1. Introduction
Over the last seven decades, starting with the demonstration of a survival benefit by treating malignant hypertension in the 1950s, epidemiological research such as the Framingham Heart Study firmly established the role of hypertension as a cardiovascular (CV) risk factor. Also, the benefits of treating lesser and lesser degrees of diastolic and systolic (BP) elevation were demonstrated in many National Institutes of Health-, Veterans Administration-, and industry-sponsored trials, including the recent SPRINT trial that assessed tighter treatment goals. Results of these studies were incorporated into management guidelines and better treatment of hypertension certainly contributed to the ∼100,000 decrease in annual CV deaths in the US from 1980 until the early 2010s [1].
However, not all the potential benefits of treating hypertension have been realized. Even in developed wealthy countries, control rates of aware and treated hypertensive subjects hover around 50% only [1]. Some reasons explaining the failure to achieve better results exceed the scope of the health care system and are more in the realm of societal interventions (e.g., health insurance, access to care, cost of medications). Others belong to the psycho-behavioral sphere (e.g., adherence and compliance with medications). In the latter, some inroads have been made by technology that enables measurements of compliance (e.g., mass spectrometry for the detection of medication metabolites in biological fluids) and by testing multidisciplinary approaches to patient care.
Even if these major issues were completely solved, there would remain two relevant additional barriers that belong in the biological realm. The first is the existence of a group of hypertensive subjects, known as the refractory resistant [2] who are not controlled despite supramaximal therapy. The second is the residual CV risk of well treated and controlled hypertensive subjects, which is higher for any BP achieved with antihypertensive therapy compared to that for the same BP as an untreated value in normotensive subjects [3].
In this opinion statement, we hypothesize about a possible role for amiloride, a blocker of the epithelial sodium channel (ENaC) in overcoming these two biological barriers.
2. Resistant and refractory hypertension, the role of ENaC
Apparent resistant hypertension (BP uncontrolled with ≥3 antihypertensive agents of different classes including a diuretic, or BP controlled with ≥4 agents) had a prevalence of 12.8% in a study of nearly half a million hypertensive subjects [4]. However, after causes of false resistance are excluded (concomitant use of pressors, overestimation of BP by poorly trained personnel, presence of the white coat phenomenon, suboptimal treatment and patient non-adherence), the actual prevalence of truly resistant hypertension may be closer to half that figure [5]. Spironolactone has become a drug of choice to treat resistant hypertension. Its beneficial effect is beyond that expected for its weak diuretic action and probably accounted for by the “inappropriate” aldosterone excess, sometimes despite renin suppression, that has been described in resistant hypertension, particularly with concomitant obesity or sleep apnea [6].
Refractory hypertension is a subset of about 5–10% of resistant hypertensive subjects who remain uncontrolled after 6 months of treatment by a hypertension specialist, usually despite 6 or more antihypertensive agents. It is more common in Blacks and in the southern US “stroke belt” and is associated with very poor prognosis, with rates of stroke and heart failure that exceed those in resistant hypertension.
Interestingly, BP reduction produced by spironolactone in refractory subjects was half that observed in resistant ones, despite their equal renin and aldosterone plasma levels [2]. In an earlier study of severe, low renin hypertension in Blacks, responses to amiloride were twice as potent as those to spironolactone [7]. Both observations suggest that there is ENaC hyperactivity in this population and that it is not entirely aldosterone dependent. Actually, up to 34% of these subjects may not respond to spironolactone at all, whereas they exhibit significant depressor responses to amiloride. They are homozygous for the variant CC allele of a SNP (rs3890011) in the CYP4A11 gene [8]. The mechanism for this entirely aldosterone-independent, ENaC hyperactivity is not fully understood, but the genetic information allows for a plausible explanation.
CYP4A11 is an ϖ-hydroxylase that oxidizes arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE). This compound has pro-hypertensive (vasoconstrictor) and antihypertensive (natriuretic) properties. A deficit of the latter is the major mechanism for salt sensitivity of BP in the Dahl SS rat. Also, a loss of function variant in another SNP of CYP4A11 (rs1126742 or T8590C), which is in linkage disequilibrium with rs3890011, has been associated with hypertension in several populations. However, the natriuretic action of 20-HETE is mediated by inhibition of NKCC at the thick ascending limb and of basolateral Na+K+ATPase, without effects on ENaC. The explanation for the association between a SNP in CYP4A11 and ENaC hyperactivity in the rs3890011 CC homozygous subjects probably resides in the complex interactions between CYP enzymes. Hence, in mice, a genetic deficiency of Cyp4a10 (a 20-HETE generating ϖ-hydroxylase) does not result in a deficit of 20-HETE (probably because of redundancy with other ϖ-hydroxylases). Rather, Cyp4a10 deficiency is associated with a marked reduction in the expression and activity of several epoxygenases, by mechanisms that are not fully understood but may involve the transcription factor PPARα [9]. Epoxygenases are CYP enzymes that synthesize epoxyeicosatrienoic acids (EETs) from arachidonic acid. Among their multiple actions, EETs are the most powerful endogenous inhibitors of ENaC. Therefore, the Cyp4a10−/− mouse has reduced synthesis of renal EETs and decreased EET-induced inactivating threonine phosphorylation of γENaC, with consequent hyperactivity of the channel and salt sensitive hypertension responsive to amiloride. Although there is no proof for a deficit of epoxygenases in the CYP4A11 rs3890011 CC homozygous Black hypertensive patients, their aldosterone-independent ENaC hyperactivity and their responsiveness to amiloride strongly suggest that a similar mechanism may underlie their hypertension. This is consistent with otherwise documented decreased levels of EETs in salt-sensitive hypertension in Dahl rats and humans [10].
The information reviewed above strongly supports the view that amiloride may have a specific role in the treatment of refractory hypertension and of resistant hypertension unresponsive to spironolactone. This is not reflected in the current guideline for management of resistant hypertension [11] or in the clinical use of this diuretic, which is relatively infrequent. The prevalence of the CYP4A11 rs3890011 CC genotype in hypertensive Blacks is higher than that of refractory hypertension among all resistant patients, suggesting that such approach may result in benefit for a significant number of resistant and refractory hypertensive patients and should be incorporated into future guidelines, at least as an alternative to lack of control of BP after use of spironolactone.
3. Residual risk after successful treatment of hypertension
It is well known that treating and controlling BP does not entirely remove the CV risk conferred by hypertension. A so-called residual risk has been demonstrated by comparing incident events in long-term studies of well treated hypertensive subjects with those in normotensive ones at the same level of BP. In PRIME (Prospective Epidemiological Study of Myocardial Infarction), a study of almost 10,000 middle-age European men followed for 10 years, treated hypertensive patients had a 50% increased risk for any CV event (46% for coronary disease, 75% for stroke and 62% for CV death), compared with normotensive subjects after adjusting for systolic BP [12].
A possible contributor to residual risk could be a deleterious effect or a diminished organ-specific benefit of otherwise effective antihypertensive agent families, consistent with results of randomized clinical trials. For example, in PRIME, residual risk for coronary events was greater for calcium channel blockers, whereas that for stroke was greater for angiotensin converting enzyme inhibitors.
Other observations suggest that residual risk is in part due to established target organ damage. Because relative risk reduction by therapy is the same at any level of calculated preexisting CV risk, the absolute risk reduction is greater the higher the CV risk. That is, more events are prevented, or a lower number-needed-to-treat is required in the groups with higher risk. However, residual risk also increases throughout the spectrum of preexisting CV risk. That is, those patients that benefit the most from treatment are nonetheless left with the largest risk for future events. The relationship between residual and preexisting risk supports a role for preexisting target organ damage in determining the former [13]. This is also supported by multiple studies describing some degree of non-reversibility in cardiac hypertrophy, fibrosis and diastolic dysfunction, arterial fibrosis and stiffness, endothelial dysfunction and vascular inflammation, and glomerular sclerosis or renal impairment, despite adequate treatment of hypertension. Actually, up to one third of optimally treated hypertensive patients have silent asymptomatic cardiac abnormalities such as left ventricular hypertrophy (29%), diastolic (21%) or systolic (6%) dysfunction, left atrial enlargement (15%), and silent myocardial ischemia (6%), with 13% of them having 3 or more of these abnormalities. It has been suggested that B-type natriuretic peptide and high sensitivity troponin should be used to screen for subjects that may benefit from full CV phenotyping and more aggressive therapy to decrease their residual risk [14].
Despite the evidence above, a study of the Framingham Offspring Cohort showed that a major component of residual risk remains unexplained [15]. This study confirmed that treated hypertensive subjects with resulting BPs above or below 140/90 mmHg had higher CV hazards than their counterparts (same BP levels) who were untreated. Investigators first calculated the hazard ratios for all groups (treated and untreated, above and below 140/90) in multivariate analyses controlled for age, sex, total and HDL cholesterol, intake of lipid-lowering medication, smoking, diabetes, aspirin use, and estimated glomerular filtration rate. Secondly, they added to the model a score derived from the preexisting burden of CV disease (left ventricular hypertrophy, systolic dysfunction, carotid ultrasound abnormality, peripheral artery disease, and microalbuminuria). In this second analysis, hazard ratios were decreased by only 3.4–20.5% in groups of progressive severity. That is, even in the group with the highest preexisting risk, ∼80% of the residual risk was not attributable to the preexisting burden of disease.
These observations raise the very plausible possibility that treatment aimed at BP reduction only targets a major marker and component of the disease (BP elevation), without however altering underlying mechanism(s) of hypertension that confer CV risk independent of BP.
4. Inflammation in angiotensin II-induced hypertension: The role of ENaC
There is mounting evidence that inflammation in hypertension may be such unaddressed CV risk factor. A role for the immune system in hypertension was suggested in the 1960s, with the demonstration that transfer of lymphocytes from rats with renal infarction produced hypertension in normal recipient rats [16]. This was followed by analogous results with transfer of splenocytes from DOCA-salt hypertension and with reverse experiments in which normotension could be conferred to recipient hypertensive rodents by immunosuppressants, thymectomy, antithymocyte serum or transplant of a thymus from a normotensive animal.
Stronger evidence emerged in 2007, with the demonstration that mice genetically lacking lymphocytes (Rag−/−) had markedly attenuated hypertension and vascular dysfunction in response to either angiotensin II (AngII) infusion or DOCA-salt treatment [17]. These effects of AngII were restored by adaptive transfer of T but not B cells. For this to occur, transferred T-cells had to express AT1 Ang II receptors and an intact NADPH oxidase, demonstrating that the effect of AngII on T-cells was a direct one and involved the generation of oxidative stress.
In addition to direct effects on T cells, AngII activates dendritic cells (DCs), the most potent antigen-presenting cells (APCs). This activation also requires NADPH oxidase and is prevented in mice lacking this enzyme [18,19]. Expression of NADPH oxidase subunits and their assembly into a functional enzyme in another model (salt-stimulated DCs) depend on the serum and glucocorticoid-regulated kinase 1 (SGK1), which is stimulated by high intracellular Na+ and also promotes assembly of the DC ENaC channels (see below). Adoptive transfer of these salt-stimulated DCs into normal mice sensitizes them to hypertension in response to subpressor AngII infusion. However, this effect is attenuated if the transferred stimulated DCs are SGK1 deficient, indicating that in addition to oxidative stress, ENaC assembly in DCs is required for AngII hypertension [20].
AngII-induced oxidative stress leads to accumulation of isolevuglandins (IsoLGs, also known as isoketals), highly reactive gamma ketoaldehydes formed from lipid peroxidation of arachidonic acid by NADPH-derived reactive oxygen species (ROS). They rapidly adduct to lysines on proteins, with their ensuing degradation into antigenic peptides. The activated DCs also produce T-cell polarizing cytokines (IL-6, IL-23, and IL-1β) which in conjunction with neoantigen presentation, lead to T-cell proliferation [19]. Whether by direct or by APC-mediated activation, CD8+ T-cells overproducing IFN-γ and TNF-α, CD4+ T-cells overproducing IL-17A and γ/δ T-cells are all involved in the hypertensive response to AngII, whereas T-regulatory cells are inhibitory.
AngII-activated T-cells migrate to and infiltrate the perivascular fat and adventitia and secrete chemokines (e.g., RANTES) and inflammatory cytokines (e.g., IFN-γ, IL-6, IL-17A, TNF-α) that stimulate neighboring vascular smooth muscle and endothelial ROS generation, collagen synthesis, fibrosis and vascular rarefaction. ROS and inhibition of eNOS by some cytokines (e.g., IL-17A and TNF-α) lead to NO deficiency and endothelial and vascular dysfunction.
In the kidney, AngII-stimulated isoLG-producing DCs, and cytokine-producing T-cells infiltrate the cortex and medulla in a manner dependent on sympathetic stimulation, as demonstrated by its blunting with renal denervation [21]. Some cytokines (e.g., IFN-γ) stimulate proximal tubular cell angiotensinogen synthesis and subsequent ACE-dependent formation of renal AngII. This local AngII stimulates renal Na+ transporters, for example direct activation of ENaC mediated by a specific cleavage of its α and γ chains [22]. Other cytokines (e.g., IL-17A, IL-6 and IFN-γ) stimulate expression of distal tubular transporters in direct fashion or via an SGK1-dependent pathway, whereas still others (e.g., TNF-α) impair natriuresis via inhibition of eNOS expression, all contributing to Na+ retention and hypertension [23]. Finally, AngII-induced intrarenal ROS production leads to renal injury, fibrosis and dysfunction.
In summary, AngII hypertension involves participation of ENaC activity at multiple steps. Traditionally, this was attributed to the systemic effect of AngII-stimulation of aldosterone release on renal ENaC. However, the observations above document a role for aldosterone-independent effects of AngII on renal and immune cell ENaC. Dependency of AngII-induced hypertension on a functional channel in APCs is an upstream occurrence in this chain of events, whereas stimulation of renal ENaC by immune-dependent generation of local renal AngII and cytokines (e.g., IL-6) are downstream consequences of immune activation.
5. Inflammation in salt-induced hypertension: The role of ENaC
About one half of human essential hypertensive patients have salt-sensitive, rather than AngII-dependent BP elevation. That is, their BP fluctuates in parallel to their salt intake or salt balance, and their renin-angiotensin system is usually suppressed with blunted responsiveness to salt loading or depletion. Renal and vascular mechanisms of salt-induced hypertension have been explored for several decades. More recently, it has been established that Na+ may exert pro-hypertensive effects via the nervous system and the gut. For example, infusion of Na+ icv or its microinjection into the organum vasculosum of the lamina terminalis increases BP and lumbar and adrenal sympathetic nerve activity [24], whereas a high salt intake alters the gut microbiome in a manner that associates with kidney damage and hypertension [25,26]. Interestingly both sympathetic activation [21] and excess dietary salt intake [25,26] have been found to produce immune cell activation.
The understanding how Na+ may lead to immune cell activation was facilitated by recent novel observations that Na+ accumulates in microdomains of the interstitium, including muscle, skin, and other organs. Such accumulation was hyperosmolar, with concentrations exceeding those of plasma by about 40 mmoL/l, in some but not all reports. Regardless of this, excess dietary salt increases interstitial Na+ (e.g., skin) without associated change in plasma Na+ [27]. Subsequent studies using 23Na magnetic resonance imaging (MRI) found that aging and hypertension are associated with accumulation of skin and skeletal muscle Na+ in humans [28].
We showed that myeloid antigen presenting cells such as monocyte-derived DCs, can sense hyperosmolar Na+ in vitro, leading to their activation and contribution to hypertension [18,20]. This occurs with Na+ concentrations well within the range present in the renal medulla. The mechanisms that lead to activation of these cells by Na+ are similar to those described above for AngII, including activation of the NADPH oxidase and formation of IsoLG-protein adducts, and they occur in rodents [18] and humans [29]. In humans, we found that high Na+, but not equiosmolar mannitol, increases monocyte production of IsoLGs and the proinflammatory cytokines IL-1β, IL-6, IL-23 and TNF-α, and enhances expression of the DC markers CD209, CD80 and CD86 [29]. Consistent with the view that high concentrations of Na+ polarize immune cells towards a proinflammatory phenotype, we have shown by flow cytometry that freshly isolated peripheral blood mononuclear cells from 70 human volunteers accumulate IsoLG-adducts and express the activation marker CD83 in a manner that parallels their skin Na+ concentration measured non-invasively with MRI [29]. We also showed that individual isoLG responses of human monocytes to Na+ exposure are highly variable. Preliminary evidence from our lab suggests that such variability depends on regulation of the DC ENaC by renal EETs, suggesting exposure to hyperosmolar Na+ in the renal medulla, and that isoLG responses may relate to the subjects’ salt sensitivity of BP (unpublished).
Our observations provide a framework to understand how Na+ contributes to hypertension via sympathetic and gut mechanisms. For example, it has been shown that efferent renal nerves mediate DC activation via IsoLG formation, which in turn promotes T cell activation and hypertension [21] that can be prevented by scavenging of IsoLGs [19]. In the kidney of hypertensive patients, we showed that macrophages and DCs accumulate at the cortico-medullary junctions, where these cells may be exposed to hyperosmolar Na+ [29]. Also, the mechanism by which excess dietary Na+ affects the gut microbiome in a proinflammatory and prohypertensive manner has been linked to lipid oxidation and IsoLGs formation in DCs [25].
Treatment of the inflammatory response to Na+ has the potential for reducing residual CV risk above and beyond effects on BP. Therefore, it is crucial to understand how DCs sense and transduce high Na+ concentration into BP elevation. We have shown that Na+-induced activation of NADPH-oxidase and IsoLG-protein adduct formation in DCs depend on the integrity of an ENaC channel composed of the α and ϒ subunits, because these effects are blocked by amiloride. Entry of Na+ via this ENaC increases its intracellular concentration and leads to its exchange for Ca2+ via the Na+/Ca2+ exchanger. Consequent increased intracellular Ca2+ activates protein kinase C, which in turn phosphorylates the NADPH oxidase subunit p47phox [18]. Also, high intracellular Na+ concentration increases expression of the salt-sensing SGK1 [20], which has several effects. SGK1 enhances expression of ENaC-γ that co-immunoprecipitates with ENaC-α indicating increased assembly of these chains into a functional channel. SGK1 also stimulates channel activity via an inactivating phosphorylation of NEDD4-2 that leads to reduced channel ubiquitination. Hence Na+ entry via ENaC produces a feed-forward loop that leads to further activation of this channel. Also, SGK1 augments NADPH oxidase subunit expression and assembly into the active enzyme which leads to oxidative stress and formation of IsoLG-protein adducts.
This sequence of events was demonstrated in mice given a pharmacological inhibitor of SGK1 or genetically engineered to lack this kinase in CD11c+ DC cells. In both circumstances, high salt-induced expression of ENaCα-γ and NADPH oxidase was prevented, mice exhibited blunted hypertensive responses to the high salt feeding phase of the employed protocol (N-Nitro-L-arginine methyl ester hydrochloride (L-NAME)/high salt model), and were protected from ensuing renal inflammation and endothelial dysfunction. Also, as mentioned in the AngII section, transfer of DCs from SGK1-deficient mice failed to elicit sustained hypertension in response to subpressor AngII in normotensive recipient mice, compared to that produced by transfer of SGK1-containing, unmanipulated DCs [20].
The reviewed data demonstrate a key role for the DC ENaC, upstream in the inflammatory response to Na+, which produces oxidative stress and target organ damage. Although not directly demonstrated in the Na+-induced hypertension model, it is likely that the ensuing activation of T-cells infiltrating the kidney determines activation of the renal ENaC, as demonstrated in AngII-induced hypertension.
6. Conclusion
There is enough evidence that the immune components of AngII- and Na+-dependent hypertension are linked to vascular and renal target organ damage and may so explain a significant portion of the residual CV risk of treated, controlled hypertensive subjects. This is analogous to the situation in atherosclerosis, in which there is now evidence for improved prognosis with immune-modulators (canakinumab) independent from changes in BP [30]. However, immunomodulators are expensive and not devoid of the risks imposed by relative immune deficiency (e.g., infection or cancer).
We have discussed here the reasons that suggest that the inexpensive and underutilized diuretic amiloride, a blocker of ENaC, may not only be beneficial in terms of BP reduction in very resistant forms of hypertension, but most importantly may be a treatment of underlying inflammation and so reduce residual CV risk of treated hypertensives.
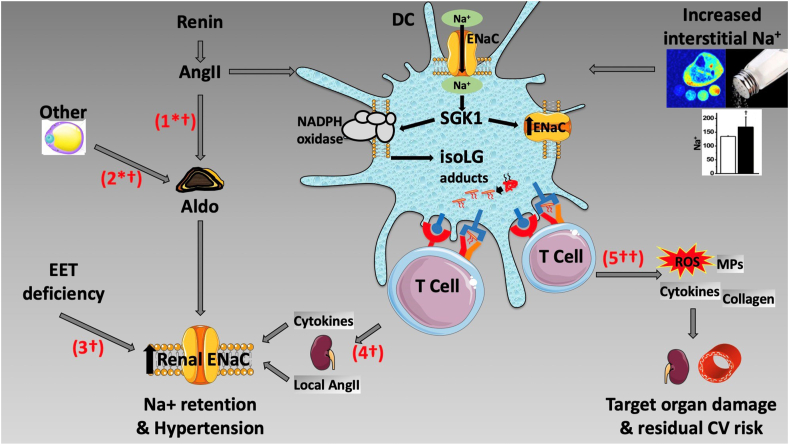
The multiple abnormalities that determine ENaC hyperactivity in essential hypertension are depicted in the cartoon (Fig. 1), separately for the renal and DC channels. Activation of the renal ENaC by systemic or by inflammatory pathways leads to Na+ retention and hypertension, whereas activation of the channel in immune cells also leads to oxidative stress and target organ damage, which are likely responsible for residual CV risk.
Fig. 1.
Pathways of involvement of the epithelial sodium channel (ENaC) in the kidney and in dendritic cells (DC). #1 and #2 depict angiotensin II (AngII)-dependent (#1) and independent (#2) aldosterone (Aldo)-stimulation of renal ENaC. “Other” stands for non-AngII stimuli of aldosterone, represented graphically by an adipocyte in view of the putative existence of adipocyte secretagogues for aldosterone production by the adrenal gland. #3 shows aldosterone-independent activation of renal ENaC by a deficiency of epoxyeicosatrienoic acids (EET), as discussed in the text. #4 and #5 represent the consequence of salt or AngII activation of DC ENaC. #4 depicts renal ENaC stimulation by T-cell infiltration of the kidney, via direct effects of cytokines on the channel and also via generation of local, renal AngII. #5 depicts the tissue-damaging effects of activation of the DC ENaC channel, mediated by the inflammatory, oxidative and pro-fibrotic effects on renal and vascular tissues. These may account for the inflammation-dependent residual cardiovascular (CV) risk of treated hypertensive subjects. The symbols next to each pathway number represent the drug(s) that may interrupt them. ∗ = spironolactone for the treatment of hypertension, † = amiloride for the treatment of hypertension, and †† = amiloride for the treatment of residual CV risk. SKG1 = serum and glucocorticoid-regulated kinase, isoLG = isolevuglandins, ROS = reactive oxygen species, MPs = metalloproteinases.
We are in the process of assessing CV outcomes in a retrospective cohort of hypertensive subjects treated with amiloride, derived from our institutional electronic medical record. If we obtain a signal for amiloride-associated reduction in CV risk in such a cohort, the ultimate proof of our hypothesis will require a prospective randomized study of amiloride therapy with prolonged follow-up.
Declaration of Competing Interest
None.
Funding
The studies of our laboratory quoted here were supported by grants R01 HL147818 and K01 HL130497 (AK), HL129941 (FE & CL), and DHL 137166 (FE).
References
- 1.Virani S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2020 update: a report from the American heart association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. https://doi:10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Acelajado M.C., Pisoni R., Dudenbostel T., Dell'Italia L.J., Cartmill F., Bin Zhang B. Refractory hypertension: definition, prevalence and patient characteristics. J. Clin. Hypertens. 2012;14:7–12. doi: 10.1111/j.1751-7176.2011.00556.x. https://doi:10.1111/j.1751-7176.2011.00556.x (Greenwich) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blacher J., Evans A., Arveiler D., Amouyel P., Ferrières J., Bingham A. On behalf of the PRIME Study Group, Residual coronary risk in men aged 50-59 years treated for hypertension and hyperlipidaemia in the population: the PRIME study. J. Hypertens. 2004;22:415–423. doi: 10.1097/00004872-200402000-00028. https://doi:10.1097/00004872-200402000-00028 [DOI] [PubMed] [Google Scholar]
- 4.Sim J.J., Bhandari S.K., Shi J., Liu I.L.A., Calhoun D.A., McGlynn E.A. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin. Proc. 2013;88:1099–1107. doi: 10.1016/j.mayocp.2013.06.017. https://doi:10.1016/j.mayocp.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigoryan L., Pavlik V.N., Hyman D.J. Characteristics, drug combinations and dosages of primary care patients with uncontrolled ambulatory blood pressure and high medication adherence. J. Am. Soc. Hypertens. 2013;7:471–476. doi: 10.1016/j.jash.2013.06.004. https://doi:10.1016/j.jash.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudenbostel T., Calhoun D.A. Use of aldosterone antagonists for treatment of uncontrolled resistant hypertension. Am. J. Hypertens. 2017;30:103–109. doi: 10.1093/ajh/hpw105. https://doi:10.1093/ajh/hpw105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha C., Eckert G.J., Ambrosius W.T., Chun T.Y., Wagner M.A., Zhao Q. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:481–487. doi: 10.1161/01.HYP.0000179582.42830.1d. https://doi:10.1161/01.HYP.0000179582.42830.1d [DOI] [PubMed] [Google Scholar]
- 8.Laffer C.L., Elijovich F., Eckert G.J., Tu W., Pratt J.H., Brown N.J. Genetic variation in CYP4A11 and blood pressure response to mineralocorticoid receptor antagonism or ENaC inhibition: an exploratory pilot study in African Americans. J Am Soc Hypertens. 2014;8:475–480. doi: 10.1016/j.jash.2014.04.011. https://doi:10.1016/j.jash.2014.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa K., Holla V.R., Wei Y., Wang W.H., Gatica A., Wei S. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J. Clin. Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. https://doi:10.1172/JCI27546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elijovich F., Milne G.L., Brown N.J., Laniado-Schwartzman M., Laffer C.L. Two pools of eicosatrienoic acids in humans: alterations in salt sensitive normotensive subjects. Hypertension. 2018;71:346–355. doi: 10.1161/HYPERTENSIONAHA.117.10392. https://doi:10.1161/HYPERTENSIONAHA.117.10392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey R.M., Calhoun D.A., Bakris G.L., Brook R.D., Daugherty S.L., Dennison-Himmelfarb C.R. Resistant hypertension: detection, evaluation, and management. A scientific statement from the American Heart Association. Hypertension. 2018;72:e53–e90. doi: 10.1161/HYP.0000000000000084. https://doi:10.1161/HYP.0000000000000084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blacher J., Evans A., Arveiler D., Amouyel P., Ferrières J., Bingham A. On behalf of the PRIME Study Group, Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME study. J. Hum. Hypertens. 2010;24:19–26. doi: 10.1038/jhh.2009.34. https://doi:10.1038/jhh.2009.34 [DOI] [PubMed] [Google Scholar]
- 13.Zanchetti A., Thomopoulos C., Parati G. Randomized controlled trials of blood pressure lowering in hypertension. A critical reappraisal. Circ. Res. 2015;116:1058–1073. doi: 10.1161/CIRCRESAHA.116.303641. https://doi:10.1161/CIRCRESAHA.116.303641 [DOI] [PubMed] [Google Scholar]
- 14.Nadir M.A., Rekhraj S., Wei L., Lim T.K., Davidson J., MacDonald T.M. Improving the primary prevention of cardiovascular events by using biomarkers to identify individuals with silent heart disease. J. Am. Coll. Cardiol. 2012;60:960–968. doi: 10.1016/j.jacc.2012.04.049. https://doi:10.1016/j.jacc.2012.04.049 [DOI] [PubMed] [Google Scholar]
- 15.Lieb W., Enserro D.M., Sullivan L.M., Vasan R.S. Residual cardiovascular risk in individuals on blood pressure-lowering treatment. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002155. https://doi:10.1161/JAHA.115.002155 e002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuda T., Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex. Rep. Biol. Med. 1967;25:257–264. PMID: 6040652. [PubMed] [Google Scholar]
- 17.Guzik T.J., Hoch N.E., Brown K.A., McCann L.A., Rahman A., Dikalov S. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. https://doi:10.1084/jem.20070657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbaro N.R., Foss J.D., Kryshtal D.O., Tsyba N., Kumaresan S., Xiao L. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017;21:1009–1020. doi: 10.1016/j.celrep.2017.10.002. https://doi:10.1016/j.celrep.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirabo A., Fontana V., de Faria A.P.C., Loperena R., Galindo C.L., Wu J. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084. https://doi:10.1172/JCI74084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Beusecum J.P., Barbaro N.R., McDowell Z., Aden L., Xiao L., Pandey A.K. High salt activates CD11c+ antigen-presenting cells via SGK (Serum Glucocorticoid Kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension. 2019;74:555–563. doi: 10.1161/HYPERTENSIONAHA.119.12761. https://doi:10.1161/HYPERTENSIONAHA.119.12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao L., Kirabo A., Wu J., Saleh M.A., Zhu L., Wang F. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ. Res. 2015;117:547–557. doi: 10.1161/CIRCRESAHA.115.306010. https://doi:10.1161/CIRCRESAHA.115.306010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen M.T.X., Lee D.H., Delpire E., McDonough A.A. Differential regulation of Na+ transporters along nephron during ANG II-dependent hypertension: distal stimulation counteracted by proximal inhibition. Am. J. Physiol. Ren. Physiol. 2013;305:F510–F519. doi: 10.1152/ajprenal.00183.2013. https://doi:10.1152/ajprenal.00183.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitzer A.L., Kirabo A. Dendritic cell A20: targeting hypertension in autoimmunity. Circ. Res. 2019;125:1067–1069. doi: 10.1161/CIRCRESAHA.119.316198. https://doi:10.1161/CIRCRESAHA.119.316198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinsman B.J., Browning K.N., Stocker S.D. NaCl and osmolarity produce different responses in organum vasculosum of the lamina terminalis neurons, sympathetic nerve activity and blood pressure. J Physiol. 2017;595:6187–6201. doi: 10.1113/JP274537. https://doi:10.1113/JP274537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson J.F., Aden L.A., Barbaro N.R., Van Beusecum J.P., Xiao L., Simmons A.J. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI insight. 2019;4 doi: 10.1172/jci.insight.126241. https://doi:10.1172/jci.insight.126241 e126241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilck N., Matus M.G., Kearney S.M., Olesen S.W., Forslund K., Bartolomaeus H. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. https://doi:10.1038/nature24628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Machnik A., Neuhofer W., Jantsch J., Dahlmann A., Tammela T., Machura K. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 2009;15:545–552. doi: 10.1038/nm.1960. https://doi:10.1038/nm.1960 [DOI] [PubMed] [Google Scholar]
- 28.Kopp C., Linz P., Dahlmann A., Hammon M., Jantsch J., Muller D.N. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. https://doi:10.1161/HYPERTENSIONAHA.111.00566 [DOI] [PubMed] [Google Scholar]
- 29.Barbaro N.R., Van Beusecum J.P., Xiao L., do Carmo L., Pitzer A., Loperena R. Sodium activates human monocytes via the NADPH oxidase and isolevuglandin formation. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa207. https://doi:10.1093/cvr/cvaa207 in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman A.M.K., MacFadyen J., Thuren T., Webb A., Harrison D.G., Guzik T.J. Effects of interleukin-1β inhibition on blood pressure, incident hypertension, and residual inflammatory risk. A secondary analysis of CANTOS. Hypertension. 2020;75:477–482. doi: 10.1161/HYPERTENSIONAHA.119.13642. https://doi:10.1161/HYPERTENSIONAHA.119.13642 [DOI] [PMC free article] [PubMed] [Google Scholar]