Abstract
Neutrophils are the key cells of our innate immune system mediating host defense via a range of effector functions including phagocytosis, degranulation, and NETosis. For this, they employ an arsenal of anti-microbial cargoes packed in their readily mobilizable granule subsets. Notably, the release of granule content is tightly regulated; however, under certain circumstances, their unregulated release can aggravate tissue damage and could be detrimental to the host. Several constituents of neutrophil granules have also been associated with various inflammatory diseases including cancer. In cancer setting, their excessive release may modulate tissue microenvironment which ultimately leads the way for tumor initiation, growth and metastasis. Neutrophils actively infiltrate within tumor tissues, wherein they show diverse phenotypic and functional heterogeneity. While most studies are focused at understanding the phenotypic heterogeneity of neutrophils, their functional heterogeneity, much of which is likely orchestrated by their granule cargoes, is beginning to emerge. Therefore, a better understanding of neutrophil granules and their cargoes will not only shed light on their diverse role in cancer but will also reveal them as novel therapeutic targets. This review provides an overview on existing knowledge of neutrophil granules and detailed insight into the pathological relevance of their cargoes in cancer. In addition, we also discuss the therapeutic approach for targeting neutrophils or their microenvironment in disease setting that will pave the way forward for future research.
Keywords: Neutrophil-derived granule cargoes, Effector functions, Tumor-associated neutrophils, Cancer therapeutics
Introduction
Neutrophils are the most abundant circulating immune cell types, constituting around 50–70% of all leukocytes in peripheral blood. They are constantly produced in the bone marrow through a process called granulopoiesis [1]. Interestingly, more than 50% of bone marrow is committed to the production neutrophils [2]. Being the first one to migrate at the site of infection, they are regarded as the first line of defense against invading pathogens. Neutrophils actively migrate from hematopoietic tissue and pass through the vasculature to reach the target site [3]. They exert their anti-pathogenic or pro-inflammatory roles via a wide range of effector functions, including phagocytosis, degranulation, and neutrophil extracellular trap (NET) formation [4]. To execute these effector functions, neutrophils are heavily equipped with a stock of toxic, anti-microbial weapons (anti-microbial peptides and lytic enzymes) stored in their distinct granule subsets. Granules are the major attribute of neutrophil effector functions and are divided into several subsets depending upon their protein content and synthesis during granulopoiesis [5]. The controlled mobilization and release of granule content allow the transformation of neutrophils from inactive circulating cells to active effector cells of the innate immune system. Following an encounter with pathogenic targets, neutrophils are primed and activated for a swift release of their granule weaponry [6]. Neutrophils can destroy pathogens intracellularly by releasing granule content into phagosomes or extracellularly into the extracellular milieu in response to stimuli which is tightly regulated [7]. On the other hand, excessive release of granule content has been implicated in collateral tissue damage [8]. Traditionally, it was considered that neutrophils are present only during the initial phase of inflammation and their sole function is to eliminate a broad spectrum of invading pathogens. However, several reports have now demonstrated their functions beyond roles in eliminating infection [1, 9]. Emerging studies also suggest their persistent influx, hyper-activation, and excessive degranulation in the pathogenesis of several maladies, including chronic respiratory diseases, rheumatoid arthritis (RA), autoimmunity, and cancer [10]. Prolonged recruitment and activation of neutrophils can reflect a state of chronic inflammation, which is now a well-recognized hallmark of cancer. Importantly, cancer patients show remarkable increase in peripheral blood neutrophil count and their infiltration in tumors. Also, substantial reports suggest diverse phenotypic and functional heterogeneity of neutrophils in cancer. Neutrophil granules are the decisive mediators of neutrophil functionality and are regarded as double-edge swords [11]. Therefore, an understanding of granule cargoes, the mode of release, and their role is critical in understanding neutrophil function in the context of cancer. Here, we comprehensively revisit the expanding roles of neutrophil-derived granule cargoes in cancer. Further, we also highlight the potential of neutrophils as substantial therapeutic target in cancer.
Neutrophil dynamics: production, egression, and migration to the target site
Granulopoiesis
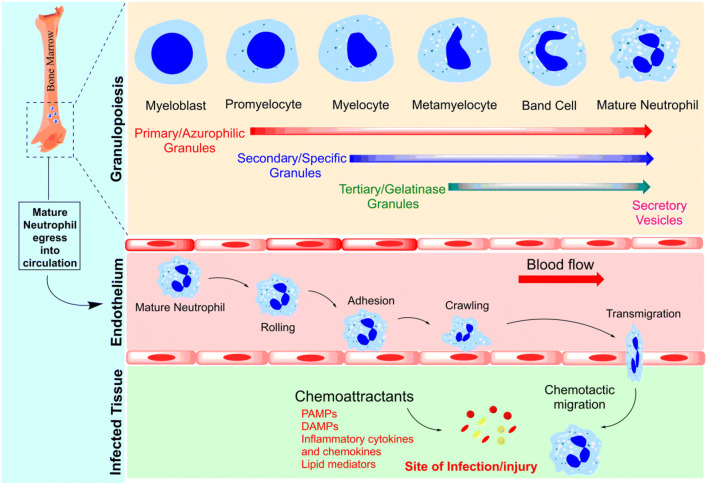
Neutrophils, eosinophils, and basophils collectively constitute a group of white blood cells defined as granulocytes. The process of production and development of granulocytes in the bone marrow is called granulopoiesis [12]. Neutrophils are the most plentiful cell population in the peripheral blood, with approximately 1011 cells produced per day [13]. They develop in the bone marrow from hematopoietic stem cells through a process involving multiple successive stages of neutrophil precursors [14] which includes myeloblast, promyelocyte, myelocyte, metamyelocyte, band cell, and segmented granulocyte [15]. The mature segmented neutrophils are then finally mobilized from bone marrow to the circulation (Fig. 1). These circulating neutrophils are non-dividing cells and have a half-life of a few hours in peripheral blood [14]. In steady-state or normal conditions, only a small percentage, out of total neutrophils residing in the bone marrow, is released into the circulation. However, during inflammation or infection, their production and release increase rapidly in a process called emergency granulopoiesis. Various transcription factors play crucial roles during neutrophil maturation, such as E26 transformation-specific family transcription factor PU.1, C/EBPα (CCAAT enhancer binding protein α), C/EBPβ, and C/EBPε. PU.1 plays a decisive role in monocyte differentiation, while C/EBP protein promotes granulocyte differentiation. C/EBPα is an integral factor in the earliest stages of granulocyte differentiation [16]. It is also considered as a master regulator of steady-state granulopoiesis because it limits the proliferation via inhibiting expression of cyclin-dependent kinases (cdk2 and cdk4) and c-Myc, whereas C/EBPβ is known to promote emergency granulopoiesis, as it does not inhibit the expression of cdk2, cdk4, or c-Myc, thus allowing proliferation of granulocytic progenitors and increasing neutrophil count in peripheral blood. Several cytokines can also modulate neutrophil production, such as G-CSF (granulocyte colony-stimulating factor), GM-CSF (granulocyte-macrophage colony-stimulating factor), IL-6 (interleukin-6), and IL-3. Increased levels of these cytokines can switch steady-state granulopoiesis to emergency granulopoiesis [17]. Among them, G-CSF is known to be the primary regulator of steady-state as well as emergency granulopoiesis. It also influences the survival, maturation, and proliferation of the cells from the granulocyte lineage. Under normal conditions, the circulating level of G-CSF is very low, but this may increase during infection, inflammation or stress. This increased G-CSF activates JAK-STAT signaling pathway and further stimulates granulocyte differentiation in concert with C/EBPβ [18, 19].
Fig. 1.
Neutrophil granulopoiesis and recruitment to the target site. Granulopoiesis is characterized by the sequential formation of neutrophil granules. Myeloblast is the first cell of committed granulopoiesis that further differentiates into promyelocyte, myelocyte, metamyelocyte, band cell, and finally into mature neutrophil. Azurophilic or primary granules are synthesized at the promyelocytic stage. Specific or secondary granules are synthesized during the myelocyte stage and then gelatinase or tertiary granules are formed during the metamyelocyte stage. Finally, secretory vesicles (SVs), which are exocytoseable membrane-bound organelles, are formed at the late stage of neutrophil maturation. Mature neutrophils now egress from the bone marrow into circulation. Upon sensing any chemoattractant, mature neutrophils actively migrate from circulation to the site of infection or injury in a process called extravasation that is a multi-step process including rolling, adhesion, crawling, and transmigration
Neutrophil egress from primary niche
Neutrophils are matured and stored in the bone marrow until they are released into the circulation [13]. Their release is tightly controlled as only 1–2% of mature neutrophils are found in the circulation while their major population remains stored in the bone marrow [20]. Various factors can trigger their release from the bone marrow, such as cytokines, chemokines, and inflammatory stimuli. Further, their mobilization depends on various receptors expressed on the surface of neutrophils such as CXCR4, CXCR2, GCSF-R, and their ligands on stromal cells. Among all, CXCR4 plays a crucial role in neutrophil retention in the bone marrow [21]. Deletion in CXCR4 shows increased release of neutrophils into the circulation, whereas high levels of CXCR4 and its ligand hold the neutrophil population in the bone marrow [22]. The major ligand for CXCR4 is stromal-derived factor 1 (SDF-1)/CXCL12, a CXC chemokine that is produced constitutively by stromal and endothelial cells of bone marrow [23]. The interaction between SDF-1 and CXCR4 maintains the neutrophil homeostasis in the bone marrow and circulation [24]. G-CSF is a principal cytokine and considered to be a potent stimulus which acts in several ways to induce neutrophil production and their release. It is known to promote neutrophil mobilization via downregulating SDF-1, thereby disrupting SDF-1 and CXCR4 interaction. In a study, the treatment of mice with G-CSF showed decreased SDF-1 production with high neutrophil mobilization from bone marrow. G-CSF treatment also reduced the surface expression of CXCR4 on myeloid cells. [25]. IL-23 produced by macrophages also increases neutrophil release through G-CSF, and therefore, the regulation of IL-23 production inhibits neutrophil release and thus maintains homeostasis of neutrophil number [26]. CXCR2 signaling on the other hand acts as CXCR4 antagonist. Upregulation of CXCR2 expression promotes neutrophil mobilization from bone marrow to the circulation [27]. Moreover, inflamed peripheral tissues produce several mediators that can influence mobilization of neutrophils from the bone marrow. For instance, in chronic peritonitis, LIX (CCL5) and MIP-2 act as ligand for CXCR2 and have shown to promote neutrophil release [28]. Similarly, in immune complex–induced arthritis, neutrophils present in the joint release LTB4, MIP-1a (CCL3), MIP-2, and IL-1b which act as potent chemoattractants [29]. Moreover, MMPs (metalloproteinases) such as MMP8 and MMP9 can cleave collagen to a peptide proline-glycine-proline (PGP), which can activate CXCR2 on neutrophils [30]. Besides this, many tumor cells can produce CXCR2, which has been associated with tumor migration and invasion [31].
Migration to the battle fronts
Neutrophils are the first cells to arrive at the site of infection or inflammation to eliminate invading pathogens. The inflamed site or damaged tissue generates a range of stimuli, such as PAMPs (pathogen-associated molecular patterns), DAMPs (damage-associated molecular patterns), lipid mediators, inflammatory cytokines, and chemokines, to initiate neutrophil migration [32, 33]. To reach at the site of inflammation, neutrophils must sense the inflammatory cues and extravasate from blood vessel into the tissues [34]. The extravasation is a multi-step process that involves rolling, adhesion, crawling, and trans-endothelial migration [35] (Fig. 1). In response to inflammatory signal, endothelial cells express P-selectin and E-selectin which are cell surface adhesion molecules. These molecules then bind to the glycosylated ligands expressed on the surface of neutrophils [36]. Neutrophils express multiple receptors such as cytokine receptors, pattern recognition receptors (PRRs), G protein-coupled receptors (GPCRs), adhesion receptors, and Fc receptors that can sense the pro-inflammatory mediators [37]. The interaction of selectins with their ligands allows the rapidly moving neutrophils to be captured from the circulation and stick to the endothelium, which is known to be a temporary interaction or fast rolling. Once the cells sense the inflammatory signal within the tissue and microvasculature, there is slowing down of the rapidly moving cells, also called slow rolling. The activated neutrophils now tightly stick on to the endothelium via spreading their pseudopods [33, 38, 39]. Neutrophils constitutively express integrins such as LFA-1(lymphocyte function-associated antigen 1) and MAC-1 (macrophage receptor 1), which bind to ICAM-1 (intercellular adhesion molecule)-1 and ICAM-2. The interaction between ICAM-1 and MAC-1 mediates the crawling of neutrophils within blood vessels. Crawling helps neutrophils to seek the most appropriate site for transmigration [40]. Trans-endothelial migration or diapedesis is the final step wherein cells breach the endothelial layer. Neutrophils can either migrate between endothelial cells (paracellular route) or through the endothelial cells (transcellular route); however, paracellular route is considered to be the efficient one [41, 42]. Neutrophil integrins such as LFA-1 and MAC-1 interact with endothelial adhesion molecules ICAM-1 and VCAM-1 (vascular cell adhesion molecule)-1 to facilitate transmigration. In addition, endothelial junctional adhesion molecules such as JAM-1, JAM-2, JAM-3, CD31, and CD99 also play crucial roles in trans-endothelial migration [43]. After passing through the endothelial cell barrier, neutrophils breach the pericyte layer and basement membrane with the help of proteases such as elastase, MMP8, and MMP9 stored in the granule subsets of neutrophils [44, 45].
Interestingly, the cell adhesion molecules also play an important role in many inflammatory processes including cancer and therefore represent key therapeutic targets [46]. Tumor cells can induce expression of E-selectin on vascular walls through the release of cytokines that stimulate E-selectin gene transcription. In addition, E-selectin also promotes tissue-specific metastasis of carcinomas. High levels of soluble form of E-selectins have been detected in serum of patients suffering from bronchial asthma, eczema, psoriasis, and allergic dermatitis, all examples of chronic inflammation [47]. During inflammation, the cytokines such as IFNγ, IL-1β, and TNFα can increase the expression of ICAM-1 and VCAM-1 [48]. Similarly, overexpression of junction adhesion molecules is also reported in many cancer types [49].
Neutrophil granules: major tools of neutrophil effector functions
Granule subsets: the vast armory
The versatile functions of neutrophils are dedicated to the different cytoplasmic granules of a mature neutrophil. They are equipped with three unique types of granules subsets namely primary (azurophilic) granules, secondary (specific) granules, and tertiary (gelatinase) granules [11]. These granules are classified as primary, secondary, and tertiary due to the fact that they are formed at different stages of neutrophils maturation (Fig. 1) [50]. The primary or azurophilic granules are the first one to be synthesized at the promyelocytic stage. They are also called as peroxidase-positive granules due to the abundance of oxidant-producing enzymes, myeloperoxidase (MPO). In addition, they also store the most toxic and proteolytic mediators, including elastase, cathepsin G, proteinase 3, azurocidin, and defensins. These proteases are capable of degrading a vast range of ECM components such as elastin, fibronectin, and type IV collagen. Next in the line are secondary or specific granules synthesized during the myelocyte stage. They include collagenase, gelatinase, lactoferrin, lysozyme, lipocalin/NGAL, and membrane receptors. Tertiary or gelatinase granules are formed during the metamyelocyte stage and include gelatinase, cathepsin, acyl transferase, and collagenase. Notably, secondary and tertiary granules share some common granule contents that are discriminated based on their densities. They are also called as peroxidase-negative granules as they lack MPO [51]. In addition, there is another granule subset called as secretory vesicles (SVs) that are actually exocytoseable membrane-bound organelles. SVs are formed at late stage of neutrophil maturation and play an important role in delivering membrane-associated receptors to cell surface [15]. Unlike the rest three granule subsets, SVs do not acquire its proteins from Golgi compartment and completely rely upon endocytosis for their formation [52]. SVs derived proteins are responsible for neutrophil extravasation from the vasculature and also for phagocytosis [5]. Interestingly, upon external stimulations, neutrophil granules are mobilized in the reverse order of their formation. SVs formed at last are the first one to be mobilized to the cell surface. Next, in the order of mobilization, are the tertiary or gelatinase granules followed by secondary or specific granules and, finally, the azurophilic granules. Primary granules majorly release their contents into phagolysosomes and undergo limited extracellular release of their toxic contents. This delay in exocytosis upon external stimulations could be due to the fact that they contain a huge cargo of toxic mediators that would have detrimental effects on surrounding tissues as well. On the contrary, secondary and tertiary granules are exocytosed most readily [53, 54].
Effector functions of neutrophils: mechanisms of host protection
Once reached to the site of infection, activated neutrophils adopt diverse mechanisms to eliminate the invading pathogens. These mechanisms include phagocytosis, degranulation, and NETs (Fig. 2). Phagocytosis is an endocytosis process, wherein phagocytic cells such as macrophages and neutrophils engulf the microbes [55]. Phagocytic cells recognize the invading microbes via the interaction of neutrophil surface receptors with the opsonic receptors present on the surface of the microbes [56]. Neutrophils employ two different receptor classes to perform phagocytosis. The first one is Fcγ receptors that include FcγRIIA (CD32) and FcγRIIIB (CD16). The second one is the complement receptors, CR1 (CD35) and CR3 (or CD11b/CD18 integrin) [57]. Microbes are internalized and enclosed within a part of the cell membrane in the form of a vacuole called a phagosome. To initiate the killing process, there is fusion of these phagosomes with the granules within the cells which is termed as phagosome maturation [58]. Phagocytosis triggers the process of oxidative burst in neutrophils which is a process of generation of reactive oxygen species (ROS) in order to kill internalized microbes. The activated neutrophils are highly efficient in generating ROS by utilizing the NADPH oxidase complex [59]. In resting neutrophils, the NADPH complex is disassembled and remains dormant. Phagocytic receptors that mediated downstream signaling trigger the assembly and activation of the NADPH complex on phagosomes [60]. Chronic granulomatous disease (CGD) is a rare genetic disorder that reveals the importance of NADPH oxidase. The disease involves a defect in the gene encoding one of the NADPH oxidase subunits resulting in the inactive form of NADPH oxidase. Therefore, CGD patients suffer from severe fungal and bacterial infections [61]. Activated NADPH complex catalyzes the formation of superoxides via transferring electrons from NADPH to O2 [62]. Superoxides can undergo further reactions to produce vast range of ROS, including H2O2 and HOCl. MPO, a heme protein localized in the azurophilic granules of neutrophils, catalyzes the reaction to generate HOCl from H2O2. HOCl is a highly toxic oxidant used by neutrophils to kill a wide range of pathogens [63].
Fig. 2.
Effector functions of neutrophils. Once reaching the battle front, neutrophils adopt diverse mechanisms to destroy pathogens, such as phagocytosis, degranulation, and NETosis. In phagocytosis, the pathogen is ingested into phagocytic vacuoles called phagosomes which become phagolysosome upon maturation. Further in the phagolysosome, the pathogen is destroyed by the action of degrading enzymes. In degranulation, neutrophils release their toxic cargo, stored in the granule subsets. During NETosis, DNA fibers equipped with granule cargo are released in the form of neutrophil extracellular traps (NETs) to entrap and kill the large microbes that cannot be ingested
During superoxide production by activating NADPH oxidase complex, the granules stored in the neutrophils fuse with the membrane and release their enzymatic contents in a process called degranulation. It is a receptor-mediated process adopted by neutrophils to kill the invading microbes via the release of toxic mediators (proteases, anti-microbial peptides and inflammatory substances [64]. Among the vast library of primary granule contents, neutrophil elastase (NE), cathepsin G, and proteinase 3, collectively called as neutrophil serine proteases, are critical for host defense. They effectively act against bacterial infections via direct killing of bacterial cells, cleaving host proteins or attenuating the bacterial virulence factors. Lactoferrin present in the secondary granules also possesses a broad range of anti-bacterial activities such as blocking the entry and adhesion of bacterial pathogens on host cells [56]. Similarly, defensins can also exert anti-microbial activities via disrupting the target cell membranes and neutralizing a range of enzymes secreted by bacteria. It can also inhibit viral transcription by blocking the intracellular signaling cascade [65]. Moreover, MMPs such as collagenases and gelatinases stored in secondary and tertiary granules are potent ECM degrading agents which facilitate neutrophils migration through basement membranes [66]. These granule contents are rich in tissue destructive proteases, and their excessive release can also cause severe tissue damage. Degranulation, therefore, is a tightly regulate process which is initiated once the receptor in the phagosomal membrane get activated and signal the granules for their mobilization and release of cargoes [67]. The molecular basis for granule exocytosis remains to be fully understood, but SNARE proteins including SNAP-23, VAMP 2, and syntaxins 4 seem to play an important role [66]. Earlier, neutrophils were known to destroy pathogens by phagocytosis, which is the engulfment of microbes or by the release of anti-microbial contents. But it was in 2004 that NETosis was recognized as another excellent method of pathogen killing adopted by neutrophils. NETs are extracellular fibers or meshes equipped with decondensed DNA, histones, and granular proteins of neutrophils such as MPO, elastase, and lactoferrin [68, 69]. Some reports propose that NETosis is adopted by neutrophils to encounter large pathogens that cannot be phagocytosed [70]. NETs entrap and neutralize microbes to promote their extracellular killing. NETosis has been categorized as suicidal NETosis and vital NETosis. Neutrophils undergo several morphological changes during suicidal NETosis which include alterations in the nucleus structure, increased permeability of nuclear and granular membranes, and inactivation of histones. These alterations then leads to chromatin expansion, mixing of chromatin and granule content, and release of NETs into the extracellular spaces through permeable plasma membrane [71, 72]. In vital NETosis, neutrophils remain functionally active after NET release [73]. NETosis can be triggered by PAMPs from microbes, auto antibodies, inflammatory mediators [64], and mitogenic stimuli like PMA (phorbol 12-myristate 13-acetate) and concanavalin A [74]. PAMPs triggered NETosis require active NADPH oxidase complex [75]; otherwise, ionomycin- and nicotine-triggered NADPH-independent NETosis is also reported which rely on mitochondrial ROS [76, 77].
Neutrophils in pathogenesis: actions against the host
Neutrophils in prevalent chronic diseases
Neutrophil number, infiltration, and activation have to be tightly controlled as dysregulated release of toxic mediators can lead to severe tissue damage and inflammation. Altered neutrophil functioning has been the cause of various diseases such as infection, cardiovascular diseases, respiratory diseases, and neuroinflammation [26] (Fig. 3). Sepsis is a life-threatening condition resulting from heightened immune response and severe tissue damage. Neutrophil reprogramming during sepsis leads to diminished recruitment of activated neutrophils at the infection site and concomitant increase in their number in circulation with enhanced release of effector molecules, thus causing tissue damage and several organ failure [78]. Neutrophils are also involved in various respiratory disorders such as chronic obstructive lung disease (COPD), adult respiratory distress syndrome (ARDS), and cystic fibrosis. Patients suffer from bronchial inflammation due to elevated recruitment of neutrophils and release of neutrophil-derived proteolytic mediators [79–82]. Atherosclerosis, a condition of narrowing of arteries due to accumulation of plague, is now considered as an inflammatory disease. Recent studies suggest an important contribution of neutrophils in triggering inflammatory response in atherosclerosis [83]. Ionita and colleagues reported a high number of neutrophils in human atherosclerotic plaques [84], whereas some studies have also reported the involvement of NETs in atherosclerosis [85]. Neutrophils migrate early to the ischemic site and are known to promote inflammation and release of proteolytic granule contents [86–88]. NETs are also thought to be involved in the thrombus formation in venous and arterial systems in conditions such as sepsis and cancer [89, 90]. In recent years, neutrophils have emerged as important participants in various systemic autoimmune diseases also. Rheumatoid arthritis (RA) is a chronic inflammatory disorder that leads to bone erosion, chronic synovitis, and joint deformity. Neutrophils are found in flocks in the synovial fluid of the affected joints of RA patients [91]. Similarly, circulating anti-citrullinated peptide autoantibodies (ACPAs) are also known to contribute to RA pathogenesis. Neutrophil-derived NETs contain citrullinated histones and serve as potential sources of autoantigens that can trigger the production of autoantibodies in RA [92]. Psoriasis is another chronic autoimmune disease characterized by skin lesions or patches. Overstimulation of neutrophils along with other immune cells such as dendritic cells, T cells, fibroblasts, melanocytes, and mast cells has been reported in psoriasis pathogenesis [93–96]. Neutrophils mobilize to the psoriatic site and trigger oxidative burst, degranulation, and NETosis, thus contributing to disease pathogenesis. Neutrophils isolated from psoriasis patients showed high ROS release compared with healthy individuals, whereas depletion of neutrophils and suppression of oxidative burst significantly relieved psoriasis patients [97]. Involvement of NETs has been reported in systemic lupus erythematosus, an autoimmune disorder wherein the body’s immune system attacks its own tissues and organs system [98, 99]. Anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis is inflammation of blood vessels in which both arteries and veins are affected. ANCA is directed against MPO and proteinase 3, which are present in the primary granules of neutrophils [100]. ANCA-stimulated neutrophils also release NETs, which are known to promote vasculitis pathogenesis [101]. Similarly, neutrophil-mediated pathogenesis has been reported in mice models of neuroinflammatory and neurodegenerative diseases such as multiple sclerosis and Alzheimer disease. Increased release of NETs is observed in the circulation of multiple sclerosis patients which results in disruption of the blood-brain barrier [102]. Moreover, high accumulation of neutrophils and NETs release has been reported around amyloid-β plaques found in brains of Alzheimer patients [103, 104].
Fig. 3.
Neutrophils in health and diseases. Neutrophils act as first line of defense and are equipped with diverse mechanisms such as phagocytosis, degranulation, and NETosis to eliminate pathogens. However, abnormal neutrophil count and function are associated with multiple diseases affecting vital organs ranging the brain, lungs, heart, liver, kidney, intestine, and bones
Neutrophils in cancer: not-so-neutral
Chronic inflammation is now well recognized as a major hallmark of cancer, and neutrophils are believed to be a central component of this process. At the site of infection, once neutrophil function is over, their clearance is essential for resolution of inflammation to maintain tissue homeostasis. But, failure in the resolution machinery and prolonged neutrophil accumulation can damage the host tissue and reflect a state of chronic inflammation [105]. According to traditional immunology, the sole function of neutrophils was in host defense, in immune modulation, and in tissue injury [12]. However, emerging research negates this traditional idea and proved that these cells function in a more complex way and display clear phenotypic and functional heterogeneity [106, 107]. Emerging studies have well documented the role of neutrophils in various chronic inflammatory disorders including cancer [107]. Though, their role in cancer was earlier ignored due to their short life span but several recent reports validate their dominant pro-tumoral role [108]. Intriguingly, an increasing number of clinical as well as preclinical observations have reported frequent accumulation of neutrophils in tumors. In clinical studies, a bulk of correlation reports have suggested poor prognosis of patients with high peripheral blood neutrophil count and high neutrophil infiltration at tumor sites leading to high neutrophil-to-lymphocyte (NLR) ratio. This state of neutrophilia has been principally observed in patients with advanced cancer [109]. For instance, in human HNSCC, enhanced neutrophil infiltration was observed in more aggressive tumors than in less aggressive tumors [110]. Also, high NLR ratios were correlated with low survival probability in patients suffering from liver, lung, colon, and pancreatic cancers [111–114]. Importantly, NLR has been introduced as a simple and inexpensive biomarker for many tumor types, including colorectal cancer [115], breast cancer [116], non-small cell lung cancer [117], and hepatocellular carcinoma [118]. Neutrophils present in the tumor microenvironment are referred to as tumor-associated neutrophils (TANs), which are further categorized as anti-tumoral (N1 type) and pro-tumoral (N2 type) and thus regarded as double-edged sword in cancer. The anti-tumoral role of neutrophils is governed by either direct tumor cell killing or indirect killing by activation of other immune cells. In direct killing, neutrophils interact with tumor cells in ADCC-dependent manner. After the physical contact, neutrophils secrete cytotoxic mediators, such as H2O2 to induce apoptosis within tumor cell [119]. Also, neutrophils can directly inhibit tumor cell proliferation and survival through production of TRAIL, a TNF superfamily member that binds to its receptor in tumor cells and induces apoptosis [120]. In indirect killing, neutrophils release various pro-inflammatory or immunostimulatory cytokines, such as TNF-α, IL-12, and chemokines such as CCL3, CXCL9, and CXCL10. These factors further facilitate recruitment and activation of other immune cells including CD8+ T cells, B cells, NK cells, and dendritic cells [121]. On the contrary, the pro-tumoral neutrophils promote tumor invasion, metastasis, and angiogenesis via releasing various factors such as oncostatin M (OSM) [122], hepatocyte growth factor (HGF) [123], neutrophil elastase (NE), and matrix metalloproteinases (MMPs) [124]. They also show strong immunosuppressive activity by releasing high levels of arginase which in turn suppress the activity of CD8+ T cells [125]. Studies conducted in mouse lung carcinoma and mesothelioma models suggest that TANs show N1 phenotype at the initial stage of tumor growth, whereas they convert into N2 phenotype with tumor progression [126]. Fridlender et al. suggested an important role of factors produced by cancer cells or other immune cells in mediating phenotypic transformation of TANs within tumor microenvironment. They were first to show the role of immunosuppressive cytokine, TGF-β in neutrophil polarization. In the presence of TGF-β, neutrophils are skewed towards an N2 phenotype. It also blocks the production of H2O2 and restricted the migration of neutrophils towards tumor cells, thus inhibiting the anti-tumoral functions of neutrophils. In contrast, the blockade of TGF-β enhances the development of neutrophils into an anti-tumoral N1 phenotype [125]. Besides TGF-β, various other cytokines have also been implicated in regulation of TANs plasticity. For instance, type I interferon can also regulate neutrophil polarization but its effect opposes to that of TGF-β. The presence of type I interferon polarizes neutrophils into an N1 phenotype, whereas impaired endogenous type I interferon signaling polarizes neutrophils into an N2 phenotype [127]. In a study with murine tumor model, IFN-β-deficient mice showed faster tumor growth, enhanced vascularization, and higher infiltration of neutrophils as compared with the wild-type mice [128]. Similarly, IL-12 is known to activate anti-tumoral N1 phenotype [129] while GCSF and IL-6 activate pro-tumoral N2 phenotype [130]. During physiological condition, neutrophils are in inactive state in circulation and get activated during any condition of infection or inflammation. Once they reach to the site of infection, they can polarize depending upon the factors present in their microenvironment. Whether these TANs can be irreversibly polarized or not is still unclear and require further studies.
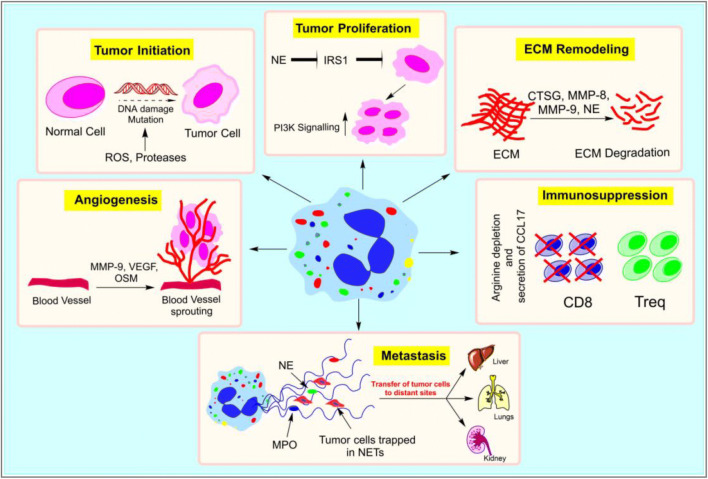
Signals from tumor cells or tumor microenvironment are known to influence infiltrating neutrophils to release their effector molecules like ROS, cytokines, chemokines, NETs, and granule contents which then impact tumorigenesis [22] (Fig. 4). Neutrophil-derived ROS can induce DNA damage and thereby enhance mutation rates contributing to increased tumor cell proliferation by deregulating tumor suppressor genes and oncogenes. Studies also correlated an increase in the number of TANs with high ROS activity and mutation rates [131, 132]. Additionally, neutrophil-derived ROS can produce a range of highly reactive mediators such as lipid hydroperoxides and epoxides which can also cause DNA damage [133]. Besides this, ROS, particularly H2O2, can act as a messenger in cell signaling which can regulate the PI3K/Akt, IKK/NF-kB, and MAPK/Erk1/2 signaling pathways in cancer [134]. In addition to ROS, neutrophils are capable of de novo synthesis and secretion of a range of cytokines and chemokines at tumor sites which not only enhances their own recruitment but also promotes the infiltration of other tumor-supportive immune cells [135]. Although neutrophils typically produce lower amounts of cytokines per cell compared with other immune cells such as macrophages, they are so abundant at inflammatory sites that their contribution to total cytokine level is quite significant [136]. IL-8, the most abundantly produced cytokine, can enhance neutrophil influx and support tumor cell proliferation by autocrine and paracrine mechanisms. Also, pro-inflammatory cytokines such as IL-1β and TNF-α can induce other cells to produce neutrophil chemoattractants and promote their extravasation. IL-6, another pro-inflammatory cytokine, released by neutrophils was reported to promote VEGF expression, thereby impacting angiogenesis. Tumor-infiltrating neutrophils can also produce cytokines such as IL-17, APRIL, BAFF, OSM, and HGF all of which have been implicated in tumor progression [137]. Furthermore, in a study, TANs showed active secretion of CCL17 which promoted recruitment of immunosuppressive regulatory T cells (Tregs) cells in the tumor microenvironment, whereas neutrophil depletion significantly reduced their recruitment to tumors [138].
Fig. 4.
Neutrophils in cancer. Neutrophils can promote tumorigenesis in several ways. NE (neutrophil elastase) can degrade insulin receptor substrate (IRS-1) and upregulate PI3K (phosphatidylinositol 3-kinase) signaling, thus inducing tumor cell proliferation. Similarly, neutrophil-derived ROS and proteases can induce DNA damage and enhance mutation rates in normal cells that can instigate initiation of tumors. Neutrophils can support tumors by stimulating tumor angiogenesis by releasing proangiogenic factors such as MMP-9, VEGF (vascular endothelial growth factor), and OSM (oncostatin M). They can also promote recruitment of immunosuppressive regulatory T cells (Treqs) cells in the tumor microenvironment. Neutrophil-derived proteolytic enzymes like MMP-8, MMP-9, NE, and CTSG (cathepsin G) can degrade a range of ECM (extracellular matrix) components, thus facilitating tumor cell migration. Further, NETs (neutrophil extracellular traps) can entrap tumor cells and aid their transfer to distant sites
Furthermore, NETs have been recognized as a new add-on to the anti-microbial action of neutrophils [139]. Released by the activated neutrophils, they are pathogen-trapping fibers equipped with chromatin and neutrophil proteolytic enzymes. Their role in cancer has recently been demonstrated [140]. They can trap the circulating tumor cells and aid their transfer to distant sites, hence acting as possible mediators of metastasis [141]. They can also impact tumorigenesis by enhancing tumor cell proliferation either by releasing granule proteases such as NE, cathepsin G, and MMP9 on the NETs, or by activating signaling machinery such as NF-kB pathway. Higher levels of NETs were found in the plasma of lung, pancreatic adenocarcinoma, and bladder cancer patients as compared with healthy controls [142]. Moreover, adverse patient outcomes are also reported to be associated with increased NETs production [143]. Besides this, granule cargoes also play a pivotal role in deciphering tumor aggressiveness which we will discuss in detail in the upcoming section.
Granule cargoes in cancer: paving the way forward
As in case of other chronic inflammatory diseases, the role of neutrophil granules seems to be important in cancer as well. Most of the granule components of neutrophils act as a salient protagonist in tumor progression (Table 1). By far, the most well-studied ones in cancer include NE, MPO, cathepsin G, MMP8, and MMP9 which play diverse pro-tumoral roles. Similarly, neutrophil-α defensins and oncostatin M can also pave the way for tumor growth and progression. In the subsequent section, we discuss the role of these granule cargoes in the context of cancer.
Table 1.
Neutrophil-derived granule cargoes and their role in different types of cancer
| Neutrophil-derived granule cargoes | Types of cancer | Role | References |
|---|---|---|---|
| Neutrophil elastase (NE) | Lung cancer | High elastase levels in serum and bronchoalveolar lavage fluid, correlated with disease progression | [153] |
| Myeloperoxidase (MPO) | Pancreatic adenocarcinoma, acute promyelocytic leukemia | MPO polymorphism leading to abnormal MPO expression | [183] [184] |
| Breast cancer | High MPO levels correlated with high cancer risk | [188] | |
| Cathepsin G (CTSG) | Breast cancer | Mediate tumor cell adhesion by stimulating E-cadherin/catenin complex formation | [209] |
| Neutrophil collagenase (MMP8) | Pancreatic adenocarcinoma, uterine cancer, head and neck squamous carcinoma cells, ovarian cancer and colorectal cancer | High expression of MMP8 correlated with tumor progression | [215–219] |
| Ovarian cancer, melanoma | MMP8 gene polymorphism associated with high cancer risks | [221, 222] | |
| Gelatinase B (MMP9) | Myxofibrosarcoma, epithelial carcinogenesis | Tumor invasion, angiogenesis, hyper-proliferation | [234, 236] |
| Neutrophil α-defensins | Renal, bladder, oral squamous cell carcinoma, and breast cancer | Over expression of α-defensins leading to tumor cell proliferation, migration and invasion | [253–256] |
| Oncostatin M (OSM) | Breast cancer | High OSM expression correlated with tumor angiogenesis and neovascularization | [262] |
Neutrophil elastase
NE is a 29-kDa serine protease of the chymotrypsin family and a key effector molecule encapsulated in the primary (azurophil) granule of neutrophils [144]. It is synthesized as a precursor in promyelocytes in bone marrow and becomes active in mature neutrophils. It is the most abundant enzyme present in neutrophils, wherein a single neutrophil contains 1 pg of NE [145]. Activated neutrophils release NE into the extracellular spaces via degranulation or NETs formation [146]. It plays an important role in mounting an inflammatory response for host defense and pathogen clearance during infection. It is also considered as an important regulator of leukocyte transmigration and emergency myelopoiesis [147]. NE hydrolyzes variety of substrates, including elastin and other ECM components such as cadherins, fibronectin, collagen, proteoglycan, lung surfactant, and growth factors [148]. In addition to its role in host defense, evidences suggest an important contribution of NE in various chronic inflammatory diseases, including cancer [149]. Upregulated activity and expression of NE have been observed in various cancer types, and its concentration is often correlated with cancer grade, stage, and survival of patients. Notably, α1-anti-trypsin, a secretory glycoprotein produced in the liver, is a natural inhibitor of NE. An imbalance in the levels of NE and α1-anti-trypsin is also associated with several diseases such as lung emphysema, RA, bronchiectasis, asthma, and chronic liver diseases. Similar imbalance has been reported in the development and progression of lung, liver, and colorectal cancers [150]. Yamashita and the group in their study showed that lung cancer patients with high elastase concentration had shorter survival and poor prognosis compared with those with low elastase levels [151]. Similar results were also observed in mice model of lung adenocarcinoma, wherein mice lacking NE showed longer survival [152]. Elevated elastase levels in serum and bronchoalveolar lavage fluid (BALF) are also correlated with disease progression in lung cancer patients [153]. Several studies have reported that NE can directly promote tumor cell proliferation by hyper activating phosphatidylinositol 3-kinase (PI3K). Clathrin-coated pit and neuropilin-1 mediate endosomal internalization of NE, which enzymatically degrades insulin receptor substrate-1 (IRS-1). It further increases the interactions between PI3K and PDGFR, a potent mitogen that triggers tumor cell proliferation [154, 155]. It is also involved in tumor cell invasion and migration by degrading ECM proteins and activating MMPs such as MMP2 and MMP3 [156, 157]. Other studies also suggest that NE promotes the release of tumor promoting factors such as VEGF, PDGF, and TGF-α into the ECM, thus directly or indirectly supporting tumor growth and progression [158].
Myeloperoxidase
MPO is found in the primary granules and makes up to 5% of neutrophil’s dry weight [159]. It is also found in monocytes, but in a much lesser amount than neutrophils and eventually lost during monocyte-to-macrophage differentiation in tissues [160, 161]. This enzyme is one of the important players in neutrophil function which is released into the ECM via degranulation, apoptosis, necrosis, and NETosis [101, 161, 162]. It acts as a powerful pro-inflammatory agent which catalyzes the formation of ROS including hypochlorous acid (HOCl), hypobromous acid (HOBr), and hypothiocyanous acid (HOSCN) [163, 164]. These intermediates are toxic agents for pathogens and act as important players in the immune response. On the contrary, their unregulated production can also damage host tissues and causes several diseases [165]. Recent reports have very well documented the role of MPO in the initiation and progression of several diseases including cardiovascular diseases [166], neurodegenerative diseases [167], RA [168], asthma [169], and cancer [170].
Genomic instability is one of the hallmarks of cancer which leads to either epigenetic alterations or mutations collectively termed as the hypermutagen state. An inefficient DNA repair system and increased sensitivity to mutagens are the salient features of this state. MPO is known to play an important role in promoting hypermutagen environment through its enzymatic actions [171]. HOCl, particularly, is known to be a potent oxidizing agent that can oxidize proteins, lipids, and DNA [172]. It can further interfere with DNA repair and promote DNA cross-links and formation of pyrimidine oxidant products. MPO-derived oxidants can also activate inhaled carcinogens such as polycyclic aromatic hydrocarbons (PAHs), leading to mutagenesis [173]. Besides DNA modifications, MPO-derived HOCL can also encourage tumor cell metastasis via activation of MMPs such as MMP2, 7, and 8 [174]. In addition, MPO-dependent activation of MMPs also contributes to vascular dysfunction leading to cardiovascular [175] and renal disorders [176]. Moreover, ROS generated by MPO is known to inhibit the activity of NK cells against tumor cells [177]. Furthermore, MPO gene polymorphism is also associated with altered MPO expression and susceptibility to cancer risks [178]. Single nucleotide polymorphism in the promoter region of MPO can affect transcription as well as translation [179]. MPO-463G/A promoter polymorphism are considered in this respect wherein G allele is associated with higher MPO gene transcription and A allele with lower gene transcription. Various reports have associated the 463A allele variant of MPO with lower risk of lung, breast, and bladder cancer [180–182], whereas 463G allele variant is known to promote pancreatic adenocarcinoma [183] and acute promyelocytic leukemia [184]. Moreover, in neurodegenerative diseases such as multiple sclerosis [185] and Alzheimer’s disease [186], G/G allele expression is correlated with a high possibility of disease development and in cystic fibrosis patients [187]; it is associated with severe lung infections.
A high MPO level in biological fluids is also correlated with several malignancies. For instance, high MPO level has been directly linked to the risk of breast cancer development and is considered as an efficient marker in premenopausal women suffering from breast cancer [188]. Similarly, patients suffering from acute myeloid leukemia (AML) showed an increase in plasma MPO levels (1.0–9514 ng/mL) as compared with the controls (3.5–20.6 ng/mL) pointing towards its clinical relevance [189]. An increased level of MPO was also detected in serum and BALF of patients suffering from COPD and lung cancer [153]. Furthermore, in a mice model of lung cancer, MPO inhibition showed a 50% reduction in MCA-induced lung carcinogenesis [190]. Thus, MPO can efficiently contribute to cancer initiation and progression; notably, its high levels can enhance cancer susceptibility.
Cathepsin G
Cathepsins are an extensive family of proteases which are known to participate in many physiological and pathological processes [191]. They are transported either to the nucleus to regulate gene expression or to the cell surface to regulate cell signaling as well as secreted outside to degrade ECM [192]. These enzymes are actively involved in development, differentiation, angiogenesis, wound healing, bone remodeling, antigen processing, and reproduction. On the contrary, they are also involved in diseases such as bronchial asthma, atherosclerosis, RA, and cancer [193–197]. They are broadly categorized into serine, aspartic, and cysteine cathepsins on the basis of the amino acids present at their active sites [198]. Neutrophils are equipped with cathepsin G (CTSG), a serine cathepsin which is stored in their azurophilic granules together with NE and proteinase 3 (PR3) [144]. Being a degradative enzyme, CTSG can kill the ingested pathogen and perform extracellular functions such as degradation of ECM, hydrolysis of host plasma proteins, and hormonal factors [199]. It can also perform immunomodulatory actions by cleaving certain chemokines and their receptors [200]. CTSG is also involved in transmigration of leukocytes at the infection site [201], platelet activation [202], conversion of angiotensin I to angiotensin II [203], and induction of airway sub mucosal gland secretion [204]. Besides its anti-microbial role, CTSG has been identified to play a very important role in inflammation, tumor growth, and progression. Pancreatic ductal adenocarcinoma (PDAC) patients showed a 2.4-fold increase in CTSG protein expression. Similarly, in patients suffering from chronic pancreatitis, a 1.9-fold upregulation of CTSG protein as compared with controls was reported [205]. CTSG is known to enhance the activity of IL-8 which is a pro-inflammatory cytokine and strong neutrophil chemoattractant. Furthermore, CTSG can also activate other pro-inflammatory cytokines such as TNF-α and IL-1β, thus contributing to inflammatory disorders. It can promote tumor cell invasion via degradation of ECM and increasing the permeability of endothelial cells [206, 207]. In another report, upregulated expression of CTSG is known to activate pro-MMP-2 and MMP-9 at the tumor-bone interface and subsequently activates TGF-β which further promotes tumor growth and bone destruction through osteoclast activation [156, 208]. In addition, it can also mediate tumor cell adhesion by stimulating E-cadherin/catenin complex formation in breast cancer cells and thus promoting tumor growth [209]. The molecular mechanism involved in these processes remains poorly understood; however, a study showed the role of IGF-1R signaling. CTSG stimulated IGF-1 release from MCF-7 cells by activating IGF-1R signaling which further promoted cell aggregation [210]. Together, these reports suggest a crucial role of CTSG in tumorigenesis and can be a potential therapeutic target.
Neutrophil collagenase
Neutrophil collagenase is also known as MMP8 or collagenase-2. It is highly expressed in neutrophils and stored as proenzyme in specific granules. Beyond the capacity to degrade ECM, MMP8 has diverse biological roles such as in innate immunity, modulation of chemokines, production of chemotactic peptides, and regulation of repair response [211–213]. Activated neutrophils quickly release MMP8 to ensure its availability at the site of infection or inflammation. Neutrophils release only 20–30% of its cellular MMP8 content into extracellular spaces, while the rest are solely localized at the cell surface. MMP activity is regulated by its inhibitor TIMP (tissue inhibitor of metalloproteinases) but, being resistant to TIMP-mediated inactivation, MMP8, majorly membrane bound, shows high efficiency in cleaving type I collagen [214]. Various studies have reported the upregulation of MMP8 in a wide range of inflammatory disorders including cancer. For instance, high expression of MMP8 was observed in tissue samples of pancreatic adenocarcinoma [215] and uterine cancer patients [216] as compared with normal tissues. Similarly, high expression of MMP8 was correlated with tumor progression as well as poor prognosis in patients with head and neck squamous carcinoma, ovarian cancer and colorectal cancer [217–219]. In hepatocellular carcinoma cells, MMP8 exhibits pro-tumorigenic effects by regulating its signaling cascade via activating the PI3K/Akt/Rac-1 pathway. It upregulates TGF-β expression, which stimulates epithelial-mesenchymal transition (EMT), thereby increasing invasion and migration of the cancer cells [220].
Importantly, various reports suggest the association of MMP8 gene polymorphism with cancer risk. For example, single nucleotide polymorphism (SNP rs11225395) in the promoter region of MMP8 gene increases the transcription rate of MMP8 which is reported to be associated with high risk of developing ovarian cancer and melanoma [221, 222]. Also, MMP8 gene polymorphism (MMP8 78K/E) is associated with high risk of bladder cancer [223]. MMP8 is also considered as potential diagnostic biomarker and therapeutic targets for various diseases. Its level can be easily quantified in oral fluids, plasma and serum. In patients with colorectal cancer, high serum MMP8 levels were correlated with increased malignancy, systemic inflammation and reduced overall survival [224, 225]. Similarly high level of MMP8 in fluids of ovarian cysts was associated with tumor development [226]. These aforementioned reports suggest a pivotal role of MMP8 in cancer; however, the tumor-promoting mechanism of MMP8 requires more extensive studies to understand the overall role of MMP8 in different steps of tumorigenesis.
MMP9/gelatinase B
MMP9 is expressed by different cell types, including neutrophils, macrophages, and mast cells. In neutrophils, it is synthesized during their maturation in the bone marrow and stored as pro-MMP9 in the secondary and tertiary granules from which it is released upon activation or chemotactic stimulations [227, 228]. In cancer microenvironment, MMP9 is known to be a major contributor to ECM degradation but it is tightly regulated by the linked TIMP-1. Interestingly, neutrophils do not produce endogenous TIMP-1 and therefore neutrophil-derived MMP9 remains active. In cancer milieu, this TIMP-1 free MMP9 is majorly known to enhance angiogenesis and tumor invasion in multiple ways such as by activating angiogenic mitogen present in matrix stores, regulating recruitment and proliferation of pericytes and mobilizing bone marrow-derived angiogenic factors to tumor stroma [229–232]. According to a study, 1.5 ng of MMP9 released by as few as 5 × 104 neutrophils can lead to a 5-fold raise in angiogenesis level suggesting its strong angiogenic potential [233].
In patients with myxofibrosarcoma, high number of neutrophils also correlated with increase in microvessel density [234]. Similarly, they were abundantly present in the angiogenic islets of tumors and dysplasia and the frequency of angiogenic response was significantly reduced upon transient depletion of the neutrophils [227]. Pahler and colleagues reported that in a transgenic mice model with impaired monocyte function, angiogenesis and tumor progression were enhanced by MMP9+ neutrophils [235]. In a mouse model for epithelial carcinogenesis (K14-HPV16), upregulation of neutrophil-derived MMP9 was associated with angiogenesis, hyperproliferation, and advancement towards more aggressive stages of cancer [236]. MMP9+ neutrophils also prevent tumor cell apoptosis and support the establishment of pulmonary tumors as shown in MMP9 knockdown mice [237].
Additionally, MMP9 shows broad catalytic activity against components of ECM. It can cleave various non-matrix proteins and activate tumor-supporting cytokines such as such as IL-8 and IL-1β [238, 239]. IL-8 acts as a potent chemoattractant for neutrophils. In a study, anti-IL-8 treatment blocked the infiltration of MMP9+ neutrophils and significantly inhibited tumor angiogenesis and invasion [240]. Neutrophils can promote tumor angiogenesis either directly via release of vesicle-stored proteolytic enzymes, growth factors, pro-angiogenic factors (VEGF, FGF), and cytokines or indirectly via activating signaling cascade. Ardi and colleagues showed that neutrophil-derived pro-MMP9 induce angiogenesis via involvement of FGF-2/FGFR-2 signaling pathway. MMP-9 activity results in increased bioavailability of FGF-2, which then becomes the essential downstream angiogenic inducer acting through its specific receptor, FGFR-2 [241]. These findings suggest that infiltrating neutrophils have a significant contribution in MMP9 levels and are critical determinant of angiogenesis and tumor invasion.
Neutrophil α-defensins
Defensins are anti-microbial peptides that belong to a unique class of cysteine-rich cationic polypeptides and play a pivotal role in innate and adaptive immunity. Based on the structure and cysteine pairing, defensins are categorized into three subfamilies: α defensins, β defensins, and θ defensins [242]. Six α-defensins are known in humans out of which four are produced by granulocytes and rest two are epithelial defensins [243]. Human neutrophil α-defensin are also defined as human neutrophil peptides (HNP). Out of the four HNPs, HNP1, HNP2, and HNP3 have potent bactericidal role and abundantly stored in the azurophil granules and make upto 5–7% of total cellular protein content in neutrophils. They lack enzymatic activity and utilize the oxygen-independent mechanism to eliminate pathogens, which makes them unique to neutrophil serine proteases [244]. HNPs move to phagolysosome upon phagocytosis or released into extracellular spaces by the activated neutrophils, which can be detected in the biological fluids [245]. In addition to potent anti-microbial activity, defensins also display cytotoxic, chemotactic and stimulatory activities towards eukaryotic cells [243]. In a healthy individual, the concentration of HNP1–3 in human plasma is approximately 40 ng/mL. and the concentration can increase several folds in various inflammatory diseases. [246]. An elevated level of neutrophil defensin is reported in various inflammatory diseases. In BALF of cystic fibrosis patients, there is 500–10,000-fold increase in the HNP concentration suggesting their crucial role in pulmonary infections. Similarly HNP-1 levels were significantly higher in the saliva of patients suffering from oral inflammation [247]. Various studies have also reported increased levels of α-defensins in several neutrophil-dominated inflammatory disorders such as idiopathic pulmonary fibrosis and meningitis [248, 249]. Furthermore, defensin also stimulates the production of IL-8 and LTB-4 by alveolar macrophages, which are potent neutrophil chemoattractants [250]. Over the past few years, an altered expression as well as secretion of α-defensin has been reported in various tumor types. Elevated HNP1 level is reported in lung tumors, oral squamous cell carcinoma, and colorectal cancer [251]. Elevated accumulation of HNP-1 was seen in the malignant human pancreatic tissue, and similar expression was also observed in vitro in human pancreatic epithelial cells [252]. HNPs are also known to promote proliferation, migration, and invasion of malignant cells in renal, bladder, oral squamous cell carcinoma, and breast cancer [253–256]. Some studies also observed expression of HNP1–3 in the endothelial cells of tumor capillaries suggesting their role in angiogenesis [253]. In addition, HNP-1 overexpression is also correlated with reduced survival in intestinal-type gastric cancer [257]. On the contrary, some studies have reported the positive role of defensin, such as cytotoxic role towards cancer cells and induction of adaptive immune response [258, 259]. Still the crucial involvement of defensins in a variety of diseases including cancer makes them a fascinating target for diagnosis and therapy which warrants further studies.
Oncostatin M
OSM is a 28-kDa glycoprotein that belongs to the IL-6 family of cytokines [260]. Also secreted by monocytes and activated T cells, neutrophils represent the predominant cells in the circulation to express OSM. It can be readily mobilized from the granule stores of activated neutrophils or can be synthesized upon stimulation with inflammatory mediators [261]. The role of neutrophil-derived OSM in cancer is still limited to a study by Queen et al. wherein they showed OSM release by neutrophils upon stimulation by breast cancer cells. This released OSM further increases VEGF secretion in breast cancer cells thus promoting tumor angiogenesis and neovascularization [262]. However, there are numerous reports of OSM in the pathogenesis of other inflammatory disorders like asthma [263], RA [264], and acute lung injury [265]. Hurst and group showed that infiltrating neutrophils were the major source of OSM in bacterial infection leading to acute inflammatory conditions [266]. OSM can impart pro-inflammatory effects by inducing adhesion and chemotaxis in neutrophils [267]. It can also induce chemokine and adhesion molecules synthesis by endothelial cells which further helps in the transmigration of neutrophils [268]. Neutrophil presence was also correlated with high levels of OSM in BALF of patients with severe pneumonia [269]. Similarly, in acute lung injury, OSM was majorly released by the infiltrating neutrophils. Grenier and colleagues showed that a combination of LPS and CSF2 treatment in vitro can induce production of OSM in neutrophils and suggested a crucial role of neutrophils in the modulation of acute inflammation [261]. Pothoven et al. showed that in patients with mucosal airway disease, neutrophils were the major source of OSM that resulted in epithelial barrier disruption. The group also reported that majority of OSM+ neutrophils expressed Arg1, which is a N2 marker, indicating that OSM+ neutrophils resemble more towards pro-tumoral phenotype of neutrophils [270]. OSM can utilize JAK/STAT, PI3K, and MAPK pathways to initiate signal transduction [266, 271, 272]. It is also known to be an effective activator of STAT1, STAT3, and STAT5 transcription factors [273]. Hence, considering the diverse role of OSM in other chronic inflammatory diseases, OSM is proposed as a putative protagonist in cancer. Further studies are warranted to understand its pathological relevance in cancer.
Targeting neutrophils: the therapeutic strategy underway
Neutrophils are not-so-neutral in cancer and their pro-tumoral side provides a rationale for development of neutrophil-targeted therapeutic interventions. Researchers across the globe are passionately exploring the strategies that could limit deleterious effects of neutrophils in cancer. One of the strategies deals with the inhibition of expansion and production of neutrophils in the bone marrow. In this context, substantial studies have targeted G-CSF, a key regulator of granulopoiesis and inducer of neutrophil production [274]. It binds to G-CSF receptors, which are highly expressed on mature neutrophils [275]. Multiple studies have reported a crucial role of G-CSF in various inflammatory ailments and high levels are correlated with disease severity [276, 277]. Thus G-CSF and its receptor could be a potential therapeutic target which can regulate neutrophil production as well as its activation [278]. This strategy has shown promising results in some preclinical models [279]. Inhibition of upstream regulator of neutrophil production is another approach to limit neutrophil production. IL-17 and IL-23 stimulate neutrophil production by regulating G-CSF [280]. Biological therapeutics targeting IL-17 and IL-23 axis has been developed for multiple inflammatory disorders [281].
Another approach targets the migration of neutrophils to the tumor sites. Chemokines play a crucial role in orchestrating neutrophil migration. Chemokines such as CXCL1 and IL-8 (CXCL8) act as potent chemoattractants for neutrophils which further activate GPCRs, particularly, CXCR1 and CXCR2. Activation of CXCR2 by IL-8 triggers neutrophil migration to the site of infection [282], whereas receptor-ligand interaction of CXCR1 is responsible for neutrophil degranulation [283]. Currently, chemokine receptor antagonists are in different stages of clinical trials. For instance, SB-656933, a CXCR2 antagonist was found effective in cystic fibrosis by reducing the levels of inflammatory biomarkers in the sputum of patients [284]. Similarly, MK-7123 (navarixin), another CXCR2 antagonist, improved lung function in COPD patients [285]. In animal models, it inhibited recruitment of neutrophils, mucus production and goblet cell hyperplasia [286]. Furthermore, neutrophil-derived molecules, playing important role in neutrophil-mediated disease pathogenesis, are emerging as potential therapeutic targets. Granule contents are the major attributes of neutrophil effector functions and their functionality can be harmonized with specific drugs targeting their production, localization or the release of key granule cargoes. For example, NE has been proposed as a potential target in various pathologies because of its unique potential to hydrolyze variety of substrates, including elastin and other ECM components such as cadherins, fibronectin, collagen, proteoglycan, lung surfactant, and growth factors [148]. Moreover, it can cleave and activate several other cytokines, such as G-CSF [287], IL-1 [288], and VEGF [289]. These attributes are known to contribute to the progression of chronic pulmonary disorders and different cancer types. Beside this, NE is known to be an independent prognostic factor in patients with lung, colon, prostate and breast cancer [290, 291]. Considering the role of NE in these pathologies, researchers have used various drugs to target NE which are currently in different phases of clinical trials. For instance, AZD9668 is an NE inhibitor which has been evaluated for its efficacy in clinical trials of various inflammatory diseases. In bronchiectasis, treatment improved the lung function with significant reduction in sputum inflammatory biomarkers [292]. Similarly, in cystic fibrosis, the patients showed reduced sputum inflammatory biomarkers including IL-6 and RANTES, together with urinary desmosine; however, the treatment had no effect on lung function [293]. Sivelestat, another NE inhibitor has been approved in Japan [294] which is known to inhibit neutrophil activation, reduce inflammation in the lungs, and induce competitive inhibition of neutrophils [295]. Sivelestat was also effective in reducing tumor growth in murine models of prostate and colorectal cancer [296, 297]. Similarly, granule contents like MPO and MMP9 are also gaining attention as potential therapeutic targets. However, the granule-mediated signaling pathways in various cancer types are poorly understood. Only few studies have been reported which shows the mechanistic pathway elucidated in cancer cell lines (Table 2) and require more extensive studies. If unraveled, these signaling mechanisms will uncover potential therapeutic targets. Additionally, NETs have also recently established their niche in sustaining tumorigenesis. Hence, targeting NETs can also be an easy and applicable strategy in cancer therapeutics. In some cases, NETs formation depends upon the active NADPH oxidase complex, thus targeting this enzyme could also inhibit NETosis. In experimental mice, DNA targeting enzyme, DNase I, was found effective against various inflammatory disease. Therefore, targeting NETs with DNase I can also be considered as a therapeutic option [140]. Another excellent therapeutic strategy could be the manipulation of neutrophil function in tumor microenvironment. TGF-β promotes N2 polarization of neutrophils, which initiates the pro-tumoral response [125]. Anti-TGF-β therapy could stimulate a robust anti-tumor response by eliciting activation of N1 neutrophils. Fresolimumab (an anti-TGF-β monoclonal antibody) and galunisertib (a TGFβR1 kinase inhibitor) are TGF-β pathway inhibitors which can promote the development of neutrophils with anti-tumor (N1type) potential [298, 299]. Table 3 shows the list of antagonists affecting neutrophils or their microenvironment that are under different phases of clinical trials.
Table 2.
Potential signaling pathways of neutrophil-derived granule cargoes in different cancer types
| Neutrophil-derived granule cargoes | Cancer type | Signaling pathway involved | Pro-tumoral effects | References |
|---|---|---|---|---|
| Neutrophil elastase (NE) |
Lung cancer Oral cancer |
PDGF Signaling, Src/PI3K-Dependent Akt Signaling | Increased tumor cell proliferation and migration | [154] [300] |
| Myeloperoxidase (MPO) | T cell acute lymphoblastic leukemia | p38 MAPK and NF-kB signaling | Production of superoxides and increased degranulation of neutrophils | [301] [302] |
| Cathepsin G (CTSG) | Breast cancer | IGF-1R signaling | Promote tumor cell aggregation | [210] |
| Neutrophil collagenase (MMP8) | Hepatocellular carcinoma | PI3K/Akt/Rac-1 signaling | Stimulate epithelial-mesenchymal transition (EMT) | [220] |
| Oncostatin M (OSM) | Skin squamous cell carcinoma | JAK/STAT, PI3K, MAPK signaling | Promote tumor angiogenesis and neovascularization | [271, 272, 303] |
Table 3.
List of drugs targeting neutrophils or their microenvironment and the mode of action
| Drug | Mode of action | Condition/disease | Clinical phase | Clinical Trials.gov identifier |
|---|---|---|---|---|
|
AZD9668 Alvelestat (MPH966) Sivelestat |
Inhibitor of neutrophil elastase |
Type 2 diabetes mellitus Cystic fibrosis Bronchiectasis Bronchiolitis obliterans syndrome Respiratory distress syndrome Adult acute lung injury |
Phase II Phase II Phase II Phase II Phase II |
|
| GSK3196165 (otilimab) | Neutralizes the action of GM-CSF, thus incapable of binding to its targeted cells. | Rheumatoid arthritis | Phase III | NCT03980483 |
| CAM-3001 (mavrilimumab) | Competitive antagonist of GM-CSF signaling | Rheumatoid arthritis | Phase II | NCT01050998 |
| Pirfenidone | Reduced neutrophil infiltration via inhibiting TNF-α | Idiopathic pulmonary fibrosis | Phase III | NCT01366209 |
| Navarixin (MK-7123) | Antagonist of CXCR2, reduces neutrophil chemotaxis | Solid tumors | Phase II | NCT03473925 |
|
SB-656933 SB-656933-AAA |
Selective CXCR2 antagonist, inhibits CXCL1-induced CD11b on peripheral blood neutrophils |
Colitis, ulcerative Pulmonary disease, chronic obstructive |
Phase II Phase I |
|
| Navarixin (MK-7123, SCH 527123) | Antagonist of human CXCR1 and CXCR2, inhibits neutrophil recruitment, MPO release |
Neutrophilic asthma COPD |
Phase II Phase II |
|
|
Danirixin (GSK1325756) QBM076 |
CXCR2 antagonist, decreases neutrophil activation and transmigration to site of inflammation. |
Virus diseases COPD COPD |
Phase II Phase II Phase II |
|
| SX-682 | CXCR1 and CXCR2 antagonist | Metastatic melanoma | Phase I | NCT03161431 |
| CCX168 (Avacopan) | Selective complement C5a receptor inhibitor | ANCA-associated vasculitis | Phase III | NCT02994927 |
| RV1729 | Phosphoinositide 3-kinase δ and γ isoform inhibitor | COPD | Phase I | NCT02140346 |
| AZD1236 | MMP-9 and MMP-12 inhibitor | COPD | Phase II | NCT00758706 |
| AZD7986 | Reversible inhibitor of DPP1, it inhibits the formation of active neutrophil serine proteases during neutrophil maturation | Healthy subjects | Phase I | NCT02653872 |
| Nemiralisib (GSK2269557) | Phosphoinositide 3-kinase δ inhibitor | COPD | Phase II | NCT03345407 |
| Ixekizumab | Selectively binds and neutralizes IL-17A thus inhibit IL-17A mediated neutrophil recruitment | Psoriasis, arthritic | Phase III | NCT01695239 |
| Secukinumab | Target IL-17A | Chronic plaque psoriasis | Phase III | NCT01358578 |
We are very well aware of the fact that neutrophils are crucial for host defense against microbial infections. Hence, their elimination or complete inhibition of their effector functions could not be the appropriate therapeutic strategy. This would cause neutropenia leading to a risky state of immunosuppression and render patients susceptible to secondary infections. Accordingly, better characterization of neutrophils, targeting tumor-promoting subsets, and limiting the neutrophil effector functions is the need of the hour for developing precise therapeutic interventions without detrimental side effects.
Conclusion and future perspectives
Effector functions of neutrophils largely depend upon their granule cargoes and their regulated release. Together with a new realization of their role, which extends beyond just anti-microbial actions, neutrophil granules and their cargoes are emerging as key players in many chronic inflammatory disorders including cancer. While much is known about granule cargoes and their functions in host defense, the process governing granule formation, packaging of cargoes and the signaling mechanisms regulating their release is fragmentary and needs to be unraveled in a more systematic way. A better understanding of these processes will aid in delineating the functional heterogeneity of neutrophils in tumor setting. Currently, drugs that can target neutrophils or their microenvironment in various inflammatory diseases are in the different stages of clinical trials. However, in cancer therapeutic, it is still limited by the emerging plasticity and heterogeneity of neutrophils in tumor conditions. How to specifically target the disease-promoting phenotype still remains a quest. Future studies are required to identify novel markers so that we can target specific neutrophil subsets in cancer and progress towards developing neutrophil subtype-specific therapeutics. This would aim to suppress the “against the host actions” of neutrophils while favoring their “against the pathogen actions.” Nonetheless, the growing interest in neutrophil biology will soon unravel this enigma and neutrophil-directed therapeutics without compromising the host immunity would become a better approach in cancer therapeutics.
Funding
The authors, Kavita Rawat and Saima Syeda, received from the University Grant Commission (UGC) of the Government of India financial support in the form of UGC-SRF.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annual Review of Immunology. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MJ. Role of neutrophils in systemic autoimmune diseases. Arthritis Research & Therapy. 2013;15(5):1–9. doi: 10.1186/ar4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvie EA, Huttenlocher A. Neutrophils in host defense: new insights from zebrafish. Journal of Leukocyte Biology. 2015;98(4):523–537. doi: 10.1189/jlb.4MR1114-524R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 5.Rørvig S, Østergaard O, Heegaard NH, Borregaard N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. Journal of Leukocyte Biology. 2013;94(4):711–721. doi: 10.1189/jlb.1212619. [DOI] [PubMed] [Google Scholar]
- 6.Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiological Reviews. 2019;99(2):1223–1248. doi: 10.1152/physrev.00012.2018. [DOI] [PubMed] [Google Scholar]
- 7.Borregaard N, Sørensen OE, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends in Immunology. 2007;28(8):340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Worthen G, Haslett C, Rees A, Gumbay R, Henson J, Henson P. Neutrophil-mediated pulmonary vascular injury. The American Review of Respiratory Disease. 1987;136:19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Pang Z, Wang G, Guan X, Fang K, Wang Z, et al. (2017) Advanced role of neutrophils in common respiratory diseases. Journal of immunology research; 2017. [DOI] [PMC free article] [PubMed]
- 10.Weiss SJ. Tissue destruction by neutrophils. New England Journal of Medicine. 1989;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 11.Mollinedo F. Neutrophil degranulation, plasticity, and cancer metastasis. Trends in Immunology. 2019;40(3):228–242. doi: 10.1016/j.it.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11(3):e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekkering S, Torensma R. Another look at the life of a neutrophil. World Journal of Hematology. 2013;2(2):44–58. doi: 10.5315/wjh.v2.i2.44. [DOI] [Google Scholar]
- 14.Kim M-H, Yang D, Kim M, Kim S-Y, Kim D, Kang S-J. A late-lineage murine neutrophil precursor population exhibits dynamic changes during demand-adapted granulopoiesis. Scientific Reports. 2017;7(1):1–15. doi: 10.1038/srep39804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang P, Li Y, Xie Y, Liu Y (2019) Different faces for different places: heterogeneity of neutrophil phenotype and function. Journal of Immunology Research;2019. [DOI] [PMC free article] [PubMed]
- 16.Coffelt SB, Carlin LM, Mackey JB. Neutrophil maturity in cancer. Frontiers in Immunology. 2019;10:1912. doi: 10.3389/fimmu.2019.01912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cain DW, Snowden PB, Sempowski GD, Kelsoe G. Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PloS One. 2011;6(5):e19957. doi: 10.1371/journal.pone.0019957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna-Gupta, A., & Berliner, N. (2018). Granulocytopoiesis and monocytopoiesis. Hematology: Elsevier, 321–33. e1.
- 19.Rotrosen D, Gallin JI. Disorders of phagocyte function. Annual Review of Immunology. 1987;5(1):127–151. doi: 10.1146/annurev.iy.05.040187.001015. [DOI] [PubMed] [Google Scholar]
- 20.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Frontiers in Physiology. 2018;9:113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day RB, Link DC. Regulation of neutrophil trafficking from the bone marrow. Cellular and Molecular Life Sciences. 2012;69(9):1415–1423. doi: 10.1007/s00018-011-0870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends in Immunology. 2010;31(8):318–324. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. The Journal of Immunology. 2008;181(8):5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17(4):413–423. doi: 10.1016/S1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 26.Németh, T., Sperandio, M., & Mócsai, A. (2020). Neutrophils as emerging therapeutic targets. Nature Reviews Drug Discovery, 1–23. [DOI] [PubMed]
- 27.Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on neutrophil function in severe inflammation. Frontiers in Immunology. 2018;9:2171. doi: 10.3389/fimmu.2018.02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends in Immunology. 2011;32(10):452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, et al. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity. 2010;33(2):266–278. doi: 10.1016/j.immuni.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, et al. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. The Journal of Immunology. 2008;180(8):5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffer T, Ma D. The emerging role of chemokine receptor CXCR2 in cancer progression. Translational Cancer Research. 2016;5(Suppl 4):S616–SS28. doi: 10.21037/tcr.2016.10.06. [DOI] [Google Scholar]
- 32.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nature Reviews Immunology. 2016;16(6):378. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mócsai A, Walzog B, Lowell CA. Intracellular signalling during neutrophil recruitment. Cardiovascular Research. 2015;107(3):373–385. doi: 10.1093/cvr/cvv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ley K. Integration of inflammatory signals by rolling neutrophils. Immunological Reviews. 2002;186(1):8–18. doi: 10.1034/j.1600-065X.2002.18602.x. [DOI] [PubMed] [Google Scholar]
- 35.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nature Medicine. 2011;17(11):1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyun YM, Hong CW. Deep insight into neutrophil trafficking in various organs. Journal of Leukocyte Biology. 2017;102(3):617–629. doi: 10.1189/jlb.1RU1216-521R. [DOI] [PubMed] [Google Scholar]
- 37.Futosi K, Fodor S, Mócsai A. Reprint of neutrophil cell surface receptors and their intracellular signal transduction pathways. International Immunopharmacology. 2013;17(4):1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Kunkel EJ, Ley K. Distinct phenotype of E-selectin–deficient mice: E-selectin is required for slow leukocyte rolling in vivo. Circulation Research. 1996;79(6):1196–1204. doi: 10.1161/01.RES.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 39.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 40.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. The Journal of Experimental Medicine. 2006;203(12):2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodfin A, Voisin M-B, Beyrau M, Colom B, Caille D, Diapouli F-M, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nature Immunology. 2011;12(8):761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nourshargh S, Renshaw SA, Imhof BA. Reverse migration of neutrophils: where, when, how, and why? Trends in Immunology. 2016;37(5):273–286. doi: 10.1016/j.it.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Voisin M-B, Nourshargh S. Neutrophil transmigration: emergence of an adhesive cascade within venular walls. Journal of Innate Immunity. 2013;5(4):336–347. doi: 10.1159/000346659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovascular Research. 1996;32(4):687–698. doi: 10.1016/S0008-6363(96)00063-6. [DOI] [PubMed] [Google Scholar]
- 45.Kang T, Yi J, Guo A, Wang X, Overall CM, Jiang W, et al. Subcellular distribution and cytokine-and chemokine-regulated secretion of leukolysin/MT6-MMP/MMP-25 in neutrophils. Journal of Biological Chemistry. 2001;276(24):21960–21968. doi: 10.1074/jbc.M007997200. [DOI] [PubMed] [Google Scholar]
- 46.Bendas G, Borsig L (2012) Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. International Journal of Cell Biology;2012. [DOI] [PMC free article] [PubMed]
- 47.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opinion on Therapeutic Targets. 2007;11(11):1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Frontiers in Immunology. 2019;10:1078. doi: 10.3389/fimmu.2019.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solimando A, Brandl A, Mattenheimer K, Graf C, Ritz M, Ruckdeschel A, et al. JAM-A as a prognostic factor and new therapeutic target in multiple myeloma. Leukemia. 2018;32(3):736–743. doi: 10.1038/leu.2017.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Cabec V, Cowland JB, Calafat J, Borregaard N. Targeting of proteins to granule subsets is determined by timing and not by sorting: the specific granule protein NGAL is localized to azurophil granules when expressed in HL-60 cells. Proceedings of the National Academy of Sciences. 1996;93(13):6454–6457. doi: 10.1073/pnas.93.13.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood, The Journal of the American Society of Hematology. 1997;89(10):3503–3521. [PubMed] [Google Scholar]
- 52.Borregaard N, Kjeldsen L, Rygaard K, Bastholm L, Nielsen M, Sengeløv H, et al. Stimulus-dependent secretion of plasma proteins from human neutrophils. The Journal of Clinical Investigation. 1992;90(1):86–96. doi: 10.1172/JCI115860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sengeløv H, Kjeldsen L, Diamond MS, Springer TA, Borregaard N. Subcellular localization and dynamics of Mac-1 (alpha m beta 2) in human neutrophils. The Journal of Clinical Investigation. 1993;92(3):1467–1476. doi: 10.1172/JCI116724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borregaard N, Lollike K, Kjeldsen L, Sengeløv H, Bastholm L, Nielsen MH, et al. Human neutrophil granules and secretory vesicles. European Journal of Haematology. 1993;51(4):187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 55.Döhrmann S, Cole JN, Nizet V. Conquering neutrophils. PLoS Pathogens. 2016;12(7):e1005682. doi: 10.1371/journal.ppat.1005682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teng, T.-S., Ji, A.-L., Ji, X.-Y., & Li, Y.-Z. (2017). Neutrophils and immunity: from bactericidal action to being conquered. Journal of immunology research, 2017. [DOI] [PMC free article] [PubMed]
- 57.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Laboratory Investigation. 2000;80(5):617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 58.Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochemical Journal. 2002;366(3):689–704. doi: 10.1042/bj20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee WL, Harrison RE, Grinstein S. Phagocytosis by neutrophils. Microbes and Infection. 2003;5(14):1299–1306. doi: 10.1016/j.micinf.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Allen L-AH, Criss AK. Cell intrinsic functions of neutrophils and their manipulation by pathogens. Current Opinion in Immunology. 2019;60:124–129. doi: 10.1016/j.coi.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holland SM. Chronic granulomatous disease. Clinical Reviews in Allergy & Immunology. 2010;38(1):3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 62.Babior BM. NADPH oxidase: an update. Blood, The Journal of the American Society of Hematology. 1999;93(5):1464–1476. [PubMed] [Google Scholar]
- 63.Odobasic D, Kitching AR, Holdsworth SR (2016) Neutrophil-mediated regulation of innate and adaptive immunity: the role of myeloperoxidase. Journal of Immunology Research;2016. [DOI] [PMC free article] [PubMed]
- 64.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annual Review of Pathology: Mechanisms of Disease. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suarez-Carmona M, Hubert P, Delvenne P, Herfs M. Defensins:“simple” antimicrobial peptides or broad-spectrum molecules? Cytokine & Growth Factor Reviews. 2015;26(3):361–370. doi: 10.1016/j.cytogfr.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Mollinedo F (2003) Human neutrophil granules and exocytosis molecular control.
- 67.Kjeldsen L, Sengelov H, Lollike K, Nielsen M, Borregaard N (1994) Isolation and characterization of gelatinase granules from human neutrophils. [PubMed]
- 68.Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nature Reviews Molecular Cell Biology. 2014;15(2):135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 69.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 70.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nature Immunology. 2014;15(11):1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122(16):2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 72.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. The Journal of Cell Biology. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nature Medicine. 2012;18(9):1386–1393. doi: 10.1038/nm.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amulic B, Knackstedt SL, Abed UA, Deigendesch N, Harbort CJ, Caffrey BE, et al. Cell-cycle proteins control production of neutrophil extracellular traps. Developmental Cell. 2017;43(4):449–62.e5. doi: 10.1016/j.devcel.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nature Chemical Biology. 2011;7(2):75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 76.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proceedings of the National Academy of Sciences. 2015;112(9):2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hosseinzadeh A, Thompson PR, Segal BH, Urban CF. Nicotine induces neutrophil extracellular traps. Journal of Leukocyte Biology. 2016;100(5):1105–1112. doi: 10.1189/jlb.3AB0815-379RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Current Opinion in Infectious Diseases. 2012;25(3):321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 79.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Molecular Medicine. 2011;17(3–4):293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fcγ receptors are essential in a mouse model of transfusion-related acute lung injury. The Journal of Clinical Investigation. 2006;116(6):1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meijer M, Rijkers GT, Van Overveld FJ. Neutrophils and emerging targets for treatment in chronic obstructive pulmonary disease. Expert Review of Clinical Immunology. 2013;9(11):1055–1068. doi: 10.1586/1744666X.2013.851347. [DOI] [PubMed] [Google Scholar]
- 82.Laval J, Ralhan A, Hartl D. Neutrophils in cystic fibrosis. Biological Chemistry. 2016;397(6):485–496. doi: 10.1515/hsz-2015-0271. [DOI] [PubMed] [Google Scholar]
- 83.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circulation Research. 2012;110(6):875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 84.Ionita MG, van den Borne P, Catanzariti LM, Moll FL, de Vries J-PP, Pasterkamp G, et al. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(9):1842–1848. doi: 10.1161/ATVBAHA.110.209296. [DOI] [PubMed] [Google Scholar]
- 85.Döring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125(13):1673–1683. doi: 10.1161/CIRCULATIONAHA.111.046755. [DOI] [PubMed] [Google Scholar]
- 86.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. Journal of Molecular and Cellular Cardiology. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 87.Mizuma A, Yenari MA. Anti-inflammatory targets for the treatment of reperfusion injury in stroke. Frontiers in Neurology. 2017;8:467. doi: 10.3389/fneur.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. Journal of Experimental Medicine. 2017;214(11):3293–3310. doi: 10.1084/jem.20170689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kimball AS, Obi AT, Diaz JA, Henke PK. The emerging role of NETs in venous thrombosis and immunothrombosis. Frontiers in Immunology. 2016;7:236. doi: 10.3389/fimmu.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proceedings of the National Academy of Sciences. 2012;109(32):13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Csepregi JZ, Orosz A, Zajta E, Kása O, Németh T, Simon E, et al. Myeloid-specific deletion of mcl-1 yields severely neutropenic mice that survive and breed in homozygous form. The Journal of Immunology. 2018;201(12):3793–3803. doi: 10.4049/jimmunol.1701803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Apel F, Zychlinsky A, Kenny EF. The role of neutrophil extracellular traps in rheumatic diseases. Nature Reviews Rheumatology. 2018;14(8):467–475. doi: 10.1038/s41584-018-0039-z. [DOI] [PubMed] [Google Scholar]
- 93.Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M (2018) Scanning the immunopathogenesis of psoriasis. International journal of molecular sciences;19(1):179. [DOI] [PMC free article] [PubMed]
- 94.Hwang ST, Nijsten T, Elder JT. Recent highlights in psoriasis research. Journal of Investigative Dermatology. 2017;137(3):550–556. doi: 10.1016/j.jid.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 95.Harvima IT, Nilsson G, Suttle M-M, Naukkarinen A. Is there a role for mast cells in psoriasis? Archives of Dermatological Research. 2008;300(9):461–478. doi: 10.1007/s00403-008-0874-x. [DOI] [PubMed] [Google Scholar]
- 96.Prinz JC. Melanocytes: target cells of an HLA-C* 06: 02–restricted autoimmune response in psoriasis. Journal of Investigative Dermatology. 2017;137(10):2053–2058. doi: 10.1016/j.jid.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 97.Ikeda S, Takahashi H, Suga Y, Eto H, Etoh T, Okuma K, et al. Therapeutic depletion of myeloid lineage leukocytes in patients with generalized pustular psoriasis indicates a major role for neutrophils in the immunopathogenesis of psoriasis. Journal of the American Academy of Dermatology. 2013;68(4):609–617. doi: 10.1016/j.jaad.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 98.Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nature Reviews Rheumatology. 2011;7(12):691–699. doi: 10.1038/nrrheum.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Science Translational Medicine. 2011;3(73):73ra20–73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proceedings of the National Academy of Sciences. 1990;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nature Medicine. 2009;15(6):623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aubé B, Lévesque SA, Paré A, Chamma É, Kébir H, Gorina R, et al. Neutrophils mediate blood–spinal cord barrier disruption in demyelinating neuroinflammatory diseases. The Journal of Immunology. 2014;193(5):2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- 103.Baik SH, Cha M-Y, Hyun Y-M, Cho H, Hamza B, Kim DK, et al. Migration of neutrophils targeting amyloid plaques in Alzheimer’s disease mouse model. Neurobiology of Aging. 2014;35(6):1286–1292. doi: 10.1016/j.neurobiolaging.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer’s disease–like pathology and cognitive decline via LFA-1 integrin. Nature Medicine. 2015;21(8):880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 105.Pham CT. Neutrophil serine proteases fine-tune the inflammatory response. The International Journal of Biochemistry & Cell Biology. 2008;40(6–7):1317–1333. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. Journal of Leukocyte Biology. 2017;102(2):343–349. doi: 10.1189/jlb.5MR1216-508R. [DOI] [PubMed] [Google Scholar]
- 107.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nature Reviews Cancer. 2016;16(7):431. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 108.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528(7582):413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schmidt H, Bastholt L, Geertsen P, Christensen IJ, Larsen S, Gehl J, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. British Journal of Cancer. 2005;93(3):273–278. doi: 10.1038/sj.bjc.6602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trellakis S, Bruderek K, Dumitru CA, Gholaman H, Gu X, Bankfalvi A, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. International Journal of Cancer. 2011;129(9):2183–2193. doi: 10.1002/ijc.25892. [DOI] [PubMed] [Google Scholar]
- 111.Halazun K, Aldoori A, Malik H, Al-Mukhtar A, Prasad K, Toogood G, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. European Journal of Surgical Oncology (EJSO) 2008;34(1):55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 112.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer. The Journal of Thoracic and Cardiovascular Surgery. 2009;137(2):425–428. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 113.Walsh S, Cook E, Goulder F, Justin T, Keeling N. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. Journal of Surgical Oncology. 2005;91(3):181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 114.An X, Ding P-R, Li Y-H, Wang F-H, Shi Y-X, Wang Z-Q, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15(6):516–522. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 115.Malietzis G, Giacometti M, Kennedy RH, Athanasiou T, Aziz O, Jenkins JT. The emerging role of neutrophil to lymphocyte ratio in determining colorectal cancer treatment outcomes: a systematic review and meta-analysis. Annals of Surgical Oncology. 2014;21(12):3938–3946. doi: 10.1245/s10434-014-3815-2. [DOI] [PubMed] [Google Scholar]
- 116.Krenn-Pilko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, Kapp KS, et al. The elevated preoperative derived neutrophil-to-lymphocyte ratio predicts poor clinical outcome in breast cancer patients. Tumor Biology. 2016;37(1):361–368. doi: 10.1007/s13277-015-3805-4. [DOI] [PubMed] [Google Scholar]
- 117.Peng B, Wang Y-H, Liu Y-M, Ma L-X. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. International journal of clinical and experimental medicine. 2015;8(3):3098. [PMC free article] [PubMed] [Google Scholar]
- 118.Halazun KJ, Hardy MA, Rana AA, Woodland DC, IV, Luyten EJ, Mahadev S, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Annals of Surgery. 2009;250(1):141–151. doi: 10.1097/SLA.0b013e3181a77e59. [DOI] [PubMed] [Google Scholar]
- 119.Sionov RV, Assi S, Gershkovitz M, Sagiv JY, Polyansky L, Mishalian I, et al. Isolation and characterization of neutrophils with anti-tumor properties. JoVE (Journal of Visualized Experiments) 2015;100:e52933. doi: 10.3791/52933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brincks EL, Risk MC, Griffith TS. Seminars in cancer biology. Amsterdam: Elsevier; 2013. PMN and anti-tumor immunity—the case of bladder cancer immunotherapy. [DOI] [PubMed] [Google Scholar]
- 121.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33(5):949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 122.Brandau S, Dumitru CA, Lang S. Seminars in immunopathology. Berlin: Springer; 2013. Protumor and antitumor functions of neutrophil granulocytes. [DOI] [PubMed] [Google Scholar]
- 123.Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Research. 2003;63(6):1405–1412. [PubMed] [Google Scholar]
- 124.Dumitru CA, Lang S, Brandau S. Seminars in cancer biology. Amsterdam: Elsevier; 2013. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. [DOI] [PubMed] [Google Scholar]
- 125.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunology, Immunotherapy. 2013;62(11):1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pylaeva E, Lang S, Jablonska J. The essential role of type I interferons in differentiation and activation of tumor-associated neutrophils. Frontiers in Immunology. 2016;7:629. doi: 10.3389/fimmu.2016.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of Clinical Investigation. 2010;120(4):1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. The Journal of Immunology. 2011;186(2):807–815. doi: 10.4049/jimmunol.1001483. [DOI] [PubMed] [Google Scholar]
- 130.Yan B, Wei J-J, Yuan Y, Sun R, Li D, Luo J, et al. IL-6 cooperates with G-CSF to induce protumor function of neutrophils in bone marrow by enhancing STAT3 activation. The Journal of Immunology. 2013;190(11):5882–5893. doi: 10.4049/jimmunol.1201881. [DOI] [PubMed] [Google Scholar]
- 131.Sandhu JK, Privora HF, Wenckebach G, Birnboim HC. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. The American Journal of Pathology. 2000;156(2):509–518. doi: 10.1016/S0002-9440(10)64755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weitzman SA, Gordon LI (1990) Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. [PubMed]
- 133.Bartsch H, Nair J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2005;591(1–2):34–44. doi: 10.1016/j.mrfmmm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 134.Liou G-Y, Storz P. Reactive oxygen species in cancer. Free Radical Research. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews Immunology. 2011;11(8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 136.Nathan C. Neutrophils and immunity: challenges and opportunities. Nature Reviews Immunology. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 137.Tecchio C, Scapini P, Pizzolo G, Cassatella MA, editors. On the cytokines produced by human neutrophils in tumors. Seminars in cancer biology; 2013: Elsevier. [DOI] [PubMed]
- 138.Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, et al. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17—a new mechanism of impaired antitumor immunity. International Journal of Cancer. 2014;135(5):1178–1186. doi: 10.1002/ijc.28770. [DOI] [PubMed] [Google Scholar]
- 139.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. The Journal of Clinical Investigation. 2013;123(8):3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. (2016) Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Science translational medicine;8(361):361ra138-361ra138. [DOI] [PMC free article] [PubMed]
- 141.Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, et al. Tumor exosomal RNAs promote lung pre-metastatic niche formation by activating alveolar epithelial TLR3 to recruit neutrophils. Cancer Cell. 2016;30(2):243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 142.Oklu R, Sheth RA, Wong KH, Jahromi AH, Albadawi H. Neutrophil extracellular traps are increased in cancer patients but does not associate with venous thrombosis. Cardiovascular Diagnosis and Therapy. 2017;7(Suppl 3):S140. doi: 10.21037/cdt.2017.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Richardson J, Hendrickse C, Gao-Smith F, Thickett D (2017) Neutrophil extracellular trap production in patients with colorectal cancer in vitro. International journal of inflammation;2017. [DOI] [PMC free article] [PubMed]
- 144.Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90(2):227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 145.Wiedow, O., Muhle, K., Streit, V., & Kameyoshi, Y. (1996). Human eosinophils lack human leukocyte elastase, Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease., 1315(3), 185–187. [DOI] [PubMed]
- 146.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. Journal of Cell Biology. 2010;191(3):677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Young RE, Thompson RD, Larbi KY, La M, Roberts CE, Shapiro SD, et al. Neutrophil elastase (NE)-deficient mice demonstrate a nonredundant role for NE in neutrophil migration, generation of proinflammatory mediators, and phagocytosis in response to zymosan particles in vivo. The Journal of Immunology. 2004;172(7):4493–4502. doi: 10.4049/jimmunol.172.7.4493. [DOI] [PubMed] [Google Scholar]
- 148.Yamanouchi H, Fujita J, Hojo S, Yoshinouchi T, Kamei T, Yamadori I, et al. Neutrophil elastase: alpha-1-proteinase inhibitor complex in serum and bronchoalveolar lavage fluid in patients with pulmonary fibrosis. European Respiratory Journal. 1998;11(1):120–125. doi: 10.1183/09031936.98.11010120. [DOI] [PubMed] [Google Scholar]
- 149.Gaida MM, Steffen TG, Günther F, Tschaharganeh DF, Felix K, Bergmann F, et al. Polymorphonuclear neutrophils promote dyshesion of tumor cells and elastase-mediated degradation of E-cadherin in pancreatic tumors. European Journal of Immunology. 2012;42(12):3369–3380. doi: 10.1002/eji.201242628. [DOI] [PubMed] [Google Scholar]
- 150.Sun Z, Yang P. Role of imbalance between neutrophil elastase and α1-antitrypsin in cancer development and progression. The Lancet Oncology. 2004;5(3):182–190. doi: 10.1016/S1470-2045(04)01414-7. [DOI] [PubMed] [Google Scholar]
- 151.Yamashita J-i, Tashiro K, Yoneda S, Kawahara K, Shirakusa T. Local increase in polymorphonuclear leukocyte elastase is associated with tumor invasiveness in non-small cell lung cancer. Chest. 1996;109(5):1328–1334. doi: 10.1378/chest.109.5.1328. [DOI] [PubMed] [Google Scholar]
- 152.Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase–mediated degradation of IRS-1 accelerates lung tumor growth. Nature Medicine. 2010;16(2):219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vaguliene N, Zemaitis M, Lavinskiene S, Miliauskas S, Sakalauskas R. Local and systemic neutrophilic inflammation in patients with lung cancer and chronic obstructive pulmonary disease. BMC Immunology. 2013;14(1):36. doi: 10.1186/1471-2172-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gregory AD, Hale P, Perlmutter DH, Houghton AM. Clathrin pit-mediated endocytosis of neutrophil elastase and cathepsin G by cancer cells. Journal of Biological Chemistry. 2012;287(42):35341–35350. doi: 10.1074/jbc.M112.385617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yamauchi T, Kaburagi Y, Ueki K, Tsuji Y, Stark GR, Kerr IM, et al. Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1,-2, and-3, their association with p85 phosphatidylinositol 3-kinase (PI3-kinase), and concomitantly PI3-kinase activation via JAK2 kinase. Journal of Biological Chemistry. 1998;273(25):15719–15726. doi: 10.1074/jbc.273.25.15719. [DOI] [PubMed] [Google Scholar]
- 156.Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. Journal of Cellular Physiology. 2001;189(2):197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 157.Okada Y, Nakanishi I. Activation of matrix metalloproteinase 3 (stromelysin) and matrix metalloproteinase 2 (‘gelatinase’) by human neutrophil elastase and cathepsin G. FEBS Letters. 1989;249(2):353–356. doi: 10.1016/0014-5793(89)80657-X. [DOI] [PubMed] [Google Scholar]
- 158.Wada Y, Yoshida K, Tsutani Y, Shigematsu H, Oeda M, Sanada Y, et al. Neutrophil elastase induces cell proliferation and migration by the release of TGF-α, PDGF and VEGF in esophageal cell lines. Oncology Reports. 2006;17(1):161–167. [PubMed] [Google Scholar]
- 159.Klebanoff SJ. Myeloperoxidase: friend and foe. Journal of Leukocyte Biology. 2005;77(5):598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 160.Bos, A., Wever, R., & Roos, D. (1978). Characterization and quantification of the peroxidase in human monocytes, Biochimica et Biophysica Acta (BBA)-Enzymology., 525(1), 37–44. [DOI] [PubMed]
- 161.Van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxidants & Redox Signaling. 2009;11(11):2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 162.Loria V, Dato I, Graziani F, Biasucci LM (2008) Myeloperoxidase: a new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators of inflammation;2008. [DOI] [PMC free article] [PubMed]
- 163.Pattison DI, Davies MJ, Hawkins CL. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radical Research. 2012;46(8):975–995. doi: 10.3109/10715762.2012.667566. [DOI] [PubMed] [Google Scholar]
- 164.Rayner BS, Love DT, Hawkins CL. Comparative reactivity of myeloperoxidase-derived oxidants with mammalian cells. Free Radical Biology and Medicine. 2014;71:240–255. doi: 10.1016/j.freeradbiomed.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 165.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood, The Journal of the American Society of Hematology. 1998;92(9):3007–3017. [PubMed] [Google Scholar]
- 166.Koeth RA, Haselden V, Tang WW. Myeloperoxidase in cardiovascular disease. Advances in clinical chemistry. 62: Elsevier; 2013. p. 1–32. [DOI] [PubMed]
- 167.Ray R, Katyal A. Myeloperoxidase: bridging the gap in neurodegeneration. Neuroscience & Biobehavioral Reviews. 2016;68:611–620. doi: 10.1016/j.neubiorev.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 168.Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, et al. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology. 2012;51(10):1796–1803. doi: 10.1093/rheumatology/kes193. [DOI] [PubMed] [Google Scholar]
- 169.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119(5):1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 170.Khan AA, Alsahli MA, Rahmani AH. Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Medical Sciences. 2018;6(2):33. doi: 10.3390/medsci6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Trush MA, Kensler TW. An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radical Biology and Medicine. 1991;10(3–4):201–209. doi: 10.1016/0891-5849(91)90077-G. [DOI] [PubMed] [Google Scholar]
- 172.Winterbourn CC. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology. 2002;181:223–227. doi: 10.1016/S0300-483X(02)00286-X. [DOI] [PubMed] [Google Scholar]
- 173.Trush A, Esterline R, Mallet W, Mosebrook D, Twerdok L. Further evidence for the role of myeloperoxidase in the activation of benzo[A]pyrene-7,8-dihydrodiol by polymorpho-nuclear leukocytesm. Biological Reactive Intermediates IV: Springer; 1991. pp. 399–401. [DOI] [PubMed] [Google Scholar]
- 174.Cui N, Hu M, Khalil RA (2017). Biochemical and biological attributes of matrix metalloproteinases. Progress in molecular biology and translational science. 147: Elsevier; p. 1–73. [DOI] [PMC free article] [PubMed]
- 175.Friedrichs K, Baldus S, Klinke A. Fibrosis in atrial fibrillation–role of reactive species and MPO. Frontiers in Physiology. 2012;3:214. doi: 10.3389/fphys.2012.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Porubsky S, Schmid H, Bonrouhi M, Kretzler M, Malle E, Nelson PJ, et al. Influence of native and hypochlorite-modified low-density lipoprotein on gene expression in human proximal tubular epithelium. The American Journal of Pathology. 2004;164(6):2175–2187. doi: 10.1016/S0002-9440(10)63775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.El-Hag A, Clark R. Down-regulation of human natural killer activity against tumors by the neutrophil myeloperoxidase system and hydrogen peroxide. The Journal of Immunology. 1984;133(6):3291–3297. doi: 10.4049/jimmunol.133.6.3291. [DOI] [PubMed] [Google Scholar]
- 178.Ding G, Liu F, Feng C, Xu J, Ding Q. Association between the myeloperoxidase gene polymorphisms and the susceptibility to prostate cancer: a case–control study in a Chinese population. Actas Urológicas Españolas (English Edition) 2013;37(2):79–82. doi: 10.1016/j.acuroe.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 179.Feyler A, Voho A, Bouchardy C, Kuokkanen K, Dayer P, Hirvonen A, et al. Point: myeloperoxidase− 463G→ A polymorphism and lung cancer risk. Cancer Epidemiology and Prevention Biomarkers. 2002;11(12):1550–1554. [PubMed] [Google Scholar]
- 180.Le Marchand L, Seifried A, Lum A, Wilkens LR. Association of the myeloperoxidase− 463G→ A polymorphism with lung cancer risk. Cancer Epidemiology and Prevention Biomarkers. 2000;9(2):181–184. [PubMed] [Google Scholar]
- 181.Ahn J, Gammon MD, Santella RM, Gaudet MM, Britton JA, Teitelbaum SL, et al. Myeloperoxidase genotype, fruit and vegetable consumption, and breast cancer risk. Cancer Research. 2004;64(20):7634–7639. doi: 10.1158/0008-5472.CAN-04-1843. [DOI] [PubMed] [Google Scholar]
- 182.Hung RJ, Boffetta P, Brennan P, Malaveille C, Gelatti U, Placidi D, et al. Genetic polymorphisms of MPO, COMT, MnSOD, NQO1, interactions with environmental exposures and bladder cancer risk. Carcinogenesis. 2004;25(6):973–978. doi: 10.1093/carcin/bgh080. [DOI] [PubMed] [Google Scholar]
- 183.Wheatley-Price P, Asomaning K, Reid A, Zhai R, Su L, Zhou W, et al. Myeloperoxidase and superoxide dismutase polymorphisms are associated with an increased risk of developing pancreatic adenocarcinoma. Cancer: Interdisciplinary International Journal of the American Cancer Society. 2008;112(5):1037–1042. doi: 10.1002/cncr.23267. [DOI] [PubMed] [Google Scholar]
- 184.Reynolds WF, Chang E, Douer D, Ball ED, Kanda V. An allelic association implicates myeloperoxidase in the etiology of acute promyelocytic leukemia. Blood, The Journal of the American Society of Hematology. 1997;90(7):2730–2737. [PubMed] [Google Scholar]
- 185.Nagra RM, Becher B, Tourtellotte WW, Antel JP, Gold D, Paladino T, et al. Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. Journal of Neuroimmunology. 1997;78(1–2):97–107. doi: 10.1016/S0165-5728(97)00089-1. [DOI] [PubMed] [Google Scholar]
- 186.Reynolds WF, Rhees J, Maciejewski D, Paladino T, Sieburg H, Maki RA, et al. Myeloperoxidase polymorphism is associated with gender specific risk for Alzheimer’s disease. Experimental Neurology. 1999;155(1):31–41. doi: 10.1006/exnr.1998.6977. [DOI] [PubMed] [Google Scholar]
- 187.Reynolds WF, Sermet-Gaudelus I, Gausson V, Feuillet M-N, Bonnefont J-P, Lenoir G, et al. (2006) Myeloperoxidase promoter polymorphism− 463G is associated with more severe clinical expression of cystic fibrosis pulmonary disease. Mediators of inflammation;2006. [DOI] [PMC free article] [PubMed]
- 188.He C, Tamimi RM, Hankinson SE, Hunter DJ, Han J. A prospective study of genetic polymorphism in MPO, antioxidant status, and breast cancer risk. Breast Cancer Research and Treatment. 2009;113(3):585–594. doi: 10.1007/s10549-008-9962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jilani I, Vincenti T, Faraji H, Giles FJ, Estey E, Kantarjian HM, et al. Clinical relevance of circulating myeloperoxidase (MPO) in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) 2004. [Google Scholar]
- 190.Rymaszewski AL, Tate E, Yimbesalu JP, Gelman AE, Jarzembowski JA, Zhang H, et al. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers. 2014;6(2):1111–1127. doi: 10.3390/cancers6021111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen S, Dong H, Yang S, Guo H. Cathepsins in digestive cancers. Oncotarget. 2017;8(25):41690. doi: 10.18632/oncotarget.16677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Duncan EM, Muratore-Schroeder TL, Cook RG, Garcia BA, Shabanowitz J, Hunt DF, et al. Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell. 2008;135(2):284–294. doi: 10.1016/j.cell.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Roshy S, Sloane BF, Moin K. Pericellular cathepsin B and malignant progression. Cancer and Metastasis Reviews. 2003;22(2–3):271–286. doi: 10.1023/A:1023007717757. [DOI] [PubMed] [Google Scholar]
- 194.Joyce JA, Baruch A, Chehade K, Meyer-Morse N, Giraudo E, Tsai F-Y, et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5(5):443–453. doi: 10.1016/S1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 195.Vasiljeva O, Reinheckel T, Peters C, Turk D, Turk V, Turk B. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Current Pharmaceutical Design. 2007;13(4):387–403. doi: 10.2174/138161207780162962. [DOI] [PubMed] [Google Scholar]
- 196.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. The EMBO Journal. 2001;20(17):4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zavašnik-Bergant T, Turk B. Cysteine cathepsins in the immune response. Tissue Antigens. 2006;67(5):349–355. doi: 10.1111/j.1399-0039.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 198.Thomas, E. L., Lehrer, R. I., & Rest, R. F. (1988). Human neutrophil antimicrobial activity. Reviews of Infectious Diseases, S450–S4S6. [DOI] [PubMed]
- 199.Wiedow O, Meyer-Hoffert U. Neutrophil serine proteases: potential key regulators of cell signalling during inflammation. Journal of Internal Medicine. 2005;257(4):319–328. doi: 10.1111/j.1365-2796.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 200.Bank U. Ansorge S. More than destructive: neutrophil-derived serine proteases in cytokine bioactivity control. Journal of Leukocyte Biology. 2001;69(2):197–206. doi: 10.1189/jlb.69.2.197. [DOI] [PubMed] [Google Scholar]
- 201.Von Dobschuetz E, Hoffmann T, Messmer K. Inhibition of neutrophil proteinases by recombinant serpin Lex032 reduces capillary no-reflow in ischemia/reperfusion-induced acute pancreatitis. Journal of Pharmacology and Experimental Therapeutics. 1999;290(2):782–788. [PubMed] [Google Scholar]
- 202.Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR. Cathepsin G activates protease-activated receptor-4 in human platelets. Journal of Biological Chemistry. 2000;275(10):6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- 203.Reilly C, Tewksbury D, Schechter N, Travis J. Rapid conversion of angiotensin I to angiotensin II by neutrophil and mast cell proteinases. Journal of Biological Chemistry. 1982;257(15):8619–8622. doi: 10.1016/S0021-9258(18)34171-1. [DOI] [PubMed] [Google Scholar]
- 204.Nadel J (1991). Role of Mast Cell and Neutrophil Proteases in Airway Secretion1–3. [DOI] [PubMed]
- 205.Pan S, Chen R, Stevens T, Bronner MP, May D, Tamura Y, et al. (2011) Proteomics portrait of archival lesions of chronic pancreatitis. PloS one;6(11):e27574. [DOI] [PMC free article] [PubMed]
- 206.Shamamian P, Pocock BJ, Schwartz JD, Monea S, Chuang N, Whiting D, et al. Neutrophil-derived serine proteinases enhance membrane type-1 matrix metalloproteinase–dependent tumor cell invasion. Surgery. 2000;127(2):142–147. doi: 10.1067/msy.2000.101155. [DOI] [PubMed] [Google Scholar]
- 207.Pintucci G, Iacoviello L, Castelli MP, Amore C, Evangelista V, Cerletti C, et al. Cathepsin G-induced release of PAI-1 in the culture medium of endothelial cells: a new thrombogenic role for polymorphonuclear leukocytes? The Journal of Laboratory and Clinical Medicine. 1993;122(1):69–79. [PubMed] [Google Scholar]
- 208.Wilson TJ, Nannuru KC, Singh RK. Cathepsin G–mediated activation of pro–matrix metalloproteinase 9 at the tumor-bone Interface promotes transforming growth factor-β signaling and bone destruction. Molecular Cancer Research. 2009;7(8):1224–1233. doi: 10.1158/1541-7786.MCR-09-0028. [DOI] [PubMed] [Google Scholar]
- 209.Kudo T, Kigoshi H, Hagiwara T, Takino T, Yamazaki M, Yui S (2009) Cathepsin G, a neutrophil protease, induces compact cell-cell adhesion in MCF-7 human breast cancer cells. Mediators of inflammation;2009. [DOI] [PMC free article] [PubMed]
- 210.Morimoto-Kamata R, Yui S. Insulin-like growth factor-1 signaling is responsible for cathepsin G-induced aggregation of breast cancer MCF-7 cells. Cancer Science. 2017;108(8):1574–1583. doi: 10.1111/cas.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Van Lint P, Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine & Growth Factor Reviews. 2006;17(4):217–223. doi: 10.1016/j.cytogfr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 212.Gutiérrez-Fernández A, Inada M, Balbín M, Fueyo A, Pitiot AS, Astudillo A, et al. Increased inflammation delays wound healing in mice deficient in collagenase-2 (MMP-8) The FASEB Journal. 2007;21(10):2580–2591. doi: 10.1096/fj.06-7860com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lin M, Jackson P, Tester AM, Diaconu E, Overall CM, Blalock JE, et al. Matrix metalloproteinase-8 facilitates neutrophil migration through the corneal stromal matrix by collagen degradation and production of the chemotactic peptide Pro-Gly-Pro. The American Journal of Pathology. 2008;173(1):144–153. doi: 10.2353/ajpath.2008.080081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Owen CA, Hu Z, Lopez-Otin C, Shapiro SD. Membrane-bound matrix metalloproteinase-8 on activated polymorphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. The Journal of Immunology. 2004;172(12):7791–7803. doi: 10.4049/jimmunol.172.12.7791. [DOI] [PubMed] [Google Scholar]
- 215.Jones LE, Humphreys MJ, Campbell F, Neoptolemos JP, Boyd MT. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: increased expression of matrix metalloproteinase-7 predicts poor survival. Clinical Cancer Research. 2004;10(8):2832–2845. doi: 10.1158/1078-0432.CCR-1157-03. [DOI] [PubMed] [Google Scholar]
- 216.Ueno H, Yamashita K, Azumano I, Inoue M, Okada Y. Enhanced production and activation of matrix metalloproteinase-7 (matrilysin) in human endometrial carcinomas. International Journal of Cancer. 1999;84(5):470–477. doi: 10.1002/(SICI)1097-0215(19991022)84:5<470::AID-IJC4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 217.Moilanen M, Pirilä E, Grenman R, Sorsa T, Salo T. Expression and regulation of collagenase-2 (MMP-8) in head and neck squamous cell carcinomas. The Journal of Pathology. 2002;197(1):72–81. doi: 10.1002/path.1078. [DOI] [PubMed] [Google Scholar]
- 218.Stadlmann S, Pollheimer J, Moser P, Raggi A, Amberger A, Margreiter R, et al. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. European Journal of Cancer. 2003;39(17):2499–2505. doi: 10.1016/j.ejca.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 219.Verspaget H, Kubben F, Tschesche H, Verheijen J, Hanemaaijer R, Lamers C. Matrix metalloproteinases increase with colorectal cancer progression. Fibrinolysis Proteolysis. 1999;13:38. [Google Scholar]
- 220.Qin G, Luo M, Chen J, Dang Y, Chen G, Li L, et al. Reciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Letters. 2016;374(1):85–95. doi: 10.1016/j.canlet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 221.Arechavaleta-Velasco F, Cuevas-Antonio R, Dominguez-Lopez P, Estrada-Moscoso I, Imani-Razavi FS, Zeferino-Toquero M, et al. Matrix metalloproteinase-8 promoter gene polymorphisms in Mexican women with ovarian cancer. Medical Oncology. 2014;31(8):132. doi: 10.1007/s12032-014-0132-3. [DOI] [PubMed] [Google Scholar]
- 222.Debniak T, Jakubowska A, Serrano-Fernández P, Kurzawski G, Cybulski C, Chauhan SR, et al. Association of MMP8 gene variation with an increased risk of malignant melanoma. Melanoma Research. 2011;21(5):464–468. doi: 10.1097/CMR.0b013e3283485fdd. [DOI] [PubMed] [Google Scholar]
- 223.Kader AK, Shao L, Dinney CP, Schabath MB, Wang Y, Liu J, et al. Matrix metalloproteinase polymorphisms and bladder cancer risk. Cancer Research. 2006;66(24):11644–11648. doi: 10.1158/0008-5472.CAN-06-1212. [DOI] [PubMed] [Google Scholar]
- 224.Väyrynen JP, Vornanen J, Tervahartiala T, Sorsa T, Bloigu R, Salo T, et al. Serum MMP-8 levels increase in colorectal cancer and correlate with disease course and inflammatory properties of primary tumors. International Journal of Cancer. 2012;131(4):E463–EE74. doi: 10.1002/ijc.26435. [DOI] [PubMed] [Google Scholar]
- 225.Sirniö P, Tuomisto A, Tervahartiala T, Sorsa T, Klintrup K, Karhu T, et al. High-serum MMP-8 levels are associated with decreased survival and systemic inflammation in colorectal cancer. British Journal of Cancer. 2018;119(2):213–219. doi: 10.1038/s41416-018-0136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stenman M, Paju A, Hanemaaijer R, Tervahartiala T, Leminen A, Stenman U-H, et al. Collagenases (MMP-1,-8 and-13) and trypsinogen-2 in fluid from benign and malignant ovarian cysts. Tumor Biology. 2003;24(1):9–12. doi: 10.1159/000070655. [DOI] [PubMed] [Google Scholar]
- 227.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences. 2006;103(33):12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, et al. Gelatinase B functions as regulator and effector in leukocyte biology. Journal of Leukocyte Biology. 2001;69(6):851–859. doi: 10.1189/jlb.69.6.851. [DOI] [PubMed] [Google Scholar]
- 229.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. Journal of the National Cancer Institute. 2002;94(15):1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 230.Mira E, Lacalle RA, Buesa JM, de Buitrago GG, Jiménez-Baranda S, Gómez-Moutón C, et al. Secreted MMP9 promotes angiogenesis more efficiently than constitutive active MMP9 bound to the tumor cell surface. Journal of Cell Science. 2004;117(9):1847–1857. doi: 10.1242/jcs.01035. [DOI] [PubMed] [Google Scholar]
- 231.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biology. 2000;2(10):737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gao D, Nolan D, McDonnell K, Vahdat L, Benezra R, Altorki N, et al. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2009;1796(1):33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proceedings of the National Academy of Sciences. 2007;104(51):20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mentzel T, Brown L, Dvorak H, Kuhnen C, Stiller K, Katenkamp D, et al. The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Archiv. 2001;438(1):13–22. doi: 10.1007/s004280000327. [DOI] [PubMed] [Google Scholar]
- 235.Pahler JC, Tazzyman S, Erez N, Chen Y-Y, Murdoch C, Nozawa H, et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10(4):329–IN2. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow–derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/S0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Acuff HB, Carter KJ, Fingleton B, Gorden DL, Matrisian LM. Matrix metalloproteinase-9 from bone marrow–derived cells contributes to survival but not growth of tumor cells in the lung microenvironment. Cancer Research. 2006;66(1):259–266. doi: 10.1158/0008-5472.CAN-05-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-α and leaves RANTES and MCP-2 intact. Blood, The Journal of the American Society of Hematology. 2000;96(8):2673–2681. [PubMed] [Google Scholar]
- 239.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. The American Journal of Pathology. 2011;179(3):1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. Journal of Biological Chemistry. 2009;284(38):25854–25866. doi: 10.1074/jbc.M109.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Reviews Immunology. 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 243.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annual Review of Immunology. 1993;11(1):105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 244.Droin N, Hendra J-B, Ducoroy P, Solary E. Human defensins as cancer biomarkers and antitumour molecules. Journal of Proteomics. 2009;72(6):918–927. doi: 10.1016/j.jprot.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 245.Welling MM, Hiemstra PS, van den Barselaar MT, Paulusma-Annema A, Nibbering PH, Pauwels E, et al. Antibacterial activity of human neutrophil defensins in experimental infections in mice is accompanied by increased leukocyte accumulation. The Journal of Clinical Investigation. 1998;102(8):1583–1590. doi: 10.1172/JCI3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Panyutich AV, Voitenok NN, Lehrer RI, Ganz T. An enzyme immunoassay for human defensins. Journal of Immunological Methods. 1991;141(2):149–155. doi: 10.1016/0022-1759(91)90141-2. [DOI] [PubMed] [Google Scholar]
- 247.Mizukawa N, Sugiyama K, Ueno T, Mishima K, Takagi S, Sugahara T. Defensin-1, an antimicrobial peptide present in the saliva of patients with oral diseases. Oral Diseases. 1999;5(2):139–142. doi: 10.1111/j.1601-0825.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 248.Ashitani J, Mukae H, Nakazato M, Ihi T, Mashimoto H, Kadota J, et al. Elevated concentrations of defensins in bronchoalveolar lavage fluid in diffuse panbronchiolitis. European Respiratory Journal. 1998;11(1):104–111. doi: 10.1183/09031936.98.11010104. [DOI] [PubMed] [Google Scholar]
- 249.Panyutich AV, Panyutich EA, Krapivin VA, Baturevich EA, Ganz T. Plasma defensin concentrations are elevated in patients with septicemia or bacterial meningitis. The Journal of Laboratory and Clinical Medicine. 1993;122(2):202–207. [PubMed] [Google Scholar]
- 250.van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS. Defensins: key players or bystanders in infection, injury, and repair in the lung? Journal of Allergy and Clinical Immunology. 1999;104(6):1131–1138. doi: 10.1016/S0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- 251.Zou H, Harrington JJ, Sugumar A, Klatt KK, Smyrk TC, Ahlquist DA. Detection of colorectal disease by stool defensin assay: an exploratory study. Clinical Gastroenterology and Hepatology. 2007;5(7):865–868. doi: 10.1016/j.cgh.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 252.Pausch T, Adolph S, Felix K, Bauer AS, Bergmann F, Werner J, et al. (2018) Antimicrobial peptide human neutrophil peptide 1 as a potential link between chronic inflammation and ductal adenocarcinoma of the pancreas. Pancreas;47(5):561–567. [DOI] [PubMed]
- 253.Müller CA, Markovic-Lipkovski J, Klatt T, Gamper J, Schwarz G, Beck H, et al. Human α-defensins HNPs-1,-2, and-3 in renal cell carcinoma: influences on tumor cell proliferation. The American Journal of Pathology. 2002;160(4):1311–1324. doi: 10.1016/S0002-9440(10)62558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Holterman DA, Diaz JI, Blackmore PF, Davis JW, Schellhammer PF, Corica A, et al., editors. Overexpression of α-defensin is associated with bladder cancer invasiveness. Urologic Oncology: Seminars and Original Investigations; 2006: Elsevier. [DOI] [PubMed]
- 255.Lundy FT, Orr DF, Gallagher JR, Maxwell P, Shaw C, Napier SS, et al. Identification and overexpression of human neutrophil α-defensins (human neutrophil peptides 1, 2 and 3) in squamous cell carcinomas of the human tongue. Oral Oncology. 2004;40(2):139–144. doi: 10.1016/S1368-8375(03)00142-8. [DOI] [PubMed] [Google Scholar]
- 256.Li J, Zhao J, Yu X, Lange J, Kuerer H, Krishnamurthy S, et al. Identification of biomarkers for breast cancer in nipple aspiration and ductal lavage fluid. Clinical Cancer Research. 2005;11(23):8312–8320. doi: 10.1158/1078-0432.CCR-05-1538. [DOI] [PubMed] [Google Scholar]
- 257.Balluff B, Rauser S, Meding S, Elsner M, Schöne C, Feuchtinger A, et al. MALDI imaging identifies prognostic seven-protein signature of novel tissue markers in intestinal-type gastric cancer. The American Journal of Pathology. 2011;179(6):2720–2729. doi: 10.1016/j.ajpath.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nature Immunology. 2005;6(6):551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 259.McKeown ST, Lundy FT, Nelson J, Lockhart D, Irwin CR, Cowan CG, et al. The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral Oncology. 2006;42(7):685–690. doi: 10.1016/j.oraloncology.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 260.Malik N, Kallestad J, Gunderson N, Austin S, Neubauer M, Ochs V, et al. Molecular cloning, sequence analysis, and functional expression of a novel growth regulator, oncostatin M. Molecular and Cellular Biology. 1989;9(7):2847–2853. doi: 10.1128/mcb.9.7.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Grenier A, Dehoux M, Boutten A, Arce-Vicioso M, Durand G, Gougerot-Pocidalo M-A, et al. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood. The Journal of the American Society of Hematology. 1999;93(4):1413–1421. [PubMed] [Google Scholar]
- 262.Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Research. 2005;65(19):8896–8904. doi: 10.1158/0008-5472.CAN-05-1734. [DOI] [PubMed] [Google Scholar]
- 263.Simpson JL, Baines KJ, Boyle MJ, Scott RJ, Gibson PG. Oncostatin M (OSM) is increased in asthma with incompletely reversible airflow obstruction. Experimental Lung Research. 2009;35(9):781–794. doi: 10.3109/01902140902906412. [DOI] [PubMed] [Google Scholar]
- 264.Cross A, Edwards SW, Bucknall RC, Moots RJ. Secretion of oncostatin M by neutrophils in rheumatoid arthritis. Arthritis and Rheumatism. 2004;50(5):1430–1436. doi: 10.1002/art.20166. [DOI] [PubMed] [Google Scholar]
- 265.Grenier A, Combaux D, Chastre J, Gougerot-Pocidalo MA, Gibert C, Dehoux M, et al. Oncostatin M production by blood and alveolar neutrophils during acute lung injury. Laboratory Investigation. 2001;81(2):133–141. doi: 10.1038/labinvest.3780220. [DOI] [PubMed] [Google Scholar]
- 266.Hurst SM, McLoughlin RM, Monslow J, Owens S, Morgan L, Fuller GM, et al. Secretion of oncostatin M by infiltrating neutrophils: regulation of IL-6 and chemokine expression in human mesothelial cells. The Journal of Immunology. 2002;169(9):5244–5251. doi: 10.4049/jimmunol.169.9.5244. [DOI] [PubMed] [Google Scholar]
- 267.Kerfoot SM, Raharjo E, Ho M, Kaur J, Serirom S, McCafferty D-M, et al. Exclusive neutrophil recruitment with oncostatin M in a human system. The American Journal of Pathology. 2001;159(4):1531–1539. doi: 10.1016/S0002-9440(10)62538-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Modur V, Feldhaus MJ, Weyrich AS, Jicha DL, Prescott SM, Zimmerman GA, et al. Oncostatin M is a proinflammatory mediator. In vivo effects correlate with endothelial cell expression of inflammatory cytokines and adhesion molecules. The Journal of Clinical Investigation. 1997;100(1):158–168. doi: 10.1172/JCI119508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Deboux M, Fierobe L, Grenier A, Toueg M, Malas V, Durand G, et al., editors. Elevated levels of oncostatin M and leukemia inhibitory factor in bronchoalveolar lavage fluid of patients with pneumonia. AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE; 1999: AMER LUNG ASSOC 1740 BROADWAY, NEW YORK, NY 10019 USA.
- 270.Pothoven KL, Norton JE, Suh LA, Carter RG, Harris KE, Biyasheva A, et al. (2017) Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. Journal of Allergy and Clinical Immunology;139(6):1966-78. e9. [DOI] [PMC free article] [PubMed]
- 271.Korzus E, Nagase H, Rydell R, Travis J. The mitogen-activated protein kinase and JAK-STAT signaling pathways are required for an oncostatin M-responsive element-mediated activation of matrix metalloproteinase 1 gene expression. Journal of Biological Chemistry. 1997;272(2):1188–1196. doi: 10.1074/jbc.272.2.1188. [DOI] [PubMed] [Google Scholar]
- 272.Badache A, Hynes NE (2001) Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Research;61(1):383–391. [PubMed]
- 273.Pothoven KL, Schleimer RP. The barrier hypothesis and Oncostatin M: restoration of epithelial barrier function as a novel therapeutic strategy for the treatment of type 2 inflammatory disease. Tissue Barriers. 2017;5(3):e1341367. doi: 10.1080/21688370.2017.1341367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Eyles JL, Roberts AW, Metcalf D, Wicks IP. Granulocyte colony-stimulating factor and neutrophils—forgotten mediators of inflammatory disease. Nature Clinical Practice Rheumatology. 2006;2(9):500–510. doi: 10.1038/ncprheum0291. [DOI] [PubMed] [Google Scholar]
- 275.Nicola N, Metcalf D. Binding of 125I-labeled granulocyte colony-stimulating factor to normal murine hemopoietic cells. Journal of Cellular Physiology. 1985;124(2):313–321. doi: 10.1002/jcp.1041240222. [DOI] [PubMed] [Google Scholar]
- 276.Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nature Reviews Rheumatology. 2009;5(10):554. doi: 10.1038/nrrheum.2009.178. [DOI] [PubMed] [Google Scholar]
- 277.Nakamura H, Ueki Y, Sakito S, Matsumoto K, Yano M, Miyake S, et al. High serum and synovial fluid granulocyte-colony stimulating factor (G-CSF) concentrations in patients with rheumatoid arthritis. Clinical and Experimental Rheumatology. 2000;18(6):713–718. [PubMed] [Google Scholar]
- 278.Campbell IK, Leong D, Edwards KM, Rayzman V, Ng M, Goldberg GL, et al. Therapeutic targeting of the G-CSF receptor reduces neutrophil trafficking and joint inflammation in antibody-mediated inflammatory arthritis. The Journal of Immunology. 2016;197(11):4392–4402. doi: 10.4049/jimmunol.1600121. [DOI] [PubMed] [Google Scholar]
- 279.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25(6):846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 280.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22(3):285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 281.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nature Reviews Immunology. 2014;14(9):585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Baggiolini M. Chemokines in pathology and medicine. Journal of Internal Medicine. 2001;250(2):91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 283.Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL–8 receptor types on PMN. Journal of Leukocyte Biology. 2009;86(3):529–543. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- 284.Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, Tal-Singer R, et al. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. Journal of Cystic Fibrosis. 2013;12(3):241–248. doi: 10.1016/j.jcf.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 285.Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER, et al. CXCR2 antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2015;191(9):1001–1011. doi: 10.1164/rccm.201405-0992OC. [DOI] [PubMed] [Google Scholar]
- 286.Chapman RW, Minnicozzi M, Celly CS, Phillips JE, Kung TT, Hipkin RW, et al. A novel, orally active CXCR1/2 receptor antagonist, Sch527123, inhibits neutrophil recruitment, mucus production, and goblet cell hyperplasia in animal models of pulmonary inflammation. Journal of Pharmacology and Experimental Therapeutics. 2007;322(2):486–493. doi: 10.1124/jpet.106.119040. [DOI] [PubMed] [Google Scholar]
- 287.Hunter MG, Druhan LJ, Massullo PR, Avalos BR. Proteolytic cleavage of granulocyte colony-stimulating factor and its receptor by neutrophil elastase induces growth inhibition and decreased cell surface expression of the granulocyte colony-stimulating factor receptor. American Journal of Hematology. 2003;74(3):149–155. doi: 10.1002/ajh.10434. [DOI] [PubMed] [Google Scholar]
- 288.Henry CM, Sullivan GP, Clancy DM, Afonina IS, Kulms D, Martin SJ. Neutrophil-derived proteases escalate inflammation through activation of IL-36 family cytokines. Cell Reports. 2016;14(4):708–722. doi: 10.1016/j.celrep.2015.12.072. [DOI] [PubMed] [Google Scholar]
- 289.Kurtagic E, Jedrychowski MP, Nugent MA. Neutrophil elastase cleaves VEGF to generate a VEGF fragment with altered activity. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2009;296(3):L534–LL46. doi: 10.1152/ajplung.90505.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Akizuki M, Fukutomi T, Takasugi M, Takahashi S, Sato T, Harao M, et al. Prognostic significance of immunoreactive neutrophil elastase in human breast cancer: long-term follow-up results in 313 patients. Neoplasia. 2007;9(3):260–264. doi: 10.1593/neo.06808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sato T, Takahashi S, Mizumoto T, Harao M, Akizuki M, Takasugi M, et al. Neutrophil elastase and cancer. Surgical Oncology. 2006;15(4):217–222. doi: 10.1016/j.suronc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 292.Stockley R, De Soyza A, Gunawardena K, Perrett J, Forsman-Semb K, Entwistle N, et al. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respiratory Medicine. 2013;107(4):524–533. doi: 10.1016/j.rmed.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 293.Elborn JS, Perrett J, Forsman-Semb K, Marks-Konczalik J, Gunawardena K, Entwistle N. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. European Respiratory Journal. 2012;40(4):969–976. doi: 10.1183/09031936.00194611. [DOI] [PubMed] [Google Scholar]
- 294.Aikawa N, Ishizaka A, Hirasawa H, Shimazaki S, Yamamoto Y, Sugimoto H, et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulmonary Pharmacology & Therapeutics. 2011;24(5):549–554. doi: 10.1016/j.pupt.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 295.Hagio T, Matsumoto S, Nakao S, Abiru T, Ohno H, Kawabata K. Elastase inhibition reduced death associated with acid aspiration-induced lung injury in hamsters. European Journal of Pharmacology. 2004;488(1–3):173–180. doi: 10.1016/j.ejphar.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 296.Ho A-S, Chen C-H, Cheng C-C, Wang C-C, Lin H-C, Luo T-Y, et al. (2014) Neutrophil elastase as a diagnostic marker and therapeutic target in colorectal cancers. Oncotarget;5(2):473. [DOI] [PMC free article] [PubMed]
- 297.Lerman I, de la Luz G-HM, Rangel-Moreno J, Chiriboga L, Pan C, Nastiuk KL, et al. Infiltrating myeloid cells exert protumorigenic actions via neutrophil elastase. Molecular Cancer Research. 2017;15(9):1138–1152. doi: 10.1158/1541-7786.MCR-17-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. Journal for Immunotherapy of Cancer. 2018;6(1):47. doi: 10.1186/s40425-018-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, et al. (2014) Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PloS one;9(3):e90353. [DOI] [PMC free article] [PubMed]
- 300.Deryugina E, Carré A, Ardi V, Muramatsu T, Schmidt J, Pham C, et al. (2020) Neutrophil elastase facilitates tumor cell intravasation and early metastatic events. Iscience;23(12). [DOI] [PMC free article] [PubMed]
- 301.Lau D, Mollnau H, Eiserich JP, Freeman BA, Daiber A, Gehling UM, et al. Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proceedings of the National Academy of Sciences. 2005;102(2):431–436. doi: 10.1073/pnas.0405193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Schoonbroodt S, Legrand-Poels S, Best-Belpomme M, Piette J. Activation of the NF-κB transcription factor in a T-lymphocytic cell line by hypochlorous acid. Biochemical Journal. 1997;321(3):777–785. doi: 10.1042/bj3210777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Simonneau M, Frouin E, Huguier V, Jermidi C, Jégou JF, Godet J, et al. Oncostatin M is overexpressed in skin squamous-cell carcinoma and promotes tumor progression. Oncotarget. 2018;9(92):36457. doi: 10.18632/oncotarget.26355. [DOI] [PMC free article] [PubMed] [Google Scholar]