Abstract
Purpose:
Due to the widespread use of computerized tomography, the diagnosis of small renal cancers (3 cm or less) within the T1a classification continues to increase. Current treatment of these tumors includes radical nephrectomy, partial nephrectomy and thermal ablation. We used the SEER (Surveillance, Epidemiology, and End Results) Program to compare treatment modalities for these cancers based on 1 cm increments in tumor size. We examined overall survival, cancer specific survival, survival from cardiovascular disease and race based treatment disparities.
Materials and Methods:
In the SEER database we identified 17,716 renal cancers 3 cm or less diagnosed from 2005 to 2010 treated with radical nephrectomy, partial nephrectomy or thermal ablation. Overall survival, cancer specific survival and cardiovascular survival were determined for each treatment group, and then substratified by size in centimeters, tumor grade, age, geographical location and ethnicity. Survival was analyzed using Kaplan-Meier methods, multivariate proportional hazards models and a propensity score weighted approach.
Results:
Overall survival, cancer specific survival and cardiovascular survival were better for partial nephrectomy than radical nephrectomy in all circumstances. Thermal ablation showed equivalent overall survival to partial nephrectomy for tumors 2 cm or less. Notably, radical nephrectomy for renal tumors 3 cm or less was applied in a disparately larger number of black patients (OR 1.63, 95% CI 1.47–1.81) and Hispanic patients (OR 1.28, 95% CI 1.14–1.44).
Conclusions:
Radical nephrectomy should be avoided for all tumors 3 cm or less. For renal cancers 2 cm or less partial nephrectomy and thermal ablation are equally effective. For tumors 2.1 to 3 cm partial nephrectomy is better than thermal ablation. We identified significant racial treatment disparities that negatively impact survival in black and Hispanic patients.
Keywords: kidney neoplasms, ablation techniques, nephrectomy
Editor’s Note:
This article is the first of 5 published in this issue for which category 1 CME credits can be earned. Instructions for obtaining credits are given with the questions on pages 1326 and 1327.
Historically, radical nephrectomy was the standard of care for all renal masses, with partial nephrectomy reserved for extenuating circumstances such as solitary kidney or the presence of bilateral tumors. With time, it became clear that PN was associated with a lower incidence of adverse renal outcomes1,2 while maintaining equivalent oncologic outcomes for T1a renal cancers in general (ie 4 cm or less).3 This became of even greater importance in light of recent medical data showing that chronic kidney disease in the general population leads to a decrease in cardiovascular survival.4 Accordingly, nephron sparing treatment with PN or thermal ablation (cryoablation or radio frequency ablation) has become more widely used during the last decade.5 Indeed, PN has become the recommended standard of care for clinical T1a renal cancers. However, RN remains a recognized alternative standard of care if PN, in the opinion of the treating urologist, is not technically feasible. In contrast, while less invasive than PN, thermal ablation, due to its lower effectiveness, is generally limited to T1a renal cancers in patients with multiple comorbidities who would be at higher risk for treatment with PN.6
Despite the increasing acceptance of PN as the standard therapy for small renal cancers, RN continues to be commonly used.5 This may be due to several factors. PN is more technically challenging and has a higher complication rate than RN. TA is a relatively new technique and often requires a joint effort between urologists and interventional radiologists. Also, there is controversy as to whether PN confers a better overall survival than RN for T1a renal cancers. Indeed, there is 1 randomized trial of renal cancers 5 cm or less (ie T1a and small T1b cancers) that showed better OS with RN than PN with no difference in CSS or progression.7 Of note, this trial was criticized for being underpowered and for having a high PN to RN crossover. In contrast, there have been multiple population based studies that have claimed superiority of PN over RN.8–10 A recent comprehensive meta-analysis has also corroborated better cancer outcomes with PN.11 While these studies have focused on varying aspects of survival for RN vs PN, to our knowledge no study has examined all 3 treatment modalities and all 3 outcome measures (ie OS, CSS and CVS) in a patient cohort further subdivided by tumor size in 1 cm increments up to 3 cm, as well as tumor grade, age, geographic location and patient ethnicity.
Of importance, earlier studies using the SEER database focused on 1991 data, when PN was predominantly used in patients with extenuating circumstances (ie solitary kidney, compromised renal function etc) and TA was too new to be included in any SEER based studies. Furthermore, in these studies all T1a renal cancers were lumped together over all age groups and ethnicities.
We hypothesize that the ideal treatment for T1a renal cancers 3 cm or less varies based on centimeter increments in tumor size (ie less than 1 cm, 1.1 to 2 cm, 2.1 to 3 cm). Furthermore, we are concerned that given that PN and TA are relatively recent and resource intensive procedures, a disproportionate number of disadvantaged or indigent patients may not be provided with these alternatives to RN. In this study we examine the most recent data from the SEER database to compare the treatment of small renal cancers (3 cm or less) with RN, PN and TA stratified by age, ethnicity, geography, tumor grade and tumor size between 2005 and 2010.
METHODS
Data Source and Study Population
The SEER Program of the National Cancer Institute contains approximately 97% of all incident cancer cases from cancer registries and these registries cover approximately 28% of the U.S. population. The SEER Program registries collect data on demographics, primary tumor site, tumor morphology and stage, first course of treatment, followup for vital status and cause of death. Of note, this study only deals with small renal cancer and not small renal masses, and the SEER database only includes histologically proven renal cancers. Our analysis data include cases diagnosed from 2005 to 2010 in 18 SEER registries.
Consecutive adult patients (age at diagnosis 18 years old or older) diagnosed with SRC between January 1, 2005 and December 31, 2010 were identified using the ICD-O-3 site code C649. Cases from autopsy or death certificate only were excluded from analysis. Patients who did not receive any surgical therapy were also excluded. All renal cancer pathological types were included in the analysis. The final analytic data set included 17,716 patients with small renal cancer who were treated with PN, RN or TA.
Variables and Statistical Analysis
Treatment was identified for each patient using the surgery for primary site variable provided in the SEER data, including partial nephrectomy, radical nephrectomy and thermal ablation. Other covariates included patient demographic and tumor characteristics. Patient race/ethnicity was categorized into the 5 groups of white, black, Hispanic, Asian/Pacific Islander or other/unknown. Age at diagnosis was used as a continuous variable or categorical variable with the 4 groups of less than 50 years, 50 to 59, 60 to 69 and 70 or greater. Registry region included the 4 categories of Central (Detroit, Iowa, Utah, Kentucky and Louisiana), Eastern (Connecticut, Georgia and New Jersey), Western (Hawaii, New Mexico, Seattle and Alaska) and California. Tumor characteristics included tumor grade (grade I-II, III-IV, unknown), size (1.0 cm or less, 1.1 to 2.0 cm, 2.1 to 3.0 cm), histology (clear cell or others) and tumor stage (localized, regional, distant or unknown).
Frequency distributions of patient demographic and clinical characteristics were analyzed using the chi-square or Fisher exact test for categorical variables in bivariate analysis. The time trend of treatments during the study period was presented. The survival probability of the general U.S. population was estimated from U.S. life table (2010) using the gender and race mix of the study population. The overall survival of the age matched general population was compared to the study cohort and to its specific treatment. After examining the proportional odds assumption and model fit, a multinomial logistic regression model was chosen to perform multivariate analysis for treatment outcome, which has 3 surgery categories. To examine effects of racial disparity on surgery choice the odds of treatment with RN or TA were compared to PN for races after controlling for other demographic and tumor characteristics.
After verifying the proportionality assumption, the Cox proportional hazards model was fitted to evaluate the effects of each predictor on survival. Possible interaction terms of main effects were also tested by comparing a reduced model to the full model. Patient followup was defined as the time between diagnosis date and death from any cause/kidney cancer/cardiovascular disease or last followup date. Multivariate Cox models were also used on subsets of age at diagnosis, tumor size and grade categories. Furthermore, survival in treatment groups was compared using the propensity score approach with inverse probability of treatment weights.12,13 The multinomial logistic regression model was chosen for discrete choice modeling to predict treatment category. Predictors in the multinomial logistic regression model included all demographic and clinical characteristic covariates. Patients were weighted by the inverse probability of receiving the treatment that they actually received. The weights were then used in the Cox model along with other predictors.
Overall, kidney cancer specific and cardiovascular specific survival in treatment groups was estimated using Kaplan-Meier methods and compared using the log-rank test in survival graphs. All statistical tests were 2-sided and p <0.05 was considered significant. All analyses were performed on SAS® 9.2.
RESULTS
Between 2005 and 2010, 20,065 patients were diagnosed with SRC. Of these patients 17,716 had treatment with PN, RN or TA. The table shows the baseline characteristics of the study population. From 2005 to 2010 the proportion of patients undergoing PN and TA increased while the proportion of patients undergoing RN decreased from 51.1% to 30.5% (fig. 1). The overall survival of the U.S. population compared to those undergoing treatment for SRC is shown in figure 2.
Figure 1.
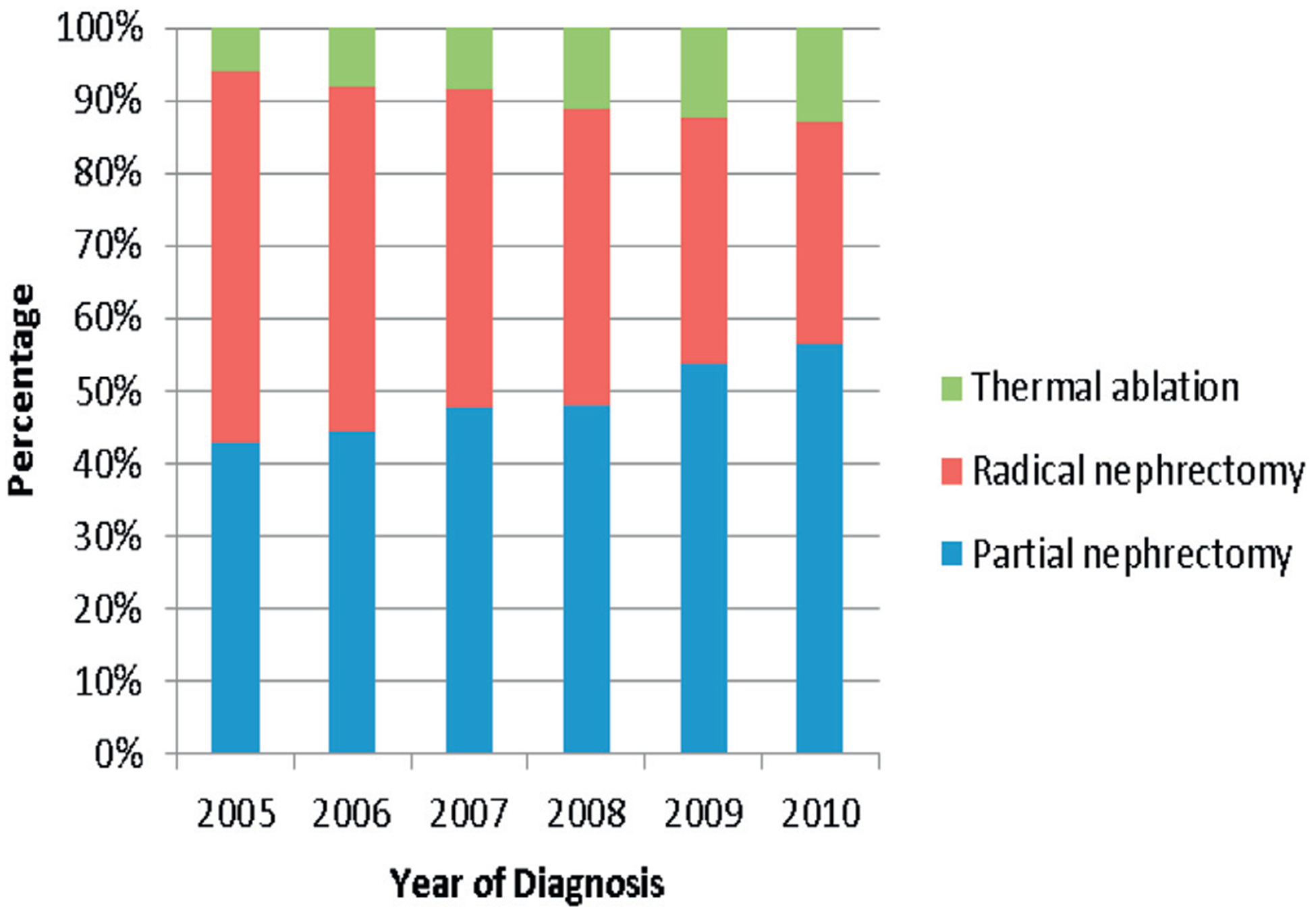
Percentage of each treatment group during study period, 2005 to 2010.
Figure 2.
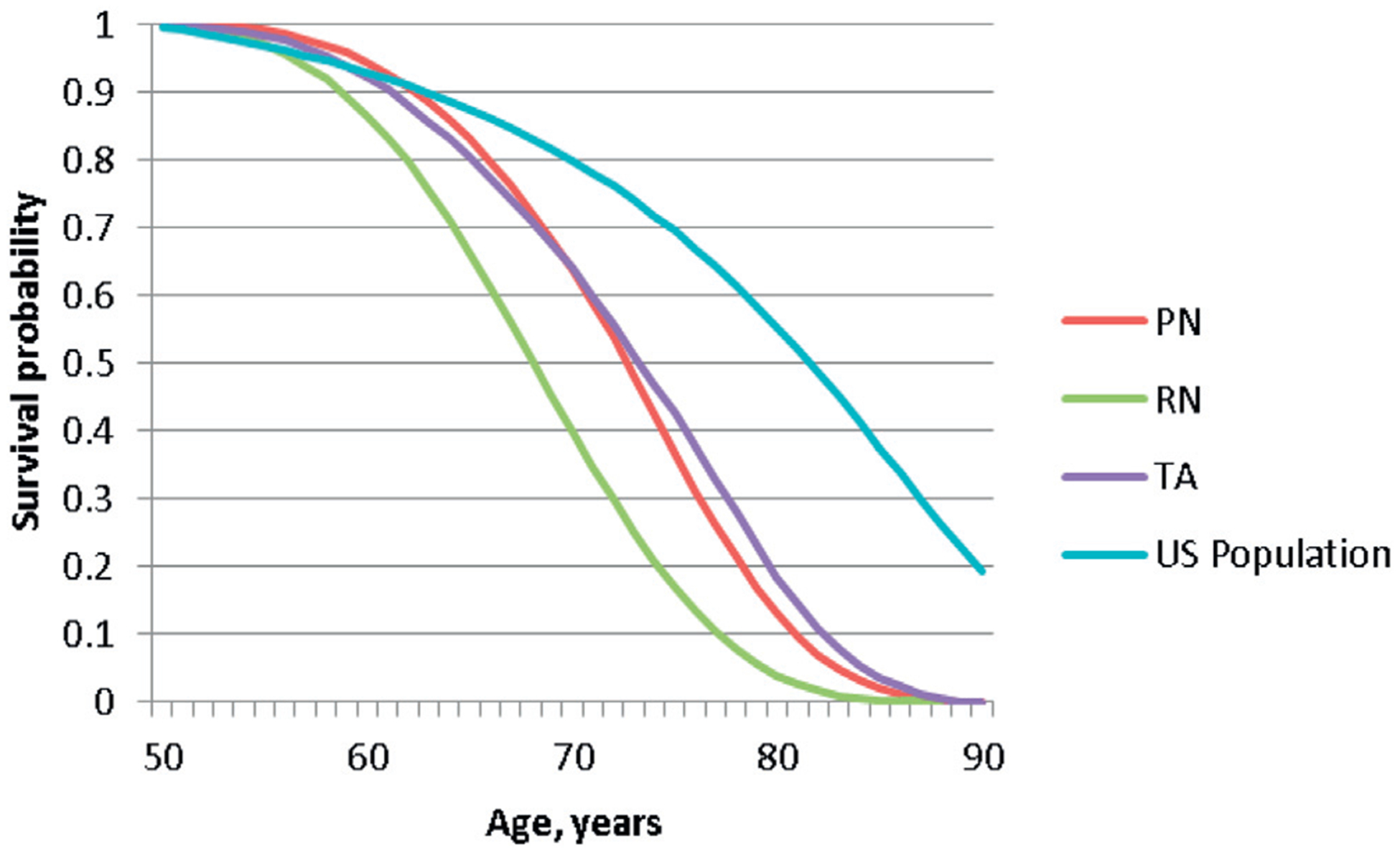
OS for each treatment group compared to U.S. population, by age.
The odds of undergoing RN vs PN were analyzed in a multivariate multinomial logistic model. Black patients had a significantly higher chance of undergoing RN over PN compared to white patients (OR 1.63, 95% CI 1.47–1.81). This difference, albeit smaller, was also noted for Hispanic patients (OR 1.28, 95% CI 1.14–1.44). Patients who were Asian/Pacific Islander and those classified as other race had an equal chance of undergoing PN vs RN compared to white patients.
The Cox model and the propensity score approach showed similar results for survival analysis. Overall survival for RN was significantly worse than for PN (HR 2.00, 95% CI 1.79–2.23, p <0.0001 in Cox model) and TA (HR 1.56, 95% CI 1.31–1.85, p <0.0001). For cancer specific survival, RN was worse than PN (HR 2.24, 95% CI 1.73–2.92, p <0.0001) but equal to TA (Cox model p=0.053, propensity score approach p=0.76). Cardiovascular survival was significantly poorer for RN than PN (HR 2.10, 95% CI 1.62–2.72, p <0.0001) and TA (HR 1.63, 95% CI 1.10–2.43, p=0.0158).
A multivariate Cox proportional hazard model for the subset analyses showed that OS for patients who had PN was significantly better than RN for all age groups, all tumor size categories and all Fuhrman grades (all p <0.05). Overall survival for TA was equal to that for PN in patients younger than 50 years (p=0.543) and those age 60 to 69 (p=0.077). Patients treated with PN had a better overall survival than those treated with TA for age 50 to 59 (HR 2.95, 95% CI 1.84–4.74, p <0.0001) and older than 70 years (HR 1.52, 95% CI 1.21–1.91, p=0.0004). Overall survival for TA and PN was the same for tumors 2 cm or less but it was significantly less for TA in tumors 2 to 3 cm (HR 1.67, 95% CI 1.34–2.07, p <0.0001, fig. 3). For grade I to II tumors OS was better for partial nephrectomy than thermal ablation (HR 1.57, 95% CI 1.24–2.01, p=0.0002), but it was equal for grade III to IV tumors (p=0.57). Overall, cancer specific and cardio vascular specific survival graphs are shown in figure 4.
Figure 3.
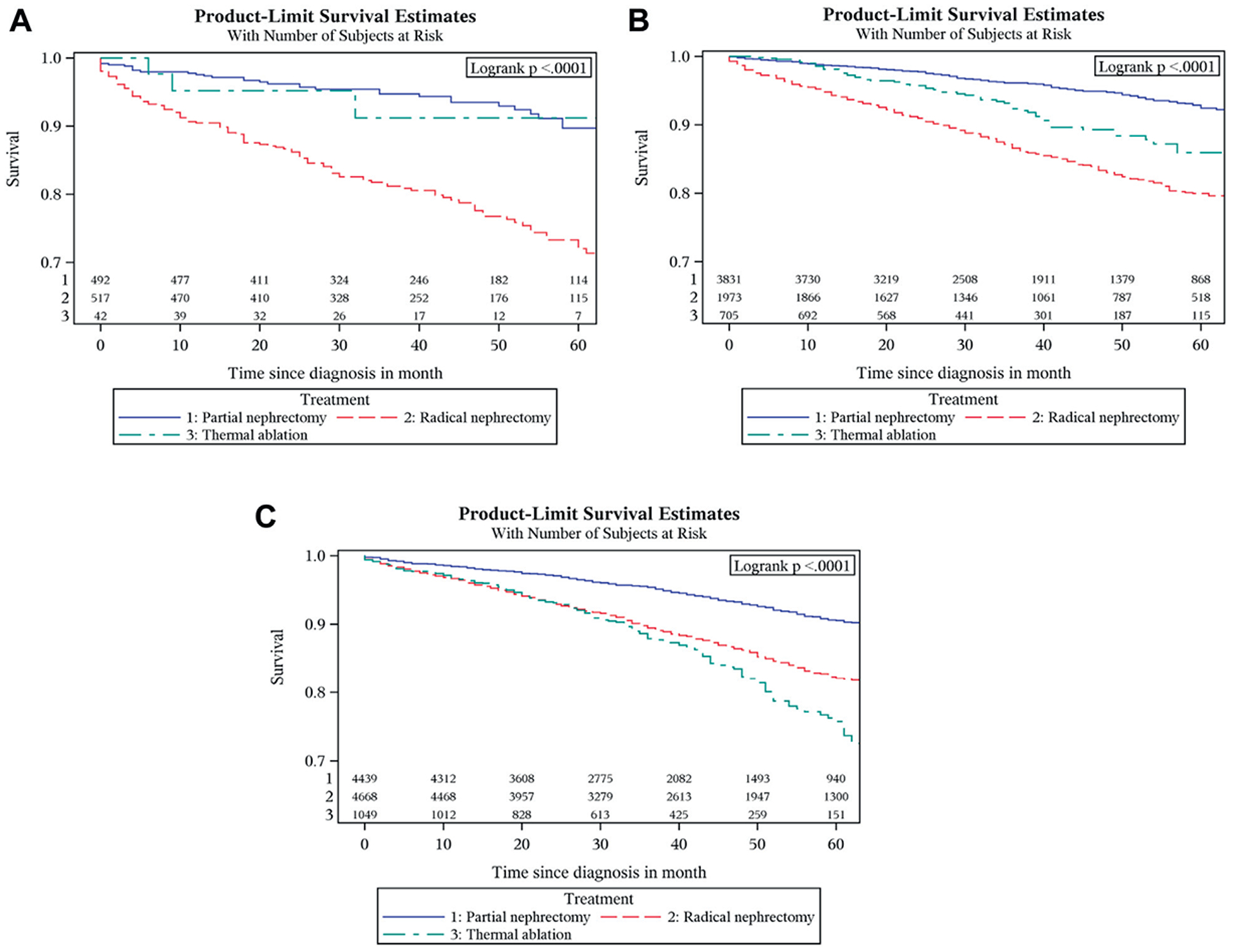
OS graphs by treatment for tumors 1 cm or less (A), 1.1 to 2 cm (B) and 2.1 to 3 cm (C)
Figure 4.
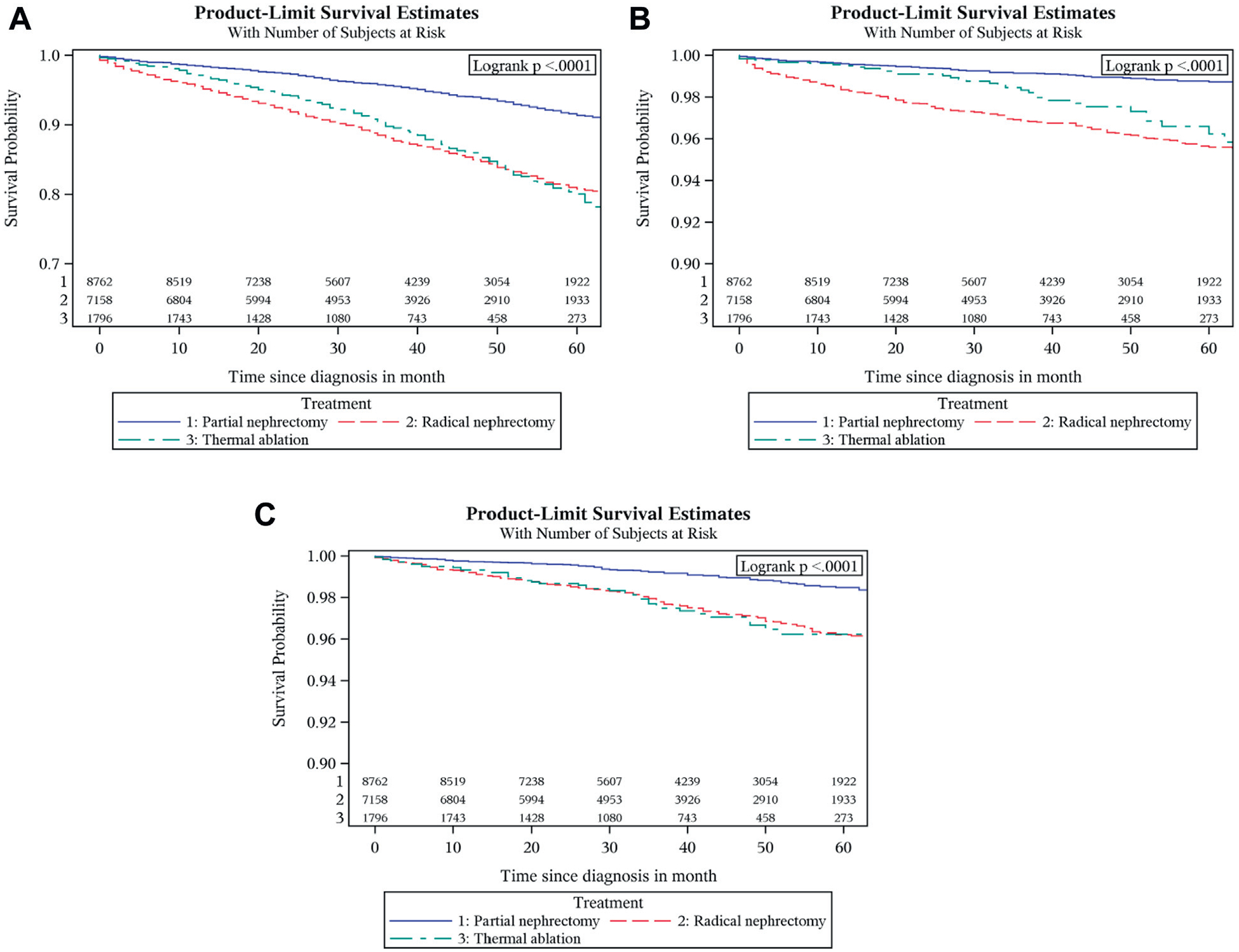
OS (A), CSS (B) and CVS (C) by treatment type
DISCUSSION
To our knowledge this is the first review of the SEER database with regard to 1) the use of TA as a treatment modality for T1a renal cancers 3 cm or less, 2) the subclassification of treatment modalities for T1a 3 cm or less disease based on 1 cm increments in tumor size, and 3) treatment disparities based on ethnicity. While PN and TA are becoming more commonly used in the treatment of T1a renal cancers 3 cm or less, nonetheless, in 2010, 30% of these small tumors were still treated with RN. As noted in earlier studies, our data demonstrate improved overall survival for PN over RN. Indeed, for all age groups, all tumor sizes and all grades of tumor, PN was superior to RN. In addition, TA resulted in survival similar to that of PN for patients with T1a tumors 2 cm or less. Furthermore, radical nephrectomy also resulted in an inferior CSS compared to partial nephrectomy, and was associated with a poorer CVS than partial nephrectomy.
Especially concerning given the improved outcomes for all T1a renal cancers 3 cm or less with PN vs RN is the racial disparity in treatment rendered. Black patients were 63% more likely and Hispanic patients 28% more likely to have RN than white patients for these small tumors. Of note, this disparity was not regional. It is unclear from our data if this difference reflects an issue of access to care, as SEER does not provide data on providers/place of therapy (ie primary, secondary or tertiary hospital). Also, the SEER database subdivides the population based on region (ie Central, Eastern, Western or California). However, a more detailed state by state analysis is not available.
Our results show promise for the use of thermal ablation for T1a renal cancers 2 cm or less. TA has traditionally been reserved for older, sicker patients in light of studies showing that TA results in a higher rate of local recurrence than PN.14,15 However, refinements in the technology and delivery of TA have led to improvements such that more recent studies have shown TA to have rates of local progression similar to PN for these small renal cancers.16 Despite this disparity between earlier and more current TA studies, the old and the more recent studies reveal that TA (specifically cryoablation) is equivalent to PN when one assesses for metastasis-free survival. In contrast, our data fall somewhere in between, in that they show equal survival for TA and PN only in patients with renal cancer 2 cm or less, but decreased survival for TA when dealing with tumors 2.1 to 3 cm. It is unclear at this time whether the differences seen reflect the expected higher incidence of preexisting comorbidities for the TA population vs that for the PN population, or problems with the treatment itself when approaching larger lesions.
Of interest, in the current SEER database the patients with renal cancer, despite the small tumor size, had poorer survival than their contemporaries without renal cancer regardless of tumor size, grade or treatment. Reasons for this disparity have yet to be elucidated. However, in this regard, an earlier study demonstrated that there may be an inherent selection bias in the SEER data, where patients treated with PN showed better survival than bladder cancer controls as well as noncancer controls.17 However, this study used data from 1992 to 2007, which represents a different era of treatment for SRC, with PN comprising only 25% of the total patients in the sample and TA not being recorded. To avoid this we focused on the more recent data from 2005 to 2010.
There are limitations to our data. Most importantly, the SEER database does not contain comorbidity data and obtaining these data is further hampered by the fact that the SEER database is not linked to Medicare claims. We attempted to account for this deficiency to some extent by using propensity score matching, with age as a reflection of preexisting comorbidity, and tumor size and grade as markers for cancer severity. In addition, the SEER database fails to differentiate between radio frequency ablation and cryoablation, nor does it distinguish between percutaneous vs laparoscopic TA, thereby further confounding a clear picture of the impact of TA.
One area of interest that we were not able to evaluate with this study was the role of active surveillance. Multiple series have shown the safety of active surveillance for clinical T1a renal masses for patients who are poor surgical candidates.18,19 We are in favor of this approach or one in which renal biopsy is performed before offering treatment in order to eliminate the 20% of patients with a small renal mass that is indeed benign.
CONCLUSIONS
Current American Urological Association guidelines state that radical nephrectomy is an alternate standard of care to PN for T1a renal cancer.6 Our data demonstrate that, to the contrary, radical nephrectomy should not be a recommended option given its poorer outcomes for overall survival, cardiovascular survival and cancer specific survival. Partial nephrectomy and thermal ablation are equally effective for tumors 2 cm or less, and partial nephrectomy alone is best for tumors 2 to 3 cm. Finally, we identified a significant racial disparity with regard to the increased use of radical nephrectomy in the black and Hispanic populations.
Baseline characteristics of the study population
| No. Overall (%) | No. No Surgery (%) | No. PN (%) | No. RN (%) | No. Needle Ablative (%) | |
|---|---|---|---|---|---|
| Totals | 20,065 (100) | 2,349 (11.7) | 8,762 (43.7) | 7,158 (35.7) | 1,796 (9.0) |
| Age at diagnosis: | |||||
| Less than 50 | 3,730 (18.6) | 137 (3.7) | 2,148 (57.6) | 1,299 (34.8) | 146 (3.9) |
| 50–59 | 4,797 (23.9) | 291 (6.1) | 2,429 (50.6) | 1,790 (37.3) | 287 (6.0) |
| 60–69 | 5,601 (27.9) | 477 (8.5) | 2,524 (45.1) | 2,077 (37.1) | 523 (9.3) |
| 70+ | 5,937 (29.6) | 1,444 (24.3) | 1,661 (28.0) | 1,992 (33.6) | 840 (14.1) |
| Gender: | |||||
| M | 12,299 (61.3) | 1,436 (11.7) | 5,459 (44.4) | 4,296 (34.9) | 1,108 (9.0) |
| F | 7,766 (38.7) | 913 (11.8) | 3,303 (42.5) | 2,862 (36.9) | 688 (8.9) |
| Yr of diagnosis: | |||||
| 2005 | 2,622 (13.1) | 289 (11.0) | 1,000 (38.1) | 1,191 (45.4) | 142 (5.4) |
| 2006 | 3,004 (15.0) | 307 (10.2) | 1,199 (39.9) | 1,280 (42.6) | 218 (7.3) |
| 2007 | 3,229 (16.1) | 363 (11.2) | 1,371 (42.5) | 1,252 (38.8) | 243 (7.5) |
| 2008 | 3,601 (17.9) | 418 (11.6) | 1,531 (42.5) | 1,294 (35.9) | 358 (9.9) |
| 2009 | 3,913 (19.5) | 486 (12.4) | 1,847 (47.2) | 1,161 (29.7) | 419 (10.7) |
| 2010 | 3,696 (18.4) | 486 (13.1) | 1,814 (49.1) | 980 (26.5) | 416 (11.3) |
| Registry region: | |||||
| Central | 5,567 (27.7) | 646 (11.6) | 2,437 (43.8) | 2,044 (36.7) | 440 (7.9) |
| Eastern | 5,630 (28.1) | 584 (10.4) | 2,590 (46.0) | 2,003 (35.6) | 453 (8.0) |
| Western | 1,907 (9.5) | 263 (13.8) | 795 (41.7) | 669 (35.1) | 180 (9.4) |
| California | 6,961 (34.7) | 856 (12.3) | 2,940 (42.2) | 2,442 (35.1) | 723 (10.4) |
| Race: | |||||
| White | 14,466 (72.1) | 1,652 (11.4) | 6,465 (44.7) | 4,972 (34.4) | 1,377 (9.5) |
| Black | 2,462 (12.3) | 382 (15.5) | 892 (36.2) | 1,014 (41.2) | 174 (7.1) |
| Hispanic | 1,953 (9.7) | 185 (9.5) | 854 (43.7) | 770 (39.4) | 144 (7.4) |
| Asian/Pacific Islander | 879 (4.4) | 90 (10.2) | 407 (46.3) | 305 (34.7) | 77 (8.8) |
| Other | 305 (1.5) | 40 (13.1) | 144 (47.2) | 97 (31.8) | 24 (7.9) |
| Tumor size (cm): | |||||
| 1.0 or Less | 1,182 (5.9) | 131 (11.1) | 492 (41.6) | 517 (43.7) | 42 (3.6) |
| 1.1–2.0 | 7,379 (36.8) | 870 (11.8) | 3,831 (51.9) | 1,973 (26.7) | 705 (9.6) |
| 2.1–3.0 | 11,504 (57.3) | 1,348 (11.7) | 4,439 (38.6) | 4,668 (40.6) | 1,049 (9.1) |
| Tumor grade: | |||||
| I or II | 12,201 (60.8) | 238 (2.0) | 6,333 (51.9) | 4,840 (39.7) | 790 (6.5) |
| III or IV | 3,077 (15.3) | 93 (3.0) | 1,422 (46.2) | 1,460 (47.4) | 102 (3.3) |
| Unknown | 4,787 (23.9) | 2,018 (42.2) | 1,007 (21.0) | 858 (17.9) | 904 (18.9) |
| Tumor histology: | |||||
| Clear cell | 9,848 (49.1) | 322 (3.3) | 4,876 (49.5) | 3,928 (39.9) | 722 (7.3) |
| Nonclear cell | 10,217 (50.9) | 2,027 (19.8) | 3,886 (38.0) | 3,230 (31.6) | 1,074 (10.5) |
| Tumor stage: | |||||
| Localized | 18,346 (91.4) | 1,648 (9.0) | 8,396 (45.8) | 6,562 (35.8) | 1,740 (9.5) |
| Regional | 868 (4.3) | 53 (6.1) | 333 (38.4) | 473 (54.5) | 9 (1.0) |
| Distant | 572 (2.9) | 417 (72.9) | 25 (4.4) | 118 (20.6) | 12 (2.1) |
| Unknown | 279 (1.4) | 231 (82.8) | 8 (2.9) | 5 (1.8) | 35 (12.5) |
Abbreviations and Acronyms
- CSS
cancer specific survival
- CVS
cardiovascular survival
- OS
overall survival
- PN
partial nephrectomy
- RN
radical nephrectomy
- SRC
small renal cancer
- TA
thermal ablation
REFERENCES
- 1.McKiernan J, Simmons R, Katz J et al. : Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 2002; 59: 816. [DOI] [PubMed] [Google Scholar]
- 2.Huang WC, Levey AS, Serio AM et al. : Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006; 7: 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau W, Blute M, Weaver A et al. : Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 2000; 75: 1236. [DOI] [PubMed] [Google Scholar]
- 4.Chronic Kidney Disease Prognosis Consortium, Matsuhita K, van der Velde M et al. : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Villalta JD, Meng MV et al. : Evolving practice patterns for the management of small renal masses in the USA. BJU Int 2012; 110: 1156. [DOI] [PubMed] [Google Scholar]
- 6.Campbell SC, Novick AC, Belldegrun A et al. : Guideline for management of the clinical T1 renal mass. J Urol 2009; 182: 1271. [DOI] [PubMed] [Google Scholar]
- 7.Van Poppel H, Da Pozzo L, Albrecht W et al. : A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011; 59: 543. [DOI] [PubMed] [Google Scholar]
- 8.Huang WC, Elkin EB, Levey AS et al. : Partial nephrectomy versus radical nephrectomy in patients with small renal tumors–is there a difference in mortality and cardiovascular outcomes? J Urol 2009; 181: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan HJ, Norton EC, Ye Z et al. : Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA 2012; 307: 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DC, Schonlau M, Litwin MS et al. : Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer 2008; 112: 511. [DOI] [PubMed] [Google Scholar]
- 11.Kim SP, Thompson RH, Boorjian SA et al. : Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol 2012; 188: 51. [DOI] [PubMed] [Google Scholar]
- 12.Curtis LH, Hammill BG, Eisenstein EL et al. : Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care, suppl., 2007; 45: S103. [DOI] [PubMed] [Google Scholar]
- 13.Harder V, Stuart E and Anthony J: Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 2010; 15: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choueiri TK, Schutz FA, Hevelone ND et al. : Thermal ablation vs surgery for localized kidney cancer: a Surveillance, Epidemiology, and End Results (SEER) database analysis. Urology 2011; 78: 93. [DOI] [PubMed] [Google Scholar]
- 15.Kunkle DA, Egleston BL and Uzzo RG: Excise, ablate or observe: the small renal mass dilemma–a meta-analysis and review. J Urol 2008; 179: 1227. [DOI] [PubMed] [Google Scholar]
- 16.Thompson RH, Atwell T, Schmit G et al. : Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2015; 67: 252. [DOI] [PubMed] [Google Scholar]
- 17.Shuch B, Hanley J, Lai J et al. : Overall survival advantage with partial nephrectomy: a bias of observational data? Cancer 2013; 119: 2981. [DOI] [PubMed] [Google Scholar]
- 18.Pierorazio PM, Johnson MH, Ball MW et al. : Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. Eur Urol 2015; 68: 408. [DOI] [PubMed] [Google Scholar]
- 19.Lane BR, Abouassaly R, Gao T et al. : Active treatment of localized renal tumors may not impact overall survival in patients aged 75 years or older. Cancer 2010; 116: 3119. [DOI] [PubMed] [Google Scholar]


