Abstract
Purpose
Adoptive transfer of activated autologous tumor-infiltrating lymphocytes (TIL) can mediate complete, durable regressions in patients with metastatic melanoma (MM). Responding patients generally do not have significant changes in non-cutaneous RECIST targets before 30–60 days following TIL infusion, and complete responses are often not confirmed for 1–2 years. There is a critical need for a biomarker that can provide early information regarding the likelihood and duration of a response to enable rational decisions about altering therapy. We wished to evaluate the role of ctDNA in separating responding from non-responding patients.
Experimental Design
We studied BRAF V600E ctDNA levels by a sensitive allele specific PCR assay in 388 serum samples from 48 patients who received TIL immunotherapy at the National Cancer Institute, and correlated differences in the dynamic patterns of their ctDNA measurements with response outcomes.
Results
A strong correlation was found between the presence or absence of an early serum peak of V600E ctDNA, and the likelihood of an objective response. Furthermore, patients that developed an early ctDNA peak and cleared their serum of V600E ctDNA were highly likely to achieve a complete response over the next 1–2 years. Patients that showed no peak of V600E ctDNA failed to achieve an objective response, with one exception.
Conclusion
We show that the dynamic changes occurring in BRAF V600E ctDNA levels within the first month following T-cell transfer immunotherapy in MM can be used to rapidly identify responding from non-responding patients, potentially allowing clinicians to make critical treatment-related decisions in a more timely manner. These data also suggest that the majority of tumor killing by TIL occurs very early after the initiation of therapy.
Keywords: ctDNA, T-cell transfer immunotherapy, melanoma, biomarkers, BRAF V600E
INTRODUCTION
Metastatic malignant melanoma is a devastating disease with an overall 5 year survival rate of less than 5% with conventional chemotherapy. Targeted agents, such as BRAF inhibitors, can mediate cancer regressions though the duration of responses is often short. Recently, immune checkpoint inhibitors have been developed that show promise in early trials, with 5 year survivals of approximately 20% being reported for ipilimumab (anti-CTLA-4) (1). Early trials with nivolumab and pembrolizumab (anti-PD1) in stage IV melanoma patients show even higher objective response rates than those reported with anti-CTLA-4 therapy (2, 3), and the durability of the responses is being evaluated.
Cell transfer immunotherapy is another promising immunotherapeutic approach to cancer. We and others (4–7) have shown that cell therapy using tumor infiltrating lymphocytes (TIL) is potentially curative in as many as 22% of patients with stage IV metastatic melanoma. In our study, 56% of all patients showed an objective response to this potent therapy (7).
Most TIL immunotherapy trials apply Response Evaluation Criteria In Solid Tumors (RECIST) to formally assess responses (8). This is accomplished by following representative measurable target lesions, usually by radiologic scans (preferably CT or MRI). Responding patients generally do not have significant changes in non-cutaneous RECIST targets before 30–60 days post TIL infusion, and complete responses are often not confirmed for 1–2 years. Thus, there is a critical need for a biomarker that can provide early information regarding the likelihood and duration of a response to enable rational decisions about altering therapy.
Circulating tumor DNA (ctDNA) is a promising new biomarker that is being investigated in multiple tumor types (9, 10). Numerous studies have shown that tumor derived ctDNA can be identified in the circulation of patients with a variety of cancers (11–16), and that changes in the levels of ctDNA can be used to monitor disease course, treatment responses, and recurrences (13–16). Changes in the ctDNA levels are presumed to reflect changes in the overall tumor burden over time (14).
Up to 50% of malignant melanomas harbor a specific V600E mutation in the BRAF gene, creating an ideal target for ctDNA studies (17). In stage III–IV melanoma, BRAF V600E ctDNA has been reported in 39–83% of patients with V600E positive melanoma (11, 18, 19), and recently there have been several studies showing dynamic changes in BRAF V600E ctDNA levels during the treatment course of patients undergoing biochemotherapy (18), targeted biologic therapies (20–22), and immune checkpoint therapies (23, 24).
In this study, we asked whether changes in circulating BRAF V600E ctDNA can provide information that is helpful in evaluating a patient’s response to TIL immunotherapy. The results suggest that early monitoring of ctDNA during the course of TIL therapy can identify responding patients, and potentially can be used to assess the adequacy of therapy.
MATERIALS AND METHODS
Patients and Serum Samples
Between 2000–2007, ninety-three patients with metastatic melanoma were enrolled in 3 consecutive NCI TIL trials (7). The clinical courses of these patients are described in detail in this manuscript. All tumor specimens were studied by pyrosequencing for BRAF and NRAS mutation analysis. Blood was collected before and during the patient’s treatment, and at every follow-up visit using standard procedures. Serum was prepared, aliquoted in 1–2 ml volumes, and stored at −80°C. All patients signed an informed consent approved by the Institutional Review Board of the National Cancer Institute.
Cell free (cf) DNA Extraction and Analyses
cfDNA was isolated from 1–2 ml serum samples using the QIAamp Circulating Nucleic Acid Kit (Qiagen Inc, Valencia, CA) according to the manufacturer’s instructions. cfDNA was quantified using a BRAF WT reference standard curve assay generated with serial dilutions of commercial placenta DNA (supplemental Fig. S1).
For BRAF V600E mutation detection, two separate duplex Competitive Allele-Specific TaqMan PCR (castPCR™) designs were employed to detect the BRAF V600E mutation and the BRAF WT reference. Both the BRAF V600E mutation and the BRAF V600 WT reference assay included an Internal Positive Control (IPC) to monitor PCR inhibition, and to assist in quality control of the reactions. The BRAF V600E assay specificity was determined to be 100%, with an analytical sensitivity of approximately 0.05% allele frequency (supplemental Fig. S1).
castPCR™ reactions were carried out in duplicate in 20 ul volumes that included 2x Genotyping Master Mix, 10x Mutation Assay or 10x Reference Assay, 0.4 ul of Exogenous IPC template, 2 ul of Exogenous IPC Mix, and 5 ul of cfDNA. PCR was carried out on a ViiA7 real-time PCR system. The BRAF mutant Allele Frequency (AF) was calculated with Mutation Detector Software (all assays, reagents, instrument, and software were purchased from Life Technologies, Carlsbad, CA). The mutant Allele Frequency is defined as the fraction of mutant alleles divided by the total number of mutant and wild type alleles expressed as a percentage.
Statistics
The analysis of the ctDNA pattern categories was performed using an exact Kruskal-Wallis test. The probability of survival as a function of time was determined using the Kaplan-Meier method. The statistical significance of the difference between Kaplan-Meier curves was determined by the log-rank test, using the Holm-Sidak method for adjustment for multiple comparisons.
RESULTS
Mutant BRAF V600E ctDNA can be detected in >90% of BRAF V600E stage IV melanoma patients
Forty eight of 93 patients with stage IV metastatic melanoma who were treated in the Surgery Branch of the National Cancer Institute with TIL immunotherapy between 2000 and 2007 were found to have melanomas with BRAF V600E mutations, using pyrosequencing (supplemental Fig. S2). Detailed clinical data from these trials were reported by Rosenberg et al in 2011 (7). The subset of 48 BRAF V600E melanoma patients included 10 complete responders (CR), 17 partial responders (PR), and 21 non-responders (NR), as assessed by RECIST criteria. Four hundred and two archived cryopreserved serum samples from these 48 patients were retrieved. Fourteen samples yielded insufficient DNA for analysis and/or failed the castPCR reaction. The remaining 388 evaluable samples (an average of 8.0 samples/patient) from different time points) were studied for the presence of BRAF V600E ctDNA by castPCR, as described in the Methods and supplemental Fig. S1. Of these 48 patients, 44 (91.7%) were found to have V600E ctDNA in at least one time point during the course of their disease; V600E ctDNA was not detected in 4 patients (1 CR with 11 samples and 3 NR with 3 or 4 samples each). Total DNA recovery was highly variable ranging from 2 to 388 ng/ml serum (median, 10 ng/ml) (supplemental Fig. S1).
Early dynamic changes in BRAF V600E ctDNA are correlated with response to TIL immunotherapy
To investigate a potential relationship between the early dynamic changes of V600E ctDNA and clinical response, we evaluated a subgroup of the 48 BRAF V600E positive patients who had a serum sample prior to treatment and had at least 2 serum samples available from the first month following TIL infusion, with one of those samples being from the first two weeks. Nine of the 48 patients did not meet these criteria, and were excluded from the remainder of the analysis (supplemental Fig. S3).
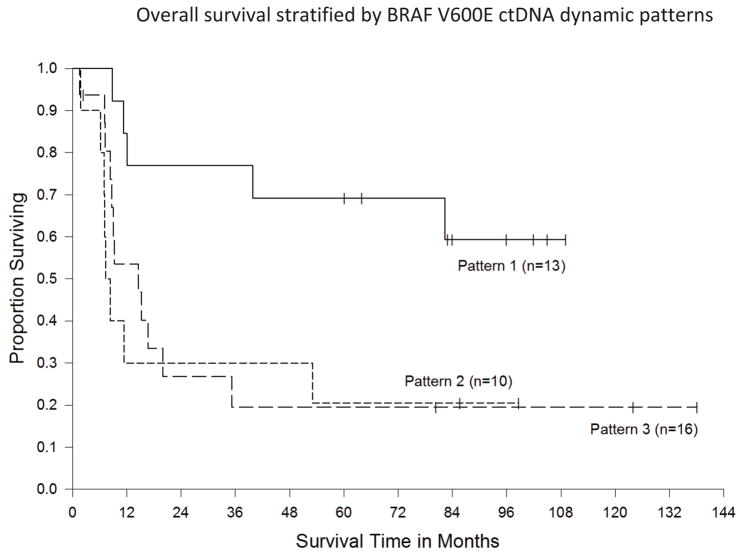
Of the 39 informative patients, there were 10 in the CR group, 14 in the PR group, and 15 in the NR group. To evaluate the changes in ctDNA, we established several definitions to categorize the patterns of dynamic change detected. We defined a peak of V600E ctDNA as greater than or equal to twice the level of the preceding time point. If the preceding time point was zero, we required that the V600E allele frequency be at least 0.1% to qualify as a peak, as the detection limit of the assay was 0.05%. We defined clearing of V600E ctDNA when a patient had undetectable levels of V600E ctDNA in two consecutive serum samples taken at least 30 days apart. Initial clearing was defined as the first time point at which BRAF V600E ctDNA was undetectable. With these definitions, we were able to group patients into three pattern categories: pattern 1, early peaks of V600E ctDNA followed by clearing (13 cases); pattern 2, early peaks of V600E ctDNA without clearing (10 cases); and pattern 3, no significant peaks with or without clearing (16 cases). There was a strong correlation between these 3 basic V600E ctDNA patterns and the 3 RECIST categories as assessed by the exact Kruskal-Wallis test (p = 0.0001) (Table 1). The overall survival of the three groups is shown in Fig. 1. Log rank test indicates a statistically significant difference among the survival curves (p = 0.02). A pairwise multiple comparison test (Holm-Sidak) showed a statistically significant survival difference between pattern 1 patients and pattern 2 patients (p=0.03), as well as between pattern 1 and pattern 3 patients (p=0.02), while there is no difference in survival between the pattern 2 and pattern 3 patients (p=0.59).
Table 1.
Correlation of BRAF V600E ctDNA dynamic patterns with RECIST category
| DETECTION PATTERNS* | CR | PR | NR | Totals |
|---|---|---|---|---|
| Pattern 1: Early peak with clearing | 9 | 4 | 0 | 13 |
| Pattern 2: Early peak without clearing | 0 | 4 | 6 | 10 |
| Pattern 3: No or minimal peak with or without clearing | 1 | 6 | 9 | 16 |
| 39 |
p = 0.0001 by exact Kruskal-Wallis test
Fig. 1.
Kaplan-Meier curves of overall survival stratified by BRAF V600E ctDNA dynamic patterns.
We recognized that any systemic treatment that patients received following TIL therapy may have impacted their overall survival. To be certain that the survival advantage of the patients that demonstrated pattern 1 ctDNA kinetics was not due to subsequent therapy, we reviewed their post TIL treatment. Of the 13 patients in this group, 9 achieved a complete response and of these, 7 received no additional therapy for melanoma and were alive and free of disease at last follow up. One of remaining 2 patients died after developing metastatic ovarian cancer, and the second expired after developing progressive melanoma and being lost to clinical followup. The remaining 4 patients achieved a partial response to cell transfer therapy. One of these patients was alive following resection of a brain metastasis (no additional treatment), and 3 had died of progressive melanoma. Two of the latter received additional TIL therapy, both surviving less than a year and the third received no additional therapy. Thus, the overall survival advantage of this group is unlikely to have been significantly affected by subsequent therapy.
Details of the individual patients are reported below according to pattern category.
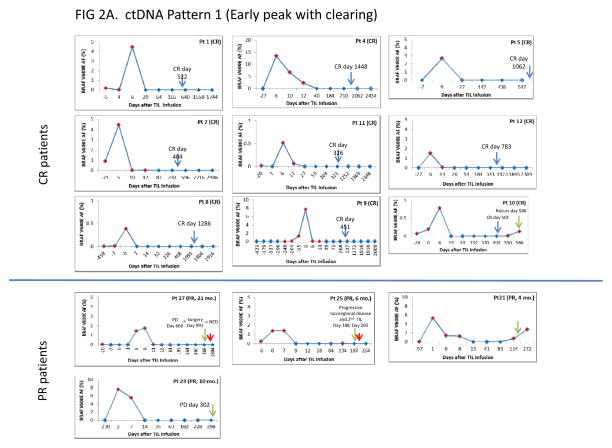
Pattern 1 (early peak, with clearing; Fig. 2A)
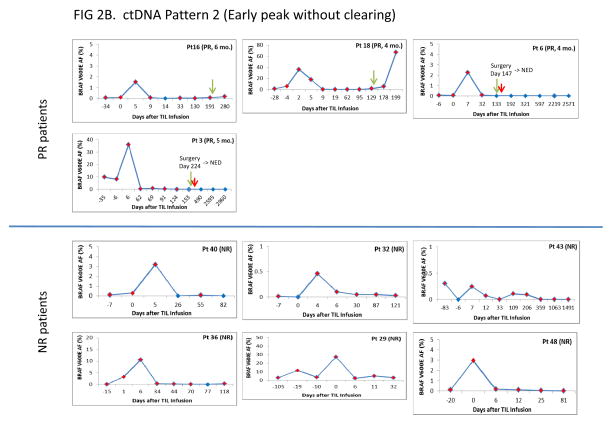
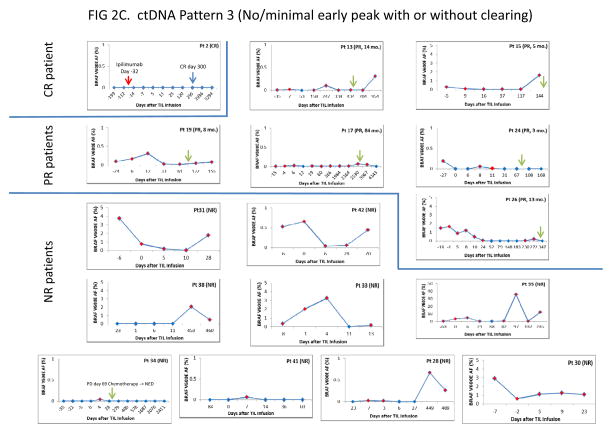
Fig. 2A, B, C.
Time courses of BRAF V600E ctDNA following TIL immunotherapy in 39 treated patients. A. Pattern 1 patients (Early peak with clearing). B. Pattern 2 patients (Early peak without clearing). C. Pattern 3 patients (No/minimal early peak with or without clearing). Each pattern category is subdivided according to RECIST category as described in the text, and shown on the left side of the figure. For each patient, time is shown on the X axis and BRAF V600E mutant allele frequency is shown on the Y axis. The mutant Allele Frequency is defined as the fraction of mutant alleles divided by the total number of mutant and wild type alleles expressed as a percentage, and was calculated by the ViiA7 Mutation Detector Software. The red diamonds indicate detection of BRAF V600E ctDNA. The blue diamonds indicate no detection at that time point. Blue arrows in Figure 2A indicate the date of confirmed CR by RECIST criteria. Green arrows in Figures 2A, 2B and 2C indicate the date when progressive disease was determined by RECIST criteria. Red arrows indicate a procedure or change in therapy.
Thirteen patients from the NCI TIL trials showed early BRAF V600E ctDNA peaks followed by clearing (pattern 1). Nine patients were complete responders (CR) and 4 were partial responders (PR) by standard RECIST criteria. No non-responder showed this pattern.
The mean time to CR in the 9 responders was 753 days (range 326–1448 days). All 9 patients showed early peaks of ctDNA followed by clearing of their serum (initial clearing 7–87 days; mean 33 days, median 27 days). Seven of the 9 CR patients showed a V600E ctDNA serum peak between days 5 and 9. The other two (cases 8 and 9) showed peaks at day 0 with initial clearing of their mutant DNA by days 39 and 7, respectively. The last positive V600E ctDNA measurement in all 9 patients was at day 14, (including the patient who showed initial clearing at day 87). Thus, it is likely that initial clearing is taking place even earlier. Interestingly, case 10 was declared a CR by RECIST just 49 days before he sero-converted to BRAF V600E positivity, which presaged his recurrence one month later.
All 4 of the Pattern 1 PR patients experienced early initial clearing of V600E ctDNA by day 26, similar to the 9 Pattern 1 CR patients. One of the four (case 27) progressed after 991 days with a single brain metastasis that was treated successfully by surgical excision. This patient has remained without evidence of disease (NED) throughout the followup period. The three other PR patients (cases 25, 21, and 23) progressed after 6, 4, and 10 months, respectively. BRAF V600E ctDNA was detected prior to clinical progression in 2 of the three cases (cases 21 and 25). The third patient (case 23) progressed approximately one month after the last evaluable serum sample.
Pattern 2 (Early peak, without clearing; Fig. 2B)
Ten patients showed early V600E ctDNA peaks without clearing of their serum. Four were PR patients who showed peak V600E ctDNA between days 2 and 7, but failed to clear BRAF V600E ctDNA for more than one consecutive time point prior to progression. Two of these patients (cases 6 and 3) progressed with single organ recurrences, were treated successfully by surgery, and both have remained clinically NED and V600E ctDNA negative for over 8 years. Six Pattern 2 patients were non-responders (cases 40, 32, 43, 36, 29, 48). Four of these 6 patients (cases 40, 32, 43, 36) showed peaks between days 4 and 6, and two (cases 29, 48) showed day 0 peaks.
Pattern 3 (No or minimal early peak, with or without clearing; Fig. 2C)
There were 16 patients that fell into this pattern category, including 1 CR patient, 6 PR patients, and 9 NR patients. The single CR patient (case 2) is unusual in that although he had a BRAF V600E positive melanoma with visceral metastases at trial entry, he did not show V600E ctDNA during throughout his treatment and followup. This patient is also unique among the CR patients in that he received partial treatment with ipilimumab 32 days before receiving his TIL therapy, which had to be interrupted due to toxicity.
Six PR patients failed to develop significant early peaks. Four of these patients (13, 15, 17, 19) failed to clear their serum of mutant DNA, while two (cases 24 and 26) showed clearing of their serum. In one of the later two, progressive disease was preceded by reappearance of V600E ctDNA.
Seven of the 9 NR patients (cases 31, 42, 30, 38, 28, 35, 33) did not develop V600E DNA peaks and all failed to clear their serum in their followup samples (Pattern 3). One of the 7 patients (case 38) who had primarily subcutaneous disease showed no mutant V600E ctDNA until 1 year following TIL infusion when the patient developed visceral metastases. The remaining 2 NR patients (cases 34 and 41) had barely detectable V600E ctDNA at only a single time point (days 4 and 7, respectively). These were not considered to be significant peaks due to their very low allele frequencies (<0.1).
DISCUSSION
Adoptive T-cell transfer is a promising new therapy for treatment of malignant melanoma with over 50% of patients showing objective responses and 20% developing complete durable remissions. A major challenge in the management of patients on TIL protocols is identifying patients who are not responding and those who are likely to progress at early time points during their therapy to enable rational decisions concerning the need to alter therapy. To date there are no effective biomarkers reported that are helpful in determining response.
In this study, we evaluated BRAF V600E ctDNA levels in 48 patients who received TIL immunotherapy at our institution between 2000 and 2007. To determine whether changes in V600E ctDNA could be used to gain predictive information about the likelihood of response, we focused on a subgroup of 39 patients that had samples at early time points. In so doing, we found a strong correlation between the presence or absence of an early serum peak of V600E ctDNA, and the likelihood of an objective response. Furthermore, patients that developed an early ctDNA peak and cleared their serum of V600E ctDNA (Pattern 1) after TIL infusion were much more likely to achieve a CR over the next 1–2 years, than those that did not clear their serum (Pattern 2). Patients that showed no peak of V600E DNA (Pattern 3) uniformly failed to achieve an objective response with one exception discussed below.
All patients who achieved a CR developed a peak of mutant V600E ctDNA early during their TIL treatment with one exception, and all showed early initial clearing of mutant DNA in their serum (median, day 27). Since the last positive ctDNA measurement in all CR cases was at day 14 post TIL infusion, the median time of clearing we report is likely to be conservative. The one CR patient who did not develop a V600E ctDNA peak was case 2. This patient received a partial course of anti-CTLA-4 treatment that was terminated one month before his TIL infusion due to the development of drug-related pancreatitis. It is possible that this course of ipilumumab, closely preceding TIL infusion, may have resulted in a reduction of the patient’s tumor load, preventing the detection of circulating V600E ctDNA at later time points.
All but one of the CR patients continued to show no evidence of V600E ctDNA during followup studies, as long as 8 years. The single exception was case 10 who achieved a CR by RECIST criteria at day 501 following TIL infusion, but who sero-converted to V600E positivity shortly thereafter, just 36 days before he experienced a clinical relapse. Reappearance of V600E ctDNA following confirmed clearing also heralded clinical progression in three other patients that were classified as PR (cases 21, 25, 26). These cases are illustrative of the potential of ctDNA analysis in monitoring relapse, and corroborate data from other studies in various tumor types (14, 16, 20).
Early peaks of mutant BRAF V600E ctDNA were detected in 64% (24/39) of cases, in all RECIST categories [CR (90%)>PR (57%) >NR (40%)], and in most patients the peak occurred between days 5 and 9. This early peak of ctDNA suggests that destruction of tumor by transferred TIL occurs very rapidly after TIL infusion. Six patients in all RECIST categories (2 CR, 2 PR, and 2 NR) showed peaks of V600E ctDNA at day 0. This is prior to a possible effect due to the infused TIL. It is not clear why these patients showed this early peak, but their kinetic patterns could have been influenced by idiosyncratic effects of the preparative chemotherapy or radiotherapy. Five of the 6 received TBI as part of their TIL preconditioning on day −1, and it may be that some patients have radiosensitive tumors that can lead to an early peak release of DNA. Day 0 peaks occurred in all RECIST response groups and patients with day 0 peaks did not have improved survivals compared with patients with peaks occurring following TIL infusion (data not shown), suggesting that whatever is responsible for this early peak does not determine response by itself, and likely involves complex factors.
Our analysis is limited in that it is a retrospective study of available stored serum samples from non-uniform time points, and patients were heavily pretreated with a variety of biologic and chemotherapeutic modalities prior to their TIL treatment. Although there are many studies in which serum samples have been analyzed, it is generally accepted that the preferable blood product for the analysis of ctDNA level is plasma, due to the potential dilutional effect caused by DNA release from lysed normal circulating cells (25).
Despite these limitations several important conclusions can be drawn from our data. First and probably most important is that patients who did not develop a peak of V600E ctDNA within the first 2 weeks following TIL infusion uniformly did not respond to their treatment, with the exception of patient 2 who had received antecedent anti-CTLA therapy. Clearly, identification of non-responders within two weeks of TIL infusion is highly desirable, and would allow clinicians to consider therapy modification before patients are declared treatment failures by traditional evaluation. Secondly, patients who developed a peak of V600E ctDNA, but did not clear ctDNA in the first two months of followup, all recurred. Such patients should be carefully watched for evidence of disease progression by both classical evaluation and rising ctDNA levels. Further studies will determine whether these patients should receive additional therapy and at what time during their treatment. Thirdly, those patients who develop early peaks and also clear their V600E ctDNA within 1–2 months are highly likely to develop a CR. This information could provide the patient with some additional level of comfort. Finally, two-thirds of TIL treated patients developed early peaks of V600E ctDNA, most within 10 days after TIL infusion, suggesting that the transferred lymphocytes are identifying their targets and are effective in killing.
ctDNA measurements are generally thought to reflect the overall tumor burden at any given time. As such ctDNA levels have been used to monitor the course of disease, response to therapy, progression of disease, and recurrence of disease (14–16, 18). Most investigators have assessed response to therapy by measuring the ctDNA level before the initiation of therapy and at a limited number of time points during, or after the therapy has been completed. In this study, the availability of multiple serial samples at early time points allowed us to visualize the efficacy of the TIL therapy in real time. The initial burst of BRAF V600E ctDNA levels almost certainly reflects on-target recognition and subsequent killing of melanoma cells resulting in the release of DNA into the circulation, as the time course is similar to that seen in mouse tumors treated with TILs (26). Thus, increases in the level of ctDNA are to be expected initially in the first week after therapy as the TIL find and destroy their target, with subsequent rapid decrease and clearing of ctDNA in patients who go on to achieve a CR. These data suggest that the impact of cell transfer therapy occurs rapidly after cell infusion.
In the current retrospective study we did not have sufficient material to further investigate the biological conclusion suggesting that peak tumor killing occurs in the first 5–10 days following TIL infusion. If peak ctDNA is, in fact, a reflection of peak cell death, one might expect that other biomarkers of cell death such as LDH, high mobility group box-1 (HMGB1) protein, and S100B, may also be correlated with the peak release of mutant BRAF DNA from the melanoma cells. These additional biomarkers may provide complementary information in evaluating TIL effectiveness. As a melanoma-associated marker, S100b would be of particular interest to follow, however, S100b elevations have also been associated with kidney and liver injury, and have also been postulated to occur with dendritic cell activation (27, 28). Unlike BRAF V600E ctDNA, none of these cellular proteins are tumor specific, and confounding factors affecting the patient’s clinical course could affect any of these analytes. Furthermore, none of these biomarkers are likely to have both the high specificity and sensitivity of melanoma specific ctDNA that is needed to monitor persistent tumor or tumor recurrences.
In conclusion, this study is the first one to provide data indicating that early kinetic changes in ctDNA levels may be predictive of the anti-tumor activity of tumor infiltrating lymphocytes, and of the long-term outcome of the patients undergoing this therapy. Prospective studies involving larger numbers of patients will be required to fully assess the ability of ctDNA to predict response (or lack of response), prior to detecting significant clinical or radiographical changes in patients receiving this therapy. The findings in this study may be generalizable to other T-cell directed and immune mediated therapies.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Tumor infiltrating lymphocyte (TIL) immunotherapy is a potentially life-saving treatment for patients with stage IV malignant melanoma MM, but there are currently no early response biomarkers that can identify and predict patients who will respond to this therapy. The current study suggests that circulating tumor-derived DNA (ctDNA) can provide early information to assist in identifying responding and non-responding patients as early as two weeks after initiation of therapy, potentially allowing clinicians to modify or change treatment protocols accordingly. These findings may be generalizable to other T-cell transfer immunotherapeutic approaches. Additionally, this study provides valuable information on the tempo of the anti-tumor response to cell transfer therapy.
Acknowledgments
We wish to acknowledge Mr. Donald White and Dr. Seth Steinberg who assisted with the statistical analysis of the data, and Dr. Hye-Jung Chung for critically reading the manuscript.
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015 Jun 25;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014 Apr 1;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010 May 1;16(9):2646–55. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 5.Itzhaki O, Levy D, Zikich D, Treves AJ, Markel G, Schachter J, et al. Adoptive T-cell transfer in melanoma. Immunotherapy. 2013 Jan;5(1):79–90. doi: 10.2217/imt.12.143. [DOI] [PubMed] [Google Scholar]
- 6.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012 Dec 15;18(24):6758–70. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011 Jul 1;17(13):4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Patel KM, Tsui DW. The translational potential of circulating tumour DNA in oncology. Clin Biochem. 2015 Oct;48(15):957–61. doi: 10.1016/j.clinbiochem.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015 Jan;61(1):112–23. doi: 10.1373/clinchem.2014.222679. [DOI] [PubMed] [Google Scholar]
- 11.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014 Feb 19;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 2014 Apr;20(4):430–5. doi: 10.1038/nm.3511. [DOI] [PubMed] [Google Scholar]
- 13.Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O’Connell A, Messineo MM, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014 Mar 15;20(6):1698–705. doi: 10.1158/1078-0432.CCR-13-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008 Sep;14(9):985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012 May 30;4(136):136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 16.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013 Mar 28;368(13):1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 17.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, et al. A landscape of driver mutations in melanoma. Cell. 2012 Jul 20;150(2):251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinozaki M, O’Day SJ, Kitago M, Amersi F, Kuo C, Kim J, et al. Utility of circulating B-RAF DNA mutation in serum for monitoring melanoma patients receiving biochemotherapy. Clin Cancer Res. 2007 Apr 1;13(7):2068–74. doi: 10.1158/1078-0432.CCR-06-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yancovitz M, Yoon J, Mikhail M, Gai W, Shapiro RL, Berman RS, et al. Detection of mutant BRAF alleles in the plasma of patients with metastatic melanoma. J Mol Diagn. 2007 Apr;9(2):178–83. doi: 10.2353/jmoldx.2007.060135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray ES, Rizos H, Reid AL, Boyd SC, Pereira MR, Lo J, et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget. 2015 Sep 22; doi: 10.18632/oncotarget.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanmamed MF, Fernandez-Landazuri S, Rodriguez C, Zarate R, Lozano MD, Zubiri L, et al. Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem. 2015 Jan;61(1):297–304. doi: 10.1373/clinchem.2014.230235. [DOI] [PubMed] [Google Scholar]
- 22.Santiago-Walker A, Gagnon R, Mazumdar J, Casey M, Long GV, Schadendorf D, et al. Correlation of BRAF Mutation Status in Circulating-Free DNA and Tumor and Association with Clinical Outcome across Four BRAFi and MEKi Clinical Trials. Clin Cancer Res. 2015 Oct 7; doi: 10.1158/1078-0432.CCR-15-0321. [DOI] [PubMed] [Google Scholar]
- 23.Lipson EJ, Velculescu VE, Pritchard TS, Sausen M, Pardoll DM, Topalian SL, et al. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer. 2014;2(1):42. doi: 10.1186/s40425-014-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsao SC, Weiss J, Hudson C, Christophi C, Cebon J, Behren A, et al. Monitoring response to therapy in melanoma by quantifying circulating tumour DNA with droplet digital PCR for BRAF and NRAS mutations. Sci Rep. 2015;5:11198. doi: 10.1038/srep11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vallee A, Marcq M, Bizieux A, Kouri CE, Lacroix H, Bennouna J, et al. Plasma is a better source of tumor-derived circulating cell-free DNA than serum for the detection of EGFR alterations in lung tumor patients. Lung Cancer. 2013 Nov;82(2):373–4. doi: 10.1016/j.lungcan.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Palmer DC, Chan CC, Gattinoni L, Wrzesinski C, Paulos CM, Hinrichs CS, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci U S A. 2008 Jun 10;105(23):8061–6. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamberg AP, Korse CM, Bonfrer JM, de Gast GC. Serum S100B is suitable for prediction and monitoring of response to chemoimmunotherapy in metastatic malignant melanoma. Melanoma Res. 2003 Feb;13(1):45–9. doi: 10.1097/00008390-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Molina RNJ, Filella X, Castel T, Ballesta AM. S-100 protein serum levels in patients with benign and malignant diseases: false-positive results related to liver and renal function. Tumour Biol. 2002 Jan-Feb;23(1):39–44. doi: 10.1159/000048687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.