Abstract
Background
The release of miRNAs in tissue fluids significantly recommends its use as non-invasive diagnostic biomarkers for the progression and pathogenesis of mild cognitive impairment (MCI) in aged patients.
Objective
The potential role of circulated miRNAs in the pathogenesis of MCI and its association with cellular oxidative stress, apoptosis, and circulated BDNF, Sirtuin 1 (SIRT1), and dipeptidyl peptidase-4 (DPP4) were evaluated in older adults with MCI.
Methods
A total of 150 subjects aged 65.4±3.7 years were recruited in this study. The participants were classified into two groups: healthy normal (n=80) and MCI (n=70). Real-time PCR analysis was performed to estimate the relative expression of miRNAs; miR-124a, miR-483-5p, miR-142-3p, and miR-125b, and apoptotic-related genes Bax, Bcl-2, and caspase-3 in the sera of MCI and control subjects. In addition, oxidative stress parameters; MDA, NO, SOD, and CAT; as well as plasma DPP4 activity, BDNF, SIRT1 levels were colorimetrically estimated.
Results
The levels of miR-124a and miR-483-5p significantly increased and miR-142-3p and miR-125b significantly reduced in the serum of MCI patients compared to controls. The expressed miRNAs significantly correlated with severe cognitive decline, measured by MMSE, MoCA, ADL, and memory scores. The expression of Bax, and caspase-3 apoptotic inducing genes significantly increased and Bcl-2 antiapoptotic gene significantly reduced in MCI subjects compared to controls. In addition, the plasma levels of MDA, NO, and DPP4 activity significantly increased, and the levels of SOD, CAT, BDNF, and SIRT1 significantly reduced in MCI subjects compared to controls. The expressed miRNAs correlated positively with NO, MDA, DPP4 activity, BDNF, and SIRT-1, and negatively with the levels of CAT, SOD, Bcl-2, Bax, and caspase-3 genes.
Conclusion
Circulating miR-124a, miR-483-5p, miR-142-3p, and miR-125b significantly associated with severe cognitive decline, cellular oxidative stress, and apoptosis in patients with MCI. Thus, it could be potential non-invasive biomarkers for the diagnosis of MCI with high diagnostic performance.
Keywords: circulating miRNAs, MCI, biomarkers, cellular oxidative stress, apoptosis, real-time RT-PCR
Introduction
Older ages clinically manifested by mild cognitive impairment (MCI) which is considered as an intermediate stage between the expected cognitive decline of normal aging and the more serious progressive decline of dementia. Subjects with MCI had a higher risk to progress to dementia compared with healthy controls.1–3 Previous studies showed that the progression rates from MCI to dementia significantly increased from 5.4% and 11.7% per year.4–7
The incidence of MCI has no significant factors even it was linked in most cases by heart problems, blood pressure, and diabetes.7 However, problems with memory, language, thought, routine MCI diagnosis, hypertension, and judgment were significantly increased in older adults with MCI.8
However, routine MCI diagnosis including clinical observation neuropsychological exam, neuroimaging, genetic testing, and neurochemical bodily may be supportive to postpone or prevent the subsequent progression to dementia.8–10 However, their routine use is unfeasible in the clinical setting due to its difficulty, invasiveness, and inconvenience to obtain data. Thus, the search for rapid and non-invasive diagnostic biomarkers is required to improve MCI diagnosis.
Previous research studies showed that increased dipeptidyl peptidase-4 (DPP4) activity and reduced both brain-derived neurotrophic factor (BDNF) and Sirtuin 1 (SIRT1) in peripheral circulation might all play pathogenetic roles in subjects with MCI.11–18 The changes in these physiological biomarkers are significantly associated with cellular oxidative stress and inflammation of MCI patients.11 In MCI, both DPP4 activity and BDNF significantly correlated with cellular oxidative stress and inflammation,19–21 oxidative stress imbalance and inflammation lead to cellular mitochondrial dysfunction, disruption of cellular homeostasis, and progressive neurodegeneration by cell death or apoptosis.22–26 Previous studies showed that aging promotes neuronal apoptosis via increasing the expression of caspase-3, Bax, and reduction in the expression of Bcl-2 genes.27,28 Thus, oxidative stress and inflammation accelerated aging and faster progression of neurodegenerative diseases particularly MCI and dementia.22–31 In addition to, with aging, brain parenchyma was impacted by cellular oxidative stress and potentially by TNF alpha deleterious action which significantly affects upon cognitive function.23–25
Circulating miRNAs are short non-coding RNAs showed to be associated with many physiological, cellular, and molecular developments, which occurred in normal and diseased cells, including MCI and dementia.32–34
The release of miRNAs in tissue fluids; serum or plasma or saliva inactive state significantly recommends its use as non-invasive diagnostic biomarkers for MCI.32–35 Several miRNAs were reported to be significantly associated with MCI and were potential biomarkers for the diagnosis of MCI.34–39 However, its association with cellular oxidative stress, apoptosis, and metabolic MCI parameters remains to be elucidated. The present study aimed to evaluate the potential role of circulated miRNAs in the pathogenesis of MCI and its association with cellular oxidative stress, apoptosis, and circulated BDNF, 1 (SIRT1, and dipeptidyl peptidase-4 (DPP4) in older adults with MCI.
Materials and Methods
Subjects
A total of 160 subjects aged 65.4±3.7 years were invited to this study. Only 150 of the subjects agreed to participate and classified according to the diagnosis of mild cognitive impairment (MCI) into two groups; healthy normal (n=80) and MCI (n=70). Elders with severe psychiatric illness, endocrine, immune, eating disorders, poor hearing and vision, and nervous system diseases, and taking glucocorticoid medication that could interfere cognitive ability measurements were already excluded from both cases and controls by investigating their past medical history. Ten subjects were excluded from this study (four refused participation, three with nervous system diseases, and three received glucocorticoid medication). The study protocol was reviewed according to the ethical guidelines of the 1975 Declaration of Helsinki and approved by the ethical committee of Rehabilitation Research Chair (RRC), King Saud University, Kingdom of Saudi Arabia, under file number (ID: RRC-2019-028) and signed informed consent forms were received from all subjects prior data collection.
Assessment of Cognitive Performance
A well-trained research neurologists performed the cognitive and functional status of all participants according to Petersen’s criteria (Table-1) 38 and by using the Activity of Daily Living scale (ADL), MiniMental State Examination (MMSE), and Montreal Cognitive Assessment (MoCA) as previously reported.39–46 The MMSE and the MoCA are the most widely used cognitive screening instruments for MCI which covers various areas of cognitive domains.47,48 The score of the MMSE (≤27) and that of the MoCA (<26) were taken together to evaluate cognitive impairment[48]. Thus, recruited participants were divided into normal control (n=80) and the MCI (n=70) group. The demographics and baseline characteristics of participants are shown in (Table-2).
Table 1.
Assessment of Cognitive Performance According to Petersen’s Criteria
| Criteria of MCI | Control Group (n=80) | MCI Group (n=70) |
|---|---|---|
| Memory complaint by participant or family (Yes/No) | No | Yes |
| Normal activities of daily living (20 items; score <26) | >26 | <26 |
| Normal general cognitive function (A: MMSE score between 20 and 27 [cutoff points for illiterate (≤ 20), primary school (≤23) and secondary school and above (≤ 27)]; B: MoCA score <26) | MMSE:> 27 MoCA: >26 |
MMSE: ≤27 MoCA: <26 |
| Objective impairment scores | >1.5 SD | <1.5 SD |
| The Clinical Dementia Rating scale | No | 0–0.5 |
Table 2.
The Demographics and Baseline Characteristics of Participants
| Parameters | Control Group | MCI Group |
|---|---|---|
| N | 80 (53.33%) | 70 (46.66%) |
| Male/Female | 50/30 | 40/30 |
| Age (years) | 65.3 ± 3.5 | 64.9 ± 4.1 |
| BMI (kg/m2) | 19.9± 2.6 | 24.1± 3.6 b |
| Waist (cm) | 77.3± 9.3 | 98.1 ± 6.3 b |
| Hips (cm) | 86.9 ± 7.5 | 92.6 ± 18.3 b |
| WHR | 0.89±0.11 | 1.4±0.16 b |
| Education (years) | 10.2 ± 0.8 | 5.3 ± 0.6b |
| Lifestyle factors, % Working Exercising regularly |
96.5 88.4 |
89.5a 76.1a |
Notes: Values are expressed as mean ±SD; Significance at p <0.05. ap <0.01, bp <0.001.
Assessment of Nitric Oxide (NO)
Serum accumulated NO was estimated as the stable end products nitrite and nitrate as previously reported.43,46 In this experiment, cadmium reagent performed to convert nitrate to nitrite which then measured by spectrophotometric assay using the Griess reagents sulfanilamide, HCl and N-naphthylethylenediamine.47–51 The absorbance of nitrite concentrations was taken at 545 nm.47–51 The concentration of the accumulated NO calculated according to the following formula:
NO concentration (μmol/L.) = {[At-Ab/As-Ab× Cons of S/V.serm used ×1000]}
Assessment of Plasma Malonaldehyde (MDA)
Plasma Lipid peroxide MDA was estimated as a measure of cellular lipid peroxidation in all subjects by using a reversed-phase high-performance liquid chromatography (HPLC/PDA Shimadzu®) using an analytic column C-18 (Phenomenex×150mm×4.6 mm, 10 µm) as previously reported.49–52 In this method, acidic thiobarbituric acid reacted with plasma MDA at 90◦C for 1 h, protein removal by centrifugation, filtered, and finally, the colored complex detected spectrophotometrically at 532 nm. MDA levels were expressed as nmol of MDA/mg protein.49–52
Assessment of Antioxidant Enzymes
In this test, catalase (CAT) and superoxide dismutase (SOD) activity were estimated by a spectrophotometer analysis as previously reported.49–56 All enzymatic assays were conducted in triplicate, corrected by hemoglobin content, and expressed as U/g of hemoglobin.51
Assessment of Plasma DPP4 Activity, BDNF, Sirtuin 1 (SIRT1) Levels
Plasma DPP4 activity was performed as previously described.11,32,54–57 In addition, brain-derived neurotrophic factor (BDNF) and sirtuin 1 (SIRT1) were estimated in the serum of all participants by immune assay (ELISA) technique,57 using the human BDNF Quantikine Kit (Catalog no: DBD00, R&D System, Minneapolis, MN, USA) and human SIRT1 ELISA Kit (Catalog no: E94912Hu, USCN Life Science, Wuhan, China). The results were performed in duplicates and were used for statistical analyses.57
Real-Time RT-PCR Analysis of Circulating miRNAs and Apoptotic Genes
Extraction of RNA and Synthesis of cDNA
For each participant, the miRNease isolation kit (Qiagen, Hilden, Germany) was used to extract total RNA from serum samples. A reverse-transcription polymerase chain reaction (RTPCR) was applied to analyze total RNA in all serum samples. Then, a complementary DNA (cDNA) was generated using reverse-transcription miScriptII RT kits (Qiagen), and the levels of miRNAs were evaluated by optical density.11
Real-Time RT-PCR Analysis
The primers of circulating miRNAs; miR-124a, miR-483-5p, miR-142-3p, and miR-125b (Applied Biosystems, Foster City, CA, USA), in addition to primer sequences of the apoptotic genes (Bax, Bcl-2, and caspase-3) (Table 3), were used to screen the expression of miRNAs and apoptotic genes in the plasma of all participants by using a quantitative real-time RT-PCR.11 The average copy number of the resultant PCR components was normalized according to the GAPDH gene which was used as an internal housekeeping gene.32 In the PCR process, templets of respective cDNA subjected to four thermal phases; primary denaturation phase (I) (at 94°C for 2 minutes); denaturation phase (II) (at 94°C for 30 seconds); annealing phase (III) (at 59°C for 30 seconds), and amplification phase (IV) (at 72°C for 30 seconds). The PCR phases (II–IV) proceed for 45 cycles and all reactions were measured in a triplicated manner.32
Table 3.
Neuropsychological Test Scores and the Cognitive Status of the Participants (n=15; Mean ±SD)
| Parameters | Control Group | MCI Group | t | p-Value |
|---|---|---|---|---|
| MMSE | 28.4 ± 3.3 | 21.3 ± 2.5 | 12.8 | <0.001 |
| MoCA | 22.7±2.9 | 27.9±5.4 | 18.9 | <0.001 |
| ADL | 22.1±3.7 | 28.6±10.3 | 19.7 | <0.001 |
| IADL | 8.3±0.2 | 6.9±0.3 | 15.3 | <0.001 |
| Wechsler Memory Scale – Revised- | <0.001 | |||
| VPA | 10.3±2.8 | 6.9±3.1 | 12.5 | |
| VR | 12.8±4.3 | 8.5±2.7 | 10.4 | |
| IM | 7.2±2.9 | 4.5±3.1 | 11.9 | |
| PR | 17.0±3.9 | 12.9±2.9 | 16.9 | |
| Wechsler Adult Intelligence scale – Chinese revision | <0.001 | |||
| S | 19.6±3.8 | 12.8±5.8 | 18.9 | |
| R | 14.1±3.7 | 8.1±2.8 | 16.9 | |
| DSC | 38.2±8.6 | 24.2±10.3 | 12.8 | |
| PC | 10.4±1.9 | 9.4±1.3 | 15.8 | |
| BDs | 79.3±24.3 | 89.8±31.6 | 14.7 | |
| V | 17.2±3.7 | 14.5±4.1 | 8.5 |
Note: Values are expressed as mean ±SD.
Abbreviations: MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; ADL, activity of daily living; IADL, instrumental activities of daily living; VPA, verbal paired associates; VR, visual reproduction; IM, immediate memory; PR, picture recall; S, similarities; R, arithmetic; DSC, digit symbol-coding; PC, picture completion; BDs, block design(s); V, vocabulary.
Statistical Analysis
Power calculations of the selected sample size of 150 subjects showed to give an estimated power of 95% and a significance level of 0.05 with an expected frequency of 10.5%.
An SPSS statistical program (SPSS, IBM Statistics V.17) was used to analyze all data produced in this study. The data of continuous variables are expressed as mean±SD. The frequency differences between the groups were analyzed by using a non-parametric test (Mann–Whitney–Wilcoxon test) and the χ2 test, respectively. In all groups, two independent sample t-tests were used for comparison between the studied variables such as cognitive score (dependent variable), expression levels of miRNAs, apoptotic genes, oxidative stress parameters, plasma DPP4 activity, BDNF, and sirtuin 1 (SIRT1) levels (independent variables). In addition, multiple stepwise regressions and Pearson’s correlation analyses were used to estimate the associations between cognitive function status and the studied independent variables in older subjects with MCI and in controls. All tests were two-tailed; because of multiple assessments, results were only considered statistically significant at a value of p <0.05.
Results
A total of 150 older adults were recruited in this study. Based on Petersen’s criteria (Table 1), mild cognitive impairment (MCI) was predicted in 46.66% of the participants. The participants classified into two groups; healthy control (n=80) and MCI group (70). In subjects with MCI, adiposity parameters; BMI, Waist, hips, and WHR were significantly increased (p=0.001) compared to healthy controls (Table 2). In addition, education scores, and lifestyle factors (working and regular exercise) significantly reduced (P=0.001) in MCI compared to normal controls (Table 2).
Cognitive function and neuropsychological scores of all subjects were estimated in this study (Table 3). Mini-mental state scores (MMSE) and active daily living scores (ADL & IADL) significantly (P=0.001) increased and the Montreal cognitive assessment scores significantly (P=0.001) reduced in older adults with MCI compared to normal controls (Table 3).
Mean neuropsychological test scores measured by memory and adult intelligence scales are listed in Table 3. The t-test was used to compare neuropsychological status and cognition function between normal and MCI groups. The two groups were significantly different (P=0.001) in neuropsychological as well as cognitive scores as shown in (Table 3).
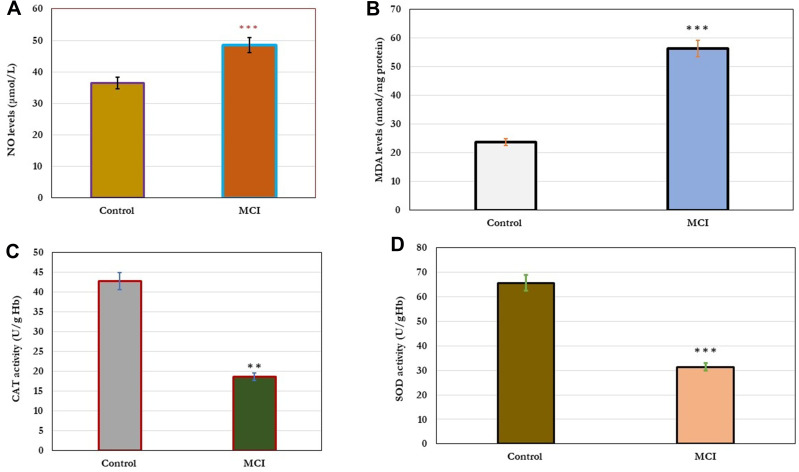
In this study, the effect of cellular oxidative stress on cognitive function was estimated (Figure 1). The results showed a significant increase (p=0.001) in the levels of cellular NO and MDA in older adults with MCI compared to healthy controls (A&B). In addition, a significant decrease (p=0.001) in the levels of antioxidant enzymes; CAT and SOD in older adults with MCI compared to healthy controls. The change in the cellular oxidative-defensive system closely correlated (P=0.001) with the status of cognitive function. In subjects with MCI, serum levels of NO and MDA correlated positively with MMSE, MoCA, ADL, and memory scores, and negatively correlated with reduced activity of antioxidant enzymes; CAT and SOD, respectively (Table 4).
Figure 1.
Changes in cellular oxidative stress; NO and MDA (A and B) and antioxidant enzymes; Cat and SOD the levels (C and D) in healthy control (n=80) and older adults with MCI (n=70). The results showed significant increase (p=0.001) in the levels of cellular NO and MDA in older adults with MCI compared to healthy controls (A and B). In addition, significant decrease (p=0.001) in the levels of antioxidant enzymes; CAT and SOD in older adults with MCI compared to healthy controls. Significance of the comparison was evaluated by Mann–Whitney-Wilcoxon test and sample t-test, * * p≤ 0.01, * * *p≤ 0.001.
Abbreviations: MCI, mild cognitive impairment; NO, nitric oxide; MDA, malonaldehyde, SOD, superoxide dismutase, CAT, catalase enzyme.
Table 4.
Correlations Between DPP4 Activities, BDNF, SIRT-1, Celluar Oxidative Stress, Apoptosis, and Relative Expression of miRNAs vs Cognitive Parameters
| Characteristics | Cognitive Function (n=70) | |||||||
|---|---|---|---|---|---|---|---|---|
| MMSEa | MoCAa | ADLa | Memory Scoresa | |||||
| r | P | r | P | r | P | r | P | |
| DPP4 activity (nmol/min/mL) | −0.320 | <0.001 | −0.291 | < 0.001 | −0.31 | <0.001 | −0.24 | <0.001 |
| BDNF (ng/mL) | 0.192 | <0.001 | 0.23 | <0.001 | 0.18 | <0.001 | 0.15 | <0.001 |
| SIRT1(ng/mL) | 0.31 | <0.001 | 0.45 | <0.001 | 0.27 | <0.001 | 0.34 | <0.001 |
| NO | 0.35 | <0.001 | 0.28 | <0.001 | 0.29 | <0.001 | 0.47 | <0.001 |
| MDA | 0.23 | <0.001 | 0.36 | <0.001 | 0.51 | <0.001 | 0.14 | <0.001 |
| CAT | −0.32 | <0.001 | −0.39 | <0.001 | −0.25 | <0.001 | −0.31 | <0.001 |
| SOD | −0.54 | 0.001 | −0.48 | <0.001 | −0.37 | <0.001 | −0.43 | <0.001 |
| Bax | 0.21 | <0.001 | 0.18 | <0.001 | 0.26 | <0.001 | 0.26 | <0.001 |
| Bcl-2 | −0.45 | <0.001 | −0.49 | <0.001 | −0.53 | <0.001 | −0.78 | <0.001 |
| Caspase-3 | 0.48 | <0.001 | 0.21 | <0.001 | 0.23 | <0.001 | 0.19 | <0.001 |
| miR-124a | 0.251 | <0.001 | 0.362 | <0.001 | 0.182 | <0.001 | 0.135 | <0.001 |
| miR-483-5p | 0.314 | <0.001 | 0.217 | <0.001 | 0.321 | <0.001 | 0.158 | <0.001 |
| miR-142-3p | −0.242 | <0.001 | −0.342 | <0.001 | −0.124 | <0.001 | −0.314 | <0.001 |
| miR-125b | −0.254 | <0.001 | −0.227 | <0.001 | −0.351 | <0.001 | −0.318 | <0.001 |
Note: aP-value determined by partial correlation analysis with respect to the DPP4 activity, BDNF, SIRT-1, cellular oxidative stress, apoptosis, and relative expression of miRNAs adjusted for age, BMI, gender, education level, exercise levels, and working.
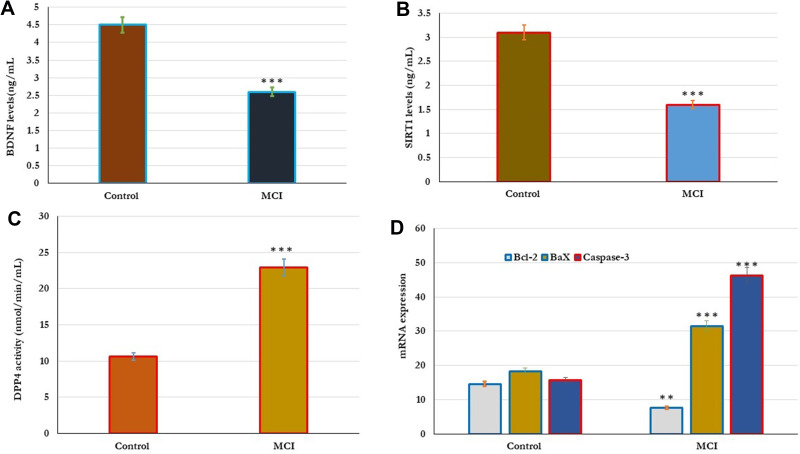
In addition, the influence of cellular apoptosis on cognitive function was reported (Figure 2). In this experiment, the cellular expression of Bcl-2, Bax, and caspase-3 genes was examined in all subjects. In Older adults with MCI, the expression levels of both Bax and caspase-3 genes significantly (p=0.001) increased, and the expression levels of the Bcl-2 gene significantly (p=0.01) reduced in comparison with the results of healthy controls (Figure 2D). The expression of cellular apoptotic genes correlated with the cognitive function. Cognitive function scores of MMSE, MoCA, ADL, and memory correlated positively with the expression of Bax and caspase-3, and negatively with the expressed Bcl-2 gene (Table 4).
Figure 2.
Metabolic changes in the DPP4 activity, BDNF, SIRT-1, and apoptotic genes Bcl-2, Bax, and caspase-3 in healthy control (n=80) and older adults with MCI (n=70). (A–D). The results showed that the levels of BDNF, SIRT-1 significantly reduced and the levels of DPP4 activity significantly increased in older subjects with MCI compared to healthy controls (A–C). In addition, the expression of apoptotic genes was significantly reported in all subjects. In Older adults with MCI, the expression levels of both Bax and caspase-3 genes significantly (p=0.001) increased, and the expression levels of Bcl-2 gene significantly (p=0.01) reduced in comparison with the results of healthy controls (D). Significance of the comparison was evaluated by Mann–Whitney–Wilcoxon test and sample t-test, **p≤ 0.01 ***p≤ 0.001.
Abbreviation: MCI, mild cognitive impairment.
The correlation between metabolic changes in the serum levels of DPP4 activity, BDNF and SIRT-1 were estimated in control and MCI subjects (Figure 2). As shown in Figure 2, the results showed that the levels of BDNF, SIRT-1 significantly reduced and the DPP4 activity significantly increased in older subjects with MCI compared to healthy controls (2A,2Band2C). The levels of DPP4 activity, BDNF and SIRT-1 were significantly associated with cognitive status. Cognitive function; MMSE, MoCA, ADL, and memory scores correlated positively (p=0.001) with serum BDNF and SIRT-1, and negatively with serum DPP4 activity as shown in Table (4). Similarly, in subjects with MCI physiological changes in the serum levels of DPP4 activity, BDNF and SIRT-1 were intercorrelated with cellular oxidative and apoptosis. The serum levels of DPP4 activity, BDNF, and SIRT-1 correlated positively with CAT and SOD activity, Bcl-2 gene expression, and negatively with cellular NO, MDA, and expressed Bax, and caspase-3 genes (Table 5).
Table 5.
Correlations Between DPP4 Activities, BDNF, and SIRT-1 vs Celluar Oxidative Stress and Apoptosis in Older Adults with MCI
| Characteristics | Cognitive-Related Metabolic Parametersa | |||||
|---|---|---|---|---|---|---|
| DPP4 Activity | BDNF | SIRT1(ng/mL) | ||||
| r | P | r | P | r | P | |
| NO | −0.128 | <0.01 | −0.32 | <0.01 | −0.56 | <0.01 |
| MDA | −0.18 | <0.001 | −0.23 | <0.001 | −0.58 | <0.001 |
| CAT | 0.89 | <0.001 | 0.76 | <0.001 | 0.81 | <0.001 |
| SOD | 0.48 | <0.001 | 0.71 | <0.001 | 0.56 | <0.001 |
| Bax | −0.31 | <0.001 | −0.43 | <0.001 | −0.48 | <0.001 |
| Bcl-2 | 0.36 | <0.001 | 0.51 | <0.001 | 0.42 | <0.001 |
| Caspase-3 | −0.87 | <0.001 | −0.96 | <0.001 | −0.74 | <0.001 |
Note: aP-value determined by partial correlation analysis with respect to the DPP4 activity, BDNF, DBR, cellular oxidative stress, and apoptosis adjusted for age, BMI, gender, education level, exercise levels, and working among older adults.
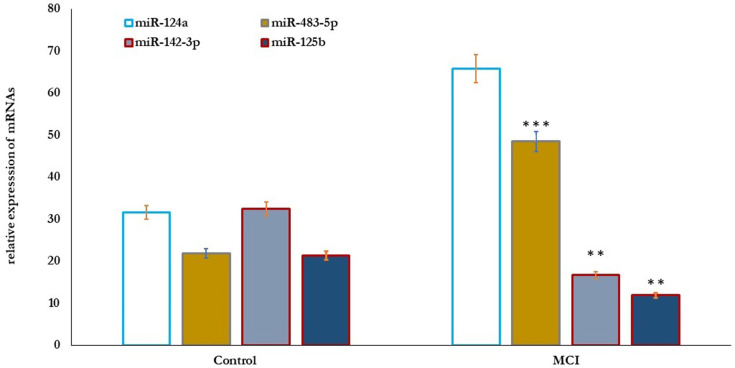
Also, the correlation between MicroRNAs’ differential expression with cognitive function in older adults was studied (Figure 3). The results showed that the relative expression of miR-124a and miR-483-5p significantly increased (P=0.001), and miR-142-3p and miR-125b significantly reduced (P=0.01) in older adults with MCI compared to healthy controls (Figure 3). Cognitive function scores of MMSE, MoCA, ADL, and memory correlated positively with the expression levels of miR-124a and miR-483-5p, and negatively with the expressed miR-142-3p, and miR-125b, respectively (Table 4).
Figure 3.
MicroRNAs’ differential expression profile in healthy control (n=80) and older adults with MCI (n=70). The results showed that the relative expression of miR-124a and miR-483-5p significantly increased (P=0.001), and miR-142-3p, and miR-125b significantly reduced (P=0.01) in older adults with MCI compared healthy controls. Significance of the comparison was evaluated by Mann–Whitney–Wilcoxon test and sample t-test, **p≤ 0.01, ***p≤ 0.001.
Abbreviation: MCI, mild cognitive impairment.
Expressed microRNAs positively correlated with cellular oxidative stress parameters; NO and MDA and negatively with deficient activities of antioxidative enzymes; CAT and SOD, respectively (Table 6). In subjects with MCI, the relative expression of microRNAs correlated positively with cognitive physiological changes in the serum levels of DPP4 activity, BDNF, and SIRT-1 (p=0.001) and negatively with the expressed cellular apoptosis genes Bcl-2, Bax, and caspase-3 (Table 6).
Table 6.
Correlations Between DPP4 Activities, BDNF, SIRT-1, Celluar Oxidative Stress, and Apoptosis vs Relative Expression of miRNAs in Older Adults with MCI
| Characteristics | miRNAs Relative Expressiona | |||||||
|---|---|---|---|---|---|---|---|---|
| miR-124a | miR-483-5p | miR-142-3p | miR-125b | |||||
| r | P | r | P | r | P | r | P | |
| DPP4 activity (nmol/min/mL) | 0.12 | <0.01 | 0.24 | <0.002 | 0.38 | < 0.002 | 0.145 | <0.001 |
| BDNF (ng/mL) | 0.21 | <0.01 | 0.31 | <0.01 | 0.28 | < 0.01 | 0.31 | <0.001 |
| SIRT1(ng/mL) | 0.45 | <0.01 | 0.57 | <0.01 | 0.49 | < 0.01 | 0.49 | <0.01 |
| NO | 0.37 | <0.001 | 0.42 | <0.001 | 0.36 | < 0.001 | 0.76 | <0.001 |
| MDA | 0.18 | <0.001 | 0.23 | <0.001 | 0.42 | < 0.001 | 0.27 | <0.001 |
| CAT | −0.51 | <0.001 | −0.62 | <0.001 | −0.54 | < 0.001 | −0.63 | <0.001 |
| SOD | −0.85 | <0.001 | −0.82 | <0.001 | −0.47 | < 0.001 | −0.84 | <0.001 |
| Bax | −0.45 | <0.001 | −0.36 | <0.001 | −0.56 | < 0.001 | −0.46 | <0.001 |
| Bcl-2 | −0.17 | <0.001 | −0.42 | <0.001 | −0.68 | < 0.001 | −0.91 | <0.001 |
| Caspase-3 | −0.75 | <0.001 | −0.53 | <0.001 | −0.47 | < 0.001 | −0.43 | <0.001 |
Note: aP-value determined by partial correlation analysis with respect to the DPP4 activity, BDNF, DBR, cellular oxidative stress, and apoptosis adjusted for age, BMI, gender, education level, exercise levels, and working among older adults with MCI.
Discussion
Mild cognitive impairment (MCI) was predicted in 46.66% of the study population. Molecular-based assays confirmed that circulating miR-124a, miR-483-5p, miR-142-3p, and miR-125b were significantly associated with severe cognitive decline, oxidative stress, and apoptosis in patients with MCI.
In this study, participants with MCI recorded higher MMSE, ADL, IADL, and lower MoCA scores compared to healthy controls. In addition, neuropsychological status, especially poor memory and intelligence, was significantly reported among MCI subjects.
MCI was reported in the aged people, whereas 5.4% and 11.7% per year of MCI significantly at higher risks to develop to severe dementia.4–6 Many problems in memory, language, thought, and judgment were significantly reported in patients with MCI which affects on their daily activities.2,8
Cellular oxidative stress was significantly associated with the severity of cognition impairment particularly in older adults with MCI.32,58–62 DNA damage (8-oxo-2ʹ-deoxyguanosine; 8-oxodGuo), lipid peroxidation (malonaldehyde; MDA), and other cellular oxidative parameters significantly reported in higher ranges in the brain tissues, serum, plasma, and cerebrospinal fluid (CSF) patients with MCI.58–61
In the current study, oxidative stress markers MDA and NO significantly increased, and CAT and SOD antioxidant defense activity significantly reduced in MCI compared to healthy-aged individuals. The deficient cellular antioxidant activity and increased free radical oxidative stress significantly associated with memory loss, language, thought, and judgment of subjects with MCI. The results showed that low cognitive performance was associated with both elevated MDA and NO levels and decreased SOD and CAT activity in subjects with MCI.
Previous reports showed that accumulation of cellular oxidative free radicals (ROS) significantly produces degeneration of brain neurons,61–65 vascular lesions,66 which consequently lead to cognitive decline and dementia in old age. It was reported that in brain tissue, ROS generated from microglia and astrocytes are proposed to control synaptic and nonsynaptic communications between neurons and glia. Thus, the release of ROS radicals in higher quantity promotes neurodegeneration and memory loss via processes of neuroinflammation and cell death.62 Consistent with our results, MDA as lipid peroxide product was significantly reported in the serum of subjects with brain disorders such as MCI.36,63 Also, it was reported that MDA and related lipid peroxides considered promising peripheral biomarkers during brain cases with white matter abnormalities. This may be related to higher lipid contents in both the axonal membranes and myelin sheaths of the brain.63,64
NO synthesized by the enzyme NOS from neurons of the brain and spinal cord.63–67 It functions to maintain cellular vascular tone, neurotransmitter function, and mediation of cellular defense in normal cases.68 However, NO was considered a neurotoxic agent when it produced at higher levels for brain microglia and might play a role in neurodegeneration.68–72 Thus, in subjects with brain disorders such as MCI, Alzheimer’s, and Parkinson’s disease thought to be associated with higher production of cellular NO induced by NOS enzyme activity.73
In addition, like our results, dysregulation of antioxidant enzymes such as CAT and SOD was proposed to play a role in cellular oxidative stress associated with age-related pathologies, especially cognitive decline.71–75 Whereas, a combined measurement of oxidative status with antioxidant potential was prospectively associated with the process of neurodegeneration and could be estimated as signs of cognitive decline among older ages.76–82
Previous research studies showed that the accumulation of ROS free radicals activates neural cell apoptosis during brain development.77–80 A set of caspases enzymes such as caspase-3 and Bax were expressed to induce apoptosis and stimulate inflammation in the nervous system which leads to brain neurodegeneration.79,80
In this study, we are trying to explore the potential mechanism of cellular apoptosis involved in neurologic injury associated with cognitive problems in patients with MCI. Thus, apoptotic genes, Bcl-2, Bax, and caspase-3, were estimated in all participants by using real-time PCR analysis. The results showed that the expression of Bax and caspase-3 apoptotic genes significantly upregulated (increased) and the expression levels of the Bcl-2 antiapoptotic gene significantly down-regulated (reduced) in the patients with MCI compared to healthy controls. The expressed apoptotic genes significantly correlated with the score of cognitive function among MCI patients. Cognitive function scores of MMSE, MoCA, ADL, and memory correlated positively with apoptotic genes Bax and caspase-3, and negatively with the expressed Bcl-2 antiapoptotic gene. The results signify the role of apoptosis in the pathogenesis of MCI.
Previously, it was reported that caspase enzymes especially caspases 3 and 7 activate regular apoptosis during brain development, neurodegeneration, and progressive dismantling of neuronal circuits in brain regions which inturn mediate memory functions. Thus, an abnormal increase in apoptosis via oxidative stress or inflammation significantly leads to deficiency in memory functions in older ages.81,82 Caspase 3 activation by intrinsic and extrinsic apoptotic pathways showed to be the most vital event associated with neuronal cell death in most chronic neurodegenerative conditions.81,82 The hippocampus is an important brain region responsible for learning and memory and higher exposure to free radical oxidative stress and apoptosis leads to significant abnormality in learning and memory among MCI patients.83–85
In this study, it was found that the levels of BDNF and SIRT-1 were significantly reduced and the enzyme DPP4 activity significantly increased in the serum of MCI patients compared to healthy controls. The changes in these neurometabolic parameters were significantly associated with the scores of cognitive measurements; MMSE, MoCA, ADL, and memory scores.
In patients with dementia (AD) and brain disorders, the levels of BDNF in serum showed to be associated with cognitive decline.86–89 Matched to our results, BDNF significantly decreased in patients with MCI and AD,87 and that higher levels of BDNF are required to protect future recurrence of brain disorders such as AD.86 Also, SIRT-1 was significantly reduced in patients with MCI in relation to healthy control subjects. The increased levels of human SIRT1 were significantly associated with neuroprotection and longevity.90,91 Thus, both BDNF and SIRT1 were considered as conceivable candidate genes contributing to MCI and AD.92,93
In this study, the levels of DPP4 activity, BDNF, and SIRT-1 in the serum of MCI patients correlated positively with CAT and SOD activity, Bcl-2 gene expression, and negatively with cellular NO, MDA, and expressed Bax, and caspase-3 genes. The increased enzyme DPP4 activity among our MCI patients was significantly associated with others who reported an increase in the levels of plasma DPP4 activity in MCI patients and concluded that DPP4 activity was mutually influenced by increased cellular free radical oxidative stress.94 Moreover, the lower levels of DPP4 activity significantly improved cognitive function via the enhancement of inflammation, oxidative stress and reducing or suppression of apoptosis.16–18 It was reported previously that increased DPP4 activity promotes the development of oxidative stress and inflammation,95 which was significantly involved in the progression and pathogenesis of cognitive dysfunction.1,90 Thus, decreased BDNF and increased DPP4 activities in the blood circulation of our MCI patients showed to have a pathogenetic role in the development of cognitive impairment as previously reported, and then it could be used as a prognostic biomarker for MCI.57,97,98
miRNAs are non-coding short cellular RNAs significantly expressed with cells and freely liberated in peripheral blood circulation, and easily identified in urine, plasma, serum, and cerebrospinal fluids. It has multicellular functions particularly the regulation of gene expression. Previous studies reported the expression of miRNAs in the brain and associated with the regulation of neuronal plasticity, function, and development. It was reported previously that most neurodevelopment disorders or neurodegenerative diseases are significantly associated with abnormality or dysfunction in miRNAs transcription.13,99,100 In general, about half of all protein-coding genes identified to be regulated by microRNAs which significantly reduce the abnormality or fluctuations in protein expression.101,102
Thus, in this study, we are trying to understand the role of miRNAs in the development of neuropsychiatric disorders associated with cognitive impairment in patients with MCI.
In this study, real-time PCR analysis was performed to estimate microRNAs’ differential expression in control and patients with MCI. It was found that the relative expression levels of miR-124a and miR-483-5p significantly increased and miR-142-3p and miR-125b significantly reduced in the serum of older adults with MCI compared to healthy controls. The data also showed that relative expression of miRNAs; miR-124a, miR-483-5p, miR-142-3p, and miR-125b correlated with the scores of cognitive function among MCI patients.
Similarly, increased peripheral miR-146a and miR-483-5p levels were previously shown to associate with the severity of cognitive impairment in subjects with MCI and to predict the conversion to dementia,31,36,103 thus both miR-486-5p and miR-483-5p were the most significant indicators of MCI among older adults. Matched to our results, the decline in the levels of miR–125b and miR-142-3p significantly associated with the scores of cognitive impairment in patients with MCI and AD,104–107 and could be used as a useful noninvasive biomarker for older adults with MCI. In addition, higher specificity (68.3%) and a sensitivity of 80.8% with quiet priority and significant correlation with the Mini-Mental State Examination (MMSE) were reported for expressed miR-125b in patients with dementia.108,109
In the current study, correlation analysis interestingly showed that expressed microRNAs were significantly associated with cellular oxidative stress and apoptotic inducing genes. The expression of microRNAs correlated positively with oxidative stress parameters, NO and MDA, and negatively with the reduced activities of antioxidative enzymes; CAT and SOD as well as expression levels of cellular apoptosis genes Bcl-2, Bax, and caspase-3. Previously, miR 125b, miR 146a, and other related miRNAs showed an association with neuropathology, apoptosis, oxidative stress, and other neurodegeneration of the human central nervous system.35 In addition, the relative expression of miR-124a and miR 125b correlated with cellular aging processes such as oxidative stress, age-related antioxidant dysfunction, senescence, and apoptosis.35,110
In the previous differential correlation analysis, plasma miR-125b with multiple miRNAs pairs showed to have higher accuracy for MCI detection.111 It was downregulated in the serum of patients with dementia.112 miR-125b promotes cellular apoptosis via regulating the function of a tumor suppressor gene (p53) which significantly associated with controlling diseases, aging, and metabolism particularly in brain neurodegeneration in AD.113–115 It was reported that miR 125b enhances neuronal apoptosis and Tau phosphorylation in patients with Alzheimer’s disease.116 Our results matched with others which showed the expression of miRNAs; miR-124a, miR-483-5p, miR-142-3p, and miR-125b significantly associated with more severe cognitive decline in patients with MCI via promoting cellular oxidative and apoptosis.117
The expression patterns of serum microRNAs; miR-124a, miR-483-5p, miR-142-3p, and miR-125b correlated positively with DPP4 activity, BDNF, and SIRT-1 in the serum of MCI patients. The proposed correlation may proceed via the indirect influence of expressed microRNAs on neuronal oxidative stress and apoptosis in patients with MCI. Whereas increased DPP4 activity and decline of both BDNF, and SIRT-1 in the serum of MCI patients resulted in the enhancement of inflammation, oxidative stress, and apoptosis which was significantly implicated in the pathophysiology of cognitive decline.19,54,55,118 In addition, many expressed microRNAs showed to target SIRT-1; thus, dysfunction or abnormal transcription of these miRNAs by higher oxidative stress and apoptosis may lead to neurodegenerative disorders.97,119,120
In order to have a full understanding of differentially expressed miR-124a, miR-483-5p, miR-142-3p, and miR-125b and its exact correlation with DPP4 activity, BDNF, and SIRT-1 in the serum of MCI patients, bioinformatics analysis of their target genes are necessary. Moreover, DPP4 activity, BDNF, SIRT-1, and its related genes showed to be associated with apoptosis, oxidative stress, inflammatory, and neural differentiation.16,32,121–123 More molecular-based tests such as luciferase assays are commonly used as a reporter to assess the transcriptional activity in intact cells. The most common applications of these gene assays are to examine the regulation of transcriptional activities by promoters and transcription factors. These assays have also been adapted for testing the effect of miRNA-mediated, posttranscriptional regulation on target genes. For many human genes, this test is achieved by engineering a luciferase gene construct containing the predicted miRNA targeting sequence from the target gene (often located in the 3-UTR).17,18,124–128 Thus, in our study, the potential interaction between expressed miR-124a, miR-483-5p, miR-142-3p, and miR-125b and its exact correlation with DPP4 activity, BDNF, and SIRT-1 in the serum of MCI patients could be explained on the basis of miRNA targeting sequence from the target genes of DPP4 activity, BDNF, and SIRT-1 using luciferase assay; however, larger cohort sample size is required which could be evaluated in future studies.
Conclusion
Our results indicated that circulating miR-124a, miR-483-5p, miR-142-3p, and miR-125b were potential biomarkers for diagnosis of MCI and significantly associated with severe cognitive decline, oxidative stress, and apoptosis in patients with MCI. The detection of circulating miR-124a, miR-483-5p, miR-142-3p, and miR-125b might serve as a new non-invasive biomarker for MCI with high diagnostic performance. However,future experimental studies based upon bioinformatics analysis were required to confirm the diagnostic value of these circulating miRNAs, as well as their regulation mechanisms in the pathogenesis of MCI in aged patients.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research, King Saud University for funding through Vice Deanship of Scientific Research Chairs.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Jiang T, Yu JT, Tan L. Novel disease-modifying therapies for Alzheimer’s disease. J Alzheimers Dis. 2012;31:475–492. doi: 10.3233/JAD-2012-120640 [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. doi: 10.1111/joim.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59 [DOI] [PubMed] [Google Scholar]
- 4.Serrano CM, Dillon C, Leis A, Taragano FE, Allegri RF. Mild cognitive impairment: risk of dementia accord ing to subtypes. Actas Esp Psiquiatr. 2013;41:330–339. [PubMed] [Google Scholar]
- 5.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tervo S, Kivipelto M, Hanninen T, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17:196–203. doi: 10.1159/000076356 [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tighe SK, OishiK, Mori S, et al. Diffusion tensor imaging of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s dementia. J Neuropsychiatry Clin Neurosci. 2012;24:484–488. doi: 10.1176/appi.neuropsych.11120375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amlien IK, Fjell AM, Walhovd KB, et al. Mild cognitive impairment: cerebrospinal fluid tau biomarker pathologic levels and longitudinal changes in white matter integrity. Radiology. 2013;266:295–303. doi: 10.1148/radiol.12120319 [DOI] [PubMed] [Google Scholar]
- 11.Zheng T, Qin L, Chen B, et al. Association of plasma DPP4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: results from the GDMD study in China. Diabetes Care. 2016;39:1594–1601. doi: 10.2337/dc16-0316 [DOI] [PubMed] [Google Scholar]
- 12.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin XY, Cao C, Cawley NX, et al. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease: a meta-analysis study (N=7277). Mol Psychiatry. 2017;22:312–320. doi: 10.1038/mp.2016.62 [DOI] [PubMed] [Google Scholar]
- 14.Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50:431–438. doi: 10.1007/s00125-006-0537-4 [DOI] [PubMed] [Google Scholar]
- 15.Herskovits AZ, Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81:471–483. doi: 10.1016/j.neuron.2014.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 17.Nagata T, Shinagawa S, Nukariya K, Yamada H, Nakayama K. Association between BDNF polymorphism (Val66Met) and executive function in patients with amnestic mild cognitive impairment or mild Alzheimer disease. Dement Geriatr Cogn Disord. 2012;33(266–272):684. doi: 10.1159/000339358 [DOI] [PubMed] [Google Scholar]
- 18.Bonda DJ, Lee HG, Camins A, et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considera tions. Lancet Neurol. 2011;10:275–279. doi: 10.1016/S1474-4422(11)70013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol. 2013;12:125. doi: 10.1186/1475-2840-12-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x [DOI] [PubMed] [Google Scholar]
- 21.Zheng TP, Yang F, Gao Y, et al. Increased plasma DPP4 activities predict new-onset atherosclerosis in association with its proinflammatory effects in Chinese over a four year period: a prospective study. Atherosclerosis. 2014;235:619–624. doi: 10.1016/j.atherosclerosis.2014.05.956 [DOI] [PubMed] [Google Scholar]
- 22.Baierle M, Nascimento SN, Moro AM, et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxid Med Cell Longev. 2015;2015:804198. doi: 10.1155/2015/804198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaman AM, Uzoni A, Popa-Wagner A, Andrei A, Petcu EB. The role of oxidative stress in etiopathogenesis of chemotherapy induced cognitive Impairment (CICI)-”Chemobrain”. Aging Dis. 2016;7(3):307–317. doi: 10.14336/AD.2015.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermann DM, Doeppner TR, Popa-Wagner A. Opportunities and limitations of vascular risk factor models in studying plasticity-promoting and restorative ischemic stroke therapies. Neural Plast. 2019;Nov(2019):9785476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zagrean AM, Hermann DM, Opris I, Zagrean L, Popa-Wagner A. Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. Therapeutic Implications Front Neurosci. 2018;6(12):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AGriñan-Ferré C, Corpas R, Puigoriol-Illamola D, Palomera-Avalos V, Sanfeliu C, Pallàs M. Understanding epigenetics in the neurodegeneration of alzheimer’s disease: SAMP8 mouse model. J Alzheimers Dis. 2018;62:943–963. doi: 10.3233/JAD-170664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung HY, Kim DH, Lee EK, et al. Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis. 2019;10:367–382. doi: 10.14336/AD.2018.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen KS, Smith C. Ageing-associated oxidative stress and inflammation are alleviated by products from grapes. Oxidative Med Cell Longev. 2016;2016:1–12. doi: 10.1155/2016/6236309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escobar KA, Cole NH, Mermier CM, VanDusseldorp TA. Autophagy and aging: maintaining the proteome through exercise and caloric restriction. Aging Cell. 2019;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pourmemar E, Majdi A, Haramshahi M, Talebi M, Karimi P, Sadigh-Eteghad S. Intranasal cerebrolysin attenuates learning and memory impairments in D-galactose-induced senescence in mice. Exp Gerontol. 2017;87:16–22. doi: 10.1016/j.exger.2016.11.011 [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Feng L, Li J, et al. The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging-dependent cognitive deficits. Behav Brain Res. 2017;334:155–162. doi: 10.1016/j.bbr.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 32.Xie B, Zhou H, Zhang R, et al. Serum miR-206 and miR-132 as potential circulating biomarkers for mild cognitive impairment. J Alzheimers Dis. 2015;45(3):721–731. doi: 10.3233/JAD-142847 [DOI] [PubMed] [Google Scholar]
- 33.Muller M, Kuiperij HB, Versleijen AA, et al. Validation of microRNAs in cerebrospinal fluid as biomarkers for different forms of dementia in a multicenter study. J Alzheimers Dis. 2016;52:1321–1333. doi: 10.3233/JAD-160038 [DOI] [PubMed] [Google Scholar]
- 34.Najaraj S, Laskowska-Kaszub K, Dębski KJ, et al. Profile of 6 microRNA in blood plasma distinguish early stage Alzheimer’s disease patients from non-demented subjects. Oncotarget. 2017;8:16122–16143. doi: 10.18632/oncotarget.15109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez B, Peplow PV. MicroRNAs as diagnostic and therapeutic tools for Alzheimer’s disease: advances and limitations. Neural Regen Res. 2019;14(2):242–255. doi: 10.4103/1673-5374.244784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miya Shaik M, Tamargo IA, Abubakar MB, Kamal MA, Greig NH, Gan SH. The role of microRNAs in Alzheimer’s Disease and their therapeutic potentials. Genes (Basel). 2018;9(4):174. doi: 10.3390/genes9040174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melo CA, Melo SA. Biogenesis and physiology of microRNAs In: Fabbri M, editor. Non-Coding RNAs and Cancer. NewYork, NY, USA: Springer; 2014:5–24. [Google Scholar]
- 38.Deng S, Wang H, Jia C, et al. MicroRNA-146a induces lineage-negative bone marrow cell apoptosis and senescence by targeting polo-like kinase 2 expression. Arterioscler Thromb Vasc Biol. 2017;37:280–290. doi: 10.1161/atvbaha.116.308378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansari A, Maffioletti E, Milanesi E, et al. miR-146a and miR-181a are involved in the progression of mild cognitive impairment to Alzheimer’s disease. Neurobiol Aging. 2019;82:102–109. doi: 10.1016/j.neurobiolaging.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 40.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(599):1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sullivan K. Estimates of interrater reliability for the Logical Memory subtest of the Wechsler Memory Scale- Revised. J Clin Exp Neuropsychol. 1996;18:707–712. doi: 10.1080/01688639608408293 [DOI] [PubMed] [Google Scholar]
- 42.Liu DH, Zhang XQ, Chen B, et al. Neurophysiologic tests to distinguish MCI and mildAD. J Brain Nerv Dis. 2008;16:223–226. [Google Scholar]
- 43.Dai XY, Ryan JJ, Paolo AM, Harrington RG. Factor analysis of the mainland Chinese version of the Wechsler Adult Intelligence Scale (WAIS-RC) in a brain-damaged sample. Int J Neurosci. 1990;55:107–111. doi: 10.3109/00207459008985956 [DOI] [PubMed] [Google Scholar]
- 44.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. THE Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 45.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 46.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 47.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 48.Porteri C, Frisoni GB. Biomarker-based diagnosis of mild cognitive impairment due to Alzheimer’s disease: how and what to tell. A kickstart to an ethical discussion. Front Aging Neurosci. 2014;6:41. doi: 10.3389/fnagi.2014.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godfrey M. Majid DSA: renal handling of circulating nitrates in anesthetized dogs. Am J Physiol Renal Physiol. 1998;275(1):F68–F73. doi: 10.1152/ajprenal.1998.275.1.F68 [DOI] [PubMed] [Google Scholar]
- 50.Tarpey MM, Fridovich I. Methods of detection of vascular reactive species: nitric oxide, super oxide, hydrogen peroxide, and peroxy nitrate. Circ Res. 2001;89:236–244. doi: 10.1161/hh1501.094365 [DOI] [PubMed] [Google Scholar]
- 51.Sravani PV, Babu NK, Gopal KV, et al. Determination of oxidative stress in vitiligo by measuring superoxide dismutase and catalase levels in vitiliginous and non-vitiliginous skin. Indian J Dermatol Venereol Leprol. 2009;3:268–271. [DOI] [PubMed] [Google Scholar]
- 52.Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–152. doi: 10.1016/0009-8981(91)90067-M [DOI] [PubMed] [Google Scholar]
- 53.Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126. [DOI] [PubMed] [Google Scholar]
- 54.Gabr SA, Al-Ghadir AH. Role of cellular oxidative stress and cytochrome c in the pathogenesis of psoriasis. Arch Dermatol Res. 2012;304(6):451–457. doi: 10.1007/s00403-012-1230-8 [DOI] [PubMed] [Google Scholar]
- 55.Gabr SA, Alghadir AH. Prediction of fibrosis in hepatitis C patients: assessment using hydroxyproline and oxidative stress biomarkers. Virusdisease. 2014;25(1):91–100. doi: 10.1007/s13337-013-0182-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng T, Gao Y, Baskota A, Chen T, Ran X, Tian H. Increased plasma DPP4 activity is predictive of prediabetes and type 2 diabetes onset in Chinese over a four-year period: result from the China National Diabetes and metabolic disorders study. J Clin Endocrinol Metab. 2014;99:E2330–334. doi: 10.1210/jc.2014-1480 [DOI] [PubMed] [Google Scholar]
- 57.Zheng T, Chen T, Liu Y, Gao Y, Tian H. Increased plasma DPP4 activity predicts new-onset hypertension in Chinese over a 4-year period: possible associations with inflammation and oxidative stress. J Hum Hypertens. 2015;29:424–429. doi: 10.1038/jhh.2014.111 [DOI] [PubMed] [Google Scholar]
- 58.Al-Rawaf HA, Alghadir AH, Gabr SA. MicroRNAs as biomarkers of pain intensity in patients with chronic fatigue syndrome. Pain Pract. 2019;19(8):848–860. doi: 10.1111/papr.12817 [DOI] [PubMed] [Google Scholar]
- 59.Safari F, Hosseini H, Bayat M, Ranjbar A. Synthesis and evaluation of antimicrobial activity, cytotoxic and proapoptotic effects of novel spiro-4H-pyran derivatives. RSC Adv. 2019;9(43):24843–24851. doi: 10.1039/C9RA03196K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bermejo P, Martin-Arag´on S, Benedi J, et al. Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from mild cognitive impairment. FreeRadic Res. 2008;42:162–170. [DOI] [PubMed] [Google Scholar]
- 61.Markesbery WR, Lovell MA. DNA oxidation in Alzheimer's disease. Antioxidants Redox Signal. 2006;8:2039–2045. doi: 10.1089/ars.2006.8.2039 [DOI] [PubMed] [Google Scholar]
- 62.Nunomura A, Honda K, Takeda A, Hirai K, Zhu X, SmithMA PG. Oxidative damage to RNA in neurodegenerative diseases. J Biomed Biotechnol. 2006;(2006):1–6. doi: 10.1155/JBB/2006/82323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Delibas N, Ozcankaya R, Altuntas I. Clinical importance of erythrocyte malondialdehyde levels as a marker for cognitive deterioration in patients with dementia of Alzheimer type: a repeated study in 5-year interval. Clin Biochem. 2002;32:137–141. doi: 10.1016/S0009-9120(02)00287-4 [DOI] [PubMed] [Google Scholar]
- 64.Keller JN, Schmitt FA, Scheff SW, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA [DOI] [PubMed] [Google Scholar]
- 65.Popa-Wagner A, Mitran S, Sivanesan S, Chang E, AM Buga. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev. 2013;2013:963520. doi: 10.1155/2013/963520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cervellati C, Cremonini E, Bosi C, et al. Systemic oxidative stress in older patients with mild cognitive impairment or late onset Alzheimer’s disease. Curr Alzheimer Res. 2013;10(4):365–372. doi: 10.2174/1567205011310040003 [DOI] [PubMed] [Google Scholar]
- 68.Wei G, Dawson VL, Zweier JL. Role of neuronal and endothelial nitric oxide synthase in nitric oxide generation in the brain following cerebral ischemia. Biochimica et Biophysica Acta. 1999;1455:23–24. doi: 10.1016/S0925-4439(99)00051-4 [DOI] [PubMed] [Google Scholar]
- 69.Milstien S, Sakai N, Brew BJ, et al. Cerebrospinal fluid nitrite/nitrate levels in neurological diseases. J Neuro Chem. 1994;63(3):1178–1180. doi: 10.1046/j.1471-4159.1994.63031178.x [DOI] [PubMed] [Google Scholar]
- 70.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(suppl1):S193–201. doi: 10.1038/sj.bjp.0706458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-X [DOI] [PubMed] [Google Scholar]
- 72.Moro MA, Darley-Usmar VM, Goodwin DA, et al. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci USA. 1994;91:6702–6706. doi: 10.1073/pnas.91.14.6702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogers J, Luber-Narod J, Styren S, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9(4):339–349. doi: 10.1016/S0197-4580(88)80079-4 [DOI] [PubMed] [Google Scholar]
- 74.Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer’s disease. Arch Pharm Res. 2010;33:1539–1556. [DOI] [PubMed] [Google Scholar]
- 75.Marcus DL, Thomas C, Rodriguez C, et al. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol. 1998;150:40–44. doi: 10.1006/exnr.1997.6750 [DOI] [PubMed] [Google Scholar]
- 76.Omar RA, Chyan YJ, Andorn AC, Poeggeler B, Robakis NK, Pappolla MA. Increased expression but reduced activity of antioxidant enzymes in Alzheimer’s disease. J Alzheimers Dis. 1999;1:139–145. doi: 10.3233/JAD-1999-1301 [DOI] [PubMed] [Google Scholar]
- 77.Ortiz GG, Pacheco Mois´es FP, Mireles-Ram´ırez M, et al. Oxidative stress: love and hate history in central nervous system. Adv Protein Chem Struct Biol. 2017;108:1–31. [DOI] [PubMed] [Google Scholar]
- 78.Revel F, Gilbert T, Roche S, et al. Influence of oxidative stress biomarkers on cognitive decline. J Alzheimers Dis. 2015;45:553–560. doi: 10.3233/JAD-141797 [DOI] [PubMed] [Google Scholar]
- 79.Irizarry MC, Raman R, Schwarzschild MA, et al. Plasma urate and progression of mild cognitive impairment. Neurodegener Dis. 2009;6:23–28. doi: 10.1159/000170883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aquilano K, Baldelli S, Ciriolo MR. Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol. 2014;5:196. doi: 10.3389/fphar.2014.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nicotera P. Caspase requirement for neuronal apoptosis and neurodegeneration. IUBMB Life. 2000;49:421–425. doi: 10.1080/152165400410272 [DOI] [PubMed] [Google Scholar]
- 82.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476 [DOI] [PubMed] [Google Scholar]
- 83.Shah AJ, Epport K, Azen, et al. Progressive declines in neurocognitive function among survivors of hematopoietic stem cell transplantation for pediatric hematologic malignancies. J Ped Hematol Oncol. 2008;30:411–418. doi: 10.1097/MPH.0b013e318168e750 [DOI] [PubMed] [Google Scholar]
- 84.D’Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. Trends Neurosci. 2012;35:700–709. doi: 10.1016/j.tins.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 85.Robertson GS, Crocker SJ, Nicholson DW, Schulz JB. Neuroprotection by the inhibition of apoptosis. Brain Pathol. 2000;10:283–292. doi: 10.1111/j.1750-3639.2000.tb00262.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028 [DOI] [PubMed] [Google Scholar]
- 87.Liang HW, Qiu SF, Shen J, et al. Genistein attenuates oxidative stress and neuronal damage following transient global cerebral ischemia in rat hippocampus. Neurosci Lett. 2008;438:116–120. doi: 10.1016/j.neulet.2008.04.058 [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez-Martinez E, Martinez F, Espinosa-Garcia MT, Maldonado P, Rivas-Arancibia S. Mitochondrial dysfunction in the hippocampus of rats caused by chronic oxidative stress. Neuroscience. 2013;252:384–395. doi: 10.1016/j.neuroscience.2013.08.018 [DOI] [PubMed] [Google Scholar]
- 89.Aisen PS. Serumbrain-derived neurotrophic factor and the risk for dementia. JAMA. 2014;311:1684–1685. doi: 10.1001/jama.2014.3120 [DOI] [PubMed] [Google Scholar]
- 90.Gezen-Ak D, Dursun E, Hanagasi H, et al. BDNF, TNFalpha, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’sdisease or mild cognitive impairment. J Alzheimers Dis. 2013;37:185–195. doi: 10.3233/JAD-130497 [DOI] [PubMed] [Google Scholar]
- 91.Laske C, Stellos K, Hoffmann N, et al. Higher BDNF serum levels predict slower cognitive decline in Alzheimer’s disease patients. Int J Neuropsychopharmacol. 2011;14:399–404. doi: 10.1017/S1461145710001008 [DOI] [PubMed] [Google Scholar]
- 92.Faria MC, Gonc¸alves GS, Rocha NP, et al. Increased plasma levels of BDNF and inflammatory markers in Alzheimer’sdisease. J Psychiatr Res. 2014;53:166–172. doi: 10.1016/j.jpsychires.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 93.Jeong H, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2011;18:159–165. doi: 10.1038/nm.2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang C, et al. Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through PI3K/AKT/mTOR and AMPK/SIRT1/FOXO3pathways. Sci Rep. 2017;7:41082. doi: 10.1038/srep41082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen B, Zheng T, Qin L, et al. Strong association between plasma dipeptidyl peptidase-4 activity and impaired cognitive function in elderly population with normal glucose tolerance. Front Aging Neurosci. 2017;9:247. doi: 10.3389/fnagi.2017.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zheng T, Baskota A, Gao Y, Chen T, Tian H, Yang F. Increased plasma DPP4 activities predict new-onset hyperglycemia in Chinese over a four year period: possible associations with inflammation. Metabolism. 2015;64:498–505. doi: 10.1016/j.metabol.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 97.Zheng T, Liu H, Qin L, et al. Oxidative stress-mediated influence of plasma DPP4 activity to BDNF ratio on mild cognitive impairment in elderly type 2 diabetic patients: results from the GDMD study in China. Metabolism. 2018b;87:105–112. doi: 10.1016/j.metabol.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 98.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246–255. doi: 10.1016/S2213-8587(13)70088-3 [DOI] [PubMed] [Google Scholar]
- 99.Swomley AM, Butterfield DA. Oxidative stress in Alzheimer disease and mild cognitive impairment: evidence from human data provided by redox proteomics. Arch Toxicol. 2015;89:1669–1680. doi: 10.1007/s00204-015-1556-z [DOI] [PubMed] [Google Scholar]
- 100.Zheng T, Qin L, Chen B, et al. Association of plasma dpp4 activity with mild cognitive impairment in elderly patients with type 2 diabetes: results from the GDMD study in China. Diabetes Care. 2016b;39:1594–1601. [DOI] [PubMed] [Google Scholar]
- 101.Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35(5):325–334. doi: 10.1016/j.tins.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saba R, Schratt GM. MicroRNAs in neuronal development, function and dysfunction. Brain Res. 2010;1338:3–13. doi: 10.1016/j.brainres.2010.03.107 [DOI] [PubMed] [Google Scholar]
- 103.Konovalova J, Gerasymchuk D, Parkkinen I, Chmielarz P, Domanskyi A. Interplay between MicroRNAs and oxidative stress in neurodegenerative diseases. Int J Mol Sci. 2019;20(23):6055. doi: 10.3390/ijms20236055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843 [DOI] [PubMed] [Google Scholar]
- 105.Schmiedel JM, Klemm SL, Zheng Y, et al. Gene expression. MicroRNA Control of Protein Expression Noise Science. 2015;348:128–132. [DOI] [PubMed] [Google Scholar]
- 106.Denk J, Boelmans K, Siegismund C, Lassner D, Arlt S, Jahn H. MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s disease. PLoS One. 2015;10:e0126423. doi: 10.1371/journal.pone.0126423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kayano M, Higaki S, Satoh JI, et al. Plasma microRNA biomarker detection for mild cognitive impairment using differential correlation analysis. Biomark Res. 2016;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lusardi TA, Phillips JI, Wiedrick JT, et al. MicroRNAs in human cerebrospinal fluid as biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2017;55:1223–1233. doi: 10.3233/JAD-160835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jia LH, Liu YN. Downregulated serum miR-223 serves as biomarker in Alzheimer’s disease. Cell Biochem Funct. 2016;34:233–237. doi: 10.1002/cbf.3184 [DOI] [PubMed] [Google Scholar]
- 110.Hong H, Li Y, Su B. Identification of circulating miR-125b as a potential biomarker of Alzheimer’s Disease in APP/PS1 transgenic mouse. J Alzheimers Dis. 2017;59(4):1449–1458. doi: 10.3233/JAD-170156 [DOI] [PubMed] [Google Scholar]
- 111.Tan L, Yu JT, Liu QY, et al. Circulating miR-125b as a biomarker of Alzheimer’s disease. J Neurol Sci. 2014;336(1–2):52–56. doi: 10.1016/j.jns.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 112.Guan B, Li Q, Shen L, et al. and Li XH: microRNA-205 directly targets Krüppel-like factor 12 and is involved in invasion and apoptosis in basal-like breast carcinoma. Int J Oncol. 2016;49:720–734. doi: 10.3892/ijo.2016.3573 [DOI] [PubMed] [Google Scholar]
- 113.Jiang M, Xiang Y, Wang D, et al. Dysregulated expression of miR-146a contributes to age-related dysfunction of macrophages. Aging Cell. 2012;11:29–40. doi: 10.1111/j.1474-9726.2011.00757.x [DOI] [PubMed] [Google Scholar]
- 114.Keller A, Backes C, Haas J, et al. disease micro RNAs using next-generation sequencing. Alzheimers Dement. 2016;12:565–576. doi: 10.1016/j.jalz.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 115.Wu Y, Xu J, Xu J, et al. Lower serum levels of miR-29c-3p and miR-19b-3p as biomarkers for Alzheimer’s disease. Tohoku J Exp Med. 2017;242:129–136. doi: 10.1620/tjem.242.129 [DOI] [PubMed] [Google Scholar]
- 116.Perluigi M, Barone E, Di Domenico F, Butterfield DA. Aberrant protein phosphorylation in Alzheimer disease brain disturbs pro-survival and cell death pathways. Biochim Biophys Acta. 2016;1862:1871–1882. doi: 10.1016/j.bbadis.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 117.Le MT, Teh C, Shyh-Chang N, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862–876. doi: 10.1101/gad.1767609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147 [DOI] [PubMed] [Google Scholar]
- 119.Ma X, Liu L, Meng J. MicroRNA-125b promotes neurons cell apoptosis and Tau phosphorylation in Alzheimer’s disease. Neurosci Lett. 2017;661:57–62. doi: 10.1016/j.neulet.2017.09.043 [DOI] [PubMed] [Google Scholar]
- 120.Krek A, Grun D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536 [DOI] [PubMed] [Google Scholar]
- 121.Prozorovski T, Schulze-Topphoff U, Glumm R, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10:385–394. doi: 10.1038/ncb1700 [DOI] [PubMed] [Google Scholar]
- 122.Im H-I, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends Neurosci. 2012;35:325–334. doi: 10.1016/j.tins.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strum JC, Johnson JH, Ward J, et al. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol Endocrinol. 2009;23:1876–1884. doi: 10.1210/me.2009-0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee ST, Chu K, Jung KH, et al. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann Neurol. 2012;72:269–277. doi: 10.1002/ana.23588 [DOI] [PubMed] [Google Scholar]
- 125.Jin Y, Chen Z, Liu X, Zhou X. Evaluating the microRNA targeting sites by luciferase reporter gene assay. Methods Mol Biol. 2013;936:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nicolas FE. Experimental validation of microRNA targets using a luciferase reporter system. Methods Mol Biol. 2011;732:139–152. [DOI] [PubMed] [Google Scholar]
- 127.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dai Y, Zhou X. Computational methods for the identification of microRNA targets. Open AccessBioinform. 2010;2:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]