Abstract
Oculodentodigital dysplasia (ODDD) is a rare congenital disorder characterised by developmental abnormalities of the eye, dentition and digits of the hands and feet, with neurological symptoms reported in 30% of individuals. Dental anomalies associated with ODDD include enamel hypoplasia and subsequent caries, microdontia, missing teeth, amelogenesis imperfecta, pulp stones and delayed tooth development. Here, we describe the comprehensive dental management of a 3-year-old girl who presented with rapid deterioration of the primary dentition due to generalised enamel hypomineralisation. Conservative, comprehensive restorative management was performed under general anaesthesia. Within 6 months, further breakdown of the remaining unrestored enamel was noted. This case documents the challenges of conservative management in dental anomalies that are not well documented due to the extreme rarity of the disorder.
Keywords: dentistry and oral medicine, genetics, mouth
Background
Oculodentodigital dysplasia (ODDD; OMIM 164200) is a rare congenital disorder characterised by developmental abnormalities of the eyes, dentition and digits of the hands and feet, with neurological symptoms observed in 30% of individuals.1 ODDD is usually an autosomal-dominant condition caused by a heterozygous mutation in the gap junction alpha1 gene (GJA1: OMIM 121014) on chromosome 6q22.31, which encodes connexin-43 (CX43).2 Autosomal-recessive ODDD cases with nonsense mutations in exon 2 of the GJA2 gene have also been reported.3 For familial cases of ODDD, the sex ratio is approximately 1:1, while there is a female preponderance for sporadic cases, with a male to female ratio of 6:15.1
The hallmark feature of the syndrome is complete, bilateral syndactyly of the fourth and fifth fingers (syndactyly type III),4 camptodactyly of the fifth finger and involvement of the third finger, and missing toe phalanges may also be present.5 Ophthalmological features include microphthalmia, microcornea, glaucoma and hypertelorism or hypotelorism with small palpebral fissures.6 Craniofacial findings are typically a wide, depressed nasal bridge with hypoplastic alae nasi and anteverted nostrils, along with dry and sparse hair and eyebrows.4 There are also reports of cleft lip and/or palate, conductive hearing loss, cognitive impairment and changes in subcortical white matter on MRI with a range of neurological symptoms.7 Dental anomalies in ODDD include enamel hypoplasia (40%) and subsequent caries, microdontia (21%), missing teeth (7%), amelogenesis imperfecta (2%), pulp stones (2%) and delayed tooth development (2%).1 To the author’s knowledge, there are no published reports of enamel hypomineralisation in children with ODDD.
The case reported herein describes the comprehensive dental management of a 3-year-old girl who presented with rapid deterioration of the primary dentition due to generalised enamel hypomineralisation.
Case presentation
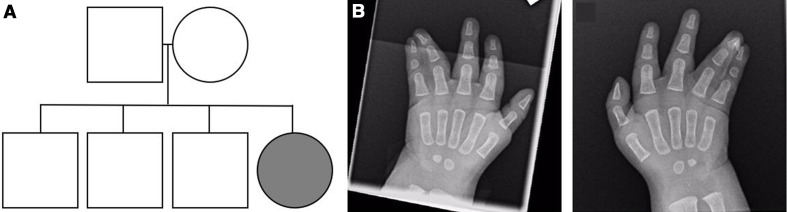
The proband was a 3-year-old girl and regular attender of the dental clinic. She had been delivered at 38+6 weeks gestation by elective caesarean section, with a birth weight of 3460 g. The proband’s parents were non-consanguineous and healthy, with an unremarkable family history (figure 1A). Clinical and radiographic examinations revealed bilateral complete simple syndactyly of fourth and fifth fingers (figure 1B), and the proband underwent surgical intervention under general anaesthesia for syndactyly release and skin grafting of both hands at 13 months of age. The infant proband also presented with micrognathia, low-set ears, hypertelorism, hypertrophic alae nasi and a small thymus on chest radiographs. At 12 months age, the head circumference was 44 cm (approximately 25th percentile) and a mild brachiocephalic cranial vault and widely patent anterior fontanelle were noted. Cardiac examination found grade 1 systolic murmur, with no radiation and a normal pericardium, and the echocardiogram showed a small patent foramen ovale, intact ventricular septum and structurally and functionally normal cardiac valves. Ophthalmic examination revealed epicanthic folds, small palpebral apertures, high upper eyelids, bilateral small iris, microphthalmia and microcornea of 8 mm in both eyes. The morphological presentation of the infant proband was consistent with previous reports of ODDD.
Figure 1.
Pedigree and radiographs of the proband at 6 months of age. (A) Pedigree of the family. The proband is indicated with an arrow. (B) Clinical radiographs revealed bilateral syndactyly of the fourth and fifth fingers.
By 2 months of age, the proband had experienced three separate episodes of choking and cyanosis, with a sleep study revealing baseline low saturation with central apnoeas to low 80% oxygen saturation. A videofluoroscopic swallowing study found subtle nasopharyngeal reflux, delayed swallow initiation with microaspiration on thin fluids. The proband was on home oxygen until 1 year and 3 months, at which stage the central sleep apnoea ceased as confirmed by a normal polysomnogram. Respiratory and feeding difficulties have not previously been reported to be a manifestation of ODDD.
Cerebral MRI at 1 month of age proved largely within normal limits. The initial sonographic finding of ventricular striate vascular calcification was not confirmed on subsequent imaging and was deemed of non-specific clinical relevance. Mild neurodevelopmental delay was noted at 12 months of age. By 18 months, mucoid effusion of both middle ears was found and Armstrong ventilation tubes were placed under general anaesthesia. Postoperative review found subsequent improvement in both hearing and language development of the proband.
Genetic investigation included bidirectional DNA Sanger sequencing of the GJA1 gene. DNA was extracted from the proband specimen and PCR was used to amplify the indicated exons plus additional flanking non-coding sequence. The PCR products were cleaned and cycle sequencing was carried out using an AMI Big Dye Terminator V.3.0 kit (Applied Biosystems, Foster City, California, USA). PCR products were resolved by electrophoresis on an ABI 3730xl capillary sequencer (Applied Biosystems). Analysis of the GJA1 gene identified a mutation in one copy of this gene (p.Lys134Glu), which has been reported in other individuals with ODDD. A chromosome array was also performed, which revealed a duplication on chromosome 2p21 within the LRPPRC gene. Mutations in both copies of the gene are known to cause autosomal-recessive Leigh syndrome (OMIM 220111), a form of cytochrome c oxidase deficiency.8 It is assumed that the proband would therefore be a carrier of autosomal-recessive Leigh syndrome.
The proband presented for an initial oral examination at 5 months of age. She had no erupted deciduous teeth, and a high arch palate was noted. The proband returned for dental examination on a 3-monthly recall. At 8 months, the lower A teeth (71, 81) were present, and by 12 months the upper A and B teeth (52, 51, 61, 62) were present. At 1 year and 7 months, the deciduous teeth had erupted, as expected for age, and the upper right deciduous lateral incisor (52) had experienced an unexplained enamel fracture on the buccal surface. By 1 year and 10 months, 20 deciduous teeth had erupted with diffuse, severe generalised hypomineralisation of the enamel, but no clinically observable caries. Each tooth was scored 11 (diffuse opacities) according to the European Academy of Paediatric Dentistry (EAPD) criteria.9 An enamel fracture on the upper left deciduous lateral incisor (62) was noted, as well as mild attrition of enamel on the occlusal surfaces of the deciduous first molars (54, 64, 74 and 84). Oral soft tissues including the gingiva, tongue, buccal mucosa and frenae were unremarkable. The proband was monitored every 3 months and showed no significant posteruptive breakdown at age 2 years and 10 months.
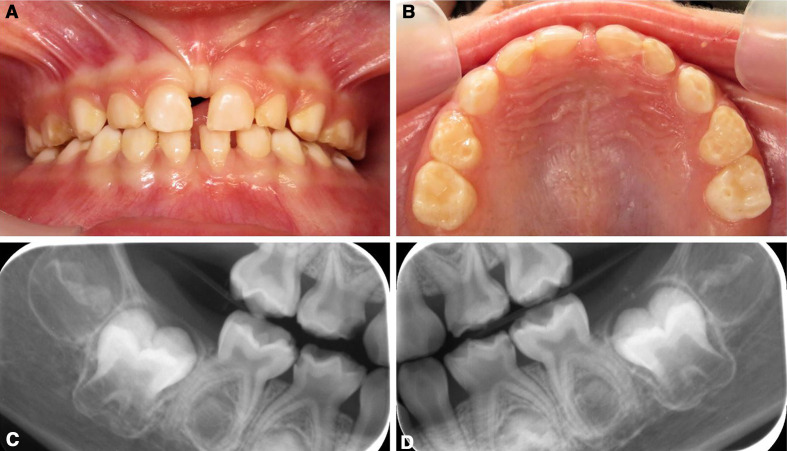
Oral examination at age 3 years and 3 months revealed extensive loss of enamel on 12 primary teeth (figure 2A–D). At this time, the proband had toothbrushing by a parent once a day in the morning with 500 ppm fluoridated toothpaste. The proband showed age-appropriate eating and drinking, as determined by a speech pathologist, but reported a recent history of pain when eating ice cream. At age 2 years and 8 months, the proband had developed a tremor and subsequent MRI investigation confirmed generalised hypomyelination. A recent ophthalmic examination found an increase in visual impairment, with full-time glasses wear necessary for both the right (+7.0 dioptres sphere (DS)) and left (+5.5 DS) eyes.
Figure 2.
Clinical photographs and radiographs of deciduous teeth of the proband preoperatively. (A, B) Frontal and upper clinical photographs at age 3 years 3 months. Diffuse, white hypomineralised areas are generalised and enamel deterioration can be observed. (C, D) Bitewing radiographs of the right and left primary molars show loss of enamel and large pulp chambers.
Treatment
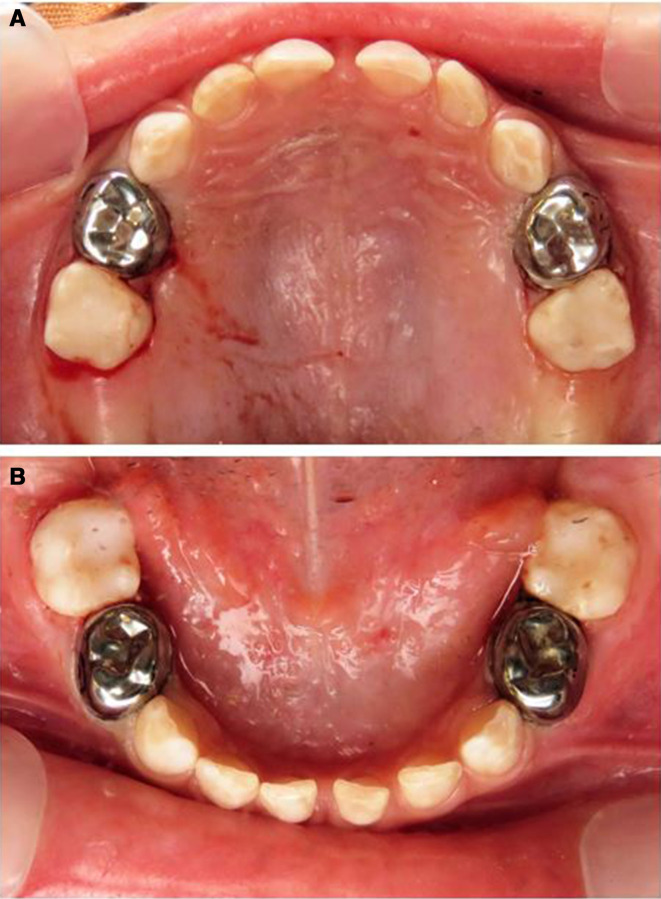
Dental treatment was performed under general anaesthesia. Restoration of all first primary molars was performed with stainless steel crowns (SSC). Fissures of the second primary molars were sealed with UltraSeal XT (Ultradent, Utah, USA), a light-cured composite resin sealant material (figure 3A, B). Conservative management was chosen without prophylactic full coverage of second primary molars or anterior teeth.
Figure 3.
Clinical photographs of deciduous teeth of the proband immediately postoperative. (A, B) All first primary molars were restored with stainless steel crowns and the second molars with fissure sealants.
Outcome and follow-up
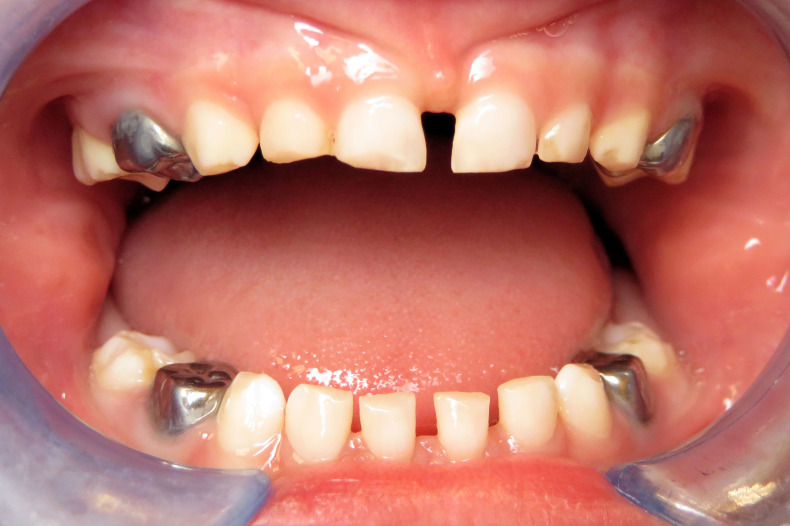
A 3-month follow-up was recommended to check restorations and continue monitoring of unrestored dentition. At the follow-up appointment 5 months postoperatively, further enamel loss was noted on unrestored deciduous anterior teeth and second primary molars (figure 4). The proband remained asymptomatic and was tolerating cold foods, but rapid deterioration of the enamel was apparent.
Figure 4.
Clinical photograph of deciduous teeth of the proband 5 months postoperatively. Attrition and enamel breakdown of unrestored anterior teeth had occurred.
DISCUSSION
The primary aims of dental treatment for this proband were to preserve arch integrity and to resolve pain on eating. Enamel hypoplasia has been reported as a dental feature in 40% of individuals with ODDD;1 case reports have documented extensive caries secondary to enamel hypoplasia10 11 and histological changes to the dentine of permanent teeth including hypocalcification.12 The proband presented with no clinical enamel hypoplasia but with diffuse, generalised opacities, consistent with enamel hypomineralisation and category 11 of the EAPD diagnostic criterial.9 The proband’s oral hygiene was well maintained by the parent even with limited cooperation in the dental setting. Although no caries were noted, extensive enamel breakdown had occurred in the short period of time between 2 years 10 months and 3 years 3 months of age. Rapid deterioration of the enamel occurred within the first 2 years of tooth eruption.
In the present case, SSCs were used for the first primary molars with the most enamel breakdown to protect against further enamel deterioration. The second permanent molars were heavily sealed with composite material to provide protection to the occlusal surface. This initial treatment plan was conservative and did not include full coverage composite resin restoration of the anterior teeth or SSC coverage of the second primary molars. Management for molar-incisor hypomineralisation (MIH), as described previously, depends on the severity of the structural defect, the age of the individual and the expectations of the individual and parent.13 14 Conservative restorative management was chosen due to the proband’s excellent maintained oral hygiene, regular attendance for dental examinations and no history of caries.
At the 5-month follow-up, oral hygiene continued to be well maintained and the parent reported initial resolution in apparent sensitivity to cold foods, which eventually returned after approximately 3 months. The second primary molars and the unrestored anterior teeth showed further attrition and breakdown of enamel, suggestive of a defect in the structural properties of the enamel. Alteration of the structure properties of enamel in MIH is well documented,15 with a reduction in the elastic modulus and hardness values for enamel presenting with hypomineralisation.
In this case, conservative dental management of this case was not adequate to resolve the ongoing rapid deterioration of hypomineralised enamel. There are no guidelines for an evidence-based approach to the management of such dental anomalies. To the authors’ knowledge, this is the first case report to document a case of widespread enamel hypomineralisation in a proband with ODDD without caries involvement. The management of these cases may require prophylactic full-coverage restorations to prevent any future deterioration of enamel.
Learning points.
This is the first report of generalised enamel hypomineralisation in a young child with oculodentodigital dysplasia (ODDD), a rare genetic disorder.
Rapid deterioration of enamel occurred despite regular dental attendance.
Conservative restorative management was performed under general anaesthesia, but within 6 months, further deterioration of unrestored enamel had occurred.
Dental management of individuals with ODDD may require prophylactic full-coverage restorations prior to enamel breakdown.
Acknowledgments
This case report was seen at the Department of Paediatric Dentistry, Women’s and Children’s Hospital, Adelaide, South Australia. I would like to acknowledge that the restorative dental treatment was provided by Dr Sam Gue. I would also like to thank the child and their family for their consent to publish this interesting report.
Footnotes
Contributors: EDJ obtained consent, performed the literature review and wrote the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Paznekas WA, Karczeski B, Vermeer S, et al. Gja1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat 2009;30:724–33. 10.1002/humu.20958 [DOI] [PubMed] [Google Scholar]
- 2.Paznekas WA, Boyadjiev SA, Shapiro RE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet 2003;72:408–18. 10.1086/346090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taşdelen E, Durmaz CD, Karabulut HG. Autosomal recessive oculodentodigital dysplasia: a case report and review of the literature. Cytogenet Genome Res 2018;154:181–6. 10.1159/000489000 [DOI] [PubMed] [Google Scholar]
- 4.Judisch GF, Martin-Casals A, Hanson JW, et al. Oculodentodigital dysplasia. four new reports and a literature review. Arch Ophthalmol 1979;97:878–84. 10.1001/archopht.1979.01020010436007 [DOI] [PubMed] [Google Scholar]
- 5.Gorlin RJ, MISKIN LH, St GEME JW. Oculodentodigital dysplasia. J Pediatr 1963;63:69–75. 10.1016/S0022-3476(63)80304-2 [DOI] [PubMed] [Google Scholar]
- 6.Rajic D, DeVeber L. Hereditary oculodentoosseous dysplasia. Annales de radiologie - radiologie clinique radiobiologie, 1966. [Google Scholar]
- 7.Loddenkemper T, Grote K, Evers S, et al. Neurological manifestations of the oculodentodigital dysplasia syndrome. J Neurol 2002;249:584–95. 10.1007/s004150200068 [DOI] [PubMed] [Google Scholar]
- 8.Mootha VK, Lepage P, Miller K, et al. Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proc Natl Acad Sci U S A 2003;100:605–10. 10.1073/pnas.242716699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghanim A, Elfrink M, Weerheijm K, et al. A practical method for use in epidemiological studies on enamel hypomineralisation. Eur Arch Paediatr Dent 2015;16:235–46. 10.1007/s40368-015-0178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean JA, Jones JE, Vash BW. Dental management of oculodentodigital dysplasia: report of case. ASDC J Dent Child 1986;53:131–4. [PubMed] [Google Scholar]
- 11.Aminabadi NA, Pourkazemi M, Oskouei SG, et al. Dental management of oculodentodigital dysplasia: a case report. J Oral Sci 2010;52:337–42. 10.2334/josnusd.52.337 [DOI] [PubMed] [Google Scholar]
- 12.Scheutzel P. Oculodentodigital syndrome: report of a case. Dentomaxillofac Radiol 1991;20:175–8. 10.1259/dmfr.20.3.1808004 [DOI] [PubMed] [Google Scholar]
- 13.Lygidakis NA, Wong F, Jälevik B, et al. Best clinical practice guidance for clinicians dealing with children presenting with Molar-Incisor-Hypomineralisation (miH): an EAPD policy document. Eur Arch Paediatr Dent 2010;11:75–81. 10.1007/BF03262716 [DOI] [PubMed] [Google Scholar]
- 14.Mathu-Muju K, Wright JT. Diagnosis and treatment of molar incisor hypomineralization. Compend Contin Educ Dent 2006;27:604–10. [PubMed] [Google Scholar]
- 15.Elhennawy K, Manton DJ, Crombie F, et al. Structural, mechanical and chemical evaluation of molar-incisor hypomineralization-affected enamel: a systematic review. Arch Oral Biol 2017;83:272–81. 10.1016/j.archoralbio.2017.08.008 [DOI] [PubMed] [Google Scholar]