Abstract
The objective of this study was to identify the carotenoids imparting the orange colour to the rind, and pale yellow color to the core, of selected smear-ripened cheeses. The cheeses investigated were Charloe, Ashbrook, Taleggio, and Limburger, and were sourced from artisanal markets. Samples of the rind and core were extracted using non-polar solvents, followed by saponification to hydrolyze triglycerides to remove fatty acids, and to release carotenoid esters. Extracts were tested using ultra-high pressure liquid chromatograph-diode array detector-high resolution mass spectrometry (UHPLC-DAD-MS and -MS/MS), and identities of α- and β-carotene, lycopene, and β-cryptoxanthin confirmed with authentic standards. β-Carotene was the predominant species in both the rind and core, absorbing ~70% of the signal at 450 nm in all cheese extracts tested, as well as minor quantities of β-cryptoxanthin and α-carotene. Carotenoids unique to the rind included lycopene as well as the rare bacterial carotenoids previously identified in bacterial isolates of cheeses (i.e. decaprenoxanthin, sarcinaxanthin, and echinenone). This is the first detailed characterisation of carotenoids extracted directly from smear-ripened cheeses, and reveals that smear-ripened cheese can contribute both provitamin A carotenoids as well as C50 carotenoids to the human diet.
Keywords: Ashbrook, Charloe, Taleggio, Limburger, high performance liquid chromatography-mass spectrometry
1. Introduction
The diversity of cheese available in the market is influenced by various factors such as type of milk, season and process of manufacture, starter and adjunct cultures, ripening conditions, etc. (Almena-Aliste & Mietton, 2014). Biochemical factors like glycolysis, lypolysis, proteolysis, loosing or retaining of moisture, pH, oxygen permeability etc. highly contribute to flavor development (Manzo et al., 2019). Cheese can be classified based on the type of coagulation i.e., acid, rennet and a combination of heat and acid. Smear-ripened cheeses are rennet-coagulated cheeses with orange-red-brown colour on the surface, which is a characteristic feature of this variety of cheeses. Examples of smear-ripened cheeses include Limburger, Tilsit, Taleggio, Livarot, and Brick. Young smear-ripened cheeses are often inoculated with old brine, known as ‘old-young’ smearing. This process transfers microbes present in the brine solution to the surface of young cheeses (Cogan, 2014). Intermittent washing of the cheese surface with the brine solution results in an even distribution and development of the smear. Cultures deliberately added or originating as adventitious microbes during manufacturing and ripening, such as Debaryomyces hansenii, Brevibacterium linens and Geotrichum candidum, may also contribute colour to the finished product of some smear-ripened cheese like Limburger and Taleggio (Giuffrida et al., 2020).
The development of a smear on the cheese is reflected by an initial dominance of yeasts, including D. hansenii, Saccharomyces, Candida, and Kluyveromyces, to a level of~108cfu/g of cheese within 7 days of manufacture. These yeasts are responsible for the initial metabolic activities observed, driving functions such as the de-acidification of the cheese surface, whereby pH increases due to metabolism of lactate to CO2 and H2O (Mounier et al., 2006). These yeasts also induce the synthesis of some growth factors, like pantothenic acid, which promote the growth of less acid-tolerant but more proteolytic and salt tolerant Gram-positive bacteria, e.g., Micrococcus, Corynebacterium, Glutamicibacter, Staphylococcus, Brevibacterium and others (Bockelmann, 2002). Some non-salt tolerant yeasts may lyse during the early stages of ripening, releasing peptides, nucleotides, and vitamins (Sheehan 2007). Sources for these bacteria found in cheese include milk, brine, vats, ripening rooms and wooden shelves. The interactions between the aforementioned surface microbiota significantly contribute to flavor development through the diffusion of the products of enzymatic activities into the cheese core. These interactions are also important for developing the characteristic coloured surface of smear-ripened cheeses, where this colour is due to carotenoids produced by surface microbes (Corsetti, Rossi, & Gobbetti, 2001).
There are more than 1190 naturally occurring carotenoids originating from plants, algae and bacteria (Yabuzaki, 2017). Because animals are incapable of synthesizing carotenoids, they source carotenoids through their diet. The majority of carotenoids found in foods are C40 tetraterpenoids, with an extensive conjugated double bond system reflecting the yellow, orange and red colours we associate with these compounds (Rodriguez-Amaya & Kimura, 2004). There is a wide range of health benefits associated with carotenoids. A subset of the “carotenes” class (i.e. those carotenoids composed strictly of hydrogen and carbon) with at least one unsubstituted β-ionone ring act as precursors for vitamin A. Vitamin A is an essential nutrient that plays a key role in cell differentiation, optimal functioning of the immune system, vision, and in adipose tissue regulation (Blaner, 2019; Britton, 1993; Harrison & Kopec, 2018). Other non-provitamin A carotenoids have been associated with health benefits as well, including the reduced risk of macular degeneration and improved cognitive function that are associated with the carotenoid lutein (Johnson et al., 2008; Sabour-Pickett, Nolan, Loughman, & Beatty, 2012), and the protection against certain types of cancer and cardiovascular disease that is associated with the carotenoid lycopene (Giovannucci, 2005; Sesso, Buring, Norkus, & Gaziano, 2004; Story, Kopec, Schwartz, & Harris, 2010). Carotenoids also have a variety of industrial applications as food colorants, nutrient supplements, animal feed, and in cosmetics and pharmaceutical products (Mathews-Roth, 1993; Mortensen, 2006; Nolan et al., 2016; Pickworth, Loerch, Kopec, Schwartz, & Fluharty, 2012). Smear-ripened cheeses may contribute to the dietary intake of common carotenoids in humans, and may also be a unique source for novel carotenoids specific to yeasts and bacteria particular to this family of cheeses. To further investigate these possibilities there is merit in identifying the carotenoids found on the surface of smear-ripened cheeses.
Various studies have endeavored to determine the type of carotenoids produced by the biomass of specific microbial isolates from the smear of smear-ripened cheeses (Giuffrida et al., 2015; Guyomarc’h, Binet, & Dufosse, 2000; Krubasik et al., 2001; Netzer et al., 2010; Tao, Yao, & Cheng, 2007). Formerly, Brevibacterium was regarded as being a major contributor, due to an ability to produce orange coloured C40 carotenoids like isorenieratene, 3’-hydroxy-isorenieratene, and 3’3’-dihydroxy-isorenieratene (Guyomarc’h et al., 2000; Kohl, Achenbach, & Reichenbach, 1983). Later it was established that the contribution of B. linens to the orange colour of smear cheeses could only be possible when the species is present at ≥ 5% of the total microbial population (Bockelmann, 2002), which rarely occurs. Recent advances in molecular methods such as cloning and sequencing were used to characterize the diverse microbial biodiversity of the smear surface, and C50 carotenoid production was identified in bacterial species related to smear bacteria. These C50 carotenoid-producing species, which occupy the majority of the cheese surface by the end of ripening, are Micrococcus, Glutamicibacter, Dietzia, and Corynebacterium (Giuffrida et al., 2015; Heider et al., 2012; Netzer et al., 2010; Tao et al., 2007). Interactions between these bacteria and other bacteria, yeasts, and casein hydrolysates from cheese (Bockelmann, 2002), under different pH, NaCl concentrations and temperature (Masoud & Jakobsen, 2003, 2005), can also influence colour development.
Despite this indirect evidence, and despite the existence of a number of strategies to extract carotenoids from the cheese matrix (Higuchi & Peterson, 1946; Hulshof, van Roekel-Jansen, van de Bovenkamp, & West, 2006; Lucas, Coulon, Agabriel, Chilliard, & Rock, 2008; Ollilainen, Heinonen, Linkola, Varo, & Koivistoinen, 1988), few reports have successfully directly identified the carotenoids present within smear-ripened cheeses (Galaup et al., 2007, 2015). Thus, the aim of the current research was to identify and compare the carotenoid profiles of a variety of commercially available smear-ripened cheeses. To facilitate this, a hybrid combination of the previously published non-polar liquid-liquid extraction methods was used to remove carotenoids from the cheese rind and core, and extracts were analyzed via ultra-high performance liquid chromatography-diode array detection-high resolution mass spectrometry (UHPLC-DADMS and UHPLC-DAD-MS/MS).
2. Materials and Methods
2.1. Chemicals
Optima grade formic acid and β-carotene standard (≥ 97% purity) were purchased from Sigma-Aldrich (St. Louis, MO). A lycopene standard was isolated and purified following a previously published method (Kopec et al., 2010). β-Cryptoxanthin (≥ 97% purity) was purchased from Extrasynthese (Genay, France). Lutein (≥ 95% purity), zeaxanthin (≥ 98% purity), and α-carotene (≥ 95% purity) were purchased from Cayman Chemical (Ann Arbor, MI). Optima grade methanol, HPLC grade acetone, hexane, and methyl tert-butyl ether (MTBE), and ACS grade potassium hydroxide were purchased from Fisher Scientific (Pittsburgh, PA). Double-deionized water was obtained from a Millipore Q-Plus filtration system.
2.2. Samples
Smear-ripened cheeses (Figure 1) were purchased at local artisanal markets. Cheeses were chosen on the basis of their origin and type of milk used. Ashbrook (from a producer in Reading, VT) and Charloe (from a producer in Defiance, OH) are made from raw cow’s milk. Taleggio, a protected designation of origin cheese (from a producer in Lombardy, Italy), and Limburger, a style of cheese originating from Belgium (from a producer in Monroe, WI), were made from pasteurized cow’s milk.
Figure 1.
Photos of the smear-ripened cheeses investigated: (A) Ashbrook, (B) Charloe, (C) Limberger, (D) Taleggio
2.3. Extraction of carotenoids from the cheese matrix
A figure of the experimental design is shown in Figure 2. Rind (2 g) was carefully scraped from the cheese exterior with a depth of ~2 mm, or a core sample (2 g) taken from the interior, were blended with 10 mL of methanol using a homogenizer (PowerGen 500, Fisher Scientific, San Diego, CA), in a 50 mL falcon tube. Samples were probe sonicated for 30 s on ice, followed by centrifugation at 2147 × g (3000 rpm) for 10 min. The supernatant was decanted into a separate 50 mL glass vial, and to the remaining cheese pellet, 10 mL of acetone/hexane (1:1) was added. This mixture was again sonicated and centrifuged as described, with the supernatant decanted into the 50 mL glass vial containing the methanol. This step was repeated until no color remained in the cheese pellet (approximately 5 times). One mL of saturated aq. NaCl solution was added to the pooled supernatant, followed by mixing and centrifugation for 5 min, to induce phase separation. Hexane aliquots (2 mL) were placed in glass vials and saponified by adding 2 mL of a 20% methanolic KOH solution and stirred vigorously for 2 h at 4 °C. After saponification, the hexane layer was dried under argon gas and stored at −20 °C for not more than 24 h before analysis. Samples were reconstituted in 1:1 MTBE/methanol and vortexed for 30 s before UHPLC-DAD-MS analysis.
Figure 2.
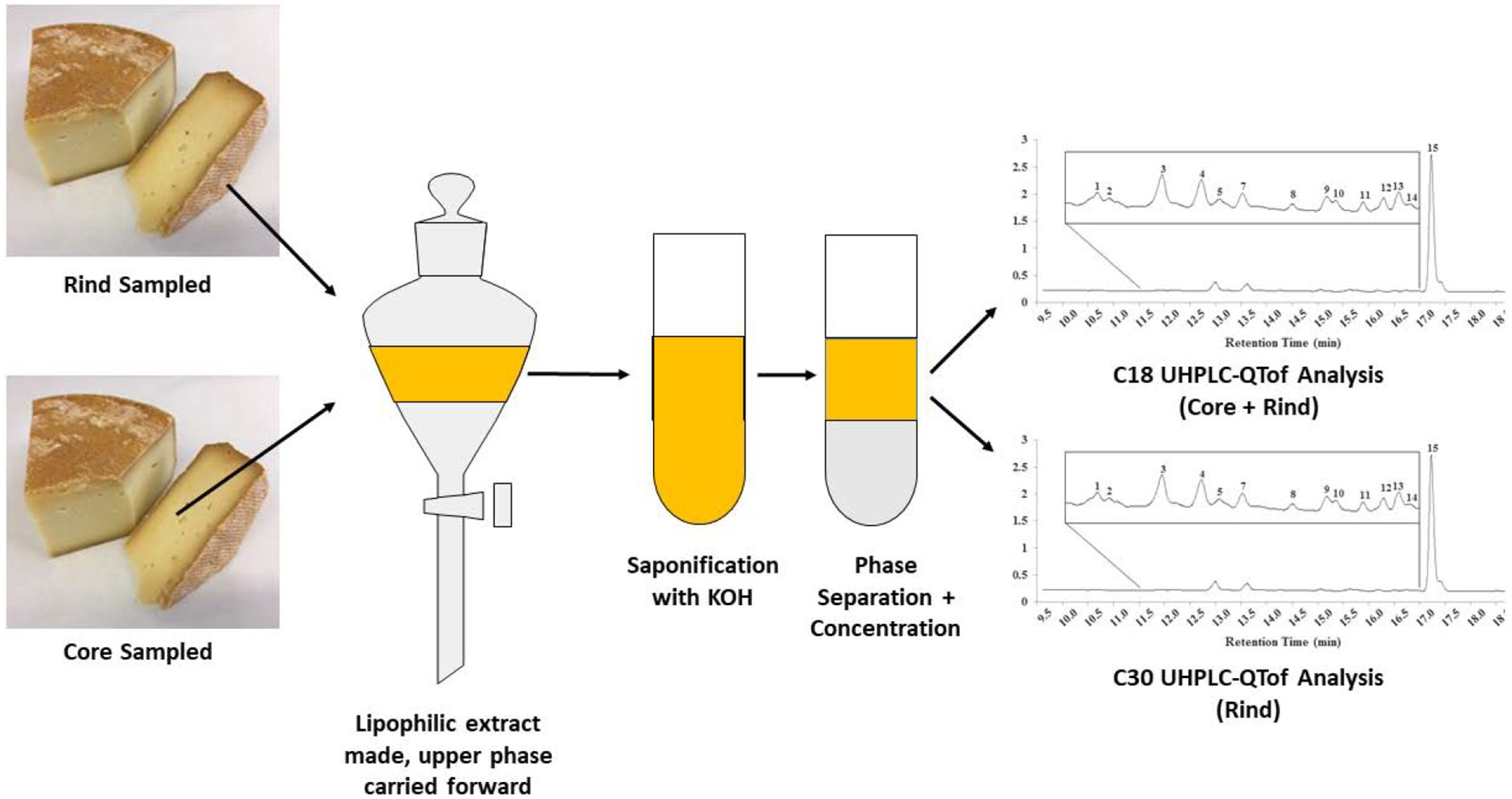
Experimental scheme employed to study carotenoids in the rind and core of smear-ripened cheeses
2.4. UHPLC-DAD-MS and MS/MS analyses
Separation of the cheese extracts was performed on an Agilent UHPLC 1290, either with an Agilent Eclipse XDB-C18 column, 150mm × 4.6 mm i.d., 5 μm particle size, or with a YMC C30 column, 150 mm × 4.6 mm i.d., 3 μm particle size. The composition of solvent A was 80:20 methanol/water and B was 78:20:2 MTBE/methanol/water, with 0.1% (v/v) formic acid added to both solvents as a modifier. The gradient was as follows: beginning at 5% B at 0 min, with a linear increase to 95% B over 18 min, holding for 3 min at 95% B, and immediately returning and holding at 5% B over 3 min. A flow rate of 1 mL/min with a column temperature of 40 °C was used. The UHPLC was interfaced with a QTof 6545 (Agilent Technologies, Santa Clara, CA) using an atmospheric pressure chemical ionization (APCI) probe operated in both positive and negative modes. Additional source settings were as follows: source gas = N2,gas temperature = 350 °C, vaporizer temperature = 350 °C, desolvation gas flow = 8 L/min, nebulizer gas = 241 kPA. The mass spectra were acquired with a scan range of m/z from 100 to 700. Authentic standards of α-carotene, β-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin were used to identify these carotenoids in the samples. A tangerine tomato extract rich in phytoene, phytofluene, and neurosporene was used as a pseudo-standard to determine if these carotenoids were present in the cheeses. The remaining carotenoids were tentatively identified based upon anticipated retention times relative to the known carotenoids in the sample, as well as UV-Vis spectra, MS precursors, and MS/MS product ions coinciding with those reported in the literature.
3. Results
3.1. Carotenoids identified in rind extract
HPLC-DAD chromatograms of the rind extracts of all four cheeses at 450 nm as separated on the C18 column are shown in Figure 3. The same extracts were also separated on the C30 column, as shown in supplementary Figure S1. To further characterize the cheese rind carotenoids, MS/MS fragmentation was performed using APCI ionization in both positive and negative modes. The UV-Visible spectra, retention order, the primary precursor ion (m/z), and product fragments (m/z) are shown in Table 1.
Figure 3.
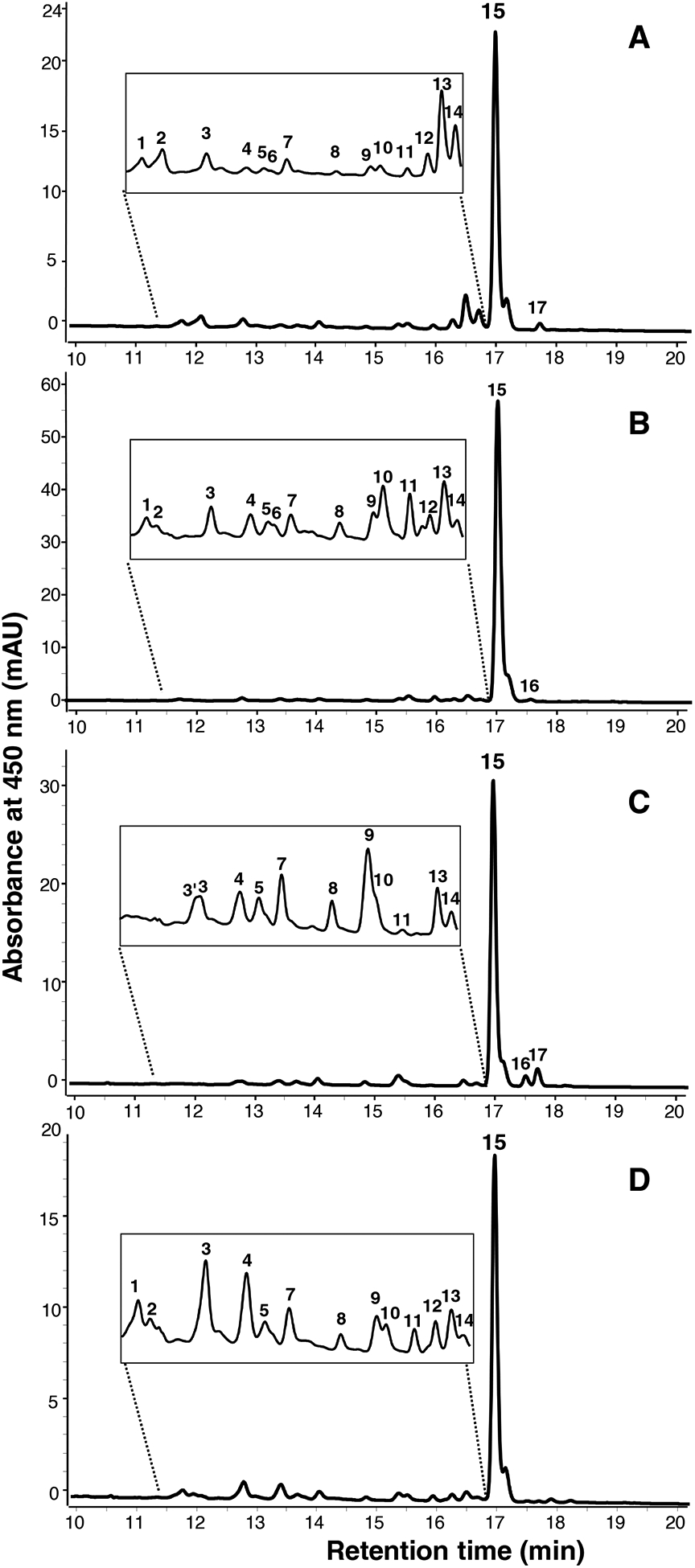
UHPLC-DAD chromatogram at 450 nm of non-polar rind extracts following saponification and concentration, as separated on a C18 column. (A) Ashbrook, (B) Charloe, (C) Limberger, (D) Taleggio. Peak numbers correspond to carotenoids noted in Table 1.
Table 1.
Carotenoids identified in core and rind extracts from smear-ripened cheeses.
| Peaka | RT (min) | Compound | UV-Vis spectra (nm) | Precursor Ion(s) (m/z)c | Precursor ion species | Product ions (m/z) produced with collision energy = 10 V | |||
|---|---|---|---|---|---|---|---|---|---|
| Ashbrook (A) | Charloe (C) | Limburger (L) | Taleggio (T) | ||||||
| 1 | 11.7 | decaprenoxanthinf,i | 414,438b, 470 | 705.5602 687.5569 |
[M+H]+ [M+H-H2O]+ |
669.6, 595.4, 613.5 | 669.6, 595.4 | 669.6, 595.4, 547.5 | |
| 2 | 11.9 | sarcinaxanthinf,i | 416,440b, 470 | 705.5602 687.6606 |
[M+H]+ [M+H-H2O]+ |
669.4, 595.4, 613.3 | 669.6 | 669.6 | |
| 2’ | 12.0 | cis-sarcinaxanthinf,i | 416, 440b, 470 | 687.6572 | [M+H]+ | 669.4, 595.4, 473.1 | - | - | - |
| 3’,d | 12.70 | unknown | 420,440b, 478 (C,L) | 569.4650 | [M+H]+ | ND | 551.5, 459.3, 205.15, 313.2 | ||
| 3 | 12.74 | unknown | 458 round spectra (A,T) | ND | ND | ND | ND | ||
| 4d | 13.4 | β-cryptoxanthine,j | 422, 452b, 474 | 553.4398 535.4283 |
[M+H]+ [M-H2O+H]+ |
535.4 | ND | 135.1 | 461.2, 135.1 |
| 5d | 13.6 | cis-β-cryptoxanthinf,j | 426, 450b, 474 | 553.4393 535.4278 |
[M+H]+ [M-H2O+H]+ |
535.4, 497.3, 135.1 | 535.4, 497.2, 135.1 | 535.4, 497.2 | 535.45, 497.2 |
| 6d | 13.7 | unknown | 444b, 472 (C,A,T); 452 round spectra (L) | ND | ND | ND | ND | ND | ND |
| 7d | 14.2 | echinenonef,i | 468b | 551.4229 | [M+H]+ | 203.1, 495.2, 255.2, 459.3 | 203.1, 495.2 | 203.1, 495.2, 255.2 | 203.1, 495.2, 255.2 |
| 8 | 15.0 | sarcinaxanthin or saprenoxanthin esterf,i | 412, 436b, 468 | 705.5602 | [M+H-FA]+ | 687.7 | ND | 687.4 | ND |
| 9d | 15.6 | mono-epoxy-β-carotenef,j | 402, 428b, 458 | 553.4370 | [M+H]+ | 535.4,461.3, 205.1, 177.1 | 535.4, 461.3, 205.1, 177.1 | 535.4, 461.3, 205.1, 177.1 | 535.4, 461.3, 205.1,177.1 |
| 10d | 15.7 | α-cryptoxanthin esterg,e,j | 420, 446b, 472 | 553.4359 535.5011 |
[M+H-FA]+ [M-H2O-FA+H]+ |
ND | ND | ND | ND |
| 11 | 16.2 | β-cryptoxanthin esterg,j | 422, 452b, 476 | 553.4955 535.4895 |
[M+H-FA]+ [M-H2O-FA+H]+ |
ND | ND | ND | ND |
| 12 | 16.5 | cis-lycopene tentatively co-eluting with 1lavuxanthin or decaprenoxanthin esterg,i | 414, 438, 470b, 500 (A,C,T) | 537.4438 705.5602 |
[M+H]+ [M+H-FA]+ |
ND | ND | ND | |
| 13 | 16.7 | all-trans-lycopenee,i | 444, 472b, 504 | 537.4382 | [M+H]+ | 457.3, 413.3, 119.0, 177.1 | ND | 457.3, 177.1 | 537.4 |
| 14 | 16.9 | cis-lycopenee,i | 342h 360h 440, 466b, 494 | 537.4382 | [M+H]+ | 413.3,177.1, 119.0 | ND | ND | ND |
| 15d | 17.2 | carotene (both β- and α-e,j) | 424, 452b, 478 | 537.4382 | [M+H]+ | 457.3, 445.3, 413.3, 255.2, 177.1, 137.1 | 457.3, 413.3, 177.1, 137.1 | 457.3, 413.3, 255.2, 177.1, 137.1 | 457.3, 413.3, 255.2, 177.1, 137.1 |
| 16 | 17.7 | decaprenoxanthin or sarcinaxanthin ester coeluting with another speciesg,i | 470b (C,L) | 705.5602 255.2324 |
[M+H-FA]+ [FA]− |
ND | ND | ||
| 17 | 17.9 | decaprenoxanthin or sarcinaxanthin ester coeluting with anotherspeciesg,i | 285, 476b (A,L) | 705.5602 | [M+H-FA]+ | ND | ND | ||
Denotes λmax
The primary precursor ion detected using atmospheric pressure chemical ionization (APCI) operated in positive ion mode
Carotenoids also identified in cheese cores
Identity confirmed with authentic standards (i.e. retention time, UV-Vis spectra, MS and MS/MS spectra consistent with standard)
Retention order, UV-Vis spectra, and MS precursor, product ions consistent with literature
Available information (retention order and/or UV-Vis spectra and/or MS precursor) consistent with literature
cis peak.
carotenoids sourced from bacteria
carotenoids sourced from milk
Abbreviations: A = Ashbrook, C = Charloe, L = Limberger, ND = not detected, due to lack of signal, or presence of co-eluting species interfering with spectra, RT = retention time, T = Taleggio
The rind carotenoid profiles of all cheeses were very similar, with ~17 compounds eluting between 11.7–17.7 min. Regardless of cheese type, ~80% of all peak area at 450 nm was determined to be a mix of α- and β-carotene (see Figure 3), as confirmed with authentic standards using the C18 method. Further analysis with the C30 column revealed that 15% of the total carotene peak was α-carotene, with the remaining 85% as β-carotene (Figure S1). Both trans- and cis-lycopene, co-eluting with a flavuxanthin (an intermediate in the biosynthesis pathway of decaprenoxanthin/sarcinoxanthin (Heider et al., 2012; Netzer et al., 2010), were also observed in the rind samples. Likewise, the C50 carotenoids decaprenoxanthin and sarcinaxanthin (both free or esterified) were observed in the rind (but not core) extracts all cheeses tested. Decaprenoxanthin comprised ~0.8% and sarcinaxanthin ~1.45% of the entire signal at 450 nm. Although they share the same precursor m/z, the relative differences in UV-visible spectra (i.e., 2 nm hypochromic shift, Figure 4) and retention order, provided the tentative assignments (Enzell & Francis, 1969; Giuffrida et al., 2015; Norgard, Francis, Jensen, & Liaaen-Jensen, 1970). Fragment ions for decaprenoxanthin were m/z 687.5 [M+H-H2O]+ representing the loss of water, m/z 613.5 [M+H-92]+ representing the loss of toluene, m/z 595.4 [M+H-18–92]+, m/z 547.5 [M+H-158]+ corresponding to the loss of dimethylcylodecapentaene (Arpin, Liaaen-Jensen, & Trouilloud, 1972; Enzell & Francis, 1969), and m/z 669.6 [M+H-35]+, a fragment also reported by Giuffrida et al., 2015, but whose identity has not been determined.
Figure 4.
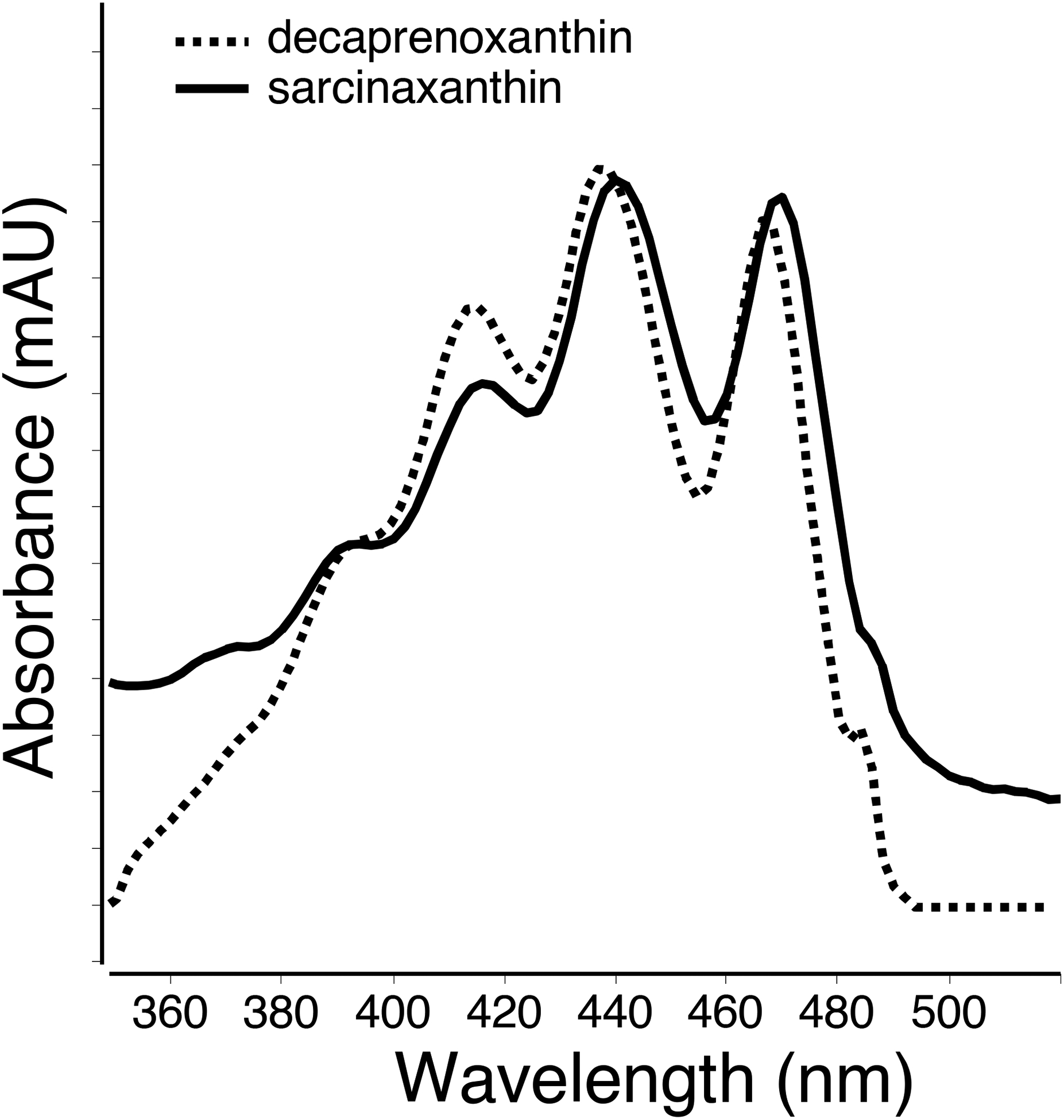
UV-Vis spectra of decaprenoxanthin (dashed line) and sarcinoxanthin (solid line) observed in the cheese rind extract.
With regards to sarcinaxanthin, the precursor consisting of a water loss was more intense than the protonated species. Fragments ions m/z 595.4 [M+H-18–92]+ and m/z 473.1 [M+H-92–140]+ are consistent with a toluene loss (Liaaen-Jensen & Hertzberg, 1968). Similarly, analytes with UV-Vis spectra and precursor m/z of 705.5 [M+H]+ were observed eluting later in the method (see Table 1, peaks 8, 16, 17) suggesting mono- and di- esterified decaprenoxanthin and sarcinoxanthin were present in the rind sample, but the precursor ion signal in negative mode was not strong enough to identify the fatty acid moiety attached.
3.2. Carotenoids identifed in core extracts
HPLC-DAD chromatogram profiles obtained from the C18 separation of the 2 g of core extracts are presented in Figure 5, utilizing the same procedure as rind sample detailed above. Provitamin A carotenes (both α- and β-carotene listed as peak 15 in Table 1) were the dominant carotenoid class observed in core samples, absorbing ~98% of all signal at 450 nm. Minor compounds included β-cryptoxanthin, cis-β-carotene and β-cryptoxanthin, and tentatively epoxy-β-cryptoxanthin, an unknown, echinenone, epoxy-β-carotene, at negligible levels.
Figure 5.
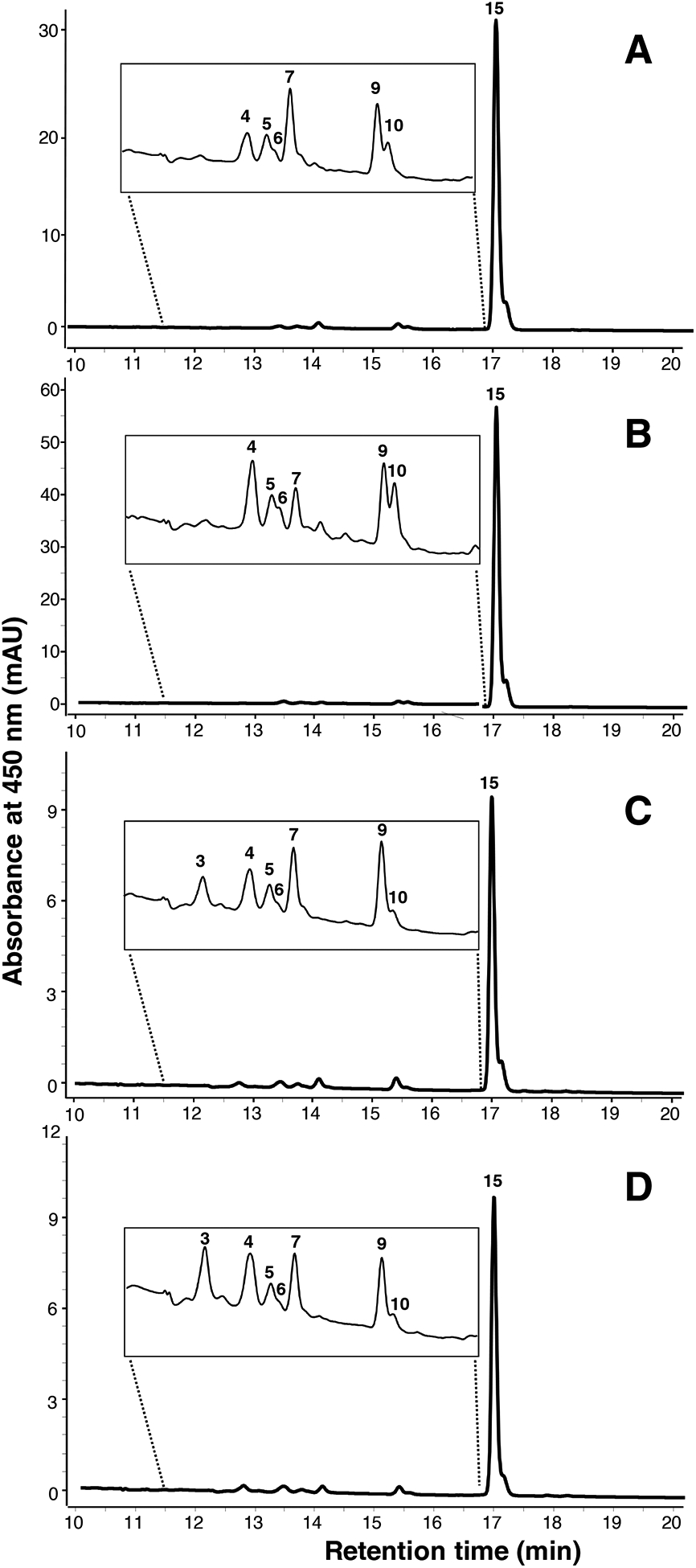
UHPLC-DAD chromatogram at 450 nm of non-polar core extracts following saponification and concentration, as separated on a C18 column. (A) Ashbrook, (B) Charloe, (C) Limberger, (D) Taleggio. Peak numbers correspond to carotenoids noted in Table 1.
3.3. Minor carotenoids identified in rind and core extracts
Echinenone was observed in all four cheeses, with the UV-Vis spectrum, precursor, and productions consistent with literature values (van Breemen, Dong, & Pjkovic, 2012). Likewise, UV-Vis, precursor and product ions were consistent with epoxy-β-carotene in all four cheeses (De Rosso & Mercadante, 2007), with fragment m/z 461.3 [M+H-92]+ revealing a toluene loss and fragment of m/z 205.1 corresponding to the epoxy group at the end of a polyene chain, and fragment m/z 177.1 corresponding to 9–10 bond cleavage (Enzell & Francis, 1969; van Breemen et al., 2012).
Esters of α-and β-cryptoxanthin were identified via UV-Vis spectra, elution times relative to one another (i.e. α- eluting before β-cryptoxanthin ester (peak 11)), and precursor ions matching β-cryptoxanthin standard (precursor signal was too low for fragmentation).
Other compounds remain unknown. Peak 3’ was observed in Limburger and at much lower concentrations in Charloe. Peak 3 at 12.74 min was observed in Ashbrook, Limburger, and Taleggio, and whose UV-Vis spectral fine features included a rounded peak at a λmax = 458 nm.
4. Discussion
A detailed investigation of the carotenoids present in smear-ripened cheeses was performed. Carotenoids identified in both rind and core samples included the pro-vitamin A carotenoids, most dominantly β-carotene, as well as α-carotene, β- and α-cryptoxanthin. Previous studies suggests that the influence of the seasonal milk, feed provided to cows, manufacturing and ripening processes, pasteurization, and standardization of milk will influence the levels of carotenes (e.g. β-carotene, α-carotene) xanthophyll (e.g. lutein, zeaxanthin, α- and β-cryptoxanthin) in milk and in milk products (Higuchi, Price, & Peterson, 1946; Higuchi & Peterson, 1946; Hulshof et al., 2006; Lucas et al., 2008). In contrast, certain carotenoids were only observed in the rind samples, including decaprenoxanthin, sarcinaxanthin, and their esters, as well as cis- and trans- lycopene. Both lycopene and the C50 carotenoids identified in the rind reflect a red hue, in contrast to the orange reflected by provitamin A carotenoids found both in the core and the rind, and are likely partially responsible for the darker color of the rind.
With the vast difference in milk sources, location of cheese producers, and differences in cheese styles articulated in the introduction, it was surprising that there was such limited variation between the cheese rind and core profiles, respectively, observed in this analysis. However, this result is consistent with a previous work which identified only seven distinct carotenoid profiles of from 114 bacterial strains individually isolated from a selection of French smear-ripened cheeses (Galaup et al., 2005).
Carotenoid extraction from the cheese matrix proved to be challenging. The very large quantity of lipid in the extract did not permit analysis without saponification, and utilizing this strategy increased sample concentration by ~10 fold, permitting carotenoids to be observed in the chromatograms. Saponification protocols of (Ollilainen et al., 1988) and (Jacobo-Velázquez & Hernández-Brenes, 2012) were tested, but these methods did not improve our sensitivity. Ultimately, the lipid remaining in the saponified extract produced large quantities of matrix interference which hampered MS ionization. This issue, combined with a low abundance in our samples, caused some carotenoids to remain only tentatively identified or un-identified. It should also be noted that even with temperature control, the saponification and sequential concentration steps may have also produced the epoxides and cis-carotenoids observed. Likewise, although strong saponification conditions were used, multiple esters were observed, suggesting saponification was only partially completed.
C50 bacterial carotenoids (i.e., decaprenoxanthin, sarcinaxanthin) were tentatively identified in the rind, as well as their presumed esters. Decaprenoxanthin was initially isolated from Agromyces mediolanus (formerly known as Flavobacterium dehydrogenes) (Goldstein, Halpern, & Robert, 1967). Later a detailed biosynthetic pathway was identified in Corynebacterium glutamicum (Krubasik et al., 2001). Three-pronged UV-Visible spectra consistent with C50 carotenoids were also identified in the smear-ripened cheese varieties Maroilles, Epoisses, and Mont d’Or (Galaup et al., 2007). Further efforts were made by Giuffrida et al. (Giuffrida et al., 2015) to identify these compounds synthesized by one of these bacteria derived from the smear-ripened cheese, Glutamicibacter arilaitensis. These authors reported the co-occurrence of decaprenoxanthin and sarcinaxanthin, as identified via UV-Visible spectra, and MS precursor and high molecular weight MS/MS product ions extracted from this bacterial species (Giuffrida et al., 2015). Micrococcus luteus, a halophile, has also been reported to produce sarcinaxanthin (Netzer et al., 2010). These bacteria are abundant in the rind of smear ripened cheeses (Bockelmann, 2002), either sourced from brine, old-young smear, or from the house microbiota. Saprenoxanthin has the same base structure as the symmetric decaprenoxanthin and sarcinaxanthin, but with one ε-ring and one γ-ring. Genes related to the production of these three C50 carotenoids were identified in C. glutamicum (Netzer et al., 2010), a reason to suspect the presence of saprenoxanthin in these extracts. A mass and spectra consistent with flavuxanthin, an intermediate in the biosynthesis of sarcinaxanthin, was also observed (Netzer et al., 2010). Collectively, the identification of C50 carotenoids in all four cheeses suggest that the microbiome developed on the surface of these cheese as part of smear is likely contributing to the development of these carotenoids.
Brevibacterium linens is usually added as an adjunct secondary culture during Limburger and Taleggio manufacture, but we did not identify any C40 carotenoids uniquely produced by this bacterium in these cheese extracts. Echinenone (tentatively identified in the core and rind) and lycopene (in the rind only) were believed to be the only C40 carotenoids of bacterial origin identified in all cheeses. Dietzia sp., known to be present on the surface of smear-ripened cheeses, produces echinenone, and can also produce the C50 carotenoid called “C.p. 450” and the C40 carotenoid canthaxanthin (absent from our mixture). The other C40 carotenes and xanthophylls observed are likely to be sourced from milk, as only mutant strains of bacteria have been found to produce significant concentrations of these species. Lycopene is an intermediate in the biosynthetic pathway of almost all carotenoids, including decaprenoxanthin and sarcinaxanthin, hence its origin in bacteria that produce these carotenoids is highly likely (Krubasik et al., 2001; Netzer et al., 2010). Thus, our data suggests that not only the end products of the pathways play a role in colour development of the smear cheese surface, but also the intermediates. We also screened for and did not find other carotenoids anticipated in these samples, i.e. phytoene, phytofluene, neurosporene, bacterioruberin, bisanhydrobacterioruberin, torularhodin, torulene (produced by coryneform bacteria), and agelaxanthin (from Brevibacterium) (Giuffrida et al., 2020).
Identifying the carotenoids of smear-ripened cheeses helps us to determine their contribution to provitamin A carotenoid delivered in the human diet. Indeed, the bioavailability of carotenoids is higher when consumed in conjunction with lipid-rich foods (Unlu, Bohn, Clinton, & Schwartz, 2005), including dairy lipids (Goltz, Campbell, Chichumroonchokchai, Failla, & Ferruzzi, 2012). Furthermore, research has suggested that co-consuming lipid with a provitamin A rich meal aids in conversion to vitamin A in the intestine of humans who are considered “low converters” (Kopec et al., 2014). Additionally, C50 carotenoids are found sparingly in the human diet, and these results demonstrate that they may be obtained from this family of cheeses. The novel C50 structure has strong antioxidant properties (Miller, Sampson, Candeias, Bramley, & Rice-Evans, 1996; Naguib, 2000), and could serve as a skin protectant, either through dietary means or through applications in sunscreens and other light protecting cosmetics (Heider et al., 2014; Osawa et al., 2010). Their advantages in human health have not been extensively studied, and this area is ripe for further work.
5. Conclusions
To our knowledge, this is the first detailed study of carotenoids present in Ashbrook, Charloe, Limburger, and Taleggio cheese extracts. The provitamin A carotenoid β-carotene was the dominant species in all samples tested, followed by α-carotene and minor quantities of epoxy-β-carotene, and β-cryptoxanthin. Rind samples specifically contained carotenoids which reflect a red hue, including lycopene and the C50 bacterial carotenoids decaprenoxanthin and sarcinaxanthin. These bacterial carotenoids are plausibly synthesized by coryneform bacteria such as C. glutamicum, G. arilaitensis, etc., and their interactions with other microbes, casein hydrolysates, and the cheese environment may lead to the development of these rich surface colours classically associated with smear-ripened cheeses. Future research on these cheeses should focus on quantitative analysis, as well as further identification of minor species in the absence of the cheese matrix (i.e., by testing biological isolates grown independent of the cheese matrix).
Supplementary Material
Highlights.
Carotenoids from four smear-ripened cheeses were extracted and profiled.
Provitamin A carotenoids predominated in both rind and core samples
Lycopene and C50 bacterial carotenoids were unique to rind samples
Similar carotenoids profiles were observed between all smear-ripened cheeses tested
Acknowledgements
This work was funded by the Teagasc Agriculture and Food Development Authority (RMIS 6485) and also by the Teagasc Walsh Fellowship Overseas Training Scheme. The UHPLCDAD-MS analyses were partially supported by NIH Award Number P30 CA016058, OSU, and the OSUCCC. BRY Jonnala is in receipt of the Teagasc Walsh Fellowship No. 2014040.
Abbreviations:
- DAD
diode array detector
- MS
mass spectrometry
- MTBE
methyl tert-butyl ether
- QTof
quadrupole time-of-flight mass spectrometer
- MS/MS
tandem mass spectrometry
- UHPLC
ultra-high pressure liquid chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Almena-Aliste M, & Mietton B (2014). Cheese classification, characterization, and categorization: A global perspective. Microbiology Spectrum, 2(1), CM-0003–2012. [DOI] [PubMed] [Google Scholar]
- Arpin N, Liaaen-Jensen S, & Trouilloud M (1972). Bacterial Carotenoids XXXVIII C50-Carotenoids 9. Isolation of decaprenoxnathin mono- and di- glucoside from a Arthrobacter sp. Acta Chemica Scandinavica, 26, 2194. [DOI] [PubMed] [Google Scholar]
- Blaner WS (2019). Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacology and Therapeutics, 197, 153–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockelmann W (2002). Development of defined surface starter cultures for the ripening of smear cheeses. International Dairy Journal, 12(2–3), 123–131. [Google Scholar]
- Britton G (1993). Structure and Nomenclature of Carotenoids. Carotenoids, Vol. 1A, Isolation and Analysis, 27–70. [Google Scholar]
- Cogan TM (2014). Smear-Ripened Cheeses. Encyclopedia of Food Microbiology, 1, 421–426. [Google Scholar]
- Corsetti A, Rossi J, & Gobbetti M (2001). Interactions between yeasts and bacteria in the smear surface-ripened cheeses. International Journal of Food Microbiology, 69(1–2), 1–10. [DOI] [PubMed] [Google Scholar]
- De Rosso VV, & Mercadante AZ (2007). Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. Journal of Agricultural and Food Chemistry, 55(13), 5062–5072. [DOI] [PubMed] [Google Scholar]
- Enzell CR, & Francis G. w. (1969). Mass spectrometric studies of carotenoids. A Survey of Fragmentation Reactions. Acta Chemica Scandinavica, 23, 727–750. [DOI] [PubMed] [Google Scholar]
- Galaup P, Flamin C, Carlet E, & Dufossé L (2005). HPLC analysis of the pigments produced by the microflora isolated from the “Protected Designation of Origin” French red-smear soft cheeses Munster, Epoisses, Reblochon and Livarot. Food Research International, 38(8–9), 855–860. [Google Scholar]
- Galaup P, Gautier A, Piriou Y, Villeblanche A. de, Valla A, & Dufossé L (2007). First pigment fingerprints from the rind of French PDO red-smear ripened soft cheeses Epoisses, Mont d’Or and Maroilles. Innovative Food Science and Emerging Technologies, 8(3), 373–378. [Google Scholar]
- Galaup P, Sutthiwong N, Leclercq-Perlat MN, Valla A, Caro Y, Fouillaud M, Guérard F, & Dufossé L (2015). First isolation of Brevibacterium sp. pigments in the rind of an industrial red-smear-ripened soft cheese. International Journal of Dairy Technology, 68(1), 144–147. [Google Scholar]
- Giovannucci E (2005). Tomato products, lycopene, and prostate cancer: A review of the epidemiological literature. The Journal of Nutrition, 135(8), 2030S–2031S. [DOI] [PubMed] [Google Scholar]
- Giuffrida D, Monnet C, Laurent F, Cacciola F, Oteri M, Le Piver M, Caro Y, Donato P, Mondello L, Roueyre D, & Dufossé L (2020). Carotenoids from the ripening bacterium Brevibacterium linens impart color to the rind of the French cheese, Fourme de Montbrison (PDO). Natural Product Research, 34(1), 10–15. [DOI] [PubMed] [Google Scholar]
- Giuffrida D, Sutthiwong N, Dugo P, Donato P, Cacciola F, Girard-Valenciennes E, Le Mao Y, Monnet C, Fouillaud M, Caro Y, & Dufossé L (2015). Characterisation of the C50 carotenoids produced by strains of the cheese-ripening bacterium Arthrobacter arilaitensis. International Dairy Journal, 55, 10–16. [Google Scholar]
- Goldstein I, Halpern B, & Robert L (1967). Identity of the C50-carotenoid dehydrogenans P439 and sarcinaxantin. Nature, 44–47. [Google Scholar]
- Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, & Ferruzzi MG (2012). Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Molecular Nutrition and Food Research, 56(6), 866–877. [DOI] [PubMed] [Google Scholar]
- Guyomarc’h F, Binet A, & Dufossé L (2000). Production of carotenoids by Brevibacterium linens: Variation among strains, kinetic aspects and HPLC profiles. Journal of Industrial Microbiology and Biotechnology, 24(1), 64–70. h [Google Scholar]
- Harrison EH, & Kopec RE (2018). Digestion and intestinal absorption of dietary carotenoids and vitamin A In Physiology of the Gastrointestinal Tract (Vol. 6, pp. 1133–1151). [Google Scholar]
- Heider SAE, Peters-Wendisch P, Netzer R, Stafnes M, Brautaset T, & Wendisch VF (2014). Production and glucosylation of C50 and C40 carotenoids by metabolically engineered Corynebacterium glutamicum. Applied Microbiology and Biotechnology, 98(3), 1223–1235. [DOI] [PubMed] [Google Scholar]
- Heider SAE, Peters-Wendisch P, & Wendisch VF (2012). Carotenoid biosynthesis and overproduction in Corynebacterium glutamicum. BMC Microbiology, 12(198), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K, & Peterson WH (1946). The carotene and vitamin A contents of Wisconsin cheese. Journal of Dairy Science, 29(11), 789–793. [Google Scholar]
- Higuchi K, Price WV, & Peterson WH (1946). Relation of corn and alfalfa silage to the quality of cheese and its carotene and vitamin A content. Journal of Dairy Science, 29(3), 157–162. [Google Scholar]
- Hulshof PJM, van Roekel-Jansen T, van de Bovenkamp P, & West CE (2006). Variation in retinol and carotenoid content of milk and milk products in The Netherlands. Journal of Food Composition and Analysis, 19(1), 67–75. [Google Scholar]
- Jacobo-Velázquez DA, & Hernández-Brenes C (2012). Stability of avocado paste carotenoids as affected by high hydrostatic pressure processing and storage. Innovative Food Science and Emerging Technologies, 16, 121–128. [Google Scholar]
- Johnson EJ, Mcdonald K, Caldarella SM, Chung H, Troen AM, & Snodderly DM (2008). Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutritional Neuroscience, 11(2), 75–83. [DOI] [PubMed] [Google Scholar]
- Kohl W, Achenbach H, & Reichenbach H (1983). The pigments of Brevibacterium linens: Aromatic carotenoids. Phytochemistry, 22(1), 207–210. [Google Scholar]
- Kopec RE, Cooperstone JL, Schweiggert RM, Young GS, Harrison EH, Francis DM, Clinton SK, & Schwartz SJ (2014). Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high–β-carotene tomato sauce and from carrots. The Journal of Nutrition, 144(8), 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec RE, Riedl KM, Harrison EH, Curley RWC Jr., Hruszkewycz DP, Clinton SK, & Schwartz SJ (2010). Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. Journal of Agricultural and Food Chemistry, 58(6), 3290–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubasik P, Takaichi S, Maoka T, Kobayashi M, Masamoto K, & Sandmann G (2001). Detailed biosynthetic pathway to decaprenoxanthin diglucoside in Corynebacterium glutamicum and identification of novel intermediates. Archives of Microbiology, 176(3), 217–223. 10.1007/s002030100315 [DOI] [PubMed] [Google Scholar]
- Liaaen-Jensen S, & Hertzberg S (1968). Bacterial carotenoids XXVII. C50-carotenoids structure determination of dehydrogenase P439. Acta Chemica Scandinavica, 22, 1171–1186. [DOI] [PubMed] [Google Scholar]
- Lucas A, Coulon JB, Agabriel C, Chilliard Y, & Rock E (2008). Relationships between the conditions of goat’s milk production and the contents of some components of nutritional interest in Rocamadour cheese. Small Ruminant Research, 74(1–3), 91–106. [Google Scholar]
- Manzo N, Santini A, Pizzolongo F, Aiello A, Marrazzo A, Meca G, Durazzo A, Lucarini M, & Romano R (2019). Influence of ripening on chemical characteristics of a traditional Italian cheese: Provolone del Monaco. Sustainability, 11(9), 2520. [Google Scholar]
- Masoud W, & Jakobsen M (2003). Surface ripened cheeses: The effects of Debaryomyces hansenii, NaCl and pH on the intensity of pigmentation produced by Brevibacterium linens and Corynebacterium flavescens. International Dairy Journal, 13(2–3), 231–237. [Google Scholar]
- Masoud W, & Jakobsen M (2005). The combined effects of pH, NaCl and temperature on growth of cheese ripening cultures of Debaryomyces hansenii and Coryneform bacteria. International Dairy Journal, 15(1), 69–77. [Google Scholar]
- Mathews-Roth M (1993). Carotenoids in erythropoietic protoporphyria and other photosensitivity diseases. Annals New York Academy of Sciences, 691(1), 127–138. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Sampson J, Candeias LP, Bramley PM, & Rice-Evans CA (1996). Antioxidant properties of carotenes and xanthophylls. FEBS Letters, 384(3), 240–242. [DOI] [PubMed] [Google Scholar]
- Mortensen A (2006). Carotenoids and other pigments as natural colorants. Pure and Applied Chemistry, 78(8), 1477–1491. [Google Scholar]
- Mounier J, Irlinger F, Leclercq-Perlat MN, Sarthou AS, Spinnler HE, Fitzgerald GF, & Cogan TM (2006). Growth and colour development of some surface ripening bacteria with Debaryomyces hansenii on aseptic cheese curd. Journal of Dairy Research, 73(4), 441–448. [DOI] [PubMed] [Google Scholar]
- Naguib YMA (2000). Antioxidant activities of astaxanthin and related carotenoids. Journal of Agricultural and Food Chemistry, 48, 1150–1154. [DOI] [PubMed] [Google Scholar]
- Netzer R, Stafsnes MH, Andreassen T, Goksøyr A, Bruheim P, & Brautaset T (2010). Biosynthetic pathway for γ-cyclic sarcinaxanthin in Micrococcus luteus: Heterologous expression and evidence for diverse and multiple catalytic functions of C50 carotenoid cyclases. Journal of Bacteriology, 192(21), 5688–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JM, Meagher KA, Howard AN, Moran R, Thurnham DI, & Beatty S (2016). Lutein, zeaxanthin and meso-zeaxanthin content of eggs laid by hens supplemented with free and esterified xanthophylls. Journal of Nutritional Science, 5, e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard S, Francis G. w., Jensen A, & Liaaen-Jensen S (1970). Bacterial Carotenoids XXXIV C50—Carotenoids. A C50-Carotenoid-D-glucoside from Sarcina lutea. Acta Chemica Scandinavica, 24, 1460–1462. [DOI] [PubMed] [Google Scholar]
- Ollilainen V, Heinonen M, Linkola E, Varo P, & Koivistoinen P (1988). Carotenoids and retinoids in Finnish foods: Dairy products and eggs. Journal of Food Composition and Analysis, 1(2), 178–188. 10.1016/0889-1575(88)90022-1 [DOI] [PubMed] [Google Scholar]
- Osawa A, Ishii Y, Sasamura N, Morita M, Kasai H, Maoka T, & Shindo K (2010). Characterization and antioxidative activities of rare C50 carotenoids-sarcinaxanthin, sarcinaxanthin monoglucoside, and sarcinaxanthin diglucoside- obtained from Micrococcus yunnanensis. Journal of Oleo Science, 59(12), 653–659. [DOI] [PubMed] [Google Scholar]
- Pickworth CL, Loerch SC, Kopec RE, Schwartz SJ, & Fluharty FL (2012). Concentration of pro-vitamin A carotenoids in common beef cattle feedstuffs. Journal of Animal Science, 90(5), 1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Amaya BD, & Kimura M (2004). Carotenoids in Foods In Handbook for Carotenoid Analysis (pp. 2–7). [Google Scholar]
- Sabour-Pickett S, Nolan JM, Loughman J, & Beatty S (2012). A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Molecular Nutrition and Food Research, 56(2), 270–286. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Norkus EP, & Gaziano JM (2004). Plasma lycopene, other carotenoids, and retinol and the risk of cardiovascular disease in women. American Journal of Clinical Nutrition, 79(1), 47–53. [DOI] [PubMed] [Google Scholar]
- Sheehan JJ (2007). Section 142: What organisms grow on the surface of smear cheese? Cheese Problems Solved, Ed. McSweeney PLH, pp 291–292. [Google Scholar]
- Story EN, Kopec RE, Schwartz SJ, & Harris GK (2010). An update on the health effects of tomato lycopene. Annual Review of Food Science and Technology, 1, 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Yao H, & Cheng Q (2007). Genes from a Dietzia sp. For synthesis of C40 and C50 β-cyclic carotenoids. Gene, 386(1–2), 90–97. [DOI] [PubMed] [Google Scholar]
- Unlu NZ, Bohn T, Clinton SK, & Schwartz SJ (2005). Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. The Journal of Nutrition, 135(3), 431–436. [DOI] [PubMed] [Google Scholar]
- van Breemen RB, Dong L, & Pajkovic ND (2012). Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids. International Journal of Mass Spectrometry, 312, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuzaki J (2017). Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database, 2017, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


