Abstract
Background:
Adiponectin, an adipocytokine, plays an important role in the development of Type 2 Diabetes Mellitus (T2DM) in obese and cardiovascular disease patients, with few studies having observed low plasma concentrations. Persistent low-grade inflammation, an important feature in T2DM and obesity, bears an indirect influence on insulin resistance and insulin secretion and is reflected by increased plasma levels of C-reactive protein (CRP). Thus, low levels of anti-inflammatory cytokine, adiponectin, depicts that inflammation could be the link between T2DM, obesity and adiponectin. Since these factors need to be explored to prevent or adequately treat T2DM, especially among Indian diabetics, this study was undertaken. Also of interest was to assess its salivary concentrations.
Aim:
This study aimed to assess serum and salivary adiponectin levels in newly diagnosed T2DM individuals along with postprandial blood sugar (PPBS) and glycosylated hemoglobin (HbA1C) and high-sensitivity-CRP (hs-CRP).
Materials and Methods:
Serum and salivary levels of adiponectin, PPBS, HbA1c and hs-CRP were assessed in 30 newly diagnosed T2DM (Group I) individuals and compared with 30 healthy individuals (Group II, healthy control). Glucose oxidase peroxidase, automatic analyzer, turbidimetric immunoassay and ELISA methods were adopted for PPBS, HbA1c, hs-CRP and adiponectin estimation.
Results:
Statistically significant decrease in mean serum (16.93 ± 3.86) and salivary (24.96 ± 8.21) adiponectin levels, were observed in Group I compared to Group II individuals with a p value of 0.00 and 0.04 respectively. In Group I individuals a significant p value of 0.02 was noted only between salivary adiponectin and PPBS. None of the other parameters correlated significantly with serum adiponectin levels.
Conclusion:
Decreased serum and salivary adiponectin levels in T2DM furthered the importance of its role in Indian T2DM. Decreased salivary adiponectin levels probably reflected salivary hypofunction. This being the preliminary study in saliva, more studies are required to emphasize its role both as a diagnostic marker and as an anti-inflammatory cytokine in T2DM.
Keywords: Adiponectin, saliva, serum, type 2 diabetes mellitus
INTRODUCTION
Type 2 diabetes mellitus (T2DM) formerly known as noninsulin-dependent DM, is the most common form of DM characterized by hyperglycemia, insulin resistance (IR) and relative insulin deficiency accompanied by a greater or lesser impairment in the metabolism of carbohydrates, lipids and proteins. Globally, as of 2011, an estimated 366 million people were diabetic, with T2DM making up about 90% of the cases, and this figure is estimated to cross 552 million by 2030.[1]
A newly discovered adipocytokine, i.e., adiponectin by stimulating oxidation in muscle and decreasing IR in the liver indicated its possible involvement in the development of T2DM (proven in both human and animal studies).[2] Reduced adiponectin levels are mainly due to the low levels of high molecular weight (HMW) hexameric form of the gene which is present in patients with cardiovascular disease or IR. These gene mutations impair the formation of HMW adiponectin hexamers resulting in less release of adiponectin from adipocytes.[3] Decreased levels of plasma adiponectin can cause decreased glucose uptake, increased gluconeogenesis and decreased fatty acid oxidation in the skeletal muscles and the liver. The decrease of fatty acid oxidation causes the increase of free fatty acids, following increase of IR, which leads to further decrease in glucose uptake. The decrease in glucose uptake and the increase of neoglucogenesis ultimately results in increase of plasma glucose and T2DM. Oxidative stress in T2DM individuals also results in increased IR and decreased adiponectin production.[4]
Adiponectin acts to increase insulin sensitivity, fatty acid oxidation, as well as energy expenditure and reduces the production of glucose by the liver. It sensitizes the effects of insulin in liver without affecting peripheral glucose disposal. Thus, its deficiency might contribute to the development of T2DM.[5]
Low plasma adiponectin concentrations were observed in obesity, T2DM and in patients with coronary artery disease where it was predictive of prospective diabetes.[6] Circulatory levels of adiponectin were found to correlate negatively with IR, serum triglycerides, fasting serum insulin and fasting plasma glucose concentrations.
Saliva is such sample, which is abundant, noninvasive and very easily obtained without any harm to the patient. Several analytes are present in saliva in amounts that relate to blood.[7] The relationship between protein levels in saliva and plasma makes saliva an attractive diagnostic tool that could be used as an alternative to blood in tests measuring biomarkers.
Thus, few abroad and only three Indian studies have recorded decreased serum adiponectin levels in T2DM, but none were in newly diagnosed T2DM individuals. Only two abroad studies[2,8] and none in the Indian population have explored the salivary adiponectin levels.
MATERIALS AND METHODS
Our study included 30 individuals with newly diagnosed T2DM and age-matched 30 healthy nondiabetic individuals, who served as controls (Healthy Control [HC]) [Table – I]. Newly diagnosed diabetic individuals (T2DM) (Group-I) were selected from the Endocrinology Outpatient Department, Narayana Super Specialty Hospital. They were diagnosed as having T2DM by the endocrinologist and had laboratory values of postprandial blood sugar (PPBS) >140 mg/dl and glycosylated hemoglobin (HbA1c) >6.5 (as per the criteria established by the Expert Committee on Diagnosis and Classification of Diabetes Mellitus [1998]) [Table – I]).[1] The healthy individuals (HC) (Group-II) were chosen from the outpatient pool of department of oral medicine. They were assessed for PPBS and HbA1c levels. Those with PPBS values<140 mg/dl and HbA1c levels<6.5 were included in the study [Table – I]. Exclusion criteria of tobacco smoking, oral lesions other than dental caries, gingivitis and periodontitis were considered common to both groups while the individuals of T2DM group were excluded if they presented with diabetic complications (retinopathy, peripheral neuropathy and nephropathy), systemic illnesses (other than T2DM) and malignancies. Ethical approval for the present study was obtained from the Institutional Ethical Committee.
Table 1.
Study characteristics
| Baseline Data | Cases (n=30) T2DM | Control (n=30) HC | |||
|---|---|---|---|---|---|
| AGE^ | 47.73 | 8.71 | 47.40 | 7.33 | |
| PPBS | ≥140mg/dl | <140mg/dl | |||
| HbA1c | ≥6.5 | < 6.5 | |||
| Gender | Female | 13 | 43% | 14 | 47% |
| Male | 17 | 57% | 16 | 53% | |
^ mean (SD) as summary measure. T2DM - Type-II Diabetes Mellitus ; HC - Healthy control
Serum collection
A volume of 4 ml of peripheral venous blood was drawn from the antecubital fossa by venipuncture method, using a 20-gauge needle with a 5-ml syringe. The blood was then transferred to the vacutainer tubes and immediately transferred to the laboratory. The blood sample was allowed to clot at room temperature and after 1 h, serum was separated from the blood by centrifuging at 3,000 rpm for 15 min. 0.5 ml of serum was utilized for PPBS by glucose oxidase-peroxidase method (Kit-M/S Excel Diagnostics Pvt. Ltd., India) and HbA1C estimation (Kit-Nycocard, India) was performed in an automatic analyzer (BIO-RAD D-10-HPLC ANALYZER) only for Group-II individuals. The serum of all individuals from both groups was stored in Eppendorf tubes for adiponectin and high-sensitivity-C-reactive protein (hs-CRP) estimation.
Saliva collection
Following the blood collection, unstimulated whole salivary samples (spit technique) were collected in a sterile graduated plastic container for 10 min, for each patient under standardized conditions. The collected salivary sample containers were stored at −20°C in cold storage room.
High sensitivity-C-reactive protein estimation
It was done using hs-CRP Turbidimetric Immunoassay method (Kit-Particle Enhanced Immuno Turbidimetry, ARKRAY Health Care Pvt. Ltd., India).
Assessment of serum and salivary adiponectin
Adiponectin levels were assessed with HUMAN ADIPONECTIN ELISA KIT (Chongqing Biospes Co., Ltd, China (Catalog No.: BEK1196), using Enzyme-linked Immunosorbent assay method. Anti-adiponectin polyclonal antibody was precoated onto 96-well plates. Moreover, the biotin-conjugated anti-adiponectin polyclonal antibody was used as detection antibody. The standards, test samples and biotin-conjugated detection antibody were added to the wells subsequently, followed by washing it with wash buffer. Then, avidin-biotin-peroxidase complex was added, and unbound conjugates were washed away with wash buffer solution. Then, TMB substrates were used to visualize HRP enzymatic reaction. TMB was catalyzed by HRP to produce a blue color product which was changed into yellow after adding acidic stop solution. The density of yellow was proportional to the adiponectin amount of sample captured in plate. O. D. absorbance was read at 450 nm in a microplate reader, and then adiponectin concentration was calculated.
Statistical analysis
Descriptive statistics and analysis were performed in IBM SPSS Version 22.0, IBM, Armonk, NY, US. For continuous variables, the data values were plotted as mean + standard deviation. Student's t-test was used to test the mean difference between two groups. Pearson's correlation test was considered to test the correlation between the groups. All the results with a p value of < 0.05 were considered as statistically significant.
RESULTS
Serum adiponectin levels were significantly lowered in Group I with a mean value of 16.93 ± 3.86, compared to Group II with a mean value of 25.33 ± 1.48 [Table – II]. It was statistically significant with a p value of 0.00 [Table – II]. Even the salivary adiponectin levels decreased in Group I individuals with a mean value of 24.96 ± 8.21 compared to Group II individuals with a mean value of 29.82 ± 9.62 and was significant with a p value of 0.04 [Table – II]. On correlating (Pearson correlation coefficient test), the adiponectin levels between serum and saliva, a pearson correlation co-efficient of 0.25 with a p value of 0.183 was observed, which was not significant [Graph-I].
Table 2.
Mean Values of Salivary & Serum Adiponectin of both Groups (T2DM & HC)
| ADIPONECTIN | Cases (n=30) T2DM | Control (n=30) HC | Mean difference | 95% Confidence Interval of the difference | P | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Lower | Upper | |||
| SERUM ADIPONECTIN | 16.93 | 3.86 | 25.33 | 1.48 | -8.41 | -9.92 | -6.9 | 0.00* |
| SALIVARY ADIPONECTIN | 24.96 | 8.21 | 29.82 | 9.62 | -4.86 | -9.48 | -0.24 | 0.04* |
T2DM - Type-II Diabetes Mellitus ; HC - Healthy control. *Statistically significant result (P<0.05)
Graph 1.
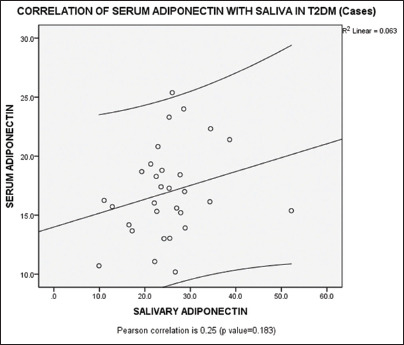
Correlation of serum adiponectin with salivary adiponectin in T2DM (Group – I) individuals
Group I (T2DM) individuals exhibited statistically significant (p value < 0.01) increase in mean values of PPBS (150.33 ± 11.17), HbA1c (7.28 ± 1.16) and hs-CRP (2.45 ± 1.48) compared to Group II (HC) individuals (104.13 ± 12.45), (5.23 ± 0.5) and (1.1 ± 0.53), respectively [Table – III]. Upon establishing a correlation using Pearson correlation test, none of the parameters (PPBS (p value 0.261), HbA1c (p value 0.954) and hs-CRP (p value 0.660)) correlated significantly with that of serum adiponectin [Table - IV]. However a p value of 0.020 (i.e p value < 0.05) was observed on correlating salivary adiponectin with PPBS with a Pearson correlation coefficient of -0.422. No significant correlation was noted between salivary adiponectin and HbA1c (p value 0.924) and hs-CRP (p value 0.686) [Table - V].
Table 3.
Mean Values of PPBS, HbA1c and Hs-CRP of both Groups (T2DM & HC)
| Parameters | Cases (n=30) T2DM | Control (n=30) HC | Mean difference | 95% Confidence Interval of the difference | p | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Lower | Upper | |||
| PPBS | 150.33 | 11.17 | 104.13 | 12.45 | 46.2 | 40.09 | 52.31 | 0.00* |
| HbA1C | 7.28 | 1.16 | 5.23 | 0.5 | 2.05 | 1.59 | 2.51 | 0.00* |
| Hs-CRP | 2.45 | 1.48 | 1.1 | 0.53 | 1.35 | 0.77 | 1.93 | 0.00* |
T2DM - Type-II Diabetes Mellitus ; HC - Healthy control. *Statistically significant result (p<0.01), PPBS: Postprandial blood sugar, HsCRP: Highsensitivity Creactive protein, HbA1C: Glycated hemoglobin
Table 4.
Correlation of Serum Adiponectin with other parameters In T2DM individuals (Group - I)
| Cases (n=30) | CORRELATION VALUE (Pearson Correlation co-efficient) | p |
|---|---|---|
| SERUM ADIPONECTIN & PPBS | -0.212 | 0.261 |
| SERUM ADIPONECTIN & HBA1C | 0.011 | 0.954 |
| SERUM ADIPONECTIN & HS-CRP | -0.084 | 0.660 |
None of the variable is significantly correlated with Serum Adiponectin
Table 5.
Correlation of Salivary Adiponectin with other Parameters in T2DM individuals (Group - I)
| Cases (n=30) | CORRELATION VALUE (Pearson Correlation co-efficient) | p |
|---|---|---|
| SALIVARY ADIPONECTIN & PPBS | -0.422 | 0.020* |
| SALIVARY ADIPONECTIN & HBA1C | -0.018 | 0.924 |
| SALIVARY ADIPONECTIN & HS-CRP | -0.077 | 0.686 |
*Statistically significant result (p<0.05)
DISCUSSION
Diabetes mellitus has earned a place among those noncommunicable diseases which have emerged as the leading causes of death globally, killing more people each year than all other causes combined.[9] As per the International Diabetes Federation, the number of individuals with T2DM is on the rise in almost every country. With currently, 387 million people with diabetes across the world, it is expected to rise to a whooping figure of 592 million in 2035.[10] The need of the hour is early detection of DM, so that appropriate treatment can be initiated at an early stage itself, preventing morbidity and early mortality. Hence, the race is on, not only limited to identifying the presence of modifiable risk factors such as obesity and physical inactivity but also to explore those biomarkers both in serum and saliva which could not only serve as an adjunct to the routine PPBS or HbA1C or oral glucose tolerance tests but also help in linking those risk factors to the disease.
The present study focused on identifying whether serum and salivary adiponectin (ACRP30) levels could serve as a diagnostic marker, in newly diagnosed T2DM individuals belonging to an age range 30–60 years, by correlating with their PPBS and HbA1c levels and also comparing it with age-matched healthy nondiabetic (HC) individuals. The inflammatory link between T2DM and adiponectin was also studied by correlating adiponectin with hs-CRP levels, an inflammatory marker in T2DM individuals.
Newly diagnosed diabetes mellitus individuals (T2DM) were chosen to nullify the effects of anti-diabetic medicines on serum and salivary adiponectin values if any and also to avoid the effect of systemic complications of T2DM on serum adiponectin values.
Statistically significant decreased serum adiponectin levels was noted in newly diagnosed T2DM (Group-I) compared to control group (HC). This was in accordance with all the six studies done in previously diagnosed T2DM [Table - VI].[2,5,11,12,13,14] Some studies related to adiponectin and other adipocytokines in T2DM have considered obesity-related anthropometric measurements also as a parameter.[2,12,14] Although reports claim that these adipocytokines are related to obesity, many Indians belong to the lean sector or show central obesity.[2,6,15]
Table 6.
Studies showing Serum adiponectin levels in T2DM individuals compared to Healthy Controls (HC)
| STUDIES | T2DM | HC |
|---|---|---|
| Supanee Thanakun et.al | 8.09±14.01 | 17.96±46.84 |
| Christian Weyer et.al | 7.6±2.6 | 10.2±4.3 |
| Makota Daimon et.al | 8.01±2.55 | 9.06±2.41 |
| Chamukuttan Snehalatha et.al | 11.3±5.5 | 16.7±7.6 |
| Kikkuko Hotta et.al | 6.6±0.4 | 7.9±0.5 |
| Valsamakis et.al | 2.9±5.5 | 3.4±6.6 |
| Present study in newly diagnosed T2DM individuals | 16.93±3.86 | 25.33±1.48 |
T2DM: Type 2 diabetes mellitus, HC: Healthy controls
Similar to the serum adiponectin levels which lowered, salivary adiponectin levels too decreased significantly in T2DM individuals compared to Healthy Controls, in newly diagnosed T2DM (Group-I) compared to control group (HC). This was in accordance with only one study of SupaneeThanakun et al's (2014)[2] observation of decreased mean levels (1.05 ± 6.48) in T2DM as compared to controls (7.91 ± 6.23) and contrary to that of Jaedicke et al's (2011)[8] observation of increased mean levels (2.0 ± 2.7 in T2DM v/s 1.4 ± 1.6 in HC). But Jaedicke et al (2011)[8] also considered the gingival and periodontal status as they were commonly seen inflammatory conditions in T2DM individuals and attributed the increase in salivary adiponectin levels to the diseased state.[8] Saliva though is considered as an ultrafiltrate of plasma, owing to the age, postmenopausal effects, repertoire of oral microorganisms, salivary pH and flow rate, diet, maintenance of oral hygiene and a plethora of other factors, the salivary constituents especially the proteins may show variations. In T2DM as a result of the involvement of parenchyma of the salivary glands and salivary gland hypofunction, the salivary flow rates decrease thereby concentrating the salivary proteins.[16,17] This could explain the variations in salivary protein levels recorded in other studies including that of ours. A positive correlation between serum and salivary adiponectin levels was noted, though not significant.
It is a golden rule that both PPBS and HbA1C levels get increased in diabetic state,[18,19,20,21] which was also a significant (p value < 0.01) finding in the present study. Thus, they have been quoted as the standalone diagnostic tests for DM.[20,21] Although the statistically significant higher mean values of PPBS and HbA1C affirmed the diabetic status in Group-I individuals, none of these parameters showed a significant correlation (p value > 0.05) with serum adiponectin levels. This was in contrast to that of Weyer et al's (2001) observation.[11] Further though the correlation between serum adiponectin and HbA1C, attempted only in our study remained insignificant, more studies in this regard could be helpful in ascertaining adiponectin as a diagnostic marker for T2DM.
The hs-CRP levels too remained statistically significant (p value < 0.01) in the T2DM group, which is again an important finding as noted by Pan et al., Pfützner and Forst, Ebersole et al., Tutuncu et al.[4,22,23,24] Although the CRP levels tend to increase in diabetic state, their association still remains questionable, as some studies which reported increased CRP levels in T2DM individuals, did not confirm it as a diagnostic marker.[4] Studies exploring other inflammatory markers, such as interleukin (IL)-1, IL-6, IL-8, transforming growth factor β1, tumor necrosis factor-α linked it to subclinical inflammatory end-organ damage in diabetes.[25,26,27,28,29] Thus in our study though we noted that both hs-CRP and adiponectin levels increased in T2DM individuals they were not significantly correlating. Added to this remains the fact that adiponectin is an anti-inflammatory cytokine. Hence more studies need to lend credence to this fact.
Our study remains the first study to correlate salivary adiponectin with the serum parameters of PPBS, HbA1C and hs-CRP in T2DM individuals. Surprisingly only PPBS yielded a significant correlation with a p value = 0.020, which was not observed with serum adiponectin.
CONCLUSION
Lowered Serum and salivary adiponectin levels in T2DM individuals definitely reflected their diabetic status, though the inflammatory link could not be proved and the periodontal status and obesity of T2DM individuals were not considered. Thus, both the serum and the salivary adiponectin assay holds promise and needs to be done considering large populations, more inflammatory markers, periodontal disease and obesity related parameters. Our study is the first preliminary salivary adiponectin study in the Indian population. However its correlation with serum adiponectin levels and other parameters like PPBS, HbA1C and hs-CRP needs to be established further, so that saliva being easily available could be used as an alternative to blood in the diagnosis and disease monitoring of T2DM patients. The limitations could be a lack of availability of these biomarkers in routine pathology laboratories, compounded by the associated increased expenses and assay standardization along with the complexities of the oral environment and the salivary factors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Thanakun S, Watanabe H, Thaweboon S, Izumi Y. Comparison of salivary and plasma adiponectin and leptin in patients with metabolic syndrome. Diabetol Metab Syndr. 2014;6:19. doi: 10.1186/1758-5996-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerre-Millo M. Adiponectin: An update. Diabetes Metab. 2008;34:12–8. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Pan A, Wang Y, Yuan JM, Koh WP. High-sensitive C-reactive protein and risk of incident type 2 diabetes: A case-control study nested within the Singapore Chinese health study. BMC Endocr Disord. 2017;17:8. doi: 10.1186/s12902-017-0159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hamodi Z, Al-Habori M, Al-Meeri A, Saif-Ali R. Association of adipokines, leptin/adiponectin ratio and C-reactive protein with obesity and type 2 diabetes mellitus. Diabetol Metab Syndr. 2014;6:99. doi: 10.1186/1758-5996-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saraheimo M, Forsblom C, Fagerudd J, Teppo AM, Pettersson-Fernholm K, Frystyk J, et al. Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care. 2005;28:1410–4. doi: 10.2337/diacare.28.6.1410. [DOI] [PubMed] [Google Scholar]
- 7.Akuailou EN, Vijayagopal P, Imrhan V, Prasad C. Measurement and validation of the nature of salivary adiponectin. Acta Diabetol. 2013;50:727–30. doi: 10.1007/s00592-012-0388-z. [DOI] [PubMed] [Google Scholar]
- 8.Jaedicke KM, Wassall RR, Taylor JJ, Preshaw PM. 857 Adipokine Concentrations in Patients with Type 2 Diabetes IADR General Session. 2011 [Google Scholar]
- 9.Global Status Report on Non-communicable Diseases 2010. World Health Organization; 2011. [Last accessed on 2014 Jun 14]. Available from: http://wwwwhoint/nmh/publications/ncd_report_full2010/_en/ [Google Scholar]
- 10.International Diabetes Federation. IDF Diabetes Atlas Update Poster. 6th ed. Brussels, Belgium: International Diabetes Federation; 2014. [Google Scholar]
- 11.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 12.Daimon M, Oizumi T, Saitoh T, Kameda W, Hirata A, Yamaguchi H, et al. Decreased serum levels of adiponectin are a risk factor for the progression to type 2 diabetes in the Japanese population: The funagata study. Diabetes Care. 2003;26:2015–20. doi: 10.2337/diacare.26.7.2015. [DOI] [PubMed] [Google Scholar]
- 13.Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A, et al. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian Indians. Diabetes Care. 2003;26:3226–9. doi: 10.2337/diacare.26.12.3226. [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Amado FM, Ferreira RP, Vitorino R. One decade of salivary proteomics: Current approaches and outstanding challenges. Clin Biochem. 2013;46:506–17. doi: 10.1016/j.clinbiochem.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Lasisi TJ, Fasanmade AA. Salivary flow and composition in diabetic and non-diabetic subjects. Niger J Physiol Sci. 2012;27:79–82. [PubMed] [Google Scholar]
- 18.Thorand B, Löwel H, Schneider A, Kolb H, Meisinger C, Fröhlich M, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: Results from the MONICA Augsburg cohort study, 1984-1998. Arch Intern Med. 2003;163:93–9. doi: 10.1001/archinte.163.1.93. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 20.20 Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health. 2015;73:43. doi: 10.1186/s13690-015-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swetha NK. Comparison of fasting blood glucose & post prandial blood glucose with HbA1c in assessing the glycemic control. Int J Healthc Biomed Res. 2014;2:134–9. [Google Scholar]
- 22.Pfützner A, Forst T. High-sensitivity C-reactive protein as cardiovascular risk marker in patients with diabetes mellitus. Diabetes Technol Ther. 2006;8:28–36. doi: 10.1089/dia.2006.8.28. [DOI] [PubMed] [Google Scholar]
- 23.Ebersole JL, Kryscio RJ, Campbell C, Kinane DF, McDevitt J, Christodoulides N, et al. Salivary and serum adiponectin and C-reactive protein levels in acute myocardial infarction related to body mass index and oral health. J Periodontal Res. 2017;52:419–27. doi: 10.1111/jre.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tutuncu Y, Satman I, Celik S, Dinccag N, Karsidag K, Telci A, et al. Acomparison of hs-CRP levels in new diabetes groups diagnosed based on FPG, 2-hPG, or hbA1c criteria. J Diabetes Res. 2016;2016:5827041. doi: 10.1155/2016/5827041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhuri S, Dutta D, Sen A, Chowdhury IH, Mitra B, Mondal LK, et al. Role of N-ε- carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol Vis. 2013;19:100–13. [PMC free article] [PubMed] [Google Scholar]
- 26.Choudhuri S, Chowdhury IH, Das S, Dutta D, Saha A, Sarkar R, et al. Role of NF-κB activation and VEGF gene polymorphisms in VEGF up regulation in non-proliferative and proliferative diabetic retinopathy. Mol Cell Biochem. 2015;405:265–79. doi: 10.1007/s11010-015-2417-z. [DOI] [PubMed] [Google Scholar]
- 27.Choudhuri S, Mandal LK, Paine SK, Sen A, Dutta D, Chowdhury IH, et al. Role of hyperglycemia-mediated erythrocyte redox state alteration in the development of diabetic retinopathy. Retina. 2013;33:207–16. doi: 10.1097/IAE.0b013e318256202e. [DOI] [PubMed] [Google Scholar]
- 28.Mandal LK, Choudhuri S, Dutta D, Mitra B, Kundu S, Chowdhury IH, et al. Oxidative stress-associated neuroretinal dysfunction and nitrosative stress in diabetic retinopathy. Can J Diabetes. 2013;37:401–7. doi: 10.1016/j.jcjd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Choudhuri S, Dutta D, Chowdhury IH, Mitra B, Sen A, Mandal LK, et al. Association of hyperglycemia mediated increased advanced glycation and erythrocyte antioxidant enzyme activity in different stages of diabetic retinopathy. Diabetes Res Clin Pract. 2013;100:376–84. doi: 10.1016/j.diabres.2013.03.031. [DOI] [PubMed] [Google Scholar]


