Summary
Humoral immunity is an essential component of the protective immune response to flavivirus infection. Typically, primary infection generates a robust neutralizing antibody response that mediates viral control and protection. It is becoming increasingly apparent that secondary infection with a closely related flavivirus strain can result in immunological cross-reactivity; however, the consequences to infection outcome remain controversial. Since its introduction to Brazil in 2015, Zika virus (ZIKV), has caused an epidemic of fetal congenital malformations within the Americas. Because ZIKV is a mosquito-borne flavivirus with a high degree of sequence and structural homology to Dengue virus (DENV), the role of immunological cross-reactivity in ZIKV and DENV infections has been of great concern In this review, we highlight contemporary findings that implicate a role for flavivirus antibodies in mediating protection, contributing to pathogenesis, and seeding the human placenta.
Keywords: Zika virus, Dengue virus, cross-reactive antibodies, vertical transmission
In Brief:
The influence of cross-reactive antibodies on flavivirus pathogenesis remains a controversial topic. In this review, Zimmerman et al. discuss the epidemiologic and experimental data highlighting the potential role of cross-reactive flavivirus antibodies on enhancement of systemic Dengue and Zika virus infection as well as Zika virus infection of the placenta.
Introduction
The Flaviviridae family of positive-sense RNA viruses comprises a diverse group of mosquito-borne viruses (Dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), and Zika virus (ZIKV)) and tick-borne viruses (tick-borne encephalitis virus (TBEV)) responsible for a wide variety of clinical diseases in humans. DENV is a self-limiting, acute viral infection responsible for approximately 50-100 million apparent and 300 million inapparent infections per year. DENV exists as four genetically distinct serotypes (DENV1-4) that co-circulate within endemic regions, including the tropics of Central and South America, sub-Saharan Africa, India, and Southeast Asia (Bhatt et al., 2013). Recent reports have detailed the increasing geographic distribution of DENV with the emergence of established autochthonous cases in the Mediterranean countries of Europe as well as the southern United States, including Florida and Texas (Fredericks and Fernandez-Sesma, 2014; Gossner et al., 2018). The major vectors for transmission of DENV are Aedes aegypti and Aedes albopictus, biting mosquitoes that live in tropical or subtropical regions near dense human populations (Carrington and Simmons, 2014). Primary DENV infections are generally asymptomatic or subclinical and provide lifelong immunity to subsequent infections with the homologous serotype. However, secondary infection with heterologous DENV serotypes can manifest as “severe dengue,” a collection of serious sequelae caused by increased capillary permeability shortly after defervescence. This can lead to severe hemorrhage, hypovolemic shock, and end-organ damage with fatal outcomes occurring in 20% of those without supportive medical care (Guzman and Harris, 2015). Symptomatic dengue fever and severe DENV infections can also lead to long-lasting arthralgias, arthropathies, and memory loss at least two years following acute illness (Garcia et al., 2011).
ZIKV has recently emerged in the Americas and continues to be a significant public health concern in many regions co-endemic with DENV. Like DENV, ZIKV is primarily transmitted by infected Aedes spp. mosquitoes; however, ZIKV can also be transmitted between persons through sexual contact or blood transfusion (Brasil et al., 2016a; de Araujo et al., 2018; Nogueira et al., 2018). While the majority of ZIKV infections in adults are asymptomatic (~80%), symptomatic cases can present with mild febrile illness characterized by headache, rash, fever and conjunctivitis, sometimes with severe neurological sequalae (Brasil et al., 2016b). ZIKV exhibits a diverse tropism, infiltrating numerous immunologically privileged regions within the body. These include the male and female reproductive organs, adult and fetal central nervous systems, peripheral nervous system, urinary tract, as well as the structural and neurologic portions of the eye (Fig. 1) (Carroll et al., 2017; Figueiredo et al., 2019; Miner and Diamond, 2017; Oh et al., 2017; Retallack et al., 2016; Tabata et al., 2016). The most devastating complications of ZIKV occur during infection of the placenta and vertical transmission to the developing fetus, leading to adverse pregnancy outcomes including spontaneous abortion and fetal brain abnormalities. Approximately 30% of congenitally infected fetuses exhibit morphological abnormalities by ultrasound (e.g. microcephaly or brain calcifications) whereas the vast majority exhibit no overt clinical manifestations at birth (Brasil et al., 2016a). While microcephaly is associated with direct infection of neural stem and progenitor cells (Miner and Diamond, 2016), neonates with normal head circumference at birth may exhibit neurodevelopmental abnormalities during the first year of life (Heald-Sargent and Muller, 2017; Kapogiannis et al., 2017; Mulkey et al., 2018). Indeed, recent reports of infants with congenital ZIKV infection revealed neurodevelopmental abnormalities in the absence of overt microcephaly, including vision loss, epilepsy and delays in age-appropriate developmental skills (Cardoso et al., 2019; Lopes Moreira et al., 2018; Rice et al., 2018; Wheeler et al., 2018).
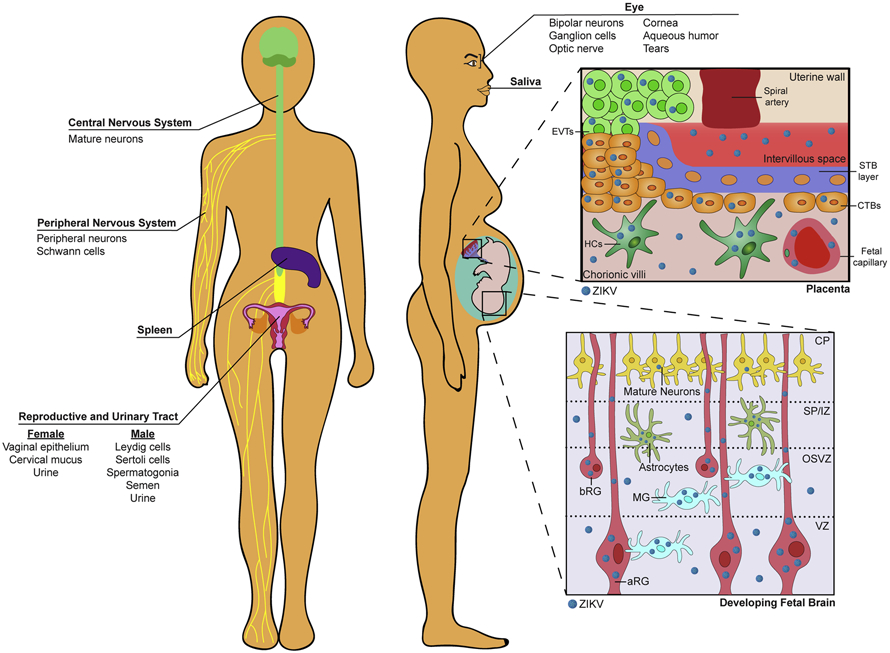
Figure 1. Sites of viral tropism during ZIKV infection.
Human and mouse studies have identified ZIKV infection of mature neurons of the adult brain as well as peripheral neurons and myelinating Schwann cells of the peripheral nervous system. ZIKV can also infect primary human cortical samples within numerous cells types of the developing fetal brain: in the apical radial glia (aRG) progenitor cells of the ventricular zone (VZ); microglia (MG), astrocytes, and basal radial glia (bRG) of the outer subventricular zone (OSVZ) and subcortical plate (SP). Limited ZIKV infection has also been detected in mature neurons of the cortical plate (CP). ZIKV can also be detected in the vaginal epithelium and cervical mucus. Notably, ZIKV infection of the placenta was found in invading villous trophoblasts, cytotrophoblasts, Hofbauer cells, and fetal endothelial cells in human placental cell culture and ex vivo chorionic villous explant studies. ZIKV was also found in Leydig cells and Sertoli cells of the male gonads as well as spermatogonia and semen in male subjects. ZIKV has been detected within the urine and saliva of non-human primates and humans. ZIKV also infects the retinal (bipolar neurons, ganglion cells, optic nerve) structures of the eye in mice and was found in the aqueous humor and tears in humans. ZIKV infection has also been identified within the spleen.
In this review, we will examine the virus-specific and cross-reactive antibody responses elicited during primary and secondary DENV and ZIKV infection. We also highlight studies which provide evidence for and against the role of cross-reactive antibody-mediated enhancement of flavivirus infection observed through human epidemiologic studies and animal model systems. We also expand upon a non-canonical mechanism of cross-reactive antibodies that appear to facilitate vertical transmission of ZIKV infection across the placental barrier. Finally, we explore recent advances in vaccines and biologic therapeutics designed to protect against ZIKV infection.
Human antibody responses to DENV and ZIKV
Upon infection, the flavivirus positive-sense RNA genome is directly translated into a single polyprotein and post-translationally cleaved to generate three structural proteins, capsid (C), pre-membrane (prM), and envelope (E) and seven nonstructural proteins, NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5. The non-structural proteins function as the replication complex to synthesize viral RNA as well as comprise auxiliary functions to antagonize host innate immune signaling pathways. The structural genes comprise the viral particle, of which E and prM proteins allow for attachment to cellular receptors, facilitating viral fusion and entry into the cell. Because of the outward orientation of E on mature viral particles, infected hosts generate an antibody (Ab) response to this protein to neutralize the virus (Rodenhuis-Zybert et al., 2015).
Primary infection with DENV generates a robust Ab response targeting the E domain I (EDI), EDII, and EDIII of the original DENV serotype with minimal cross-reactivity to heterologous serotypes. EDI/EDII-specific Abs, including ones targeting the EDII fusion loop, comprise a majority of the response but display poor neutralizing activity while the EDIII-specific Abs, although lower in quantity, show superior neutralization activity (Beltramello et al., 2010; Flipse and Smit, 2015). These cross-reactive, poorly neutralizing DENV Abs produced by primary DENV infection have been implicated in antibody-dependent enhancement (ADE) and severe dengue cases during secondary DENV infection (Guzman and Harris, 2015). Highly neutralizing Abs were found to target the complex quaternary epitopes spanning multiple domains across adjacent E protein dimers (Beltramello et al., 2010; de Alwis et al., 2011). In another study, depletion of E-protein specific antibodies from primary DENV-2 and DENV-3-immune sera with whole DENV-2 and DENV-3 virions, not recombinant E protein coated beads, eliminated the neutralization capacity towards the same DENV serotype (de Alwis et al., 2012). In contrast to primary DENV infection, secondary DENV infection generates a broader breadth of cross-reactive antibodies against the E-protein, as well as prM and NS1. Immature DENV virions can also elicit a potent antibody response during secondary DENV infection with 20-35% of the Abs targeting prM (Rodenhuis-Zybert et al., 2015). Unlike primary DENV infections, secondary DENV infection typically provides long-lasting immunity to subsequent infection with homotypic and heterotypic serotypes through the generation of both potently neutralizing type-specific and cross-reactive antibodies (Patel et al., 2017).
Serum procured from DENV-naïve patients with primary ZIKV infection contained weakly neutralizing antibodies to ZIKV EDI, EDII and EDIII. Similar to DENV, depletion of recombinant E protein-specific antibodies elicited by primary ZIKV infection did not significantly reduce neutralization capacity of the patient sera, suggesting that ZIKV-specific antibodies also target complex quaternary E protein epitopes (Collins et al., 2019). Analysis of immune sera from ZIKV-infected patients also showed similar neutralization across African, Asian, and South American ZIKV strains, suggesting that, in contrast to DENV, only a single serotype of ZIKV exists (Dowd et al., 2016). The ZIKV and DENV envelope protein share ~54% amino acid sequence homology, resulting in antibody cross-reactivity with sera from dengue-infected patients (Priyamvada et al., 2016). Because of the structural similarities between ZIKV and DENV and their co-circulation within similar endemic regions, understanding how previous DENV immunity can shape the ZIKV-specific antibody response is of paramount interest. Rogers et al. demonstrated that the plasmablast response in DENV-experienced ZIKV infected patients showed clonal expansion and levels of somatic hypermutation higher than DENV-naïve ZIKV-infected donors and comparable to secondary DENV infections (Rogers et al., 2017). A longitudinal study using pediatric Nicaraguan cohort demonstrated that previous DENV immunity minimally shaped the breadth and specificity of ZIKV-specific memory B cell responses. Similar to previous work, activation of cross-reactive memory B cell responses between ZIKV/DENV was high during early convalescence and waned to ZIKV-specific antibody levels by late convalescence (Andrade et al., 2019).
Cross-reactive antibody responses between DENV and ZIKV: Protective vs Pathogenic
The influence of previous flavivirus immunity on flavivirus pathogenesis was first described in 1977 by Scott Halstead following the observation that DENV4-immune serum and serum from infants of DENV2-infected mothers enhanced in vitro DENV infection in cultured blood leukocytes (Halstead and O'Rourke, 1977; Marchette et al., 1979). In vivo studies assessing the role of DENV-specific passive immunity on DENV enhancement in macaques and Thai infants (Halstead, 1979; Kliks et al., 1988) were also instrumental in our early understanding of antibody-dependent enhancement (ADE) of flavivirus infection. ADE occurs when sub-neutralizing levels of cross-reactive antibodies weakly bind to structurally similar viruses, allowing for enhanced viral entry into normally non-permissive FcγR-bearing cells. The enhanced viral burden and resulting immune response is considered the primary cause of the increased inflammation and devastating symptoms of severe dengue disease (Halstead, 2003). Numerous epidemiologic studies have assessed whether pre-existing DENV antibodies facilitate the manifestation of either apparent dengue infection or severe dengue disease during secondary heterotypic DENV infection. Early prospective cohort studies in Thailand identified a correlation with age (>10 years), not DENV seropositivity, that distinguished apparent (symptomatic) vs. inapparent (asymptomatic or subclinical) infections. However, 12.5% (7 of 56) of children with secondary DENV infections and 0% (0 of 47) with primary DENV infection were hospitalized with dengue hemorrhagic fever (Burke et al., 1988). Another pediatric cohort in Sri Lanka also reported that patients with pre-existing monotypic neutralizing DENV antibodies were more likely to develop apparent dengue fever over those with heterotypic cross-reactive neutralizing antibodies (Corbett et al., 2015). In a large retrospective serologic study in Santiago de Cuba, distinct chronologic DENV epidemics paired with thorough viral surveillance demonstrated that 92.1% (4,608 of 5,003) patients presenting with overt dengue fever and 98.5% (191 of 193) patients that developed severe dengue disease during the 1997 DENV-2 epidemic were seropositive for DENV-1 antibodies (Guzman et al., 2000). More recently, a large prospective study in a Nicaraguan pediatric cohort suggests that previous dengue immunity, particularly within the 1:20 to 1:80 titer range, results in higher risk of severe dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) as a result of ADE (Katzelnick et al., 2017). A recent meta-analysis pooling data from numerous cohort and cluster studies corroborated these epidemiologic findings, determining that DENV immune status directly affects the clinical outcomes during secondary DENV infection greater than one year after initial infection (Clapham et al., 2017). Phase III safety and efficacy studies on Sanofi Pasteur’s Dengvaxia, a live-attenuated tetravalent yellow fever chimeric DENV vaccine, have also demonstrated that vaccinated seronegative children have a higher relative risk of acquiring severe dengue infection. Conversely, dengue seropositive children who received the vaccine showed lower incidence rates of hospitalization and decreased disease severity caused by secondary DENV infection (Sridhar et al., 2018). However, it remains poorly understood how Dengvaxia-induced cross-reactive antibody responses affect subsequent infection with ZIKV and other co-circulating flaviviruses.
Utilizing the same Nicaraguan pediatric cohort mentioned earlier, recent studies have found that previous dengue immunity protected against symptomatic ZIKV infection, but did not mitigate the risk of primary ZIKV infection (Gordon et al., 2019). Pre-existing dengue immunity has been postulated to influence the incidence of congenital Zika syndrome (CZS) or spontaneous abortion during ZIKV infection in pregnant mothers. Serologic analysis of a Brazilian cohort of pregnant women revealed that 50% (7 of 14) DENV-IgG negative women and 43.8% (43 of 98) DENV-IgG positive women had abnormal birth outcomes (Halai et al., 2017). Another study reported that increased ZIKV-specific neutralizing antibody titers, not differences in DENV EDIII-specific antibody titers, were found in ZIKV-infected pregnant women with confirmed CZS when compared to case controls (Robbiani et al., 2019). Generalized linear model analysis determining factors differentiating CZS cases and controls showed that multi-typic dengue immunity (neutralizing antibodies to ≥2 DENV serotypes) were protective against CZS (Pedroso et al., 2019). Additional prospective studies that stratify pregnant patients based on monotypic and heterotypic DENV infection as well as DENV antibody titers are necessary to fully grasp the impact of cross-reactive DENV antibodies on vertical transmission and adverse pregnancy outcomes in ZIKV-infected pregnant mothers.
DENV and ZIKV disease severity in flavivirus-naive individuals
The presence of sub-neutralizing levels of DENV cross-reactive antibodies inducing ADE of infection is considered the primary mechanism behind severe dengue disease in patients with secondary DENV infections. However, primary DENV infection can develop into apparent dengue fever or severe dengue disease in flavivirus seronegative individuals. European traveler studies found that adult patients without prior flavivirus immunity correlated with severe DENV disease (34%; 8 of 22) or spontaneous bleeding (29%; 5 of 17) upon acquiring primary DENV infection (Wichmann et al., 2007). A prospective Thai cohort reported that 23% of patients with primary DENV infection developed dengue hemorrhagic fever compared to 53% of those with secondary DENV infections (Vaughn et al., 2000). Notably, a large majority of cases (31 of 32) which manifested as severe dengue were caused by primary infection with DENV-1 and DENV-3. However, during a prospective Cuban cohort study during a DENV-2 outbreak in 1997, a small frequency of patients (2 of 193) with primary DENV infection developed severe dengue symptoms. While humoral immune status between cohorts remains the chief hypothesis, the discrepancies in apparent infection/severe dengue rates between cohorts have been attributed to genetic variation between different ethnicities, HLA-allele polymorphisms, cohort age ranges, and nutritional status (Coffey et al., 2009).
Due to the recent emergence of ZIKV as well as the co-circulation of ZIKV and DENV in endemic countries, prospective epidemiologic studies assessing the possible connections amid congenital Zika syndrome (CZS) and flavivirus-naïve or DENV-seropositive pregnant mothers remains limited. During the height of the Brazilian ZIKV epidemic from 2016-2017, 94 pregnant women who gave birth to infants with or without microcephaly were tested for ZIKV infection as well as presence of preexisting DENV immunity. Approximately 16% (5 of 30) of the microcephalic cases had confirmed primary ZIKV infection while 63% (19 of 30) had confirmed previous DENV and ZIKV plaque reduction neutralization test (PRNT) titers, indicating that previous DENV immunity is not a prerequisite for ZIKV entry into the fetal compartment (de Araujo et al., 2016). Another study from Brazil corroborated these findings by observing even higher rates among dengue-IgG negative, ZIKV-infected pregnant women (50%; 7 of 14) present with abnormal birth outcomes compared to DENV-IgG positive cases (43.8%; 43 of 98) (Halai et al., 2017). However, additional prospective epidemiologic studies as well as ex vivo or animal model experimentation is needed to fully dissect the complex risk factors and pathways through which ZIKV can seed and infect the fetal compartment.
Cross reactive antibodies in animal models of systemic ZIKV ADE
Mouse and non-human primate (NHP) model systems have been utilized to study the influence of DENV humoral immunity on ZIKV pathogenesis and severity of infection. Due to the inability of ZIKV to evade murine type I IFN signaling, particularly STAT2-mediated innate immunity, deletion of murine Stat2 or insertion of human STAT2 are necessary for studying ZIKV pathogenesis in mouse systems (Gorman et al., 2018; Grant et al., 2016). Passive immunization provided by human DENV and WNV convalescent serum followed by ZIKV challenge caused enhancement of ZIKV replication in multiple target organs and increased mortality in pregnant and non-pregnant Stat2−/− mice (Bardina et al., 2017; Brown et al., 2019). Conversely, when immunocompetent 129Sv/ev mice were pre-treated with DENV cross-reactive monoclonal antibodies (mAbs) and challenged with ZIKV, no enhanced disease was demonstrated (Stettler et al., 2016). A caveat to these studies is the use of human serum and purified IgG within mice to induce ADE of ZIKV infection. Murine cells also do not express FcγRIIa, FcγRIIc, or FcγRIIIa, all of which are found in human cells and can participate in binding Fc regions of viral immune complexes, allowing for ADE (Bruhns, 2012). Past studies have also affirmed that human IgG binds less efficiently to murine FcγRs, but, later studies have shown that human IgG binding to murine FcγRs is comparable to murine IgG (Langerak et al., 2019). Given these limitations, the impact of ADE in contributing to worsened ZIKV disease severity remains unclear.
Conversely, antibodies produced from primary ZIKV infection have been reported to augment secondary DENV2 infections. Pre-administration of cross-reactive ZIKV mAbs to DENV-2 infected immunodeficient AG129 mice, a ZIKV permissive mouse model lacking alpha/beta IFN and gamma IFN receptors, led to severe symptoms and increased lethality by day 5 compared to non-antibody treated controls (Stettler et al., 2016). Using mice with type I IFN signaling specifically ablated in macrophages, ZIKV-experienced dams displayed increased viral burden and higher mortality attributed to increased production of the proinflammatory cytokine TNF-α when challenged with primary DENV infection (Fowler et al., 2018). In another study, rhesus macaques were infected with ZIKV subcutaneously and subsequently infected with DENV-2 and monitored for an enhanced infection phenotype. The ZIKV-immune macaques showed higher levels of DENV-2 viremia, larger pro-inflammatory cytokine responses, and notably, a strong anamnestic cross-reactive B cell response (George et al., 2017). Given the co-circulation of DENV and ZIKV in endemic areas as well as the further development of DENV and ZIKV vaccines, further evaluation of the cross-protective and pathogenic effects of virus or vaccine-mediated antibody responses is of utmost importance.
The human placenta: a new target for pathogenic flaviviruses
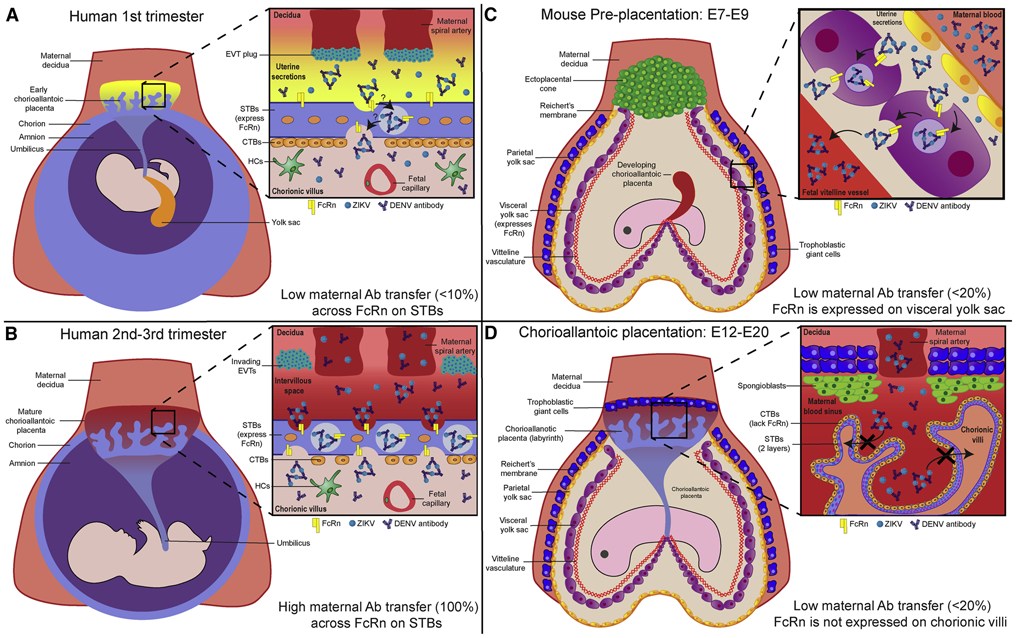
Established by the 3rd week of gestation, the human placenta is the sole physical and immunologic barrier between the maternal and fetal bloodstreams. For the duration of pregnancy, it is responsible for transporting nutrients, solutes, and waste between the mother and developing fetus (Coyne and Lazear, 2016). The functional unit of the human placenta are chorionic villi, tree-like structures anchored to the maternal decidua or floating within the intervillous space. These villi are primarily hemomonochorial with an apical syncytiotrophoblast (STB) layer overlaying a basolateral progenitor cytotrophoblast (CTB) layer. The STB layer comprises the first barrier of defense for the placenta against invading pathogens. Beneath the STB and CTB layers lies the villous stroma which contains numerous cell types, including stromal fibroblasts, villous macrophages (Hofbauer cells (HCs)), and fetal endothelial cells comprising the fetal vasculature. During the first trimester, extravillous trophoblasts (EVTs), which invade the decidua and anchor villi, form plugs on the maternal spiral arteries, inhibiting the oxygen-rich maternal blood from entering the intervillous space. Without maternal blood during the first trimester, the villi are instead bathed in uterine secretions that are phagocytosed by STBs to provide nutrition to the fetus (Fig. 2A). Signifying the beginning of the second trimester, the EVT plug dissolves and the flow of blood from the spiral arteries into the intervillous space is uninhibited, bathing the chorionic villi in maternal blood (Ander et al., 2019). During this time, maternal IgG also appears in the intervillous space and transcytoses across the STB layer into the villous stroma using the neonatal Fc receptor (FcRn) (Fig. 2B). This provides ample passive immunity to the developing fetus throughout the duration of pregnancy and up to six months after birth (Simister and Story, 1997).
Figure 2. Differences in barrier structure and antibody translocation between human and mouse placentae.
A) By the third week of gestation, the human chorioallantoic placenta has invaded the maternal decidua and established the chorionic villous system for nutrient exchange. The chorionic villi are composed of a fused syncytiotrophoblast (STB) layer with underlying progenitor cytotrophoblasts (CTBs) that can regenerate the STB layer or develop into extravillous trophoblasts (EVTs). At this point in pregnancy, EVTs plug the maternal spiral arteries, hindering maternal blood from contacting the chorionic villi. Instead, the chorionic villi are bathed in uterine secretions to provide nutrition. Unlike the second and third trimesters, transfer of maternal IgG across the STB layer into the villous stroma is low (<10% maternofetal transfer efficiency) B) Dissolution of the EVT plugs and flooding of the intervillous space with maternal blood signifies the start of the second trimester. At approximately the 13th week of gestation until term, maternal IgG and immune complexes readily bind to the neonatal Fc receptor on the STB surface and are transcytosed into the villous stroma with 100% maternofetal transfer efficiency. Once in the villous stroma, these antibodies and complexes can be phagocytosed by resident Hofbauer cells (HCs) or taken up fetal endothelial cells to enter the fetal circulation. C) In contrast to humans, the mouse has two different placentae throughout gestation. Around embryonic age 7-9 (E7-9), the mouse is surrounded by trophoblastic giant cells and the vitelline yolk sac (VYS) placenta. The VYS placenta is comprised of an outer endodermal cell layer with a mesodermal vitelline vasculature network underneath. The VYS endodermal cell layer expresses FcRn and allows for modest levels of passive antibody transfer from the uterine secretions to the developing fetal mouse (<20% maternofetal transfer efficiency). At this point, the labyrinthine chorioallantoic placenta is not fully established D) Around E12, the enveloping trophoblastic giant cells now divert blood through the spongiotrophoblast layer into the fully formed labyrinthine chorioallantoic placenta, containing interwoven villous structures perfused with maternal blood. Distinct from humans, the chorionic villi of the mouse trichorioallantoic placenta contain an outermost CTB layer with two inner STBs layer. In contrast to the VYS placenta, neither the CTB nor STB layers express FcRn and cannot transfer maternal antibodies to the fetus.
ZIKV infection of the human placenta
Despite the innate and immunologic defenses provided by the placenta and the mother (Coyne and Lazear, 2016), ZIKV is hypothesized to bypass the highly antiviral STB layer and seed the fetal compartment through both a placental route via invading EVTs and a paraplacental route through the amniochorion (Tabata et al., 2016). During ZIKV infection in vivo, histopathologic findings show ZIKV antigen expression and extensive damage within the chorionic villi, including edema, placental infarctions, stromal calcifications, HC hypercellularity, and sclerosis (Hirsch et al., 2018; Martines et al., 2016). Despite these pathologic findings, other groups have reported minimal inflammation coupled with HC hypercellularity in the placenta during ZIKV infection in vivo (Rosenberg et al., 2017). We and others have also demonstrated that, once the STB layer has been circumvented, ZIKV preferentially targets and productively infects HCs within the villous stroma (Bhatnagar et al., 2017; Jurado et al., 2016; Quicke et al., 2016; Tabata et al., 2016; Zimmerman et al., 2018). In one study, ZIKV RNA was isolated from placental tissues in 75% of women who experienced adverse pregnancy outcomes, and ZIKV replicating negative-sense RNA was found localized to HCs in 50% of the women with positive ZIKV RT-PCR results. ZIKV was also found to persist in the placenta for over 200 days post maternal onset of symptoms (Bhatnagar et al., 2017). Emerging epidemiological observations estimate that between 20-50% of pregnant women with possible ZIKV exposure had detectable ZIKV RNA in the placenta (Reagan-Steiner et al., 2017). Recent studies from our group and others have also determined that cross-reactive dengue virus (DENV) antibodies can bind to ZIKV and greatly enhance ZIKV infection in both HCs and mid-gestation chorionic villous explant tissues (Hermanns et al., 2018; Priyamvada et al., 2016; Zimmerman et al., 2018). Because DENV and ZIKV are co-endemic and pregnant women living in these areas are often seropositive for DENV, the degree to which cross-reactive antibody responses affect ZIKV pathogenesis, especially within the placenta, remains incompletely understood.
New Paradigm: Viral transcytosis using the placental FcRn pathway?
With the growing number of new flavivirus infections emerging in non-endemic regions, the impact of cross-reactive antibodies on the clinical severity of secondary infection with structurally similar flaviviruses continues to remain controversial. More recently, understanding how cross-reactive antibodies facilitate viral transport across immunologically privileged barriers, particularly the placenta, has become an emerging field of interest. After the transfer of maternal IgG across the placenta through FcRn, HCs reside in the villous stroma and express high levels of FcγRs, allowing them to bind and phagocytose IgG immune complexes with high affinity. HCs are highly permissive to ZIKV infection and are the primary target of infection in ZIKV-infected pregnant mothers within the placenta (Bhatnagar et al., 2017; Quicke et al., 2016; Zimmerman et al., 2018). Several congenital viruses, including hCMV and HIV-1, have been implicated in utilization of maternal antibodies to transcytose through the trophoblast layer and enter the fetal compartment (Maidji et al., 2006; Toth et al., 1994). Using mid-gestation explants, immune complexes generated from DENV cross-reactive antibodies bound to ZIKV were found to cross the human placental barrier in an FcRn-dependent manner (Zimmerman et al., 2018). Human mid-gestation placental explants are a powerful system for studying viral infections within the normal architecture of the placenta; however, they have limited lifespans (<10 days), lack normal blood perfusion, and can be problematic to procure. Given the limitations of the human ex vivo explant system, pregnant animal model systems have been utilized to try and recapitulate the human placenta to study the influence of DENV immunity on viral seeding of the placenta during secondary ZIKV infection.
Recently, Brown et al. evaluated the effects of DENV cross-reactive antibodies on ZIKV infection in both a pregnant mouse model system and in human ex vivo placental explants. In their study, pregnant mice were infected with ZIKV in the presence of purified DENV immune plasma. They observed that increased fetal resorption, increased infection of the placenta, and placental damage in ZIKV-infected pregnant mice inoculated with DENV immune plasma at embryonic age day 5.5 (E5.5). This effect was diminished if the pregnant mice were infected at an advanced gestational age, E10.5 (Brown et al., 2019). In another study, Rathore and colleagues demonstrated that previous DENV immunity or administration of the pan-flavivirus binding antibody, 4G2, in pregnant mice caused enhanced infection in the fetus, causing fetal resorption and limited cortical growth. They also determined that increased fetal ZIKV infection was facilitated by translocation of viral immune complexes across the placenta by murine FcRn (Rathore et al., 2019). One limitation of these studies is the embryonic age, E6.5-E7, at which the pregnant DENV- or 4G2-experienced mice were inoculated with ZIKV. Infection of pregnant mice at E6.5-7, when the development of the vitelline yolk sac placenta is variable and the chorioallantoic placenta has not yet formed. In contrast to the singular monochorial placenta of humans, fetal mice obtain nutrients from two separate placenta, the vitelline yolk sac (VYS) and labyrinthine placenta, at different times during gestation. The VYS arises at embryonic age E7-E9 and is comprised of a single-cell layer of endodermal epithelium with underlying mesodermal vitelline vasculature bathed in uterine secretions containing maternal IgG (Cross et al., 1994; Kim et al., 2009). Unlike humans, approximately 20% of IgG transfer is facilitated across the yolk sac through murine FcRn while a majority of IgG transfer is absorbed in the mouse gut postnatally through breastmilk (Ander et al., 2019) (Fig 2C). Around E12 of pregnancy, the trichorioallantoic labyrinthine placenta is fully functional with two outer layers of CTBs and one inner layer of STBs, neither of which express FcRn (Kim et al., 2009; Latvala et al., 2017). (Fig. 2D). To more accurately model human FcRn (hFcRn) expression and trafficking of flavivirus immune complexes within the murine placenta, future animal studies could utilize the transgenic humanized mouse strains Tg32 and Tg276, which express copious amounts of human FcRn within different layers of the labyrinthine placenta (Latvala et al., 2017). Additionally, the innate immune functionality of the murine STB layer, which has been shown to halt bacterial and viral infections through the impermeability of the STB syncytium as well as type III IFN signaling, is not present at these early gestational ages (Bayer et al., 2016; Chen et al., 2017; Jagger et al., 2017; Robbins et al., 2010; Zeldovich et al., 2013). This suggests that the trophoblasts layers found in the murine chorioallantoic, but not VYS, placenta should be considered in future studies for accurately recapitulating mechanisms of placental translocation by invading pathogens observed in humans.
Because of their high level of homology to humans, non-human primates, particularly rhesus macaques, have been used extensively to understand transplacental ZIKV infection and subsequent neurological birth defects within the developing fetus. NHPs utilize a bidiscoidal, hemomonochorial placenta that expresses FcRn on the STB surface, allowing for >80% efficiency of maternofetal IgG transfer during pregnancy (Latvala et al., 2017; Pentsuk and van der Laan, 2009). Recent work has shown that early ZIKV infection (<7 wks) of pregnant rhesus macaques initiates infection of the placenta and causes widespread neuropathology in infected fetuses (Martinot et al., 2018). In contrast, macaques infected later in pregnancy (>12 weeks) showed minimal placental ZIKV infection despite detectable ZIKV RNA within the fetal meninges. Concurrent findings also showed that ZIKV infection in pregnant macaques altered the hemodynamics of maternofetal blood flow downstream of decidual spiral artery fibrin deposition as well as villous damage, including numerous infarctions and calcifications (Hirsch et al., 2018). Previous work has shown that rhesus macaques exposed to DENV-1 or DENV-2, despite causing serologic enhancement of ZIKV infection in vitro, did not show enhanced viremia nor clinical severity upon challenge with ZIKV (Pantoja et al., 2017). However, it is not clear whether previous dengue humoral immunity enhances in vivo viral seeding of the fetal compartment during subsequent ZIKV infection in NHPs. Given the growing literature suggesting that cross-reactive DENV mAbs augment ZIKV transcytosis and infection in human placental chorionic explants (Hermanns et al., 2018; Zimmerman et al., 2018), the effects of previous dengue immunity on ZIKV infection in pregnant rhesus macaques warrants further investigation.
Vaccines for DENV and ZIKV
With the concern of potential ADE effects caused by vaccine-elicited antibody responses, passive administration of mAbs has been used as a potential prophylactic measure against ZIKV infection. Infusion of rhesus macaques with a cocktail of three engineered ZIKV neutralizing mAbs with LALA mutations, all of which target domains II and III of the E protein (EDII and EDIII) but cannot bind to FcγRs, conferred sterilizing immunity upon ZIKV challenge. Moreover, these ZIKV mAbs maintained high neutralizing concentrations up to 42 days post-infusion (Magnani et al., 2017). Keeffe and colleagues also showed that administration of two potently neutralizing DENV cross-reactive ZIKV mAbs, including their non-FcγR binding variants, bind the lateral ridge of EDIII and provide moderate protection while eliminating escape mutant variants in NHPs (Keeffe et al., 2018). Despite providing immunity in non-pregnant NHPs, administration of a triple antibody cocktail to pregnant rhesus macaques previous to ZIKV challenge did not prevent vertical transmission to the fetus (Magnani et al., 2018).
Due to a lack of approved flavivirus-specific antiviral therapeutics, numerous groups have studied various ZIKV vaccine platforms to confer immunologic protection in both mice and NHPs. Lipid nanoparticle (LNP) mRNA vaccines have been widely tested during the ZIKV epidemic due to its non-infectivity and capacity to express high levels of viral protein. ZIKV prM-E mRNA LNP vaccines have been shown induce high levels of neutralizing ZIKV-specific IgG production as well as antiviral CD4+ T cell responses in both immunocompetent and immunocompromised mice. Mutation in the fusion loop epitope in the ZIKV mRNA LNP vaccine alleviated potential DENV enhancement effects without compromising protection from ZIKV challenge (Richner et al., 2017). Low dose administration of ZIKV prM-E mRNA LNP vaccine to rhesus macaques also exhibited sterilizing immunity and complete protection from subcutaneous challenge with ZIKV (Pardi et al., 2017). Recent work has also shown that vaccines with ZIKV prM/M-E engineered into low-circulation AdC7 andRhAd52 adenovirus constructs can provide single-dose protection in IFNAR−/− mice lacking the type I interferon receptor and rhesus macaques, respectively (Abbink et al., 2017; Xu et al., 2018). High levels of humoral protection against ZIKV infection were also observed in both pregnant Ifnar−/− mice and their pups after inoculation of dams with the adenovirus-based ZIKV vaccines, RhAd52.M-Env and Ad26.M-Env (Larocca et al., 2019). Live attenuated vaccines (LAVs) induce robust and durable adaptive immune responses but carry risk of viral reversion and pathogenesis, especially in immunocompromised and pregnant patients. To ameliorate this concern, one group developed a live-attenuated ZIKV vaccine with trans-complemented wild-type capsid protein while encoding a mutant capsid protein, allowing for only one round of replication (Xie et al., 2018). Upon ZIKV challenge 28 days post-immunization, viremia was not detectable in either the mother or fetus in pregnant A129 mice. Because of the presence of ZIKV non-structural proteins within the LAV construct, the viral protection in these pregnant mice were attributed to the high neutralizing antibody titers and robust CD4+ and CD8+ T cell responses.
Conclusions
Considering the co-circulation of both DENV and ZIKV within endemic regions, understanding how pre-existing flavivirus immunity affects the pathogenesis of secondary flavivirus infection remains of utmost importance. While serious complications from primary DENV infections can occur, increasing epidemiologic and experimental evidence continues to solidify the role of pre-existing DENV antibodies in mediating severe dengue disease. Due to the recent emergence of ZIKV in 2015 and limited epidemiologic studies, whether cross-reactive antibodies elicited from a primary DENV or ZIKV infection ameliorate or exacerbate subsequent flavivirus infection is a multifactorial issue that remains unresolved. While the epidemiological evidence does not yet support the concept that pre-existing dengue immunity worsens the clinical severity of ZIKV infection in the mother or fetus, our group and others have evaluated the ability of ZIKV to utilize the FcRn-mediated transcytosis pathway to gain access and seed the placenta. While the model systems used in these studies have their respective limitations, the information gleaned from this work has furthered our insight and sparked many questions regarding the impact of cross-reactive flavivirus antibodies on ZIKV pathogenesis in the placenta. How does ZIKV survive the low pH environment during transcytosis? Do cross-reactive antibodies bind to specific ZIKV epitopes, stabilizing ZIKV as it crosses the STB layer? What impact will the widespread use of multivalent DENV and ZIKV vaccines have on the cross-reactive antibody repertoire and potential transcytosis across the placenta in pregnant women? Hopefully, the growing interest in antibody-mediated vertical transmission of ZIKV will stimulate further in vivo studies in pregnant NHPs or women to dissect the immunological and molecular mechanisms of ZIKV transcytosis across the placenta.
Acknowledgments
Funding: This research is funded in part by National Institutes of Health grants U19AI083019 (M.S.S), U01AI131566 (M.S.S.), 2U19AI057266 (M.S.S.), 5U19AI057266-13REVIS Supplement (M.S.S and J.W), National Institutes of Health’s Office of the Director, Office of Research Infrastructure Programs, P51OD011132, Children’s Healthcare of Atlanta (M.S.S.), Emory Vaccine Center (M.S.S. and J.W.), and The Georgia Research Alliance (M.S.S). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: The authors declare no competing interests.
References
- Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, Kirilova M, Peterson R, Li Z, Nanayakkara O, et al. (2017). Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ander SE, Diamond MS, and Coyne CB (2019). Immune responses at the maternal-fetal interface. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade P, Gimblet-Ochieng C, Modirian F, Collins M, Cardenas M, Katzelnick LC, Montoya M, Michlmayr D, Kuan G, Balmaseda A, et al. (2019). Impact of pre-existing dengue immunity on human antibody and memory B cell responses to Zika. Nat Commun 10, 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, et al. (2017). Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr., Cherry S, Sadovsky Y, and Coyne CB (2016). Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe 19, 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, et al. (2010). The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LB, Suzuki T, Ritter J, Keating MK, Hale G, et al. (2017). Zika Virus RNA Replication and Persistence in Brain and Placental Tissue. Emerg Infect Dis 23, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. (2013). The global distribution and burden of dengue. Nature 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Pereira JP Jr., Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai UA, Salles TS, et al. (2016a). Zika Virus Infection in Pregnant Women in Rio de Janeiro. N Engl J Med 375, 2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, Siqueira AM, de Mendonca MC, Nogueira RM, de Filippis AM, et al. (2016b). Guillain-Barre syndrome associated with Zika virus infection. Lancet 387, 1482. [DOI] [PubMed] [Google Scholar]
- Brown JA, Singh G, Acklin JA, Lee S, Duehr JE, Chokola AN, Frere JJ, Hoffman KW, Foster GA, Krysztof D, et al. (2019). Dengue Virus Immunity Increases Zika Virus-Induced Damage during Pregnancy. Immunity 50, 751–762 e755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhns P (2012). Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119, 5640–5649. [DOI] [PubMed] [Google Scholar]
- Burke DS, Nisalak A, Johnson DE, and Scott RM (1988). A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg 38, 172–180. [DOI] [PubMed] [Google Scholar]
- Cardoso TF Jr., Santos RSD, Correa RM, Campos JV, Silva RB, Tobias CC, Prata-Barbosa A, Cunha A, and Ferreira HC (2019). Congenital Zika infection: neurology can occur without microcephaly. Arch Dis Child 104, 199–200. [DOI] [PubMed] [Google Scholar]
- Carrington LB, and Simmons CP (2014). Human to mosquito transmission of dengue viruses. Front Immunol 5, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll T, Lo M, Lanteri M, Dutra J, Zarbock K, Silveira P, Rourke T, Ma ZM, Fritts L, O'Connor S, et al. (2017). Zika virus preferentially replicates in the female reproductive tract after vaginal inoculation of rhesus macaques. PLoS Pathog 13, e1006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Liang Y, Yi P, Xu L, Hawkins HK, Rossi SL, Soong L, Cai J, Menon R, and Sun J (2017). Outcomes of Congenital Zika Disease Depend on Timing of Infection and Maternal-Fetal Interferon Action. Cell Rep 21, 1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham HE, Cummings DAT, and Johansson MA (2017). Immune status alters the probability of apparent illness due to dengue virus infection: Evidence from a pooled analysis across multiple cohort and cluster studies. PLoS Negl Trop Dis 11, e0005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey LL, Mertens E, Brehin AC, Fernandez-Garcia MD, Amara A, Despres P, and Sakuntabhai A (2009). Human genetic determinants of dengue virus susceptibility. Microbes Infect 11, 143–156. [DOI] [PubMed] [Google Scholar]
- Collins MH, Tu HA, Gimblet-Ochieng C, Liou GA, Jadi RS, Metz SW, Thomas A, McElvany BD, Davidson E, Doranz BJ, et al. (2019). Human antibody response to Zika targets type-specific quaternary structure epitopes. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, de Silva AD, and de Silva AM (2015). Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis 211, 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, and Lazear HM (2016). Zika virus - reigniting the TORCH. Nat Rev Microbiol 14, 707–715. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, and Fisher SJ (1994). Implantation and the placenta: key pieces of the development puzzle. Science 266, 1508–1518. [DOI] [PubMed] [Google Scholar]
- de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brien JD, Tsai WY, et al. (2011). In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis 5, e1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE Jr., et al. (2012). Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109, 7439–7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo TVB, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo APL, Valongueiro S, de Albuquerque M, Souza WV, Braga C, et al. (2016). Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 16, 1356–1363. [DOI] [PubMed] [Google Scholar]
- de Araujo TVB, Ximenes RAA, Miranda-Filho DB, Souza WV, Montarroyos UR, de Melo APL, Valongueiro S, de Albuquerque M, Braga C, Filho SPB, et al. (2018). Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis 18, 328–336. [DOI] [PubMed] [Google Scholar]
- de Oliveira WK, de Franca GVA, Carmo EH, Duncan BB, de Souza Kuchenbecker R, and Schmidt MI (2017). Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. Lancet 390, 861–870. [DOI] [PubMed] [Google Scholar]
- Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith ARY, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, et al. (2016). Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep 16, 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo CP, Barros-Aragao FGQ, Neris RLS, Frost PS, Soares C, Souza INO, Zeidler JD, Zamberlan DC, de Sousa VL, Souza AS, et al. (2019). Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat Commun 10, 3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flipse J, and Smit JM (2015). The Complexity of a Dengue Vaccine: A Review of the Human Antibody Response. PLoS Negl Trop Dis 9, e0003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AM, Tang WW, Young MP, Mamidi A, Viramontes KM, McCauley MD, Carlin AF, Schooley RT, Swanstrom J, Baric RS, et al. (2018). Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice. Cell Host Microbe 24, 743–750 e745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks AC, and Fernandez-Sesma A (2014). The burden of dengue and chikungunya worldwide: implications for the southern United States and California. Ann Glob Health 80, 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia G, Gonzalez N, Perez AB, Sierra B, Aguirre E, Rizo D, Izquierdo A, Sanchez L, Diaz D, Lezcay M, et al. (2011). Long-term persistence of clinical symptoms in dengue-infected persons and its association with immunological disorders. Int J Infect Dis 15, e38–43. [DOI] [PubMed] [Google Scholar]
- George J, Valiant WG, Mattapallil MJ, Walker M, Huang YS, Vanlandingham DL, Misamore J, Greenhouse J, Weiss DE, Verthelyi D, et al. (2017). Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci Rep 7, 10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Gresh L, Ojeda S, Katzelnick LC, Sanchez N, Mercado JC, Chowell G, Lopez B, Elizondo D, Coloma J, et al. (2019). Prior dengue virus infection and risk of Zika: A pediatric cohort in Nicaragua. PLoS Med 16, e1002726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MJ, Caine EA, Zaitsev K, Begley MC, Weger-Lucarelli J, Uccellini MB, Tripathi S, Morrison J, Yount BL, Dinnon KH 3rd, et al. (2018). An Immunocompetent Mouse Model of Zika Virus Infection. Cell Host Microbe 23, 672–685 e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossner CM, Ducheyne E, and Schaffner F (2018). Increased risk for autochthonous vector-borne infections transmitted by Aedes albopictus in continental Europe. Euro Surveill 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sanchez-Seco MP, Evans MJ, Best SM, et al. (2016). Zika Virus Targets Human STAT2 to Inhibit Type I Interferon Signaling. Cell Host Microbe 19, 882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, and Harris E (2015). Dengue. Lancet 385, 453–465. [DOI] [PubMed] [Google Scholar]
- Guzman MG, Kouri G, Valdes L, Bravo J, Alvarez M, Vazques S, Delgado I, and Halstead SB (2000). Epidemiologic studies on Dengue in Santiago de Cuba, 1997. Am J Epidemiol 152, 793–799; discussion 804. [DOI] [PubMed] [Google Scholar]
- Halai UA, Nielsen-Saines K, Moreira ML, de Sequeira PC, Junior JPP, de Araujo Zin A, Cherry J, Gabaglia CR, Gaw SL, Adachi K, et al. (2017). Maternal Zika Virus Disease Severity, Virus Load, Prior Dengue Antibodies, and Their Relationship to Birth Outcomes. Clin Infect Dis 65, 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB (1979). In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis 140, 527–533. [DOI] [PubMed] [Google Scholar]
- Halstead SB (2003). Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60, 421–467. [DOI] [PubMed] [Google Scholar]
- Halstead SB, and O'Rourke EJ (1977). Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265, 739–741. [DOI] [PubMed] [Google Scholar]
- Heald-Sargent T, and Muller W (2017). Zika Virus: A Review for Pediatricians. Pediatr Ann 46, e428–e432. [DOI] [PubMed] [Google Scholar]
- Hermanns K, Gohner C, Kopp A, Schmidt A, Merz WM, Markert UR, Junglen S, and Drosten C (2018). Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerg Microbes Infect 7, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch AJ, Roberts VHJ, Grigsby PL, Haese N, Schabel MC, Wang X, Lo JO, Liu Z, Kroenke CD, Smith JL, et al. (2018). Zika virus infection in pregnant rhesus macaques causes placental dysfunction and immunopathology. Nat Commun 9, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger BW, Miner JJ, Cao B, Arora N, Smith AM, Kovacs A, Mysorekar IU, Coyne CB, and Diamond MS (2017). Gestational Stage and IFN-lambda Signaling Regulate ZIKV Infection In Utero. Cell Host Microbe 22, 366–376 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, Wu M, Lindenbach BD, Abrahams VM, Guller S, et al. (2016). Zika virus productively infects primary human placenta-specific macrophages. JCI Insight 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapogiannis BG, Chakhtoura N, Hazra R, and Spong CY (2017). Bridging Knowledge Gaps to Understand How Zika Virus Exposure and Infection Affect Child Development. JAMA Pediatr 171, 478–485. [DOI] [PubMed] [Google Scholar]
- Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, Balmaseda A, and Harris E (2017). Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeffe JR, Van Rompay KKA, Olsen PC, Wang Q, Gazumyan A, Azzopardi SA, Schaefer-Babajew D, Lee YE, Stuart JB, Singapuri A, et al. (2018). A Combination of Two Human Monoclonal Antibodies Prevents Zika Virus Escape Mutations in Non-human Primates. Cell Rep 25, 1385–1394 e1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Mohanty S, Ganesan LP, Hua K, Jarjoura D, Hayton WL, Robinson JM, and Anderson CL (2009). FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J Immunol 182, 2583–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliks SC, Nimmanitya S, Nisalak A, and Burke DS (1988). Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 38, 411–419. [DOI] [PubMed] [Google Scholar]
- Langerak T, Mumtaz N, Tolk VI, van Gorp ECM, Martina BE, Rockx B, and Koopmans MPG (2019). The possible role of cross-reactive dengue virus antibodies in Zika virus pathogenesis. PLoS Pathog 15, e1007640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca RA, Mendes EA, Abbink P, Peterson RL, Martinot AJ, Iampietro MJ, Kang ZH, Aid M, Kirilova M, Jacob-Dolan C, et al. (2019). Adenovirus Vector-Based Vaccines Confer Maternal-Fetal Protection against Zika Virus Challenge in Pregnant IFN-alphabetaR(−/−) Mice. Cell Host Microbe 26, 591–600 e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latvala S, Jacobsen B, Otteneder MB, Herrmann A, and Kronenberg S (2017). Distribution of FcRn Across Species and Tissues. J Histochem Cytochem 65, 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes Moreira ME, Nielsen-Saines K, Brasil P, Kerin T, Damasceno L, Pone M, Carvalho LMA, Pone SM, Vasconcelos Z, Ribeiro IP, et al. (2018). Neurodevelopment in Infants Exposed to Zika Virus In Utero. N Engl J Med 379, 2377–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani DM, Rogers TF, Beutler N, Ricciardi MJ, Bailey VK, Gonzalez-Nieto L, Briney B, Sok D, Le K, Strubel A, et al. (2017). Neutralizing human monoclonal antibodies prevent Zika virus infection in macaques. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani DM, Rogers TF, Maness NJ, Grubaugh ND, Beutler N, Bailey VK, Gonzalez-Nieto L, Gutman MJ, Pedreno-Lopez N, Kwal JM, et al. (2018). Fetal demise and failed antibody therapy during Zika virus infection of pregnant macaques. Nat Commun 9, 1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidji E, McDonagh S, Genbacev O, Tabata T, and Pereira L (2006). Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol 168, 1210–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchette NJ, Halstead SB, O'Rourke T, Scott RM, Bancroft WH, and Vanopruks V (1979). Effect of immune status on dengue 2 virus replication in cultured leukocytes from infants and children. Infect Immun 24, 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martines RB, Bhatnagar J, de Oliveira Ramos AM, Davi HP, Iglezias SD, Kanamura CT, Keating MK, Hale G, Silva-Flannery L, Muehlenbachs A, et al. (2016). Pathology of congenital Zika syndrome in Brazil: a case series. Lancet 388, 898–904. [DOI] [PubMed] [Google Scholar]
- Martinot AJ, Abbink P, Afacan O, Prohl AK, Bronson R, Hecht JL, Borducchi EN, Larocca RA, Peterson RL, Rinaldi W, et al. (2018). Fetal Neuropathology in Zika Virus-Infected Pregnant Female Rhesus Monkeys. Cell 173, 1111–1122 e1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo ASO, Chimelli L, and Tanuri A (2017). Congenital Zika Virus Infection: Beyond Neonatal Microcephaly-Reply. JAMA Neurol 74, 610–611. [DOI] [PubMed] [Google Scholar]
- Miner JJ, and Diamond MS (2016). Understanding How Zika Virus Enters and Infects Neural Target Cells. Cell Stem Cell 18, 559–560. [DOI] [PubMed] [Google Scholar]
- Miner JJ, and Diamond MS (2017). Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 21, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey SB, Vezina G, Bulas DI, Khademian Z, Blask A, Kousa Y, Cristante C, Pesacreta L, du Plessis AJ, and DeBiasi RL (2018). Neuroimaging Findings in Normocephalic Newborns With Intrauterine Zika Virus Exposure. Pediatr Neurol 78, 75–78. [DOI] [PubMed] [Google Scholar]
- Nogueira ML, Nery Junior NRR, Estofolete CF, Bernardes Terzian AC, Guimaraes GF, Zini N, Alves da Silva R, Dutra Silva GC, Junqueira Franco LC, Rahal P, et al. (2018). Adverse birth outcomes associated with Zika virus exposure during pregnancy in Sao Jose do Rio Preto, Brazil. Clin Microbiol Infect 24, 646–652. [DOI] [PubMed] [Google Scholar]
- Oh Y, Zhang F, Wang Y, Lee EM, Choi IY, Lim H, Mirakhori F, Li R, Huang L, Xu T, et al. (2017). Zika virus directly infects peripheral neurons and induces cell death. Nat Neurosci 20, 1209–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja P, Perez-Guzman EX, Rodriguez IV, White LJ, Gonzalez O, Serrano C, Giavedoni L, Hodara V, Cruz L, Arana T, et al. (2017). Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 8, 15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, et al. (2017). Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 543, 248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Longo P, Miley MJ, Montoya M, Harris E, and de Silva AM (2017). Dissecting the human serum antibody response to secondary dengue virus infections. PLoS Negl Trop Dis 11, e0005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso C, Fischer C, Feldmann M, Sarno M, Luz E, Moreira-Soto A, Cabral R, Netto EM, Brites C, Kummerer BM, et al. (2019). Cross-Protection of Dengue Virus Infection against Congenital Zika Syndrome, Northeastern Brazil. Emerg Infect Dis 25, 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentsuk N, and van der Laan JW (2009). An interspecies comparison of placental antibody transfer: new insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res B Dev Reprod Toxicol 86, 328–344. [DOI] [PubMed] [Google Scholar]
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. (2016). Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A 113, 7852–7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O'Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, et al. (2016). Zika Virus Infects Human Placental Macrophages. Cell Host Microbe 20, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, and Petersen LR (2016). Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med 374, 1981–1987. [DOI] [PubMed] [Google Scholar]
- Rathore APS, Saron WAA, Lim T, Jahan N, and St John AL (2019). Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci Adv 5, eaav3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Steiner S, Simeone R, Simon E, Bhatnagar J, Oduyebo T, Free R, Denison AM, Rabeneck DB, Ellington S, Petersen E, et al. (2017). Evaluation of Placental and Fetal Tissue Specimens for Zika Virus Infection - 50 States and District of Columbia, January-December, 2016. MMWR Morb Mortal Wkly Rep 66, 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Mancia Leon WR, Krencik R, Ullian EM, Spatazza J, et al. (2016). Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A 113, 14408–14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Galang RR, Roth NM, Ellington SR, Moore CA, Valencia-Prado M, Ellis EM, Tufa AJ, Taulung LA, Alfred JM, et al. (2018). Vital Signs: Zika-Associated Birth Defects and Neurodevelopmental Abnormalities Possibly Associated with Congenital Zika Virus Infection - U.S. Territories and Freely Associated States, 2018. MMWR Morb Mortal Wkly Rep 67, 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. (2017). Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 169, 176. [DOI] [PubMed] [Google Scholar]
- Robbiani DF, Olsen PC, Costa F, Wang Q, Oliveira TY, Nery N Jr., Aromolaran A, do Rosario MS, Sacramento GA, Cruz JS, et al. (2019). Risk of Zika microcephaly correlates with features of maternal antibodies. J Exp Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JR, Skrzypczynska KM, Zeldovich VB, Kapidzic M, and Bakardjiev AI (2010). Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog 6, e1000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenhuis-Zybert IA, da Silva Voorham JM, Torres S, van de Pol D, and Smit JM (2015). Antibodies against immature virions are not a discriminating factor for dengue disease severity. PLoS Negl Trop Dis 9, e0003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TF, Goodwin EC, Briney B, Sok D, Beutler N, Strubel A, Nedellec R, Le K, Brown ME, Burton DR, et al. (2017). Zika virus activates de novo and cross-reactive memory B cell responses in dengue-experienced donors. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AZ, Yu W, Hill DA, Reyes CA, and Schwartz DA (2017). Placental Pathology of Zika Virus: Viral Infection of the Placenta Induces Villous Stromal Macrophage (Hofbauer Cell) Proliferation and Hyperplasia. Arch Pathol Lab Med 141, 43–48. [DOI] [PubMed] [Google Scholar]
- Simister NE, and Story CM (1997). Human placental Fc receptors and the transmission of antibodies from mother to fetus. J Reprod Immunol 37, 1–23. [DOI] [PubMed] [Google Scholar]
- Sridhar S, Luedtke A, Langevin E, Zhu M, Bonaparte M, Machabert T, Savarino S, Zambrano B, Moureau A, Khromava A, et al. (2018). Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med 379, 327–340. [DOI] [PubMed] [Google Scholar]
- Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. (2016). Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353, 823–826. [DOI] [PubMed] [Google Scholar]
- Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, and Pereira L (2016). Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission. Cell Host Microbe 20, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth FD, Mosborg-Petersen P, Kiss J, Aboagye-Mathiesen G, Zdravkovic M, Hager H, Aranyosi J, Lampe L, and Ebbesen P (1994). Antibody-dependent enhancement of HIV-1 infection in human term syncytiotrophoblast cells cultured in vitro. Clin Exp Immunol 96, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, et al. (2000). Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181, 2–9. [DOI] [PubMed] [Google Scholar]
- Wheeler AC, Ventura CV, Ridenour T, Toth D, Nobrega LL, Silva de Souza Dantas LC, Rocha C, Bailey DB Jr., and Ventura LO (2018). Skills attained by infants with congenital Zika syndrome: Pilot data from Brazil. PLoS One 13, e0201495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann O, Gascon J, Schunk M, Puente S, Siikamaki H, Gjorup I, Lopez-Velez R, Clerinx J, Peyerl-Hoffmann G, Sundoy A, et al. (2007). Severe dengue virus infection in travelers: risk factors and laboratory indicators. J Infect Dis 195, 1089–1096. [DOI] [PubMed] [Google Scholar]
- Xie X, Kum DB, Xia H, Luo H, Shan C, Zou J, Muruato AE, Medeiros DBA, Nunes BTD, Dallmeier K, et al. (2018). A Single-Dose Live-Attenuated Zika Virus Vaccine with Controlled Infection Rounds that Protects against Vertical Transmission. Cell Host Microbe 24, 487–499 e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Song Y, Dai L, Zhang Y, Lu X, Xie Y, Zhang H, Cheng T, Wang Q, Huang Q, et al. (2018). Recombinant Chimpanzee Adenovirus Vaccine AdC7-M/E Protects against Zika Virus Infection and Testis Damage. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldovich VB, Clausen CH, Bradford E, Fletcher DA, Maltepe E, Robbins JR, and Bakardjiev AI (2013). Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog 9, e1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MG, Quicke KM, O'Neal JT, Arora N, Machiah D, Priyamvada L, Kauffman RC, Register E, Adekunle O, Swieboda D, et al. (2018). Cross-Reactive Dengue Virus Antibodies Augment Zika Virus Infection of Human Placental Macrophages. Cell Host Microbe 24, 731–742 e736. [DOI] [PMC free article] [PubMed] [Google Scholar]