Summary
The advancement of serial cryoFIB/SEM offers an opportunity to study large volumes of near-native, fully hydrated frozen cells and tissues at voxel sizes of 10 nm and below. We explored this capability for pathologic characterization of vitrified human patient cells by developing and optimizing a serial cryoFIB/SEM volume imaging workflow. We demonstrate profound disruption of subcellular architecture in primary fibroblasts from a Leigh syndrome patient harboring a disease-causing mutation in USMG5 protein responsible for impaired mitochondrial energy production.
Keywords: volume imaging, CryoFIB/SEM, CryoSEM, block face, Leigh syndrome, USMG5, mitochondria disease
Graphical Abstract
Highlights
-
•
Developed and optimized a serial cryoFIB/SEM volume imaging workflow
-
•
Visualized the 3D structure of an entire cell under native conditions
-
•
Revealed a disruption of cellular structures in primary LS patient fibroblasts
-
•
Demonstrated the potential for clinical phenotyping of pathogenic tissues
Serial cryoFIB/SEM offers new opportunities for structural analysis of cells and tissues under near-native conditions. Zhu et al. developed and optimized a serial cryoFIB/SEM volume imaging workflow for visualization of entire vitreous cells in 3D, and demonstrated its potential for clinical and pathological applications with primary Leigh syndrome patient cells.
Introduction
Cryoelectron tomography (cryoET), with subtomogram averaging, has emerged as a powerful method for visualizing heterogeneous structures and in situ specimens at subnanometer resolutions (Himes and Zhang, 2018; Sutton et al., 2020; Zhang, 2019). However, due to limited penetrance of the electron beam in thicker regions of cells (Lucic et al., 2013; Wang et al., 2012), its utility is limited to very thin samples (<300 nm), such as thin regions of the cell periphery or cell lamella by cryo-focused ion beam (cryoFIB) thinning. On the other hand, serial FIB/scanning electron microscopy (SEM) has been rapidly adopted as a technique for generating large 3D volumes of cells and tissue constituents, which have been fixed (cryo or chemically), dehydrated, resin-embedded, and stained for imaging contrast (Kizilyaprak et al., 2019; Schirra and Zhang, 2014; Steyer et al., 2019). Its application to vitreous biological samples, namely serial cryoFIB/SEM, involves many challenges associated with low-contrast (no staining) and low-dose (radiation sensitive) imaging. Examples of serial cryoFIB/SEM showed its potential for studying whole-mount plunge-frozen and high-pressure frozen cells and tissues (Akiva et al., 2019; Schertel et al., 2013; Sviben et al., 2016; Vidavsky et al., 2015, 2016; Wu et al., 2020). We now explore this new capability for pathologic characterization of Leigh syndrome (LS) patient cells harboring a disease-causing mutation in USMG5 protein responsible for impaired mitochondrial energy production.
The primary role of mitochondria is to generate energy in cells through mitochondrial oxidative phosphorylation (OXPHOS) (Lake et al., 2015). OXPHOS deficiency leads to mitochondrial diseases, including LS, a devastating neurological disorder and the most common mitochondrial disease in children (Sofou et al., 2014). LS is genetically heterogeneous with more than 90 nuclear or mitochondrial genes implicated in its pathogenesis (Chang et al., 2020; McCormick et al., 2018). Virtually all of these genes encode the mitochondrial respiratory complex machinery required for energy generation through OXPHOS (Barca et al., 2018), including those regulating the structure and assembly of complex V (ATP synthase). Classical transmission electron microscopy of thin tissue sections from LS patients is typically used to diagnose mitochondrial disease, revealing abnormality of the structure of mitochondria (Lee et al., 2016). Disease-causing mutations, such as (T8993G-1) in cytochrome c oxidase (complex IV) and in SURF1 (a complex IV protein) were shown to lead to ultrastructural changes in mitochondria and, in the case of SURF1, also aggregation of abnormal intracellular inclusions (Makino et al., 2000; Pronicki et al., 2008). Recently a genetic study identified a novel pathogenic mutation (c.87 + 1G > C), in the USMG5 gene that results in autosomal recessive LS (Barca et al., 2018). The mutation abolishes the canonical GT splice site donor of exon 4 of USMG5 and produces aberrant transcripts that are degraded via nonsense-mediated decay with >90% loss of USMG5 expression (Barca et al., 2018). USMG5, also known as DAPIT (diabetes-associated protein in insulin-sensitive tissues), is a constituent of complex V required for its dimerization. Complex V ordinarily exists as a dimeric supercomplex required to shape the mitochondrial cristae, enabling efficient flow of the protons needed to fuel ATP synthesis. Recent cryoET of thin peripheral regions of LS patient cells harboring this USMG5 gene mutation revealed significant disturbances in mitochondrial crista (Siegmund et al., 2018). The effect of the USMG5 mutation on the level of whole-cell and subcellular architecture, however, has not been investigated.
Here, we developed and optimized a workflow using serial cryoFIB/SEM to study whole plunge-frozen primary fibroblast cells from a healthy individual and from an LS patient carrying the homozygous mutation in the USMG5 gene previously shown to impair mitochondria cristae structure and ATP synthesis (Siegmund et al., 2018). The resulting 3D volumes of patient and control cells demonstrate a profound disruption of cellular and subcellular structures in LS patient cells. Compared with conventional serial FIB/SEM of stained and resin-embedded samples, serial cryoFIB/SEM offers a much faster (without a lengthy dehydration and embedding process during sample preparation) and close-to-native technique for phenotypic characterization of whole cells or tissue, which could be exceedingly useful in clinical settings.
Results
A Workflow for 3D Volume Imaging of Near-Native Cells and Tissues
To investigate the phenotypic impact of a specific USMG5 gene mutation (c.87 + 1G > C) on cellular and subcellular structures in a near-native state, we cultured the primary fibroblast cells isolated from an LS patient and from a healthy individual on gold EM grids, which were subsequently plunge-frozen (Figure 1). Serial cryoFIB sectioning and cryoSEM imaging of frozen-hydrated primary fibroblast cells were performed using a Zeiss Crossbeam 550 instrument (Table S1). To maximize the cryoSEM image contrast and balance between resolution and total volume and time, we tested a number of FIB and SEM parameters, including pixel spacing, FIB slice thickness, FIB and SEM probe currents, acceleration potential, SEM dwell time, and average line count. Using a lateral pixel spacing of 10.5 nm for SEM imaging and a FIB slice thickness of 21.0 nm, an entire patient fibroblast cell was sliced through and 2018 slices were imaged at 4,096 × 3,072 pixels in about ~17.5 h, resulting in a total volume of 58,789 μm3 (Video S1). For the control fibroblast cell, a similar voxel size was used, but a reduced raster of 3,072 × 1,150 pixels was used for imaging in total 575 slices. A total volume of 4,062 μm3 was obtained within ~5.5 h for the control cell (Video S2). Detailed parameters are listed in Table S1. The density profile plots indicate that the resolution of cryoSEM images is at least 37 nm with 10 nm pixel size under the image conditions specified (Figure S1). The actual resolution might be assessed using a crystalline material.
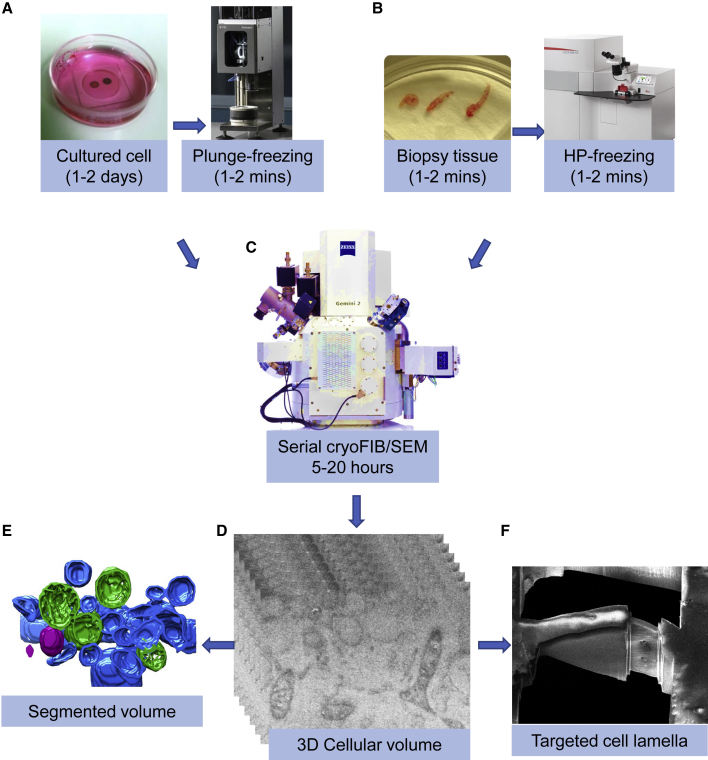
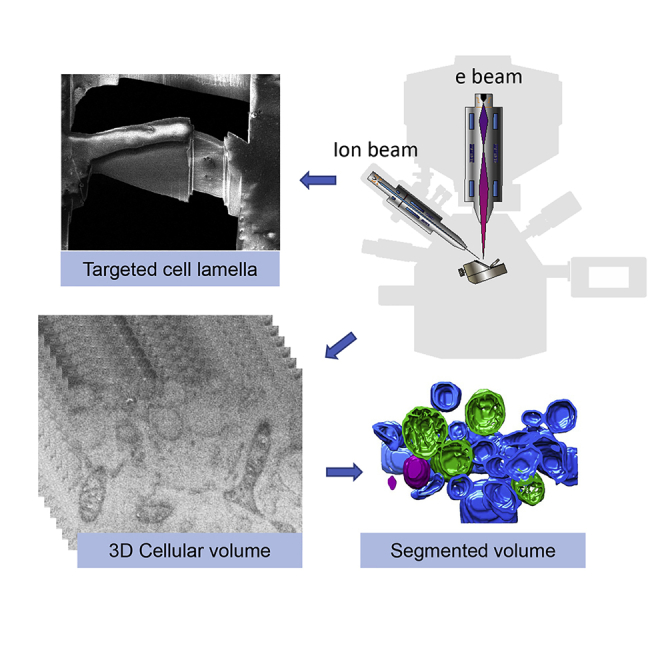
Figure 1.
A Workflow for Serial cryoFIB/SEM Volume Imaging of Near-Native Cells and Tissues
(A) Cells cultured on EM grids are subjected to plunge freezing.
(B) Needle biopsy can be performed to extract tissues within 1–2 min, and immediately subjected to high pressure freezing.
(C) Serial cryoFIB/SEM is performed automatically for 5–20 h, depending on the volume to be imaged, dwell time, and average line count.
(D) A stack of 2D cryoSEM images enclosing the volume of the cell.
(E) 3D segmentation of the cell volume.
(F) Targeted cell lamella preparation on the intracellular region of interest identified by serial cryoFIB/SEM.
Serial cryoFIB/SEM Volume Imaging of Control and Patient Fibroblasts
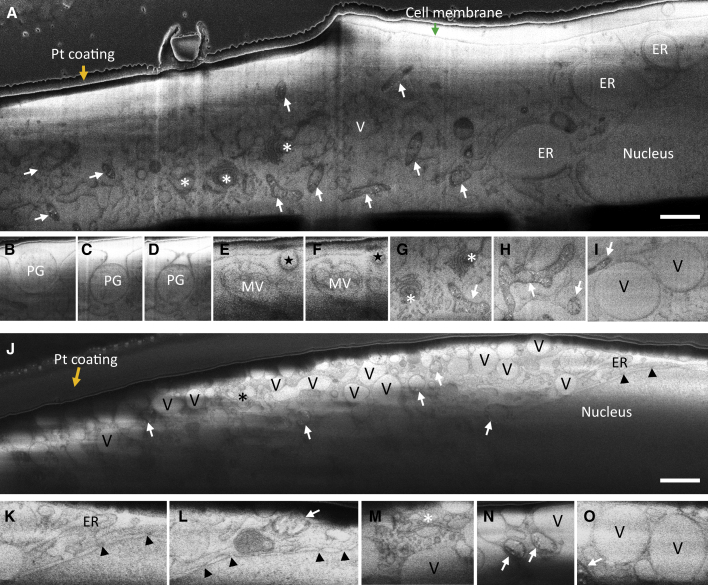
Figure 2 shows cryoSEM images of representative slices from both patient and control cells. We noted a contrast imbalance between the lower part and the upper part of the cross-sectioned patient cell (Figure 2J). Since the cryoSEM image contrast is related to local surface potentials, the median potential (or threshold) can be different depending on the environment and local charge dissipation, such as the top cell region being close to the cold deposited platinum precursor layer and bottom part being in the vicinity of the support film and grid bars. In both cells, subcellular structures are clearly visible, especially membrane-enclosed subcellular compartments (Figures 2A and 2J), including the nucleus. Notably, serial cryoFIB/SEM provides unambiguous visualization of nuclear pores in human cells (Figures 2J–2L, arrowheads), which had not been achieved previously using this method. In the control cell, individual cellular organelles can be identified based on their distinct morphologies, including the cell membrane, a phagosome (Figures 2B–2D, PG, in three consecutive slices), endoplasmic reticulum, multivesicular bodies (Figures 2E and 2F, MV), Golgi (Figure 2G, ∗), mitochondria (Figures 2G–2I, white arrows), vacuole-like membranous structures (Figures 2I and 2V), and the cell nucleus. The LS patient fibroblast cell, however, shows substantial cytoarchitectural derangements, with the interior of the cell largely occupied by vacuolated structures of indeterminate origin. Of the residual identifiable structures, including mitochondria and Golgi, organelles are significantly decreased in volume and displayed gross morphological abnormalities. For example, the Golgi apparatus lacks extended membrane stacks (Figures 2J and 2M). More remarkably, compared with the complex shape and network of mitochondria in the control cell (Figures 2A, 2G, and 2H; Video S2), nearly all patient mitochondria are roughly round with minimal cristae (Figures 2J, 2L, and 2N; Video S1), consistent with our previous cryoET analyses (Siegmund et al., 2018). This suggests that the architecture responsible for energy metabolism in the patient cells is compromised, consistent with the earlier biochemical characterization of these primary fibroblasts (Barca et al., 2018). The patient cells also grow substantially slower than the control cells.
Figure 2.
Serial cryoFIB/SEM of Frozen-Hydrated Primary Cells from Control and Patient Fibroblasts
(A) A representative cryoSEM image from a stack of 575 serial micrographs recorded from a control fibroblast cell cultured on an EM grid.
(B–I) An image gallery of subcellular structures and organelles observed in the control cell, including three consecutive slices of a phagosome entering the cell (B–D), two consecutive slices of an endosome (E and F, star), a multivesicular body (E and F, MV), Golgi complexes (G, asterisks), tubular-shaped mitochondria (G and H, arrow), and vacuoles (I and V).
(J) A representative cryoSEM image from a stack of 2018 serial micrographs recorded from a patient fibroblast cell cultured on an EM grid.
(K–O) An image gallery of subcellular structures and organelles observed in the patient cell, showing endoplasmic reticulum (K, ER), nuclear pores (K and L, arrowheads), Golgi complex (M, asterisk), mitochondria (L and N, arrows), and vacuoles (M, O, and V). Arrows, mitochondria; asterisks, Golgi; stars, endosome; PG, phagosome; MV, multivesicular body; V, vacuoles; arrowheads, nuclear pore; orange arrows, platinum GIS coating; green arrow, cell membrane; ER, endoplasmic reticulum. Scale bars, 1 μm.
3D Reconstruction and Segmentation of Control and Patient Cells
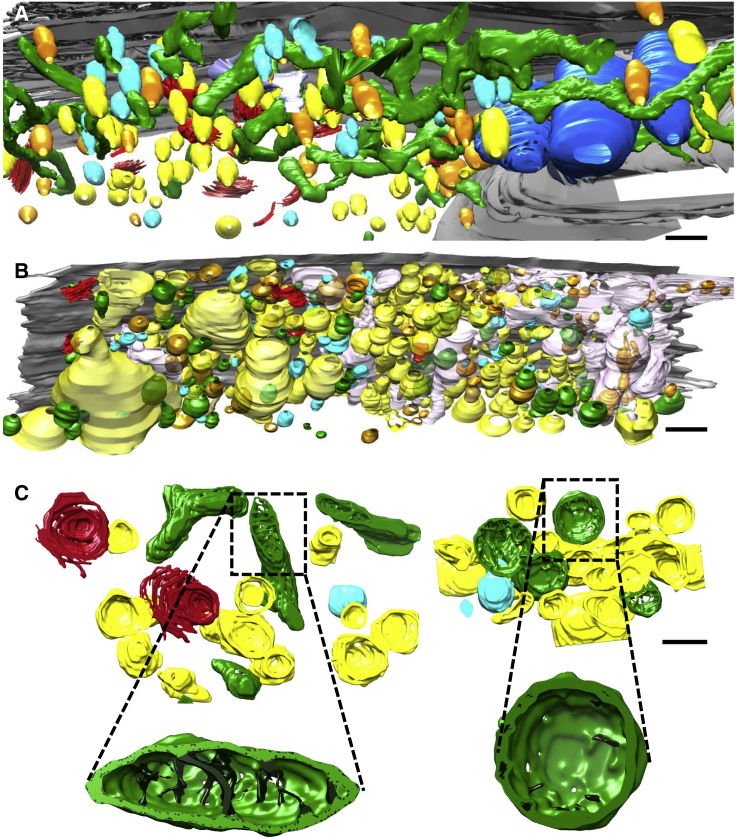
For further analysis, we have performed 3D reconstruction and segmentation of the serial cryoFIB/SEM volume data for both patient and control cells (Figures 3A and 3B; Videos S1 and S2). In the volume rendering of the control cell, an extended network of oblate tubular-shaped mitochondria is evident (Figure 3A, green; Video S3), whereas the patient cell shows mitochondria that are discrete and mostly round or oval-shaped individuals (Figure 3B, green; Video S4). Some individual mitochondria appear in close association with one another in both control and patient cells (Figure S2). The size of vacuoles (Figure 3B, yellow) within the patient cell are also remarkably larger than those of the control cell, more abundant and densely packed (Figures 2A, 2J, 3A, and 3B). The overall volume of mitochondria and number of each organelle in patient and control cells are compared in Figure S3. To analyze the structural details of organelles, a small region of the cell was cropped and segmented semi-automatically, as shown in Figures 3C and 3D (Videos S5 and S6). We can appreciate the drastic differences in the morphology of mitochondria and the shape and distribution of cristae between patient and control cells. Cristae structure is severely disturbed in the patient cell, appearing sparse in number and short, as previously observed by cryoET of limited regions of the cell periphery (Siegmund et al., 2018). The dramatic impact of complex V's failure to dimerize due to a specific USMG5 gene mutation on overall cellular architecture and organelle structures in LS patient cells is now more fully appreciated in the greater context of the whole cell through in situ large volume imaging.
Figure 3.
3D Reconstruction and Segmentation of Control and Patient Cells
(A and B) Surface rendering of segmented volumes of control (A) and patient (B) fibroblast cells. Green, mitochondria; red, Golgi, yellow, vacuoles; orange, dense vesicles; cyan, partially dense vesicles; blue, ER.
(C) Segmentation of a small representative volume from control (left) and patient (right) fibroblasts. Inserts are enlarged views of a single mitochondrion in control (left) and patient (right) cells. Cristae are shown in dark green. Scale bars, 1 μm (A and B) and 500 nm (C).
Discussion
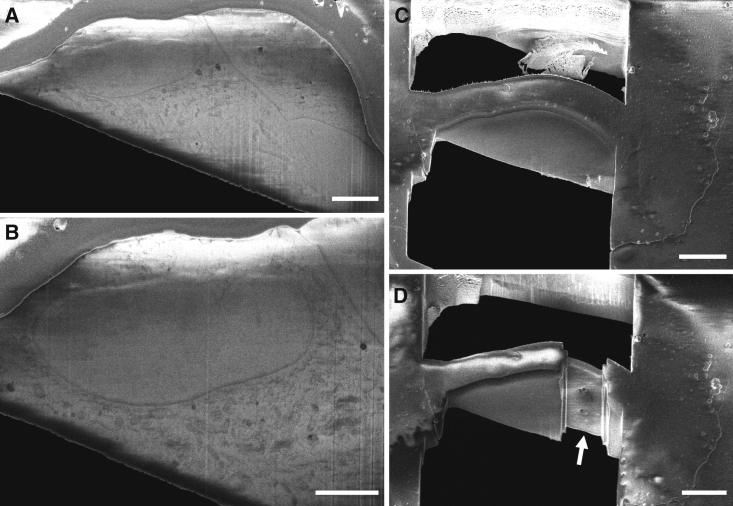
The majority of imaging studies on LS disease have been understandably focused on mitochondria. Nevertheless, our work demonstrates dramatic phenotypic changes to LS patient cells that extend beyond mitochondria to alter most, if not all, organelles within the cell, in particular a substantial accumulation of vacuoles. This is consistent with a recent study with PARL-deficient (Parl−/−) mice exhibiting a Leigh-like syndrome, where, in addition to mitochondrial structural changes, similar intracellular vacuolization was observed in a response to alterations in complex III (Spinazzi et al., 2019). Using emerging serial cryoFIB/SEM technology, we captured and visualized an entire frozen-hydrated mammalian cell in 3D. More importantly, we applied this capability to studies of human disease cellular processes. This revealed a profound disruption of cellular and subcellular structures in a primary LS patient fibroblast cells. Such whole-cell volume phenotypic characterization of cells and tissues in situ, at the near-native state, offers an opportunity to improve our understanding of diseases beyond LS and potentially provides new means for clinical use, from diagnosis to treatment. The potential of combining large-scale cryo-volume imaging using serial cryoFIB/SEM, followed by cryoFIB lamella preparation of the specific region of interest identified through serial cryoFIB/SEM (Figure 4), with cryoET imaging of the targeted lamella at a high resolution on the exact same object, is especially exciting.
Figure 4.
Targeted Cell Lamella Preparation by Serial cryoFIB/SEM
(A and B) Two representative cryoSEM images of cryoFIB block face, 320 nm apart.
(C and D) CryoSEM images of cell lamella preparation at the same region shown in (B). Arrow points to the thin lamella. Scale bars, 5 μm (A and B) and 10 μm (C and D).
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological Samples | ||
| Fetal bovine serum | Sigma-Aldrich | 12103C |
| Chemicals, Peptides, and Recombinant Proteins | ||
| MEM Vitamin solution | Thermo Fisher | 11120037 |
| Antibiotic-antimycotic | Thermo Fisher | 15240062 |
| Dulbecco’s minimal essential media (DMEM) | Thermo Fisher | 11965092 |
| Fibronectin | Sigma-Aldrich | 33016015 |
| Deposited Data | ||
| Serial cryoFIB/SEM raw images | This Paper | EMPIAR-10515 |
| Experimental Models: Cell Lines | ||
| Primary skin fibroblasts from a patient carrying a homozygous USMG5 mutation (C.87+1G>C, 1 base pair after the end of Exon 3) | Siegmund et al., 2018 | N/A |
| Primary control cells from healthy human subjects | Siegmund et al., 2018 | N/A |
| Software and Algorithms | ||
| IMOD | Kremer et al., 1996 | https://bio3d.colorado.edu/imod/ |
| MATLAB | MathWorks | https://www.mathworks.com/products/matlab.html |
| Chimera | Pettersen et al., 2004 | https://www.cgl.ucsf.edu/chimera/ |
| TrakEM2 | Cardona et al., 2012 | https://imagej.net/TrakEM2 |
| Other | ||
| Quantifoil Gold R2/2 grids, 300 mesh | Quantifoil | Q350AR2 |
| Glass-bottom culture dishes | MatTek Corporation | P35G-1.5-7-C |
Resource Availability
Lead Contact
Further information and requests should be directed and will be fulfilled by the Lead Contact, Prof. Peijun Zhang, (peijun@strubi.ox.ac.uk).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The raw Serial cryoFIB/SEM images during this study has been deposited at EMPIAR (https://www.ebi.ac.uk/pdbe/emdb/empiar/) with the access code EMPIAR-10515.
Experimental Model and Subject Details
Ethics Statement
The research received approval from the MRC Regulatory Support Centre.
Cell Lines
Skin fibroblasts from a patient carrying a homozygous USMG5 mutation (C.87+1G>C, 1 base pair after the end of Exon 3), and control cells from both male and female healthy human subjects are provided by Dr. Michio Hirano, Columbia University. Cell lines have not been authenticated.
Primary Cell Cultures
Cells were cultured in Dulbecco’s minimal essential media (DMEM) (Thermo Fisher, Waltham, MA, USA) supplemented with 15% fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA), 1% vitamin solution and 1% antibiotic-antimycotic (Thermo Fisher) as described earlier(Siegmund et al., 2018).
Methods Details
Sample Preparation
All experiments were conducted on cells cultured for <15 passages. Cells were plated onto gold R2/2 Quantifoil finder EM grids (Quantifoil Micro Tools GmbH, Jena, Germany) at density of 0.5-1 × 105 cells/ml (total 2 ml culture) in glass-bottom culture dishes (MatTek Corporation, Ashland, MA). The gold EM grids were coated with 50 μg/ml fibronectin (Sigma-Aldrich) and sterilized under UV light for 2 hours before use. For the control cells, after 48 hours culture, the grids were blotted with a filter paper and plunge-frozen into liquid ethane for rapid vitrification using an FEI Vitrobot (FEI, Hillsboro, OR) at ~100% humidity. Patient cells grew slowly and were cultured for 5 days before plunge-freezing.
Serial cryoFIB/SEM
For patient cells, the plunge-frozen EM grid was mounted in a Leica Vacuum Cryo Manipulation (VCM) preparation box (Leica Microsystems GmbH, Vienna, Austria) on a Leica cryo-holder for freeze-fracturing under cryogenic conditions. The TEM grid was held down on the flat top surface by using the clamp. The sample holder was transferred into a Leica ACE 600 cryo-sputter coater using a Leica VCT500 sample shuttle (Leica Microsystems, Vienna, Austria). At -154°C, the sample was sputter-coated with a 4 nm thick tungsten layer. The samples were then transferred into a ZEISS Crossbeam 550 FIB-SEM (Carl Zeiss Microscopy GmbH, Oberkochen, Germany). The cryo-stage temperature was maintained at -155°C and the system vacuum was 1.6×10-6 mbar. For areas containing cells, a cold deposition of platinum precursor material was achieved by opening the gas valve for 45 seconds. For cold deposition, the gas reservoir temperature was 28°C, and the distance between the gas capillary and the sample was about 3 mm.
First, a viewing channel for SEM imaging was milled using a FIB-milling probe current of 7 nA. The resulting cross-section was polished with a FIB probe current of 3 nA. For serial sectioning and imaging, a FIB probe current of 700 pA was used, and the FIB slice thickness was 21 nm. SEM images using InLens SE detection with a SEM probe current of 35 pA, a SEM acceleration potential of 2.3 keV and a dwell time of 100 ns were recorded. The lateral pixel spacing for SEM imaging was 10.5 nm and the image size was 4096 × 3072 pixels. Line Average with a line average count N = 61 was used for noise reduction.
The control cell sample (bare-TEM grid clipped into an autogrid ring) was mounted on a pre-tilted Leica sample holder for on-grid-thinning (Leica Microsystems GmbH, Vienna, Austria). After sputter-coating and transfer into the Crossbeam 550 FIB/SEM, a cold deposition of platinum precursor was done following the same procedure as above. The Crossbeam 550 system pressure was 8×10-7 mbar. A FIB probe current of 700 pA and a FIB slice thickness of 20 nm were used for serial sectioning and imaging. The lateral pixel spacing for SEM imaging was 10 nm and the imaging box was reduced to 3072 × 1150 pixel since the cell width is much larger than its height. For SEM imaging, InLens SE detection, a SEM probe current of 59 pA, a SEM acceleration potential of 1.9 keV and a dwell time of 200 ns were used. We employed a line average count N = 19 was used for noise reduction.
For cryoSEM imaging, the acceleration potential influences sample charging and is chosen in order to minimize sample charging and charging artefacts at interfaces. Due to the different mounting geometry, sample charging conditions are affected, and the acceleration potential is varied to optimize for imaging conditions. Since a charging and beam sensitive sample is imaged, short dwell times are advantageous and the total electron dose (beam current ∗ dwell time ∗ line average count) is restricted to minimize beam damage. By variation of how a specific electron dose is provided the imaging conditions are optimized especially near interfaces.
Local Reconstruction and Subvolume Segmentation
The raw SEM images were first aligned and a 3D volume generated using IMOD(Kremer et al., 1996). Subvolumes with representative features were cropped out from each aligned image stack (300×200 pixel × 40 slices or ~3.2 × 2.1 × 0.84 μm for patient cell and 500×400 pixel×40 slices or ~5.0 × 4.0 × 0.8 μm for control cell). The alignment between images was refined using an in-house Matlab script based on the Imregister function. To make objects smooth, 19 additional images were generated, with a linear interpolation, and inserted between two successive image slices with a home-made Matlab script. Using PixelAnnotationTool available online (https://github.com/abreheret/PixelAnnotationTool/releases), specific organelles, such as mitochondria, Golgi etc., were labeled manually from the cropped images as masks, which were then used to extract the image data from the corresponding region separately. These segmented image volumes were displayed in Chimera(Pettersen et al., 2004).
Global Image Alignment and 3D Modelling
The region containing the cell content was masked using Chimera software(Pettersen et al., 2004) with a boundary contour that was generated in 3dmod to just include the cell. The masked image slices (237 slices from the control cell and 218 slices from the patient cell) were first aligned with the Tiltxcorr(Kremer et al., 1996) program in IMOD using cross-correlation to determine the X and Y translations between successive image slices. The coarse-aligned image stacks were registered further using a SIFT-based algorithm(Lowe, 2004) adapted to run on TrakEM2 plugin for FIJI(Cardona et al., 2012). The post-registration images were exported into 3dmod(Kremer et al., 1996) for further manual segmentation. Chimera was used for display of segmented 3D models.
Quantification and Statistical Analysis
The parameters of serial cryoFIB/SEM in Table S1 are optimal imaging conditions. Resolution assessment was done through density profile plot in Digital Micrograph (Gatan Inc.) software and presented in Figure S1. The numbers for specific organelles were counted from the segmented cells and presented in Figure S3. The volumes for segmented mitochondria were calculated in IMOD and presented in Figure S3.
Acknowledgments
We thank Drs. Teresa Brosenitsch and Luiza Mendonça for critical reading of the manuscript. We are grateful to Dr. Michio Hirano for providing the control and patient cells. We also thank Drs. James Gilchrist for technical support, and Drs. Tao Ni, Min Xu, Julika Radecke, and Andrew Howe for helpful discussions about segmentation. We thank Dr. Saskia Mimietz-Oeckler and Andreas Halladay, Leica Microsystems, for technical support. This work was supported by the Department of Defense grants PR141292 (to Z.F.) and PR192466 (to Z.F.), the NIH P50 grant AI150481 (to P.Z.), the UK Wellcome Trust Investigator Award 206422/Z/17/Z (to P.Z.), and the UK Biotechnology and Biological Sciences Research Council grant BB/S003339/1 (to P.Z.).
Author Contributions
P.Z. conceived and designed the experiments. J.N. cultured cells and X.F. prepared cryo-specimens. A.S., L.C.Z.-D., and M.L.M.-F. performed serial cryoFIB/SEM. Y.Z., P.P.G., A.M.W., and D.S. performed segmentation and, with P.Z., analyzed data. Y.Z. and P.Z. wrote the paper with support from other authors.
Declaration of Interests
The authors declare no competing interests.
Published: October 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.str.2020.10.003.
Supplemental Information
References
- Akiva A., Nelkenbaum O., Schertel A., Yaniv K., Weiner S., Addadi L. Intercellular pathways from the vasculature to the forming bone in the zebrafish larval caudal fin: possible role in bone formation. J. Struct. Biol. 2019;206:139–148. doi: 10.1016/j.jsb.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Barca E., Ganetzky R.D., Potluri P., Juanola-Falgarona M., Gai X., Li D., Jalas C., Hirsch Y., Emmanuele V., Tadesse S. USMG5 Ashkenazi Jewish founder mutation impairs mitochondrial complex V dimerization and ATP synthesis. Hum. Mol. Genet. 2018;27:3305–3312. doi: 10.1093/hmg/ddy231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona A., Saalfeld S., Schindelin J., Arganda-Carreras I., Preibisch S., Longair M., Tomancak P., Hartenstein V., Douglas R.J. TrakEM2 software for neural circuit reconstruction. PLoS One. 2012;7:e38011. doi: 10.1371/journal.pone.0038011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X., Wu Y., Zhou J., Meng H., Zhang W., Guo J. A meta-analysis and systematic review of Leigh syndrome: clinical manifestations, respiratory chain enzyme complex deficiency, and gene mutations. Medicine (Baltimore) 2020;99:e18634. doi: 10.1097/MD.0000000000018634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes B.A., Zhang P. emClarity: software for high-resolution cryo-electron tomography and subtomogram averaging. Nat. Methods. 2018;15:955–961. doi: 10.1038/s41592-018-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer J.R., Mastronarde D.N., MacIntosh J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kizilyaprak C., Stierhof Y.D., Humbel B.M. Volume microscopy in biology: FIB-SEM tomography. Tissue Cell. 2019;57:123–128. doi: 10.1016/j.tice.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Lake N.J., Bird M.J., Isohanni P., Paetau A. Leigh syndrome: neuropathology and pathogenesis. J. Neuropathol. Exp. Neurol. 2015;74:482–492. doi: 10.1097/NEN.0000000000000195. [DOI] [PubMed] [Google Scholar]
- Lee J.S., Kim H., Lim B.C., Hwang H., Choi J., Kim K.J., Hwang Y.S., Chae J.H. Leigh syndrome in childhood: neurologic progression and functional outcome. J. Clin. Neurol. 2016;12:181–187. doi: 10.3988/jcn.2016.12.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D.G. Distinctive image features from scale-invariant keypoints. Int. J. Comput. Vis. 2004;60:91–110. [Google Scholar]
- Lucic V., Rigort A., Baumeister W. Cryo-electron tomography: the challenge of doing structural biology in situ. J. Cell Biol. 2013;202:407–419. doi: 10.1083/jcb.201304193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino M., Horai S., Goto Y., Nonaka I. Mitochondrial DNA mutations in Leigh syndrome and their phylogenetic implications. J. Hum. Genet. 2000;45:69–75. doi: 10.1007/s100380050014. [DOI] [PubMed] [Google Scholar]
- McCormick E.M., Zolkipli-Cunningham Z., Falk M.J. Mitochondrial disease genetics update: recent insights into the molecular diagnosis and expanding phenotype of primary mitochondrial disease. Curr. Opin. Pediatr. 2018;30:714–724. doi: 10.1097/MOP.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pronicki M., Matyja E., Piekutowska-Abramczuk D., Szymanska-Debinska T., Karkucinska-Wieckowska A., Karczmarewicz E., Grajkowska W., Kmiec T., Popowska E., Sykut-Cegielska J. Light and electron microscopy characteristics of the muscle of patients with SURF1 gene mutations associated with Leigh disease. J. Clin. Pathol. 2008;61:460–466. doi: 10.1136/jcp.2007.051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertel A., Snaidero N., Han H.M., Ruhwedel T., Laue M., Grabenbauer M., Mobius W. Cryo FIB-SEM: volume imaging of cellular ultrastructure in native frozen specimens. J. Struct. Biol. 2013;184:355–360. doi: 10.1016/j.jsb.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Schirra R.T., Jr., Zhang P. Correlative fluorescence and electron microscopy. Curr. Protoc. Cytom. 2014;70:12 36 11–10. doi: 10.1002/0471142956.cy1236s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund S.E., Grassucci R., Carter S.D., Barca E., Farino Z.J., Juanola-Falgarona M., Zhang P.J., Tanji K., Hirano M., Schon E.A. Three-dimensional analysis of mitochondrial crista ultrastructure in a patient with Leigh syndrome by in situ cryoelectron tomography. Iscience. 2018;6:83. doi: 10.1016/j.isci.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofou K., De Coo I.F., Isohanni P., Ostergaard E., Naess K., De Meirleir L., Tzoulis C., Uusimaa J., De Angst I.B., Lonnqvist T. A multicenter study on Leigh syndrome: disease course and predictors of survival. Orphanet J. Rare Dis. 2014;9:52. doi: 10.1186/1750-1172-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinazzi M., Radaelli E., Horre K., Arranz A.M., Gounko N.V., Agostinis P., Maia T.M., Impens F., Morais V.A., Lopez-Lluch G. PARL deficiency in mouse causes complex III defects, coenzyme Q depletion, and Leigh-like syndrome. Proc. Natl. Acad. Sci. U S A. 2019;116:277–286. doi: 10.1073/pnas.1811938116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer A.M., Schertel A., Nardis C., Mobius W. FIB-SEM of mouse nervous tissue: fast and slow sample preparation. Methods Cell Biol. 2019;152:1–21. doi: 10.1016/bs.mcb.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Sutton G., Sun D., Fu X., Kotecha A., Hecksel C.W., Clare D.K., Zhang P., Stuart D.I., Boyce M. Assembly intermediates of orthoreovirus captured in the cell. BioRxiv. 2020 doi: 10.1101/2020.06.10.144998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviben S., Gal A., Hood M.A., Bertinetti L., Politi Y., Bennet M., Krishnamoorthy P., Schertel A., Wirth R., Sorrentino A. A vacuole-like compartment concentrates a disordered calcium phase in a key coccolithophorid alga. Nat. Commun. 2016;7:11228. doi: 10.1038/ncomms11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidavsky N., Addadi S., Schertel A., Ben-Ezra D., Shpigel M., Addadi L., Weiner S. Calcium transport into the cells of the sea urchin larva in relation to spicule formation. Proc. Natl. Acad. Sci. U S A. 2016;113:12637–12642. doi: 10.1073/pnas.1612017113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidavsky N., Masic A., Schertel A., Weiner S., Addadi L. Mineral-bearing vesicle transport in sea urchin embryos. J. Struct. Biol. 2015;192:358–365. doi: 10.1016/j.jsb.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Wang K., Strunk K., Zhao G., Gray J.L., Zhang P. 3D structure determination of native mammalian cells using cryo-FIB and cryo-electron tomography. J. Struct. Biol. 2012;180:318–326. doi: 10.1016/j.jsb.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.H., Mitchell P.G., Galaz-Montoya J.G., Hecksel C.W., Sontag E.M., Gangadharan V., Marshman J., Mankus D., Bisher M.E., Lytton-Jean A.K.R. Multi-scale 3D cryo-correlative microscopy for vitrified cells. Structure. 2020 doi: 10.1016/j.str.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P. Advances in cryo-electron tomography and subtomogram averaging and classification. Curr. Opin. Struct. Biol. 2019;58:249–258. doi: 10.1016/j.sbi.2019.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw Serial cryoFIB/SEM images during this study has been deposited at EMPIAR (https://www.ebi.ac.uk/pdbe/emdb/empiar/) with the access code EMPIAR-10515.