Abstract
The aim of the present study is to evaluate the association of serum uric acid (UA) levels with the risk of incident hypertension among different age groups in men and women using a single large Japanese general cohort. The present study is based on annual health check-up program in Gunma, Japan. We studied 12,029 participants (mean age, 48 ± 9 years old; 31% women) free of prevalent cardiovascular disease and hypertension at baseline (2009). Hypertension was defined by self-report, hypertensive medication use, or measured BP > 140/90 mmHg at each visit. Discrete proportional hazards regression model was used to evaluate the association of UA level at baseline with incident hypertension through 2012 adjusted for age, gender, baseline blood pressure, and other CVD risk factors among different age decade groups in men and women. During follow-up of 3 years, 12% of the cohort (n = 1457) developed hypertension. UA was strongly associated with incident hypertension in the multivariable model in all participants. In age-stratified analysis, participants below 50 years of age in men had the significant association of UA with incident hypertension, whereas participants above 50 years did not. In women, participants above 40 years had the significant association, whereas participants below 40 years did not. The present data suggest that UA level is an independent predictor for incident hypertension among middle aged men below 50 years old and middle aged and the elderly women above 40 years.
Keywords: Blood pressure, Hypertension, Uric acid
1. Introduction
Hypertension is an important public health challenge because of its high frequency and concomitant risks of cardiovascular morbidity and mortality [1]. However, hypertension cases are mainly of essential hypertension, and the etiology of its onset remains unclear [2]. It is important to elucidate the pathophysiological mechanisms underlying hypertension and to discover new approaches for identifying individuals prone to hypertension development.
Serum uric acid (UA) have been proposed as a risk factor for incident hypertension [3], [4], [5], [6], as well as cardiovascular disease [7]. Previous studies also have demonstrated the blood pressure is lowered by UA-lowering drugs [8]. However, there have been controversial results especially in the elderly regarding the blood pressure control by UA-lowering drug [9]. Also, attenuation of UA effect on blood pressure with increasing age has been reported [3]. Taken together, the impact of UA with incident hypertension might differ in different age groups.
There is no study that assesses the effect of UA on incident hypertension among different age groups in both men and women. Thus, the aim of the present study is to assess the prospective association of serum UA levels with the risk of incident hypertension among different age groups in men and women using a single large Japanese general cohort.
2. Method
2.1. Study population
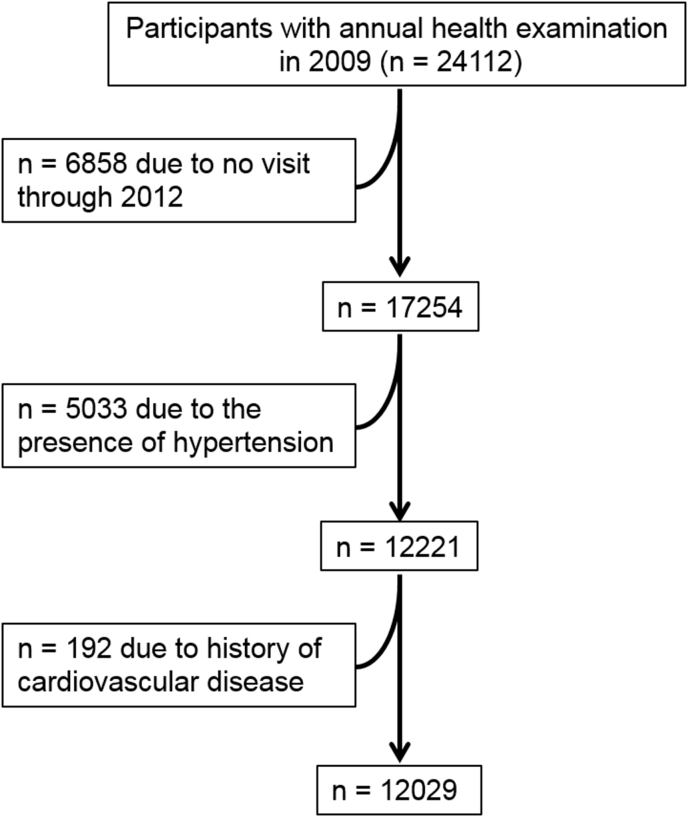
The present study is retrospective observational study based on annual health check-up program in Gunma, Japan. As shown in Fig. 1, we retrospectively screened 24,112 individuals >20 years old who had health check-up program at baseline (2009). Of these participants, we excluded participants who have missing data and those without follow-up visit through 2012. We also excluded participants who had baseline hypertension, defined as a systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or use of any antihypertensive medication, and prevalent cardiovascular disease. Finally, a total of 12,029 participants free of prevalent hypertension and cardiovascular disease were included for the present study.
Fig. 1.
Flow chart of the study participants.
Compared with excluded participants, included participants were younger and less likely to be less cardiovascular risk factors (data not shown). The institutional ethics review boards of Gunma Chuo Hospital approved this study with waiver of consent.
2.2. Outcomes
We had two follow-up examinations in 2011 and 2012 annually. Resting systolic (SBP) and diastolic blood pressures (DBP) were measured in the seated position using an automated oscillometric sphygmomanometer. We defined incident hypertension as a SBP≧140 mmHg, DBP≧90 mmHg, or use of any antihypertensive medication, or self-reported hypertension during any of the follow-up examinations.
2.3. CVD risk factors assessment
During baseline examination, all participants completed standardized questionnaires to provide information about demographic variables, smoking history, and medication use for hypertension, diabetes, and dyslipidemia. Glucose and lipids were measured after a 12-h fast. Diabetes mellitus was defined as fasting glucose ≥126 mg/dl or use of insulin or oral hypoglycemic medication. Estimated glomerular filtration rate (eGFR) from serum creatinine concentrations using the Chronic Kidney Disease (CKD) Epidemiology Collaboration 2012 equation.
2.4. Statistical analysis
Continuous variables are shown as mean ± SD unless otherwise specified and categorical variables are shown as percentages. Comparisons between participants with or without incident hypertension were performed using student's t-test and Mann-Whitney U test for normally and non-normally distributed data, respectively. Oneway ANOVA was used for comparison of continuous variables among more than 3 groups. Categorical variables are presented as frequencies and percentages and analyzed using χ2 tests.
We used discrete proportional hazards (complementary log-log) regression models to estimate hazard ratios (HRs) for baseline serum UA levels with incident hypertension [10]. This model is similar to a Cox proportional hazards model, but is able to account for the time between the discrete time visits because the exact date of the development hypertension is unknown. Thus, this method is suitable for data based on annual check-up program in the present study. We show the HR for a 1 mg/dl increase of UA levels. Cox regression models using sex-specific UA quartile instead of continuous uric acid levels was also performed. We also conducted analyses stratified by age tertile groups (<40 years, 40–49 years, 50–59 years, and >60 years) in men and women. Model 1 adjusted for demographic factors (age, gender, height, and weight) and Model 2 adjusted for conventional cardiovascular risk factors (systolic blood pressure, diabetes mellitus, smoking status, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and eGFR) adding to Model 1.
A 2-tailed p value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata version 14.0 (Stata Corp LP, College Station, TX).
3. Results
3.1. Participant characteristics
Demographic and clinical parameters at baseline for participants is presented in Table 1. The study population was 41% female with mean age of 48 ± 9 years. The mean serum UA at baseline was 5.2±1.4 mg/dl (5.9±1.2 for male, and 4.3±0.9 for female). A total of 1457 participants (12%) experienced incident hypertension over an average of 3 years follow-up. Participants with incident hypertension were older and more likely to be male, diabetic, and active smokers, and more likely to have increased body mass index, LDL cholesterol and blood pressures, and decreased HDL compared to participants without events. UA levels were greater in participants with incident hypertension among both gender. Participants with higher UA levels were more likely to have greater cardiovascular risk profile (Supplemental Table 1). Participants in the higher age group had higher blood pressure. In men, older participants had lower serum UA, whereas older women had higher UA, compared to younger participants (Supplemental Table 2).
Table 1.
Baseline characteristics stratified by incident hypertension.
| All participants |
no HTN |
HTN |
p value | |
|---|---|---|---|---|
| (n = 12029) | (n = 10572) | (n = 1457) | ||
| Age, years | 48 (9) | 47 (9) | 51 (9) | <0.001 |
| Female, % | 41 | 43 | 27 | <0.001 |
| Height, cm | 165 (9) | 165 (9) | 166 (8) | <0.001 |
| Weight, kg | 62 (11) | 61 (11) | 65 (12) | <0.001 |
| BMI, kg m−2 | 22.6 (3.1) | 22.4 (3.0) | 23.7 (3.3) | <0.001 |
| Current Smoking, % | 33 | 32 | 37 | <0.001 |
| LDL, mg dl−1 | 127 (33) | 126 (33) | 132 (33) | <0.001 |
| HDL, mg dl−1 | 65 (17) | 65 (17) | 62 (16) | <0.001 |
| eGFR, ml min−1 per 1.73 m2 | 84.1 (13.9) | 84.3 (13.8) | 82.4 (14.2) | <0.001 |
| Fasting Glucose, mg dl−1 | 93 (18) | 92 (16) | 98 (26) | <0.001 |
| SBP, mmHg | 119 (11) | 118 (11) | 128 (8) | <0.001 |
| DBP, mmHg | 75 (8) | 74 (8) | 81 (6) | <0.001 |
| Uric acid, mg dl−1 | 5.2 (1.4) | 5.2 (1.3) | 5.7 (1.4) | <0.001 |
| Male | 5.9 (1.2) | 5.9 (1.2) | 6.1 (1.3) | <0.001 |
| Female | 4.3 (0.9) | 4.3 (0.9) | 4.6 (1.0) | <0.001 |
Values are mean (SD) or %. BMI indicates body mass index; LDL, low density lipoprotein; HDL, high density lipoprotein; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; HTN, hypertension.
3.2. Relationship of uric acid with incident hypertension
Discrete proportional hazards models for all participants are presented in Table 2. In univariate analysis, UA was associated with incident hypertension in all participants. This association of UA with incident hypertension was maintained after adjusting for CVD risk factors (HR, 1.14; 95% CI, 1.09 to 1.20; p < 0.001; per 1 mg/dl increase for UA). Hazard ratio for highest sex-specific quartile group for UA was 1.44 (95%CI; 1.25–1.67) compared with lowest quartile. Similar results were obtained in models using diastolic blood pressure, or both systolic and diastolic blood pressure instead of systolic blood pressure (data not shown). In sex-stratified analysis, HR of women in association of UA with incident hypertension had trend to be higher that of men (HR 1.23 for women, 1.10 for men in multivariable models), but there was no significant interaction of UA with sex in its association with incident hypertension using multiplicative interaction terms (p = 0.08, Table 3)
Table 2.
Hazard ratios of the uric acid for incident hypertension.
| no.of events | Unadjusted |
Model1 |
Model2 |
|
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Continuous | ||||
| total n = 12029 | 1457 | 1.27 (1.23–1.32)* | 1.21 (1.15–1.27)* | 1.14 (1.09–1.20)* |
| Categorical | ||||
| Q1 (n = 3406) | 334 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Q2 (n = 2869) | 295 | 1.05 (0.90–1.23) | 1.05 (0.90–1.23) | 0.98 (0.84–1.15) |
| Q3 (n = 2893) | 365 | 1.30 (1.12–1.51)* | 1.33 (1.15–1.54)* | 1.21 (1.04–1.41)† |
| Q4 (n = 2861) | 463 | 1.72 (1.50–1.98)* | 1.74 (1.51–2.00)* | 1.44 (1.25–1.67)* |
Hazard ratios are indicated per 1 mg/dl increase for continuous uric acid level, and each quartile compared to lowest quartile. Adjustment was performed for the following risk factors: model 1 = adjusted for age, gender, height, and weight; model 2 = model 1 + systolic blood pressure, diabetes, smoking, LDL cholesterol, HDL cholesterol, and eGFR.
Q1 indicates lower quartile for uric acid; Q4, highest quartile; HR, hazard ratio; other abbreviation as in Table 1.
*p < 0.001, †p < 0.05.
Table 3.
Age stratified analysis for the association of UA with incident hypertension in men and women.
| n | no. of incident hypertension | HR | 95%CI | p | ||
|---|---|---|---|---|---|---|
| Male | ||||||
| Total | 7,112 | 1,057 | 1.1 | 1.04 | 1.17 | <0.001 |
| age subgroups | ||||||
| <40 years | 1,769 | 148 | 1.14 | 0.98 | 1.32 | 0.084 |
| 40-50 years | 2,654 | 378 | 1.17 | 1.07 | 1.28 | 0.001 |
| 50-60 years | 1,881 | 360 | 1.07 | 0.96 | 1.19 | 0.201 |
| ≧60 years | 808 | 171 | 1.03 | 0.9 | 1.18 | 0.686 |
| Female | ||||||
| Total | 4,917 | 400 | 1.23 | 1.1 | 1.38 | <0.001 |
| age subgroups | ||||||
| <40 years | 937 | 31 | 1.05 | 0.66 | 1.67 | 0.66 |
| 40-50 years | 1,902 | 130 | 1.29 | 1.07 | 1.56 | 0.009 |
| 50-60 years | 1,436 | 148 | 1.21 | 1 | 1.47 | 0.053 |
| ≧60 years | 642 | 91 | 1.34 | 1.04 | 1.73 | 0.023 |
Hazard ratios are indicated per 1 mg/dl increase for uric acid level. Adjustment was performed for the following risk factors: model 1 = adjusted for age, gender, height, and weight; model 2 = model 1 + systolic blood pressure, diabetes, smoking, LDL cholesterol, HDL cholesterol, and eGFR.
Q1 indicates lower quartile for uric acid; Q4, highest quartile; HR, hazard ratio; other abbreviation as in Table 1.
3.3. Age stratified analysis in men and women
In analyses stratified by baseline age category, we observed that the significant association of UA with incident hypertension among participants below 50 years of age in men, whereas not among participants above 50 years, in multivariable models, respectively (Table 3). In women, there was significant association of UA with incident hypertension among participants above 40 years), whereas not among participants below 40 years (Table 3). In the models using diastolic blood pressure, or both systolic and diastolic blood pressure instead of systolic blood pressure, the associations in 40–50 years in men and above 60 years old in men maintained, whereas the associations in below 40 years in men and below 60 years in women were absent (data not shown).
4. Discussion
The present study evaluates the association of serum UA levels with incident hypertension at short-term (3 years) follow up in a large Japanese general population, using discrete proportional hazards regression models based on annual health check-up data. UA was a significant predictor for incident hypertension independent of other risk factors including baseline blood pressure in the overall population. When we evaluated this association of serum UA with incident hypertension according to different age groups (<40, 40–49, 50–59, ≧60 years), it was only significant in the non-elderly population under 50 years old for men, whereas it was significant in the middle age and the elderly population above 40 years old for women.
The relationship between serum UA and incident hypertension has been explored extensively at short term and long term in different ethnicity [3], [6], [11], [12]. We found that serum UA was associated with incident hypertension at short-term follow-up (3 years) in the Japanese population. The strength of the association was modest in our study (a 14% increased hazard ratios of hypertension incidence 1 mg/dl increment in UA) in the multivariable model and consistent with previous reports from Framingham study that is similar to participants characteristics (mean age 48.7 years old) and follow-up period (4 years). Several mechanisms between serum UA and hypertension have been noted. In experimental studies, hyperuricemia induces renal vascular in renal vasoconstriction through a reduction in nitric oxide, with activation of renin-angiotensin system [13], [14].
The present study demonstrated that sex specific association of serum UA with incident hypertension in different age groups: in men, higher serum UA was associated with incident hypertension only in participants with under 50 years of age, not in those with above 50 years, whereas in women, that association was significant in participants with over 40 years of age. In regards to men, the results in the present study is consistent with the previous study that showed the positive relationship of UA with blood pressure only in the non-elderly in cross-sectional analysis [15]. There are several potential explanations for the attenuation of the relationship of UA with hypertension in the elderly men. Previous studies showed that the effects of serum UA might be more evident at a younger age [3], [16]. Higher background rate of the hypertension incidence with increasing age or other risk factors might have contributed to a reduction in the relationship of UA with hypertension [3].
Several previous studies demonstrated that the cardiovascular risk in women increases in postmenopause period [17], [18], [19], because estrogen has protective effects on vessel function through nitric oxide release and an antiproliferative effect on smooth muscle cells [17]. These estrogen's effect might lessen the impact of UA on blood pressure progression, and reduction of these estrogen's effect around postmenopause period might be the one of the reason for the significant association of serum UA with incident hypertension in women after middle age in the present study.
Our study has limitations. The definition for incident hypertension is based on annual physical examination visit. Because annual health examination is in part dependent of own health consciousness in each participants, the participants at baseline represent a relatively healthy sample who take care of their health. Thus, generalization of the study results is limited by selection and survival bias. Second, we cannot exclude the possibility that participants took lower uric acid medication because we only evaluated the medication for hypertension, diabetes, and dyslipidemia in the present study. The strength of our study is a large sample size and repeated measures of cardiovascular risk factors including uric acid.
5. Conclusion
In the present study based on annual health check-up, higher uric acid is independently associated with greater risk of incident hypertension in the Japanese general population. The present study also confirmed the sex-specific effect on the relationship of serum uric acid levels with incident hypertension in different age groups; baseline serum uric acid level could be an independent predictor among middle aged men below 50 years and middle aged and the elderly women above 40 years. It is important to detect the age/gender groups which would obtain maximal benefit from the treatment of hyperuricemia for the prevention and treatment of hypertension. Future prospective studies and clinical trials are needed to determine whether uric acid is a potential screening and therapeutic target.
Conflict of interest
None declared.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijchy.2019.100009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Whelton P.K., He J., Appel L.J. Primary prevention of hypertension: clinical and public health advisory from the national high blood pressure education program. J. Am. Med. Assoc. J. Am. Med. Assoc. 2002;288(15):1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 2.Rossi G.P., Bernini G., Caliumi C. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol. 2006;48(11):2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 3.Sundstrom J., Sullivan L., D'Agostino R.B., Levy D., Kannel W.B., Vasan R.S. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45(1):28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 4.Perlstein T.S., Gumieniak O., Williams G.H. Uric acid and the development of hypertension: the normative aging study. Hypertension. 2006;48(6):1031–1036. doi: 10.1161/01.HYP.0000248752.08807.4c. [DOI] [PubMed] [Google Scholar]
- 5.Grayson P.C., Kim S.Y., LaValley M., Choi H.K. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res. 2011;63(1):102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takase H., Kimura G., Dohi Y. Uric acid levels predict future blood pressure and new onset hypertension in the general Japanese population. J. Hum. Hypertens. 2014;28(9):529–534. doi: 10.1038/jhh.2013.143. [DOI] [PubMed] [Google Scholar]
- 7.Feig D.I., Kang D.-H., Johnson R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feig D.I., Soletsky B., Johnson R.J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. J. Am. Med. Assoc. J. Am. Med. Assoc. 2008;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siu Y.P., Leung K.T., Tong M.K., Kwan T.H. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am. J. Kidney Dis. Official J. Nat. Kidney Foundation. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 10.van Ballegooijen A.J., Kestenbaum B., Sachs M.C. Association of 25-hydroxyvitamin D and parathyroid hormone with incident hypertension: MESA (Multi-Ethnic Study of Atherosclerosis) J. Am. Coll. Cardiol. 2014;63(12):1214–1222. doi: 10.1016/j.jacc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan E., Kwoh C.K., Schumacher H.R., Kuller L. Hyperuricemia and incidence of hypertension among men without metabolic syndrome. Hypertension. 2007;49(2):298–303. doi: 10.1161/01.HYP.0000254480.64564.b6. [DOI] [PubMed] [Google Scholar]
- 12.Yang T., Chu C.H., Bai C.H. Uric acid concentration as a risk marker for blood pressure progression and incident hypertension: a Chinese cohort study. Metab. Clin. Exp. 2012;61(12):1747–1755. doi: 10.1016/j.metabol.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Mazzali M., Hughes J., Kim Y.G. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 14.Mazzali M., Kanellis J., Han L. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am. J. Physiol. Renal Physiol. 2002;282(6):F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 15.Lee J.J., Ahn J., Hwang J. Relationship between uric acid and blood pressure in different age groups. Clin Hypertens. 2015;21:14. doi: 10.1186/s40885-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feig D.I., Johnson R.J. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42(3):247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi R., Grimaldi T., Origliani G., Fantini G., Coppi F., Modena M.G. Menopause and cardiovascular risk. Pathophysiol. Haemostasis Thrombosis. 2002;32(5–6):325–328. doi: 10.1159/000073591. [DOI] [PubMed] [Google Scholar]
- 18.Kannel W.B., Hjortland M.C., McNamara P.M., Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann. Intern. Med. 1976;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 19.Nabulsi A.A., Folsom A.R., White A. Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. N. Engl. J. Med. 1993;328(15):1069–1075. doi: 10.1056/NEJM199304153281501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.