.003) and male sex (OR 8.87 (1.91–41.26), p = 0.005), whereas Charlson index ≥3 (OR 0.06 (0.01–0.69), p = 0.024) was associated with a lower incidence. Also, in severe pneumonia, an independent predictor was an estimated glomerular filtrate ≤ 60 ml/min/m2 (OR 28.14 (2.13–371.3, p = 0.011), whereas male sex (OR 4.95 (0.97–25.27), p = 0.055), and Charlson index ≥3 (OR 0.05 (0.002–1.08), p = 0.056) approached statistical significance.
After adjustment for confounding factors, only a higher imaging score during acute infection phase was associated with the persistence of radiological lung involvement, both in the global cohort and in patients with severe pneumonia (OR 1.66 (1.30–2.11) and 1.68 (OR 1.28–2.196), p<0.001, respectively).
Quality of life
The median decrease of 9 points on the EuroQol VAS shows a worsening in the perception of health after the COVID infection. PCS patients, compared to those without sequelae, showed a more frequently an impact on their quality of life after infection, 66.9% versus 43.2%, p = 0.0001, respectively.
Discussion
The present study confirmed a high incidence of persistent symptoms in patients with COVID-19 (around 50%), 10–14 weeks after disease onset. These symptoms were mostly mild general (fatigue), respiratory (dyspnea) or neurological complaints, but were not found to be associated with other pathological findings in the examinations performed. Radiological and spirometric changes were observed in less than 25%. PCS entails a high psychological distress influencing the quality of life. However, respiratory and neurological symptoms, drastically improve 16–18 weeks after disease onset. Neither the baseline characteristics of the patients nor the COVID-19 disease features were associated with the persistence of these symptoms in the global cohort, whereas in the severe pneumonia group, extension of lung involvement and higher heart rate at admission, were independent predictors of post-COVID syndrome. Renal insufficiency and male sex were associated with changes in spirometry, whereas higher imaging score during acute infection was associated with the persistence of lung involvement.
The available data regarding the incidence and evolution of postCOVID alterations are scarce and heterogeneous. Halpin et al.2 , Tenforde et al.3 and Carvalho-Schneider et al.9, reported the results of a structured telephone interview about symptoms after acute infection phase. Halpin et al. evaluated 100 patients (32 ICU), a mean of 48 days postdischarge from hospital; fatigue was the most commonly reported symptom by 72% and 60.3%, followed by breathlessness (65.6% and 42.6%) and psychological distress (46.9% and 23.5%), with a drop in EQ5D in 68.8% and 45.6%, ICU group vs hospital ward, respectively.2. Tendforde et al. reported a series of 292 patients with mild COVID-19 (without hospital admission), 94% reported experiencing one or more symptoms at the time of testing (cough 43%, fatigue 35%, or shortness of breath 29%)[3]. Finally, Carvalho-Schneider et al.9 showed that up to 2 months after symptom onset, two thirds of 150 adults with non-critical COVID-19 had complaints, mainly anosmia/ageusia, dyspnea or asthenia.
Carfi et al.4 and Wang et al.5, reported the results of face-to-face evaluation after hospital discharge. In a population of 143 patients (5% invasive ventilation) assessed a mean of 60.3 (SD, 13.6) days after onset of the first COVID-19 symptom, 12.6% were completely free of any COVID- 19–related symptom, while 32% had 1 or 2 symptoms and 55% had 3 or more, as fatigue (53.1%), dyspnea (43.4%), joint pain (27.3%) and chest pain (21.7%);4 a worsened quality of life was observed in 44.1%. Wang et al. evaluated 131 COVID-19 patients (median age 49, 52.6% severe pneumonia) weekly up to 4 weeks. At discharge, 40.4% had symptoms (mainly cough 29.0%, fatigue 7.6%, expectoration 6.1%)[5]. These symptoms progressively declined in the 4 weeks after discharge, with only 9.1% symptomatic patients, without differences depending on the severity of the initial condition. Townsend et al.10 reported the persistence of fatigue in 52.3% (67/128) a median of 10 weeks after initial COVID-19, irrespective of severity of initial illness (44.5% managed as outpatients), highlighting the importance of following all patients diagnosed with COVID, not merely those who required hospitalization.
Regarding pulmonary dysfunction, Zhao et al.11 reported that in 55 patients, 3 months after discharge, 64% had persistent symptoms and 71% radiologic abnormalities and 25% decreased diffusion lung capacity. In another study, Huang et al.12, of 57 patients evaluated 30 days after discharge, found decreased lung diffusion capacity (53%) and diminished respiratory muscle strength (49%). Finally, van den Borst et al.13 showed that the lung diffusion capacity was below normal range in 42% (40/97) of discharged patients, 13 weeks after onset of SARS-CoV-2 symptoms; but only 57% of the invited patients attended to aftercare facility.
Our initial assessment did not include the diffusion capacity. The evaluation was designed for a general assessment of respiratory function, through clinical features (symptoms, oxygen saturation), chest radiography and spirometry, as a screening test. We plan more specific tests such as diffusion capacity and high-resolution computed tomography, in a subsequent evaluation of patients with positive screening (persistence of respiratory symptoms, changes in chest radiography or spirometry). This approach is in accordance with a recent published guidance of respiratory follow-up of patients with COVID-19 pneumonia,14 looking for a rational use of resources. It is important to highlight that most of the patients with spirometry alterations in our series had a mild involvement, without clear clinical impact.
The extent of emotional and behavioral concerns and general distress of those affected by COVID-19 has yet to be determined. In this sense, the reported series have shown that PCS leads to a deterioration in the quality of life in 2/3 of the patients.
In summary, the full range of the duration and severity of post-acute COVID-19 is currently unknown. A consensus is needed regarding when and how to classify manifestations in the post-acute period, considering many of these features can resolve with time and their prevalence therefore depends on the time of evaluation. In an attempt to standardize our current understanding of post-acute COVID-19, Amenta et al.15 propose that the post-acute period for COVID-19 starts 3 weeks after symptom onset. Also, they propose classifying post-acute manifestations into 3 categories: (1) residual symptoms that persist after recovery from acute infection; (2) organ dysfunction that persists after initial recovery; and (3) new symptoms or syndromes that develop after initial asymptomatic or mild infection. The homogenization of the nomenclature, would facilitate the comparison between future series.
The strength of our study is based on four main points, which in turn are the main criticisms to the series published [6]: 1. The representativeness of the sample (wide clinical spectrum of severity and representative of comorbidity in western countries); 2. The re-evaluation time, longer than any published series; 3. Structured face-to-face assessment intervention, including blood test, chest radiographs, pulmonary function tests, and quality of life evaluation, allowing a systematic search for residual objective abnormalities after COVID-19, beyond those perceived by patients; 4. Analysis of the characteristics of acute infection associated with persistent symptoms.
Limitations include possible undetected pre-COVID abnormalities in the patients, the exclusion of some patients with severe comorbidity, the absence of imaging to evaluate myocardial involvement, the lack of study of the diffusion capacity of the lung for carbon monoxide (DLCO), the relatively small sample size to detect minor associations, some identified statistical associations with changes in spirometry may not be explained by biological plausibility and the single-center design, as well as the need for long-term follow-up.
These findings support the need for a multidisciplinary approach to the care of this vulnerable population, to help better define this new “Post-COVID syndrome” and lay the groundwork to efficiently conducting therapeutic intervention studies and designing future follow-up plans. Longer longitudinal observational studies will be critical to elucidate the health consequences attributable to COVID-19.
Funding
No external funding was received. The information of this article have not been presented in any meeting(s).
Access to data
O.M-P and E.M have full access to the data and are the guarantor for the data.
Contribution
We encourage authors to disclose their personal contribution to the research and article (Writing – Original Draft: E.M. and O.M-P.; Writing – Review & Editing: E.M., O.M-P., V.B., M.A., J-M.L., J.G., J-M.R., J.A., S.A., R.S., P.R-T., I.G., A.S., A.A., P.G.; Conceptualization: E.M. and O.M-P.; Investigation: E.M., O.M-P., J-M.R., V.B., X.P., M.A., J-M.L., J.G., J.A., S.A., R.S., P.R-T., I.G., A.S., A.A., P.G.; Methodology: E.M. and O.M-P.; Formal Analysis: O.M-P; Project Administration: E.M; Funding Acquisition: not applicable.)
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
The members of COVID19-ALC research group are: Esperanza Merino, Joan Gil, Vicente Boix, Ximo Portilla, Oscar Moreno-Pérez, Mariano Andrés, Jose-Manuel Leon-Ramirez, Santos Asensio, Cleofé Fernandez, Alfredo Candela, Mª del Mar García, Rosario Sánchez, Diego Torrus, Sergio Reus, Pilar González, Silvia Otero, Jose M Ramos, Beatriz Valero, Alex Scholz, Antonio Amo, Héctor Pinargote, Paloma Ruiz, Raquel García-Sevila, Ignacio Gayá, Violeta Esteban, Isabel Ribes, Julia Portilla, Cristina Herreras, Alejando Cintas, Alicia Ferradas, Ana Martí, Blanca Figueres, Marcelo Giménez, María-Ángeles Martínez, María-Mar García-Mullor, María Angeles Martínez, Irene Calabuig, Marisa Peral, Ernesto Tovar, M Carmen López, Paloma Vela, Pilar Bernabeú, Ana Yuste, José Ponce, Bertomeu Massuti, Vicente Climent, Vicente Arrarte, Fernando Torres, Laura Valverde, Laura Delegido, Cristina Cambra, Miriam Sandín, Teresa Lozano, Amaya García-Fernández, Alejandro Do Campo, Eduardo Vergara, Nicolás López, Elena Elvira, Fátima López, Fernando Dahl, Blanca Serrano, Sarai Moliner, Carmina Díaz, Dolores Castaño, Beatriz López; Antonio Picó, Joaquín Serrano, Sol Serrano, María Marín-Barnuevo, María Díaz, Cristina Gilabert, Estela Martínez, Elena Vivó, Noelia Balibrea, Miguel Perdiguero, Carolina Mangas, Lucía Medina, Oscar Murcia, María Rodríguez, Rodrigo Jover, Javier López, Marina Morillas, Mercedes Khartabil, Cristina Gil, Carlos Salazar, Eva Vera, Helena López, Vanesa Rodríguez, Sandra Baile, Norma Guerra, Mar Blanes, Jaime Guijarro, José Carlos Pascual, Iris Gonzalez, Pedro Sanso, José Manuel Ramos, Jaime Javaloy, Clara Llopis, Olga Coronado, Esther García, Gonzalo Rodríguez, Paola Melgar, Mariano Franco, Félix Lluís, Carmen Zaragoza, Cándido Alcaraz, Ana Carrión, Celia Villodre, Emilio Ruiz de la Cuesta, Cristina Alenda, Francisca Peiró, María Planelles, Laura Greco, Sandra Silvia, Antonio Francia, Iván Verdú, Juan Sales, Ana Palacios, Hortensia Ballester, Antonio García-Valentín, Marta Márquez, Eva Canelo, Andrea Juan, Elena Vives, Andrea Revert, Gonzalo Fuente, Ester Nofuentes, Carolina Mangas, Eva Vera, Alicia Ferradas, Helena López, Cristian Herrera, Beatriz López, Marina Morillas, Vanesa Rodríguez, Mercedes Khartabil, Mario Giménez, Ernesto Tovar, Estela Martínez, Lucia Medina, Sandra Baile, Carlos Salazar, Norma Guerra, Sarai Moliner, Mari-Carmen López-González, Blanca Figueres.
References
- 1.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA 2020; . [DOI] [PMC free article] [PubMed]
- 2.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., Walshaw C., Kemp S., Corrado J., Singh R., Collins T., O'Connor R.J., Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2020; . [DOI] [PubMed]
- 3.Tenforde M.W., Kim S.S., Lindsell C.J., Billig Rose E., Shapiro N.I., Files D.C., Gibbs K.W., Erickson H.L., Steingrub J.S., Smithline H.A., Gong M.N., Aboodi M.S., Exline M.C., Henning D.J., Wilson J.G., Khan A., Qadir N., Brown S.M., Peltan I.D., Rice T.W., Hager D.N., Ginde A.A., Stubblefield W.B., Patel M.M., Self W.H., Feldstein L.R. IVY network investigators, CDC COVID-19 response team, IVY network investigators. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carfì A., Bernabei R., Landi F. Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Xu H., Jiang H., Wang L., Lu C., Wei X., Liu J., Xu S. Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM Mon J Assoc Phys. 2020;113:657–665. doi: 10.1093/qjmed/hcaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg P., Arora U., Kumar A., Wig N. The “post-COVID” syndrome: how deep is the damage? J Med Virol. 2020 doi: 10.1002/jmv.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., Li Y., Guan W., Sang L., Lu J., Xu Y., Chen G., Guo H., Guo J., Chen Z., Zhao Y., Li S., Zhang N., Zhong N., He J. China medical treatment expert group for COVID-19. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180:1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litmanovich D.E., Chung M., R Kirkbride R., Kicska G., P Kanne J. Review of chest radiograph findings of COVID-19 pneumonia and suggested reporting language. J Thorac Imaging 2020; . [DOI] [PubMed]
- 9.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., Flament T., Ferreira-Maldent N., Bruyère F., Stefic K., Gaudy-Graffin C., Grammatico-Guillon L., Bernard L. Follow-up of adults with non-critical COVID-19 two months after symptoms’ onset. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 2020; .
- 10.Townsend L., Dyer A.H., Jones K., Dunne J., Mooney A., Gaffney F., O'Connor L., Leavy D., O'Brien K., Dowds J., Sugrue J.A., Hopkins D., Martin-Loeches I., Ni Cheallaigh C., Nadarajan P., McLaughlin A.M., Bourke N.M., Bergin C., O'Farrelly C., Bannan C., Conlon N. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y.M., Shang Y.M., Song W.B., Li Q.Q., Xie H., Xu Q.F., Jia J.L., Li L.M., Mao H.L., Zhou X.M., Luo H., Gao Y.F., Xu A.G. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., Zhou X., Liu X., Huang X., Yuan S., Chen C., Gao F., Huang J., Shan H., Liu J. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Borst B., Peters J.B., Brink M., Schoon Y., Bleeker-Rovers C.P., Schers H., van Hees H.W.H., van Helvoort H., van den Boogaard M., van der Hoeven H., Reijers M.H., Prokop M., Vercoulen J., van den Heuvel M. Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis Off Publ Infect Dis Soc Am 2020; .
- 14.George P.M., Barratt S.L., Condliffe R., Desai S.R., Devaraj A., Forrest I., Gibbons M.A., Hart N., Jenkins R.G., McAuley D.F., Patel B.V., Thwaite E., Spencer L.G. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75:1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 15.Amenta E.M., Spallone A., Rodriguez-Barradas M.C., El Sahly H.M., Atmar R.L., Kulkarni P.A. Post-acute COVID-19: an overview and approach to classification. Open Forum Infect Dis [Internet] 2020 [cited 2020 Dec 13]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7665635/. [DOI] [PMC free article] [PubMed]