Abstract
A 19-year-old man was admitted with a 2-week history of continuous cough along with a day history of acute onset unsteadiness and hiccups. Given the current pandemic, he was initially suspected to have COVID-19, however he tested negative on two occasions. Subsequent brain magnetic resonance imaging (MRI)confirmed a small left acute and subacute lateral medullary infarction with chest X-ray suggesting aspiration pneumonia with right lower lobe collapse. This is a distinctive case of posterior circulation stroke presenting with a new continuous cough in this era of COVID-19 pandemic. We anticipate based on MRI findings that his persistent cough was likely due to silent aspiration from dysphagia because of the subacute medullary infarction. It is therefore imperative that healthcare workers evaluate people who present with new continuous cough thoroughly to exclude any other sinister pathology. We should also be familiar with the possible presentations of posterior circulation stroke in this pandemic era.
Keywords: stroke, infectious diseases
Background
Undoubtedly in the current pandemic, a new continuous cough has become a red flag symptom attributed to COVID-19. Cough might occasionally be seen in neurological diseases. However, it is now being recognised that there is an increasing association of symptoms of cough and neurological diseases with cases of COVID-19 manifesting as acute stroke, confusion and encephalopathy.1 As we discover more COVID-19-related neurological diseases, we should also recognise COVID-19 mimics secondary to neurological diseases.
Posterior circulation stroke is a notable cause of dysphagia and in some cases could manifest solely as respiratory symptoms of sore throat, cough and dysphagia.2
Case presentation
A 19-year-old, non-smoker, normally fit and well man presented to our hospital during the first quarter of this year with a 2-week history of continuous cough. There was no history of fever, recent travel or contact with anyone with COVID-19. However, he had been in self-isolation because of this symptom.
A day before presentation, he developed a sudden onset disequilibrium, sense of vertigo with associated nausea and several episodes of vomiting while standing. He described a history of left facial paraesthesia and left-sided neck pain prior to his hospital presentation with some difficulty in clearing his throat in the preceding days, which became apparent during detailed history taking. No prior vascular risk factor was identifiable.
There was no family history of stroke or cardiovascular disease, no history of significant childhood disease and his younger sibling was healthy. There were no high-risk behaviours or recreational drug use.
Detailed neurological examination revealed subtle left-sided incoordination of both upper and lower limbs and horizontal gaze evoked nystagmus. There was evident dysarthria and impairment in swallowing due to impaired laryngeal elevation. There were no features suggestive of Horner’s syndrome. No visual field deficit was identified and there were no other apparent cranial nerve deficits. Power was normal in all limbs with no objective sensory impairment.
From the history of cough in this current pandemic, the patient was initially suspected to have COVID-19. Therefore, he was isolated and swabbed for COVID-19. However, his symptoms coupled with detailed examination were suggestive of a brainstem lesion. The sudden onset of the neurological symptoms leaned towards a vascular aetiology. However, given age and lack of vascular risk factors for stroke, other differentials were also considered initially.
Investigations
Routine blood tests including initial inflammatory markers were normal. Cholesterol and venous glucose were unremarkable. ECG demonstrated sinus rhythm. Transthoracic echocardiography was within normal limits with no thrombus in left ventricle, no obvious valvular lesions and with no flow seen through the atrial septum.
The patient had initial COVID-19 nasal swab screen which was negative. Chest X-ray on admission was within normal limits. Computed Tomography(CT) scan of the brain and CT angiography (CTA) of the neck and brain were normal with no evidence of dissection. MRI of the brain, however, revealed diffusion restriction in left lateral medulla. Interestingly, there was mismatch between the two diffusion weighted imaging(DWI) (b0 and b1000 sequences, respectively) and apparent diffusion coefficient (ADC) sequences (figure 1) thereby hinting at the probability of an acute and subacute ischaemic change. The radiological changes suggested that an initial event may have triggered the cough and a subsequent extension caused significant neurological deficit to persuade the patient come into hospital.
Figure 1.
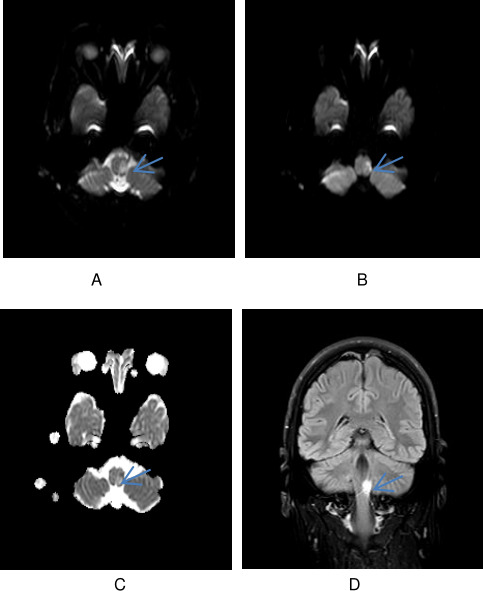
(A) DWI b0 image of the lesion in the lateral part of the left medulla. (B) DWI b1000 image of the lesion in the lateral part of the left medulla. (C) ADC map demonstrating the variable diffusion restriction in the lesion suggestive of acute on a subacute infarct. (D) Coronal fluid attenuated inversion recovery (FLAIR) image showing an infarct in the left medulla.
On the third day of admission, patient became febrile and had worsened respiratory distress. Repeat chest radiograph demonstrated collapse consolidation of the right lower lobe (figure 2). A repeat COVID-19 nasal swab was sent which also came out as negative. A COVID-19 antibody test during the time of presentation was not in vogue.
Figure 2.
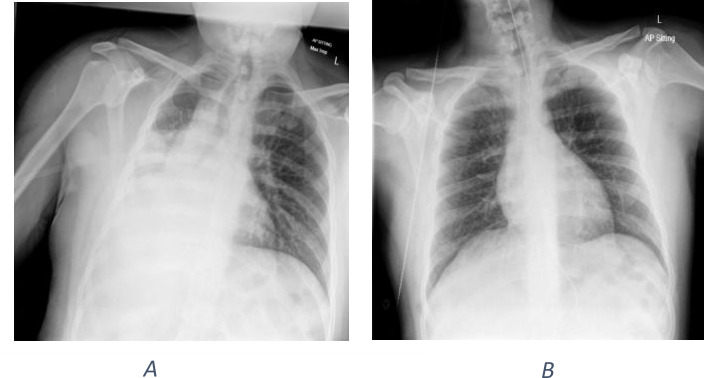
(A) Chest radiograph done at the time of respiratory distress, showing consolidation and collapse in the right lower lung zones initially suspicious for COVID-19. (B) Chest radiograph after 3 days of antibiotics for the treatment of aspiration pneumonia, showing significant improvement in the chest radiograph.
Vasculitis and thrombophilia screen along with check for HIV were negative.
Differential diagnosis
In the assessment of neurological disease, localisation of a neurological finding is important before determining the cause. The examination findings were suggestive of a brainstem lesion and subsequently MRI confirmed a lateral medullary infarct. In the young population especially with no risk factors, vertebral artery dissection (VAD) is a common cause of posterior circulation ischaemic (POCI) stroke.3 Given the history of cough, which could precipitate VAD, and the history of left-sided neck pain, this was a strong differential.4 5 However, CTA did not confirm a VAD or any intracranial or extracranial vessel abnormality.
It is worth noting that the standard techniques for imaging the arteries that are commonly available are CTA, magnetic resonance angiography (MRA) and digital subtraction angiography (DSA). It is known that these techniques although can reveal abnormalities of the vessel lumen, but do not readily distinguish vessel wall pathology. There is therefore a growing need for direct visualisation of the vessel wall with high-resolution intracranial vessel wall MR imaging (VW-MR imaging). Intracranial VW-MR imaging is likely a useful adjunct to conventional imaging to differentiate causes of intracranial arterial narrowing such as intracranial atherosclerotic plaque, vasculitis, reversible cerebral vasoconstriction syndrome and arterial dissection to name a few and may also help to identify symptomatic, non-stenotic disease of the intracranial arteries.6 However, dedicated VW-MR imaging is not available at many centres and is currently not in practice at our institution due to difficulties in image acquisition but may be available in the not-so-distant future.
Cardioembolic stroke is also a possibility in this case with 40% of posterior circulation strokes deemed to have a cardioembolic origin.3 However, a 72-hour ambulatory ECG monitoring and echocardiogram did not reveal any paroxysmal atrial fibrillation or structural heart disease
Our patient is still undergoing further investigations like formal bubble echo and if initial tests are inconclusive and the stroke is deemed cryptogenic, he may need referral for consideration of an implantable loop recorder to look for paroxysmal atrial fibrillation. Previous studies indicate that atrial fibrillation is more likely to be detected with an insertable cardiac monitor than with conventional follow-up in patients with a recent cryptogenic stroke.7
Treatment, outcome and follow-up
The patient was admitted to the stroke unit. He was not thrombolysed as his initial National Institutes of Health Stroke Scale Score was 2 on review by the stroke physician. Also, initial diagnosis was a bit unclear in view of his staggered symptoms.
He was given high-dose aspirin for the treatment of ischaemic stroke, and then was switched to low-dose clopidogrel after 2 weeks. He was formally assessed by the speech and language team the following day. Given he had reduced laryngeal sensation, elevation and penetration, which would be a high risk for aspiration, a nasogastric tube was inserted to support his nutrition and medications. His symptom of nausea was controlled with regular metoclopramide which was overlapped with gabapentin to also treat his hiccups.
On the third day, the patient deteriorated with fever, hypoxia and shortness of breath. A chest radiograph demonstrated a right lower lobe collapse and consolidation likely from aspiration. He was treated with intravenous amoxicillin, extensive chest physiotherapy and oxygen therapy. He made an excellent recovery on ward-based active treatment within the next few days.
At the time of the early pandemic, the team revisited the patient’s presentation on whether he may have had COVID-19-related ischaemic stroke. However, clinical and radiological features were more in keeping with an aspiration secondary to dysphagia typically associated with brain stem strokes. Our patient underwent a CT angiogram and this was unremarkable with no obvious suggestion of vasculopathy and clinical, radiological along with inflammatory parameters improved rapidly following treatment for clinical and radiological aspiration.
The patient has recovered completely from his illness. He is currently on normal diet and fluids. He is independent in mobility and all activities of daily living. He has returned to his work and is under follow-up in the outpatient stroke clinic.
Discussion
POCI strokes are responsible for close to 30% of strokes in the UK.8 These involve vertebrobasilar system and can present with several brainstem syndrome including lateral medullary syndrome, also known as posterior inferior cerebellar artery (PICA) or Wallenberg syndrome. Typical constellation of lateral medullary syndrome features include dizziness, ataxia, crossed sensory signs, nystagmus, dysphagia and Horner’s syndrome, while some may also develop intractable hiccups.9 Lateral medullary infarcts can be caused by ischaemia involving the vertebral artery, PICA artery and the lateral or medial branch of the PICA artery.3 However, it is of note that most of the patients do not present with the full constellation of the syndrome depending on the branches of PICA affected and degree of collateral supply.3
Patients with lateral medullary infarct could present rarely with isolated dysphagia and aspiration pneumonia.2 One must be vigilant about atypical presentations of a posterior circulation stroke to facilitate prompt evaluation and treatment of patients to avert fatal and disabling complications especially in the current COVID-19 pandemic.10
This pandemic era has interestingly revealed the unlikely harmony between respiratory and neurological diseases.11 Several COVID-19 cases are being linked with diverse neurological symptoms like confusion, acute cerebrovascular disease, encephalitis and encephalopathy.1 12 13 It has been postulated that this might be a result of the presence of ACE-2 receptors in the brain.14 It is also known that neurological diseases could present with respiratory symptoms like shortness of breath. This often is seen in neuromuscular diseases like myasthenia gravis causing restrictive lung pathology.
Current literature has suggested the probability of 7·6-fold increase in the odds of stroke with COVID-19 compared with influenza. The mechanisms in COVID-19 include a hypercoagulable state from systemic inflammation and cytokine storm, postinfectious immune-mediated responses and direct viral-induced endotheliitis or endotheliopathy, potentially leading to angiopathic thrombosis along with cardiomyopathy to name a few.15 16 One study found 13.5% of patients with COVID-19 had neurological disorder (1.9% of which had stroke), which was associated with an increased risk of in-hospital mortality (HR 1.38, 95% CI 1.17 to 1.62).17 A recent report of six consecutive patients with acute ischaemic stroke and COVID-19 suggested that all patients had large vessel occlusion with markedly elevated D-dimer levels, often with a period of delay from infection to stroke.18
In our index patient, there was no evidence of COVID-19 infection after two negative swabs to suggest any causal association with his stroke. Although COVID-19 antibody was unavailable at that period to check for previous exposure, recent systematic review and meta-analysis suggests that currently available evidence does not favour the continued use of the existing point-of-care serological tests for COVID-19 antibody testing.19 However, this may be useful in diagnosing COVID-19 in patients with late presentation, prolonged symptoms or negative results from reverse transcription PCR tests.13
Although, there have been cases linking isolated dysphagia with lateral medullary infarcts, that of a POCI stroke presenting with a new continuous cough is rarely reported.2 In our index case, the initial history of cough preceding the more acute symptom of disequilibrium correlated with the MRI finding of acute and subacute infarcts in the medulla. This further validates our hypothesis that the subacute medullary infarct must have been the precipitant of the cough right from the start. In addition, there are also known case reports of patients with cough who were found to have silent aspirations from impaired swallowing confirmed on investigation. This difficulty with swallowing was not often apparent on history or examination.20 This might explain the delayed presentation.
It should be noted that a sinister pathology like POCI stroke in patients who present with new continuous cough unrelated to COVID-19 might not be unravelled early enough as people follow self-isolation guidance from the government. The patients who may also present to the emergency department might not be evaluated extensively enough to exclude other serious pathologies due to human factors and bias.
Therefore, as we triage patients who present with new continuous cough in the emergency department, we must recognise the COVID-19 mimics especially when there are neurological signs in those patients. In a person with new cough and neurological signs, we must think about a POCI stroke as a differential while also keeping in mind COVID-19-related neurological disease.
Learning points.
Health workers must extensively evaluate patient with new cough and exclude sinister causes of cough unrelated to COVID-19.
In individuals with recurrent cough and new neurological signs, a brainstem cause must be considered.
We must recognise the atypical presentation of posterior circulation strokes in this current pandemic. Cough and hiccups could be a feature of lateral medullary infarction.
Footnotes
Contributors: MW: managed the patient, conceived the idea of the case report, was involved in planning, literature search and referencing for the case report, took consent from the patient, wrote the initial draft of the case report, revised the case report and approved the final draft of the case report. PB: managed the patient, conceived the idea of the case report, was involved in planning of the case report, wrote the initial draft of case report, revised the case report and approved the final draft of the case report and is the guarantor. SD: managed the patient, was involved in planning the case report, did literature search, revised and critically analysed the initial draft and approved the final draft of the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 2020;12:e7352. 10.7759/cureus.7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García Carretero R, Romero Brugera M, Rebollo-Aparicio N, et al. Dysphagia and aspiration as the only manifestations of a stroke. BMJ Case Rep 2016;2016. 10.1136/bcr-2015-213817. [Epub ahead of print: 11 Feb 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merwick Áine, Werring D. Posterior circulation ischaemic stroke. BMJ 2014;348:g3175. 10.1136/bmj.g3175 [DOI] [PubMed] [Google Scholar]
- 4.Nomura M, Kannuki S, Kuwayama K, et al. A patient with Wallenberg's syndrome induced by severe cough. J Clin Neurosci 2004;11:179–82. 10.1016/S0967-5868(03)00139-5 [DOI] [PubMed] [Google Scholar]
- 5.Mehdi E, Aralasmak A, Toprak H, et al. Craniocervical dissections: radiologic findings, pitfalls, mimicking diseases: a pictorial review. Curr Med Imaging Rev 2018;14:207–22. 10.2174/1573405613666170403102235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38:218–29. 10.3174/ajnr.A4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 8.Flossmann E, Rothwell PM. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain 2003;126:1940–54. 10.1093/brain/awg197 [DOI] [PubMed] [Google Scholar]
- 9.Sampath V, Gowda MR, Vinay HR, et al. Persistent hiccups (singultus) as the presenting symptom of lateral medullary syndrome. Indian J Psychol Med 2014;36:341–3. 10.4103/0253-7176.135397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huff JS. Stroke mimics and chameleons. Emerg Med Clin North Am 2002;20:583–95. 10.1016/S0733-8627(02)00012-3 [DOI] [PubMed] [Google Scholar]
- 11.Polkey MI, Lyall RA, Moxham J, et al. Respiratory aspects of neurological disease. J Neurol Neurosurg Psychiatry 1999;66:5–15. 10.1136/jnnp.66.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolaji P, Kukoyi B, Ahmad N, et al. Extensive cerebral venous sinus thrombosis: a potential complication in a patient with COVID-19 disease. BMJ Case Rep 2020;13. 10.1136/bcr-2020-236820. [Epub ahead of print: 11 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson J, Richter A, Deeks J. Testing for SARS-CoV-2 antibodies. BMJ 2020;370:m3325. 10.1136/bmj.m3325 [DOI] [PubMed] [Google Scholar]
- 14.Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fifi JT, Mocco J. COVID-19 related stroke in young individuals. Lancet Neurol 2020;19:713–5. 10.1016/S1474-4422(20)30272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaschetto R, Cena T, Sainaghi PP, et al. Cerebral nervous system vasculitis in a Covid-19 patient with pneumonia. J Clin Neurosci 2020;79:71–3. 10.1016/j.jocn.2020.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized COVID-19 patients in New York City. Neurology 2020:10.1212/WNL.0000000000010979. 10.1212/WNL.0000000000010979 [DOI] [Google Scholar]
- 18.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry 2020;91:889–91. 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisboa Bastos M, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ 2020;24:m2516 10.1136/bmj.m2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa C, Drozdz D, Jesus P, et al. Pharyngeal swallowing phase and chronic cough. Int Arch Otorhinolaryngol 2013;16:502–8. 10.7162/S1809-97772012000400012 [DOI] [PMC free article] [PubMed] [Google Scholar]


