Abstract
Inconsistencies in studies of chronic psychosocial stress and hypertension may be explained by the use of stress markers greatly influenced by circadian rhythm and transient stressors. We assessed whether hair cortisol, a marker that captures systemic cortisol over months, was independently associated with hypertension. We measured hair cortisol and blood pressure in 75 consecutive participants in the Survey of the Health of Wisconsin, using an ELISA test. Individuals with values ≥ median (78.1 pg/mg) were considered exposed. We used approximate Bayesian logistic regression, with a prior odds ratio of 1.0–4.0, to quantify the multivariate-adjusted hair cortisol-hypertension association. Participants' average age was 46.9 years; 37.3% were male; and 25.3% were hypertensive. Hypertension prevalence was 2.23 times higher in exposed (95% CI: 1.69–3.03). This finding was unlikely explained by differential measurement errors, since we conducted blinded measurements of exposure and outcome. Sensitivity analyses showed the association was unlikely explained by an unmeasured confounder, survival bias, or reverse causality bias. Findings suggest elevated hair cortisol is a risk factor for hypertension. Although feasible, the clinical value of hair cortisol as a tool for hypertension risk stratification or for monitoring the effect of chronic psychosocial stress management interventions is still uncertain.
Keywords: Blood pressure, Chronic psychosocial stress, Cortisol, Hair cortisol, Hypertension
1. Introduction
Chronic psychosocial stress (CPS) has been proposed as a potential risk factor for the development of hypertension [1]. CPS could increase the risk of hypertension through a sustained exposure to increased levels of glucocorticoids (cortisol), which leads to downregulation of the glucocorticoid receptor and cortisol resistance, diminished capacity of the immune system's response to cortisol's anti-inflammatory actions, mild chronic inflammation, intra-abdominal accumulation of visceral fat, increased salt retention, and insulin resistance [2], [3], [4]. However, available evidence of a link between CPS and hypertension is inconsistent [5], with some [6], [7], [8], but not all [9], [10] studies documenting an association.
A lack of accurate markers of CPS significantly contributes to its uncertain role in the development of hypertension. Most studies of the CPS-hypertension association have relied on questionnaires that measure stress indirectly. Unfortunately, stress questionnaires are unreliable, because factors that define CPS – environmental stressors, perceptions of inability to cope, and behavioral and biological responses to stressors [11], [12] – vary across individuals and time [8]. Other studies have relied on biological markers of CPS, such as blood, urinary, and salivary levels of cortisol and catecholamines [13]. However, these markers are highly influenced by daily physiological fluctuations (circadian rhythms) and transient stressors [11]. Hair cortisol (HC) has been recently proposed as a reliable alternate biological marker of CPS [11], [14]. Blood cortisol accumulates into hair as hair grows and provides a retrospective measure of cortisol secretion over several months [15]. Therefore, HC captures systemic cortisol levels over longer periods of time than blood, urine, or saliva, and is not highly influenced by circadian rhythms and transient events [11], [14], [16]. Although HC is increasingly accepted as a relevant biomarker for CPS in clinical studies, it has not been previously used to investigate a possible CPS-hypertension association.
2. Methods
We conducted a cross-sectional study, nested within the 2016 Survey of the Health of Wisconsin (SHOW), an annual health survey in a random sample of Wisconsin residents [17]. We enrolled eligible SHOW participants as they came in, until completing the target sample size. Eligible participants had at least 1.5 inches of hair on the back of the scalp and were between 30 and 60 years old. We excluded pregnant women, individuals working night shifts, using topical, oral, or injected corticosteroids, and those with hair treatments that could affect HC concentration (dyeing or chemical or heat treatment to make the hair straight or curly). The study was approved by the institutional review board of the University of Wisconsin-Madison. All participants provided written informed consent.
A hair sample, typically a 1/8” bundle, of the most proximal 3 cm of hair was collected from the back of the head and used for cortisol extraction. Cortisol content in these samples corresponds to average blood cortisol during the last three months [15]. All processing of hair samples and cortisol quantification was conducted at the Wisconsin State Laboratory of Hygiene. Hair sample washing, drying, homogenization, and extraction were based on methods outlined by Meyer et al. [18]. In general, >7 mg of hair was required for accurate quantitation of cortisol, though depending upon cortisol levels, hair masses as low as 5 mg were viable. A coarsely chopped hair sample was rinsed twice with HPLC grade isopropanol and dried at room temperature under a HEPA hood. Dried hair was weighed to the nearest 0.01 mg, put into 2.0 mL Sarstedt plastic tubes and homogenized by bead-beating for 5 min (Mini-Beadbeater 8, Biospec Products, 3.2 mm chrome steel balls). Cortisol was then extracted by incubating/shaking the powdered hair samples in 1.5 mL of HPLC grade methanol on a shaker table (200 rpm) for 22 h at room temperature. QC samples included cortisol-spiked tubes, cortisol-spiked hair samples, method blanks, and hair duplicates (when sufficient hair mass was available). Samples were then centrifuged at 10,000 rpm and 1.0 mL of supernatant was removed and aliquoted to a clean vial. The methanol was evaporated to dryness under nitrogen at 50 °C in an EvapTrap© and then each sample was reconstituted with 0.25 mL of phosphate buffered saline [16]. Sample extracts were frozen at −20 °C until quantification of cortisol via ELISA (Alpco 11-CORHU-E01-SLV) (sensitivity of 1 ng/mL). For purposes of analysis, we dichotomized HC as above or below 78.1 pg/mg (the median value in the study sample).
Self-perceived stress was measured using the short-form version of the Depression Anxiety Stress Scales (DASS-21) [19]. The DASS-21 is a self-reported 4-point Likert-type measure of three dimensions of mental health: depression, anxiety, and stress. The DASS-21 has excellent psychometric properties in older adults [20]. Individuals with a DASS-21 stress score of 15 or higher were categorized as being under high stress [21].
Blood pressure (BP) was measured using an oscillometric device (Omron Model HBP-1300, Omron Healthcare, Lake Forest, IL) after a five-minute rest period in a sitting position and following standard procedures. This device has been validated and exceeds the minimum requirements of the international validation protocol of the British Hypertension Society and the Association for the Advancement of Medical Instrumentation [22]. Three BP measurements were taken, with one minute between measurements, and the average of the last two was used in the analysis. Participants were considered hypertensive if they had a systolic BP ≥ 140 mmHg, or diastolic BP ≥ 90 mmHg, or took prescribed antihypertensive drugs.
For the analysis, age was dichotomized as <50 years and ≥50 years. Abdominal obesity was defined as waist circumference ≥96.8 cm in White women and ≥102.1 cm in White men [23]. Women having >7 alcohol drinks/week and men having >14 drinks/week were considered heavy drinkers [24].
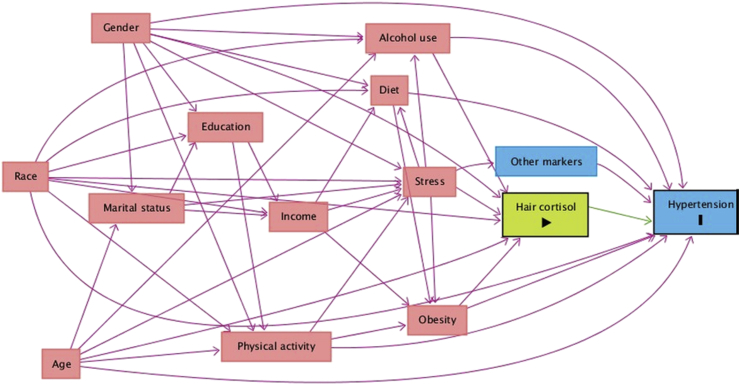
We drew a directed acyclic graph (DAG) and used Pearl's back-door criterion [25], as implemented in DAGitty [26], to select a minimally sufficient adjustment set (MSAS) of variables that would allow us to identify an unconfounded effect of HC on hypertension. Conditioning on the variables included in the MSAS blocks all non-causal pathways between HC levels and hypertension, without blocking causal pathways. We built the DAG (Fig. 1) by identifying all known factors that are independently associated with either hypertension or HC levels, and then adding any variable that was a common cause of at least two variables already included in the DAG. The MSAS included age, gender, race, obesity, and alcohol use. We did not adjust for race because all but one of the participants were White. All models included the MSAS variables plus an age-by-gender interaction term, based on a well-documented difference in the age-related rise in BP in men and women.
Fig. 1.
A causal DAG representing the relationship between hair cortisol and hypertension. Variables enclosed in circle are those in the minimally sufficient adjustment set (MSAS) of variables that would allow identifying an unconfounded effect of hair cortisol levels on hypertension.
Concerned about sparse data bias [27], due to the limited number of participants in our study, we decided a priori to use Bayesian analysis to improve the precision of the estimate of the association between HC and hypertension. On average, this approach results in estimates that are more accurate and have better predictive ability than frequentist approaches [27]. Specifically, we used approximate Bayesian logistic regression via penalized likelihood estimation with data augmentation, to estimate the association between HC and hypertension [28]. We first fitted a model without a prior odds ratio (OR) for the effect of HC (i.e., a standard frequentist logistic regression model). Then we fitted a model with a prior OR for the effect of HC on hypertension, with a log-normal distribution and bounds of 1.0 and 4.0. Prior OR values for MSAS variables were obtained from a logistic regression analysis of data from the National Health and Nutrition Examination Survey 1999–2004. We used bootstrapping, with 1000 replications, to obtain 95% credible intervals (CI) without making assumptions regarding the distribution of the estimated OR [29].
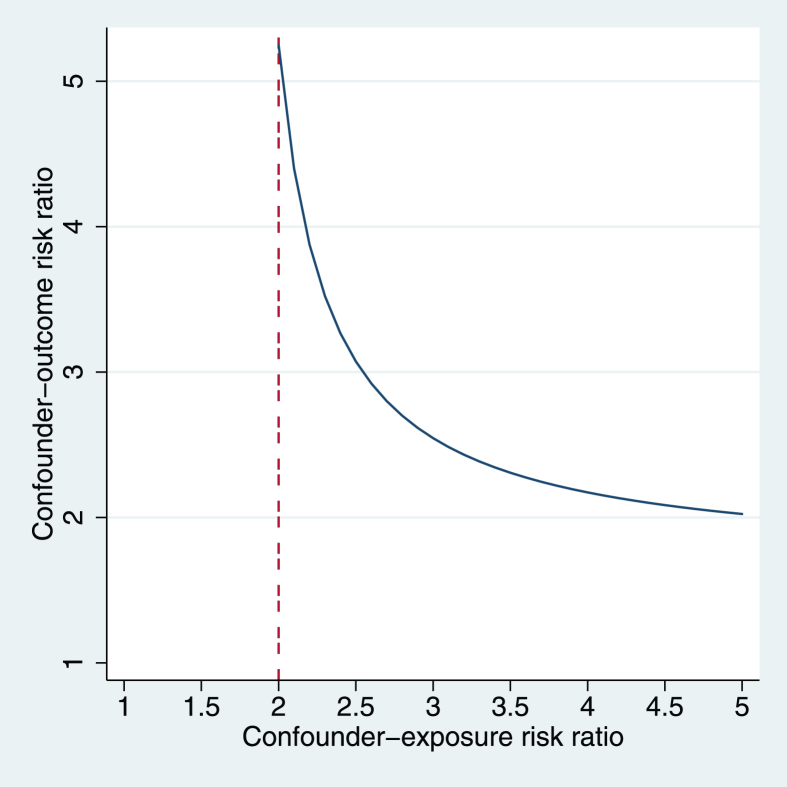
We conducted sensitivity analyses to assess study assumptions and potential sources of bias. First, we conducted an analysis with HC prior OR bounds of 0.72 and 3.10. These correspond to the smallest and largest OR reported in previous studies of CPS and hypertension [8], [30]. This relaxed the assumption of an OR between 1 and 4, used in the main analysis. Second, we used an assumption-free bounding method [31] to estimate how strong the joint associations (i.e. risk ratios) between an unmeasured confounder and exposure, and unmeasured confounder and outcome would have to be, so that the unmeasured confounder would explain away the observed effect of HC on hypertension. Then, we calculated risk ratios of high cortisol levels using correlations [32], [33] reported in a meta-analysis of basic determinants of HC [34] and compared them with the bounding risk ratio that would explain away the effect of HC. This allowed us to judge how likely it was for an unmeasured confounder to explain away the observed effect of HC on hypertension. Third, we conducted an analysis excluding individuals who were aware that they were hypertensive, to account for the possibility that high levels of HC were a consequence of a diagnosis of hypertension, instead of a cause.
All analyses were conducted using Stata 15.0 (StataCorp 1985–2017, College Station, TX).
3. Results
We studied 75 individuals, 37.3% male and 25.3% hypertensive, with an average age of 46.9 years and a mean HC level of 78.1 pg/mg (Table 1). Risk factors for hypertension (male gender, older age, heavy drinking, and abdominal obesity) were more likely in individuals with HC levels above the median.
Table 1.
Average risk factors for hypertension, mean blood pressure, and hypertension prevalence by hair cortisol level (95% confidence intervals).
| Risk Factor | High hair cortisol (>78.1 pg/mg) | Low hair cortisol (≤78.1 pg/mg) | All |
|---|---|---|---|
| Mean age (years) | 48.3 (45.1, 51.5) | 45.6 (42.2, 49.0) | 46.9 (44.6, 49.2) |
| Age >50 years (%) | 54.1 (36.9, 70.5) | 44.7 (28.6, 61.7) | 49.3 (37.6, 61.1) |
| Male (%) | 37.8 (22.5, 55.2) | 36.8 (21.8, 54.0) | 37.3 (26.4, 49.3) |
| Abdominal obesity (%) | 54.1 (36.9, 70.5) | 47.4 (31.0, 64.2) | 50.7 (38.9, 62.4) |
| Mean DASSa | 11.3 (10.3, 12.4) | 11.9 (10.7, 13.2) | 11.6 (10.8, 12.5) |
| Heavy drinking (%)b | 13.9 (4.7, 29.5) | 13.2 (4.4, 28.1) | 13.5 (6.7, 23.5) |
| Mean blood pressure | |||
| Systolic (mm Hg) | 123.1 (118.2, 127.9) | 119.5 (116.2, 122.8) | 121.3 (118.4, 124.2) |
| Diastolic (mm Hg) | 77.9 (74.5, 81.4) | 74.1 (71.1, 77.0) | 76.0 (73.7, 78.2) |
| Hypertensionc (%) | 32.4 (18.0, 49.8) | 18.4 (7.7, 34.3) | 25.3 (16.0, 36.7) |
Depression Anxiety Stress Scales; one individual with missing data.
>14/7 drinks/week for men/women; three individuals with missing data.
Systolic/diastolic blood pressure ≥140/90 mm Hg or taking antihypertensive medication.
When a prior OR ranging from 1 to 4 was used in the Bayesian analysis, the crude prevalence of hypertension was 2.04 (95% credible interval –CI: 1.43, 2.73) times higher in individuals with high HC than in those with lower HC levels (Table 2). After adjusting for the MSAS variables, the prevalence odds ratio increased to 2.07 (95% CI: 1.52, 2.80). Further adjustment for self-perceived stress increased the prevalence odds ratio to 2.23 (95% CI: 1.68, 3.04). Using a prior OR ranging from 0.72 to 3.10, as suggested by previous studies, decreased the adjusted OR by 19%. However, the 95% CI still supported a minimum increase of 21% in hypertension prevalence among individuals with high HC (OR = 1.68, 95% CI: 1.21, 2.33). Similar analyses, with and without adjustment for HC levels, resulted in a prevalence odds ratio of 1.22 (95% CI: 0.95, 1.60) for high level of self-perceived stress.
Table 2.
Odds ratios of hypertension by hair cortisol level and type of analysis.
| Type of Analysis | Limits for prior odds ratio | Odds ratio | 95% Credible interval |
|---|---|---|---|
| Frequentist | |||
| Crude | 1/∞, ∞ | 2.13 | (0.73, 6.20) |
| Adjusteda | 1/∞, ∞ | 2.35 | (0.71, 7.80) |
| Adjusteda + stress | 1/∞, ∞ | 3.60 | (0.93, 13.98) |
| Bayesian | |||
| Crude | 1.00, 4.00 | 2.04 | (1.43, 2.73) |
| Adjusteda | 1.00, 4.00 | 2.07 | (1.52, 2.80) |
| Adjusteda + stress | 1.00, 4.00 | 2.23 | (1.68, 3.04) |
| Crude | 0.72, 3.10 | 1.67 | (1.16, 2.30) |
| Adjusteda | 0.72, 3.10 | 1.68 | (1.21, 2.33) |
| Adjusted + stressb | 0.72, 3.10 | 1.81 | (1.33, 2.51) |
Adjusted for gender, age, alcohol intake, and abdominal obesity.
Further adjusted for self-perceived stress.
The sensitivity analysis for confounding showed that an unmeasured confounder would have to increase the probability of having high HC by ≥ 100% to explain away the observed HC-hypertension association (Fig.2). An unmeasured confounder with weaker effects on the risk of high cortisol level could still nullify the HC-hypertension association, but only if it also increased the risk of hypertension more than five-fold.
Fig. 2.
Area above the line corresponds to the joint values of the (unmeasured) confounder-exposure and confounder-outcome risk ratios that would make the lower limit of the 95% credible interval <1 and explain away the effect of hair cortisol on hypertension.
Main determinants of HC increased the probability of having HC levels above the median by 1.39–1.98 times (Table 3).
Table 3.
Relative effects (odds ratios) of main determinants of hair cortisol on the risk of having hair cortisol levels above the mean.
| Determinant | Correlationa | Odds ratio |
|---|---|---|
| Cortisol awakening response (salivary) | 0.185 | 1.98 |
| Mean diurnal salivary cortisol | 0.179 | 1.93 |
| Single time point salivary cortisol level | 0.148 | 1.72 |
| Overall post-awakening salivary cortisol | 0.129 | 1.60 |
| Body mass index | 0.134 | 1.63 |
| Waist to hip circumference ratio | 0.132 | 1.62 |
Correlation coefficients come from a meta-analysis of observational studies [1].
When individuals who were aware of their hypertensive status were excluded from the analysis, the adjusted OR for HC decreased to 1.95 (95% CI: 1.53, 2.36).
4. Discussion
In this study, individuals with high HC, a biological marker of CPS, were twice more likely to be hypertensive than those with low HC. Moreover, there was a 95% probability of the prevalence of hypertension being 68%–204% higher among individuals with high HC. This association persisted after adjusting for variables selected on substantive grounds and on inferential rules aimed to identify a minimum set of variables needed to control for confounding bias [25]. Adjustment for self-perceived stress and abdominal obesity defined by World Health Organization's cut points, resulted in non-substantial changes in the HC-hypertension association.
The impact of potential biases and assumptions in our results should be carefully considered before interpreting our findings. Bayesian analysis constitutes a tradeoff between bias and precision, but greatly improves estimation and prediction accuracy [35]. Indeed, the Bayesian OR estimate in our study (OR = 2.23) was closer to 1 than the frequentist OR estimate (3.60), but its precision was about eight times higher. The increased precision results from the formal incorporation of existing knowledge on the effects of risk factors for hypertension, in the form of a plausible range of values for the OR (i.e. in the form of a prior OR) [35]. In contrast to the Bayesian approach, frequentist analyses disregard existing knowledge. In fact, the frequentist analysis in our study is equivalent to a Bayesian analysis conducted under the assumption that the effect of high HC on hypertension could take any value, from extremely protective (prior OR = 1/∞) to extremely harmful (prior OR = ∞). Based on substantive knowledge on hypertension risk factors, such extreme effects are untenable.
Of course, the validity of our Bayesian OR estimate depends on the validity of the prior OR used in the analysis. Fortunately, we selected bounds for the prior OR that are consistent with current knowledge on hypertension. We selected a lower bound of OR = 1 because there does not seem to be a biological mechanism by which CPS or high levels of circulating cortisol would decrease the risk of hypertension. On the other hand, we selected an upper bound of OR = 4, because we considered unlikely that an effect of CPS on hypertension would be stronger than the risk of progression to hypertension among individuals with systolic/diastolic BP of 120-129/80–84 mmHg, after accounting for other risk factors [36]. Moreover, we also conducted analyses where priors were the lower (OR = 0.72) and upper (OR = 3.10) bounds of ORs reported in previous studies using salivary cortisol, blood cortisol or self-perceived stress as markers of CPS [8], [30]. These informative prior ORs were selected before looking at the data, and both lead to consistent findings.
Errors in the measurement of HC could have also induced bias. However, it is unlikely these errors resulted in a positive association between HC and hypertension. Laboratory tests were conducted on de-identified samples and without knowledge of BP level or hypertensive status, and there is no reason to believe hair quality and mass were associated with the latter variables. In consequence, any errors in HC measurements were likely similar in hypertensive and non-hypertensive participants. On average, such errors would weaken the HC-hypertension association. Moreover, there were no substantive changes in the HC-hypertension association (OR = 2.34; 95% CI 1.88, 2.83) when participants with HC levels below the limit of detection (n = 17), mostly due to limited hair sample mass, were excluded from the analysis. Inter-individual variation in the efficiency of incorporation of blood cortisol into hair may be another source of error in the measurement of the exposure. However, this error should be independent of hypertension status (i.e. non-differential) and in average would have weakened the HC-hypertension association.
Our study only included individuals who have survived up to the time of recruitment into SHOW. This could result in selection bias because CPS and hypertension are both associated with increased mortality. However, this bias cannot explain the association observed in this study. Indeed, if a randomly selected survivor had CPS, she would more likely be normotensive than hypertensive. Thus, in a cross-sectional sample, which only includes survivors, the CPS-hypertension correlation would be negative, and the corresponding OR would be underestimated. On the other hand, CPS and hypertension were measured at the same time and it is not possible to ensure CPS preceded the development of hypertension. This could be the case, for instance, if CPS developed because participants became aware they had hypertension. This explanation also seems unlikely, because the CPS-hypertension OR (1.95; 95% CI: 1.54, 2.39) changed little when we excluded individuals who knew they were hypertensive (n = 14) from the analysis.
We used a DAG (Fig. 1) and mathematical rules to identify a MSAS needed to correct for confounding bias. Unfortunately, this approach does not account for bias due to unknown confounders. However, according to our sensitivity analysis, only an unknown confounder that at least doubles the risk of having high HC could explain away the observed CPS-hypertension association. Estimates derived from a meta-analysis of factors associated with HC [34] suggest only other measures of systemic cortisol may have such an strong association with HC levels (Table 3, Fig. 2). This bound for the unknown confounder-HC association is predicated on the likely assumption that the unknown confounder will not increase the risk of hypertension more than fivefold. Current knowledge on risk factors for hypertension indicates that such strong unknown risk factors are unlikely.
Findings from previous studies of the CPS-hypertension association have been inconsistent. Those studies were cross-sectional, and CPS was found to be associated with hypertension in some [7], [8], [30], but not all of them [10], [37]. This lack of consistency could be partly explained by the use of CPS markers (i.e. cortisol in saliva, urine, and blood) that reflect short lasting adaptive response to stress and/or circadian variability. These short period exposures are unlikely to be related to a chronic stress response and the development of sustained elevated blood pressure. These limitations also apply to self-reported questionnaire measurements of CPS, as they evaluate individual beliefs and feelings, usually over a period of a month or less, and may be compromised by individual differences in awareness of affective states and by social desirability and retrospection bias [38], [39]. Even though HC only measures CPS levels over a period of months, it is still a better measure than the above-stated markers. Indeed, studies in adult populations are heterogeneous regarding the relationship between HC and self-perceived stress [40].
Multiple pathways may be involved in the development of CPS-related hypertension. Experimental studies in animal models and healthy adult volunteers indicate that prolonged stressors (social status disruption/stressful life events) result in continued exposure to high levels of hypothalamic pituitary adrenal axis (HPA) hormones, such as cortisol, which lead to glucocorticoid receptor resistance [4]. This leads to a diminished capacity of the immune system's response to the anti-inflammatory actions of cortisol, and a chronic pro-inflammatory state [3], [41], [42] which in turn leads to the development of obesity [43], [44] and hypertension [45]. Moreover, elevated systemic cortisol may lead to vascular endothelial dysfunction. High levels of cortisol inhibit the expression of inducible nitric oxide synthase, decrease the availability of endothelial nitric oxide, and increase regional vascular resistance, which could in turn lead to BP elevation and hypertension [46].
Our findings suggest that CPS, as evaluated through HC levels, results in a stable increase in systemic cortisol levels that eventually leads to glucocorticoid resistance and to the development of hypertension. However, this finding should be further evaluated in larger prospective cohort studies. HC levels provide an etiologically relevant measure of exposure to CPS, and reduce the variability associated with self-reported measurements and measures of cortisol in urine, saliva, and blood samples. Although potentially feasible, the clinical value of HC as a tool for risk stratification or for monitoring the effect of CPS management interventions is still uncertain and should be evaluated in future studies.
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number P30AG017266. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
None.
Acknowledgements
We would like to thank the study participants, the staff at the Survey of the Health of Wisconsin, and the Wisconsin State Hygiene Laboratory for their help with the study. The authors thank the staff of the Environmental Toxicology Program at the Wisconsin State Laboratory of Hygiene, in particular Dawn Perkins and Jocelyn Hemming, and their graduate intern, Martha Longley, for cortisol method development and for the hair cortisol measurements.
Contributor Information
L.E. Bautista, Email: lebautista@wisc.edu.
P.K. Bajwa, Email: bajwa@wisc.edu.
M.M. Shafer, Email: mmshafer@wisc.edu.
K.M.C. Malecki, Email: kmalecki@wisc.edu.
C.A. McWilliams, Email: camcwilliams@wisc.edu.
A. Palloni, Email: palloni@ssc.wisc.edu.
References
- 1.Liu M.-Y., Li N., Li W.A., Khan H. Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol. Res. 2017;39(6):573–580. doi: 10.1080/01616412.2017.1317904. [DOI] [PubMed] [Google Scholar]
- 2.Mocayar Marón F.J., Ferder L., Saraví F.D., Manucha W. Hypertension linked to allostatic load: from psychosocial stress to inflammation and mitochondrial dysfunction. Stress. 2018:1–13. doi: 10.1080/10253890.2018.1542683. [DOI] [PubMed] [Google Scholar]
- 3.Quax R.A., Manenschijn L., Koper J.W. Glucocorticoid sensitivity in health and disease. Nat. Rev. Endocrinol. 2013;9(11):670–686. doi: 10.1038/nrendo.2013.183. [DOI] [PubMed] [Google Scholar]
- 4.Cohen S., Janicki-Deverts D., Doyle W.J. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(16):5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparrenberger F., Cichelero F.T., Ascoli A.M. Does psychosocial stress cause hypertension? A systematic review of observational studies. J. Hum. Hypertens. 2008;23:12. doi: 10.1038/jhh.2008.74. [DOI] [PubMed] [Google Scholar]
- 6.Kang A.W., Dulin A., Nadimpalli S., Risica P.M. Stress, adherence, and blood pressure control: a baseline examination of Black women with hypertension participating in the SisterTalk II intervention. Prevent. Med. Rep. 2018;12:25–32. doi: 10.1016/j.pmedr.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobe S.W., Kiss A., Sainsbury S., Jesin M., Geerts R., Baker B. The impact of job strain and marital cohesion on ambulatory blood pressure during 1 Year: the double exposure study. Am. J. Hypertens. 2007;20(2):148–153. doi: 10.1016/j.amjhyper.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Markovitz J., Matthews K., Whooley M., Lewis C., Greenlund K. Increases in job strain are associated with incident hypertension in the CARDIA study. Ann. Behav. Med. 2004;28(1):4–9. doi: 10.1207/s15324796abm2801_2. [DOI] [PubMed] [Google Scholar]
- 9.Agyei B., Nicolaou M., Boateng L., Dijkshoorn H., van den Born B.-J., Agyemang C. Relationship between psychosocial stress and hypertension among Ghanaians in Amsterdam, The Netherlands – the GHAIA study. BMC Public Health. 2014;14(1):692. doi: 10.1186/1471-2458-14-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kivimäki M., Head J., Ferrie J.E. Hypertension is not the link between job strain and coronary heart disease in the Whitehall II study. Am. J. Hypertens. 2007;20(11):1146–1153. doi: 10.1016/j.amjhyper.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Russell E., Koren G., Rieder M., Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37(5):589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S., Janicki-Deverts D., Miller G.E. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 13.Walker B.R., Soderberg S., Lindahl B., Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J. Intern. Med. 2000;247(2):198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- 14.Staufenbiel S.M., Penninx B.W.J.H., Spijker A.T., Elzinga B.M., van Rossum E.F.C. Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology. 2013;38(8):1220–1235. doi: 10.1016/j.psyneuen.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Stalder T., Kirschbaum C. Analysis of cortisol in hair -- State of the art and future directions. Brain Behav. Immun. 2012;26(7):1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Sauve B., Koren G., Walsh G., Tokmakejian S., Van Uum S.H. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin. Investig. Med. 2007;30(5):E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 17.Nieto F.J., Peppard P., Engelman C. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Public Health. 2010;10(1):785. doi: 10.1186/1471-2458-10-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer J., Novak M., Hamel A., Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. J. Vis. Exp. JoVE. 2014;(83):50882. doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry J.D., Crawford J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2011;44(2):227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 20.Crawford J.R.. J.D. Henry, The Depression Anxiety Stress Scales (DASS): normative data and latent structure in a large non-clinical sample. Br. J. Clin. Psychol. 2003;42:111–131. doi: 10.1348/014466503321903544. [DOI] [PubMed] [Google Scholar]
- 21.Lovibond S.H., Lovibond P.F. 2nd. Ed. Psychology Foundation; Sidney: 1995. Manual for the Depression Anxiety Stress Scales. [Google Scholar]
- 22.Meng L., Zhao D., Pan Y. Validation of Omron HBP-1300 professional blood pressure monitor based on auscultation in children and adults. BMC Cardiovasc. Disord. 2016;16(1):9. doi: 10.1186/s12872-015-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrera V.M., Casas J.P., Miranda J.J. Interethnic differences in the accuracy of anthropometric indicators of obesity in screening for high risk of coronary heart disease. Int.J Obes.(Lond). 2009;33:568–576. doi: 10.1038/ijo.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Department of Health and Human Services and U.S. Department of Agriculture . eighth ed. 2015–2020. Dietary Guidelines for Americans. Available at: https://health.gov/dietaryguidelines/2015/guidelines/.2015. [Google Scholar]
- 25.Pearl J. Cambridge University Press; New York: 2000. Causal Diagrams and the Identification of Causal Effects. Causality: Models, Reasoning, and Inference; pp. 65–106. [Google Scholar]
- 26.Textor J., Hardt J., Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S., Mansournia M.A., Altman D.G. Sparse data bias: a problem hiding in plain sight. Br. Med. J. 2016;353(i1981) doi: 10.1136/bmj.i1981. [DOI] [PubMed] [Google Scholar]
- 28.Discacciati A., Orsini N., Greenland S. Approximate Bayesian logistic regression via penalized likelihood by data augmentation. Stata J. 2015;15(3):712–736. [Google Scholar]
- 29.Efron B. Nonparametric estimates of standard error: the Jackknife, the bootstrap and other methods. Biometrika. 1981;68(3):589–599. [Google Scholar]
- 30.Schnall P.L., Pieper C., Schwartz J.E. The relationship between 'job strain,' workplace diastolic blood pressure, and left ventricular mass index: results of a case-control study. JAMA. 1990;263(14):1929–1935. doi: 10.1001/jama.1990.03440140055031. [DOI] [PubMed] [Google Scholar]
- 31.Ding P., VanderWeele T.J. Sensitivity analysis without assumptions. Epidemiology. 2016;27(3):368–377. doi: 10.1097/ede.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. Converting among effect sizes. In: Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R., editors. Introduction to Meta-Analysis. John Willey & Sons; New York: 2009. [Google Scholar]
- 33.Bonett D.G. Transforming odds ratios into correlations for meta-analytic research. Am. Psychol. 2007;62(3):254–255. doi: 10.1037/0003-066X.62.3.254. [DOI] [PubMed] [Google Scholar]
- 34.Stalder T., Steudte-Schmiedgen S., Alexander N. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. doi: 10.1016/j.psyneuen.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Greenland S., Mansournia M.A. Penalization, bias reduction, and default priors in logistic and related categorical and survival regressions. Stat. Med. 2015;34(23):3133–3143. doi: 10.1002/sim.6537. [DOI] [PubMed] [Google Scholar]
- 36.Vasan R.S., Larson M.G., Leip E.P., Kannel W.B., Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358(9294):1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 37.Fauvel J.P., M'Pio I., Quelin P., Rigaud J.-P., Laville M., Ducher M. Neither perceived job stress nor individual cardiovascular reactivity predict high blood pressure. Hypertension. 2003;42(6):1112–1116. doi: 10.1161/01.HYP.0000102862.93418. [DOI] [PubMed] [Google Scholar]
- 38.Mauss I.B., Levenson R.W., McCarter L., Wilhelm F.H. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- 39.Feldman P.J., Cohen S., Lepore S.J., Matthews K.A., Kamarck T.W., Marsland A.L. Negative emotions and acute physiological responses to stress2. Ann. Behav. Med. 1999;21(3):216–222. doi: 10.1007/BF02884836. [DOI] [PubMed] [Google Scholar]
- 40.Gerber M., Endes K., Brand S. In 6- to 8-year-old children, hair cortisol is associated with body mass index and somatic complaints, but not with stress, health-related quality of life, blood pressure, retinal vessel diameters, and cardiorespiratory fitness. Psychoneuroendocrinology. 2017;76:1–10. doi: 10.1016/j.psyneuen.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Rohleder N. Variability in stress system regulatory control of inflammation: a critical factor mediating health effects of stress. Expert Rev. Endocrinol. Metab. 2011;6(2):269–278. doi: 10.1586/eem.11.8. [DOI] [PubMed] [Google Scholar]
- 42.Rohleder N., Marin T.J., Ma R., Miller G.E. Biologic cost of caring for a cancer patient: dysregulation of pro- and anti-inflammatory signaling pathways. J. Clin. Oncol. 2009;27(18):2909–2915. doi: 10.1200/JCO.2008.18.7435. [DOI] [PubMed] [Google Scholar]
- 43.Engström G., Hedblad B., Stavenow L., Lind P., Janzon L., Lindgärde F. Inflammation-sensitive plasma proteins are associated with future Weight gain. Diabetes. 2003;52(8):2097–2101. doi: 10.2337/diabetes.52.8.2097. [DOI] [PubMed] [Google Scholar]
- 44.Hamer M., Stamatakis E. Inflammation as an intermediate pathway in the association between psychosocial stress and obesity. Physiol. Behav. 2008;94(4):536–539. doi: 10.1016/j.physbeh.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Bautista L.E., Vera L.M., Arenas I.A., Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J. Hum. Hypertens. 2005;19(2):149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 46.Whitworth J.A., Schyvens C.G., Zhang Y., Andrews M.C., Mangos G.J., Kelly J.J. The nitric oxide system in glucocorticoid-induced hypertension. J. Hypertens. 2002;20(6):1035–1043. doi: 10.1097/00004872-200206000-00003. [DOI] [PubMed] [Google Scholar]