Abstract
Background
Severe asymptomatic hypertension (SAH) is associated with significant health cost, morbidity and mortality.
Aim
Establish the nationwide prevalence, trends and associated sociodemographic characteristics of SAH among patients with hypertension in the USA.
Methods
We utilized the National Health and Nutrition Examination data collected over five survey cycles (2007–2016). Included were participants aged 20–80 years with self-reported diagnosis of hypertension. SAH was defined as having a mean systolic blood pressure (SBP) ≥180 mmHg and/or mean diastolic blood pressure (DBP) ≥120 mmHg at the time of examination. The Chi square test was used to compare prevalence across different categories. Associations between sociodemographic variables and SAH were assessed using multivariate binary logistic regression.
Results
The prevalence of SAH among patients with hypertension is 2.15% (95% CI 1.80–2.56), mainly explained by isolated mean SBP≥180 mmHg (86% of all cases), with no statistically significant change between 2007: 2.66% (95% CI 2.10–3.36) and 2016:2.61% [95% CI 1.73–3.94), p-trend = 0.17. Increasing age (OR 1.07, 95% CI 1.04–1.09), NH Blacks (OR 2.20, 95% CI 1.37–3.54), BMI< 25 (OR 2.52, 95% CI 1.48–4.28), lack of health insurance OR 4.92% (95% CI 2.53–9.54) and never married individuals (OR = 2.59%, 95% CI 1.20–5.60) were more likely to have SAH, comparatively. There was no significant association between duration of hypertension and SAH.
Conclusion
The prevalence of SAH in the USA is 2.15% and has been stable over the past decade. Our study underscores the importance of identifying barriers to screening and treatment of hypertension which is a major treatable risk factor for cardiovascular disease.
Keywords: Hypertension, NHANES, Prevalence
1. Introduction
Cardiovascular diseases constitute a major global health concern responsible for the highest rate of premature disability and death worldwide [1]. Hypertension remains the strongest risk factor for cardiovascular diseases such as stroke, ischemic heart disease and heart failure [2,3]. In 2010, 31.1% of adults globally were living with hypertension, representing a population of about one billion [1]. In 2018, 49.69% of US adults 20 years and older were living with hypertension, corresponding to 115 million persons [4].
Severe hypertension is defined as systolic blood pressure (SBP) ≥ 180 mmHg and/or a diastolic blood pressure (DBP) ≥120 mmHg. About three-quarters of these persons are usually asymptomatic and without any acute or impending change in target organ damage or dysfunction; termed severe asymptomatic hypertension (SAH). The most recent US guidelines no longer make this distinction in classifying hypertension. However, the European Society of Cardiology defines this severity of blood pressure as Grade 3 hypertension [6], underscoring its importance and highlighting classification challenges. Non-adherence to therapy among patients with hypertension remains the leading predisposing factor for hypertensive crises [[5], [6], [7], [8], [9], [10]]. However, the absence of discernible clinical or laboratory end organ damage does not necessarily equate SAH to a purely benign state and thus must not be overlooked. Treatment for SAH consists in resumption of antihypertensive medications for non-adherent patients; intensification of antihypertensive therapy in adherent patients; and initiation of therapy for newly diagnosed patients [[11], [12], [13]], often without the need for hospitalization.
While it is common knowledge that appropriate treatment of hypertension remains the best preventive approach of hypertensive crises, a significant proportion of patients will eventually experience hypertensive crises [5,6,14,15]. Though there is considerable literature on the incidence of hypertensive crises in emergency departments, there is limited data on the community prevalence and sociodemographic characteristics. We aim to assess the community prevalence and trend of SAH and its determinants among participants with hypertension in the USA from 2007 to 2016.
2. Materials and methods
2.1. Survey design
The NHANES (National Health And Nutrition Examination Survey), conducted by the Centers for Disease Control and Prevention/National Center for Health, is a nationally representative survey on the health and nutritional status of non-institutionalized US population. It utilizes a multistage probability sampling design and collects information from approximately 5000 persons per year. Detailed information on the NHANES survey is available from the survey documentation [16].
2.2. Data collection
Survey participants were interviewed in their homes to ascertain demographic characteristics (age, gender, level of education, ethnicity, marital status, place of birth, health insurance, and smoking status among others); and comorbidities (diabetes, hypertension, age at diagnosis of hypertension and treatment status [whether issued a prescription for antihypertensive pills or not], and dyslipidemia). Questions on disease conditions were generally followed by 4 categorical response options (“Yes” “No” “Refuse” “Don't know”). Persons who responded “Refuse” and “Don't know” were classified as “No”, which in most instances constituted less than 0.1% of all respondents. Persons who reported having smoked ≥100 cigarettes were classified as smokers. Body Mass Index (BMI) was calculated from measured weight and height and grouped into 3 categories (BMI<25.00, BMI 25.00–29.99, BMI≥30.00). The family poverty index (PIR) was calculated by dividing the total family income by the poverty threshold, as defined by the US census bureau, adjusting for family size at the time of the interview [17]. Family PIR was grouped into three categories (PIR<1.00, PIR 1.00–2.99 and PIR≥3.00).
Persons who had been told by their physicians as having hypertension on at least two separate occasions and/or reported being on antihypertensive medications were considered to have hypertension. At mobile examination centers (MEC), up to four separate blood pressure measurements were obtained using standardized protocols. Mean systolic (SBP) and diastolic (DBP) blood pressures were then computed and persons with mean SBP ≥ 180 mmHg and/or a mean DBP ≥120 mmHg were classified as having SAH. Persons with SBP <180 mmHg and DBP <120 mmHg were considered as not having SAH. All study questionnaires, exact question wording and response options are freely accessible [16]. Informed consent was obtained from all participants, and the institutional review board of the National Center for Health Statistics approved the protocol.
2.3. Statistical analysis
Relevant datasets from five survey cycles (2007–2016) were combined and analytical weights computed in keeping with analytic guidelines [18] to generate disease prevalence estimates representative of the USA civilian, noninstitutionalized population. Included in our analysis were participants≥20 years with previous diagnosis of hypertension or on antihypertensive pills. Participants were classified into two groups depending on the presence or absence of severe asymptomatic hypertension. The Chi square test for categorical variables was used to compare prevalence across the two categories. Trends of SAH was evaluated using binary logistics regression. Determinants of SAH were assessed using multivariate binary logistic regression. Analysis was done using STATA 16 (STATA IC 16.1, College Station, TX: StataCorp LP) with two-tailed p values < 0.05 considered statistically significant.
3. Results
3.1. Participant characteristics
A total of 50,588 individuals participated in the NHANES surveys from 2007 to 2016. Of these, 29,201 (57.7%) persons were in the age range 20–80 years, with a median age of 49 years (IQR 34 to 64). 9785 persons reported having been diagnosed of hypertension. About 75% of participants with hypertension were ≥50 years with a median duration since diagnosis of 9 years (IQR = 4–17), of which 91% reported having been issued prescriptions for antihypertensive pills. Most participants were married, US born, NH Whites and 56% had at least some form of college education. About 90% of study participants had health insurance while 17% lived in poverty. The prevalence of smoking, dyslipidemia and diabetes was 51%, 58% and 21%, respectively (Table 1).
Table 1.
Characteristics of study participants and prevalence of severe asymptomatic hypertension.
| Variable | Categories | All (%) (n = 9785) | Hypertensive Urgency (%) |
Odds ratio | p-valuea | |
|---|---|---|---|---|---|---|
| Yes (n = 281) | No (n = 9504) | |||||
| Age (Years) | Overall (Median-IQR) | 59 (49–70) | 64 (55–80) | 59 (49–69) | 2.09 (1.75–2.49) | <0.001 |
| 20 to <35 | 6.223 | 1.272 | 6.328 | 0.43 (0.13–1.38) | <0.001 | |
| 35 to <50 | 19.94 | 12.62 | 20.0 | 1.32 (0.84–2.06) | ||
| 50 to <65 | 37.56 | 29.61 | 37.72 | 1.65 (1.08–2.52) | ||
| ≥65 | 36.28 | 56.50 | 35.85 | 3.25 (1.72–2.54) | ||
| Duration of hypertension (Years) | Overall (median-IQR) | 9 (4–17) | 12 (6–22) | 9 (4–17) | NA | 0.01 |
| 0 to <5 | 28.42 | 17.97 | 28.64 | 1.30 (0.84–2.00) | 0.006 | |
| 5 to <10 | 22.18 | 18.23 | 22.26 | 1.69 (1.06–2.68) | ||
| ≥10 | 49.40 | 63.80 | 49.10 | 2.65 (2.11–2.65) | ||
| Gender | Male | 46.13 | 40.69 | 46.25 | 1.84 (1.33–2.55) | 0.29 |
| Female | 53.87 | 59.31 | 53.75 | 2.30 (1.80–2.94) | ||
| Educational status | Less than HS | 19.72 | 26.2 | 19.58 | 2.76 (2,06–3.69) | 0.012 |
| HS or GED | 24.30 | 28.39 | 24.21 | 2.43 (1.62–3.64) | ||
| Some college or Associate degree | 32.18 | 33.98 | 32.14 | 2.20 (1.52–3.17) | ||
| College and above | 23.80 | 11.44 | 24.07 | 1.00 (0.66–1.51) | ||
| Ethnicity | Hispanic | 9.00 | 9.065 | 12.30 | 2.83 (2.02–3.96) | 0.001 |
| NH Black | 14.18 | 14.37 | 14.37 | 3.4 (2.70–4.28) | ||
| NH White | 71.04 | 70.80 | 59.91 | 1.77 (1.32–2.36) | ||
| Others | 5.789 | 5.76 | 4.403 | 1.60 (0.86–2.95) | ||
| Marital status | Never married | 9.134 | 13.53 | 9.04 | 3.10 (1.94–4.91) | <0.001 |
| Married | 63.43 | 44.24 | 63.84 | 1.46 (1.12–1.90) | ||
| Divorce | 15.75 | 20.94 | 15.64 | 2.78 (1.75–4.40) | ||
| Widowed | 11.69 | 21.29 | 11.49 | 3.81 (2.72–5.31) | ||
| Place of Birth | USA | 87.60 | 83.07 | 87.70 | 1.96 (1.41–2.73) | 0.16 |
| Non-USA | 12.40 | 16.93 | 12.30 | 2.83 (1.91–4.17) | ||
| Health Insurance | Yes | 89.60 | 79.15 | 89.82 | 1.84 (1.51–2.26) | <0.001 |
| No | 10.40 | 20.85 | 10.18 | 4.19 (2.68–6.49) | ||
| Poverty Index Ratio | <1 | 17.24 | 30.53 | 16.98 | 3.31 (2.25–4.85) | 0.002 |
| 1 to 3 | 42.05 | 38.84 | 42.11 | 1.73 (1.38–2.16) | ||
| ≥3 | 40.71 | 30.62 | 40.90 | 1.41 (0.92–2.14) | ||
| Body Mass Index | <25 | 15.92 | 30.63 | 15.62 | 3.92 (2.99–5.13) | <0.001 |
| 25–30 | 31.93 | 32.87 | 31.91 | 2.10 (1.46–3.00) | ||
| ≥30 | 52.14 | 36.5 | 52.47 | 1.43 (0.99–2.05) | ||
| Smoking | Yes | 51.06 | 51.24 | 51.05 | 2.10 (1.60–2.75) | 0.97 |
| No | 48.94 | 48.76 | 48.95 | 2.08 (1.61–2.70) | ||
| Dyslipidemia | Yes | 58.74 | 57.84 | 58.76 | 1.93 (1.42–2.63) | 0.85 |
| No | 41.26 | 42.16 | 41.24 | 2.01 (1.55–2.59) | ||
| Diabetes | Yes | 21.94 | 26.25 | 21.84 | 2.49 (1.83–3.38) | 0.26 |
| Hypertension treatment | No | 78.06 | 73.75 | 78.16 | 1.96 (1.52–2.53) | |
| Yes | 90.89 | 96.67 | 90.77 | 2.22 (1.83–2.71) | 0.06 | |
| No | 9.11 | 3.33 | 9.23 | 0.78 (0.25–2.38) | ||
HS: High school; GED: General education diploma; USA: United States of America.
p-values obtained from comparison of prevalence of severe asymptomatic hypertension across categories.
3.2. Prevalence of severe asymptomatic hypertension and trend
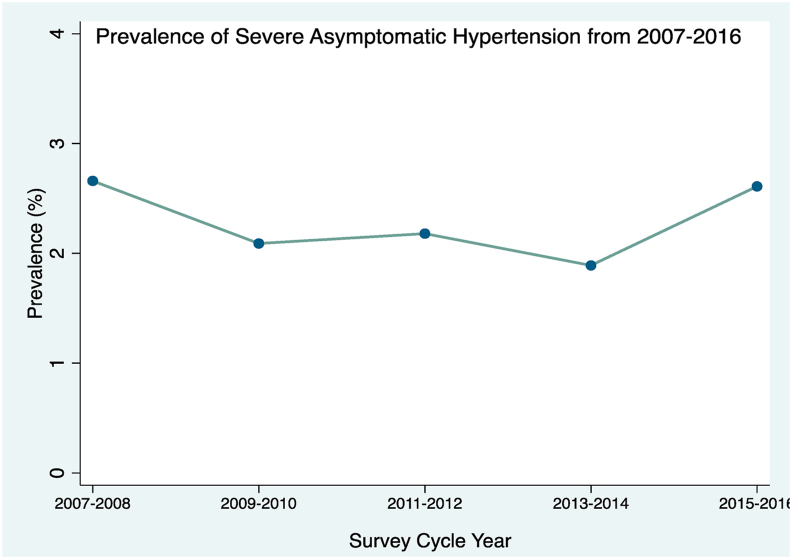
The prevalence of hypertension among persons ≥20 years was 31.8%, corresponding to 72 million (95% CI 67–76 million) persons. The prevalence of severe asymptomatic hypertension was 2.15% (95% CI 1.80–2.56), corresponding to an absolute head count of 1.39 million (95% CI 1.15–1.63million) persons. The proportion of SAH explained by an isolated elevated SBP, isolated elevated DBP and by both elevated SBP and DBP was 85.8%, 7.3% and 6.9%, respectively. The prevalence of severe asymptomatic hypertension remained stable throughout the study period, 2.66% (95% CI 2.10–3.36) in 2007 and 2.61% (95% CI 1.73–3.94) in 2016, with p-trend of 0.17 as shown on Fig. 1.
Fig. 1.
Line plot showing prevalence of Severe Asymptomatic Hypertension by survey year (ptrend = 0.17).
In univariate models, severe asymptomatic hypertension was significantly and positively associated with increasing age and duration of hypertension. Participants with less than high school certificate, NH Blacks, widow (er)s, persons without health insurance and individuals living in poverty also had significantly higher prevalence compared to their respective counterparts. There was no statistically significant difference in prevalence of SAH by categories of smoking, dyslipidemia, diabetes, place of birth, antihypertensive treatment status and gender.
In a multivariate model, older persons, NH Blacks, participants with BMI<25 kg/m2, never married and those without health insurance were more likely to have SAH, comparatively. NH Blacks were 2.20 (95% CI 1.37–3.54) times likely to have SAH compared to NH Whites. Also, persons without health insurance were 4.92 (95% CI 2.53–9.54) times likely to have SAH compared to those with health insurance. Persons with BMI <25 kg/m2 (normal weight) were 2.52 (95% CI 1.48–4.28) times likely to have SAH compared to those with BMI≥30 kg/m2 (obese). On a similar note, persons who had never been married were more likely to have SAH compared to those who were married or living together. There were no statistically significant associations between poverty, duration of hypertension and current treatment status and SAH. Full display of associations between socio-demographic characteristics and select comorbidities with SAH are shown on Table 2.
Table 2.
Factors associated with severe asymptomatic hypertension.
| Variable | Categories | OR (95% CI) | p-value |
|---|---|---|---|
| Age | 1.07 (1.04–1.09) | <0.001 | |
| Gender | Female | 1 | |
| Male | 1.23 (0.73–2.07) | 0.430 | |
| Ethnicity | NH whites | 1 | |
| Hispanics | 1.77 (0.92–3.42) | 0.090 | |
| NH Blacks | 2.20 (1.37–3.54) | 0.001 | |
| Others | 0.68 (0.30–1.57) | 0.363 | |
| Educational level | First degree and above | 1 | |
| Less than high school | 1.48 (0.72–3.06) | 0.290 | |
| High school or GED | 2.05 (1.02–4.14) | 0.045 | |
| Some college/associate degree | 2.01 (1.02–3.94) | 0.043 | |
| Marital Status | Married or living with partner | 1 | |
| Never married | 2.59 (1.2–5.6) | 0.016 | |
| Divorce or separated | 1.77 (0.92–3.4) | 0.084 | |
| Widowed | 1.59 (0.83–3.05) | 0.161 | |
| Health Insurance | Yes | 1 | |
| No | 4.92 (2.53–9.54) | <0.001 | |
| Poverty index ratio (PIR) | PIR≥3 | 1 | |
| less than 1 | 1.18 (0.6–2.33) | 0.619 | |
| PIR 1 to <3 | 0.67 (0.38–1.18) | 0.162 | |
| Body mass index (in kg/m2) | BMI≥30 | 1 | |
| BMI less than 25 | 2.52 (1.48–4.28) | 0.001 | |
| BMI 25 to less than 30 | 1.39 (0.77–2.50) | 0.268 | |
| Antihypertensives | Yes | 1 | |
| No | 0.54 (0.12–2.4) | 0.421 | |
| Duration of hypertension | 1.00 (0.99–1.02) | 0.707 |
OR = Odds ratio, CI = Confidence interval, NH = Non-Hispanic, GED = General education diploma.
4. Discussion
Using cumulative nationally representative data spanning a decade, we report a nationwide community prevalence of severe asymptomatic hypertension among persons with hypertension of 2.15% in the USA. This prevalence remained stable from 2007 through 2016. Older persons, NH Blacks, individuals with BMI<25, never married, individuals with higher levels of education and participants without health insurance were more likely to have SAH than their respective counterparts.
Our prevalence of 2.15%, corresponding to about 1.3 million individuals, is similar to the 2.0% reported by Caligiuri et al. in a hypertension awareness campaign in Canada [19]. Though with similar prevalence, the health awareness campaign included all-comers who were willing to participate in the study in contrast to the subset with hypertension in the current study. Our prevalence is however lower than the modal 5% reported by other authors [8,20,21]. This discrepancy is partly explained by the fact that these studies were carried out in health care settings, capturing both patients with severe asymptomatic hypertension and emergency, contrary to NHANES study that is conducted in a non-healthcare setting that captured participants with SAH. This suggests that a significant proportion of hypertensive patients live in a chronic state of severe hypertension and thus more likely to present with long term hypertension related complications as opposed to hypertensive emergencies.
Studies have shown disparate results with regards to outcomes of hypertensive crises with some studies showing a benign course with low rate of MACE and others showing increased event rates. In one study by Patel et al., SAH when diagnosed in a clinic setting is associated with a low rate of conversion to hypertensive emergency and onset of major adverse cardiovascular event within 6 months [21]. However increased event rates of MACE have been observed in hypertensive crises seen in those presenting as emergency or needing hospitalization [22]. The stable prevalence of severe asymptomatic hypertension is despite the fact that there has been a trend in improved hypertension control [23]. There appears to be an unmet need in identifying individuals with SAH in the community underscoring the need for identifying the responsible factors and interventions in this subgroup.
We observed that participants without health insurance are five times likely to harbor severe asymptomatic hypertension. Lack of health care coverage can be an impedance to seek medical care and also afford medications. Nonadherence to prescribed medical therapy is the main predisposing factor for hypertensive crises, with laboratory confirmation through measurements of drug metabolites [6,8]. While medication acquisition is no guarantee for treatment adherence, it is certainly an undeniable pre-requisite. Finally, persons without health insurance are less likely to follow up with care providers for treatment review as well as adhering to recommended dietary and lifestyle modifications [24]. Interventions like “polypill” have been studied in underserved population with benefits observed in terms of reduction in blood pressure and low density lipoprotein cholesterol [25]. Consistent with prior studies, older age, NH blacks and never married individuals were more likely to have hypertensive crises [15,[26], [27], [28]].
Hypertensives with lower BMI were more likely to have SAH compared to obese participants. This observation, though counterintuitive, is not entirely novel and clear reasons remain poorly elucidated. However, previous authors have reported an obesity paradox in which obese individuals had a somewhat better cardiovascular disease profile comparatively. Possible explanations include increased disease awareness, decreased global sympathetic activity and increased access to healthcare services among others [[29], [30], [31]]. Also, we observed a nonlinear association between level of education and SAH which runs counter to the widely reported increased burden and poor control rates in less educated individuals [4]. Being a cross sectional study, this might be explained by residual confounding. Also, this may suggest that while a higher proportion of less educated individuals have poorly controlled blood pressure, they are largely below the range for SAH. We encourage researchers to consider exploring the associations between obesity and level of education and SAH in future projects.
4.1. Strengths and limitations
Though the first nationally representative study on the community prevalence of SAH among patients with hypertension, lack of clinical and laboratory data might have led to misclassification of a few participants with end organ damage. However, it is very unlikely that persons with hypertensive emergency would have remained asymptomatic to show up at MEC for appointments and complete the data collection procedures. Also, hypertension status was self-reported which could lead to misclassification. Furthermore, participants were examined once and thus we are unable to establish if the severely elevated blood pressure was a transient observation (maybe related to the whitecoat phenomenon) or a persistent state. Notwithstanding, the use of standardized BP measuring procedures with up to 4 values allowed for accurate determination of blood pressure and classification of patients. Despite these limitations, we have been able to identify subgroups with disproportionately higher burden of severe asymptomatic hypertension. Studies to identify barriers and the impact of interventions both from health policy and health care provider standpoint are needed.
5. Conclusion
The community prevalence of SAH among patients with hypertension in the USA is 2.15% and has remained stable over of the past decade. Increasing age, never married individuals, persons without health insurance, NH Blacks, individuals with BMI <25 kg/m2 and higher level of education persons harbor disproportionately higher prevalence of SAH. Interventions aimed at reducing the burden of severe hypertension at the community level are much needed.
Author contributions
Conceptualization: Ebad Ur Rahman, Mehiar El-Hamdani, Adee ElHamdani
Statistical Analysis: Muchi Ditah Chobufo, Kanaan Mansoor
Writing – original draft - Ebad Ur Rahman, Farah Fatima, Mohamed Suliman
Writing – review and editing – Ebad Ur Rahman, Sudarshan Balla
Supervision: Sudarshan Balla
Funding
None.
Declaration of competing interest
None.
References
- 1.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., Amann M., Anderson H.R., Andrews K.G., Aryee M., Atkinson C., Bacchus L.J., Bahalim A.N., Balakrishnan K., Balmes J., Barker-Collo S., Baxter A., Bell M.L., Blore J.D., Blyth F., Bonner C., Borges G., Bourne R., Boussinesq M., Brauer M., Brooks P., Bruce N.G., Brunekreef B., Bryan-Hancock C., Bucello C., Buchbinder R., Bull F., Burnett R.T., Byers T.E., Calabria B., Carapetis J., Carnahan E., Chafe Z., Charlson F., Chen H., Chen J.S., Cheng A.T.-A., Child J.C., Cohen A., Colson K.E., Cowie B.C., Darby S., Darling S., Davis A., Degenhardt L., Dentener F., Des Jarlais D.C., Devries K., Dherani M., Ding E.L., Dorsey E.R., Driscoll T., Edmond K., Ali S.E., Engell R.E., Erwin P.J., Fahimi S., Falder G., Farzadfar F., Ferrari A., Finucane M.M., Flaxman S., Fowkes F.G.R., Freedman G., Freeman M.K., Gakidou E., Ghosh S., Giovannucci E., Gmel G., Graham K., Grainger R., Grant B., Gunnell D., Gutierrez H.R., Hall W., Hoek H.W., Hogan A., Hosgood H.D., Hoy D., Hu H., Hubbell B.J., Hutchings S.J., Ibeanusi S.E., Jacklyn G.L., Jasrasaria R., Jonas J.B., Kan H., Kanis J.A., Kassebaum N., Kawakami N., Khang Y.-H., Khatibzadeh S., Khoo J.-P., Kok C., Laden F., Lalloo R., Lan Q., Lathlean T., Leasher J.L., Leigh J., Li Y., Lin J.K., Lipshultz S.E., London S., Lozano R., Lu Y., Mak J., Malekzadeh R., Mallinger L., Marcenes W., March L., Marks R., Martin R., McGale P., McGrath J., Mehta S., Mensah G.A., Merriman T.R., Micha R., Michaud C., Mishra V., Mohd Hanafiah K., Mokdad A.A., Morawska L., Mozaffarian D., Murphy T., Naghavi M., Neal B., Nelson P.K., Nolla J.M., Norman R., Olives C., Omer S.B., Orchard J., Osborne R., Ostro B., Page A., Pandey K.D., Parry C.D.H., Passmore E., Patra J., Pearce N., Pelizzari P.M., Petzold M., Phillips M.R., Pope D., Pope C.A., Powles J., Rao M., Razavi H., Rehfuess E.A., Rehm J.T., Ritz B., Rivara F.P., Roberts T., Robinson C., Rodriguez-Portales J.A., Romieu I., Room R., Rosenfeld L.C., Roy A., Rushton L., Salomon J.A., Sampson U., Sanchez-Riera L., Sanman E., Sapkota A., Seedat S., Shi P., Shield K., Shivakoti R., Singh G.M., Sleet D.A., Smith E., Smith K.R., Stapelberg N.J.C., Steenland K., Stöckl H., Stovner L.J., Straif K., Straney L., Thurston G.D., Tran J.H., Van Dingenen R., van Donkelaar A., Veerman J.L., Vijayakumar L., Weintraub R., Weissman M.M., White R.A., Whiteford H., Wiersma S.T., Wilkinson J.D., Williams H.C., Williams W., Wilson N., Woolf A.D., Yip P., Zielinski J.M., Lopez A.D., Murray C.J.L., Ezzati M., AlMazroa M.A., Memish Z.A. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A global brief on hypertension | A global brief on Hyper tension. 2013. www.who.int (accessed March 14, 2019) [Google Scholar]
- 3.Agbor V.N., Essouma M., Ntusi N.A.B., Nyaga U.F., Bigna J.J., Noubiap J.J. Heart failure in sub-Saharan Africa: a contemporaneous systematic review and meta-analysis. Int. J. Cardiol. 2018;257:207–215. doi: 10.1016/j.ijcard.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 4.Ditah M., Gayam V., Soluny J., Rahman E., Enoru S. International Journal of Cardiology Hypertension Prevalence and control of hypertension in the USA: 2017–2018. Int. J. Cardiol. Hypertens. 2020 doi: 10.1016/j.ijchy.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender S.R., Fong M.W., Heitz S., Bisognano J.D. Characteristics and management of patients presenting to the emergency department with hypertensive urgency. J. Clin. Hypertens. (Greenwich) 2006;8:12–18. doi: 10.1111/j.1524-6175.2005.04898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krämer B.K., Krämer R.M., Benck U., Krüger B. Nonadherence in patients with hypertensive emergency or hypertensive urgency. J. Clin. Hypertens. 2018:1–3. doi: 10.1111/jch.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jr G.R. Management of hypertensive crises. JAMA. 2011;266:829. [PubMed] [Google Scholar]
- 8.Overgaauw N., Alsma J., Brink A., Hameli E., Bahmany S., Peeters L.E.J., Van Den Meiracker A.H., Schuit S.C.E., Koch B.C.P., Versmissen J. Drug nonadherence is a common but often overlooked cause of hypertensive urgency and emergency at the emergency department. J. Hypertens. 2018:1. doi: 10.1097/HJH.0000000000002005. [DOI] [PubMed] [Google Scholar]
- 9.Saguner A.M., Dur S., Perrig M., Schiemann U., Stuck A.E., Burgi U., Erne P., Schoenenberger A.W. Risk factors promoting hypertensive crises: evidence from a longitudinal study. Am. J. Hypertens. 2010;23:775–780. doi: 10.1038/ajh.2010.71. [DOI] [PubMed] [Google Scholar]
- 10.Wallbach M., Lach N., Stock J., Hiller H., Mavropoulou E., Chavanon M.-L., Neurath H., Blaschke S., Lowin E., Herrmann-Lingen C., Müller G.A., Koziolek M.J. Direct assessment of adherence and drug interactions in patients with hypertensive crisis – a cross-sectional study in the Emergency Department. J. Clin. Hypertens. 2019;21:55–63. doi: 10.1111/jch.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., MacLaughlin E.J., Muntner P., Ovbiagele B., Smith S.C., Spencer C.C., Stafford R.S., Taler S.J., Thomas R.J., Williams K.A., Williamson J.D., Wright J.T. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. Hypertension. 2017;71 doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 12.Varon J. The diagnosis and treatment of hypertensive crises. Postgrad. Med. 2009;121:5–13. doi: 10.3810/pgm.2009.01.1950. [DOI] [PubMed] [Google Scholar]
- 13.Vidt D.G. Hypertensive crises: emergencies and urgencies. J. Clin. Hypertens. (Greenwich) 2004;6:520–525. doi: 10.1111/j.1524-6175.2004.03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tisdale J.E., Huang M.B., Borzak S. Risk factors for hypertensive crisis: importance of out-patient blood pressure control. Fam. Pract. 2004;21:420–424. doi: 10.1093/fampra/cmh412. [DOI] [PubMed] [Google Scholar]
- 15.Bennett N.M., Shea S. Hypertensive emergency: case criteria, sociodemographic profile, and previous care of 100 cases. Am. J. Public Health. 1988;78:636–640. doi: 10.2105/AJPH.78.6.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NHANES Questionnaire Data, (n.d.). https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire (accessed June 29, 2018).
- 17.U.C. Bureau, How the Census Bureau Measures Poverty, (n.d.). https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html (accessed January 15, 2019).
- 18.NHANES - Survey Methods and Analytic Guidelines, (n.d.). https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed April 24, 2017).
- 19.Caligiuri S.P.B., Austria J.A., Pierce G.N. Alarming prevalence of emergency hypertension levels in the general public identified by a hypertension awareness campaign. Am. J. Hypertens. 2017;30:236–239. doi: 10.1093/ajh/hpw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zampaglione B., Pascale C., Marchisio M., Cavallo-Perin P. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertens. (Dallas, Tex. 1979) 1996;27:144–147. doi: 10.1161/01.HYP.27.1.144. [DOI] [PubMed] [Google Scholar]
- 21.Patel K.K., Young L., Howell E.H., Hu B., Rutecki G., Thomas G., Rothberg M.B. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern. Med. 2016;176:981–988. doi: 10.1001/jamainternmed.2016.1509. [DOI] [PubMed] [Google Scholar]
- 22.Deshmukh A., Kumar G., Kumar N., Nanchal R., Gobal F., Sakhuja A., Mehta J.L. Effect of joint national committee VII report on hospitalizations for hypertensive emergencies in the United States. Am. J. Cardiol. 2011;108:1277–1282. doi: 10.1016/j.amjcard.2011.06.046. [DOI] [PubMed] [Google Scholar]
- 23.Egan B.M., Zhao Y., Axon R.N. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 24.Duru O.K., Vargas R.B., Kermah D., Pan D., Norris K.C. Health insurance status and hypertension monitoring and control in the United States. Am. J. Hypertens. 2007;20:348–353. doi: 10.1016/j.amjhyper.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz D., Uzoije P., Reynolds C., Miller R., Walkley D., Pappalardo S., Tousey P., Munro H., Gonzales H., Song W., White C., Blot W.J., Wang T.J. Polypill for cardiovascular disease prevention in an underserved population. N. Engl. J. Med. 2019;381:1114–1123. doi: 10.1056/NEJMoa1815359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldron F.A., Benenson I., Jones-Dillon S.A., Zinzuwadia S.N., Adeboye A.M., Eris E., Mbadugha N.E., Vicente N., Over A. Prevalence and risk factors for hypertensive crisis in a predominantly African American inner-city community. Blood Press. 2019:1–10. doi: 10.1080/08037051.2019.1568183. [DOI] [PubMed] [Google Scholar]
- 27.Shea S., Misra D., Ehrlich M.H., Field L., Francis C.K. Predisposing factors for severe, uncontrolled hypertension in an inner-city minority population. N. Engl. J. Med. 1992;327:776–781. doi: 10.1056/NEJM199209103271107. [DOI] [PubMed] [Google Scholar]
- 28.van den Born B.-J.H., Koopmans R.P., Groeneveld J.O., van Montfrans G.A. Ethnic disparities in the incidence, presentation and complications of malignant hypertension. J. Hypertens. 2006;24:2299–2304. doi: 10.1097/01.hjh.0000249710.21146.38. [DOI] [PubMed] [Google Scholar]
- 29.Esler M., Lambert G., Schlaich M., Dixon J., Sari C.I., Lambert E. Obesity paradox in hypertension: is this because sympathetic activation in obesity-hypertension takes a benign form? Hypertens. (Dallas, Tex. 1979) 2018;71:22–33. doi: 10.1161/HYPERTENSIONAHA.117.09790. [DOI] [PubMed] [Google Scholar]
- 30.Jayedi A., Shab-Bidar S. Nonlinear dose-response association between body mass index and risk of all-cause and cardiovascular mortality in patients with hypertension: a meta-analysis. Obes. Res. Clin. Pract. 2018;12:16–28. doi: 10.1016/j.orcp.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Carbone S., Canada J.M., Billingsley H.E., Siddiqui M.S., Elagizi A., Lavie C.J. Obesity paradox in cardiovascular disease: where do we stand? Vasc. Health Risk Manag. 2019;15:89–100. doi: 10.2147/VHRM.S168946. [DOI] [PMC free article] [PubMed] [Google Scholar]



 @sudarshanballa
@sudarshanballa