Abstract
Upper tract urothelial carcinoma (UTUC) is often diagnosed late and exhibits poor prognosis. Only limited data are available concerning therapeutic regimes and potential biomarkers for disease monitoring. Standard therapies often provide only insufficient treatment options. Hence, immunotherapies and complementary approaches, such as personalized neoepitope-derived multipeptide vaccine (PNMV), come into focus. In this context, genetic analysis of tumor tissue by whole exome sequencing represents an essential diagnostic step in order to calculate tumor mutational burden (TMB) and to reveal tumor-specific neoantigens. Furthermore, disease progression is essential to be monitored. Longitudinal screening of individually known mutations in plasma circulating tumor DNA (ctDNA) by the use of next-generation sequencing and digital droplet PCR (ddPCR) might be a promising method to fill this gap.
Here, we present the case of a 55-year-old man who was diagnosed with high-risk metastatic UTUC in 2015. After initial surgery and palliative chemotherapy, he developed recurrence of the tumor. Genetic analysis revealed a high TMB of 41.2 mutations per megabase suggesting a potential success of immunotherapy. Therefore, in 2016, off-label treatment with the checkpoint-inhibitor pembrolizumab was started leading to strong regression of the disease. This therapy was then discontinued due to side effects and treatment with a previously produced PNMV was started that induced strong T cell responses. During both treatments, plasma Liquid Biopsies (pLBs) were performed to measure the number of mutated molecules per mL plasma (MM/mL) of a known tumor-specific variant in the MLH1 gene by ddPCR for longitudinal monitoring. Under treatment, MM/mL was constantly zero. A few months after all therapies had been discontinued, an increase of MM/mL was detected that persisted in the following pLBs. When MRI scans proved tumor recurrence, treatment with pembrolizumab was started again leading to a rapid decrease of MM/mL in the pLB to again zero. Treatment response was then also confirmed by MRI.
This case shows that use of immunotherapy and PNMV might be a promising treatment option for patients with high-risk metastatic UTUC. Furthermore, measurement of individually known tumor mutations in plasma ctDNA by the use of pLB could be a very sensitive biomarker to longitudinally monitor disease.
Keywords: immunotherapy, vaccination, urologic neoplasms, translational medical research, tumor biomarkers
Case report
Background
Upper tract urothelial carcinoma (UTUC) is a rather rare entity of cancer with 60% of invasive carcinoma at the time of diagnosis.1 The gold standard for high-risk disease is radical nephroureterectomy2 and the established first-line therapy for metastasized UTUC is platinum-based adjuvant chemotherapy. However, many patients treated with this first-line therapy suffer from side effects and 26.5% of patients even show disease progression.3 4 In the age of next-generation sequencing, it is possible to analyze a tumor comprehensively with respect to its somatic mutations. Thus, one can gain information about tumor-specific mutations to then design individual therapies. Many recent studies have shown that tumor mutational burden (TMB) and microsatellite instability (MSI) are good predictors of response to immunotherapy.5 6 This makes UTUC carrying high TMB a promising target for immunotherapy. Indeed, the immune checkpoint inhibitor (ICI) pembrolizumab as second-line therapy showed significantly longer survival rates, regardless of the PD-L1 (programmed cell death 1 ligand 1) expression.7 This approach can be combined with other immune-stimulatory therapies, such as personalized neoepitope-derived multipeptide vaccines (PNMVs), where genetic analysis of the tumor is used to design peptides mimicking the neoantigens that the tumor is most likely to present on its surface. The synthesized peptides either bind to human leukocyte antigen class I (HLA-I) or are presented via HLA-II by antigen-presenting cells (APCs). The HLA genes are extremely polymorphic making it necessary to haplotype the individual patient’s HLA to elicit the best immune response. T cells are then supposed to be primed by interacting with the peptide-presenting APC, so that they recognize the neoantigen-presenting tumor. PNMV is likely most fitting for a patient setting with minimal residual disease to establish a sort of ‘cancer surveillance’.8 The combination of ICI and PNMV aims at priming the patient’s immune system to selectively attack cancer cells and prevent progression. However, up to date, this is largely theory and has not reached clinical practice yet. According to the US National Library of Medicine’s Clinical Trials Database, only some studies currently explore the combination treatment, none of them for UTUC.9
For the purpose of staging, clinicians mainly rely on imaging techniques. But these struggle to detect early progression of disease. As a result, UTUC and disease progression are often detected late in an advanced state, so biomarkers are urgently needed in the treatment of UTUC.1 Easy to obtain are blood samples, but there is very limited data available on blood-based biomarkers in UTUC.10 However, there are some candidates for biomarkers at stake. One promising candidate presents circulating tumor DNA (ctDNA) that can be sampled from the patient’s peripheral blood by a method called plasma Liquid Biopsy (pLB). The amount of ctDNA in the peripheral blood is thought to correlate with tumor mass. A positive correlation has been shown for various cancer types.11 12 Measurement of individually known variants in plasma ctDNA by digital droplet PCR (ddPCR) represents a sensitive method to monitor disease activity longitudinally, that may match the need for a clinical biomarker.13
Here, we introduce the case of a patient with high-risk, metastatic UTUC treated with immunotherapy as well as PNMV whose course of disease has been monitored by pLB-based ddPCR.
Case presentation
A 55-year-old man was diagnosed with high-risk UTUC of the right ureter in April 2015. Staging and grading revealed stage pT2 N1 M0 (1/1) G2 with a paraaortal lymph node metastasis. The patient underwent radical nephroureterectomy in May 2015 followed by two cycles of palliative chemotherapy (gemcitabine/carboplatin). In July 2015, the chemotherapy was discontinued due to side effects, especially urticaria.
Two months later, in September 2015, the patient developed tumor recurrence in the bladder and hence underwent transurethral resection of the bladder (TUR-B). Four days later, a CT scan revealed extended infestation of cervical, supraclavicular, retroperitoneal, and retrocrural lymph nodes and a second CT scan in March 2016 showed strong progression of the lymph node metastases (figure 1A). Later in March, a bone metastasis of the right seventh rib was discovered.
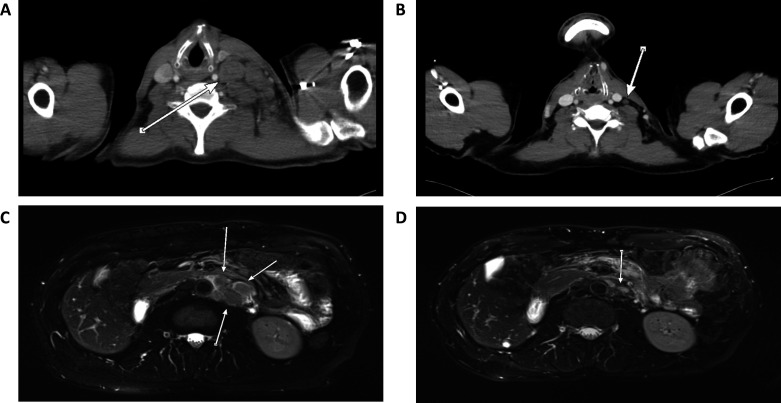
Figure 1.
CT/MRI scans at different time points over the course of the disease. (A) CT scan from March 2016 before start of initial treatment with pembrolizumab showing strong lymph node metastases (arrow). (B) CT scan from April 2018 under initial therapy with pembrolizumab showing shrinkage of the metastases (arrow) indicating great treatment response supported by mutated molecules per mL plasma (MM/mL) constantly at zero. (C) MRI scan from November 2019, 6 months after discontinuation of treatment with personalized neoepitope-derived multipeptide vaccines showing reappearance of enlarged paraaortal lymph nodes (arrows) corresponding with increasing MM/mL between 44.49 and 188.01. (D) MRI scan from February 2020, 3 months after re-initiation of treatment with pembrolizumab showing shrinkage of the enlarged lymph nodes (arrow) indicating a new treatment response corresponding with MM/mL returning to zero.
The patient’s tumor tissue was genetically analyzed by whole exome sequencing (WES) in order to potentially reveal new treatment targets. For the analysis, tissue from the TUR-B procedure from September 2015 was used. Results are displayed in online supplemental table S1. The genetic analysis showed a very high TMB of 41.2 mutations per megabase. One important tumor-specific genetic variant detected by WES analysis was the one of the MLH1 gene (MLH1 c.883A>T; p.Ser295Cys). The MLH1 gene encodes for the MutL homolog protein 1, which plays an important role in the mismatch repair (MMR). Defects in MLH1 are associated with MSI, which was also predicted in our patient. For more details of the technical methods, please see the online supplemental material.
jitc-2020-001406supp001.pdf (816KB, pdf)
Even though PD-L1 testing was not established at this time, immunotherapy was recommended based on DNA MMR deficiency and high TMB in the tumor genome, and in April 2016 off-label therapy with the ICI pembrolizumab (2 mg/kg body weight every 3 weeks) was started. The patient generally tolerated the immunotherapy well, but his fifth cycle of pembrolizumab in July 2016 was delayed for 10 days due to subclinical elevations in liver transaminases.
Furthermore, because of the high TMB, the tumor was considered likely to have many mutated antigens present on its surface providing a rationale for a treatment attempt with PNMVs. In order to produce such vaccines, the tumor specimen was analyzed by WES, followed by prediction of neoepitopes and selection of peptides (see online supplemental material). Seven major histocompatibility complex (MHC) class I peptides and three longer MHC class II peptides were chosen that were then used for the production of the personalized vaccine (table 1). The vaccine was produced as an individual healing attempt in Tuebingen, Germany, in May 2016, but following the patient’s wish was not yet applied at this time.
Table 1.
Overview of the immune monitoring results
| Peptide-specific immune respones | September 13, 2018 (V9) | March 14, 2019 (V17) | July 08, 2019 (post V18) | |||||||
| No. | Peptide sequence | Gene and somatic variant | HLA | VAF (%) | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 |
| 1 | HSYRGTGGIFK | TBL1XR1 NM_024665 c.G1462A:p.E488K |
HLA-A*03:01 | 36 | – | – | ++ SI: 4.3 (1.7%) |
+ SI: 2.1 (0.6%) |
Pool peptide 1+5 +++ SI: 7.3 (0.4%) |
Pool peptide 1+5 ++++ SI: 15.9 (6.5 %) |
| 2 | MMFRNYQRK | CHD4 NM_001273 c.G1705A:p.V569M |
HLA-A*03:01 | 38 | – | – | – | ++ SI: 3.8 (1,8%) |
– | + SI: 3.2 (0.6 %) |
| 3 | EVSAAHRAHYF | DNMT3A NM_022552.4 c.G2375A:p.R792H |
HLA-A*26:01 | 36 | +++ SI: 7.7 (1.1%) |
(+) positive tendency | + SI: 2.2 (0.6%) |
– | Pool peptide 3+7 ++++ SI: 21.6 (1.8%) |
Pool peptide 3+7 – |
| 4 | DPTASVPSM | KIAA1549 NM_001164665 c.G4753A:p.V1585M |
HLA-B*35:01 | 33 | +++ SI: 5.6 (4.3%) |
– | ++ SI: 3.7 (1.6%) |
– | Pool peptide 4+6 ++++ SI: 11.6 (0.5%) |
Pool peptide 4+6 – |
| 5 | HIPIIWATSY | PIK3C2B NM_002646 c.G1919A:p.R640H |
HLA-A*26:01 | 45 | – | – |
++++ SI: 13.1 (6,6%) |
+++ SI: 6.2 (2.9%) |
Pool peptide 1+5 +++ SI: 7.3 (0.4%) |
Pool peptide 1+5 ++++ SI: 15.9 (6.5 %) |
| 6 | VQRRAQGKLF | KEL NM_000420 c.A869T:p.E290V |
HLA-B*15:01 | 37 | – | – | – | + SI: 2.1 (1.0%) |
Pool peptide 4+6 ++++ SI: 11.6 (0.5) |
Pool peptide 4+6 – |
| 7 | AFDDKTRLV | WWC3 NM_015691 c.T662C:p.V221A |
HLA-C*04:01 | 33 | – | – | – | – | Pool peptide 3+7 ++++ SI: 21.6 (1.8%) |
Pool peptide 3+7 – |
| 8 | TGVPQSRPHIPRTQPQP | RNF43 NM_017763.4 c.C1742A:p.P581H |
Class II | 36 | ++++ SI: 39.3 (1.7%) |
– | + SI: 2.5 (0.7%) |
– | ++ SI: 4.1 (0.3 %) |
– |
| 9 | MFKGVASSQFLPKGTKT | SETD2 NM_014159.6 c.G158A:p.R53Q |
Class II | 38 | ++++ SI: 37.5 (0.9%) |
– | +++ SI: 9.5 (2.0%) |
– |
++++ SI: 35.9 (2.4%) |
– |
| 10 | DWNPHQDLHAQDRAHRI | SMARCA4 NM_001128844.1 c.A3555T:p.Q1185H |
Class II | 33 | – | – | – | – | – | – |
HLA: human leukocyte antigen type, for which binding was predicted. VAF: variant allele frequency, detection frequency of the mutated allele. The observed frequencies could be influenced by tumor content and copy number variations and do not directly equal the proportion of the mutation in the tumor. SI: Stimulation Index, x-fold increase of polyfunctional reactive T cells (at least two of the markers CD154, IFN-γ, TNF and/or IL-2 positive) in the stimulated specimen compared to the according negative control specimen. Additional information: proportion (%) of reactive T cells (IFN-γ, TNF, IL-2 and/or CD154 positive) of the CD4+ or CD8+ T cell population after in vitro amplification. Does not directly equal the frequency of specific cells in vivo. +, lightly positive; ++, positive; +++, strongly positive; ++++, very strongly positive; IFN, interferon; TNF, tumor necrosis factor; IL, interleukin.
Instead, treatment with pembrolizumab was continued and a CT scan was performed in June 2016. This scan showed strong regression of the metastases and great response to the immunotherapy (figure 1B). Additional CT scans in October 2016 and January 2017 revealed further regression of the disease and no new lesions were found at any time point.
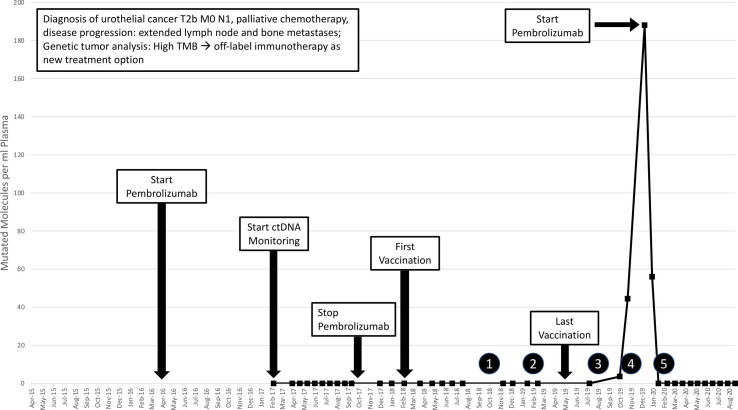
Since February 2017, pLBs were performed regularly as a potential longitudinal monitoring of the patient’s disease. From pLB-derived ctDNA, the amount of MM/mL of the tumor-specific genetic variant of the MLH1 gene was measured by ddPCR. The MLH1 variant was chosen to be measured in pLB as it is considered one of the tumor’s driver mutations and was not part of the 10 peptides used in the vaccine. Under the first course of treatment with pembrolizumab, the amount of MM/mL observed in the pLB was always zero (figure 2).
Figure 2.
Timeline of the patient’s treatment course. Starting in February 2017, MM/mL were measured via pLB and remained at undetectable levels until July 2019. Circled numbers indicated successive MRI scans: (1) and (2) no detected changes; (3) unspecific lymph node enlargement; (4) assured tumor progression and (5) tumor regression. ctDNA, circulating tumor DNA; MM/mL, mutated molecules per ml plasma; pLB, plasma Liquid Biopsy; TMB, tumor mutational burden.
Further CT scans from April 2017 until April 2018 showed stable disease. In October 2017, after the 26th cycle of pembrolizumab in total, the immune therapy was discontinued due to the development of unclear urticaria.
In February 2018, under stable disease conditions and after long discussions with the patient and his physicians, the patient decided to receive his first PNMV. Some concerns existed regarding the fact that the PNMV had been produced based on 2 year old WES data. Nonetheless, having in mind the possibility of a shifted tumor mutational profile, the PNMV was applied after long consideration as it still presented a valuable treatment option at that time. The vaccine was always injected intracutaneously in the left thigh followed by a subcutaneous injection of an adjuvant (granulocyte-macrophage colony-stimulating factor/Leukine). Over the course of 16 days, he received three more vaccinations (priming phase), followed by one vaccination every 4 weeks to 6 weeks (boosting phase).
Before starting the vaccine treatment, an initial immune monitoring had been performed in February 2017, showing no specific T cell response against any of the used peptides. After having received the ninth vaccination, a second immune monitoring was performed to gain knowledge about a first immunological response. This monitoring revealed strong CD4+ T cell responses against 4 of the 10 chosen peptides. A third and fourth immune monitoring were performed at the time of the 17th vaccination and 2 months after the 18th vaccination in March and July 2019. These revealed strong CD4+ and CD8+ T cell responses against many of the chosen peptides indicating a successful vaccination approach (table 1). Throughout the whole PNMV treatment, 10 pLBs were performed by the use of ddPCR at an average interval of 4–6 weeks to longitudinally monitor tumor burden and the amount of MM/mL was constantly zero (figure 2).
After the 18th vaccination in May 2019, the patient developed an allergic reaction with local swelling, itching, nausea and strong urge to urinate, leading to discontinuation of the vaccination therapy. At this time point, the amount of MM/mL measured in the pLB was zero and MRI scans showed no signs of tumor progression. After cessation of the vaccination therapy, the patient received no new treatment immediately. Nevertheless, pLBs (figure 2) and MRI scans for monitoring were performed regularly.
In August 2019, an MRI scan revealed unclear enlargement of a left paraaortal infrarenal lymph node. Therefore, a control MRI was scheduled for 3 months later. Meanwhile, two pLBs were performed on first and 23rd of October 2019 which showed increment of the amount of MM/mL to first 3.63 and then 44.49. In November 2019, the control MRI revealed further enlargement of the left paraaortal lymph node as well as new enlargement of a left iliacal commune lymph node (figure 1C). Therefore, the treating oncologists decided to start a new cycle of treatment with pembrolizumab. Unfortunately, before re-initiation of pembrolizumab, a new analysis of the genomic profile of the tumor tissue could not be performed because neither surgery nor another biopsy was carried out, and the amount of cell free DNA (cfDNA) in the blood, which at this time point ranged between 3.51 ng/mL plasma and 4.78 ng/mL plasma, as well as the tumor content in the cfDNA indicated by ddPCR were too low for a comprehensive sequencing approach.
When the patient received his first dose of pembrolizumab at the beginning of December 2019, the amount of MM/mL measured in the pLB was 188.01. Three weeks later, the patient received his second dose of pembrolizumab. Interestingly, at this time, the amount of MM/mL had already decreased to 56.01 suggesting a strong therapy response. On January 17, 2020, another pLB was performed revealing a further decrease of the amount of MM/mL to again zero under immune therapy and a control MRI scan performed on February 28, 2020, showed significant regression of the lymph nodes confirming a treatment response (figure 1D). Up until today, the patient remains in remission. Ten more pLBs were conducted between February 2020 and August 2020 and the amount of MM/mL measured was constantly zero (see figure 2). The latest CT and MRI scans performed on September 18, 2020, showed no signs of tumor recurrence.
Discussion
High-risk UTUC represents a type of cancer that is associated with a high morbidity and bad clinical outcomes. As of today, standard treatment options include surgery and chemotherapy, but mainly lack successful personalized targeted therapies.14
Here, we present the case of a 55-year-old man diagnosed with high-risk metastatic UTUC who developed recurrence of the disease after having undergone standard treatment. We put emphasis on the importance of genetic analysis of tumor tissue by WES in order to reveal new personalized treatment targets. In the specific case presented here, WES showed the presence of MSI and a very high TMB (41.2 mutations per megabase) of over 10 fold the mean TMB found in UTUC (2.91 mutations per megabase),15 placing it into the highest quartile of TMB in metastatic urothelial carcinoma16 17 and thereby indicating a potential treatment success of ICI.5 Therefore, in 2016, the decision was made to treat the patient with pembrolizumab. At this time point, the use of immunotherapy for the treatment of UTUC was still declared off-label, but since then several studies have shed light on the success of this type of treatment leading to FDA approval for pembrolizumab as second-line therapy for UTUC in May 2017.18 In the same year, the FDA even approved the use of immunotherapy for the treatment of MSI-high solid tumors in general, independent of their entity.19
With the goal to further enhance the immunotherapy, we also produced a PNMV based on the genetic analysis of the tumor that induced very strong CD4+ as well as CD8+ T cell responses in the patient. This type of therapeutic anti-cancer peptide vaccine is a very new approach to target tumor cells and is still considered an individual healing attempt. In Germany, there is a unique situation for individual healing attempts, which are outside of otherwise required permission, and are possible even without obtaining an ethical approval. Nevertheless, it is important to document an interdisciplinary team decision together with the patient.
Most current clinical trials investigating anti-tumor peptide vaccines focus on the use of tumor-associated antigens,20 but some very recent publications show that PNMVs might be an even more promising therapy option for many tumor entities, including those with limited other available therapies, for example, glioblastoma.21 22 Recently, some studies also reported promising results in designing neoantigen-based off-the-shelf vaccines for MMR-deficient colorectal cancer,23 24 but these vaccines are less personalized as they are based on the same neoantigens that are considered most likely to be present in MMR deficient cancers for all patients.
In our patient, the high TMB possibly led to an abundance of tumor neoantigens on the tumor cell surface that could be targeted by the vaccine. Due to the big pool of potentially eligible peptide sites, we were able to select some of very high predicted HLA haplotype affinity making this type of treatment more likely to be successful. Still, other patients treated with PNMV who had only an intermediate or even low TMB also showed response to the therapy (unpublished data) suggesting that not only the quantity of targetable tumor neoantigens seems to be important for treatment response, but also their quality. Nevertheless, more research is needed to fully investigate the effects of such vaccines in larger patient cohorts.
Unfortunately, our patient experienced tumor recurrence. We think this might be explained by an immunologic escape mechanism the tumor acquired during the vaccine therapy. This mechanism has been described for other types of anti-tumor vaccinations before, such as an EGFRvIII-targeted (epidermal growth factor receptor transcript variant 3) peptide vaccine for the treatment of glioblastoma.25 Nevertheless, to fully investigate this theory, sequencing of the current tumor tissue in comparison to the data from the previous analysis in 2015 would be crucial, which was not possible in our patient due to two main reasons: First, the amount of cfDNA in the blood was low (see lines 145 f), and second, the frequency of the MLH1 driver mutation in the pLBs indicated a tumor content of maximum ~20% in the cfDNA in one sample, which represents the lower border for tumor sequencing. Thus, a characterization of the tumor based on panel sequencing or WES of cfDNA would have only been possible with extremely limited sensitivity. These two limitations (cfDNA amount and tumor content) did not suggest a panel sequencing or WES approach to potentially bring up new relevant insights.
Even though our patient did not receive ICI and PNMV at the same time, recent studies show that combining the two types of therapies can lead to mutual reinforcement.26 27
Furthermore, monitoring of the disease progress is a very important aspect in the treatment of UTUC. To the best of our knowledge, there are currently no valid biomarkers available to longitudinally monitor a patient with this tumor entity.10 Physicians mainly rely on imaging techniques, which tend not to be very sensitive, making it hard to detect potential recurrence of the tumor early, as a recent study showed for the closely related entity of bladder cancer.28 Here, we propose the use of pLB-based measurements of individually known mutations in ctDNA as a potential biomarker for longitudinal monitoring of the disease, which goes in line with the findings of Chalfin et al from 2019.29 In our patient, these measurements have proven to be a sensitive method to detect potential tumor recurrence in a very early stage, provided that the chosen mutations are not subject to some selection pressure caused by the therapy. Most interestingly, constant increases of the amount of MM/mL were observed earlier than specific changes in MRI scans, giving first hints about an ongoing tumor recurrence 1 month before imaging techniques specifically detected it. Furthermore, after new induction of treatment with immunotherapy, MM/mL decreased again rapidly indicating a treatment success. Nevertheless, more in-depth research with a larger patient cohort needs to be conducted to fully investigate the potential of such measurements as a biomarker for UTUC and other types of cancer.
Acknowledgments
The authors thank the patient for his permission to publish this case report and for his friendly cooperation with providing necessary data. Furthermore, we thank the Center for Bioinformatics Tuebingen, Department of Computer Science, University Tuebingen, for performance of epitope prediction and human leukocyte antigen typing.
Footnotes
CB and JB contributed equally.
Contributors: CB and JB were involved in manuscript writing, data analysis and creation of figures. MS was responsible for coordination of the project. OB and DH were involved in designing the study concept. AR, CK and SKayser performed data analysis. FB was involved in bioinformatics and creation of figures. MK and SKelkenberg performed laboratory work. AS was involved in the clinical care of the patient. SB was the principal investigator. SB, MS, MF, FB and NP contributed to editing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: SB is cofounder and managing director of CeGaT GmbH. AS reports grants from Johnson & Johnson, grants from Amgen Inc, other from Bayer AG, other from CureVac, other from immatics biotechnologies GmbH, grants from immatics biotechnologies GmbH, grants from Novartis AG and grants from Karl Storz AG, other from Astellas, during the conduct of the study; personal fees from Ipsen Pharma, personal fees from Janssen, personal fees from Alere, outside the submitted work.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the upper tract urothelial carcinoma collaboration. Cancer 2009;115:1224–33. 10.1002/cncr.24135 [DOI] [PubMed] [Google Scholar]
- 2.Rouprêt M, Babjuk M, Compérat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol 2018;73:111–22. 10.1016/j.eururo.2017.07.036 [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa T, Komemushi Y, Kawai T, et al. Efficacy of post-nephroureterectomy cisplatin-based adjuvant chemotherapy for locally advanced upper tract urothelial carcinoma: a multi-institutional retrospective study. World J Urol 2017;35:1569–75. 10.1007/s00345-017-2032-6 [DOI] [PubMed] [Google Scholar]
- 4.Yoneyama T, Imai A, Hatakeyama S, et al. Sequential chemotherapy using gemcitabine + carboplatin followed by gemcitabine + carboplatin + docetaxel for advanced upper-tract urothelial cancer. Int J Clin Oncol 2015;20:1179–84. 10.1007/s10147-015-0846-z [DOI] [PubMed] [Google Scholar]
- 5.Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598–608. 10.1158/1535-7163.MCT-17-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Chang M, Chang HM, et al. Microsatellite instability: a predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol 2018;26:e15–21. 10.1097/PAI.0000000000000575 [DOI] [PubMed] [Google Scholar]
- 7.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hale DF, Clifton GT, Sears AK, et al. Cancer vaccines: should we be targeting patients with less aggressive disease? Expert Rev Vaccines 2012;11:721–31. 10.1586/erv.12.39 [DOI] [PubMed] [Google Scholar]
- 9.U.S. National Library of Medicine Clinicaltrials.gov. Available: https://clinicaltrials.gov/ct2/results?cond=UTUC
- 10.Yates DR, Catto JWF. Distinct patterns and behaviour of urothelial carcinoma with respect to anatomical location: how molecular biomarkers can augment clinico-pathological predictors in upper urinary tract tumours. World J Urol 2013;31:21–9. 10.1007/s00345-012-0946-6 [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulou E, Davilas E, Sotiriou V, et al. Cell-free DNA and RNA in plasma as a new molecular marker for prostate and breast cancer. Ann N Y Acad Sci 2006;1075:235–43. 10.1196/annals.1368.032 [DOI] [PubMed] [Google Scholar]
- 12.Frattini M, Gallino G, Signoroni S, et al. Quantitative analysis of plasma DNA in colorectal cancer patients: a novel prognostic tool. Ann N Y Acad Sci 2006;1075:185–90. 10.1196/annals.1368.025 [DOI] [PubMed] [Google Scholar]
- 13.Hufnagl C, Leisch M, Weiss L, et al. Evaluation of circulating cell-free DNA as a molecular monitoring tool in patients with metastatic cancer. Oncol Lett 2020;19:1551–8. 10.3892/ol.2019.11192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leow JJ, Liu Z, Tan TW, et al. Optimal management of upper tract urothelial carcinoma: current perspectives. Onco Targets Ther 2020;13:1–15. 10.2147/OTT.S225301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson BD, Vlachostergios PJ, Bhinder B, et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat Commun 2019;10:2977. 10.1038/s41467-019-10873-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013;499:214–8. 10.1038/nature12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76. 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzman DL, Agrawal S, Ning Y-M, et al. FDA approval summary: Atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin-containing chemotherapy. Oncologist 2019;24:563–9. 10.1634/theoncologist.2018-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite Instability-High solid tumors. Clin Cancer Res 2019;25:3753–8. 10.1158/1078-0432.CCR-18-4070 [DOI] [PubMed] [Google Scholar]
- 20.Obara W, Kanehira M, Katagiri T, et al. Present status and future perspective of peptide-based vaccine therapy for urological cancer. Cancer Sci 2018;109:550–9. 10.1111/cas.13506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilf N, Kuttruff-Coqui S, Frenzel K, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature 2019;565:240–5. 10.1038/s41586-018-0810-y [DOI] [PubMed] [Google Scholar]
- 22.Sahin U, Derhovanessian E, Miller M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017;547:222–6. 10.1038/nature23003 [DOI] [PubMed] [Google Scholar]
- 23.Kloor M, Reuschenbach M, Pauligk C, et al. A frameshift peptide Neoantigen-based vaccine for mismatch repair-deficient cancers: a phase I/IIa clinical trial. Clin Cancer Res 2020;26:4503–10. 10.1158/1078-0432.CCR-19-3517 [DOI] [PubMed] [Google Scholar]
- 24.Leoni G, D'Alise AM, Cotugno G, et al. A genetic vaccine encoding shared cancer neoantigens to treat tumors with microsatellite instability. Cancer Res 2020;80:3972–82. 10.1158/0008-5472.CAN-20-1072 [DOI] [PubMed] [Google Scholar]
- 25.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 2010;28:4722–9. 10.1200/JCO.2010.28.6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547:217–21. 10.1038/nature22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali OA, Lewin SA, Dranoff G, et al. Vaccines combined with immune checkpoint antibodies promote cytotoxic T-cell activity and tumor eradication. Cancer Immunol Res 2016;4:95–100. 10.1158/2326-6066.CIR-14-0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen E, Birkenkamp-Demtröder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by Ultra-Deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol 2019;37:1547–57. 10.1200/JCO.18.02052 [DOI] [PubMed] [Google Scholar]
- 29.Chalfin HJ, Glavaris SA, Gorin MA, et al. Circulating tumor cell and circulating tumor DNA assays reveal complementary information for patients with metastatic urothelial cancer. Eur Urol Oncol 2019. 10.1016/j.euo.2019.08.004. [Epub ahead of print: 25 Sep 2019]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001406supp001.pdf (816KB, pdf)