Abstract
This systematic review aimed at investigating longitudinal changes in human milk bioactive protein concentrations in Chinese population. Both English and Chinese databases were searched. The data were pooled into six defined lactation stages. Weighted means of protein concentrations in each stage and the statistical significance of means of different lactation stages were calculated. The data of 11 bioactive proteins were retrieved. Concentrations of sIgA, IgM, and IgG decreased sharply during the first 14 days of lactation. The levels of α‐lactalbumin, lactoferrin, and β‐casein also decreased throughout lactation. Conversely, lysozyme levels increased over lactation. The changing patterns of the serum albumin, osteopontin, and bile salt‐stimulated lipase (BSSL) were not conclusive. This study represents the most comprehensive summary of bioactive proteins in Chinese human milk. In the future, mass spectrometry‐based analysis of human milk proteomics may be used to investigate the longitudinal changes of many more bioactive proteins.
Keywords: breast milk, composition, dynamic, profile
This systematic review aims at investigating the longitudinal changes in the bioactive proteins in the human milk of the Chinese population. The concentrations of α‐lactalbumin, lactoferrin, β‐casein, and three immunoglobulins (sIgA, IgM, and IgG) decrease during lactation. Lysozyme concentrations increase during lactation.

1. INTRODUCTION
Human milk provides infants with advantages in cognitive development, defense against pathogens, digestion and absorption of nutrients, lower risk of chronic diseases in later life, etc. (Lönnerdal, 2016b). Accordingly, the World Health Organization recommends that infants should be exclusively breastfed for the first 6 months of their lives. Breastfeeding beyond 6 months—with the addition of appropriate complementary foods—is also recommended (WHO, 2003). The components in human milk contain a complex matrix of nutrients, including proteins and amino acids, lipids, lactose and oligosaccharides, vitamins, minerals, and other substances.
The proteins in human milk are beneficial to infants in two distinct ways. First, amino acids derived from proteins can be assimilated by infants as their tissue proteins, catabolized as metabolic fuels, or converted to intermediate metabolites such as ornithine and citrulline (Kalhan & Bier, 2008). Second, some proteins exhibit beneficial effects on infants as intact proteins or partially digested products (peptides). These proteins are commonly defined as bioactive proteins (Lönnerdal, 2013). Bioactive proteins offer a wide variety of functions such as facilitating nutrient digestion and absorption, modulating immune functions, and defense against pathogens. Table 1 lists bioactive proteins with known or proposed functions (Artym & Zimecki, 2013; Haschke et al., 2016; Lönnerdal, 2016a). Furthermore, it should be noted that the advancement of analytical tools has allowed an increasing number of bioactive proteins to be identified and quantified in human milk.
Table 1.
List of bioactive proteins investigated in this study
| Item | Molecular weight (kDa) | Compartment in human milk | Digestibility by infant's gut | Functions |
|---|---|---|---|---|
| α‐Lactalbumin | 14 | Whey | Partial digestion | Zn & Fe absorption; immunomodulation; prebiotics |
| Lactoferrin | 80 | Whey | No or limited digestion; intact proteins found in stool | Fe absorption; Immunomodulation; antimicrobial activity; intestinal development; prebiotics; cognitive development |
| Serum albumin | 67 | Whey | Easily digested | Unclear |
| Secretory IgA (sIgA) | 60 | Whey | No or limited digestion; intact proteins found in stool | Immunomodulation; antimicrobial activity |
| IgM | 74 | Whey | Easily digested | Immunomodulation |
| IgG | 50 | Whey | Easily digested | Immunomodulation |
| Lysozyme | 14 | Whey | No or limited digestion; intact proteins found in stool | Antimicrobial activity |
| Osteopontin | 44–75 | Whey | Partial digestion | Immunomodulation |
| Bile salt‐stimulated lipase (BSSL) | 90 | Whey | No or limited digestion; intact proteins found in stool | Lipid digestion and absorption; antimicrobial activity |
| Haptocorrin | 60 | Whey | No or limited digestion; intact proteins found in stool | Vitamin B12 absorption; antimicrobial activity |
| Milk fat globule membrane protein (MFGMP) | N/A | Mucin | N/A | Antimicrobial activity, prebiotics |
| β‐Casein | 24 | Casein | Partial digestion | Ca, Zn, and P absorption |
| κ‐Casein | 19 | Casein | Partial digestion | Antimicrobial activity |
Abbreviation: N/A, data not available.
Different bioactive proteins in human milk follow different changing patterns throughout lactation (Lönnerdal et al., 2017). It is possible that the changing patterns of bioactive proteins meet specific needs of the infants during different stages of growth and development. For example, lactoferrin is a bioactive protein that can inhibit bacterial growth and has immunomodulatory properties (Legrand, 2016; Yin et al., 2014). Its concentration is the highest in colostrum, which is consistent with the fact that infants are more vulnerable to foreign pathogens during the first week of life when compared with the rest of infancy (Levy, 2007). Therefore, understanding the changing patterns throughout lactation can shed light on the physiological functions of bioactive proteins.
Genetic and dietary factors may lead to differences in the composition of human milk among different countries (Stam et al., 2013). China has a population of more than 1.4 billion. The bioactive proteins in human milk in Chinese population have been investigated and published in original research articles in both English and Chinese. However, to our best knowledge, there is currently no systematic review that compiles the data of bioactive proteins in Chinese human milk. Compared with original research articles, systematic reviews can provide a more comprehensive summary.
The aim of this study is to investigate the longitudinal changes of human milk bioactive proteins in Chinese population. To achieve this goal, a systematic review was conducted and the statistical significance of bioactive protein levels between different lactation stages was analyzed. Our study represents the first systematic review to summarize the bioactive proteins in human milk in Chinese population.
2. METHODS
2.1. Literature screening
The PRISMA guidelines were followed (Shamseer et al., 2015). For articles published in English, the databases Pubmed, Web of Science, Taylor & Francis Online, and Springer were searched. The searching strategy of "(human OR breast) AND (milk) AND (bioactive protein OR α‐lactalbumin OR lactoferrin OR secretory IgA OR IgG OR IgM OR lysozyme OR bile salt‐stimulated lipase OR haptocorrin OR osteopontin OR β‐casein OR κ‐casein OR MFGM) AND (composition OR concentration OR content) AND (China OR Chinese)" was used. For articles published in Chinese, the databases China National Knowledge Infrastructure (CNKI; http://cnki.net/), Wanfang Data (http://www.wanfangdata.com.cn), and Chongqing VIP Information (http://qikan.cqvip.com/) were searched. Per the style of the Chinese language, the searching strategy was optimized to "(human OR breast) AND (milk) AND (effective components OR bioactive protein OR α‐lactalbumin OR lactoferrin OR secretory IgA OR IgG OR IgM OR lysozyme OR bile salt‐stimulated lipase OR haptocorrin OR osteopontin OR β‐casein OR κ‐casein OR MFGM) AND (composition OR concentration OR content)." The literature searching was completed in April 2020.
Duplicates and obviously irrelevant articles were removed after reading the titles and abstracts. The full texts of the remaining articles were screened using the inclusion and exclusion criteria listed in PICOS (Table 2). Furthermore, a quality assessment was performed for all the included articles (Table S1). The literature screening process was performed independently by two investigators (Q. R. and Y. Z.). Discrepancies were discussed in the presence of Y. X. and S. J. until consensuses were reached.
Table 2.
Inclusion and exclusion criteria for selecting articles (PICOS)
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Chinese population; healthy mothers | Non‐Chinese populations; non‐human; mothers or infants with defined diseases or disorders (premature delivery was not regarded as diseases or disorders) |
| Intervention | N/A | N/A |
| Comparator | N/A | N/A |
| Outcomes | Human milk samples; data were expressed as means or medians; lactation stages could fit into the categories of 1–7, 8–14, 15–30, 31–60, 61–90, 91–365 postnatal days | Lactation stage not specified or simply described as colostrum, transition milk, or mature milk |
| Study design | Original articles from peer‐reviewed journals; master theses or doctoral dissertations that reported original research data | Review articles; abstracts; articles without access to full‐text; milk samples were pooled together before assessment |
2.2. Data extraction and analysis
The means and medians were extracted, and the medians were converted to means as previously described (Hozo et al., 2005; Luo et al., 2015). All units were converted to mg/100 ml. The density of the human milk was assumed to be 103.2 g/100 ml for unit conversion (Neville et al., 1988). Lactation was divided into six stages—1–7, 8–14, 15–30, 31–60, 61–90, and beyond 91 postnatal days—and data within each lactation stage were pooled. Outliers were identified using Q3 + 1.5 * (Q3 − Q1) and Q1 − 1.5 * (Q3 − Q1) as upper and lower fences, respectively, and were removed from further calculation. After data cleaning, the weighted means and standard deviations (SDs) were calculated. One‐way ANOVA analyses followed by Student–Newman–Keuls tests were used to determine the statistical significance of the means of each bioactive protein in different lactation stages. Excel and SPSS (19.0) were used for data extraction and analysis. Data extraction and analysis were performed independently by two investigators (Q. R. and Y. Z.). Discrepancies were discussed in the presence of Y. X. and S. J. until consensuses were researched.
3. RESULTS
The literature screening process is shown in Figure 1. The included studies are listed in Table 3 (Affolter et al., 2016; Bruun et al., 2018; Cai et al., 2018; Chen, 2007; Chen et al., 1986; Dai & Guan, 1985; Dou et al., 1986; Elwakiel et al., 2019; Han et al., 2010; Hsu et al., 2014; Jackson et al., 2004; Jiang, 2017; Jiang et al., 1999; Li, et al., 1995; Li, et al., 1995; Liu et al., 1994, 2018, 2019; Liu & Zhang, 2005; Min, 1989; Sha et al., 2019; Shan et al., 2011; Shi et al., 2011; Urwin et al., 2013; Wang et al., 2012; Wang, 1987, 2012; Wang & Lin, 1997; Wei & Pan, 1991; Wu et al., 1995; Yang et al., 2018; Yuen et al., 2012). The included studies covered 23 out of 34 provinces in China. Furthermore, 20 of the included studies specified that milk from mothers who delivered full‐term infants was investigated, whereas the remaining 12 did not indicate the gestation age at delivery. Three of the included studies specified that milk was collected from vaginal delivery mothers, whereas the rest of the studies did not mention the types of delivery. Two studies collected foremilk, four studies collected full expression from one side, and the rest did not specify the means of milk collection (Table 3).
FIGURE 1.
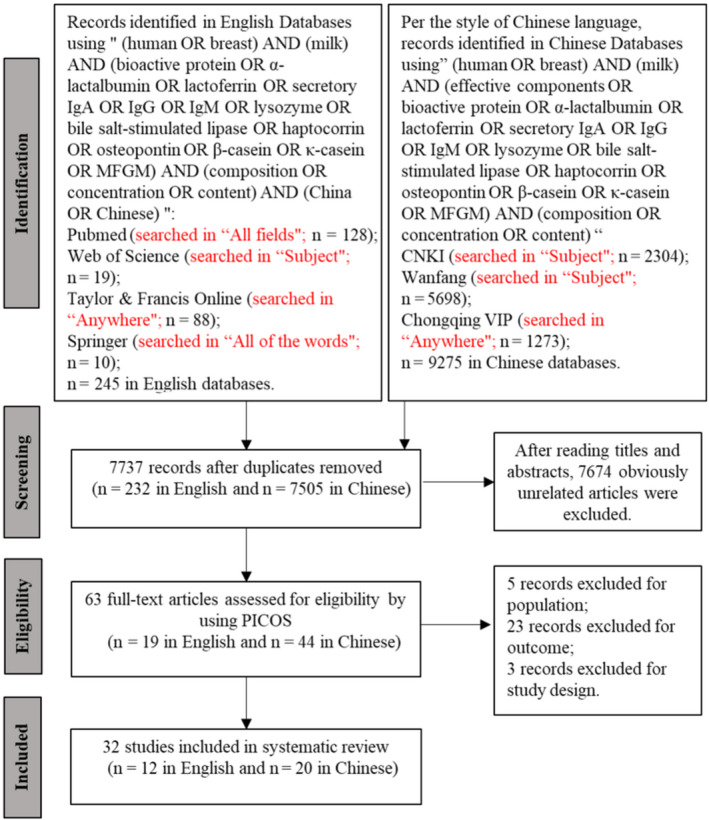
Flow diagram of the literature search process
Table 3.
List of all included studies
| Reference | Bioactive proteins reported | Quantification methods | Human milk collection location | Term/preterm | Mode of delivery | Foremilk/hindmilk/full expression |
|---|---|---|---|---|---|---|
| Dai and Guan (1985) | sIgA, IgG, IgM | Single radil immunodiffusion | Hubei | Term | N.S. | N.S. |
| Wang et al. (2012) | sIgA | ELISA | Inner Mongolia, Shanghai | Term | N.S. | N.S. |
| Wang (2012) | sIgA | Turbidimetric inhibition immuno assay | Hainan | Term | N.S. | N.S. |
| Dou et al. (1986) | Lactoferrin, sIgA, IgG, IgM, lysozyme | Single radil immunodiffusion (lactoferrin, sIgA, IgG, IgM); agar plate method (lysozyme) | Shanxi | Term | N.S. | N.S. |
| Min (1989) | sIgA, IgG, IgM | Single radil immunodiffusion | Hubei | Term | N.S. | N.S. |
| Li, Guo, et al. (1995) | sIgA, IgG, IgM | Single radil immunodiffusion | Gansu | Term | Vaginal delivery | N.S. |
| Chen (2007) | sIgA, IgG, IgM | Turbidimetric inhibition immuno assay | Chongqing | Term | N.S. | N.S. |
| Liu et al. (1994) | sIgA, IgG, IgM | Single radil immunodiffusion | Guangdong | Term | Vaginal delivery | N.S. |
| Li, Mei, et al. (1995) | sIgA, IgG, IgM | Single radil immunodiffusion | Hebei | Term | Vaginal delivery | N.S. |
| Wang and Lin (1997) | Lysozyme | Agar plate method | Hubei | N.S. | N.S. | N.S. |
| Wang (1987) | sIgA | Single radil immunodiffusion | N.S. | N.S. | N.S. | N.S. |
| Liu and Zhang (2005) | sIgA, IgG, IgM | Single radil immunodiffusion | Liaoning | Term | N.S. | N.S. |
| Wu et al. (1995) | sIgA, IgG, IgM | Single radil immunodiffusion | Hebei | Term | N.S. | N.S. |
| Wei and Pan (1991) | Lysozyme | Agar plate method | Jiangsu | N.S. | N.S. | N.S. |
| Liu et al. (2018) | Lactoferrin | ELISA | Beijing | N.S. | N.S. | Full expression |
| Jiang (2017) | β‐Casein, α‐lactalbumin | HPLC‐MS | Zhejiang, Gansu, Beijing | N.S. | N.S. | Full expression |
| Shan et al. (2011) | Lactoferrin | ELISA | Shanghai | Term | N.S. | Foremilk |
| Jiang et al. (1999) | sIgA, IgG, IgM | Turbidimetric inhibition immuno assay | Guangdong | Term | N.S. | N.S. |
| Chen et al. (1986) | sIgA | Turbidimetric inhibition immuno assay | Sichuan | Term | N.S. | N.S. |
| Han et al. (2010) | sIgA, IgG | Radioimmunoassay | Henan | N.S. | N.S. | N.S. |
| Jackson et al. (2004) | α‐Lactalbumin | HPLC‐MS | Sichuan | Term | N.S. | N.S. |
| Hsu et al. (2014) | Lactoferrin, sIgA, lysozyme | ELISA | Taiwan | Term | N.S. | N.S. |
| Liu et al. (2019) | β‐Casein, κ‐casein, α‐lactalbumin, lactoferrin, serum albumin | HPLC‐MS | Shandong, Hubei, Inner Mongolia | N.S. | N.S. | N.S. |
| Yang et al. (2018) | Lactoferrin | HPLC‐MS | Beijing, Gansu, Guangdong, Guangxi, Heilongjiang, Inner Mongolia, Shandong, Shanghai, Xinjiang, Yunnan, Zhejiang | Term | N.S. | Full expression |
| Urwin et al. (2013) | sIgA | ELISA | Jiangsu, Shandong, Hebei | N.S. | N.S. | Foremilk |
| Cai et al. (2018) | Lactoferrin | HPLC‐MS | Beijing, Shanghai, Guangdong, Heilongjiang, Zhejiang | N.S. | N.S. | N.S. |
| Yuen et al. (2012) | Lactoferrin, sIgA, lysozyme | ELISA | Hongkong | Term | N.S. | N.S. |
| Bruun et al. (2018) | Osteopontin | ELISA | Hunan | N.S. | N.S. | N.S. |
| Affolter et al. (2016) | α‐Lactalbumin, Lactoferrin, serum albumin, sIgA, IgG, IgM | LabChip GX‐II (lactoferrin, serum albumin); ELISA (sIgA, IgG, IgM) | Beijing, Jiangsu, Guangdong | Term | N.S. | Full expression |
| Shi et al. (2011) | α‐Lactalbumin, lactoferrin, serum albumin, sIgA, IgG, IgM | The MDQ capillary electrophoresis system | Inner Mongolia | N.S. | N.S. | N.S. |
| Sha et al. (2019) | BSSL | ELISA | Jiangsu | N.S. | N.S. | N.S. |
| Elwakiel et al. (2019) | α‐Lactalbumin, lactoferrin, serum albumin, osteopontin, BSSL, β‐casein, κ‐casein | LC‐MS | Inner Mongolia | Term | N.S. | N.S. |
Abbreviation: N.S., not specified.
We retrieved concentrations of 11 bioactive proteins: α‐lactalbumin (6 studies; 5 stages), lactoferrin (11 studies; 6 stages), serum albumin (4 studies; 5 stages), sIgA (20 studies; 6 stages), IgM (12 studies; 6 stages), IgG (13 studies; 6 stages), lysozyme (5 studies; 6 stages), osteopontin (2 studies; 2 stages), BSSL (2 studies; 2 stages), β‐casein (3 studies; 3 stages), and κ‐casein (2 studies; 2 stages). Four proteins accounted for more than 10% of total human milk proteins at any of the lactation stages: α‐lactalbumin, β‐casein, lactoferrin, and sIgA. α‐lactalbumin and β‐casein were the most abundant bioactive proteins, whereas sIgA represented the dominant immune globulin (Table S2).
Concentrations of 5 bioactive proteins decreased significantly during lactation (Figure 2). The levels of α‐lactalbumin and lactoferrin decreased gradually without reaching plateaus throughout lactation, except for the level of lactoferrin during the 61–90 postnatal period, which was significantly higher than either the preceding or subsequent stages. The levels of immunoglobulins were the highest during the first 7 postnatal days, decreased thereafter, and reached plateaus. For sIgA and IgM, the plateaus were reached at the 15–30 postnatal day stage. For IgG, the plateau was reached at the 8–14 postnatal day stage. The concentrations of sIgA, IgM, and IgG during the 1–7 postnatal days stage were 3, 3.5, and 16 times higher than the levels during the 8–14 postnatal day stage (Table S2).
FIGURE 2.
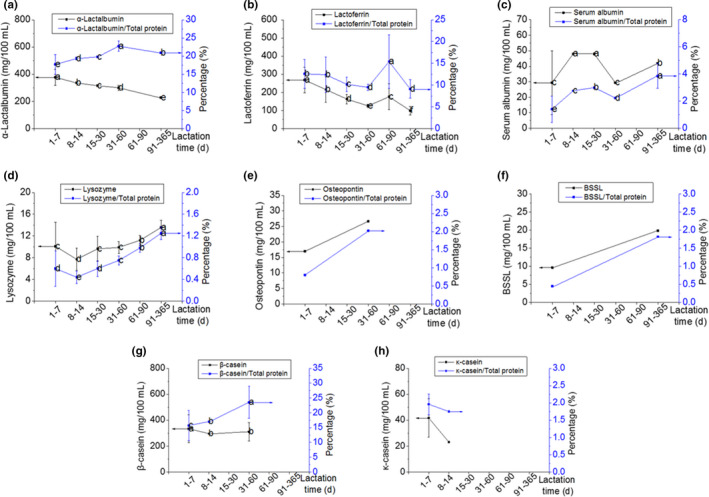
The longitudinal changes in the protein concentrations and the percentages of the total proteins of the bioactive proteins in the human milk of the Chinese population
The lysozyme levels were similar during the first 60 postnatal days—except for the 8–14 postnatal day stage—and increased gradually during the remainder of lactation (Figure 2). No clear pattern could be observed in the levels of serum albumin, whose concentrations were higher in the 8–14 and 15–30 postnatal day periods than during the preceding or subsequent stages. For bioactive proteins with data of less than four stages available, the levels of β‐casein and κ‐casein were higher during the first 7 postnatal days than in later stages. Conversely, the levels of osteopontin and BSSL in the first 7 postnatal days were lower than those in later lactation stages.
The percentages of each bioactive protein in total protein generally followed the trends in protein concentration in milk, except for α‐lactalbumin and β‐casein (Figure 2). The percentage of α‐lactalbumin in total protein increased during lactation, which was contradictory to the change in its concentration in human milk. The percentage of β‐casein in total protein during the first 7 postnatal days was lower than those in later stages, whereas its concentration in human milk was higher in the first 7 postnatal days when compared with later stages.
4. DISCUSSION
This study represents the most comprehensive overview of bioactive protein concentrations in the human milk of the Chinese population to date. We found that α‐lactalbumin, lactoferrin, sIgA, IgM, and IgG decreased whereas lysozyme increased throughout lactation. The trends of the rest of the included proteins are less conclusive due to a lack of data in the literature. Furthermore, we revealed that the concentrations of haptocorrin, MFGM proteins, and other bioactive proteins have never been reported in Chinese population.
The longitudinal changes in human milk bioactive proteins in Chinese population were compared with studies in other populations (Table S3). The trends we found are generally consistent with the findings in non‐Chinese populations with three minor exceptions. First, Lonnerdal et al. found the concentrations of serum albumin increased in the first 60 days of lactation and decreased thereafter, whereas we did not observe such a trend in our study. Second, Schack L et al. reported that osteopontin decreased over lactation, whereas we found that osteopontin in the first 7 postnatal days were lower than that in the 31–60 postnatal days period (Akpele & Bailey, 2004; Donovan, 2019; Greibe et al., 2013; Jiang & Lönnerdal, 2019; Liao et al., 2017; Lönnerdal et al., 2017; Nagatomo et al., 2004; Piemontese et al., 2012; Schack et al., 2009). Third, we observed a sudden increase in the lactoferrin concentration in 61–90 postnatal days, which has never been reported in other populations. However, lactoferrin levels in 61–90 postnatal days in Chinese human milk were only reported by two studies that used distinct methods and published in the year of 1986 and 2018 (Dou et al., 1986; Liu et al., 2018). Accordingly, the longitudinal change in lactoferrin in Chinese population needs to be further elucidated. Although insufficient number of studies available may cause the observed discrepancies in human milk bioactive proteins in different populations, we cannot rule out the possibility that genetic or dietary factors may cause the discrepancies. For instance, serum albumin in the blood is an indicator of the nutritional and hydration status (Akpele & Bailey, 2004). Therefore, it is entirely possible that the serum albumin in human milk is also related to maternal nutritional and hydration status.
The longitudinal changes in the bioactive proteins may have implications in infant nutrition and health. There is a sharp decrease in immunoglobulin (sIgA, IgM, and IgG) concentrations after the first 14 postnatal days, indicating that during the first 14 days of life, infants require their mothers to produce antibodies for the protection against pathogens. In this way, mothers may endow their infants with adaptive immune responses via human milk and protect them from pathogens that both the mothers and infants are exposed to (Levy, 2007). After 2 weeks of life, infants may be able to produce sufficient antibodies on their own and become less dependent on human milk for immunoglobulins (Gao et al., 2012). Additionally, the increase in the lysozyme concentration at the beginning of the 8–14 postnatal day stage suggests an increasing involvement of lysozyme in the protection of the infants against pathogenic bacteria.
This study was only able to reveal the longitudinal changes in 11 bioactive proteins. Previously, Lonnerdal et al. retrieved 7 bioactive proteins in their systematic review (Lönnerdal et al., 2017). Most of these proteins were quantified by immunoassays (Table 3), which were dependent on the development of specific antibodies. Moreover, the specificity and sensitivity of the antibodies used could contribute to variations among different studies. It should be noticed that more than 1,600 proteins have been identified in human milk due to the advances in mass spectrometry (Beck et al., 2015). In the future, mass spectrometry‐based proteomic analysis of human milk may be used to shed light on the longitudinal changes of many more bioactive proteins in human milk (Cao et al., 2017).
There are limitations in our study. First, the association between the means of delivery and the bioactive protein levels in human milk remains controversial (Affolter et al., 2016; Liu et al., 2019; Yang et al., 2018). Unfortunately, only three of the included studies specified the means of delivery, making it impossible for us to extract data and analyze the association between the means of delivery and the bioactive protein concentrations in human milk. Second, it is well‐known that premature infants are more prone to infections and that human milk decreases the rate of infections (Patel & Kim, 2018). Therefore, it is possible that milk from mothers who delivered premature infants may contain higher concentrations of immunomodulatory proteins such as immunoglobulins and lactoferrin (Bernloehr et al., 2016). Nevertheless, 20 out of the 32 identified article reported milk from mothers who delivered full‐term infants, whereas the rest did not specify the gestation age at delivery. Therefore, it is impossible for us to analyze differences in bioactive protein levels between milk from mothers delivered at different gestation ages.
5. CONCLUSIONS
This systematic review aimed at investigating the longitudinal changes in bioactive proteins in the human milk of the Chinese population. Data from 20 and 12 publications that were originally published in Chinese and English, respectively, were combined. The concentrations of α‐lactalbumin, lactoferrin, β‐casein, and three immunoglobulins (sIgA, IgM, and IgG) decrease during lactation. Particularly, sharp decreases are evident in the immunoglobulin concentrations during the first 14 postnatal days. Conversely, the lysozyme concentrations increase during lactation. The patterns of longitudinal changes in serum albumin, osteopontin, BSSL, and κ‐casein are less conclusive, mainly due to the limited data available. This study represents the most comprehensive report on the bioactive proteins in the human milk of the Chinese population to date.
The findings in the Chinese population are similar to those in other populations. Furthermore, it is revealed that future studies should document factors such as means of delivery, gestational age at delivery, and the protocol of milk collection to examine the association between these factors and the bioactive protein concentrations in human milk. Additionally, mass spectrometry‐based analysis of human milk proteomics may be used to investigate the longitudinal changes in many more bioactive proteins.
7. CONFLICT OF INTEREST
The authors declare no conflict of interest.
8. ETHICAL APPROVAL
This study does not involve any human or animal testing.
9.
Supporting information
Supplementary Material
6. ACKNOWLEDGMENTS
This study was supported by “Bai‐Qian‐Wan Engineering and Technology Master Project” (Grant # 2019ZX07B01), which was funded by the Government of Heilongjiang Province of the People's Republic of China.
Ren Q, Zhou Y, Zhang W, et al. Longitudinal changes in the bioactive proteins in human milk of the Chinese population: A systematic review. Food Sci Nutr.2021;9:25–35. 10.1002/fsn3.2061
Contributor Information
Wei Zhang, Email: zhangwei1@feihe.com.
Yajun Xu, Email: xuyajun@bjmu.edu.cn.
Shilong Jiang, Email: jiangshilong@feihe.com.
9.1. DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- Affolter, M. , Garcia‐Rodenas, C. , Vinyes‐Pares, G. , Jenni, R. , Roggero, I. , Avanti‐Nigro, O. , de Castro, C. , Zhao, A. I. , Zhang, Y. , Wang, P. , Thakkar, S. , & Favre, L. (2016). Temporal changes of protein composition in breast milk of Chinese urban mothers and impact of caesarean section delivery. Nutrients, 8(8), 504 10.3390/nu8080504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akpele, L. , & Bailey, J. L. (2004). Nutrition counseling impacts serum albumin levels. Journal of Renal Nutrition, 14(3), 143–148. 10.1053/j.jrn.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Artym, J. , & Zimecki, M. (2013). Milk‐derived proteins and peptides in clinical trials. Postepy Higieny I Medycyny Doswiadczalnej, 67, 800–816. 10.5604/17322693.1061635 [DOI] [PubMed] [Google Scholar]
- Beck, K. L. , Weber, D. , Phinney, B. S. , Smilowitz, J. T. , Hinde, K. , Lönnerdal, B. O. , Korf, I. , & Lemay, D. G. (2015). Comparative proteomics of human and macaque milk reveals species‐specific nutrition during postnatal development. Journal of Proteome Research, 14(5), 2143–2157. 10.1021/pr501243m [DOI] [PubMed] [Google Scholar]
- Bernloehr, C. , Bossow, S. , Armeanu, S. , Ungerechts, G. , Gregor, M. , Neubert, W. , & Bitzer, M. (2016). Lactoferrin levels in human milk after preterm and term delivery. American Journal of Perinatology, 33(11), 1085–1089. 10.1055/s-0036-1586105 [DOI] [PubMed] [Google Scholar]
- Bruun, S. , Jacobsen, L. N. , Ze, X. , Husby, S. , Ueno, H. M. , Nojiri, K. , Kobayashi, S. , Kwon, J. , Liu, X. , Yan, S. , Yang, J. , Zachariassen, G. , Chen, L. , Zhou, W. , Christensen, B. , & Sørensen, E. S. (2018). Osteopontin levels in human milk vary across countries and within lactation period: Data from a multicenter study. Journal of Pediatric Gastroenterology and Nutrition, 67(2), 250–256. 10.1097/MPG.0000000000002004 [DOI] [PubMed] [Google Scholar]
- Cai, X. , Duan, Y. , Li, Y. , Wang, J. , Mao, Y. , Yang, Z. , Zhao, X. , Zhao, Y. , Guan, Y. , & Yin, S. (2018). Lactoferrin level in breast milk: A study of 248 samples from eight regions in China. Food & Function, 9(8), 4216–4222. 10.1039/C7FO01559C [DOI] [PubMed] [Google Scholar]
- Cao, X. , Kang, S. , Yang, M. , Li, W. , Wu, S. , Han, H. , & Yue, X. (2017). Quantitative N‐glycoproteomics of milk fat globule membrane in human colostrum and mature milk reveals changes in protein glycosylation during lactation. Food & Function, 9(2), 1163–1172. 10.1039/C7FO01796K [DOI] [PubMed] [Google Scholar]
- Chen, B. (2007). Study on the anti‐infection effect of sIgA in breast milk. ChongQing Medical University. [Google Scholar]
- Chen, Y. , Zheng, D. , & Qian, Y. (1986). Dynamic observation on the milk sIgA of premature and full‐term infants. Chinese Journal of Neonatology, 1(5), 221–224. [Google Scholar]
- Dai, Q. , & Guan, H. (1985). Dynamic observation of immune bodies in the breast milk and serum of postpartum mother. Acta Universitatis Medictnae Tangji,5, 349–352. [Google Scholar]
- Donovan, S. M. (2019). Human milk proteins: Composition and physiological significance. Karger Publishers, 90, 93–101. 10.1159/000490298 [DOI] [PubMed] [Google Scholar]
- Dou, G. , Chen, M. , & Dai, W. (1986). Dynamic observation of lactoferrin, lysozyme, C3 and immunoglobulin in human milk in different lactation. Shanghai Journal of Immunology, 6(2), 98–100. [Google Scholar]
- Elwakiel, M. , Boeren, S. , Hageman, J. A. , Szeto, I. M. , Schols, H. A. , & Hettinga, K. (2019). Variability of serum proteins in Chinese and Dutch human milk during lactation. Nutrients, 11(3), 499 10.3390/nu11030499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X. , Mcmahon, R. J. , Woo, J. G. , Davidson, B. S. , Morrow, A. L. , & Zhang, Q. (2012). Temporal changes in milk proteomes reveal developing milk functions. Journal of Proteome Research, 11(7), 3897–3907. 10.1021/pr3004002 [DOI] [PubMed] [Google Scholar]
- Greibe, E. , Lildballe, D. L. , Streym, S. , Vestergaard, P. , Rejnmark, L. , Mosekilde, L. , & Nexo, E. (2013). Cobalamin and haptocorrin in human milk and cobalamin‐related variables in mother and child: A 9‐mo longitudinal study. The American Journal of Clinical Nutrition, 98(2), 389–395. 10.3945/ajcn.113.058479 [DOI] [PubMed] [Google Scholar]
- Han, L. , Pang, K. , & Liu, A. (2010). Determination of nutritional and bioactive components of woman colostrum in Zhengzhou city. Journal of Zhengzhou University (Medical Sciences), 45(1), 59–61. 10.3969/j.issn.1671-6825.2010.01.019 [DOI] [Google Scholar]
- Haschke, F. , Haiden, N. , & Thakkar, S. K. (2016). Nutritive and bioactive proteins in breastmilk. Annals of Nutrition and Metabolism, 69(2), 17–26. 10.1159/000452820 [DOI] [PubMed] [Google Scholar]
- Hozo, S. P. , Djulbegovic, B. , & Hozo, I. (2005). Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology, 5(1), 1–10. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, Y. C. , Chen, C. H. , Lin, M. C. , Tsai, C. R. , Liang, J. T. , & Wang, T. M. (2014). Changes in preterm breast milk nutrient content in the first month. Pediatrics and Neonatology, 55(6), 449–454. 10.1016/j.pedneo.2014.03.002 [DOI] [PubMed] [Google Scholar]
- Jackson, J. G. , Janszen, D. B. , Bo, L. , Lien, E. L. , Pramuk, K. P. , & Kuhlman, C. F. (2004). A multinational study of α‐lactalbumin concentrations in human milk. Journal of Nutritional Biochemistry, 15(9), 517–521. 10.1016/j.jnutbio.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Jiang, J. (2017). Study on important nutrients, bioactive factors and small molecule metabolites in breast milk. (Master), Zhejiang University, Hangzhou, Beijing. [Google Scholar]
- Jiang, J. , Fu, F. , & Yan, Y. (1999). Compare the concentrations of anti‐infective factors of human colostrum between cesarean section and normal labor. Guangdong Medical Journal, 20(4), 254–255. 10.13820/j.cnki.gdyx.1999.04.009 [DOI] [Google Scholar]
- Jiang, R. , & Lönnerdal, B. (2019). Osteopontin in human milk and infant formula affects infant plasma osteopontin concentrations. Pediatric Research, 85(4), 502–505. 10.1038/s41390-018-0271-x [DOI] [PubMed] [Google Scholar]
- Kalhan, S. C. , & Bier, D. M. (2008). Protein and amino acid metabolism in the human newborn. Annual Review of Nutrition, 28(1), 389–410. 10.1146/annurev.nutr.28.061807.155333 [DOI] [PubMed] [Google Scholar]
- Legrand, D. (2016). Overview of lactoferrin as a natural immune modulator. Journal of Pediatrics, 173, S10–S15. 10.1016/j.jpeds.2016.02.071 [DOI] [PubMed] [Google Scholar]
- Levy, O. (2007). Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nature Reviews Immunology, 7(5), 379–390. 10.1038/nri2075 [DOI] [PubMed] [Google Scholar]
- Li, L. , Guo, B. , & Guo, Z. (1995). Determination of iron, copper, total protein, fat, immunoglobulin in colostrum. Journal of Lanzhou Medical College, 21(4), 221 10.13885/j.issn.1000-2812.1995.04.014 [DOI] [Google Scholar]
- Li, S. , Mei, Z. , & Tang, Z. (1995). Observation on the content of immunoglobulin and complement in breast milk. Journal of Practical Obstetrics and Gynecology, 11(6), 308. [Google Scholar]
- Liao, Y. , Weber, D. , Xu, W. , Durbin‐Johnson, B. P. , Phinney, B. S. , & Lönnerdal, B. (2017). Absolute quantification of human milk caseins and the whey/casein ratio during the first year of lactation. Journal of Proteome Research, 16(11), 4113–4121. 10.1021/acs.jproteome.7b00486 [DOI] [PubMed] [Google Scholar]
- Liu, B. , Gu, F. , Ye, W. , Ren, Y. , & Guo, S. (2019). Colostral and mature breast milk protein compositional determinants in Qingdao, Wuhan and Hohhot: Maternal food culture, vaginal delivery and neonatal gender. Asia Pacific Journal of Clinical Nutrition, 28(4), 800. [DOI] [PubMed] [Google Scholar]
- Liu, M. , & Zhang, H. (2005). Determination and clinical significance of immunoglobulin in breast milk. Chinese Journal of Practical Gynecology and Obstetrics, 21(6), 324 10.3969/j.issn.1005-2216.2005.06.029 [DOI] [Google Scholar]
- Liu, R. , Zheng, D. , & Wang, M. (1994). Observation on the content of immunoglobulin in breast milk. Chinese Journal of Applied Clinical Pediatrics, 9, 165. [Google Scholar]
- Liu, Y. , Dong, X. , & Jiang, T. (2018). Lactoferrin concentration in human milk and its affection factors. China Food Additives, 8, 70–74. [Google Scholar]
- Lönnerdal, B. (2013). Bioactive proteins in breast milk. Journal of Paediatrics and Child Health, 49, 1–7. 10.1111/jpc.12104 [DOI] [PubMed] [Google Scholar]
- Lönnerdal, B. (2016a). Bioactive proteins in human milk: Health, nutrition, and implications for infant formulas. Journal of Pediatrics, 173, S4–S9. 10.1016/j.jpeds.2016.02.070 [DOI] [PubMed] [Google Scholar]
- Lönnerdal, B. (2016b). Human milk: Bioactive proteins/peptides and functional properties. Nestle Nutrition Institute Workshop, 86, 97–107. 10.1159/000442729 [DOI] [PubMed] [Google Scholar]
- Lönnerdal, B. , Erdmann, P. , Thakkar, S. K. , Sauser, J. , & Destaillats, F. (2017). Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. Journal of Nutritional Biochemistry, 41, 1–11. 10.1016/j.jnutbio.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Luo, D. , Wan, X. , Liu, J. , & Tong, T. (2015). Optimally estimating the sample mean from the sample size, median, mid‐range and/or mid‐quartile range. Statistical Methods in Medical Research, 27(6), 1785–1805. 10.1177/0962280216669183 [DOI] [PubMed] [Google Scholar]
- Min, X. (1989). Analysis of immune substances in early maternal milk. Shanghai Journal of Immunology, 9(1), 29–31. [Google Scholar]
- Nagatomo, T. , Ohga, S. , Takada, H. , Nomura, A. , Hikino, S. , Imura, M. , Ohshima, K. , & Hara, T. (2004). Microarray analysis of human milk cells: Persistent high expression of osteopontin during the lactation period. Clinical and Experimental Immunology, 138(1), 47–53. 10.1111/j.1365-2249.2004.02549.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville, M. C. , Keller, R. , Seacat, J. , Lutes, V. , Neifert, M. , Casey, C. , & Archer, P. (1988). Studies in human lactation: Milk volumes in lactating women during the onset of lactation and full lactation. American Journal of Clinical Nutrition, 48(6), 1375–1386. 10.1051/rnd:19870513 [DOI] [PubMed] [Google Scholar]
- Patel, A. L. , & Kim, J. H. (2018). Human milk and necrotizing enterocolitis. Seminars in Pediatric Surgery, 27(1), 34 10.1053/j.sempedsurg.2017.11.007 [DOI] [PubMed] [Google Scholar]
- Piemontese, P. , Roncada, P. , Soggiu, A. , Bonizzi, L. , Budelli, A. , Agostoni, C. , Gianni, M. , Roggero, P. , & Mosca, F. (2012). 357 Human milk bile salt‐stimulated lipase: The dynamic changes during lactation. Archives of Disease in Childhood, 97(Suppl 2), A105 10.1136/archdischild-2012-302724.0357 [DOI] [Google Scholar]
- Schack, L. , Lange, A. , Kelsen, J. , Agnholt, J. , Christensen, B. , Petersen, T. E. , & Sørensen, E. S. (2009). Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. Journal of Dairy Science, 92(11), 5378–5385. 10.3168/jds.2009-2360 [DOI] [PubMed] [Google Scholar]
- Sha, L. , Zhou, S. , Xi, Y. , Li, R. , & Li, X. (2019). The level of bile salt‐stimulated lipase in the milk of Chinese women and its association with maternal BMI. Journal of Biomedical Research, 34, 122–128. 10.7555/JBR.33.20180107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer, L. , Moher, D. , Clarke, M. , Ghersi, D. , Liberati, A. , Petticrew, M. , & Stewart, L. A. (2015). Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. BMJ, 349(1), g7647. [DOI] [PubMed] [Google Scholar]
- Shan, J. , Wang, X. , & Chen, X. (2011). Preliminary determination of lactoferrin in human breast milk. Journal of Clinical Pediatrics, 29(6), 549–551. 10.3969/j.issn.1000-3606.2011.06.013 [DOI] [Google Scholar]
- Shi, Y.‐D. , Sun, G.‐Q. , Zhang, Z.‐G. , Deng, X. , Kang, X.‐H. , Liu, Z.‐D. , Ma, Y. , & Sheng, Q.‐H. (2011). The chemical composition of human milk from Inner Mongolia of China. Food Chemistry, 127(3), 1193–1198. 10.1016/j.foodchem.2011.01.123 [DOI] [PubMed] [Google Scholar]
- Stam, J. , Sauer, P. J. J. , & Boehm, G. (2013). Can we define an infant's need from the composition of human milk? The American Journal of Clinical Nutrition, 98(2), 521S–528S. 10.3945/ajcn.112.044370 [DOI] [PubMed] [Google Scholar]
- Urwin, H. J. , Zhang, J. , Gao, Y. , Wang, C. , Li, L. , Song, P. , Man, Q. , Meng, L. , Frøyland, L. , Miles, E. A. , Calder, P. C. , & Yaqoob, P. (2013). Immune factors and fatty acid composition in human milk from river/lake, coastal and inland regions of China. British Journal of Nutrition, 109(11), 1949–1961. 10.1017/s0007114512004084 [DOI] [PubMed] [Google Scholar]
- Wang, G. X. , Zhao, H. M. , Zhu, X. X. , Yang, X. M. , Wang, H. Y. , Huang, Q. T. , & Jing, L. I. (2012). Project of hospital education in lactation practices improves breast milk siga concentration. Journal of Clinical Pediatrics, 12, 1519–1521. [Google Scholar]
- Wang, J. (2012). Impact of different delivery ways on colostrum lactation time and immunoglobulin a level. China Tropical Medicine, 12(12), 1519–1521. 10.13604/j.cnki.46-1064/r.2012.12.008 [DOI] [Google Scholar]
- Wang, M. (1987). Determination of immunoglobulin in breast milk. Central Plains Medical Journal, 6, 36–37. [Google Scholar]
- Wang, Y. , & Lin, Q. (1997). Lysozyme in breast milk and physiological diarrhea in infants. Chinese Journal of Microecology, 9(5), 32–33, 35. 10.13381/j.cnki.cjm.1997.05.010 [DOI] [Google Scholar]
- Wei, Z. , & Pan, X. (1991). Determination of lysozyme in breast milk. Acta Universitatis Medicinalis Nanjing, 11(1), 16–18. [Google Scholar]
- WHO (2003). Global strategy for infant and young child feeding. 10.1111/j.1467-8624.2011.01675.x [DOI] [PubMed] [Google Scholar]
- Wu, C. , Li, X. , & Chen, W. (1995). Determination and analysis of immunoglobulin and complement content in breast milk. Chinese Journal of Birth Health & Heredity, 3(6), 91–94. 10.13404/j.cnki.cjbhh.1995.06.042 [DOI] [Google Scholar]
- Yang, Z. , Jiang, R. , Chen, Q. I. , Wang, J. , Duan, Y. , Pang, X. , Jiang, S. , Bi, Y. E. , Zhang, H. , Lönnerdal, B. O. , Lai, J. , & Yin, S. (2018). Concentration of lactoferrin in human milk and its variation during lactation in different Chinese populations. Nutrients, 10(9), 1235 10.3390/nu10091235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, C. , Wong, J. H. , & Ng, T. B. (2014). Recent studies on the antimicrobial peptides lactoferricin and lactoferrampin. Current Molecular Medicine, 14(9), 1139–1154. 10.2174/1566524014666141015151749 [DOI] [PubMed] [Google Scholar]
- Yuen, J. W. M. , Loke, A. Y. , & Gohel, M. I. (2012). Nutritional and immunological characteristics of fresh and refrigerated stored human milk in Hong Kong: A pilot study. Clinica Chimica Acta, 413(19), 1549–1554. 10.1016/j.cca.2012.03.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


