Abstract
Purpose
Phentolamine mesylate ophthalmic solution (PMOS), applied to the eye topically, was shown previously to have beneficial effects in patients with dim light vision disturbances (DLD), including decreased pupil diameter (PD), improved best-corrected distance visual acuity (BCDVA), as well as lower intraocular pressure (IOP). The ORION-1 trial evaluated the long-term safety and efficacy of PMOS in a glaucomatous, presbyopic population.
Patients and Methods
In this randomized, double-masked, multi-center, placebo-controlled, multiple-dose Phase 2b trial, 39 patients with elevated IOP were randomized to receive one evening dose of study medication or placebo for 14 days. The primary outcome measure was mean change in diurnal IOP, and the key secondary outcome measures included changes in PD, distance-corrected near visual acuity (DCNVA), and conjunctival hyperemia.
Results
Use of 1% PMOS did not lead to a statistically significant decrease in diurnal IOP compared to placebo (P = 0.89) but trended toward a greater decrease in patients with lower IOP baselines. PMOS produced a statistically significant mean 20% PD reduction under both photopic and mesopic conditions that was sustained for 36 hours post-dosing. A statistically significant number of patients with PMOS compared to placebo demonstrated ≥1 line of improvement in photopic DCNVA at day 8 (P = 0.0018), day 15 (P = 0.0072), and day 16 (P = 0.0163), with a trend for 2- and 3-line improvements at all time points. There was no statistical difference in conjunctival hyperemia compared to placebo.
Conclusion
Although mean IOP was not lowered significantly, daily evening dosing of 1% PMOS was found to be well tolerated with no daytime conjunctival redness and demonstrated improvement in DCNVA with sustained PD reduction in a glaucomatous and presbyopic population. Smaller pupil size can have beneficial effects in improving symptoms of presbyopia and DLD, which will be the focus of further studies.
Keywords: IOP, presbyopia, night vision disturbances, dim light vision disturbances, pupil diameter, ORION-1
Introduction
Glaucoma, presbyopia, and night vision or dim light vision disturbances (DLD) are among the most common ocular disorders affecting aging populations. Glaucoma is the second leading cause of blindness worldwide after cataracts,1 while presbyopia is arguably the most common ocular ailment worldwide affecting ~1.8 billion people.2 Dim light or night vision disturbances are common sequelae of some combination of ocular aberrations, scatter and internal intraocular lens (IOL) reflections, sometimes seen after lens or corneal-based refractive surgery, as well as in patients with keratoconus, pseudophakic patients with a square edge IOL design and in other conditions.3–6
The currently available treatments for glaucoma have different limitations and risks that must be considered when tailoring the choice of treatment to a patient’s needs and preferences. First, it is not uncommon for a patient to require more than one class of drugs, and even then, many patients still do not achieve a safe target intraocular pressure (IOP).7 Second, therapeutic classes have known ocular systemic adverse effects, including beta-adrenergic antagonists with bradycardia, dyspnea, and wheezing, as well as alpha-2 agonists with dry mouth, fatigue, sedation, and dizziness.8,9 Therefore, it is important to develop IOP-lowering therapies for patients with signs of glaucoma progression despite lower IOP with current treatment and also in individuals with normotensive glaucoma (NTG), where patients show progressive structural changes in their optic nerve fibers and/or progressive visual field loss despite IOP in what may be otherwise considered a “normal range.” The Baltimore Eye Survey of 5308 patients found that 78% of eyes with primary open-angle glaucoma had screening IOP of <25 mmHg, indicating the importance of achieving efficacy at these lower IOP levels.10 Whereas the prevalence of NTG was once thought to be low, the Beaver Dam Eye Study showed that nearly one-third of glaucoma patients met the diagnostic criteria of having NTG.11 Other studies indicate that as many as two-thirds of glaucomatous patients in Japan have NTG.12
As a potential alternative therapy for this subset of patients, alpha-adrenergic antagonists, including phentolamine, have been previously shown to lower IOP in animals via decreased aqueous humor production and increased uveoscleral outflow,13 which is a secondary outflow track for aqueous humor. The primary outflow pathway is the trabecular meshwork (TM), a tissue with contractile properties and alpha-2-adrenergic receptors.14 Thus, the non-selective alpha-antagonist phentolamine may relax the TM and lower IOP via this major outflow pathway. Further impetus to explore the potential of phentolamine mesylate ophthalmic solution (PMOS) as a potential ocular antihypertensive was the observation that in a prior DLD trial, patients with normal baseline IOP treated with different concentrations of PMOS, an investigational alpha-1 and alpha-2 antagonist, showed statistical lowering of IOP after a few hours (ClinicalTrials.gov identifier: NCT01703559).15
Similar to glaucoma, presbyopia is an age-related ocular disorder, typically beginning to manifest clinically at around 40 years of age. Presbyopia is caused by inability of the aging lens to dynamically change shape, curvature, and dioptric power in an effort to focus the image of nearby objects onto the retina, often described as loss of accommodation. Presbyopia has a significantly negative impact on quality of life,16 interfering with daily activities such as reading, use of computers or hand-held devices, and seeing the dashboard of the car. Currently, there are no pharmacological therapies approved for presbyopia, but there is some evidence that decreasing pupil diameter, optimally to a size of 1.6 to 2 mm to create a “pinhole” effect, can improve visual acuity by increasing the depth of focus via pseudoaccommodation.17–19 Pupil sizes smaller than that range are subject to the detrimental effects of diffraction, decreased retinal illumination, and photon noise, negatively impacting contrast thresholds and reading performance.20
An additional unmet ocular disorder, DLD, affects patients under mesopic or scotopic conditions. DLD involves photic phenomena, such as glare, halo, and starbursts, which can be a result of a combination of ocular aberrations, light scatter, internal reflection due to the square edge conformation of IOLs, as well as the effect of multiple images simultaneously superimposed onto the retina, as in the case of multifocal IOLs. Many people who suffer from DLD are unable to comfortably drive at dusk or otherwise function visually at night when the pupil naturally dilates and aberrant light rays from the periphery enter the eye to distort images or cause glare or starburst effects.4,21,22 This large cohort includes patients with night myopia (eg, uncorrected myopia), refractive errors (eg, irregular corneal astigmatism, and hyperopia), multifocal and other IOL designs, cataracts, keratoconus, and peripheral corneal imperfections from refractive surgeries (eg, laser-assisted in situ keratomileusis [LASIK], photorefractive keratectomy [PRK], radial keratotomy [RK]).23–25 In many of these cases, DLD can be mitigated by miosis, where the smaller pupil blocks the unfocused, peripheral aberrant rays of light, selectively allowing passage of the more centrally focused rays26 or obviates internal reflections resulting from a square edge IOL design.6 A miotic change in the pupil size can be achieved by modulating one of two or both opposing sets of muscles – the iris sphincter muscles controlled by the cholinergic nervous system and the iris dilator muscles controlled by the adrenergic nervous system.27,28 An alpha-1 antagonist approach will weaken the action of the iris dilator muscle and produce a miotic effect.29
Given the co-existence of these conditions in older cohorts, this trial studied the safety and efficacy of 1% PMOS, an alpha-1 antagonist, as a drug candidate to lower IOP in glaucoma patients, and, in parallel, to modulate pupil diameter and improve visual acuity in refractive conditions such as presbyopia and DLD. The hypothesis was that at the 1% concentration:
PMOS may provide a once-daily evening therapeutic option to reduce IOP in patients with open-angle glaucoma (OAG) or ocular hypertension (OHT).
PMOS may provide a unique once-daily evening therapeutic option in presbyopic patients to durably reduce pupil diameter and improve near vision.
PMOS would improve near vision without compromising distance visual acuity.
PMOS demonstrates a well-tolerated safety profile with mild adverse effects.
Patients and Methods
The ORION-1 Phase 2 trial was a randomized, multi-center, double-masked, placebo-controlled trial of 1% PMOS applied bilaterally at night for 14 days in patients with OAG or OHT (ClinicalTrials.gov identifier: NCT03960866). The study participants were either naïve to, or were previously taking, IOP-lowering medication and had to be washed out for approximately 30 days prior to dosing.
The investigators conducted the multi-centered trial in accordance with Good Clinical Practice, the ethical principles set forth in the Declaration of Helsinki and with the US Code of Federal Regulations governing the protection of human patients. The study protocol and its amendments, and the informed consent form for all sites, were reviewed and approved by a centralized IRB (Quorum Review IRB, Seattle, WA).
Patient Selection
Patients underwent a Screening visit where they were included in the trial if they were at least 18 years of age with either OHT in both eyes or OAG in 1 eye with OHT in the fellow eye, which was previously untreated or treated with ≤2 ocular hypotensive medications. If study participants previously took IOP-lowering medications, they had to be washed out for at least 28 days, but no more than 35 days. Patients who entered the washout period returned to the study site after approximately 2 weeks for an IOP safety check and evaluation of adverse events (AEs) and concomitant medications. If, in the judgment of the investigator, there was any risk to the eye(s) of the patient or if the mean IOP during the washout was >30 mmHg, then an appropriate rescue or prior medication could be administered, and the patient was considered a screen failure. Untreated or post-washout IOP had to be ≥22 mmHg and ≤ 30 mmHg in the study eye to qualify for enrollment.
Patients were excluded if they had closed or very narrow angles (Grade 0–1, Shaffer) or angles that the investigator judged as occludable, previous ocular surgery, trauma, any pupil abnormalities, recent ocular infection in the eye, central corneal thickness in either eye ≥600 μm at Screening, or any contact lens wear within 3 days prior to and for the duration of the trial. Gonioscopy (using a direct goniolens) for grading the angle was performed at Screening unless screening criteria were available from a previous test within the last 6 months. In addition, patients were excluded if they had clinically significant systemic disease, use of systemic adrenergic or cholinergic drugs up to 30 days prior to Screening, use of systemic medications that could have an effect on IOP, or resting heart rate (HR) and blood pressure (BP) out of the normal range, or known hypersensitivity to any alpha-adrenoceptor antagonists. Each investigator also assessed the appropriateness of patient entry into this clinical trial in that no patients were entered with optic nerve or visual field signs of end-stage glaucoma or, in the investigator’s best judgment, could have risk of visual field worsening as a result of participation in this trial.
Efficacy and Safety Assessments
After Screening, the patient returned to the site at Day 1. At 8 AM, the site conducted baseline ophthalmic examinations including pupil diameter, distance-corrected near and best-corrected distance visual acuity (DCNVA and BCDVA, respectively) under photopic and mesopic conditions, conjunctival hyperemia, biomicroscopy, and then IOP measurements. IOP was the primary efficacy assessment and was measured with a Goldmann applanation tonometer using a 2-person method (1 person physically applies the tonometer, while another reads the result). Non-ophthalmic aspects of the exam included evaluations of AEs, HR, BP, and a urine pregnancy test (for women of childbearing potential only). Patient Day 1 values at 8 AM were their “baseline values.” Patients were then randomized 1:1 study medication:placebo (vehicle) and were further assessed for IOP, conjunctival redness, and AEs at 10 AM and 4 PM. After their 4 PM visit, patients received their study medication—either 1% PMOS or placebo (vehicle) eye drops (Bio-Concept Laboratories, Salem, New Hampshire, USA)—and were instructed on the correct topical ocular administration procedure. Patients were then sent home with instructions to administer their first dose of study medication at 8 PM to 10 PM that evening (Day 1) and to self-administer subsequent doses to both eyes at the same time each evening from Day 1 to Day 14. Patients, investigators, clinical site staff, and all personnel responsible for monitoring and medical evaluation of the data remained masked to treatment assignment throughout the study.
Treatment study visits occurred on Day 8 ± 1 day, Day 15 ± 1 day, and Day 16 (approximately 36 hours after last dose) with measurements at 8 AM, 10 AM, and 4 PM. IOP, pupil diameter, DCNVA, BCDVA, conjunctival redness, AE evaluations, concomitant medications, HR/BP, ophthalmoscopy, biomicroscopy, and urine pregnancy test (for women of childbearing potential only) were performed according to the protocol. BP was measured after at least 3 minutes of rest in the sitting position. If BP was outside the normal range (diastolic BP > 105 mmHg or systolic BP > 160 mmHg), it could be repeated once only following at least a 5-minute rest period in the sitting position. Further, patients were contacted by telephone on Day 22 (7 days after their last dose) to assess AEs, conjunctival redness, and concomitant medications.
Both eyes were assessed at each visit. The study eye was designated as the eye with higher IOP on Day 1 at 8 AM. If both eyes had the same IOP, then the right eye was designated as the study eye.
IOP was measured twice in both eyes using Goldmann applanation tonometry (Automated Ophthalmics, Inc., Elkridge, Maryland, USA) at each assessment and the mean value was used at each time point. Pupil diameter was measured with a pupilometer (NeurOptics NPi-200, Laguna Hills, California, USA). BCDVA was measured in each eye (right eye first) under photopic and mesopic conditions with high contrast using an Early Treatment Diabetic Retinopathy Study (ETDRS) standard chart (Precision Vision, Woodstock, Illinois, USA) 4 meters away in an Illuminator Cabinet. Only the number of letters read was collected and converted to lines as follows: 1 line = 3 to 7 letters read; 2 lines = 8 to 12 letters read; 3 lines = 13 to 17 letters read, etc. DCNVA was measured in logMAR in each eye (right eye first) under photopic and mesopic conditions with high contrast using an ETDRS visual acuity chart 2000 in a Small 914 Illuminator Cabinet placed 14 inches away (Precision Vision, Woodstock, Illinois, USA). LogMAR values were converted to lines as follows: 1 line = 1.3 logMAR; 2 lines = 1.2 logMAR, etc., to 14 lines = 0.0 logMAR, 15 lines = −0.1 logMAR, 16 lines = −0.2 logMAR, and 17 lines = −0.3 logMAR. Monocular and binocular measurements were recorded. Conjunctival hyperemia was measured with a Cornea and Contact Lens Research Unit (CCLRU) 4-point scale of none, mild, moderate, and severe.
The primary endpoint of the trial was mean change in diurnal IOP of the study eye at Day 15, defined as average of measurements at 8 AM, 10 AM, and 4 PM. Other secondary endpoints included measurements of pupil diameter, testing of DCNVA, as well as assessments of the drug’s safety profile.
Statistical Analyses
A sample size of 36 total completed patients randomized in a 1:1 ratio to the 1% PMOS and the placebo groups were planned for the trial to provide approximately 90% power to detect a difference of 3.4 mmHg between the 1% PMOS and placebo groups in the change from baseline to Day 15 in mean diurnal IOP. This calculation was based on a 2-sided t-test at the 5% level of significance (α = 0.05) and a common standard deviation of 3.0. Additionally, it was assumed that there would be approximately 10% drop-out between baseline/Day 1 and Day 15. To account for this drop-out, a total of 40 patients were targeted for randomization into the trial.
All statistical analyses and reporting were performed using the SAS® System Version 9.4. The primary and each of the continuous secondary efficacy endpoints were analyzed using analysis of covariance (ANCOVA).
Results
Demographic and Other Baseline Characteristics
The trial enrolled 39 patients across 5 clinical sites (Rochester, NY; Morrow, GA; Cleveland, OH; Mission Hills, CA; and Ann Arbor, MI) with a median age of 64 years old. Of these 39 patients, 19 were allocated to 1% PMOS and 20 were allocated to placebo. These 39 patients constitute the full analysis set, defined as all randomized patients who have received at least 11 doses of study medication without missing 2 consecutive doses and have both a Baseline and a Day 15 IOP measurement, which was used for statistical analysis of all efficacy and safety measures, except for IOP. Patients were dosed once daily at bedtime. All demographics and baseline characteristics, including age, baseline IOP, pupil diameter, and visual acuity were similar between arms (Table 1). Two patients, one from the treatment arm and one from the placebo arm, were excluded because of major protocol deviations, reducing the Per Protocol Population to 37 patients, which is the basis of the IOP statistical analysis. One patient from the Nyxol arm took two different prohibited medications, and one patient from the placebo arm had not washed out preserved artificial tears before enrolling in the study. A total of 17 patients had 35 minor protocol deviations.
Table 1.
Demographic and Baseline Characteristics of the Full Analysis Set of the ORION-1 Trial Participants
| Placebo | 1% PMOS | P-value | |
|---|---|---|---|
| N (Full Analysis Set) | 20 | 19 | |
| Age (years): Median, Mean (SD) | 64.5, 63.2 (10.35) | 61.0, 58.1 (13.23) | 0.57 |
| Gender: Female n (%) | 13 (65%) | 9 (47%) | 0.27 |
| Race: White n (%) | 14 (70%) | 11 (58%) | 0.43 |
| Characteristics | |||
| Study Eye [n (%)] | |||
| OD | 10 (50%) | 11 (58%) | 0.62 |
| OS | 10 (50%) | 8 (42%) | 0.62 |
| Baseline Mean Diurnal IOP (Study Eye) mmHg [mean (SD)] | |||
| Study Eye | 24.4 (2.10) | 24.4 (1.68) | 0.94 |
| All Eyes | 23.8 (2.24) | 23.1 (1.66) | 0.29 |
| Baseline IOP Category [n (%)] | |||
| ≥ 25 mmHg | 13 (65%) | 10 (53%) | 0.43 |
| < 25 mmHg | 7 (35%) | 9 (47%) | 0.43 |
| Mean Baseline BCDVA (SD) | |||
| Photopic logMAR (Study Eye) | 0.05 (0.11) | 0.05 (0.14) | 1.00 |
| Mesopic logMAR (Study Eye) | 0.17 (0.12) | 0.19 (0.13) | 0.62 |
| Mean Baseline DCNVA (SD) (Study Eye) | |||
| Photopic logMAR (Study Eye) | 0.28 (0.26) | 0.22 (0.18) | 0.41 |
| Mesopic logMAR (Study Eye) | 0.36 (0.21) | 0.38 (0.29) | 0.80 |
Intraocular Pressure
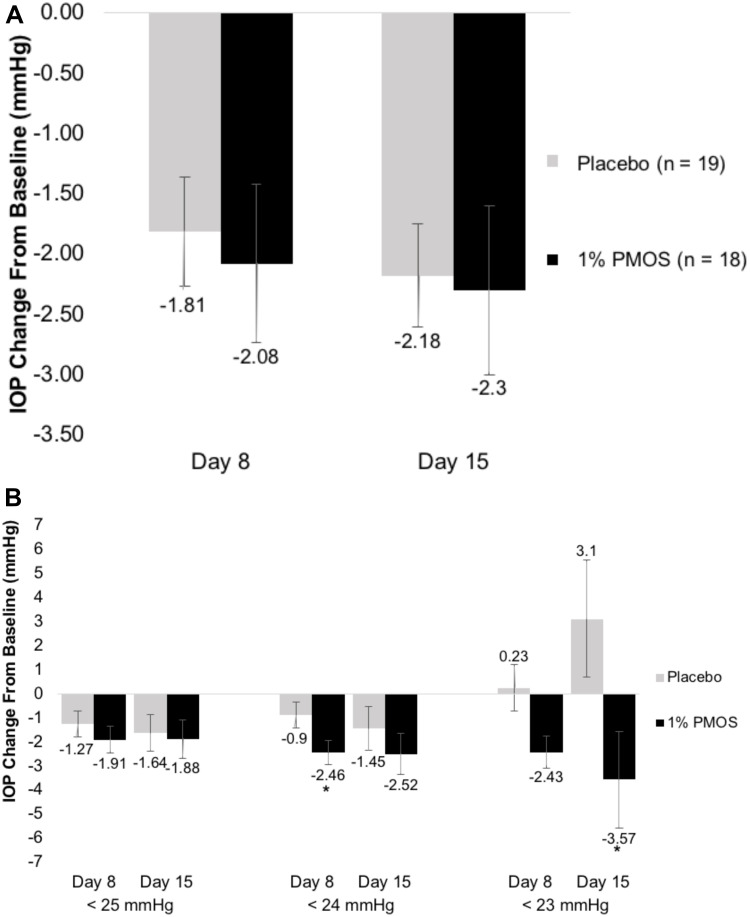
In regard to the primary endpoint, the mean reduction in diurnal IOP at Day 15 from baseline in the study eye was not significantly different between the PMOS (−2.30 mmHg) and placebo (−2.18 mmHg) arms (P = 0.89) (Figure 1A). Post hoc analyses stratifying eyes meeting different baseline IOP thresholds of <25, <24, and <23 mmHg were conducted in which PMOS demonstrated a significant IOP decrease in patients with baseline IOP < 24 mmHg at Day 8 (P = 0.049) (−2.46 mmHg in the PMOS and −.90 mmHg in the control) and a trend at Day 15 (P = 0.40) (−2.52 mmHg in the PMOS and −1.45 mmHg in the control) (Figure 1B). In patients with baseline IOP < 23 mmHg at Day 8, PMOS showed a numeric IOP decrease compared to placebo (−2.43 mmHg in PMOS and +0.23 mmHg in the control), but given the very small sample size, these results should not be over-interpreted.
Figure 1.
Mean changes in diurnal IOP after treatment. (A) Mean change in diurnal IOP at Day 8 and 15 in the study eye (n = 18 for PMOS and n = 19 for placebo) did not show statistical differences between PMOS and control groups. (B) A post hoc analysis of diurnal IOP at Day 8 and 15 in any eye with Baseline IOP < 25 mmHg (n = 11 for PMOS and n = 12 for placebo), < 24 mmHg (n = 9 for PMOS and n = 8 for placebo), and < 23 mmHg (n = 4 for PMOS and n = 2 for placebo). *Denotes P < 0.05.
Pupil Diameter
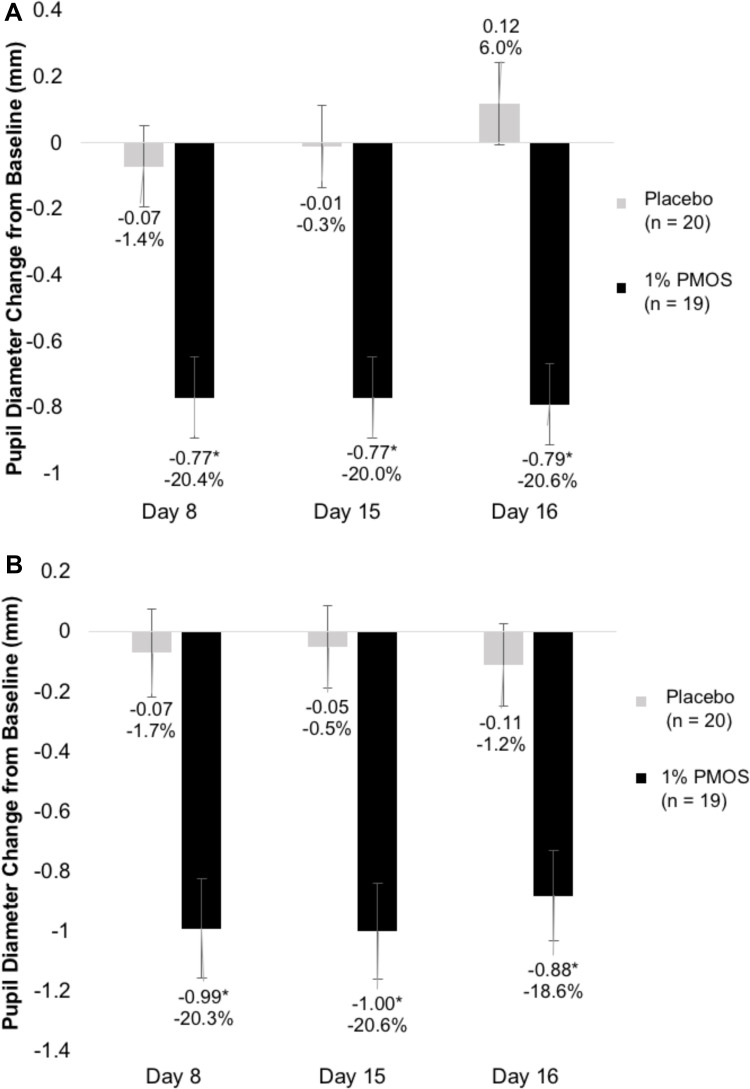
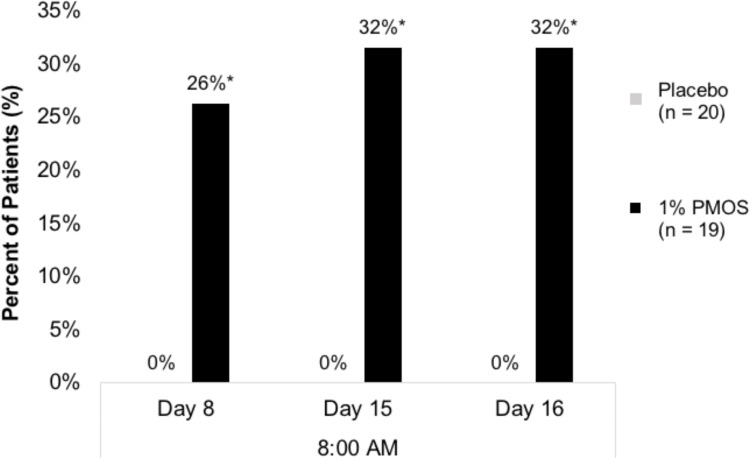
PMOS showed a consistent, approximate 20% mean reduction in pupil diameter from baseline in both photopic and mesopic conditions. At baseline, mean pupil diameter was 4.69±.95 and 3.63±.72 under photopic and mesopic conditions, respectively, and was reduced to 3.71±.81 and 2.86±.55, respectively, at the Day 8 8 AM time point. This effect was sustained for over 24 hours from the night that the last dose was given on Day 14 to the Day 16 8 AM time point (Figure 2A and B). Furthermore, at the 8 AM time points of Day 8, Day 15, and Day 16, more than 25% of patients who were given PMOS achieved ≥30% reduction in pupil diameter (P = 0.04, 0.01, and 0.01, respectively) (Figure 3). Statistically significant pupil diameter reduction from baseline was observed in the PMOS arm in the vast majority of parameters tested for study eye and fellow eye, regardless of condition (photopic, mesopic), time point (Day 8, Day 15, Day 16), or analysis method (mean pupil diameter, mean percent reduction, percent reduction category).
Figure 2.
Mean change in pupil diameter from baseline in (A) photopic and (B) mesopic conditions. *Denotes P < 0.05.
Figure 3.
Percent of patients with ≥ 30% reduction in pupil diameter at 3 time points (Day 8, Day 15, and Day 16, at 8AM). *Denotes P < 0.05.
Distance-Corrected Near Visual Acuity
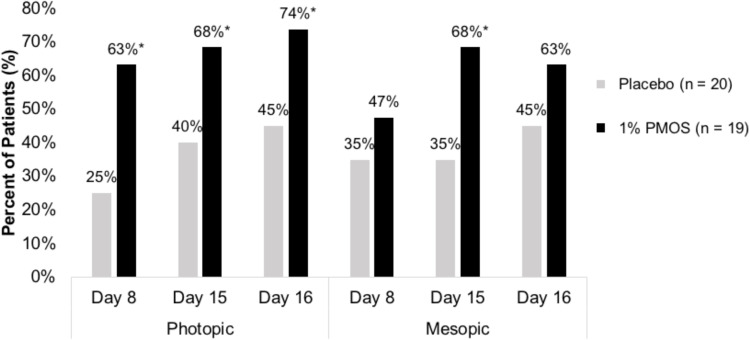
In an analysis of pooled data from the study eye and fellow eye, mean DCNVA at baseline was 0.26 logMAR. Under mesopic and photopic conditions, a statistically significant number of patients favoring the PMOS arm compared with the placebo arm achieved ≥1 line DCNVA improvement at one or more time points where patients were counted in a category if they met the reduction criteria for at least one eye (described as “best eye”) (Figure 4). Further, at Day 15 approximately 60% of patients who were given PMOS had ≥1 line of improvement in DCNVA in both photopic and mesopic conditions. There were trends towards significance favoring PMOS in DCNVA ≥ 2 lines improvement in both mesopic and photopic conditions.
Figure 4.
Percent of patients with ≥ 1 line improvement in DCNVA in photopic (left) and mesopic (right) conditions. *Denotes P < 0.05.
A post hoc analysis with ANCOVA was performed with patients who were categorized as having DCNVA ≥ 0.3 logMAR in their study eye in mesopic conditions at baseline, with mean baseline DCNVA of 0.57 logMAR for PMOS (n = 11) and 0.48 logMAR for placebo (n = 13). Statistically significant improvements in DCNVA from baseline were observed in mesopic conditions with the same −0.11 logMAR least-squares mean difference favoring PMOS compared to placebo at all time points (Day 8, Day 15, and Day 16; P < 0.02). A trend with similar mean difference favoring PMOS was observed in photopic conditions.
Best-Corrected Distance Visual Acuity
The mean baseline BCDVA for all eyes was 0.05 logMAR in photopic conditions and 0.18 logMAR in mesopic conditions across all patients. In all eyes under photopic conditions, a statistically significant number of patients favoring PMOS compared with placebo achieved ≥1 line improvement in BCDVA from baseline in the best eye at the Day 8, 8 AM time point (P = 0.03). There was only 1 patient in the PMOS arm and 2 patients in the placebo arm who had a ≥ 3-line worsening in BCDVA at Day 15 in either eye or both eyes, with 1 patient in each arm also exhibiting a worsening in BCDVA at Day 16. At all other time points or visits, there was no statistically significant difference in BCDVA for treatment or placebo patients. In all eyes under mesopic conditions, at all time points and visits, there was no statistically significant difference in BCDVA for treatment or placebo patients.
Adverse Events
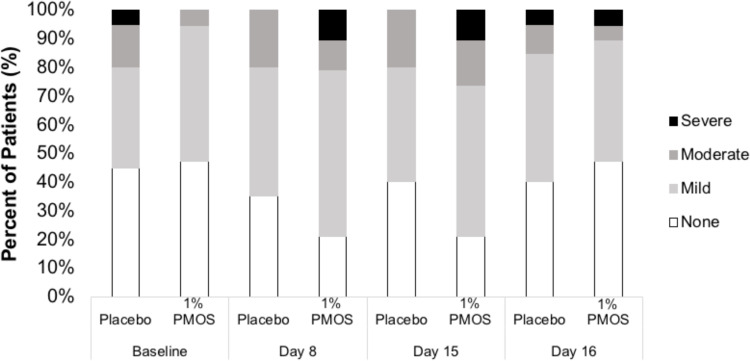
Sixteen treatment-emergent adverse events (TEAEs) were reported in 6 patients (31.6%) in the PMOS arm and 2 TEAEs in 1 patient (5.0%) in the placebo arm (P = 0.04). All TEAEs were mild in severity, with no serious TEAEs or TEAEs leading to the withdrawal of medication or trial. Of the 19 PMOS-treated patients, 3 had conjunctival hyperemia, 1 had eye pruritus, 3 had burning or pain on instillation, and 2 had mild infections of the prostate and upper respiratory tract. Of the 20 placebo-treated patients, 1 had conjunctival hyperemia. All ocular TEAEs were considered related to study medication per the investigator, whereas the non-ocular TEAEs were considered not related to treatment. Conjunctival redness scores increased numerically to some extent in the PMOS arm at Day 8 and Day 15, but these scores were not significantly different from the placebo arm at all time points (Figure 5) (P = 0.26–1.00).
Figure 5.
Conjunctival hyperemia at the 8AM assessment at 4 time points (Day 1/Baseline, Day 8, Day 15, and Day 16), measured using the CCLRU scale of none, mild, moderate, and severe.
In addition, mean systolic and diastolic BP and HR were relatively unchanged, remained within normal ranges throughout the duration of the trial, and were similar between arms. Biomicroscopic examination of other parameters, including cornea, conjunctiva (other than redness), anterior chamber, corneal fluorescein staining, as well as examination of the optic nerve, macula vessels, and periphery, showed no clinically significant abnormalities from baseline.
Discussion
Various anterior ophthalmic conditions associated with the aging population, such as glaucoma, presbyopia, and DLD, would benefit from additional or first-time pharmacologic therapies. PMOS, a proprietary, preservative-free, stable, ophthalmic formulation of phentolamine mesylate, was investigated as a potential treatment option for these ophthalmic conditions.
This Phase 2b trial was designed to explore multiple endpoints across various ophthalmic conditions, with IOP lowering as the primary endpoint and key secondary endpoints including reduction in pupil diameter and improvement in DCNVA. Although the primary endpoint of IOP lowering with once-daily evening dosing of 1% PMOS for glaucoma patients was not met in the ORION-1 trial, many other endpoints relevant for the potential use in presbyopia and DLD patients were met. These endpoints include confirming the 1% dose and evening dosing regimen of PMOS, statistically significant and durable pupil diameter reductions and near visual acuity improvements, and strengthening the safety profile with no statistically significant increase in conjunctival hyperemia compared to placebo in daytime assessments.
Intraocular Pressure
Besides the high placebo response often seen in glaucoma trials,30 another reason that may have contributed to the lack of observed efficacy of 1% PMOS in decreasing IOP was that the patient sample had an average IOP of approximately 24 mmHg, whereas a post hoc analysis of PMOS led to a reduction in IOP in both patients with baseline IOPs of <24 mmHg and DLD patients with normal baseline IOPs after several hours.27 Based on these preliminary observations, the utility of PMOS could be further explored in patients with NTG or as a second line with topical prostaglandins given the potential for greater IOP-lowering effect in patients with lower baseline IOPs. While patients with lower IOP or NTG may have independent risk factors in addition to IOP contributing to their optic neuropathy and visual field loss, the Collaborative Normotension Glaucoma Study, the Early Manifest Glaucoma trial, and other dose-dependent trials clearly support the central role of adequate IOP reduction in NTG and the rationale for further pressure reduction in those whose disease progresses despite low pressures.31–33 Also, multiple doses per day for PMOS should be considered since the IOP-lowering effects between 2 and 12 hours are still unknown. This would be similar to other IOP-lowering medications such as timolol or brimonidine that are routinely given multiple times per day to elicit their effect.34,35 The authors recognize, however, that conclusions drawn from post hoc analyses are limited in nature and should be interpreted accordingly. We believe that, despite this, the findings can provide information that can inform future studies.
Pupil Reduction and Visual Performance
Efficacy endpoints of pupil diameter reduction with 1% PMOS demonstrated a statistically significant and clinically relevant mean reduction in pupil diameter of approximately 20% at all time points under photopic and mesopic conditions. Further, the moderate miotic pupil diameter effects were maintained 10 to 20 hours later on Day 8 and Day 15 in this older population, and pupil diameter effects were sustained through 36 hours post-dose at Day 16. This is consistent with prior trials where 1% PMOS showed a decrease in pupil diameter of ~15% among a younger and middle-aged population, which lasted up to 24 hours.15 Such long-term effects might be attributed to drug binding to melanin, creating a depot and slowing drug release.36
In this trial of a presbyopia-aged cohort, over 85% of patients had DCNVA worse than 0.1 logMAR. Many of these presbyopes could benefit from expanding their depth of focus via a smaller pupil, thereby blocking unfocused peripheral rays of light and expanding their Conoid of Sturm. Relevant to presbyopia, the creation of a pinhole-sized pupil can allow the eye to better focus on near objects via the mechanism of pseudoaccommodation, resulting in less dependence on reading adds, reading glasses or contact lenses.17–19 This phenomenon is supported by the fact that 1% PMOS showed ≥1-line improvement in DCNVA from baseline as compared with placebo under different lighting conditions and at multiple time points. In addition, mean DCNVA improvements were seen with patients who had 20/40 or worse vision at baseline.
Further, there was only one patient in the PMOS group and two patients in the placebo group with loss of BCDVA in this study, suggesting that PMOS can improve near vision without compromising distance vision. Moreover, the improvement in DCNVA in this trial could potentially be expanded upon with a second miotic agent since the target optimal pinhole pupil size of 1.6 mm to 2 mm was not achieved with 1% PMOS alone.
In addition to treating presbyopia, this trial suggests that PMOS may be a potential candidate for treating patients with DLD. A smaller pupil size in DLD can mitigate optical scatter or higher order aberrations that occur when light passes through a wider optical zone of the cornea or intraocular structures, as well as internal light reflections within an IOL related to square edge design. This has been shown in prior trials where patients with DLD exhibited improvement in mesopic low-contrast distance visual acuity and contrast sensitivity after treatment with PMOS.15,37
It should be noted that, besides phentolamine, other alpha-1 adrenergic antagonists have a miotic effect and, in fact, have been shown to be safe and effective for the pharmacological reversal of mydriasis. Dapiprazole hydrochloride ophthalmic solution 0.5%, an alpha-1 adrenergic receptor antagonist, was approved by the US FDA in 1990 for the treatment of iatrogenically induced mydriasis produced by adrenergic or parasympatholytic agents.38 However, the product was withdrawn and discontinued by the manufacturer for reasons not related to safety or efficacy.39 Thymoxamine, another alpha-1 antagonist, has also shown efficacy in reducing PD from iatrogenic mydriasis but was never approved by the FDA for this purpose.40 In contrast to these selective alpha-1 adrenergic antagonists, PMOS is a non-selective alpha-1 and alpha-2 antagonist acting on adrenergic receptors and the current study provides new information as to the temporal effects on pupil size and near vision of this agent.
Safety
PMOS at 1% concentration continued to demonstrate a favorable overall safety profile, consistent with prior clinical trials.15,37 All TEAEs were mild in severity, and none led to discontinuation or withdrawal from the trial. There were no systemic side effects. Given the mechanism of PMOS as an alpha-1 adrenergic antagonist and its potential effects on hemodynamics,41 an important safety metric consistent with prior results was no change in mean systolic and diastolic BP and HR.15,37 This is in contrast with the effects of timolol, which has been shown to significantly reduce BP and HR.42
The ocular side effect of mild-to-moderate conjunctival hyperemia was expected due to the on-target vasodilatory effect of alpha-1 antagonist drugs. A new and important finding from previous trials of PMOS, however, was the lack of clinical or statistical significance in daytime conjunctival hyperemia when PMOS is dosed daily in the evening, establishing a preferred dosing regimen.
Conclusion
This trial found that 1% PMOS did not reduce IOP in patients with glaucoma or OHT. However, statistically significant decreases in pupil size and improvements in DCNVA were seen, which may be clinically relevant in ocular diseases with pupil modulation as a solution, such as presbyopia and DLD. In addition, evening dosing of PMOS was shown to be durable, exhibiting effects up to 36 hours while also ameliorating daytime redness. There were no major ocular or systemic safety issues. PMOS has been studied in patients ranging from 18 to 81 years of age and further trials should be explored to use PMOS eye drops for pupil modulation indications or potentially NTG.
Acknowledgments
Publication of this article was sponsored by Ocuphire Pharma, Inc. The authors wish to thank the patients, the staff at the trial sites, Oculos Development Services for coordination of the trial, Summit Analytical for statistics, BIA Clinical Group for clinical consultation, KCT Data for data management, Bio-Concepts Laboratory for drug product, and Xerimis for drug packaging. The principal author, JSP, presented interim findings at the virtual annual meeting for the Association for Research in Vision and Ophthalmology, May 2020, Abstract #3364450. The ARVO Annual Meeting Abstract was published in Investigative Ophthalmology & Visual Science (IOVS), an ARVO journal, in June 2020 (https://iovs.arvojournals.org/article.aspx?articleid=2768855).
Data Sharing Statement
The data sets generated and/or analyzed during the current study are not publicly available owing to the need to minimize risk to the privacy and confidentiality of research participants and ensure compliance with legal requirements for privacy and data protection but are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This multi-center trial was compliant with the principles of the Declaration of Helsinki and the International Council for Harmonisation guidelines for Good Clinical Practice; it was registered at ClinicalTrials.gov (identifier: NCT03960866). Written consent was obtained from each patient prior to any study-related procedures at Visit 1. The study protocol and its amendments, and the informed consent form for all sites, were reviewed and approved by a centralized IRB (Quorum Review IRB, Seattle, WA). The authors do not intend to share individual deidentified participant data, nor will any study documents be made available, aside from publicly available presentations and publications.
Disclosure
MPS, ARM, RMJ, KC, ATA, JEY, and SAK are or were employees, directors/officers, or paid consultants for Ocuphire Pharma. JSP, ESL, PMK, and MBM are on the medical advisory board for Ocuphire Pharma. PJH, HBD, MAA, RJSM, and SEM were principal investigators for this clinical trial funded by Ocuphire Pharma. CBS serves as Chief Medical Officer of Oculos Development Services, LLC, which was the contract research organization for this clinical trial. Dr Jay S Pepose reportspersonal fees from Acufocus, Kala Pharmaceuticals, Keeler, MG Therapeutics, Mimetogen Pharmaceuticals, Novartis, Ocunexis Therapeutics, Okogen, Stuart Pharmaceuticals, Sun Pharma, Thea Pharma, TearLab, and Ocuphire, outside the submitted work. Dr Robert J Smyth-Medina reports research study site funding from Ora. Dr Sayoko E Moroi reports grants from R01 EY022124 (PI Moroi), R21 EY030363 (MPI Musch + Moroi), R01 EY025752 (PI Komaromy), NSF 1,760,291 (MPI Argento + Moroi), Aerie Pharmaceuticals, Inc, Allergan, Ocuphire, Icare USA, Wolters Kluwer Health: Royalty for Shield’s Textbook of Glaucoma, grants from MCubed, outside the submitted work. Mr Alan R Meyer have patents US 9,789,088 B2, US 9,795,560 B2, and US 10,278,918 assigned to Ocuphire Pharma issued. Dr Konstantinos Charizanis reports a patent PCT/US2019/056324 pending. Dr Marguerite B McDonald reports personal fees from Allergan, Alcon, Bausch and Lomb, Eyevance, Novartis, Tarsus, Visus, Aperta, Ocusoft, OCULUS USA, DOMPE, BioTissue, BlephEx, Akorn, Quidel, ORCA Surgical, TearLab, J&J Vision, TearCare, NuLids, Ocuphire, STROMA, Hellas LTD, Sun Pharma, Avedro, Omeros, Scope, and Sight Sciences, during the conduct of the study; personal fees from Visus and Allergan, outside the submitted work. The authors indicated that they have no other conflicts of interest with regard to the content of this article.
References
- 1.Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 2.Fricke TR, Tahhan N, Resnikoff S, et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018;125(10):1492–1499. doi: 10.1016/j.ophtha.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 3.Eydelman M, Hilmantel G, Tarver ME, et al. Symptoms and satisfaction of patients in the patient-reported outcomes with laser in situ keratomileusis (PROWL) studies. JAMA Ophthalmol. 2017;135(1):13. doi: 10.1001/jamaophthalmol.2016.4587 [DOI] [PubMed] [Google Scholar]
- 4.Kimlin JA, Black AA, Wood JM. Nighttime driving in older adults: effects of glare and association with mesopic visual function. Invest Ophthalmol Vis Sci. 2017;58(5):2796–2803. doi: 10.1167/iovs.16-21219 [DOI] [PubMed] [Google Scholar]
- 5.de Vries NE, Webers CAB, Touwslager WRH, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37(5):859–865. doi: 10.1016/j.jcrs.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 6.Masket S, Fram NR. Pseudophakic dysphotopsia: review of incidence, cause, and treatment of positive and negative dysphotopsia. Ophthalmology. 2020. doi: 10.1016/j.ophtha.2020.08.009 [DOI] [PubMed] [Google Scholar]
- 7.Marshall LL, Hayslett RL, Stevens GA. Therapy for open-angle glaucoma. Consult Pharm J Am Soc Consult Pharm. 2018;33(8):432–445. doi: 10.4140/TCP.n.2018.432 [DOI] [PubMed] [Google Scholar]
- 8.Antonelli-Incalzi R, Pedone C. Respiratory effects of β-adrenergic receptor blockers. Curr Med Chem. 2007;14(10):1121–1128. doi: 10.2174/092986707780362853 [DOI] [PubMed] [Google Scholar]
- 9.Giovannitti JA, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62(1):31–38. doi: 10.2344/0003-3006-62.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black americans: the baltimore eye survey. Arch Ophthalmol. 1991;109(8):1090–1095. doi: 10.1001/archopht.1991.01080080050026 [DOI] [PubMed] [Google Scholar]
- 11.Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam eye study. Ophthalmology. 1992;99(10):1499–1504. doi: 10.1016/S0161-6420(92)31774-9 [DOI] [PubMed] [Google Scholar]
- 12.Shiose Y, Kitazawa Y, Tsukahara S, et al. Epidemiology of glaucoma in Japan–a nationwide glaucoma survey. Jpn J Ophthalmol. 1991;35(2):133–155. [PubMed] [Google Scholar]
- 13.Kiel JW, Reitsamer HA. Paradoxical effect of phentolamine on aqueous flow in the rabbit. J Ocul Pharmacol Ther. 2007;23(1):21–26. doi: 10.1089/jop.2006.0102 [DOI] [PubMed] [Google Scholar]
- 14.McAuliffe-Curtin D, Buckley C. Review of alpha adrenoceptor function in the eye. Eye. 1989;3(4):472–476. doi: 10.1038/eye.1989.71 [DOI] [PubMed] [Google Scholar]
- 15.Ocuphire Pharma, Inc. Double-masked parallel evaluation of the safety and efficacy of phentolamine mesylate ophthalmic solution in subjects with severe night vision disturbances. clinicaltrials.gov; 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT01703559. Accessed June22, 2020.
- 16.Associations of presbyopia with vision-targeted health-related quality of life | ophthalmology | JAMA ophthalmology | JAMA network; 2020. Available from: https://jamanetwork-com.proxy.lib.umich.edu/journals/jamaophthalmology/fullarticle/415846. [DOI] [PubMed]
- 17.Charman WNTJ The depth-of-focus of the human eye for Snellen letters. - abstract - Europe PMC; 2020. Available from: https://europepmc.org/article/med/1111286. [DOI] [PubMed]
- 18.Dexl AK, Rückl T, Seyeddain O, et al. Reading performance after implantation of a small aperture corneal inlay for the surgical correction of presbyopia: two-year follow-up of 32 patients. Invest Ophthalmol Vis Sci. 2011;52(14):847. [DOI] [PubMed] [Google Scholar]
- 19.Park SY, Choi YJ, Jung JW, et al. Clinical efficacy of pinhole soft contact lenses for the correction of presbyopia. Semin Ophthalmol. 2019;34(2):106–114. doi: 10.1080/08820538.2019.1586966 [DOI] [PubMed] [Google Scholar]
- 20.Xu R, Gil D, Dibas M, Hare W, Bradley A. The effect of light level and small pupils on presbyopic reading performance. Invest Ophthalmol Vis Sci. 2016;57(13):5656–5664. doi: 10.1167/iovs.16-20008 [DOI] [PubMed] [Google Scholar]
- 21.Brooks NO, Greenstein S, Fry K, Hersh PS. Patient subjective visual function after corneal collagen crosslinking for keratoconus and corneal ectasia. J Cataract Refract Surg. 2012;38(4):615–619. doi: 10.1016/j.jcrs.2011.11.029 [DOI] [PubMed] [Google Scholar]
- 22.Schallhorn SC, Tanzer DJ, Kaupp SE, Brown M, Malady SE. Comparison of night driving performance after wavefront-guided and conventional LASIK for moderate myopia. Ophthalmology. 2009;116(4):702–709. doi: 10.1016/j.ophtha.2008.12.038 [DOI] [PubMed] [Google Scholar]
- 23.Bidgoli S, Alio JL. Night vision disturbances following refractive surgery: causes, prevention, and treatment In: Alio JL, Azar DT editors. Management of Complications in Refractive Surgery. Springer International Publishing; 2018:163–174. doi: 10.1007/978-3-319-60561-6_21. [DOI] [Google Scholar]
- 24.Alio JL, Azar DT. Management of Complications in Refractive Surgery. Springer; 2018. [Google Scholar]
- 25.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Contact Lens Anterior Eye. 2010;33(4):157–166. doi: 10.1016/j.clae.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Martínez CE, Applegate RA, Klyce SD, McDonald MB, Medina JP, Howland HC. Effect of pupillary dilation on corneal optical aberrations after photorefractive keratectomy. Arch Ophthalmol. 1998;116(8):1053–1062. doi: 10.1001/archopht.116.8.1053 [DOI] [PubMed] [Google Scholar]
- 27.Yoshitomi T, Ito Y, Inomata H. Adrenergic excitatory and cholinergic inhibitory innervations in the human iris dilator. Exp Eye Res. 1985;40(3):453–459. doi: 10.1016/0014-4835(85)90158-7 [DOI] [PubMed] [Google Scholar]
- 28.Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. Int J Psychophysiol. 2004;52(1):77–86. doi: 10.1016/j.ijpsycho.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 29.Yu Y, Koss MC. α1A-adrenoceptors mediate sympathetically evoked pupillary dilation in rats. J Pharmacol Exp Ther. 2002;300(2):521–525. doi: 10.1124/jpet.300.2.521 [DOI] [PubMed] [Google Scholar]
- 30.Sharpe RA, Nelson LA, Stewart JA, Stewart WC. The placebo effect in early-phase glaucoma clinical trials. Curr Eye Res. 2015;40(6):653–656. doi: 10.3109/02713683.2014.946519 [DOI] [PubMed] [Google Scholar]
- 31.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 32.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol Chic Ill 1960. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268 [DOI] [PubMed] [Google Scholar]
- 33.Kim M, Kim DM, Park KH, Kim T-W, Jeoung JW, Kim SH. Intraocular pressure reduction with topical medications and progression of normal-tension glaucoma: a 12-year mean follow-up study. Acta Ophthalmol (Copenh). 2013;91(4):e270–275. doi: 10.1111/aos.12082 [DOI] [PubMed] [Google Scholar]
- 34.Iester M. Brinzolamide ophthalmic suspension: a review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin Ophthalmol Auckl NZ. 2008;2(3):517–523. doi: 10.2147/OPTH.S3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petounis A, Mylopoulos N, Kandarakis A, Andreanos D, Dimitrakoulias N. Comparison of the additive intraocular pressure-lowering effect of latanoprost and dorzolamide when added to timolol in patients with open-angle glaucoma or ocular hypertension: a randomized, open-label, multicenter study in Greece. J Glaucoma. 2001;10(4):316–324. doi: 10.1097/00061198-200108000-00012 [DOI] [PubMed] [Google Scholar]
- 36.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi: 10.1208/s12248-010-9183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ocuphire Pharma, Inc. Single dose study of phentolamine mesylate eye drops in patients with severe night vision disturbances. clinicaltrials.gov; 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT04004507. Accessed June22, 2020.
- 38.Wood JM, Garth D, Grounds G, McKay P, Mulvahil A. Pupil dilatation does affect some aspects of daytime driving performance. Br J Ophthalmol. 2003;87(11):1387–1390. doi: 10.1136/bjo.87.11.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Food and Drug Administration. Determination that REV–EYES (dapiprazole hydrochloride ophthalmic solution), 0.5%, was not withdrawn from sale for reasons of safety or effectiveness. Fed Reg. 2013;78(92):27971. [Google Scholar]
- 40.Shah B, Hubbard B, Stewart-Jones JH, Edgar DF, Turner P. Influence of thymoxamine eye-drops on the mydriatic effect of tropicamide and phenylephrine alone and in combination. Ophthal Physiol Opt J Br Coll Ophthal Opt Optom. 1989;9(2):153–155. doi: 10.1111/j.1475-1313.1989.tb00835.x [DOI] [PubMed] [Google Scholar]
- 41.Roberts G, Richardson AW, Green HD. Effects of regitine (c-7337) upon the blood flow responses to epinephrine in the innervated hind limb of the dog. J Pharmacol Exp Ther. 1952;105(4):466–476. [PubMed] [Google Scholar]
- 42.Oddone F, Rossetti L, Tanga L, et al. Effects of topical bimatoprost 0.01% and timolol 0.5% on circadian IOP, blood pressure and perfusion pressure in patients with glaucoma or ocular hypertension: a randomized, double masked, placebo-controlled clinical trial. PLoS One. 2015;10(10):e0140601. doi: 10.1371/journal.pone.0140601 [DOI] [PMC free article] [PubMed] [Google Scholar]