Abstract
Thrombocytopenia (TCP) may cause severe and life-threatening bleeding. While this may be prevented by platelet transfusions, transfusions are associated with potential complications, do not always work (platelet refractory) and are not always available. There is an urgent need for a synthetic alternative. We evaluated the ability of fibrinogen-coated nanospheres (FCNs) to prevent TCP-related bleeding. FCNs are made of human albumin polymerized into a 100-nm sphere and coated with fibrinogen. We hypothesized that FCNs would bind to platelets through fibrinogen-GPIIb/IIIa interactions, contributing to hemostasis in the setting of TCP. We used two murine models to test these effects: in the first model, BALB/c mice received 7.25 Gy total-body irradiation (TBI); in the second model, lower dose TBI (7.0 Gy) was combined with an anti-platelet antibody (anti-CD41) to induce severe TCP. Deaths in both models were due to gastrointestinal or intracranial bleeding. Addition of antiplatelet antibody to 7.0 Gy TBI significantly worsened TCP and increased mortality compared to 7.0 Gy TBI alone. FCNs significantly improved survival compared to saline control in both models, suggesting it ameliorated TCP-related bleeding. Additionally, in a saphenous vein bleeding model of antibody-induced TCP, FCNs shortened bleeding times. There were no clinical or histological findings of thrombosis or laboratory findings of disseminated intravascular coagulation after FCN treatment. In support of safety, fluorescence microscopy suggests that FCNs bind to platelets only upon platelet activation with collagen, limiting activity to areas of endothelial damage. To our knowledge, this is the first biosynthetic agent to demonstrate a survival advantage in TCP-related bleeding.
INTRODUCTION
Thrombocytopenia (TCP) is a significant problem in hematology, oncology, trauma surgery, and a number of other conditions including exposure to high-dose ionizing radiation (1). While this is currently addressed by transfusing platelets, there are a number of challenges with this strategy: 1. Transfused platelets have a short life span and will need to be given repeatedly if they are not being produced endogenously, which multiplies the risk of platelet transfusion reactions (2); 2. There is the potential of human leukocyte antigen sensitization with repeated platelet transfusions, increasing the destruction of transfused platelets (3); 3. The storage of platelet products at room temperature introduces the risks of bacterial contamination; and 4. Their short, five-day shelf life results in waste from expired units (220,00 apheresis units in 2013, 11% of those distributed), inadequate supply as hospitals try to manage inventory (13.2% of hospitals reported nonsurgical platelet needs were unmet in 2013, and 10.5% reported elective surgery was postponed due to unmet platelet needs), and high costs (approximately $517 for a leukocyte-reduced apheresis product) (4). Problems with availability may be heightened in special situations such as in the event of a radiation catastrophe (5, 6). At the same time, there is growing demand and limited supply: In 2013, 1.3 million total platelet units were transfused in the U.S., constituting a 15.4% increase from 2011, while collections decreased by 4.3% (4). Thus, there is an urgent need for synthetic substitutes.
One possible means of circumventing these challenges is the development of platelet analogs that could work “off the shelf.” We hypothesized that a novel fibrinogen-coated albumin nanosphere can serve as an effective platelet substitute for treatment of TCP. Fibrinogen-coated nanospheres (FCNs) are made of clinical-grade human albumin molecules polymerized into a spherical shape with an average diameter of 100 nm and coated with clinical-grade human fibrinogen. Fibrinogen is a key component of clotting, by binding to glycoprotein IIb/IIIa on activated platelets and promoting platelet aggregation by cross-linking adjacent activated platelets (7, 8). Earlier studies with fibrinogen-bound erythrocytes or microcapsules showed promise, shortening bleeding times (9–13), but none improved survival. At sites where platelets are actively forming wound-sealing clots, the spheres are passively trapped to form co-aggregates with the activated platelet, thus promoting the timely formation of an effective clot. In severe thrombocytopenia, we hypothesize that FCNs can promote hemostasis by amplifying the effect of activated platelets through cross-linking, preventing life-threatening bleeding, without causing spontaneous thrombosis.
In this work, using murine models of thrombocytopenia, we show that FCNs improve survival by reducing fatal hemorrhage. FCNs also shorten bleeding times, suggesting that the survival benefit comes from improvements in primary hemostasis. In a variety of flow cytometry and microfluidic assays, we demonstrate that FCNs bind to activated platelets to contribute to platelet aggregation and clot formation; however, they do not bind to inactivated platelets, suggesting that they would not cause spontaneous thrombosis. Safety is further supported by our in vivo murine data, suggesting that FCNs may be developed as a safe and effective treatment for TCP-related bleeding.
MATERIALS AND METHODS
Synthesis of FCNs and Quality Assurance
The synthesis of FCNs is described in U.S. Patent 6264988. In brief, an albumin solution is combined with ethanol and a cross-linking agent to form albumin nanospheres. A fibrinogen solution (1–2 mg/ml) containing stabilizer (sodium tetradecyl sulfate, sodium lauryl sulfate) is added to the albumin solution. Ethanol is removed by passing the solution through a renal dialysis unit. Sorbitol and caprylate are added, and the resulting solution is diluted to a concentration of 8 mg/ml before it is pasteurized at 60°C for 10 h. Control nanospheres are produced using the same protocol without the addition of fibrinogen. The mean hydrodynamic diameter of FCNs generated by this process is 112 ± 2 nm (n = 5), as measured using nanoparticle tracking analysis (Supplementary Fig. S1A and B; https://doi.org/10.1667/RADE-20-00016.S1).
Nanoparticle tracking analysis was performed using the NanoSight 500 system (Malvern, UK). Nanoparticle Brownian motion was tracked by a video camera and saved as a video file, which was then analyzed by software using the Stokes Einstein equation to calculate the nanoparticles’ hydrodynamic diameters.
The amount of fibrinogen coating on the nanospheres was measured by sandwich ELISA using a matched-pair antibody set for ELISA of human fibrinogen antigen and VisuLize™ Buffer Pak (Affinity Biologicals Inc., Ancaster, Canada) according to the manufacturer’s instructions.
Animals
Six-to-eight-week-old BALB/c mice and DsRed mice were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were housed in barrier facilities at the Duke Cancer Center Isolation Facility (Durham, NC). All mice were maintained in an animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all experimental procedures were performed in accordance with federal and state government guidelines and established institutional guidelines and protocols approved by the Institutional Animal Care and Use Committee at Duke University. Mice were euthanized when humane end points were met. The humane end points included poor posture or ambulating difficulty (e.g., tense, tucked-up, stiff gait), lost hair coat condition (e.g., ruffled fur, lack of grooming, piloerection), sudden activity level change (e.g., restlessness, pacing, reluctance to move), signs of moderate to severe pain or distress, and more than 30% weight loss.
Murine Thrombocytopenia Survival Models
BALB/c mice received single-fraction total-body irradiation (TBI) in a Mark I-68A 137Cs irradiator (JL Shepherd and Associates, San Fernando, CA) to induce persistent TCP and increase the risk of bleeding. The dose rate was 600 cGy/min. Mice were placed in a Plexiglas® holder on a rotating platform to ensure uniform exposure. Dosimetry is confirmed by our institutional quality assurance program, as described elsewhere by Yoshizumi et al. (14). In some cases, TCP was further increased by the administration of anti-CD41 (BD Pharmigen™, San Jose, CA) or anti-CD42b anti-platelet antibody (Emfret Analytics, Wuerzburg, Germany) as described elsewhere (15). Platelet-depleting anti-CD41 and and-CD42b antibodies were given intraperitoneally. Irradiations were performed on day 0, and antibody was administered on days 0, 5 and 10 to increase TCP. Necropsies were performed on all mice upon being found dead or euthanasia and tissues were examined for cause of death. For blood samples, a separate cohort of mice was used so that blood draws would not impact survival. Mice were monitored for hematopoietic recovery over time.
Bleeding Time Assays
Bleeding time in BALB/c mice was measured using two different approaches. The first assay was a modification of that reported elsewhere by Molina et al. (16), whereby the tail vein of normal or thrombocytopenic BALB/c mice was punctured using a sterile lancet. Bleeding was timed using a stopwatch, and cessation of bleeding was assessed by repeated application of 1M Whatman® Cellulose Filter Paper (Sigma-Aldrich® LLC, St. Louis, MO) to the wound until no further bleeding was noted. The platelet count was confirmed using Hemavet 950 (Drew Scientific Group, Miami Lakes, FL).
In the second assay, a saphenous vein bleeding model was used, as described elsewhere (17). BALB/c mice were injected with 0.5–2 μg/g of anti-platelet antibody 24 h prior to the assay to induce varying levels of thrombocytopenia. At 2 h prior to assay, mice were injected with either FCNs or saline only. Blood was drawn via retro-orbital bleed and CBC was measured. The mouse was anesthetized with isoflurane and hair was removed from the hind legs. A small incision was made, and the skin and fascia overlying the saphenous vein were removed. The saphenous vein was injured via introduction of a 23g lancet. Bleeding was timed using a stopwatch. The vein was re-injured upon cessation of bleeding, and the time to bleeding cessation was monitored over 30 min. Because of the semi-subjective nature of these assays, the observer in both assays was blinded to the animal’s platelet count and treatment arm (FCN vs. control).
In Vivo Imaging and Clot Formation
The mice were anesthetized with isoflurane (Butler Animal Health Supply, Dublin, OH) and injected via the tail vein with CD9-Phycoerythrin (PE) labeled platelets (18) and Alexa Fluor® 488 5-TFP-labeled FCNs. A mesenteric vein was exposed, and filter paper (1-mm diameter patch of 1M Whatman paper) saturated with 10% FeCl3 (Sigma-Aldrich) was applied for 2 min. Thrombus formation was monitored in real time under a two-photon microscope (FluoView FV1000; Olympus®, Central Valley, PA).
Human Subjects
Healthy adult donors and patients experiencing varying levels of thrombocytopenia were identified in the Duke Cancer Institute and consented to provide peripheral blood samples for research usage. Informed consent was obtained after the nature and possible consequences of the studies were explained. All samples were deidentified prior to use in the study. This study was performed in accordance with the Declaration of Helsinki after informed consent and was approved by the Duke University Institutional Review Board (IRB Pro00044019).
Preparation of Human Platelets and FCNs
Aliquots of whole blood collected in ethylenediaminetetraacetic acid (EDTA), heparin, citrate or hirudin were obtained from healthy donors and centrifuged at 210g for 15 min and the platelet-rich plasma (PRP) fraction was collected; other samples were centrifuged at 2,310g for 15 min, and the platelet-poor plasma (PPP) fraction collected. PRP was diluted with PPP to 50×106 platelets/ml (18). The platelet concentration of PRP, PPP, and mixtures was determined using the Sysmex XP-300 (Kobe, Japan). For imaging studies, platelets were labeled with phycoerythrin (PE-label) mouse antihuman CD31(1:100) (BD Biosciences, San Jose, CA). FCNs or control nanospheres (consisting of albumin only without fibrinogen) were labeled with Alexa Fluor 488 (50 μg/ml) (Thermo Fisher Scientific™ Inc., Waltham, MA) as described by the manufacturer.
FCN-Platelet Interactions
For direct visualization of platelet-FCN interactions, platelets (50 × 106 platelets/ml) were combined with FCNs (80 μg/ml) in the absence or presence of collagen I (0.20 mg/ml; Bio/Data Corp., Horsham, PA) while shaking at 500 RPM at 37°C for 10 min. Platelet-FCN interactions were visualized using an Olympus BX51WI fluorescence microscope. Platelet-FCN interactions were also observed with flow cytometry (FACSCanto™ II, BD Biosciences) using the method of Cuyper et al. (18) and analyzed using BD FACSDiva™ Software (BD Biosciences). For analysis, the appearance of double-positive events in the upper right quadrant (Q2) was quantified as a percentage of total events.
Electron Microscopy
FCNs were visualized using scanning electron microscopy (SEM). FCN preparations in the presence or absence of platelets and presence or absence of collagen were deposited onto 9 × 9-mm collagen-coated coverslips and allowed to settle for 30 min in a covered moist chamber. After a gentle wash with water, they were gently flooded with 2% glutaraldehyde in 0.1 M phosphate buffer and fixed for 30 min. FCNs were then dehydrated in a graded series of ethanol, infiltrated with liquid CO2 and dried in a critical point drier (Samdri-PVT-3D; Tousimis Research Corp., Rockville, MD), glued onto aluminum stubs, coated with platinum in a sputter coater (E 5100; Polaron Instruments Inc., Hertfordshire, England), and viewed in a scanning electron microscope (JSM 6400, JEOL USA, Inc., Peabody, MA). Digital prints were collected using Revolution (4pi Analysis Inc., Hillsborough, NC).
FCN-Endothelium Interactions
Human umbilical vein endothelial cells (HUVECs) from a pool of donors (Lonza Inc., Walkersville, MD) were seeded at 10,000 cells per well into 8-well chamber slides, in EGM-2MV media (Lonza). The wells were coated with 0.1% gelatin before use. The cells were incubated overnight at 37°C in 5% CO2 and then labeled with CellTracker™ Blue CMAC (Thermo Fisher Scientific) following the manufacturer’s protocol. After labeling, the wells were incubated with Alexa Fluor 488-labeled FCNs (5.9 mg/ml) for 1 h at 37°C in 5% CO2, and binding was visualized using an Olympus BX51WI fluorescence microscope.
Platelet Aggregation Assays
The Cellix Microfluidic System (Cellix Ltd., Dublin, Ireland) was used to evaluate aggregation under flow-based conditions. The Cellix system allows blood to be drawn through the Vena8 Fluro + biochip using the Mirus Evo Nanopump at desired flow conditions. The chip is coated with COLtest reagent containing collagen type I at a reconstituted activity equivalent of 100 μg/ml (Verum Diagnostica GmbH, Munich, Germany), which stimulates platelet adhesion and activation. Each channel was covered with 10 μl of reconstituted COLtest reagent at room temperature and left overnight at 4°C, in a humidified chamber, according to Cellix specifications. Images were taken using a Nikon® Eclipse Ti-S inverted microscope (Melville, NY) and analyzed using VenaFlux (Cellix) to measure the number of aggregates and total area of aggregation.
Testing for Disseminated Intravascular Coagulation (DIC)
BALB/c mice were injected with FCNs, after which they were anesthetized at various time points. Blood was then drawn using inferior vena cava puncture to obtain sufficient blood (500 μl) for DIC analysis (PT, PTT, TT, fibrinogen, D-dimer), after which the animals were sacrificed humanely. All reagents were purchased from Diagnostica Stago, Inc. (Parsippany, NJ) and prepared according to manufacturer’s instructions. Assays were performed on either the automated Diagnostica Stago STA R Evolution® or manual Diagnostica Stago Start® 4 according to parameters outlined in the assay kit or instrument software. All results were determined automatically. For quantitative assays, calibration curves were predetermined in accordance with assay specifications.
Testing for Spontaneous Thrombosis
BALB/c mice were injected with FCNs, after which they were sacrificed at various time points. Organs (brain, heart, lungs, liver, kidneys) were removed, and sections stained with hematoxylin and eosin (H&E) were prepared for all ten mice groups. Organs were examined for evidence of spontaneous thrombosis under the guidance of an experienced pathologist (MH).
Statistics Analysis
Platelet counts and bleeding times were compared between groups at different days using two-sided two-sample t tests. Fold changes were compared to control using two-sided one-sample t tests. Survival times are displayed by Kaplan-Meier plots, and comparisons between groups were performed using the log-rank test. Longitudinal data were compared between groups using repeated measures analysis of variance (ANOVA). A test was considered significant at P < 0.05. All statistical analyses were performed using R software.
RESULTS
FCN Treatment Improves Survival in Murine Models of Radiation-Induced Thrombocytopenia-Related Hemorrhage
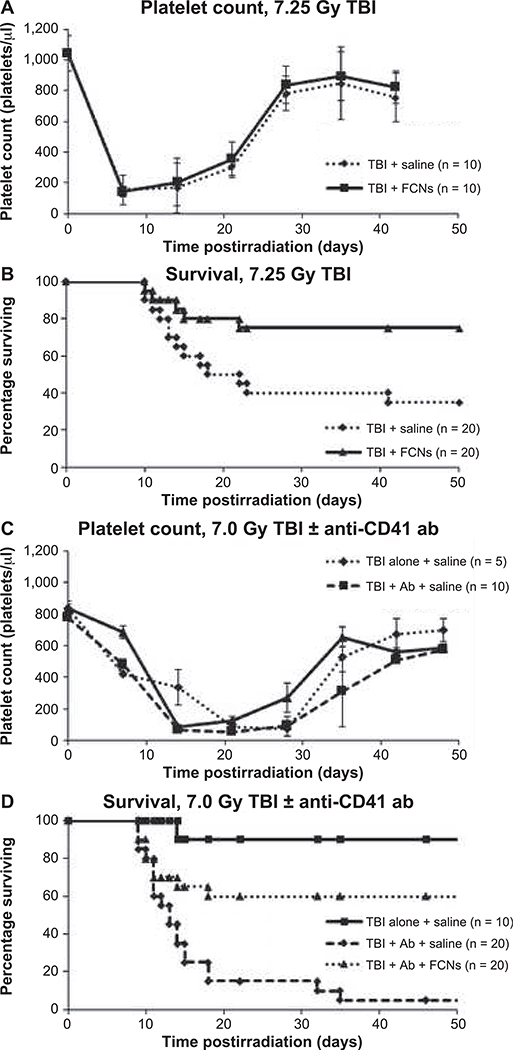
To test the hypothesis that FCNs prevent thrombocytopenia-related hemorrhage, we evaluated the effect of FCN treatment in two murine models. In the first, BALB/c mice received 7.25 Gy TBI on day 0 to induce thrombocytopenia and hemorrhage. They were subsequently treated with saline control or FCNs (8 mg/kg) on days 1, 5 and 10. Both groups were found to have similar platelet counts, suggesting that FCNs did not have an effect on cell numbers (Fig. 1A). However, survival at day 50 was significantly improved in the group receiving FCNs (75% vs. 35%, P = 0.015) (Fig. 1B).
FIG. 1.
FCNs reduce the risk of fatal hemorrhage in murine models of radiation-induced thrombocytopenia. FCNs significantly improved survival in two murine models of thrombocytopenia. In the first model BALB/c mice received 7.25 Gy TBI and then given either FCNs or saline control. Platelet counts were not significantly different between the two groups (panel A). Survival was significantly improved in the group receiving FCNs (panel B). In the second model, to look more specifically at thrombocytopenia, mice received 7.0 Gy TBI and were then treated with either saline alone, saline + anti-CD41 anti-platelet antibody, or FCNs + anti-CD41 antiplatelet antibody. Again, platelet counts were measured through the nadir and recovery. Those receiving anti-platelet antibody reached the nadir of their platelet counts more rapidly (panel C). Survival was significantly improved in the FCN-treated group (panel D).
Because radiation has many other effects in addition to thrombocytopenia, in the second model, BALB/c mice received a sublethal dose (7.0 Gy) of TBI and thrombocytopenia was worsened by administration of an anti-CD41 anti-platelet antibody. Serial platelet counts showed that mice receiving 7.0 Gy TBI with anti-CD41 antibody had significantly lower platelet counts than mice that received 7.0 Gy TBI alone (Fig. 1C), particularly during the crucial time point, day 14 postirradiation, around when most deaths occurred. Indeed, mice receiving 7.0 Gy TBI with anti-CD41 antibody had only 5% survival compared to 90% in the mice receiving TBI alone (Fig. 1D). This suggests that the radiation effect on the mouse is magnified by the antibody-induced thrombocytopenia. Administration of FCNs 8 mg/kg on days 1, 5 and 10 significantly improved survival at day 50 from 5% in the control group to 60% in the FCN-treated group (P < 0.01). All deaths were from gastrointestinal or intracranial hemorrhage as revealed by necropsy, suggesting that in this model of thrombocytopenia-related hemorrhage, FCNs improve survival by improving hemostasis.
FCN Treatment Improves Platelet Aggregation In Vitro and Bleeding Time In Vivo
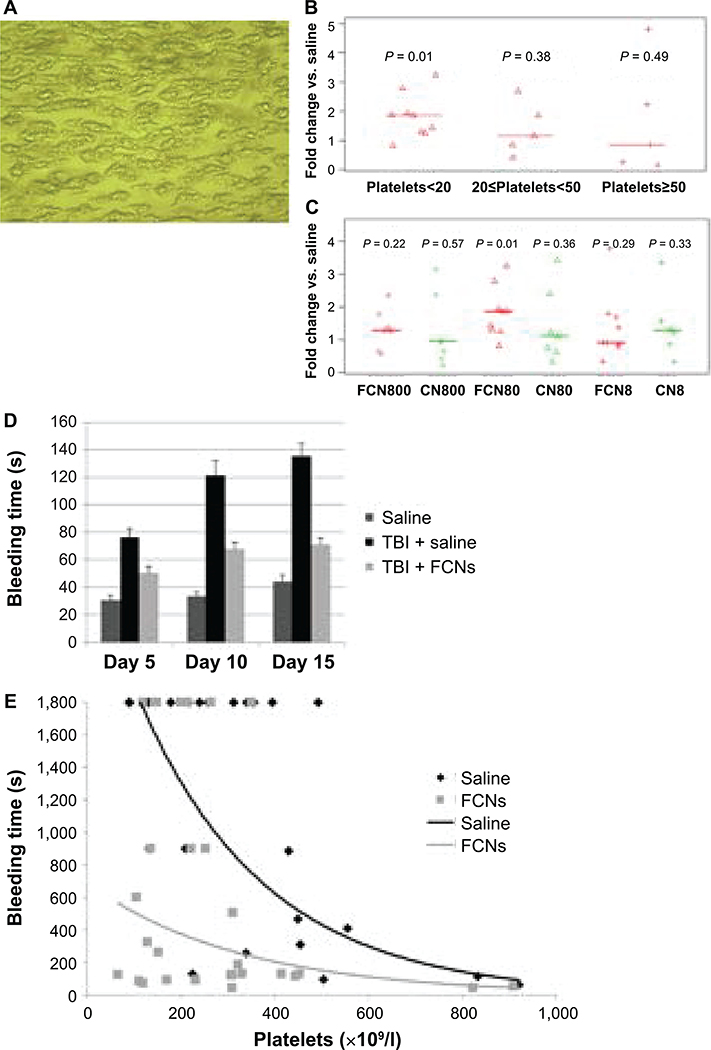
To determine how FCNs improve hemostasis, we evaluated their effect in in vitro assays. Platelet aggregation was almost doubled when evaluated using the Cellix Microfluidic System. Specifically, when collagen-induced platelet adhesion and aggregation were measured under continuous blood flow conditions, the total area of platelet aggregates (Fig. 2A) was doubled with addition of FCNs. This effect was seen under thrombocytopenic conditions (blood taken from patients with a platelet count <20,000/μl, P = 0.01), although not when the platelet count was >20,000/μl (Fig. 2B). In evaluating different concentrations, this effect was strongest with FCN dose of 80 μg/ml (Fig. 2C). Of note, improvement in aggregation was only seen by addition of FCNs and not with control (plain albumin) nanospheres.
FIG. 2.
FCNs improve bleeding time by enhancing platelet aggregation. We observed the effects of FCNs under flow-based conditions (see Materials and Methods). FCNs, control nanospheres (CNs) or saline were added to whole blood samples from patients with varying degrees of thrombocytopenia. Images were taken of platelets adhered to a collagen-coated chip (panel A) and analyzed to determine the total area of platelet aggregation (40× magnification). A scale bar was also made. No significant difference was observed in the area of aggregation when FCNs were added to samples from patients whose platelet counts were greater than 20,000/μl; however, a twofold increase in aggregation was observed when FCNs were added to samples from patients whose platelet counts were <20,000/μl (panel B). We further explored these findings by comparing FCNs, 80 μg/ml, to tenfold-higher and -lower doses, as well as to the corresponding doses of CNs. (FCN800=FCNs 800 μg/ml; FCN80=FCNs 80 μg/ml; FCN8=FCNs 8 μg/ml; CNs=plain albumin without fibrinogen coating; CN800=CNs 800 μg/ml; CN80=CNs 80 μg/ml; and CN8=CNs 8 μg/ml). FCNs, 80 μg/ml, appeared to be the most efficacious, whereas the other results were varied and inconsistent (panel C). In a tail vein bleeding model, the bleeding time was significantly shorter in mice treated with FCNs than those treated with saline at days 5, 10 and 15 postirradiation (panel D). A saphenous vein bleeding model was also used, in which mice were given anti-CD42b anti-platelet antibody to induce varying degrees of thrombocytopenia and treated with saline or FCNs before saphenous vein injury. Time to cessation of bleed was measured, with bleeding time capped at 30 min. Bleeding time was significantly shortened in the mice receiving FCNs, particularly when the platelet count was <200,000/μl (P = 0.030) or 200–400,000/μl (P = 0.046). (panel E).
An effect on platelet aggregation is supported by improvements in bleeding times in thrombocytopenic mice injected with FCNs. We demonstrated this using two murine models. In the first, BALB/c mice received 7.5 Gy TBI to induce thrombocytopenia. Bleeding time was measured using the tail vein method. The bleeding time was significantly shorter in mice treated with FCNs than those treated with saline at day 5, 10 and 15 postirradiation (Fig. 2D), although it did not completely normalize (i.e., it was still prolonged compared to nonirradiated mice). In the second model, BALB/c mice were given anti-CD42b antiplatelet antibody to induce thrombocytopenia. Bleeding time was measured using the saphenous vein method. Bleeding time was significantly shortened in the mice with platelet counts <400,000/μl (P = 0.030 for platelet counts <200,000/μl; P = 0.046 for platelet counts 200,000–400,000/μl; P = 0.168 for platelet counts 400,000–600,000/μl; and P = 0.090 for platelet counts >600,000/μl) (Fig. 2E).
FCNs Bind to Platelets In Vitro
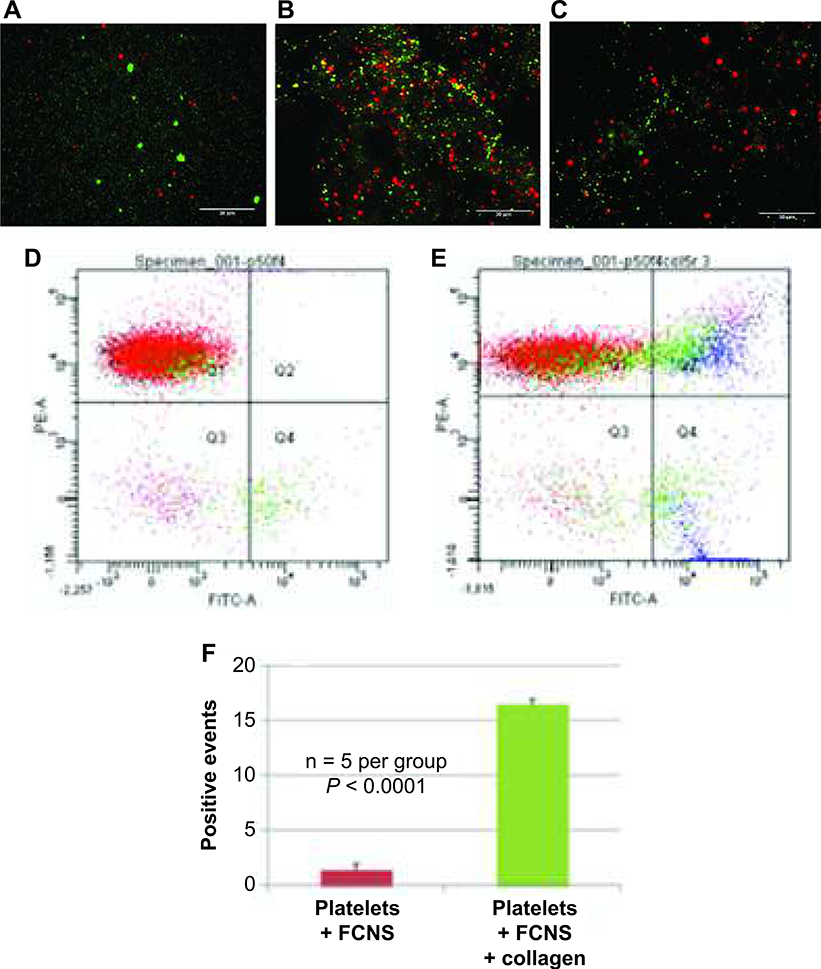
To better understand how FCN treatment improves hemostasis and to test the hypothesis that it does so by binding to platelets, we directly visualized FCN-platelet interactions using fluorescent microscopy. When FCNs (Alexa Fluor 488, green channel) and platelets (CD9-PE, red channel) were combined in vitro, they remained separate and were visualized as discrete objects (Fig. 3A). When collagen, a platelet agonist, was added, the result was clumping of FCNs and platelets (Fig. 3B), with overlay of green (FCN) and red (platelet) signals to produce yellow, suggesting that FCNs bind to activated but not resting platelets. The presence of fibrinogen is crucial to this interaction, as control nanospheres (plain albumin nanospheres without fibrinogen coating) labeled with Alexa Fluor 488 failed to produce overlapping events in the presence of platelets and collagen (Fig. 3C).
FIG. 3.
Fibrinogen-coated nanospheres (FCNs) bind to activated but not resting platelets. Fluorescence microscopy images of platelets (labeled red with CD9-PE antibody) and FCNs (labeled green with Alexa Fluor 488) show no interaction in the absence of platelet activation (panel A). The addition of collagen (a platelet agonist) shows yellow signal overlap (panel B), indicating interaction between the activated platelets and FCNs. However, control nanospheres (albumin spheres without fibrinogen coating) do not interact with platelets, even in the presence of collagen (panel C). The degree of interaction can be estimated with flow cytometry. When platelets (labeled CD9-PE) and FCNs (labeled Alexa Fluor 488) are mixed together in the absence of collagen, there are very few overlapping events, measured as double-positive events (panel D). When collagen is added, the number of double-positive events significantly increases (panel E). These data are quantified in panel F.
To quantify these interactions, we used flow cytometry. Using the same fluorescent labels as above, FCNs and platelets were combined with and without collagen, and the number of double-positive events (indicating binding between the FCNs and platelets) was measured. In the absence of collagen, very few double-positive events occurred (Fig. 3D); with the addition of collagen, the number of double-positive events was approximately 16% (Fig. 3E and F).
FCNs Bind to Endothelium
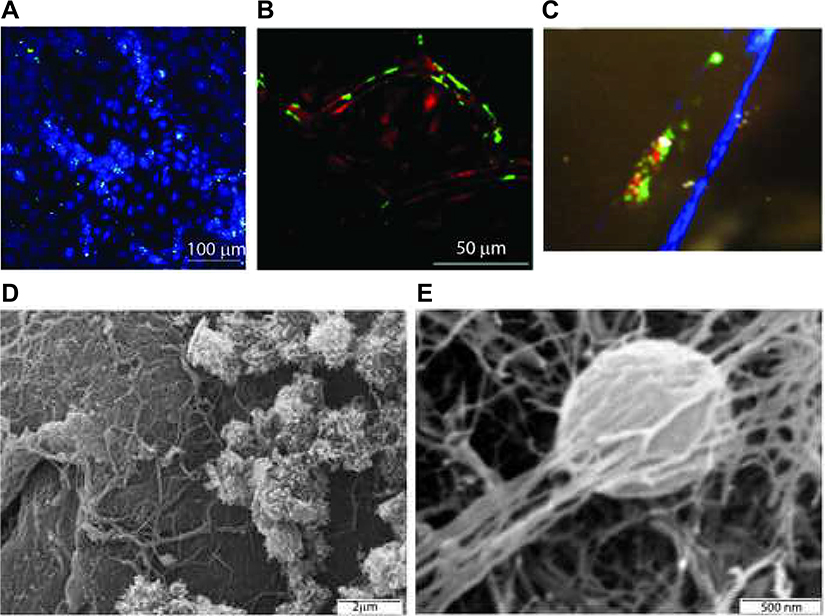
To determine if FCNs also interact with the endothelium, we incubated FCNs with human umbilical vein endothelial cells (HUVECs). We used fluorescent microscopy to visualize these interactions, labeling HUVECs (cell tracker blue CMAC) and FCNs (Alexa Fluor 488 as above). Binding was only observed where cells were located (Fig. 4A) indicating that FCNs bound specifically to the cells. We also visualized FCN-endothelial interactions in vivo. Labeled FCNs were injected into a C57BL/6 mouse expressing DsRed and imaged using two-photon microscopy. At 1 h after injection, FCNs were seen binding to the endothelium (Fig. 4B). This signal persisted for up to 48 h after injection (data not shown).
FIG. 4.
FCNs bind to endothelium. Cells grown in 8-well chamber slides were labeled with CellTracker Blue CMAC and treated with Alexa Fluor 488-labeled FCNs (Fig. 4A). Two-photon in vivo microscopy of a murine ear vessel 1 h after injection of Alexa Fluor 488-labeled FCNs showed persistent green signal lining the endothelium (panel B). Two-photon microscopy of a murine mesenteric vessel after thrombosis induced by the topical application of ferric chloride, with platelets labeled red with CD9-PE and FCNs labeled green with Alexa Fluor 488, showed co-localization of red and green signal at the site of FeCl3 application, suggesting interaction between platelets and FCNs in thrombosis formation (panel C). These interactions were supported by electron microscopy. Examination of the endothelium revealed platelet aggregates and fibrin deposition. FCNs can be visualized within this meshwork (panel D). Closer study shows a nanosphere incorporated into the fibrin clot (panel E).
FCNs Participate in Clot Formation In Vivo
To evaluate FCN-platelet-endothelial interactions in vivo, a two-photon microscopy was used to observe thrombus formation, using a ferric chloride thrombosis model. After injecting platelets (CD9-PE, red) and FCNs (Alexa Fluor 488), ferric chloride was applied topically to induce reactive oxygen species and thrombus formation. Within seconds, green (FCNs) and red (platelet) signals began to gather at the site of application, interacting with each other and the endothelium, forming a clot (Fig. 4C).
To further characterize this interaction, scanning electron microscopy was employed to visualize the structure of clots. After mice were injected with unlabeled FCNs, the inferior vena cava (IVC) was identified, removed and filleted open. Close examination of the endothelium (Fig. 4D) revealed platelet aggregates and fibrin deposition. With additional magnification, FCNs were visualized within this meshwork (Fig. 4E).
FCN Treatment does not Cause Spontaneous Thrombosis or Disseminated Intravascular Coagulation
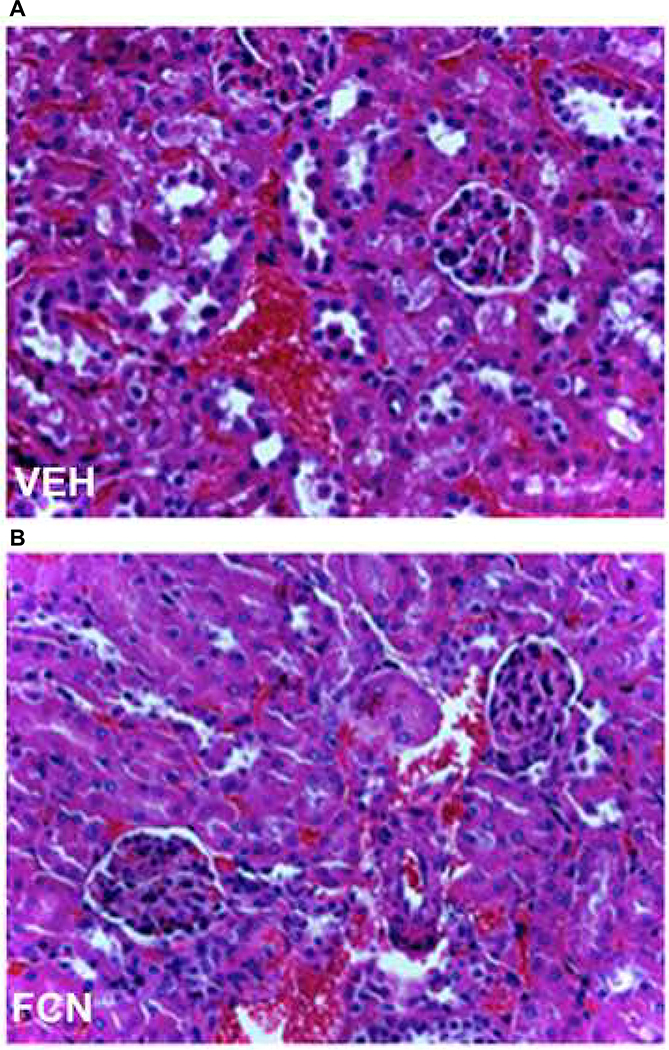
To evaluate the risk of spontaneous thrombosis and DIC, BALB/c mice were injected with saline or FCNs 8 mg/kg (n=10 per group), and blood was taken at 24–48 h for evaluation of prothrombin time, partial thromboplastin time, fibrinogen and D-dimer. No differences were detected between saline- and FCN-treated groups (Table 1). All mice were sacrificed at 24–48 h after injection, and organs (brain, heart, lungs, liver and kidneys) were processed and H&E slides examined for evidence of spontaneous thrombi; none were seen (Fig. 5).
TABLE 1.
FCNs do not Cause Disseminated Intravascular Coagulation
| Saline (24 h) | FCNs (24 h) | Saline (48 h) | FCNs (48 h) | |
|---|---|---|---|---|
| PT (s) (normal 9–12) | 11.4 ± 0.17 | 11.7 ± 0.87 | 11.5 ± 0.36 | 11.1 ± 0.33 |
| aPTT (s) (normal 20–30) | 23.6 ± 0.80 | 23.9 ± 2.27 | 21.5 ± 2.01 | 24.7 ± 3.10 |
| TT (s) (normal 16–22) | 20.1 ± 1.90 | 20.4 ± 2.80 | 18.5 ± 1.26 | 16.9 ± 1.47 |
| Fibrinogen (mg/dl) (normal 100–200) | 152.8 ± 19.4 | 116.2 ± 32.5 | 116.0 ± 41.5 | 172.6 ± 29.2 |
| D-dimer (mcg/ml) (normal 0.22) | 0.22 ± <0.0 | 0.22 ± <0.0 | 0.22 ± <0.0 | 0.22 ± <0.0 |
Notes. To evaluate safety, blood was drawn from mice treated with FCNs and examined for evidence of DIC. No differences or variations from normal (32, 33) were observed in prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), fibrinogen, or D-dimer levels at 24 and 48 h after FCN injection. The mean values of five replicates and standard deviations are shown.
FIG. 5.
FCNs do not cause spontaneous thrombosis. We examined histology from the brain, heart, lungs, kidneys and liver of mice treated with saline (kidney shown in panel A) vs. FCNs (kidney shown in panel B) in all mice (n = 10/group). No thrombi or microthrombi were found in either animal group. 100× magnification.
DISCUSSION
FCN therapy has the potential to be a safe and effective strategy for the prevention or treatment of thrombocytopenia-related bleeding. Studies showing improvements in bleeding times and platelet aggregation suggest that the impact is on primary hemostasis, and additional in vitro studies suggest that FCNs only interact with activated platelets, suggesting that it should not cause spontaneous thrombosis. The safety of FCNs is further supported by the absence of DIC or microthrombi on laboratory and histological evaluation of mice injected with FCNs.
While these findings of FCN particles have been reported elsewhere, the novelty of this current work is in the use of nanoscale particles. While other synthetic platelet substitutes have shown decreases in bleeding time, (12, 19, 20) ours is the first to show a survival benefit. The survival benefit in radiation-induced thrombocytopenia is particularly intriguing, given the lack of FDA-approved antithrombocytopenic agents in the event of a catastrophic radiation event (21).
FCNs have many favorable features compared to platelet transfusions: 1. They are ready to use as a suspension, injectable without the need of significant preparation or support, which may be advantageous in situations where a blood bank is not available; 2. Their shelf life (at least 1 year at 4°) has yet to be fully tested but already exceeds 5 days for donor platelets; 3. A smaller volume (1 ml/kg weight of patient) is needed than with platelet transfusions, decreasing the risk of volume overload (22, 23); 4. Likewise, synthetic formulation decreases the risk of transmission of infections (24); and 5. Absence of HLA antigens decreases the chance of alloimmunization and refractoriness (25–27). Furthermore, the potential of FCN therapy is yet to be determined: in addition to testing shelf life and kinetics, alternate shapes, sizes or core materials may improve the efficacy of FCN treatment.
While FCNs lack many of the functions of platelets (28), they may allow a decrease in the threshold for prophylactic platelet transfusion (e.g., 5,000 vs. 10,000 platelets/μl) (29, 30) or aid in hemostasis in the setting of platelet refractoriness. They may also serve an important role in radiation catastrophe events where platelets are not readily available.
Supplementary Material
Fig. S1. Measurement of the size distribution of nanospheres. Nanoparticles were visualized by light scattering, and a snapshot of nanoparticle Brownian motion recorded for nanoparticle hydrodynamic size measurement. Nanoparticle size distribution was normalized by the highest concentration.
Fig. S2. FCNs bind to endothelium for up to 48 h after injection. Two-photon in vivo microscopy of a murine ear vessel 48 h after injection of Alexa Fluor 488-labeled FCNs showed persistent green signal lining the endothelium.
ACKNOWLEDGMENTS
Portions of these data were presented at the annual meeting of the American Society for Blood and Marrow Transplantation and published in the conference proceedings (31). We thank Gowthami M. Arepally, Associate Professor of Medicine and Pathology, Duke University, and Dougald M. Monroe III, PhD, Professor of Medicine, University of North Carolina Chapel Hill, for their advice and assistance. We also thank Sharon Hall and Keith Klemp in the Duke Hemostasis and Thrombosis Core Laboratory for assistance with clinical laboratory assays. This work was supported by National Institute of Allergy and Infectious Diseases/National Institutes of Health grant nos. HHSN272201400034C (BJC), and U01AI133604 (NJC), U54 HL112307 (TLO, ADS), T32 HL007057-37 (ADS), Leukemia and Lymphoma Society New Idea Award NIA-8998-14 (ADS), and American Society of Hematology Research Training Award for Fellows (ADS). RCY is the owner of Fiplate, Inc. and holds U.S. Patent 6264988. The other authors declare no competing financial interests.
REFERENCES
- 1.DiCarlo AL, Poncz M, Cassatt DR, Shah JR, Czarniecki CW, Maidment BW. Medical countermeasures for platelet regeneration after radiation exposure. Report of a workshop and guided discussion sponsored by the National Institute of Allergy and Infectious Diseases, Bethesda, MD, March 22–23, 2010. Radiat Res 2011; 176:e0001–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doughty HA, Murphy MF, Metcalfe P, Rohatiner AZ, Lister TA, Waters AH. Relative importance of immune and non-immune causes of platelet refractoriness. Vox Sanguinis 1994; 66:200–5. [DOI] [PubMed] [Google Scholar]
- 3.Kim HW, Greenburg AG. Toward 21st century blood component replacement therapeutics: artificial oxygen carriers, platelet substitutes, recombinant clotting factors, and others. Artif Cells Blood Substit Immobil Biotechnol 2006; 34:537–50. [DOI] [PubMed] [Google Scholar]
- 4.Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion 2016; 56:2173–83. [DOI] [PubMed] [Google Scholar]
- 5.Hrdina CM, Coleman CN, Bogucki S, Bader JL, Hayhurst RE, Forsha JD, et al. The “RTR” medical response system for nuclear and radiological mass-casualty incidents: a functional TRiage-TReatment-TRansport medical response model. Prehosp Disaster Med 2009; 24:167–78. [DOI] [PubMed] [Google Scholar]
- 6.Planning guidance for response to a nuclear detonation. 2nd ed. Washington, DC: Federal Emergency Management Agency; 2010. [Google Scholar]
- 7.Levy JH, Welsby I, Goodnough LT. Fibrinogen as a therapeutic target for bleeding: a review of critical levels and replacement therapy. Transfusion 2014; 54:1389–405; quiz 8. [DOI] [PubMed] [Google Scholar]
- 8.Franchini M, Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus 2012; 10:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamura Y, Fujie T, Nogawa M, Maruyama H, Handa M, Ikeda Y, et al. Haemostatic effects of polymerized albumin particles carrying fibrinogen gamma-chain dodecapeptide as platelet substitutes in severely thrombocytopenic rabbits. Transfus Med 2008; 18:158–66. [DOI] [PubMed] [Google Scholar]
- 10.Rybak ME, Renzulli LA. A liposome based platelet substitute, the plateletsome, with hemostatic efficacy. Biomater Artif Cells Immobilization Biotechnol 1993; 21:101–18. [DOI] [PubMed] [Google Scholar]
- 11.Agam G, Livne AA. Erythrocytes with covalently bound fibrinogen as a cellular replacement for the treatment of thrombocytopenia. Eur J Clin Invest 1992; 22:105–12. [DOI] [PubMed] [Google Scholar]
- 12.Shukla M, Sekhon UD, Betapudi V, Li W, Hickman DA, Pawlowski CL, et al. In vitro characterization of SynthoPlate (synthetic platelet) technology and its in vivo evaluation in severely thrombocytopenic mice. J Thromb Haemost 2017; 15:375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Sen Gupta A. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials 2013; 34:3031–41. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizumi T, Brady SL, Robbins ME, Bourland JD. Specific issues in small animal dosimetry and irradiator calibration. Int J Radiat Biol 2011; 87:1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O’Donnell E, Zhao BQ, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood 2008; 111:4958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina ES, Fujita A, Sogayar MC, Demasi MA. A quantitative and humane tail bleeding assay for efficacy evaluation of antihaemophilic factors in haemophilia A mice. Haemophilia 2014; 20:e392–8. [DOI] [PubMed] [Google Scholar]
- 17.Buyue Y, Whinna HC, Sheehan JP. The heparin-binding exosite of factor IXa is a critical regulator of plasma thrombin generation and venous thrombosis. Blood 2008; 112:3234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Cuyper IM, Meinders M, van de Vijver E, de Korte D, Porcelijn L, de Haas M, et al. A novel flow cytometry-based platelet aggregation assay. Blood 2013; 121:e70–80. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi K, Hashimoto M, Ogaki S, Watanabe H, Takeoka S, Ikeda Y, et al. Effect of repeated injections of adenosine diphosphate-encapsulated liposomes coated with a fibrinogen gamma-chain dodecapeptide developed as a synthetic platelet substitute on accelerated blood clearance in a healthy and an anticancer drug-induced thrombocytopenia rat model. J Pharm Sci 2015; 104:3084–91. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto M, Taguchi K, Ogaki S, Watanabe H, Kinoshita M, Nishikawa K, et al. Pharmacokinetic properties of single and repeated injection of liposomal platelet substitute in a rat model of red blood cell transfusion-induced dilutional thrombocytopenia. J Pharm Sci 2015; 104:3968–76. [DOI] [PubMed] [Google Scholar]
- 21.Rios CI, Cassatt DR, Dicarlo AL, Macchiarini F, Ramakrishnan N, Norman MK, et al. Building the strategic national stockpile through the NIAID Radiation Nuclear Countermeasures Program. Drug Dev Res 2014; 75:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumberg N, Heal JM, Gettings KF, Phipps RP, Masel D, Refaai MA, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion 2010; 50:2738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raval JS, Mazepa MA, Russell SL, Immel CC, Whinna HC, Park YA. Passive reporting greatly underestimates the rate of transfusion-associated circulatory overload after platelet transfusion. Vox Sanguinis 2015; 108:387–92. [DOI] [PubMed] [Google Scholar]
- 24.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood 2009; 113:3406–17. [DOI] [PubMed] [Google Scholar]
- 25.Stanworth SJ, Navarrete C, Estcourt L, Marsh J. Platelet refractoriness–practical approaches and ongoing dilemmas in patient management. Br J Haematol 2015; 171:297–305. [DOI] [PubMed] [Google Scholar]
- 26.Vassallo RR. Recognition and management of antibodies to human platelet antigens in platelet transfusion-refractory patients. Immunohematology 2009; 25:119–24. [PubMed] [Google Scholar]
- 27.Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol 2008; 142:348–60. [DOI] [PubMed] [Google Scholar]
- 28.McFadyen JD, Kaplan ZS. Platelets are not just for clots. Transfus Med Rev 2015; 29:110–9. [DOI] [PubMed] [Google Scholar]
- 29.Stanworth SJ, Estcourt LJ, Llewelyn CA, Murphy MF, Wood EM. Impact of prophylactic platelet transfusions on bleeding events in patients with hematologic malignancies: a subgroup analysis of a randomized trial. Transfusion 2014; 54:2385–93. [DOI] [PubMed] [Google Scholar]
- 30.Estcourt LJ, Stanworth SJ, Doree C, Hopewell S, Trivella M, Murphy MF. Comparison of different platelet count thresholds to guide administration of prophylactic platelet transfusion for preventing bleeding in people with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation. Cochrane Database Syst Rev 2015:Cd010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung AD, Yen R, Deoliveira D, Jiao Y, Piryani S, Bernanke A, et al. Fibrinogen-coated nanospheres prevent thrombocytopenia-related bleeding. Biol Blood Marrow Transplant 2015; 21:S111–S3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Measurement of the size distribution of nanospheres. Nanoparticles were visualized by light scattering, and a snapshot of nanoparticle Brownian motion recorded for nanoparticle hydrodynamic size measurement. Nanoparticle size distribution was normalized by the highest concentration.
Fig. S2. FCNs bind to endothelium for up to 48 h after injection. Two-photon in vivo microscopy of a murine ear vessel 48 h after injection of Alexa Fluor 488-labeled FCNs showed persistent green signal lining the endothelium.