Abstract
Intraductal papillary mucinous neoplasms (IPMNs) are mucin-secreting cystic neoplasm of pancreas. They have a malignant potential. They are usually localised to the pancreas but occasionally can involve surrounding structures (1.9%–6.6%), like bile duct and duodenum, and are labelled as IPMN with invasion. Jaundice as a manifestation of IPMN is not common (4.5%). It can present as jaundice as a result of invasion of common bile duct (CBD) resulting in stricture formation or uncommonly as a result of fistulising to CBD with resultant obstruction of CBD by thick mucin secreted by this tumour. As only few cases (around 23) of mucin-filled CBD are reported in the literature. We are presenting our experience in dealing a rare case of obstructive jaundice caused by IPMN fistulising into CBD, highlighting the difficulties faced in managing such case, especially with regards to biliary drainage and what can be the optimum management in such cases.
Keywords: pancreas and biliary tract, endoscopy
Background
Mucin-filled common bile duct (CBD) is an uncommon presentation of a more common disease, intraductal papillary mucinous neoplasm (IPMN). IPMN comprises 20%–30% of all cystic tumours of pancreas.1 They have a malignant potential. They are located predominantly in head of pancreas.2 They are usually localised to the pancreas but occasionally can involve surrounding structures (1.9%–6.6%), like bile duct and duodenum, and are labelled as IPMN with invasion (the term malignant IPMN is discouraged).3
Most common presentation is pain abdomen and weight loss (75%) followed by acute/recurrent pancreatitis. Jaundice as a manifestation of IPMN is not common (4.5%). It can present as jaundice as a result of invasion of CBD, resulting in stricture formation or uncommonly as a result of fistulising to CBD with resultant obstruction of CBD by thick mucin secreted by this tumour. IPMN presenting as jaundice is a poor prognostic sign.1 4
Only few studies are available to guide appropriate management on this type of presentation of IPMN. Our experience and review of the literature showed that early surgical biliary drainage could have led to a better outcome.
Case presentation
A 60-year-old woman presented with abdominal pain and jaundice for 9 months and fever for 2 months. Abdominal pain was predominantly in epigastric region, moderate to severe in intensity, radiating to back, not related to meals. Pain used to relieve only by oral or injectable analgesics. Jaundice was progressive, non-fluctuant, associated with severe itching and also clay-coloured stool, but not associated with Gasto-Intestinal (GI) bleed, abdominal distension, encephalopathy, alternative medication intake, prior surgery or lump abdomen. Fever was high grade, associated with chills and rigour, intermittent in nature with no diurnal variation. She was a known diabetic on insulin. She had lost significant weight of around 30 kg during her illness and also she had significant loss of her appetite.
Investigations
Haemoglobin (Hb): 8.6 gm/dL, total leucocyte count (TLC): 14 300 cells/mm3 (polymorphs: 85%).
Total bilirubin: 13 mg/dL, direct: 10 mg/dL, SGOT: 13 IU/L, SGPT: 37 IU/L, ALP: 267 IU/L, total protein: 6.1 gm/dL, S. alb: 2.4 gm/dL.
S.creatinine: 3.1 mg/dL, blood urea: 69 mg/dL.
Blood culture: Escherichia coli grown.
Bile culture: E. coli and Klebsiella pneumoniae grown.
Ultrasonography (USG) abdomen: approximately 3×2.8 cm size ill-defined soft tissue lesion in head and neck region of pancreas. Pancreas was atrophic. Main Pancreatic Duct (MPD) was dilated. CBD was 20 mm in size.
Contrast-enhanced computed tomography (CECT) abdomen: enhancing cystic lesion of size 17×14 mm in periampullary region, bilobar IntraHepatic Biliary Radicle Dilatation (IHBRD), dilated CBD (20 mm) and MPD (11 mm), both communicating in pancreatic head region along with mild ascites.
Differential diagnosis
Provisional diagnosis: lower end CBD obstruction likely due to periampullary carcinoma with cholangitis.
Other differential diagnoses for mucin-filled CBD are: biliary IPMN (Borderline Intraductal Papillary Mucinous Neoplasm), biliary papillomatosis, co-occurrence of IPMN and IPMB, carcinoma head of pancreas.5 6
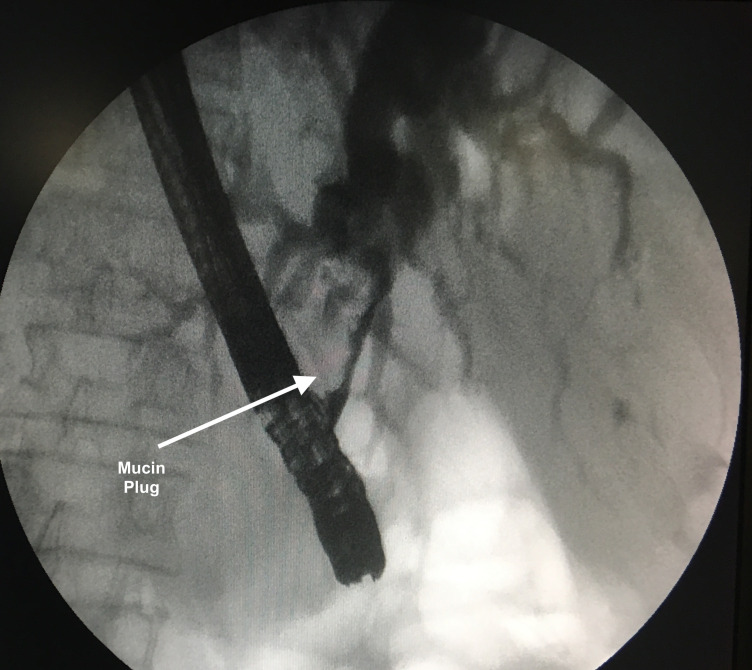
Differentiation of biliary IPMN and biliary papillomatosis from fistulising IPMN may not be possible on cross-sectional imaging and required cholangioscopy in many cases.7 Preoperative diagnosis of fistulising IPMN into CBD is rare, as in many studies described, the diagnosis was made after resection. Our diagnosis of fistulising IPMN with mucin-filled CBD was made based on CT abdomen, Endoscopic retrograde cholangiopancreatography (ERCP) and cytology. CT showed enhancing cystic lesion in periampullary region, with both CBD and MPD dilated and communicating in pancreatic head region. ERCP showed CBD opening, which was filled with mucus. Cholangiogram showed multiple irregular shaped filling defect that on balloon trawling could not be removed and the filling defects changed shape (figure 1, arrow). The cytology from mucin showed adenocarcinoma (video 1).
Figure 1.
Cholangiogram showing obstructed commonbile duct by mucin plug.
Video 1.
Patient was treated with broad-spectrum antibiotics (meropenem, ofloxacin, tramadol injections, paracetamol tablet, intravenous fluids).
ERCP: we noticed a large bulky papilla with extensive mucus extruding from it. As the selective CBD cannulation could not be done, we decided to do a fistulotomy. After fistulotomy, we could make out the CBD opening, which was filled with mucus. Cholangiogram was done and it showed multiple irregular shaped filling defect (figure 1, arrow). Balloon trawling was tried but mucus could not be removed and the filling defects changed the shape. A 10 french 10 cm straight flap stent was placed.
Outcome and follow-up
Her bilirubin came down to 2 mg/dL but started increasing again and reached 11 mg/dL after the plastic stent migrated in few days. Patient’s TLC also reached 22 500 cells/mm3.
Subsequently endoscopic Naso Biliary Drain (ENBD) was placed, which was irrigated repeatedly with N-acetylcysteine to prevent its blockage.
Unfortunately the ENBD also migrated and a Percutaneous Transhepatic Biliary Drain (PTBD) was done.
Cholangitis improved after PTBD and the patient was shifted to surgical intervention. But she could not be taken up for surgery due to her poor general condition and she succumbed to her illness.
Discussion
IPMNs are increasingly detected on CT/MRI done for other reasons. Invasion of IPMN into adjacent structures is uncommon and IPMN fistulising into CBD is rare. Frequency of adjacent organ involvement is duodenum (65.4%), stomach (19.2%), CBD (11.5%) and colon (3.8%).8 Factors responsible for fistula are relatively high pressure in the PD or invasion of IPMNs. Though IPMN fistulising to CBD is rare but frequency of jaundice in such patients is very high (97.1%).9
IPMNs are of two major types: main duct IPMN (MD-IPMN) and branch-duct IPMN (BD-IPMN).10 Since frequency of malignancy in MD-IPMN is about 70%, the current recommendation is that all MD-IPMN should be resected.11Preferred surgical options in MD-IPMN are pancreaticoduodenectomy, lateral pancreatectomy with splenectomy or total pancreatectomy.10 11 Frequency of malignancy in BD-IPMN is only 25%, and predictors of malignancy are presence of symptoms, size >3 cm and presence of mural nodules on imaging. Surgical resection is indicated only if any of these parameters are present.10 11 Surgery for BD-IPMN mostly involves standard pancreatic resections. However, middle pancreatectomy, spleen preserving lateral pancreatectomy and middle preserving pancreatectomy may be offered to selected cases. Multifocal BD-IPMN may need extended resection up to total pancreatectomy.10 11
Prognosis of IPMN with invasion is poorer than non-invasive IPMN. 5-year survival postsurgery is approximately 100% for non-invasive IPMN but only 24%–74% for invasive IPMN. Mean survival in IPMN with CBD fistula in surgical resection group was 47.9 months in comparison to 10.4 months in non-surgical group, but IPMNs still have a better survival than pancreatic adenocarcinoma.1 9 12 Like for adenocarcinoma resection, pancreaticoduodenectomy for IPMN has reasonable procedure-related morbidity, the most significant being pancreatic anastomotic leak/fistula.13 14 Two elegant papers from Italy emphasised that risk factors for pancreaticoduonectomy-related complications especially pancreatic fistula include soft pancreatic texture, pancreatic duct <3 mm, presence of jaundice, elderly age and comorbidities.13 14
In our case, we repeated failed in achieving adequate biliary drainage. We corroborated our finding with the published literature, which also showed similar results of almost universal failure of CBD stents. Even metal stents were also blocked by thick mucin.15Though we could achieve adequate drainage by PTBD, this was not the case in other studies. Achieving adequate drainage is even more difficult in cases of IPMN fistulising to CBD. Endoscopic measures and PTBD are at best a short-term measure to improve cholangitis in patients prior to surgery, but cannot be solely relied on as a definitive biliary drainage procedure. Almost all the published literature advocate early surgical drainage in such cases, that is, choledochojejunostomy.9 12 15 Surgery biliary drainage will also be the only option in many elderly patients who are unresectable because of poor performance status.
Patient’s perspective.
When I knew my mother has this disease, me and my family were very upset. We have been explained that this disease is malignant and is behaving aggressively. Though my mother could not be saved, the only satisfaction is that doctors did their best efforts.
Learning points.
The main limitation in our management was repeated endoscopic attempts at biliary drainage.
Though adequate drainage was achieved by Percutaneous Transhepatic Biliary Drain (PTBD), by that time. patient general condition became poor and patient could not be taken up for surgery.
Upfront surgical drainage could have had better outcomes.
Footnotes
Contributors: MK: drafting the article, acquisition of data or analysis and interpretation of data. US: revising it critically for important intellectual content. SS: revising it critically for important intellectual content. AD: conception and design and interpretation of data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Machado NO, Al Qadhi H, Al Wahibi K. Intraductal papillary mucinous neoplasm of pancreas. N Am J Med Sci 2015;7:160–75. 10.4103/1947-2714.157477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellano-Megías VM, Andrés CI-de, López-Alonso G, et al. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol 2014;6:311–24. 10.4251/wjgo.v6.i9.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basturk O, Hong S-M, Wood LD, et al. A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 2015;39:1730–41. 10.1097/PAS.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales-Oyarvide V, Fong ZV, Fernández-Del Castillo C, et al. Intraductal papillary mucinous neoplasms of the pancreas: strategic considerations. Visc Med 2017;33:466–76. 10.1159/000485014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren X, Zhu C-L, Qin X-F, et al. Co-Occurrence of IPMN and malignant IPNB complicated by a pancreatobiliary fistula: a case report and review of the literature. World J Clin Cases 2019;7:102–8. 10.12998/wjcc.v7.i1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SS, Kim M-H, Lee SK, et al. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer 2004;100:783–93. 10.1002/cncr.20031 [DOI] [PubMed] [Google Scholar]
- 7.Somogyi L, Dimashkieh H, Weber FL, et al. Biliary intraductal papillary mucinous tumor: diagnosis and localization by endoscopic retrograde cholangioscopy. Gastrointest Endosc 2003;57:620–2. 10.1067/mge.2003.157 [DOI] [PubMed] [Google Scholar]
- 8.Ravaud S, Laurent V, Jausset F, et al. CT and MR imaging features of fistulas from intraductal papillary mucinous neoplasms of the pancreas to adjacent organs: a retrospective study of 423 patients. Eur J Radiol 2015;84:2080–8. 10.1016/j.ejrad.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 9.Kurihara K, Nagai H, Kasahara K, et al. Biliopancreatic fistula associated with intraductal papillary-mucinous pancreatic cancer: institutional experience and review of the literature. Hepatogastroenterology 2000;47:1164–7. [PubMed] [Google Scholar]
- 10.Crippa S, Partelli S, Falconi M. Extent of surgical resections for intraductal papillary mucinous neoplasms. World J Gastrointest Surg 2010;2:347–51. 10.4240/wjgs.v2.i10.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6:17–32. 10.1159/000090023 [DOI] [PubMed] [Google Scholar]
- 12.Komo T, Oishi K, Kohashi T, et al. Pancreatobiliary fistula associated with intraductal papillary mucinous carcinoma accompanying obstructive jaundice: a case report. Int J Surg Case Rep 2018;48:126–30. 10.1016/j.ijscr.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conzo G, Gambardella C, Tartaglia E, et al. Pancreatic fistula following pancreatoduodenectomy. evaluation of different surgical approaches in the management of pancreatic stump. literature review. Int J Surg 2015;21(Suppl 1):S4–9. 10.1016/j.ijsu.2015.04.088 [DOI] [PubMed] [Google Scholar]
- 14.Mauriello C, Polistena A, Gambardella C, et al. Pancreatic stump closure after pancreatoduodenectomy in elderly patients: a retrospective clinical study. Aging Clin Exp Res 2017;29:35–40. 10.1007/s40520-016-0657-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel A, Lambiase L, Decarli A, et al. Management of the mucin filled bile duct. A complication of intraductal papillary mucinous tumor of the pancreas. J Pancreas 2005;6:255–9. [PubMed] [Google Scholar]