Abstract
Dialkyldiazirines have emerged as reagents of choice for biological photoaffinity labeling studies. The mechanism of crosslinking has dramatic consequences for biological applications where instantaneous labeling is desirable, as carbene insertions display different chemoselectivity and are much faster than competing mechanisms involving diazo or ylide intermediates. Here, deuterium labeling and diazo compound trapping experiments are employed to demonstrate that both carbene and diazo mechanisms operate in the reactions of a dialkyldiazirine motif that is commonly utilized for biological applications. For the fraction of intermolecular labeling that does involve a carbene mechanism, direct insertion is not necessarily involved, as products derived from a carbonyl ylide are also observed. We demonstrate that a strained cycloalkyne can intercept diazo compound intermediates and serve as a bioorthogonal probe for studying the contribution of the diazonium mechanism of photoaffinity labeling on a model protein under aqueous conditions.
Graphical Abstract
Photoaffinity labeling has become a ubiquitous tool in chemical biology and drug discovery.1 Aromatic diazirines, arylazides, and benzophenone derivatives have long served in photoaffinity labeling applications,1–3 and recent probes based on nitrile imines4 and photoredox catalysis5 offer new possibilities. A consideration for the design of any photoaffinity label (PAL) is the minimization of size and hydrophobicity in order to ameliorate any detrimental impact of the PAL on the function, solubility, permeability, or subcellular localization of the biological molecules being studied.6 Accordingly, dialkyldiazirines have emerged as favored probes for photoaffinity labeling in cellular experiments7–9 with applications that include probing small molecule–protein interactions,6,7 protein–protein interactions,10 nucleic acid–protein interactions,11 metabolic oligosaccharide engineering,12 lipid–protein interactions,13,14 and the study of biological membranes.15
While their physiochemical properties are advantageous for chemical biology, there are important photochemical differences between dialkyldiazirines and more traditional aromatic diazirines. Analogs of α-trifluoromethyl-α-phenyldiazirine are well established biological probes that upon irradiation give high intermolecular labeling yields via formal carbene addition even with aliphatic CH bonds,16 and because α-trifluoromethyl-α-phenylcarbene lacks α-CH bonds, it cannot participate in intramolecular α-C–H insertion processes. α-Trifluoromethyl-α-phenyldiazirine also leads to ∼35% of α-trifluoromethyl-α-phenyldiazomethane.16 While this side product undergoes photochemistry with shorter wavelength UV (∼300 nm),17 it does not absorb at the wavelengths typically used for diazirine activation (>350 nm) and displays high stability in the dark under neutral or acidic conditions.16 Photochemical reactions of dialkyldiazirines are relatively challenging to study because of the susceptibility of both alkylcarbenes and their photoexcited nitrogenous precursors to undergo intramolecular rearrangements with α-hydrogens.18 However, several dialkylcarbenes have been characterized and both singlet and triplet ground states have been observed.19
When diazirines are applied in photoaffinity labeling, it is frequently assumed that carbene insertion reactions are responsible for the bimolecular capture of biological targets, but further consideration is required with dialkyldiazirine precursors (1, Figure 1A). Like aromatic diazirines, aliphatic diazirines partially rearrange to diazo compounds upon irradiation.18 However, aliphatic diazo compounds (2) are readily protonated at neutral pH to give diazonium compounds (4) which are reactive alkylating agents.20,21 While both carbene (3) and diazonium mechanisms can lead to intermolecular labeling, the chemoselectivity of alkyldiazonium labeling is likely to differ from that of a carbene mechanism. Labeling by alkyldiazonium 4 is known to favor esterification reactions,22–25 whereas the reactions of free carbenes are less selective and include aliphatic C–H insertion as a possibility.26 Moreover, crosslinking reactions are extremely rapid for the carbene pathway,26 whereas the alkylation reactions of alkyldiazonium ions are much slower; for example, the reaction of a methanediazonium ion with water has a relatively long half-life of ∼0.4 s in water/THF solution.27,28 Thus, in biological target identification experiments, alkyldiazonium ions are likely to display a much larger labeling radius than more reactive carbene intermediates. Additionally, when alkyldiazonium ions do modify proteins they are likely to modify carboxylic acid residues,22–25 producing esters which may be subsequently hydrolyzed by esterases22–24 in live cell experiments and potentially in the proteomic workflow. Understanding the relative contributions of carbene and diazonium intermediates is therefore critical to the interpretation of biological data from photoaffinity experiments.
Figure 1.
Potential mechanisms for photoaffinity labeling with dialkyldiazirine probes. (A) Both carbene and diazonium intermediates can potentially lead to crosslinking. (B) For diazirines with pendant carbonyls, carbene mechanisms do not necessarily involve direct insertion, but may instead involve formation of ylide and oxocarbenium intermediates.
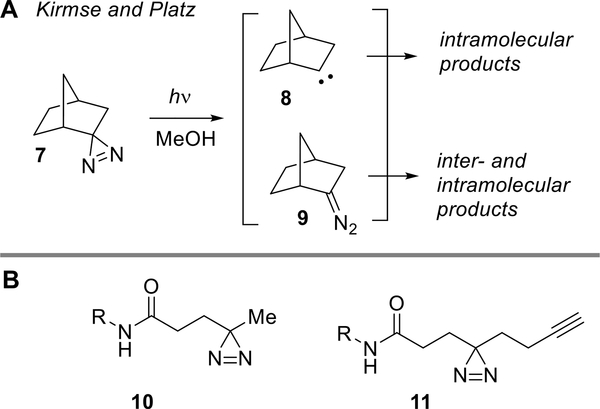
In seminal studies, Kirmse, Platz and coworkers studied the photolyses of spirobicyclic diazirines including norbornane-derived 7, which upon irradiation in MeOH gives a mixture of products that could derive from either carbene 8 or diazo compound 9 (Figure 2A).18 By studying the changes in product distribution in the presence of dipolarophile traps for diazocompounds and by analyzing levels of deuterium incorporation for photolyses in MeOD, it was possible to deduce that diazirine 7 produces both carbene 8 and diazo compound 9 in significant amounts. These studies with bicyclic diazirines not only demonstrated the importance of diazonium ions to intermolecular ether formation but also revealed a structural dependence on the contribution of carbenes to bimolecular chemistry.
Figure 2.
(A) Studies of spirobicyclic diazirines by Kirmse and Platz. (B) Structures of “linear” alkyl diazirines more commonly used in biological photoaffinity labeling.
While “linear” dialkyldiazirines have emerged as reagents of choice for photoaffinity labeling (e.g., 1012 and 11,7 Figure 2B), studies on the mechanism of intermolecular labeling are lacking for the substrate types that are most often used for biological experiments. Another mechanistic consideration for such systems is that carbonyl groups are generally used to attach the probe to a molecule of interest, which opens a pathway for carbenes to react via carbonyl ylides (5) and subsequently formed oxocarbenium ions (6) (Figure 1). Recent LC-MS/MS studies on diazirine photo crosslinking of proteins have uncovered a preference for labeling carboxylic acid residues, suggesting an important role of diazo intermediates.25,29,30 Here, we present direct evidence that both carbene and diazonium mechanisms operate in the reactions of a dialkyldiazirine motif commonly utilized for photoaffinity labeling. We demonstrate that a strained cycloalkyne can intercept diazocompound intermediates20 and serve as a bioorthogonal probe for studying the contribution of the diazonium mechanism of photoaffinity labeling on a model protein under aqueous conditions.
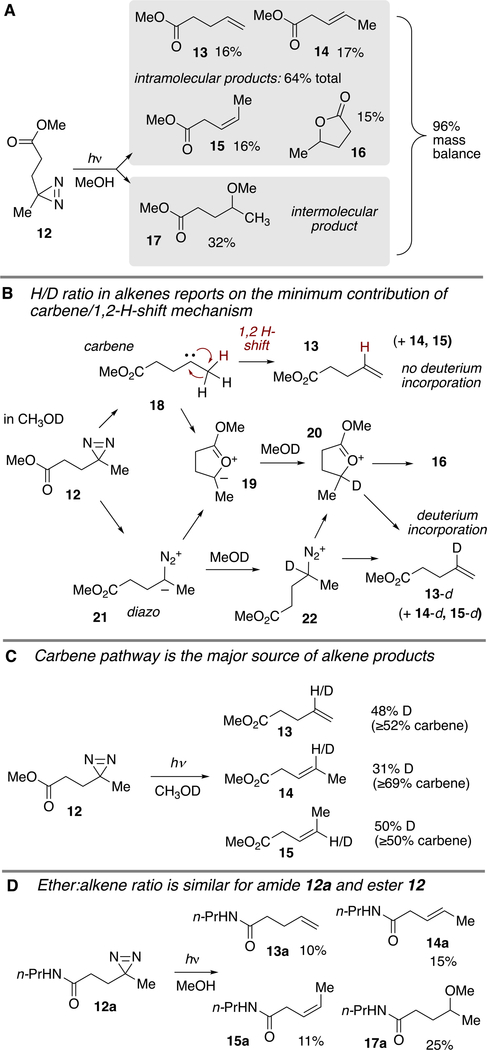
Our study began by observing the products formed when methanol solutions of diazirine 12 (60 mM) were irradiated at 350 nm in the air to produce three alkenes 13–15, lactone 16, and the methanol adduct 1731 (Figure 3A). Based on corrected GC yields there was a 64% yield of the intramolecular products 13–16 and a 32% yield of the intermolecular product 17. Alkene products 13–15 may arise via carbene 18 or via diazo 21/diazonium 22 (Figure 3B,C). For carbene 18, the alkenes can form either by 1,2 hydride (1,2 H–) shift or via the intermediacy of ylide 19 and oxocarbenium 20. Among these mechanisms, only the carbene concerted 1,2 H-shift does not involve protonation. Thus, for irradiations carried out in methanol-d, the D/H ratio reports on the contribution of the carbene 1,2 H-shift mechanism for the formation of alkenes 13–15,18 as alkenes 13-d, 14-d, and 15-d arise from protonation/elimination of diazo 21 or ylide 19. As shown in Figure 3C, the level of deuterium incorporation for 13, 14, and 15 was 48%, 31%, and 50%, respectively. Hence, the carbene concerted 1,2 H-shift mechanism is responsible for at least 50–69% of the alkene products 13–15.
Figure 3.
(A) Products from photolysis of diazirine 12. (B) Mechanistic pathways of intramolecular product formation for photolyses in MeOD leading to deuterated and non-deuterated alkenes 13–15. (C) D/H ratio serves as a reporter of the carbene 1,2 H-migration pathway. (D) The ratio of alkene:ether products from amide 12a is similar to that from ester 12.
Additionally, the diazirinyl amide 12a was prepared and irradiated in MeOH (350 nm, 2 h) and by NMR analysis was observed to give alkene (13a–15a) and ether (17a) products in 61% yield and ratios that were similar to what was observed with diazirinyl ester 12. Lactone 16 was not observed, nor was the cyclic butyrolactam, 5-methyl-1-propylpyrrolidin-2-one.32
Dipolarophiles such as fumaronitrile and diethyl fumarate participate in cycloaddition reactions with diazo compounds formed photochemically from diazirines.33 These dipolarophiles have been used as mechanistic probes in the reactions of spirocyclic diazirines,18,34 but are also potent electrophiles toward biological nucleophiles. We therefore sought to identify a bioorthogonal dipolarophile that could intercept intermediate diazo compounds under biologically relevant conditions. Cycloalkynes including the cyclooctyne BCN participate in a number of bioorthogonal reactions35 including 3 + 2 cycloadditions with diazo compounds.20 As shown in Figure 4A, the photolysis of diazirine 12 in MeOH in the presence of BCN (1.1 equiv) produces cycloadduct 23 as a mixture of diastereomers in 22% isolated yield, thus demonstrating the ability of BCN to intercept diazo intermediate 21. As depicted in Figure 4B,D, the inclusion of dipolarophile in photolyses of 12 (60 mM) in MeOD also has influence on D-incorporation for alkene products 13–15. Interception of diazo 21 by BCN is expected to lead to a decrease in the yield and the D/H ratio for alkenes 13–15. In the absence of BCN, alkene products were formed in combined 52% yield and with 31–50% D incorporation. However, the photolysis of 60 mM 12 in the presence of 70 mM BCN gave alkenes 13–15 with a yield decrease to 33% and with only 6–9% D incorporation.36 This decrease in alkene yield and D-incorporation is consistent with the capture by BCN of diazo 21, which is partly responsible for the formation of alkenes 13-d, 14-d, and 15-d. Similar decreases in yield and D-incorporation were noted for the photolysis in the presence of fumaronitrile.18
Figure 4.
(A) Preparative reaction of diazirine 12 with the cycloalkyne BCN to produce cycloadduct 23 is proposed to occur by [3 + 2] cycloaddition with intermediate diazo compound 21, which (B) influence formation of intramolecular products by decreasing the yield and level of D-incorporation for alkenes 13–15, and (C) on intermolecular chemistry by decreasing the yield of product 17. (D) Effect of product formation by dipolarophiles BCN and fumaronitrile.
As depicted in Fig 4C,D, the inclusion of dipolarophiles in photolyses of 12 also decreases the yield of bimolecular product 17. The yield of ether 17 without added dipolarophile is 29%, whereas irradiation of 12 (60 mM) with either BCN (70 mM) or fumaronitrile (200 mM) gives 17 in 14% yield. Thus, intercepting diazo intermediate 21 leads to a major reduction in bimolecular product formation. These results illustrate the importance of diazo compound protonation/alkylation for intermolecular product formation.
Persistently formed in the photolyses is lactone 16, which is proposed to arise via protonation of ylide 19 and subsequent hydrolysis of oxocarbenium 20 by adventitious water. Intermediates 19 and 20 may arise from either the carbene pathway (via cyclization of 18) or the diazo pathway (via cyclization of either 21 to 19, or 22 to 20 (Figure 3B)). Consistent with all of these mechanisms, 16 shows >99% D-incorporation when photolyses are carried out in MeOD. The formation of ylide 19 from carbene 18 provides a possible explanation for the incomplete ability of dipolarophiles to suppress D-incorporation in alkenes 13–15, as would be expected if the carbene 1,2 H-shift was the sole pathway (Figure 2).37 The formation of 19 from 18 also explains the incomplete suppression of bimolecular adduct 17 (Figure 4B,C). Thus, while carbene 18 may lead to the formation of an intermolecular product 17, this is most likely preceded by an intramolecular reaction to produce ylide 19 based on earlier conclusions that intramolecular H-shifts are generally too rapid for intermolecular reactions to compete.18,38–40 Also consistent with the formation of ylide 19 from carbene 18 is the incomplete ability of dipolarophiles to suppress the formation of lactone 16, suggesting that 16 arises from a combination of the carbene and diazo pathways.
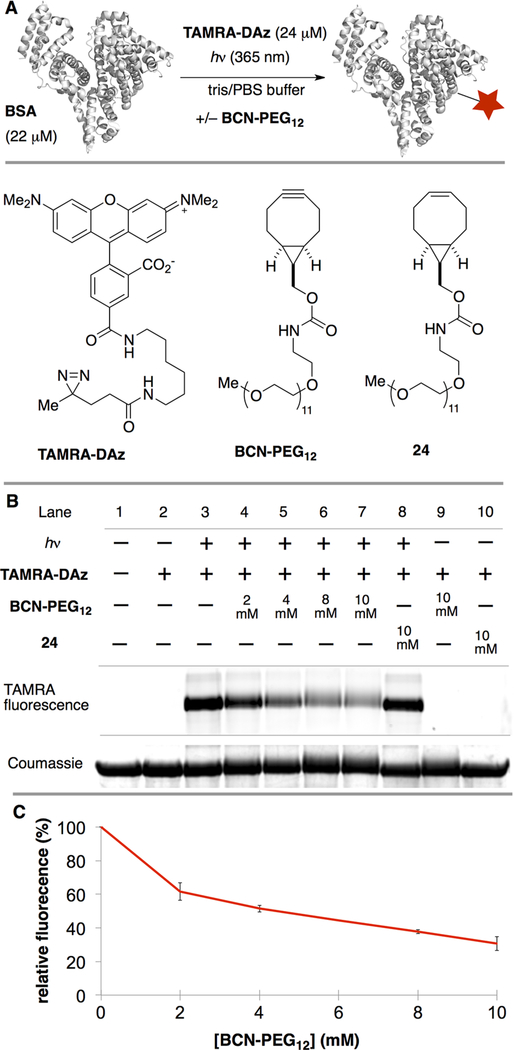
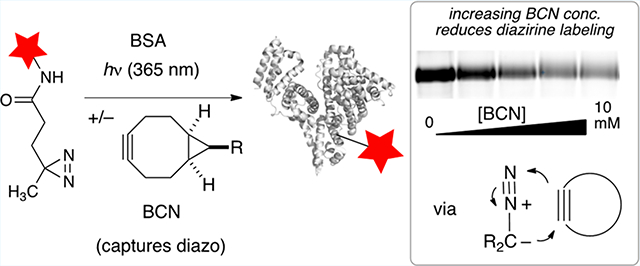
We next sought to use BCN to probe the mechanism of protein photoaffinity labeling. Rhodamines are known to non-selectively bind to bovine serum albumin (BSA), and Jewett had previously demonstrated fluorescent labeling of BSA with a rhodamine–diazirine conjugate.41 Accordingly, we prepared a fluorescent diazirine, TAMRA-DAz, and the water-soluble BCN-PEG12 (Figure 5A). BSA was reduced by DTT and alkylated with iodacetamide before usage to minimize cysteine labeling by the BCN reagent.42,43 As shown in Figure 5B, irradiating a solution of BSA (22 μM) with TAMRA-DAz (24 μM) in PBS/tris buffer for 15 min at 365 nm led to fluorescent labeling as judged by SDS-PAGE (lane 3), whereas controls without light gave no labeling (lanes 2, 9, 10). The inclusion of BCN-PEG12 (2–10 mM) in photolysis experiments with BSA led to decreased labeling by TAMRA-DAz, with up to a 69% decrease at the highest BCN-PEG12 concentration (lanes 4–7). However, no suppression of fluorescence was observed upon irradiation in the presence of 24, an analog of BCN-PEG12 that lacks the reactive alkyne (lane 8). As further evidence that the diazonium mechanism is partly responsible for protein labeling, an additional control was carried out in the presence of sodium acetate, which would be expected to decrease labeling by competing for the diazonium intermediate.22–25 As shown in Figure S1, inclusion of NaOAc (1 M) during the irradiation of TAMRA-DAz with BSA led to a 45% decrease in labeling.
Figure 5.
(A) Experiment involving BSA, TAMRA-DAz, BCN-PEG12, and 24. (B) Lane assignments in SDS-PAGE. (C) Relative fluorescence with 0 to 10 mM BCN-PEG12.
These protein labeling results are consistent with the results in methanol that show that intermolecular labeling can be suppressed by capturing diazoalkane intermediates, and support the hypothesis that the diazonium alkylation pathway is a major pathway in photoaffinity labeling. The inability to completely quench labeling by addition of BCN-PEG12 does suggest a role for the a carbene intermediates in diazirine photoaffinity labeling. However, the mechanism by which the carbenes studied here label proteins is unclear and may not involve commonly assumed carbene insertion reactions, but could instead involve the intermediacy of ylide and oxocarbenium intermediates. These mechanistic details have consequences for chemical biology applications, as alkylations of diazonium or oxocarbenium ions are much slower than carbene insertion reactions which may in turn negatively impact the radius of labeling in protein identification experiments. Additionally, reactions of diazo compounds with proteins are known to selectively react with carboxylic acid residues to give esters,22–25 which may not be stable in live cell applications due to native esterases and/or the proteomic workflow employed.22–24
The long-recognized problem of detangling the chemistry of carbenes from the reactions of their precursors remains incredibly relevant today.18,39,44–48 The field of chemical biology will benefit from future studies to develop photoaffinity tags with desirable physiochemical properties that can label biological targets via a “real” carbene insertion mechanism.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH GM132460 and Pfizer Inc. J.G.K.O. and A.J. were supported as CBI fellows through T32-GM133395, and J.G.K.O. thanks the University of Delaware for fellowship support. We thank R. Knowles and his group for sending us analytical samples of 13a and 5-methyl-1-propylpyrrolidin-2-one. Instrumentation was supported by NIH Awards P20GM104316, P30GM110758, S10OD025185, S10RR026962, and S10OD016267 and NSF Awards CHE-0840401, CHE-1229234, and CHE-1048367.
Footnotes
The authors declare the following competing financial interest(s): C.W.A. is an employee of Pfizer Inc.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.0c02714.
Experimental procedures, characterization details, and copies of 1H and 13C NMR spectra for new compounds (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.orglett.0c02714
Contributor Information
Jessica G. K. O’Brien, Department of Chemistry and Biochemistry, University of Delaware, Newark, Delaware 19716, United States
Andrew Jemas, Department of Chemistry and Biochemistry, University of Delaware, Newark, Delaware 19716, United States.
Papa Nii Asare-Okai, Department of Chemistry and Biochemistry, University of Delaware, Newark, Delaware 19716, United States.
Christopher W. am Ende, Pfizer Worldwide Research and Development, Groton, Connecticut 06340, United States.
Joseph M. Fox, Department of Chemistry and Biochemistry, University of Delaware, Newark, Delaware 19716, United States.
REFERENCES
- (1).Halloran MW; Lumb J-L Recent Applications of Diazirines in Chemical Proteomics. Chem. - Eur. J 2019, 25, 4885–4898. [DOI] [PubMed] [Google Scholar]
- (2).Mishra PK; Yoo C-M; Hong E; Rhee HW Photo-crosslinking: An Emerging Chemical Tool for Investigating Molecular Networks in Live Cells. ChemBioChem 2020, 21, 924. [DOI] [PubMed] [Google Scholar]
- (3).Dubinsky L; Krom BP; Meijler MM Diazirine based photoaffinity labeling. Bioorg. Med. Chem. 2012, 20, 554–570. [DOI] [PubMed] [Google Scholar]
- (4).Herner A; Marjanovic J; Lewandowski TM; Marin V; Patterson M; Miesbauer L; Ready D; Williams J; Vasudevan A; Lin Q 2-Aryl-5-carboxytetrazole as a New Photoaffinity Label for Drug Target Identification. J. Am. Chem. Soc. 2016, 138, 14609–14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Loh YY; Nagao K; Hoover AJ; Hesk D; Rivera NR; Colletti SL; Davies IW; MacMillan DWC Photoredoxcatalyzed deuteration and tritiation of pharmaceutical compounds. Science 2017, 358, 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).MacKinnon AK; Taunton J Target Identification by Diazirine Photo-Cross-linking and Click Chemistry. Curr. Protoc. Chem. Biol. 2009, 1, 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Li Z; Hao P; Li L; Tan CYJ; Cheng X; Chen GYJ; Sze SK; Shen H-M; Yao SQ Design and Synthesis of Minimalist Terminal Alkyne-Containing Diazirine Photo-Crosslinkers and Their Incorporation into Kinase Inhibitors for Cell- and Tissue-Based Proteome Profiling. Angew. Chem., Int. Ed. 2013, 52, 8551–8556. [DOI] [PubMed] [Google Scholar]
- (8).Das J Aliphatic Diazirines as Photoaffinity Probes for Proteins: Recent Developments. Chem. Rev. 2011, 111, 4405–4417. [DOI] [PubMed] [Google Scholar]
- (9).Chang C-F; Mfuh A; Gao J; Wu H-Y; Woo CM Synthesis of an electronically-tuned minimally interfering alkynyl photo-affinity label to measure small molecule-protein interactions. Tetrahedron 2018, 74, 3273–3277. [Google Scholar]
- (10).Murale DP; Hong SC; Haque MM; Lee J-S, Photo-Affinity Labeling (PAL) in Chemical Proteomics: a Handy Tool to Investigate Protein-Protein Interactions (PPIs). Proteome Sci. 2016, 15 DOI: 10.1186/s12953-017-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Song C-X; He C Bioorthogonal Labeling of 5-Hydroxymethylcytosine in Genomic DNA and Diazirine-Based DNA Photo-Cross-Linking Probes. Acc. Chem. Res. 2011, 44, 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bond MR; Zhang H; Kim J; Yu S-H; Yang F; Patrie SM; Kohler JJ Metabolism of Diazirine-Modified N-Acetylmannosamine Analogues to Photo-Cross-Linking Sialosides. Bioconjugate Chem. 2011, 22 (22), 1811–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Feltes M; Moores S; Schaffer JE Synthesis and characterization of diazirine alkyne probes for the study of intracellular cholesterol trafficking. J. Lipid Res. 2019, 60, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Xia Y; Peng L Photoactivatable Lipid Probes for Studying Biomembranes by Photoaffinity Labeling. Chem. Rev. 2013, 113, 7880–7929. [DOI] [PubMed] [Google Scholar]
- (15).Peng T; Hang HC Bifunctional Fatty Acid Chemical Reporter for Analyzing S-Palmitoylated Membrane Protein–Protein Interactions in Mammalian Cells. J. Am. Chem. Soc. 2015, 137, 556–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Brunner J; Senn H; Richards FM 3-Trifluoromethyl-3-phenyldiazirine. J. Biol. Chem. 1980, 255 (8), 3313–3318. [PubMed] [Google Scholar]
- (17).Chee G-L; Yalowich JC; Bodner A; Wu X; Hasinoff BB A diazirine-based photoaffinity etoposide probe for labeling topoisomerase II. Bioorg. Med. Chem. 2010, 18, 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kirmse W; Meinert T; Modarelli DA; Platz MS Carbenes and the O-H Bond: Bicycloalkylidenes. J. Am. Chem. Soc. 1993, 115, 8918–8927. [Google Scholar]
- (19).Platz MS A Perspective on Physical Organic Chemistry. J. Org. Chem. 2014, 79, 2341–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Mix KA; Aronoff MR; Raines RT Diazo Compounds: Versatile Tools for Chemical Biology. ACS Chem. Biol. 2016, 11, 3233–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).McGarrity JF In The Chemistry of Diazonium and Diazo Groups; Patai S, Ed.; Wiley: Chichester, 1978. [Google Scholar]
- (22).McGrath NA; Andersen KA; Davis AKF; Lomax JE Diazo compounds for the bioreversible esterification of proteins. Chem. Sci. 2015, 6, 752–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Mix KA; Lomax JE; Raines RT Cytosolic Delivery of Proteins by Bioreversible Esterification. J. Am. Chem. Soc. 2017, 139, 14396–14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ressler VT; Mix KA; Raines RT Esterification Delivers a Functional Enzyme into a Human Cell. ACS Chem. Biol. 2019, 14, 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Iacobucci C; Götze M; Piotrowski C; Arlt C; Rehkamp A; Ihling C; Hage C; Sinz A Carboxyl-Photo-Reactive MS-Cleavable Cross-Linkers: Unveiling a Hidden Aspect of Diazirine-Based Reagents. Anal. Chem. 2018, 90, 2805–2809. [DOI] [PubMed] [Google Scholar]
- (26).Kirmse W Carbene Chemistry, 2nd ed.; Academic Press: New York, 1971. [Google Scholar]
- (27).McGarrity JF; Smyth T Hydrolysis of Diazomethane—Kinetics and Mechanism. J. Am. Chem. Soc. 1980, 102, 7303–7308. [Google Scholar]
- (28).Pearce M The Rates of Diazomethane Formation from Methylnitrosoamides. The Stability of Diazomethane Solutions towards Aqueous Alkalis. Helv. Chim. Acta 1980, 63, 887–891. [Google Scholar]
- (29).Jumper CC; Bomgarden R; Rogers J; Etienne C; Schriemer DC High-Resolution Mapping of Carbene-Based Protein Footprints. Anal. Chem. 2012, 84, 4411–4418. [DOI] [PubMed] [Google Scholar]
- (30).Ziemianowicz DS; Bomgarden R; Etienne C; Schriemer DC Amino Acid Insertion Frequencies Arising from Photoproducts Generated Using Aliphatic Diazirines. J. Am. Soc. Mass Spectrom. 2017, 28, 2011–2021. [DOI] [PubMed] [Google Scholar]
- (31).King S Orthoester-Dependent Alcoholysis of Lactones. Facile Preparation of 4-Alkoxybutanoates and 5-Alkoxypentanoates. J. Org. Chem. 1994, 59, 2253–2256. [Google Scholar]
- (32).Nguyen ST; Zhu Q; Knowles RR PCET-Enabled Olefin Hydroamidation Reactions with N-Alkyl Amides. ACS Catal. 2019, 9 (5), 4502–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Fairfax DJ; Austin DJ; Xu SL; Padwa A Alternatives to a-Diazo Ketones for Tandem Cyclization-Cycloaddition and Carbenoid-Alkyne Metathesis Strategies. Novel Cyclic Enol-Ether Formation via Carbonyl Ylide Rearrangement Reactions. J. Chem. Soc., Perkin Trans. 1 1992, 2837–2844. [Google Scholar]
- (34).Knoll W; Mieusset J-L; Arion VB; Brecker L; Brinker UH 2H-Azirines from a Concerted Addition of Alkylcarbenes to Nitrile Groups. Org. Lett. 2010, 12, 2366–2369. [DOI] [PubMed] [Google Scholar]
- (35).Dommerholt J; Schmidt S; Temming R; Hendriks LJA; Rutjes FPJT; van Hest JCM; Lefeber DJ; Friedl P; van Delft FL Angew. Chem., Int. Ed. 2010, 49, 9422–9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).For each alkene, the decrease in yield corresponded within 3% of the expected yield based on the D/H ratio. See Table S9 in the Supporting Information.
- (37).Complete suppression of D-incorporation was observed for intramolecular products in studies by Kirmse and Platz with bicyclic diazirine 9, which lacks a carbonyl and therefore cannot form a carbonyl ylide intermediate. See ref 18.
- (38).Sugiyama MH; Celebi S; Platz MS A Significant Barrier to 1,2 Hydrogen Migration in Singlet 1-Phenylethylidene. A Laser Flash Photolysis Study. J. Am. Chem. Soc. 1992, 114, 966–973. [Google Scholar]
- (39).Modarelli DA; Morgan S; Platz MS Carbene Formation, Hydrogen Migration, and Fluorescence in the Excited States of Dialkyldiazirines. J. Am. Chem. Soc. 1992, 114, 7034–7041. [Google Scholar]
- (40).Modarelli DA; Platz MS Interception of Dimethylcarbene with Pyridine: A Laser Flash Photolysis Study. J. Am. Chem. Soc. 1991, 113, 8985–8986. [Google Scholar]
- (41).Ahad AM; Jensen SM; Jewett JC A Traceless Staudinger Reagent To Deliver Diazirines. Org. Lett. 2013, 15, 5060–5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).van Geel R; Prujin GJM; van Delft FL; Boelens WC Preventing Thiol-Yne Addition Improves the Specificity of Strain-Promoted Azide–Alkyne Cycloaddition. Bioconjugate Chem. 2012, 23, 392–398. [DOI] [PubMed] [Google Scholar]
- (43).Tian H; Sakmar TP; Huber T A simple method for enhancing the bioorthogonality of cyclooctyne reagent. Chem. Commun. 2016, 52, 5451–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Fox JM; Scacheri JEG; Jones KGL; Jones M Jr.; Shevlin PB; Armstrong B; Sztyrbicka R The Phenylcarbene Rearrangement as a Source of Real Carbenes. Tetrahedron Lett. 1992, 33, 5021–5024. [Google Scholar]
- (45).Chen N; Jones M Jr.; White WR; Platz MS Equilibrium between Homocub-1(9)-ene and Homocub-9-ylidene. J. Am. Chem. Soc. 1991, 113, 4981–4992. [Google Scholar]
- (46).Mansoor AM; Stevens IDR Hot radical effects in carbene reactions. Tetrahedron Lett. 1966, 7, 1733–1737. [Google Scholar]
- (47).Chang KT; Shechter H Roles of Multiplicity and Electronic Excitation on Intramolecular Reactions of Alkylcarbenes in Condensed Phase. J. Am. Chem. Soc. 1979, 101, 5082–5084. [Google Scholar]
- (48).Chambers GR; Jones M Jr. Intermolecular reactions of 4,4dimethylcyclohex-2-enylidene. J. Am. Chem. Soc. 1980, 102, 4516–4518. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.