Abstract
Purpose
The present study investigates the phytosynthesis of silver nanoparticles (AgNPs) using Perilla frutescens leaf extract, which acts as a reducing agent for the conversion of silver ions (Ag+) into AgNPs. P. frutescens leaf synthesized AgNPs (PF@AgNPs) were evaluated for biomedical properties including antibacterial, antioxidant and anticancer activities.
Materials and Methods
PF@AgNPs were synthesized using P. frutescens leaf extract and silver nitrate solution. The morphology and physical properties of PF@AgNPs were studied by spectroscopic techniques including, UV-Vis, FTIR, TEM, XRD, DLS, and TGA. Antibacterial activity of PF@AgNPs was evaluated by disk diffusion assay. Antioxidant activity of PF@AgNPs was checked by 2.2-diphenyl-1-picrylhydrazyl (DPPH), and 2.2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radical scavenging assays. Anticancer activity of PF@AgNPs was checked by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. Cytotoxic effects of PF@AgNPs on most susceptible cancer cell lines were observed by phase contrast microscopy.
Results
PF@AgNPs showed surface plasmon resonance peak at 461 nm. XRD pattern showed that the PF@AgNPs were face-centered cubic crystals with a mean size of 25.71 nm. TEM analysis revealed the different shapes (spherical, rhombic, triangle, and rod) of PF@AgNPs. Zeta potential value (−25.83 mV) indicated that PF@AgNPs were long-term stable and not agglomerated. A low polydispersity index value (0.389) indicated the monodispersity of PF@AgNPs. TGA revealed the high thermal stability of PF@AgNPs. PF@AgNPs exhibited maximum inhibition against Escherichia coli, followed by Bacillus subtilis and Staphylococcus aureus. PF@AgNPs showed maximum inhibition of 68.02 and 62.93% against DPPH and ABTS-free radicals, respectively. PF@AgNPs showed significant anticancer activity against human colon cancer (COLO205) and prostate adenocarcinoma (LNCaP). PF@AgNPs exhibited apoptotic effects on LNCaP cells including cell shrinkage, membrane blebbing, chromatin condensation, fragmentation of nuclei, and formation of apoptotic bodies.
Conclusion
The present study reports the successful synthesis of PF@AgNPs using P. frutescens leaf extract. The synthesized PF@AgNPs are FCC crystals, monodispersed, long-term stable, and non-agglomerated. The observed antibacterial, antioxidant, and anticancer activities demonstrate the potential biomedical applications of PF@AgNPs.
Keywords: Perilla frutescens, silver nanoparticles, antibacterial activity, antioxidant activity, anticancer activity, LNCaP
Introduction
Owing to their unequivocal properties and promising features, nanomaterials will play essential roles in the future. Nanomaterials are used in drug delivery to increase the bioavailability, solubility, and half-life of drugs, and they have been widely employed for targeted and controlled release of drugs in cancer chemotherapy. Noble metals such as gold, platinum, silver, titanium, and palladium have been used extensively to synthesize nanomaterials.1–3 Metallic nanoparticles, particularly silver nanoparticles (AgNPs), have gained importance because of their unique properties, including electronic, thermal, optical, magnetic, and catalytic attributes.3–5 AgNPs have been widely used as bio-labeling materials, polarizing filters, biosensors, signal enhancers, optical receptors, and biocatalysts, and as antibacterial, antioxidant, anticancer, antiviral, antinociceptive, larvicidal, and insecticidal agents.3–7 AgNPs have been widely employed in the preparation of medical kits, diagnostic kits, wound dressings, antiseptic creams, hand wash liquids, and bandages in the medical field because of the innate antimicrobial nature of silver.8–11 The antimicrobial properties of AgNPs render them suitable for infusion into tissue/cartilage/bone grafting materials such as collagen, polycaprolactone, and hydroxyapatite.12,13 The deodorant properties of AgNPs have been utilized in fabrics, sports shoes, and other sporting equipment.14 AgNPs have been widely used in the coatings of walls, windows, timber furniture, glass, and countertops of hospitals to reduce the growth of bacteria and fungi.15,16 The antioxidant and antimicrobial properties of AgNPs have been particularly useful in the preparation of food packing materials to increase the shelf life of foods and food ingredients.17,18
The physicochemical properties of AgNPs can be determined by their size, shape, surface area to volume ratio, and crystalline nature.1,2,5 Various chemical and physical methods have been successfully employed for size- and shape-controlled synthesis of AgNPs, including laser ablation, thermal decomposition, lithographic, aerosol, UV-radiation, γ-irradiation, ultrasonication, microwave-assisted, photochemical, sonochemical, electrochemical, polyvinyl pyrrolidine, polyol, polyaniline, tollens process, and other chemical reduction methods.4,5,19 However, chemical and physical methods for synthesis of nanoparticles involve hazardous chemicals and toxic radiations and require separate capping agents. Furthermore, toxic chemicals on the surface of AgNPs limit their biomedical applications.20 Environmentally friendly methods for biological synthesis of AgNPs, including enzymatic, microbial, and plant-mediated synthesis, have become a new research avenue in the nanotechnology field worldwide. Although enzymatic methods are eco-friendly, they involve different steps, including isolation, extraction, and purification of enzymes, and are time-consuming, arduous, and expensive. Microbial methods involve laborious culturing conditions, and the mishandling of microbes can lead to contamination and risk to human health. Synthesis of non-toxic, clean, biocompatible, and biofunctionalized AgNPs using plant extracts deserves merit as a simple, rapid, cost-effective, eco-friendly, and safe method beings.6,21,22
Plants contain secondary metabolites, such as phenolic compounds, flavonoids, triterpenoids, alkaloids, quinines, coumarins, glycosides, steroids, and anthocyanins, that confer resistance to plants against pests, insects, drought stress, and other stress conditions. These compounds are very important for humans as they possess a wide variety of bioactivities, including anticancer, antioxidant, antidiabetic, anti-obesity, antibacterial, and antiviral properties.23,24 Over the last two decades, scientists have successfully employed plant extracts and their secondary metabolites for the production of different types of metallic nanomaterials. In this simple, rapid, and cost-effective process, plant metabolites act as bioreduction agents that reduce the metal salts and metal oxides to their respective metallic nanoparticles. The plant metabolites also stabilize the nanoparticles by capping them.6,19
Perilla frutescens is a herbaceous perennial plant belonging to the family Lamiaceae. It is an important medicinal plant that is widely distributed in China, India, Korea, Japan, and Southeast Asia.25 There are wild and cultivated varieties in the northern, southwestern, and other regions in China. The leaves of P. frutescens were used as a food ingredient in China. Perilla leaves have a wide variety of natural active substances including phenylpropanoids (elemicin, isoelemicin, myristicin, and methyl isoeugenol), alkaloids (1H-indole-3-carboxylic acid and indole-3-carboxaldehyde), phenolic compounds (rosmarinic acid, protocatechuic acid, caffeic acid, vanillic acid, and ferulic acid),26 (Takano et al, 2004), flavonoids (luteolin, apigenin, scutellarein, catechin, negletein, vicenin-2, and catechin),27–29 anthocyanins (shisonin, Cis-shisonin, and malonyl shisonin), coumarins (esculetin and 6.7-dihydroxycoumarin), carotenoids, neolignans, sesquiterpenoids, fatty acids, policosanols, tocopherols, sitosterols, glucosides, peptides, benzoxepin derivatives and other constituents.26,30,31 The leaves of P. frutescens showed multiple biological functions due to the presence of abundant natural active substances. The biological functions including antidiabetic activity (α-glucosidase and aldose-reductase inhibitory activity of luteolin, apigenin, and diosmetin),28,32 anti-hyperuricemia (xanthine oxidase inhibitory activity of luteolin, negletein and scutellarin),27 antispasmodic effect of flavonoid vicenin,29 insecticidal activity of sesquiterpenoids,30 antiallergic activity (hyaluronidase inhibition by rosmarinic acid, caffeic acid, and luteolin), anti-inflammatory activity of phenylpropanoids,33 anti-depressant activity of luteolin and apigenin, hepatoprotective activity of caffeic acid and rosmarinic acid, antioxidant activity of flavonoids and polyphenols, anticancer and antimicrobial activities of triterpenoids, flavonoids, and polyphenols.31,34–36 In the present investigation, we report the phytosynthesis of AgNPs using P. frutescens aqueous leaf extract, which acts as both a reducing and stabilizing agent for AgNPs. Characterization of AgNPs was achieved using a range of spectroscopic methods. The synthesized AgNPs were evaluated for antibacterial activities against gram-negative (Escherichia coli) and gram-positive (Bacillus subtilis and Staphylococcus aureus) bacteria using a disk diffusion assay. The antioxidant activity of the AgNPs was checked by 2,2-diphenyl-1-picrylhydrazyl (DPPH), and 2,2ʹ-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assays. The anticancer activity of the PF@AgNPs was evaluated against human colon cancer (COLO205) and human prostate adenocarcinoma (LNCaP) cell lines using an MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay.
Materials and Methods
Preparation of P. frutescens Leaf Extract and Phytosynthesis of PF@AgNPs
The leaves of P. frutescens were collected from croplands near the North University of China, Taiyuan, Shanxi Province, China. Initially, P. frutescens leaves were washed thoroughly with tap water and then rinsed with distilled water for 10 to 15 min. Finally, the leaves were cleaned with double distilled water (DDW) and then dried completely under shade. The dried leaves were crushed into a fine powder. Leaf powder (10 g) was added to 100 mL of DDW in a 250 mL flask and then boiled at 50°C for 30 min.37,38 Subsequently, the solution was allowed to cool to room temperature and filtered by employing simple filtration process through Whatman No.1 filter paper. The filtrate, named as P. frutescens leaf aqueous extract, was used for the phytosynthesis of PF@AgNPs. This extract (10 mL) was added to 2 mM AgNO3 (90 mL), and the mixture was boiled at 50°C for 30 min and then incubated for 2 h in the dark.37,38 The light yellow color of the solution became dark brown after incubation.
Spectral Analysis of PF@AgNPs
To confirm phytosynthesis, the dark brown colloidal solution was analyzed using ultraviolet–visible (UV–Vis) spectroscopy (Perkin-Elmer Ltd) between 200 and 800 nm.39 After confirmation of phytosynthesis, the solution of PF@AgNPs was centrifuged for 20 min at 15,000 × g. The supernatant was removed and the pellet was redispersed in distilled water and centrifuged again for 20 min at 15,000 × g. This procedure was repeated for three times.40 Finally, the pellet was collected and dried into a fine powder that was used for further analysis. Fourier transform infrared (FTIR) analysis of PF@AgNPs was carried out at 4000–500 cm−1 using KBr pellets (Perkin-Elmer Ltd).41 The x-ray diffraction (XRD) pattern of PF@AgNPs was recorded in the 2θ range of 10° to 90° using Bruker D8 Advance powder XRD (Bruker AXS GmbH, Karlsruhe, Germany).42 Thermogravimetric analysis (TGA) and derivative thermogravimetric analysis (DTA) were performed using a TGA-1 analyzer (Mettler Toledo, Switzerland).43 The shapes and sizes of PF@AgNPs and the selected area electron diffraction (SAED) pattern of PF@AgNPs were determined using transmission electron microscopy (TEM).44 The polydispersity index (PI) and zeta potential values were recorded using dynamic light scattering (DLS; Brookhaven Instruments).45
Antibacterial Activity of PF@AgNPs
For this study, we selected both gram-negative (E. coli) and gram-positive (B. subtilis and S. aureus) pathogens. Bacterial cultures were activated 24 h prior to the assay. The antibacterial activity of PF@AgNPs was evaluated using a Kirby–Bauer disk diffusion assay.46 First, 10 mL of Luria-Bertani (LB) agar was added to Petri dishes under sterile laminar airflow (LAF). Then, 150 µL of active bacterial suspension was added to each LB agar plate. Thereafter, four sterile paper disks saturated with dimethyl sulfoxide (DMSO; control), 10 µL of PF@AgNPs, 10 µL of pristine leaf extract (PTLE), and 10 µL of streptomycin (standard antibiotic drug) were transferred onto LB plates and then incubated at 37°C for 24 h. Inhibition zones were determined by measuring the bacterial clearance around each disk in mm.
Antioxidant Activity of PF@AgNPs
Antioxidant activity of PF@AgNPs was evaluated using DPPH and ABTS scavenging assays.47,48 Ascorbic acid was used as the standard antioxidant. In DPPH assay, PF@AgNPs (20, 40, 60, 80, and 100 µg) were dissolved in 1 mL methanol in separate test tubes. Then, 2 mL DPPH stock solution (1 mM/L) was added, and the tubes were incubated in the dark for 45 min. Then, the absorbance values were recorded at 517 nm. In ABTS assay, ABTS radical working solution was prepared prior to the assay. ABTS radical working solution (0.9 mL) was added to 0.1 mL of the different concentrations (20, 40, 60, 80, and 100 µg/mL) of PF@AgNPs, shaken well for 45 sec, and incubated in the dark for 15 min, followed by measurement of the absorbance at 734 nm. DPPH/ABTS scavenging activity was calculated using the equation: % scavenging = [(control absorbance − test compound absorbance)/control absorbance] × 100.
Anticancer Activity of PF@AgNPs
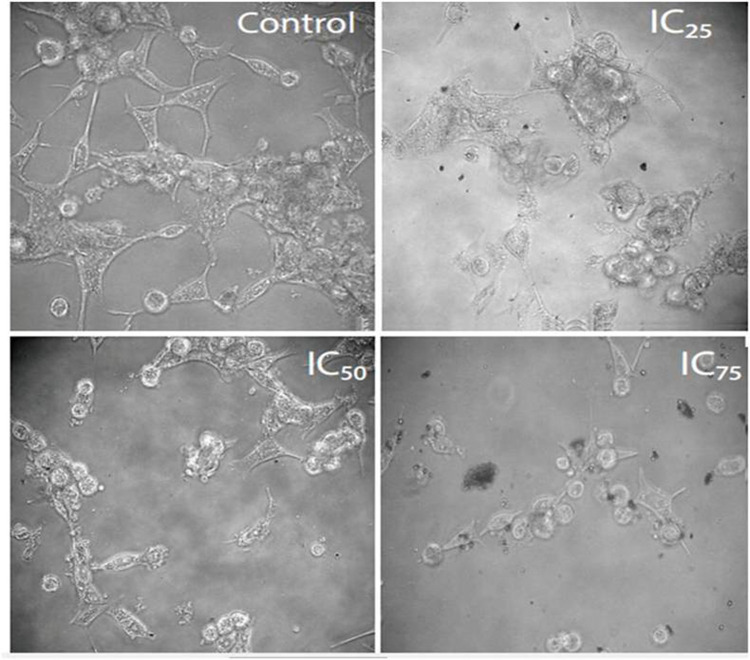
The anticancer activity of PF@AgNPs was evaluated by MTT assay using human colon cancer (COLO205) and human prostate adenocarcinoma (LNCaP) cell lines by the method of Mossman (1983).49 The cells (5 × 103) were seeded in 96 well plates, with each well containing 100 µL of medium. After overnight incubation in a 5% CO2 chamber at 37°C, exactly 100 µL of different concentrations of PF@AgNPs (10, 20, 40, 60, 80, and 100 µg/mL) were added. After 24 h, the cell viability was checked by the addition of 10 μL MTT per well and incubation for a further 3 h. The plates were centrifuged for 10 min at 1000 × g. Thereafter, the medium was discarded, and the formazan blue crystals formed were dissolved in dimethyl sulfoxide (100 μL). Then, the absorbance values were recorded at 570 nm, and the percentage of cell viability was determined. To study morphological changes, further phase-contrast images were taken of the most susceptible cell lines to PF@AgNPs at concentrations of IC25, IC50, and IC75 after 24 h of exposure.
Statistical Analysis
All the experiments were repeated for three times. Mean values were represented with standard deviation. Significance analysis between each group of data was carried out under the condition of P˂0.05. Statistical analyses involved the use of SPSS version 22.0. We have employed Origin 8.5 for the interpretation of UV-Vis, FTIR, TGA, and XRD peaks. We have employed Prism 7.0 for drawing graphs.
Results and Discussion
Phytosynthesis of PF@AgNPs
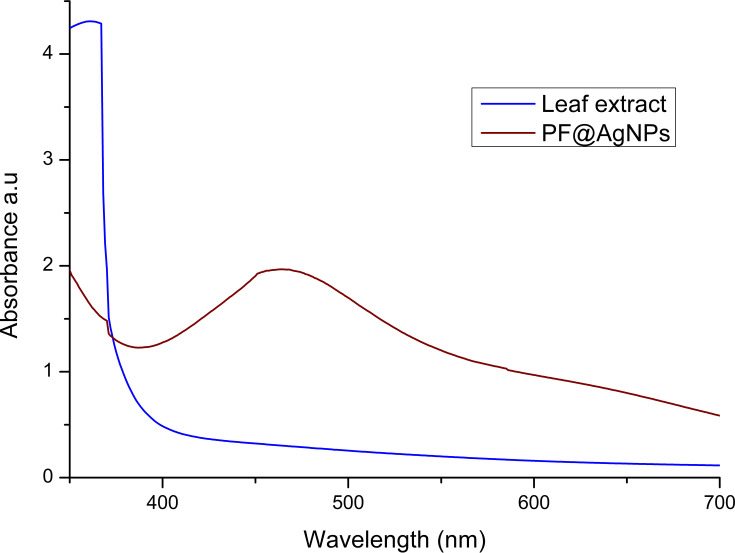
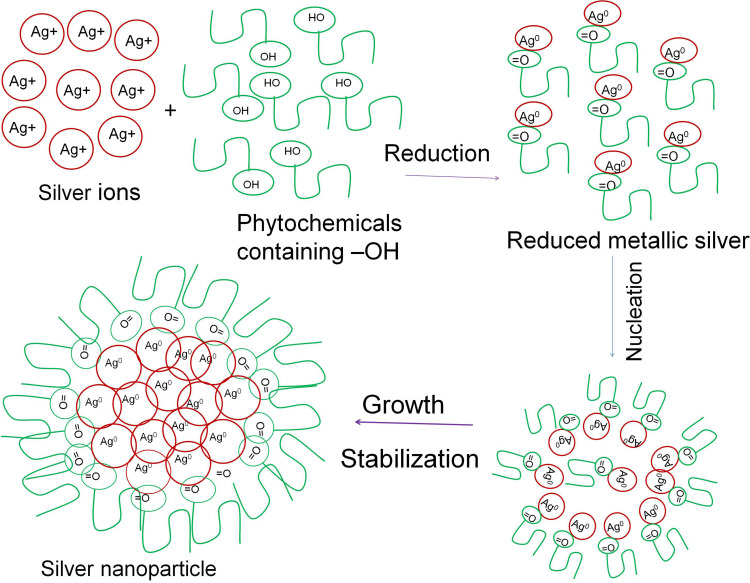
In the present study, we investigate the phytosynthesis of AgNPs using P. frutescens leaf extract. This acts as a reducing agent to convert silver ions (Ag+) into nanosilver (Ag0), named as P. frutescens leaf-synthesized silver nanoparticles (PF@AgNPs). Phytosynthesis was initially observed by a color change of the reaction solution (containing silver ions and leaf extract) from a light-yellow color to dark brown color (Figure 1) after 2 h of incubation in the dark. This color change indicates the phytosynthesis of PF@AgNPs. Phytosynthesis was further confirmed by UV–Vis analysis. The resultant UV–Vis spectrum (Figure 2) showed a hyperbolic peak between 400 and 480 nm. This peak is a characteristic absorption peak of AgNPs due to surface plasmon vibrations excited by the silver metal lattice.1,2,50 Phytochemicals such as flavonoids, triterpenoids, glycosides, and polyphenolic components might carry out the bioreduction of Ag+ to Ag0. The schematic diagram (Figure 3) depicts a possible mechanism of phytosynthesis of AgNPs that can be accomplished in three steps. The first activation step involves the reduction of silver ions (Ag+) into metallic silver atoms (Ag0). In the second step, nucleation and growth phases occur during which small adjacent metallic silver atoms coalesce into AgNPs of a definitive size and shape. The final step involves the stabilization process in which phytochemicals cap AgNPs, thus preventing their agglomeration.
Figure 1.

Color change of the reaction solution from light yellow to dark brown after 2 h indicates the formation of PF@AgNPs.
Figure 2.
UV-Vis analysis of P. frutescens leaf extract and PF@AgNPs. UV-Vis spectrum of PF@AgNPs showed surface plasmon resonance peak at 461 nm.
Figure 3.
Schematic diagram represents the possible mechanism of plant-mediated synthesis (phytosynthesis) of AgNPs.
Phytosynthesis of AgNPs has become popular owing to its economical, eco-friendly, and simple procedure. Phytosynthesized AgNPs have many advantages over chemically synthesized AgNPs (CS-AgNPs). Phytosynthesized AgNPs do not require surface coating chemicals for stabilization. In CS-AgNPs, these surface coating chemicals lead to the formation of toxic and non-biocompatible AgNPs that cannot be used for various biomedical purposes (drug delivery, tissue grafting, bone regeneration, wound dressings, dental implants, diagnostic equipment, and other medical devices) as they contain toxic/hazardous chemicals on their surface. In contrast, phytosynthesis produces clean, non-toxic, and biocompatible AgNPs that can be used for various biomedical purposes.19,22
FTIR Analysis of Phytosynthesized PF@AgNPs
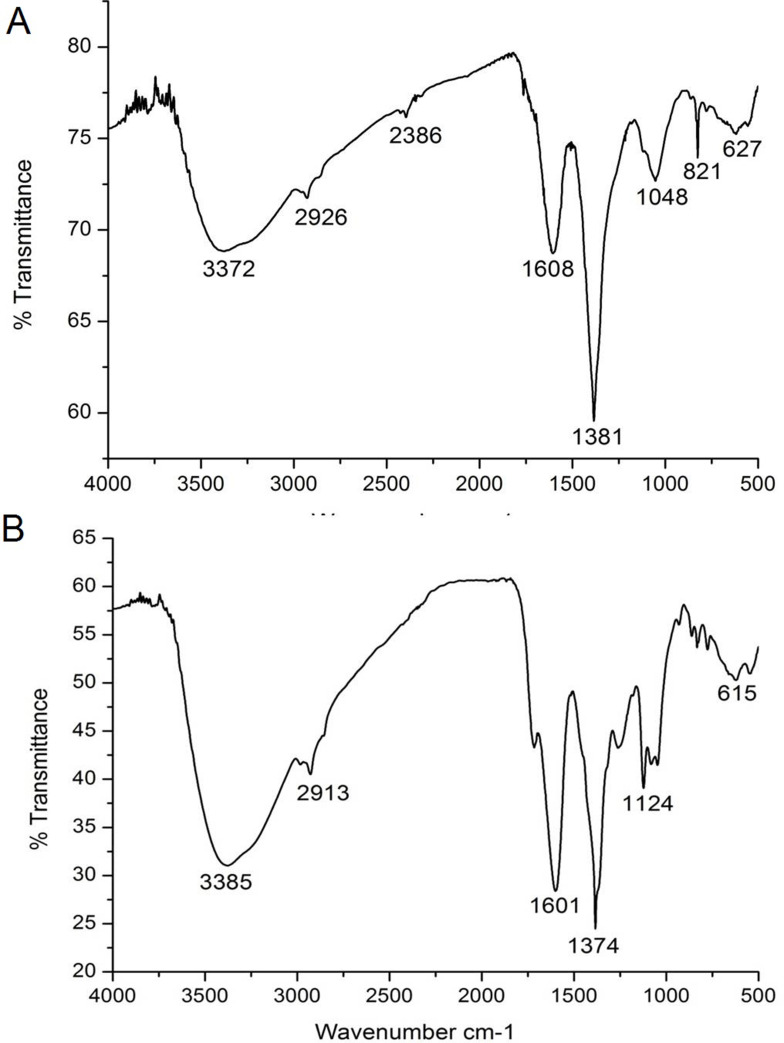
FTIR analysis was carried out to determine the major phytochemicals involved in the phytosynthesis and capping of PF@AgNPs. The FTIR spectrum (Figure 4A) of P. frutescens leaf extract showed peaks at 627, 821, 1048, 1381, 1608, 2386, 2926, and 3372 cm−1. The FTIR spectrum (Figure 4B) revealed major peaks at 615, 1124, 1374, 1601, 2913, and 3385 cm−1. The peaks at 627 and 615 cm−1 were due to the C-H group, which indicated the presence of aliphatic and aromatic compounds. The peaks at 1048 and 1124 cm−1 represented C-O (carboxylic acid) stretching vibrations of aromatic compounds. The peaks at 1381 and 1374 cm−1 indicated C-N stretching vibrations of aromatic amines.51 The peaks at 1608 and 1601 cm−1 signified the secondary amide (-N-H) group of peptide bonds. The shift in the peak from 627 to 615 cm−1revealed the participation of aromatic/aliphatic compounds in the synthesis of PF@AgNPs. The shift in the peak from 1381 to 1374 cm−1 and shift in the peak from 1608 to 1601 cm−1 revealed the involvement of proteins, which cap around the AgNPs and act as stabilizing agents. Capped AgNPs are non-agglomerated and very stable for long durations. The stability of PF@AgNPs was further explained by DLS studies. The peaks at 2926 and 2913 cm−1 were due to the methylene (-C-H) group of aromatic/aliphatic chains.52 The peaks at 3372 and 3385 cm−1 corresponded to the O-H (hydroxyl) group of flavonoids, triterpenoids, and phenolic compounds. This is a critical shift that demonstrates the involvement of polyphenolic compounds in the reduction of Ag+ ions into AgNPs.53 The disappearance of some peaks and shifting in the bands clearly demonstrate the involvement of polyphenols and proteins in the synthesis and stabilization of PF@AgNPs. Polyphenolic compounds such as rosmarinic acid, caffeic acid, protocatechuic acid, chlorogenic acid, vanillic acid, ferulic acid, gallic acid, apigenin, and luteolin have been reported in the leaves of P. frutescens, and it is plausible that these compounds may be involved in the bioreduction process.31 Phenolic acids possess a phenolic nucleus and a carboxyl group, and thus form a resonance stable phenoxy radical nucleus. Oxidation of phenolic acids leads to stable phenoxy radical formation.54 Hence, oxidation of phenolic acids by AgNO3 produces stable phenoxy radicals and reduced metallic silver atoms. The reduced metallic silver atoms join together and form a stable silver nanoparticle. A plausible mechanism of phytosynthesis and capping of AgNPs is depicted in the schematic diagram (Figure 3). Our FTIR results were consistent with previous reports, which demonstrated that polyphenolic compounds are involved in the bioreduction process and that proteins provide stability by capping them.31,51–54
Figure 4.
FTIR analysis of (A) P. frutescens leaf extract and (B) PF@AgNPs.
XRD Pattern of Phytosynthesized PF@AgNPs
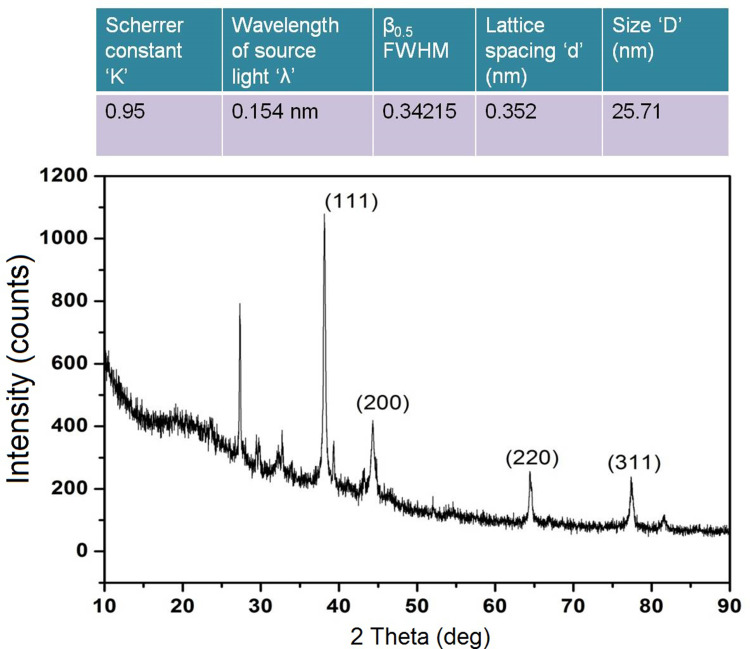
The crystal nature of metallic nanoparticles is a key factor affecting their proper biological functions. The XRD pattern of PF@AgNPs (Figure 5) showed major diffraction peaks at 38.11, 44.29, 64.39, and 77.32° that were indexed to (111), (200), (220), and (311) diffraction planes, respectively. The diffraction peaks and their respective planes revealed that the phytosynthesized PF@AgNPs were crystalline with a face-centered cubic (FCC) lattice (JCPDS File No. 4–0783). Crystallite size (nm) was determined using the Debye–Scherrer equation: crystallite size d=Kλ/β0.5cosθ, where K is the Scherrer constant ‘0.95ʹ, λ indicates source light wavelength (1.54 A°/0.54 nm), β0.5 indicates the FWHM (full width half maximum length), and θ is the Bragg angle (radians). Using the Gaussian nonlinear curve fit (NLFfit), the FWHM of the nanocrystal was determined as 0.34215°. According to the Debye–Scherrer equation, the crystallite size (d) was 25.71 nm. Our XRD results were consistent with those of previous studies. AgNPs synthesized using Tithonia diversifolia,52 Artemisia turcomanica,53 and Ziziphus Jujuba,55 are all nanocrystals with FCC structure.
Figure 5.
XRD pattern of PF@AgNPs.
TEM and DLS Analysis of Phytosynthesized PF@AgNPs
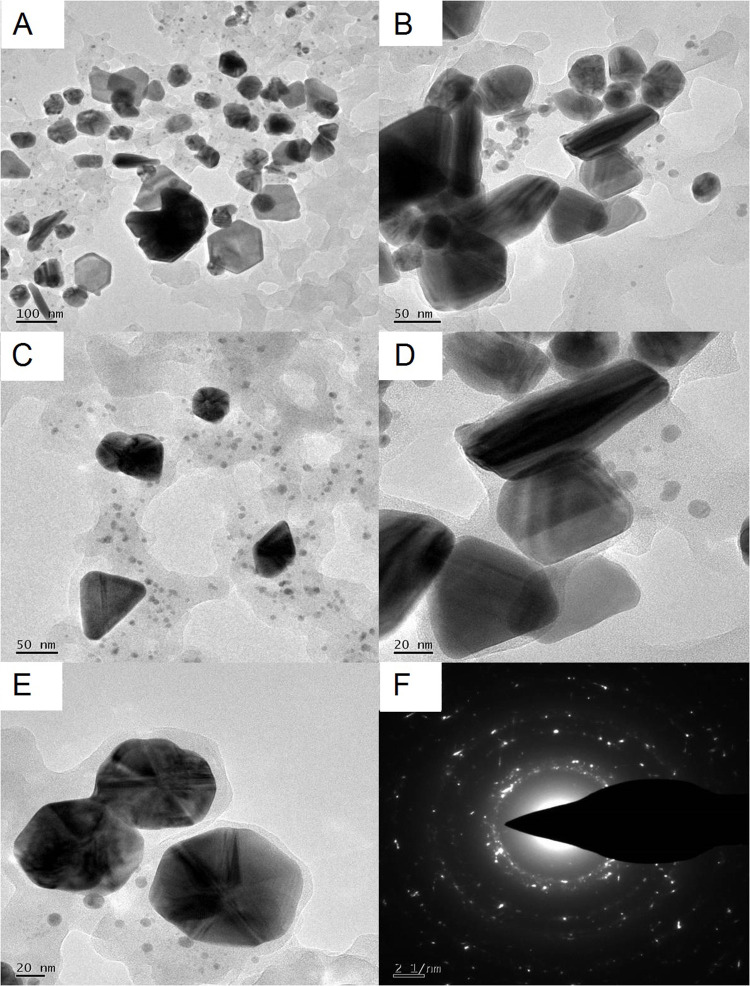
TEM micrographs (Figure 6A–E) revealed that the phytosynthesized PF@AgNPs in this study were 20–50 nm in size with various shapes, including spherical, rhombic, triangle, and rod. However, most AgNPs were spherical. TEM micrographs also showed that PF@AgNPs were monodispersed with no aggregation. The SAED pattern (Figure 6F) of the nanocrystals showed clear Debye–Scherrer rings of FCC crystal. Our TEM results were consistent with those of previous studies. The sizes of AgNPs synthesized using Artemisia turcomanica,53 Ziziphus Jujuba,55 Artemisia absinthium,56 Berberis vulgaris57 are 20–60 nm, 20–30 nm, 5–20 nm, and 30–70 nm, respectively, and all are spherical.
Figure 6.
TEM analysis PF@AgNPs (A) TEM micrograph at 100 nm shows monodispersed AgNPs with different shapes including spherical, rod, triangle, and rhombic; (B) rod-shaped, rhombic shaped, and spiral-shaped AgNPs observed at 50 nm scale; (C) triangle, rhombic and spherical shaped AgNPs at 50 nm; (D) rod-shaped and triangle-shaped AgNPs at 20 nm; (E) spherical shaped AgNPs at 20 nm; (F) SAED pattern of AgNPs.
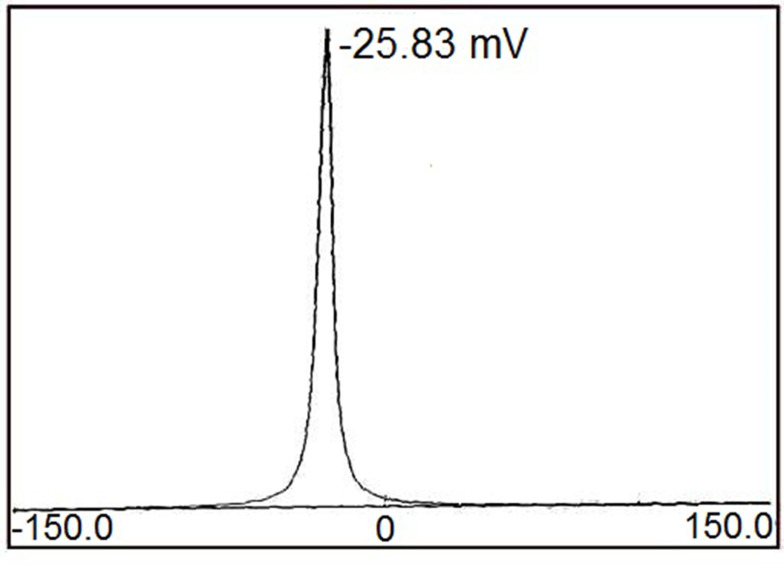
DLS studies showed that the PI value of PF@AgNPs was 0.389, which indicated that PF@AgNPs are monodispersed. The stability of PF@AgNPs was further demonstrated by zeta potential (ZP) measurement (Figure 7). The ZP value of PF@AgNPs was −25.83 mV. This high negative ZP value revealed the repulsion between the AgNPs. The repulsion indicated that PF@AgNPs are long-term stable with no aggregation. TEM micrograph clearly showed the non-aggregation of PF@AgNPs and is due to capping of PF@AgNPs by proteins or phytochemicals of P. frutescens leaf extract. Our DLS results were consistent with those of previous reports. The PI and ZP values of AgNPs synthesized using Pterodon emarginatus,58 Juglans regia,59 Punica granatum,60 were, respectively, 0.372, 0.4, and 0.54 for PI, and −23.9, −33.8, and −34.3 mV for ZP. The DLS results support the monodispersity and long-term stability of phytosynthesized PF@AgNPs.
Figure 7.
Zeta potential measurement of PF@AgNPs.
TG and DTG Analysis of Phytosynthesized PF@AgNPs
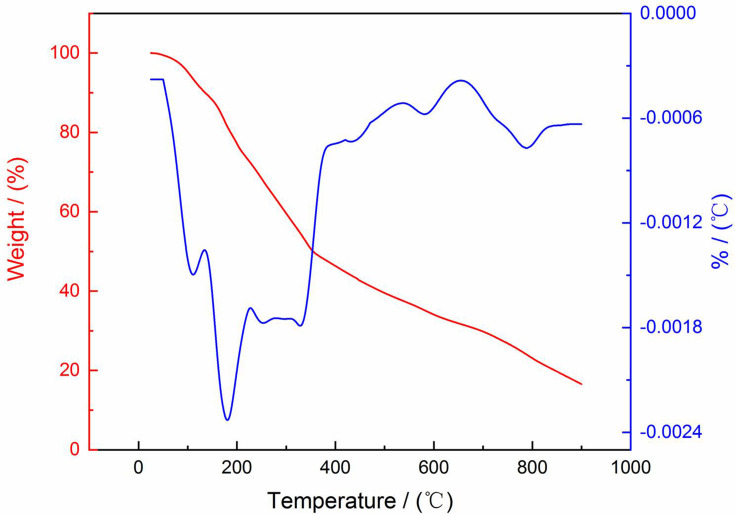
TG and DTG plots (Figure 8) were studied to determine the thermal stability of phytosynthesized PF@AgNPs. TG and DTG plots revealed that phytosynthesized PF@AgNPs were highly stable between 25 and 900°C with very low weight loss. The TG plot of PF@AgNPs revealed steady weight loss in the temperature range of 100–900°C. The DTG plot of PF@AgNPs showed that three major weight losses of 0.14, 0.24, and 0.18% were observed at 111.25, 180.75, and 328.75°C, respectively. The observed weight loss might be due to the desorption of weak conjugated phytochemicals with nanosilver. The TG and DTG results revealed that phytochemicals capped on the nanoparticles impart stability against temperature variations. These phytochemicals act as conjugated molecules on the surface of AgNPs.
Figure 8.
TG and DTG analysis PF@AgNPs.
Antibacterial Activity of PF@AgNPs
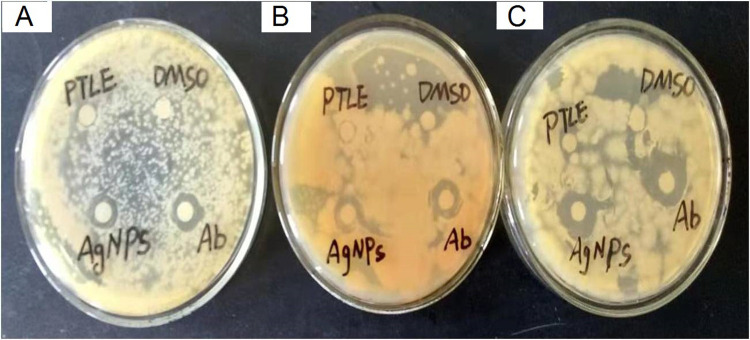
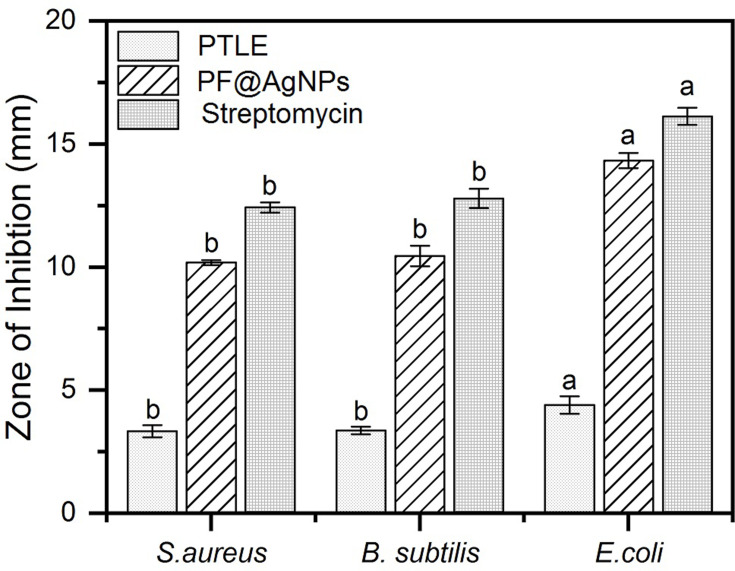
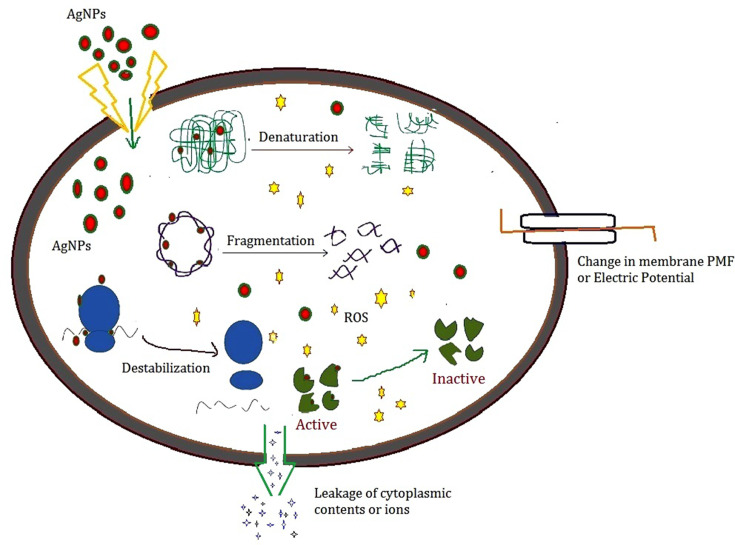
The antibacterial activity of PF@AgNPs was evaluated by a disk diffusion assay against E. coli, B. subtilis, and S. aureus. PF@AgNPs exhibited effective antibacterial activity against all the tested species and produced inhibition zones after 24 h of incubation. Figure 9 represents that PF@AgNPs produced inhibition zones of 14.4, 10.6, and 10.3 mm against E. coli, B. subtilis, and S. aureus, respectively. Antibacterial activity of the pristine leaf extract, PF@AgNPs, and streptomycin was compared by measuring the diameter of inhibition zones (Figure 10). Compared to pristine leaf extract, PF@AgNPs exhibited 2.5- to 3-fold greater antibacterial activity against all the bacteria. There are three possible reasons for the antibacterial activity of PF@AgNPs, viz., the innate antibacterial nature of colloidal silver, the flavonoids, and polyphenols of the plant leaf extract may also contribute antibacterial activity and the ultrasmall size of nanoparticles, which could easily penetrate the bacterial membrane and exhibit their action.61,62 A possible antibacterial mechanism of PF@AgNPs is represented in the schematic diagram (Figure 11). AgNPs cause disruption of the peptidoglycan layer and lead to the formation of pores on the bacterial cell wall, through which AgNPs can enter the cell and exhibit their action. AgNPs interact with genomic DNA and cause DNA fragmentation, resulting in loss of the bacterial capacity for division/replication. AgNPs bind to bacterial enzymes/proteins and cause their inactivation/denaturation, which leads to the cessation of bacterial metabolism. AgNPs bind to the 70S ribosomal-mRNA-complex, which results in dissociation of ribosomal subunits and fragmentation of mRNA, thereby preventing bacterial protein synthesis. Important ions or cytoplasmic contents can be released through the pores on the bacterial cell wall, which alters the proton motive force (PMF)/electric potential of the bacterial membrane. Collectively, these actions of AgNPs synergistically lead to bacterial cell death. In a case study on the antibacterial effect of nanosilver against E. coli as a model of gram-negative bacteria, Sondi and Salopek-Sondi (2004) reported that the antibacterial activity is a result of the AgNP-generated cell wall pits, which increase the membrane permeability and inactivate the respiratory chain.63 Jung et al (2014) reported a common antibacterial mechanism against both gram-negative and gram-positive bacteria, in which AgNPs bind with carboxyl and phosphate groups of the bacterial cell membrane, thereby altering the electric membrane potential, which causes leakage of ions/molecules and leads to cell death.64 Feng et al (2000) reported that AgNPs interact with DNA and enzymes/proteins involved in major metabolic pathways of bacteria leading to the cessation of bacterial metabolism and death of bacteria.65 The results of the present study are supported strongly by previous reports. Mohammed et al (2018) reported that Phoenix dactylifera, Ferula asafoetida, and Acacia nilotica leaf-synthesized AgNPs exhibit antibacterial activity against both gram-positive (S. aureus) and gram-negative (E. coli) bacteria.66 Elemike et al (2017) reported that pathogenic bacteria such as S. aureus, E. coli, B. subtilis, Klebsiella pneumoniae, and Pseudomonas aeruginosa were susceptible to phytosynthesized AgNPs using Costus afer.67 Oedera genistifolia leaf-synthesized AgNPs exhibit effective antibacterial activity against both gram-negative and gram-positive bacteria.68
Figure 9.
Antibacterial activity of PF@AgNPs against (A) S. aureus, (B) B. subtilis, and (C) E. coli; PTLE indicates pristine leaf extract; Ab indicates antibiotic; DMSO indicates dimethyl sulfoxide.
Figure 10.
Comparison of antibacterial activity of pristine leaf extract (PTLE), PF@AgNPs and standard antibiotic streptomycin. Results were expressed as mean ± SD (n=3). Different lowercase letters above the bars indicate significant differences (P < 0.05) by SPSS test.
Figure 11.
Scheme represents the possible mechanism of antibacterial activity of AgNPs.
In vitro Antioxidant Activity of the PF@AgNPs
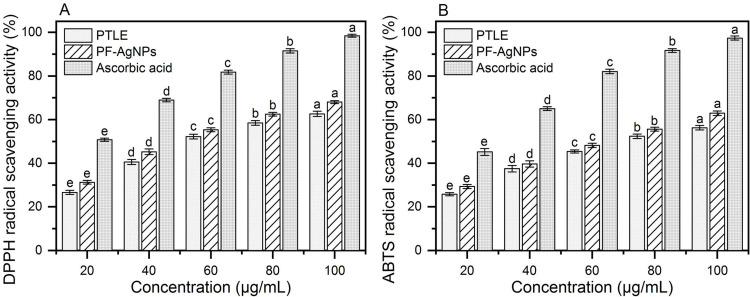
The antioxidant activity of the PF@AgNPs was evaluated using ABTS and DPPH scavenging assays. The radical scavenging capacity of PF@AgNPs was concentration-dependent and increased with increasing concentration of PF@AgNPs from 20 to 100 μg/mL (Figure 12). PF@AgNPs exhibited a maximum scavenging capacity of 68.02% against DPPH radicals with an IC50 concentration of 54.52 μg/mL. PF@AgNPs also exhibited significant ABTS radical scavenging activity with a maximum inhibition of 62.93%. The IC50 concentration of PF@AgNPs against ABTS radicals was 66.86 μg/mL. The antioxidant activity of PF@AgNPs substantiates their applications in the biomedical and nutraceutical industries. AgNPs are widely used in the preparation of food packaging materials because of their antioxidant and antimicrobial activities. Various internal and external factors cause oxidative stress through the production of reactive oxygen species. Oxidative stress is associated with different diseases, including cancer, cardiovascular, neurological, and aging-related diseases, and antioxidants protect the body by reducing oxidative stress.69 In recent years, scientists have focused on natural antioxidants because of the unknown side effects of synthetic drugs. Various phytochemicals such as polyphenols and, flavonoids, are known to play a major role in the synthesis and capping of AgNPs. Different polyphenols and flavonoids including caffeic acid, rosmarinic acid, gallic acid, ferulic acid, luteolin, apigenin, quercetin, and genistein are already reported in the aqueous extracts of P. frutescens. All of these molecules are natural antioxidants with strong radical scavenging activities.31,70,71 Thus, the natural antioxidant capacity of phytosynthesized AgNPs has become an important property for their application in the biomedical, nutraceutical, and pharmaceutical fields.
Figure 12.
Antioxidant activities of PTLE, PF@AgNPs and ascorbic acid (A) DPPH radical scavenging activity, and (B) ABTS radical scavenging activity. All the data were represented as mean ± SD (n =3) in the form of bar graph. Data were analyzed by one-way analysis of variance (ANOVA, P < 0.05). Different lowercase letters above the bars indicate significant differences (P < 0.05).
Anticancer Activity of PF@AgNPs
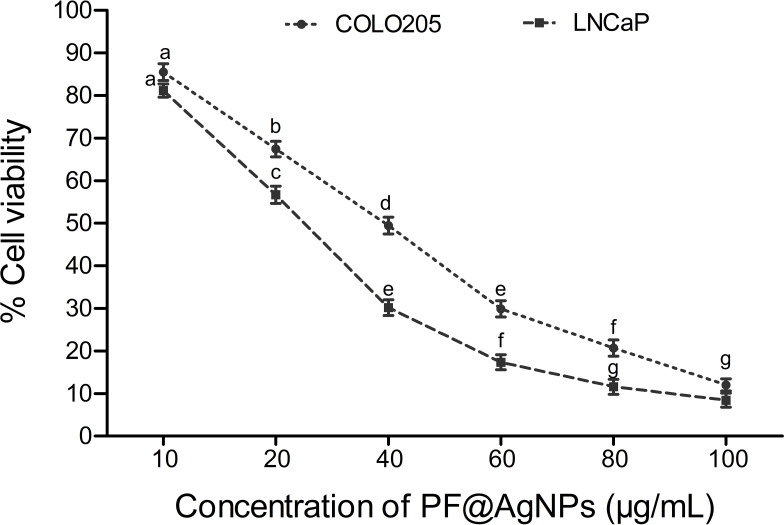
The anticancer activity of the PF@AgNPs was evaluated using the cell lines COLO205 and LNCaP. PF@AgNPs showed anticancer activity in a concentration-dependent manner. An increase in the concentration of PF@AgNPs significantly reduced the cell viability of both COLO205 and LNCaP (Figure 13). PF@AgNPs exhibited maximum inhibition of 91.6 and 87.9% cell viability of LNCaP and COLO205 cells at the highest concentration (100 μg/mL) used in this study. The IC50 concentrations of PF@AgNPs against COLO205 and LNCaP were determined as 39.28 and 24.33 μg/mL, respectively. Thus, LNCaP cells were more susceptible than COLO205 cells to the PF@AgNPs. Further morphological abnormalities in the most susceptible LNCaP cells were investigated under a phase-contrast microscope. Cells treated with IC25, IC50, and IC75 concentration of phytosynthesized AgNPs for 24 h showed noticeable morphological changes in a concentration-dependent manner (Figure 14). The morphological abnormalities include cell shrinkage, membrane destruction, chromatin condensation, protrusion of microspikes, fragmentation of nuclei, and formation of apoptotic bodies. However, the control cells showed healthy morphology with intact nuclei and no abnormalities. The results were supported by previous reports. Phytosynthesized AgNPs using Mentha arvensis and showed anticancer activity against colon cancer cell lines by exhibiting apoptotic effects, cell membrane fragmentation, and cell cycle suspension.72 Phytosynthesized AgNPs using Clinacanthus nutans showed cytotoxic effects including membrane blebbing, nuclear fragmentation, and chromatin condensation of oral squamous cancer cells.73
Figure 13.
Dose-dependent cytotoxic effects of PF@AgNPs against human colon carcinoma (COLO205) and prostate carcinoma (LNCaP) All the data were represented as mean ± SD (n =3) in the form of bar graph. Bars with different superscripts indicate significant differences (P < 0.05).
Figure 14.
Anticancer activity of PF@AgNPs against LNCaP cell lines. The effects of PF@AgNPs on morphological changes of LNCaP cells were studied after 24 h exposure at different concentrations. PF@AgNPs affected the cell viability by inducing apoptotic symptoms, including warping of cells, rounding of cells, membrane blebbing, and cell shrinkage.
Conclusions
In the present study, we report the bioreduction of silver ions into silver nanoparticles using P. frutescens aqueous leaf extract, which acts as both a reducing and capping agent for PF@AgNPs. The successful synthesis of PF@AgNPs was indicated by the color change and UV–Vis absorption peak. FTIR analysis demonstrated that polyphenols, flavonoids, and proteins play a major role in this bioreduction process. The physical properties of PF@AgNPs, such as size, shape, crystallinity, dispersity, surface charge, and stability were studied using a range of spectroscopic techniques, including TEM, XRD, DLS, and TGA. Different shapes of PF@AgNPs were detected, including spherical, triangle, rhombic, and rod shapes. The crystallite size was 25.71 nm with an FCC lattice. The high negative surface charge (−25.83 mV) indicated long-term stability with no aggregation. TEM micrograph and lower PI (0.389) value revealed the monodispersity of PF@AgNPs. PF@AgNPs were found to be thermostable with very low weight loss even at high temperatures. PF@AgNPs produced the maximum inhibition zone against E. coli, followed by B. subtilis and S. aureus. PF@AgNPs showed significant antioxidant activity by quenching DPPH and ABTS-free radicals. PF@AgNPs significantly reduced the cell viability of both COLO205 and LNCaP cancer cell lines. The apoptotic effects of PF@AgNPs in the most susceptible LNCaP cells were observed and revealed the morphological changes, including rounded up cells, chromatin condensation, protrusion of microspikes, fragmentation of nucleus, and formation of apoptotic bodies. Thus, AgNPs were successfully synthesized using P. frutescens aqueous leaf extract, and we report their characterization, and antibacterial, antioxidant, and anticancer activities. The possible mechanisms of the bioreduction process and the antibacterial activity of green synthesized AgNPs are explained.
Highlights of the Manuscript
Perilla frutescens leaf extract act as reducing and capping agent involved in the synthesis and stabilization of silver nanoparticles.
P. frutescens leaf synthesized AgNPs (PF@AgNPs) are irregular shaped, face centered cubic crystallites, monodispersed, long term stable, and non-agglomerated.
PF@AgNPs showed antibacterial activity against both gram-positive and gram-negative bacteria.
PF@AgNPs exhibited significant free radical scavenging activity on ABTS and DPPH radicals.
PF@AgNPs exhibited significant anticancer activity against human colon cancer and prostate adenocarcinoma.
PF@AgNPs showed significant cytotoxic effects including nuclear fragmentation, membrane blebbing, and chromatin condensation of prostate cancer cells.
Acknowledgments
This work was supported by Key Research and Development (R&D) Projects of Shanxi Province (201803D31060), China. We are very grateful to Dr. Hongjiao Zhang (School of Chemical Engineering Technology, North University of China) for her efforts in the statistical analysis of data, and drawing graphs.
Ethical Statement
No animals were used/harmed for the present study. The cell lines including human colon cancer (COLO205) and prostate cancer (LNCaP) cells were commercially purchased from National Centre for Cellular Sciences, Pune, India.
Disclosure
All the authors report no conflicts of interest in this work.
References
- 1.Jo DH, Kim JH, Lee TG, Kim JH. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine. 2015;11:603–1611. doi: 10.1016/j.nano.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 2.Evanoff DD, Chumanov G. Synthesis and optical properties of silver nanoparticles and arrays. Chem Phys Chem. 2005;6:1221–1231. doi: 10.1002/cphc.200500113 [DOI] [PubMed] [Google Scholar]
- 3.Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):3. doi: 10.1186/1477-3155-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Natsuki J, Natsuki T, Hashimoto Y. A review of silver nanoparticles: synthesis methods, properties and applications. Int J Mater Sci Appl. 2015;4(5):325–332. doi: 10.11648/j.ijmsa.20150.405.17 [DOI] [Google Scholar]
- 5.Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syafiuddin A, Salim MR. A review of silver nanoparticles: research trends, global consumption, synthesis, properties, and future challenges. J Chinese Chem Soc. 2017;64(7):732–756. doi: 10.1002/jccs.201700067 [DOI] [Google Scholar]
- 7.Pareek V, Gupta R, Panwar J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater Sci Eng C Mater Biol Appl. 2018;90:739–749. doi: 10.1016/j.msec.2018.04.093 [DOI] [PubMed] [Google Scholar]
- 8.Kalantari K, Mostafavi E, Afifi AM, et al. Wound dressings functionalized with silver nanoparticles: promises and pitfalls. Nanoscale. 2020;12(4):2268–2291. doi: 10.1039/c9nr08234d [DOI] [PubMed] [Google Scholar]
- 9.Furno F, Morley KS, Wong B, et al. Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? J Antimicrob Chemother. 2004;54(6):1019–1024. doi: 10.1093/jac/dkh478 [DOI] [PubMed] [Google Scholar]
- 10.Hamdan S, Pastar I, Drakulich S, et al. Nanotechnology-driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci. 2017;3(3):163–175. doi: 10.1021/acs.centsci.6b00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 2013;52(6):1636–1653. doi: 10.1002/anie.201205923 [DOI] [PubMed] [Google Scholar]
- 12.Fathi-Achachelouei M, Knopf-Marques H. Use of nanoparticles in tissue engineering and regenerative medicine. Front Bioeng Biotechnol. 2019;7:113. doi: 10.3389/fbioe.2019.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marassi V, Di Cristo L, Smith SGJ, et al. Silver nanoparticles as a medical device in healthcare settings: a five-step approach for candidate screening of coating agents. R Soc Open Sci. 2018;5(1):171113. doi: 10.1098/rsos.171113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawhney APS, Condon B, Singh KV, Pang SS, Li G, Hui D. Modern applications of nanotechnology in textiles. Textile Res J. 2008;78(8):731–739. doi: 10.1177/0040517508091066 [DOI] [Google Scholar]
- 15.Marin S, Vlasceanu GM, Tiplea RE, et al. Applications and toxicity of silver nanoparticles: a recent review. Curr Top Med Chem. 2015;15(16):1596–1604. doi: 10.2174/156802661566615041.4142209 [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Mahendra S, Alvarez PJ. Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations. ACS Nano. 2010;4(7):3580–3590. doi: 10.1021/nn100866w [DOI] [PubMed] [Google Scholar]
- 17.Tavakoli H, Rastegar H, Taherian M, Samadi M, Rostami H. The effect of nano-silver packaging in increasing the shelf life of nuts: an in vitro model. Ital J Food Saf. 2017;6(4):6874. doi: 10.4081/ijfs.2017.6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X, Deng H, Hwang HM. The current application of nanotechnology in food and agriculture. J Food Drug Anal. 2019;27(1):1–21. doi: 10.1016/j.jfda.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abbasi E, Milani M, Fekri Aval S, et al. Silver nanoparticles: synthesis methods, bio-applications and properties. Crit Rev Microbiol. 2016;42(2):173–180. doi: 10.3109/1040841X.2014.912200 [DOI] [PubMed] [Google Scholar]
- 20.Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(1):1–35. doi: 10.1080/10590500802708267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkat MA, Harshita BS, et al. Current progress in synthesis, characterization and applications of silver nanoparticles: precepts and prospects. Recent Pat Antiinfect Drug Discov. 2018;13(1):53–69. doi: 10.2174/1574891X12666171006102833 [DOI] [PubMed] [Google Scholar]
- 22.Mousavi SM, Hashemi SA, Ghasemi Y, et al. Green synthesis of silver nanoparticles toward bio and medical applications: review study. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S855–S872. doi: 10.1080/21691401.2018.1517769 [DOI] [PubMed] [Google Scholar]
- 23.Hamburger M, Hostettmann K. Bioactivity in plants: the link between phytochemistry and medicine. Phytochemistry. 1991;30:3864–3874. doi: 10.1016/0031-9422(91)83425-K [DOI] [Google Scholar]
- 24.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893 [DOI] [PubMed] [Google Scholar]
- 25.Kamei R, Fujimura T, Matsuda M, et al. A flavanone derivative from the Asian medicinal herb (Perilla frutescens) potently suppresses IgE-mediated immediate hypersensitivity reactions. Biochem Biophys Res Commun. 2017;483(1):674–679. doi: 10.1016/j.bbrc.2016.12.083 [DOI] [PubMed] [Google Scholar]
- 26.Takano H, Osakabe N, Sanbongi C, et al. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical inhibits seasonal allergic rhinoconjuncti vitis in humans. Exp Biol Med. 2004;229:247–254. doi: 10.1177/153537020422900305 [DOI] [PubMed] [Google Scholar]
- 27.Šmelcerović A, Tomović K, Šmelcerović Ž, et al. Xanthine oxidase inhibitors beyond allopurinol and febuxostat; an overview and selection of potential leads based on in silico calculated physico-chemical properties, predicted pharmacokinetics and toxicity. Eur J Med Chem. 2017;135:491–516. doi: 10.1016/j.ejmech.2017.04.031 [DOI] [PubMed] [Google Scholar]
- 28.Ha TJ, Lee JH, Lee MH, et al. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012;135(3):1397–1403. doi: 10.1016/j.foodchem.2012.05.104 [DOI] [PubMed] [Google Scholar]
- 29.Verspohl EJ, Fujii H, Homma K, Buchwald-Werner S. Testing of Perilla frutescens extract and Vicenin 2 for their antispasmodic effect. Phytomedicine. 2013;20(5):427–431. doi: 10.1016/j.phymed.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 30.You CX, Yang K, Wu Y, et al. Chemical composition and insecticidal activities of the essential oil of Perilla frutescens (L.) Britt. aerial parts against two stored product insects. Eur Food Res Technol. 2014;239(3):481–490. doi: 10.1007/s00217-014-2242-8 [DOI] [Google Scholar]
- 31.Yu H, Qiu JF, Ma LJ, Hu YJ, Li P, Wan JB. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem Toxicol. 2017;108(PtB):375–391. doi: 10.1016/j.fct.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 32.Oates PJ, Mylari BL. Aldose reductase inhibitors: therapeutic implications for diabetic complications. Expert Opin Investig Drugs. 1999;8(12):2095–2119. doi: 10.1517/13543784.8.12.2095 [DOI] [PubMed] [Google Scholar]
- 33.Jeon IH, Kim HS, Kang HJ, et al. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules. 2014;19(6):6941–6951. doi: 10.3390/molecules19066941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakazawa T, Yasuda T, Ueda J, Ohsawa K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol Pharm Bull. 2003;26(4):474–480. doi: 10.1248/bpb.26.474 [DOI] [PubMed] [Google Scholar]
- 35.Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17(3):240–243. doi: 10.1002/ptr.1115 [DOI] [PubMed] [Google Scholar]
- 36.Li HZ, Ren Z, Reddy NV, Hou T, Zhang Z. In silico evaluation of antimicrobial, antihyaluronidase and bioavailability parameters of rosmarinic acid in Perilla frutescens leaf extracts. SN Appl Sci. 2020;2:1547. doi: 10.1007/s42452-020-03323-8 [DOI] [Google Scholar]
- 37.Saifullah AS, Ahmad M, Swami BL, Ikram S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiation Res Appl Sci. 2016;9:1–7. doi: 10.1016/j.jrras.2015.06.006 [DOI] [Google Scholar]
- 38.Arokiyaraj S, Vincent S, Saravanan M, Lee Y, Young KO, Kim KH. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif Cells Nanomed Biotechnol. 2017;45(2):372–379. doi: 10.3109/21691401.2016.1160403 [DOI] [PubMed] [Google Scholar]
- 39.Singh P, Kim YJ, Singh H, et al. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanomedicine. 2015;10:2567–2577. doi: 10.2147/IJN.S72313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashour AA, Raafat D, El-Gowelli HM, El-Kamel AH. Green synthesis of silver nanoparticles using cranberry powder aqueous extract: characterization and antimicrobial properties. Int J Nanomedicine. 2015;10:7207–7221. doi: 10.2147/IJN.S87268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zia F, Ghafoor N, Iqbal M, Mehboob S. Green synthesis and characterization of silver nanoparticles using Cydonia oblong seed extract. Appl Nanosci. 2016;6:1023–1029. doi: 10.1007/s13204-016-0517-z [DOI] [Google Scholar]
- 42.Sun W, Qu D, Ma Y, Chen Y, Liu C, Zhou J. Enhanced stability and antibacterial efficacy of a traditional Chinese medicine-mediated silver nanoparticle delivery system. Int J Nanomedicine. 2014;9:5491–5502. doi: 10.2147/IJN.S71670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majeed Khan MA, Kumar S, Ahamed M, Alrokayan SA, Alsalhi MS. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res Lett. 2011;6(1):434. doi: 10.1186/1556-276X-6-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan MJ, Shameli K, Sazili AQ, Selamat J, Kumari S. Rapid green synthesis and characterization of silver nanoparticles arbitrated by curcumin in an alkaline medium. Molecules. 2019;24(4):719. doi: 10.3390/molecules24040719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhat RS, Almusallam J, Al Daihan S, Al-Dbass A. Biosynthesis of silver nanoparticles using Azadirachta indica leaves: characterization and impact on Staphylococcus aureus growth and glutathione-S-transferase activity. IET Nanobiotechnology. 2019;13(5):498–502. doi: 10.1049/iet-nbt.2018.5133 [DOI] [Google Scholar]
- 46.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493–496. doi: 10.1093/ajcp/45.4_ts.493 [DOI] [PubMed] [Google Scholar]
- 47.LI HZ, Zhang Z, Jiao X. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and rosmarinic acid from perilla leaves using response surface methodology. Food Sci Technol. 2016;36:686–693. doi: 10.1590/1678-457x.13516 [DOI] [Google Scholar]
- 48.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3 [DOI] [PubMed] [Google Scholar]
- 49.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83).90303-4 [DOI] [PubMed] [Google Scholar]
- 50.Kelly KL, Coronando E, Zhao LL, Schatz GC. The Optical properties of metal nanoparticles: the influence of size, shape and dielectric environment. J Phys Chem B. 2002;107:668–677. doi: 10.1021/jp026731y [DOI] [Google Scholar]
- 51.Das S, Das J, Samadder A, Bhattacharyya SS, Das D, Khuda-Bukhsh AR. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Colloids Surf B Biointerfaces. 2013;101:325–336. doi: 10.1016/j.colsurfb.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 52.Tran TT, Vu TT, Nguyen TH. Biosynthesis of silver nanoparticles using Tithonia diversifolia leaf extract and their antimicrobial activity. Mater Lett. 2013;105:220–223. doi: 10.1016/j.matlet.2013.04.021 [DOI] [Google Scholar]
- 53.Mousavi B, Tafvizi F, Zaker Bostanabad S. Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). Artif Cells Nanomed Biotechnol. 2018;46(sup1):499–510. doi: 10.1080/21691401.2018.1430697 [DOI] [PubMed] [Google Scholar]
- 54.Simić A, Manojlović D, Segan D, Todorović M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules. 2007;12(10):2327–2340. doi: 10.3390/121023.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gavade NL, Kadam AN, Suwarnkar MB, Ghodake VP, Garadkar KM. Biogenic synthesis of multi-applicative silver nanoparticles by using Ziziphus Jujuba leaf extract. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136(Pt B):953–960. doi: 10.1016/j.saa.2014.09.118 [DOI] [PubMed] [Google Scholar]
- 56.Ali M, Kim B, Belfield KD, Norman D, Brennan M, Ali GS. Green synthesis and characterization of silver nanoparticles using Artemisia absinthium aqueous extract–A comprehensive study. Mater Sci Eng C Mater Biol Appl. 2016;58:359–365. doi: 10.1016/j.msec.2015.08.045 [DOI] [PubMed] [Google Scholar]
- 57.Behravan M, Hossein Panahi A, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol. 2019;124:148–154. doi: 10.1016/j.ijbiomac.2018.11.101 [DOI] [PubMed] [Google Scholar]
- 58.Oliveira GZS, Lopes CAP, Sousa MH, Silva LP. Synthesis of silver nanoparticles using aqueous extracts of Pterodon emarginatus leaves collected in the summer and winter seasons. Int Nano Lett. 2019;9:109–117. doi: 10.1007/s40089-019-0265-7 [DOI] [Google Scholar]
- 59.Khorrami S, Zarrabi A, Khaleghi M, Danaei M, Mozafari MR. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int J Nanomedicine. 2018;13:8013–8024. doi: 10.2147/IJN.S189295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasiriboroumand M, Montazer M, Barani H. Preparation and characterization of biocompatible silver nanoparticles using pomegranate peel extract. J Photochem Photobiol B. 2018;179:98–104. doi: 10.1016/j.jphotobiol.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 61.Alexander JW. History of the medical use of silver. Surg Infect. 2009;10:289–292. doi: 10.1089/sur.2008.9941 [DOI] [PubMed] [Google Scholar]
- 62.Ranghar S. Nanoparticle-based drug delivery systems: promising approaches against infections. Brazilian Arch Biol Technol. 2012;57:209–222. doi: 10.1590/S1516-89132013005000011 [DOI] [Google Scholar]
- 63.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 64.Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74(7):2171–2178. doi: 10.1128/AEM.02001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52(4):662–668. doi: [DOI] [PubMed] [Google Scholar]
- 66.Elemike EE, Fayemi OE, Ekennia AC, Onwudiwe DC, Ebenso EE. Silver nanoparticles mediated by Costus afer leaf extract: synthesis, antibacterial, antioxidant and electrochemical properties. Molecules. 2017;22(5):701. doi: 10.3390/molecules22050701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohammed AE, Al-Qahtani A, Al-Mutairi A, Al-Shamri B, Aabed KF. Antibacterial and cytotoxic potential of biosynthesized silver nanoparticles by some plant extracts. Nanomaterials. 2018;8(6):382. doi: 10.3390/nano8060382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okaiyeto K, Ojemaye MO, Hoppe H, Mabinya LV, Okoh AI. Phytofabrication of silver/silver chloride nanoparticles using aqueous leaf extract of Oedera genistifolia: characterization and antibacterial potential. Molecules. 2019;24(23):4382. doi: 10.3390/molecules24234382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young I, Woodside J. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akhtar MS, Panwar J, Yeoung-Sang Y. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sus Chem Eng. 2013;1:591–602. doi: 10.1021/sc300118u [DOI] [Google Scholar]
- 71.Pratt DE. Natural antioxidants from plant material,” in phenolic compounds in food and their effects on health II. ACS Symposium Ser. 2009;507:54–71. doi: 10.1021/bk-1992-0507.ch005 [DOI] [Google Scholar]
- 72.Javed B, Mashwani ZU. Synergistic effects of physicochemical parameters on bio-fabrication of mint silver nanoparticles: structural evaluation and action against HCT116 colon cancer cells. Int J Nanomedicine. 2020;15:3621–3637. doi: 10.2147/IJN.S254402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yakop F, Abd Ghafar SA, Yong YK, et al. Silver nanoparticles Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif Cells Nanomed Biotechnol. 2018;46(sup2):131–139. doi: 10.1080/21691.401.2018.1452750 [DOI] [PubMed] [Google Scholar]